Abstract
Purpose: To determine the relationship between uric acid (UA) and nutritional and antioxidant status in hemodialysis (HD) patients, given that hyperuricemia could be an indicator of good nutritional status possibly because of the antioxidant properties of UA. Methods: Cross-sectional study with 93 patients on HD. Hyperuricemia was considered as UA ≥6.0 mg/dL in females and ≥7.0 mg/dL in males. Nutritional variables were registered. Blood samples were taken before the dialysis session to determine oxidative damage as plasma malondialdehyde (MDA) content, and antioxidant capacity measuring 2,2-diphenyl-piclrylhidrazil radical (DPPH●) scavenging activity and oxygen radical absorbance capacity (ORAC) value. Results: Patients with hyperuricemia had higher creatinine (11.9 vs. 10.5 mg/dL; p = 0.004), potassium (5.5 vs. 5.0 mg/dL; p = 0.014) levels; phase angle (5.8 vs. 4.9; p = 0.005), protein consumption (normalized protein nitrogen appearance, nPNA, 1.03 vs. 0.83; p = 0.013) than normouricemic patients. DPPH● scavenging activity was higher in hyperuricemic subjects (1.139 vs. 1.049 mM Trolox equivalents; p = 0.007); likewise, hyperuricemic subjects had less oxidant damage measured by MDA (10.6 vs. 12.7 nmol/mL; p = 0.020). Subjects with normouricemia were at higher risk of having a reactance to height (Xc/H) ratio less than 35 (OR 2.79; 95% CI, 1.1–7.017, p = 0.028); nPNA < 1.0 (OR 3.78; 95% CI, 1.4–10.2, p = 0.007), diagnosis of cachexia (OR 2.95; 95% CI, 1156–7.518, p = 0.021), potassium levels <5 (OR 2.97; 95% CI, 1.136–7.772, p = 0.023) and PA < 5.5° (OR 3.38; 95% CI, 1.309–8.749, p = 0.012.) Conclusions: Patients with hyperuricemia had higher antioxidant capacity and better nutritional status. Purines and protein restrictions in HD patients with hyperuricemia need to be reviewed individually for each patient. More studies are needed to stablish a cut point of UA levels in renal population.
Keywords: uric acid; nutritional status; renal dialysis; oxidative stress; malondialdehyde; oxygen radical absorbance capacity; 2,2-diphenyl-1-picrilhydrazyl
1. Introduction
Chronic kidney disease (CKD) is a public health problem given its epidemic nature, the complications it causes and the lack of human and material resources available for its treatment [1,2,3]. End stage renal disease (ESRD) patients are at high risk of protein energy wasting (PEW) syndrome (a pathological entity characterized by low protein and energetic deposits and systemic inflammation), and their nutritional status should be thoroughly assessed to diagnose and treat PEW in time [4,5]. The diet of renal patients on hemodialysis (HD) is restricted in different nutrients, such as potassium (K), phosphorus (P) and sodium (Na) [6,7], in addition to purines when hyperuricemia is detected [8,9]. These dietary restrictions, together with other dietary factors and HD, may increase the risk and/or aggravate PEW [10]. Uric acid (UA) is the final product of purine metabolism [11]. Hyperuricemia, defined as UA levels ≥6.0 and ≥7.0 mg/dL in women and men, respectively [12], has been related with cardiovascular (CV) events; for example, in the URRAH Project cohort, the level of uricemia above which the independent risk of CV disease may increase are 4.7 and 5.6 mg/dL for total and CV mortality, respectively [13]. Furthermore, hyperuricemia has been associated with the incidence of CKD in healthy subjects [14]; one of the reasons has been UA role as an oxidizing molecule [15]. However, there are controversial data about the latter in the HD population, in whom it also has a role as an antioxidant molecule (due role of UA) [16,17]. Recent studies [18,19,20,21] have pointed to UA as a marker of good nutritional status in subjects on HD, as it has been associated with adequate values of body mass index (BMI), handgrip strength and protein intake. However, these studies had different nutritional objectives and did not evaluate the nutritional status of patients or the presence of PEW with an adequate number of markers, as suggested by international institutions, nor did they attempt to measure oxidant damage to provide evidence of the dual role of UA in this population [22,23,24]. The objective of the present study was to establish an association between UA concentration and nutritional and antioxidant status.
2. Materials and Methods
We studied patients attending two HD units in our city. Inclusion criteria were as follows: At least three months on chronic HD; without amputations or implants or comorbidities; and without difficulties in mobility or behaviors that affected UA concentrations. Patients with a history of alcohol or drug use, liver cirrhosis, comorbidities and/or treatments (neoplasms, active autoimmune and infectious diseases or chemotherapy) that affected UA levels were excluded. To calculate sample size, we used statistical tables for odds ratio (OR) estimations according to the data of the Lee et al. study [23].
The institutional ethics committee authorized the protocol with the number 1968. All patients signed an informed consent form. Hyperuricemia was defined as serum UA ≥6.0 mg/dL in women and ≥7.0 mg/dL in men [12,25]. Dialysis efficacy was measured by Kt/V and urea reduction rate (URR) indicators. From the patients′ medical records, we obtained data about the dialysis characteristics and normalized protein nitrogen appearance (nPNA). We also conducted predialysis biochemical measurements of phosphorus, potassium, transferrin, creatinine and blood urea nitrogen (BUN).
2.1. Primary Outcomes: Antioxidant Status.
Before the beginning of the HD session, blood samples were taken (with at least 4 h fasting), directly using vascular access to analyze the markers of antioxidant capacity (2,2-diphenyl-piclrylhidrazil radical (DPPH●)) scavenging activity (a single electron transfer (SET)-based assay) and oxygen radical absorbance capacity (ORAC) value (a hydrogen atom transfer (HAT)-based assay) as well as the content of oxidative damage marker malondialdehyde (MDA). The samples were collected in tubes with the anticoagulant ethylenediamine tetraacetic acid (EDTA; 7.2 mg), centrifuged for 15 min at 175× g, placed in 1.5 mL centrifuge tubes and stored for approximately 10 days at −40 °C before the analysis. The DPPH● scavenging assay was performed according to the colorimetric method described by Koren et al. [26] and results were expressed as mM Trolox equivalents (TE): Fluorometric ORAC assay was performed according to Huang et al. [27] and data were expressed as uM TE. Finally, the spectrophotometric assay of MDA was performed according to the procedure described by Gérard-Monnier et al. [28] and the measurements were expressed as nmol MDA/mL. All determinations were obtained by using Synergy HT multi-mode microplate reader (BioTek Instruments Inc., Winooski, VT, USA).
2.2. Secondary Outcomes: Nutritional Status
After HD, nutritionists trained in anthropometric techniques assessed the nutritional status. The BMI was estimated based on the weight and height of each patient (weight scale SECA®, Germany). A Lange caliper (Beta Technologies, Santa Cruz, CA, USA) was used to measure skin folds, and a Takei dynamometer was used to measure handgrip strength by asking the patients to squeeze the dynamometer three times using the arm opposite to the vascular access (fistula or catheter) and recording the highest value. To diagnose PEW, we used the malnutrition inflammation score (MIS ≥6 points) and the criteria from the International Society of Renal Nutrition and Metabolism (ISRNM, biochemical criteria; low body weight, reduced total body fat, or weight loss; a decrease in muscle mass; and low protein or energy intakes) [10,29]. To evaluate the diet, the patients were asked to record the food they consumed on three days: One day without hemodialysis, one day with hemodialysis and one day of the weekend (the analysis was performed using the NutriKcal VO software). Bioelectrical impedance was measured using Body-Stat Quadscan 4000 and RJL equipment; we obtained bodily dielectric properties as follows: Resistance normalized per height (R/H), reactance normalized per height (Xc/H) and phase angle (PA). For the diagnosis of cachexia [30], we carried out a bioelectrical impedance vector analysis with the software BIVA-confidence and BIVA-tolerance by Piccoli et al. [31,32], using the reference values of the Mexican population [33].
2.3. Statistical Analysis
The data are reported as means ± standard deviations (SD), as medians and interquartile ranges (IQR), or as frequencies and percentages, according to the Kolmogorov–Smirnov and Shapiro–Wilk tests. Student′s t-test was used to compare the variables of independent samples. The Mann–Whitney U test and the chi-square test were used as necessary. The bivariate analysis used to estimate risks (with 95% confidence intervals) included a single-step logistic regression. A value of p < 0.05 was considered statistically significant. The statistical program SPSS v.21 (IBM Corporation, Chicago, United States of America) was used to analyze the data.
3. Results
We studied 93 patients of two HD units; 54% were men, and the median age of all patients was 40 (29–52) years. The prevalence of hyperuricemia was 72% (95% CI: 67–77%) and that of PEW, according to MIS, was 35% (95% CI: 30–40%). In the general population the time of dialysis was 215.3 ± 17.3 min, and the dialysis vintage was 39 (21–79) months; the medium-flux filter (F80) was the most common filter used for the HD session (69%) and dialysis efficacy measured by Kt/V was 1.46 (1.25–1.77) and URR was 71% (65–79). The majority of the patients had a dialysis session three days a week; an arteriovenous fistula (AVF) was the predominant type of vascular access, and the efficacy of hemodialysis, as measured by Kt/V and URR, was adequate in all patients (Table 1).
Table 1.
General characteristics in normo and hyperuricemic patients on hemodialysis.
| All Patients (n = 93) | Normouricemic Patients (n = 26) | Hyperuricemic Patients (n = 67) | p | |
|---|---|---|---|---|
| Age (years) | 40 (29–52) | 47 (31–61) | 39 (29–47) | 0.026 |
| Sex (M%) | 50 (54) | 18 (69.2) | 32 (47.8) | 0.062 |
| Comorbidities | ||||
| Diabetes mellitus n (%) | 24 (25.8) | 8 (30.8) | 16 (23.9) | 0.496 |
| Hypertension n (%) | 57 (61.3) | 18 (69.2) | 39 (58.2) | 0.355 |
| Allopurinol Consumption | ||||
| Yes n (%) | 8 (8.6) | 1 (3.8) | 7 (10.4) | 0.308 |
| Dialysis session time (min) | 215.3 ± 17.3 | 211.7 ± 15.5 | 216.7 ± 17.9 | 0.195 |
| Ultra-filtered (mL) | 2000 (1353–2500) | 2350 (1950–2564) | 2000 (1200–2300) | 0.051 |
| Dialysis vintage (months) | 39 (21–79) | 47 (24–85) | 38 (20–72) | 0.300 |
| Sessions/Week (days) | ||||
| 3 n (%) | 85 (91.4) | 25 (96.2) | 61 (91) | 0.663 |
| 2 n (%) | 7 (7.5) | 1 (3.8) | 5 (7.5) | |
| 1 n (%) | 1 (1.1) | 0 | 1 (1.5) | |
| Vascular Access | ||||
| AF n (%) | 57 (61.3) | 16 (61.5) | 41 (61.2) | 1.0 |
| Catheter n (%) | 36 (38.7) | 10 (38.5) | 26 (38.8) | 1.0 |
| Right arm n (%) | 51 (54.8) | 14 (53.8) | 37 (55.2) | 1.0 |
| Filter | ||||
| F18 n (%) | 8 (8.6) | 0 | 8 (11.9) | 0.092 |
| F80 n (%) | 69 (74.2) | 23 (88.5) | 46 (68.7) | |
| F180 n (%) | 16 (17.2) | 3 (11.5) | 13 (19.4) | |
| Dialysis Efficacy | ||||
| Kt/V | 1.46 (1.25–1.77) | 1.43 (1.18–1.67) | 1.46 (1.25–1.79) | 0.436 |
| URR (%) | 71 (65–79) | 70 (65–74) | 72 (66–81) | 0.126 |
ESRD—end stage renal disease; AF—arteriovenous fistula; F18—filter number 18; F80—filter number 80; F180—filter number 180; URR —urea reduction rate; min—minutes. Continuous variables are expressed as mean ± SD or median with interquartile range in case of non-normally distributed data, and categorical variables are expressed as a percentage. p < 0.05 was considered statistically significant.
3.1. Primary Outcomes: Antioxidant Status
The total antioxidant capacity, as measured by DPPH● scavenging, was higher in patients with hyperuricemia (1.14 vs. 1.05 EqMm of Trolox), who also showed lower oxidative damage, as measured by MDA (10.6 vs. 12.7 nmol/mL) (Table 2).
Table 2.
Plasma antioxidant capacity and oxidant damage in normo and hyperuricemic patients on hemodialysis.
| Normouricemic Patients (n = 26) | Hyperuricemic Patients (n = 67) | p | |
|---|---|---|---|
| DPPH● scavenging (mM TE) | 1.0495 ± 0.1226 | 1.1385 ± 0.1681 | 0.007 |
| ORAC (μM TE) | 2337.8 ± 370.7 | 2441.7 ± 313.7 | 0.213 |
| MDA (nmol/mL) | 12.7 ± 4.6 | 0.6 ± 13.5 | 0.020 |
DPPH●—2,2-diphenyl-1-picrylhidrazyl radical; ORAC—oxygen radical absorbance capacity; MDA—malondialdehyde; TE—Trolox equivalents. DPPH● scavenging and ORAC assays were made by duplicate and MDA determination was made once. Results are expressed as mean ± SD. p < 0.05 was considered statistically significant.
3.2. Secondary Outcomes
The values of the biochemical parameters measured in the study are shown in Table 3. Patients with hyperuricemia had higher concentrations of creatinine (11.9 vs. 10.5 mg/dL; p = 0.004), predialysis BUN (70 vs. 53 mg/dL: p < 0.001) and potassium (5.5 vs. 5.0 mg/dL; p = 0.014). In general, all patients had adequate albumin and transferrin levels, adequate nutritional status (MIS score) and adequate handgrip strength values.
Table 3.
Biochemical and nutritional parameters in normo and hyperuricemic patients on hemodialysis.
| Normouricemic Patients (n = 26) | Hyperuricemic Patients (n = 67) | p | |
|---|---|---|---|
| Biochemical | |||
| Creatinine (mg/dL) | 10.5 (8.1–11.8) | 11.9 (9.6–14.5) | 0.004 |
| Albumin (g/dL) | 3.8 ± 0.3 | 3.78 ± 0.4 | 0.771 |
| BUN (mg/dL) | 53 (41–60) | 70 (54–90) | <0.001 |
| Potassium (mg/dL) | 5.0 ± 0.9 | 5.5 ± 0.7 | 0.014 |
| Phosphorus (mg/dL) | 5.0 (3.9–6.4) | 5.1 (4.0–6.6) | 0.942 |
| Glucose (mg/dL) | 85 (72–109) | 83 (75.5–103) | 0.834 |
| Hemoglobin (mg/dL) | 9.3 ± 2.01 | 9.9 ± 2.3 | 0.218 |
| Hematocrit (%) | 30.6 (25–34) | 31.8 (26–35.6) | 0.475 |
| Transferrin (mg/dL) | 213.4 ± 45.7 | 196.7 ± 54.1 | 0.148 |
| Nutritional scores | |||
| MIS score | 4.5 (3–6) | 5 (3–6) | 0.785 |
| Normal nutrition state/Mild malnutrition n (%) | 17 (65.4) | 47 (70.1) | 0.803 |
| Moderate malnutrition n (%) | 7 (26.9) | 17 (25.4) | |
| Severe malnutrition n (%) | 2 (7.7) | 3 (4.5) | |
| PEW (MIS) n (%) | 9 (34.6) | 24 (35.8) | 0.656 |
| PEW (ISRNM) n (%) | 9 (34.6) | 17 (25.4) | 0.373 |
| Muscular functionality | |||
| Handgrip strength (kg) | 25.3 ± 5.9 | 26.7 ± 8.5 | 0.366 |
BUN—blood urea nitrogen; MIS—malnutrition inflammation score; PEW—protein energy wasting; ISRNM—International Society of Renal Nutrition and Metabolism criteria. Continuous variables are expressed as mean ± SD or median with interquartile range in case of non-normally distributed data, and categorical variables are expressed as a percentage. p < 0.05 was considered statistically significant.
The anthropometric markers of body composition indicated that subjects had an adequate BMI and a good percentage of fat and lean mass. Regarding the electrical properties of the body, patients with hyperuricemia had a higher ratio of reactance to height (Xc/H) (37.6 vs. 30.2; p = 0.004) and PA values (PA, 5.8 vs. 4.9°, p = 0.005), as well as a lower prevalence of cachexia (54%; 95% CI = 49–59%). Likewise, the nutritional intake analysis showed that patients with hyperuricemia had a higher protein intake, based on the nPNA values (1.03 vs. 0.83; p < 0.05) and the animal protein intake (37.1 vs. 32.1 g/d; p = 0.017) (Table 4).
Table 4.
Body composition and Dietary intake in normo and hyperuricemic patients on hemodialysis.
| Normouricemic Patients (n = 26) | Hyperuricemic Patients (n = 67) | p | |
|---|---|---|---|
| Anthropometrics | |||
| Dry weight (kg) | 62.2 (56.4–70.9) | 61.5 (53.9–71.6) | 0.784 |
| Height (m) | 1.60 ± 0.05 | 1.59 ± 0.09 | 0.451 |
| BMI (kg/m2) | 25 (21.3–27.4) | 24 (21.3–28.2) | 0.983 |
| AC (cm) | 27.7 (25.7–29.6) | 27.4 (25.6–31.5) | 0.625 |
| TSF (mm) | 11.5 (10–17) | 14 (11–18) | 0.192 |
| FM (%) | 27.2 (20.5–31.6) | 28.3 (20.9–32.9) | 0.602 |
| FFM (%) | 72.8 (68.4–79.5) | 71.7 (67.1–79.2) | 0.602 |
| BIA | |||
| R/H (Ω/m) | 323 (300.6–380) | 360.4 (317.6–412.2) | 0.139 |
| Xc/H (Ω/m) | 30.2 ± 10.4 | 37.6 ± 10.9 | 0.004 |
| PA (°) | 4.9 ± 1.2 | 5.8 ± 1.4 | 0.005 |
| Cachexia diagnosis n (%) | 14 (53.8) | 19 (28.4) | 0.021 |
| Dietetic | |||
| Energy (kcals/d) | 1746.5 (1544–2055) | 1746 (1293–2389) | 0.869 |
| Energy (kcals/kgAW/d) | 28.5 (22.4–39.1) | 27.4 (20.5–43.9) | 0.777 |
| Proteins (g/d) | 64.1 (43.7–82.5) | 65.3 (51.6–88.9) | 0.340 |
| Animal protein (g/d) | 32.1 (20.3–42.8) | 37.1 (30.8–54.1) | 0.017 |
| Vegetable protein (g/d) | 21.8 (19.6–28.5) | 21.8 (17.4–31.4) | 0.911 |
| Proteins (g/kgAW/d) | 1.08 (0.68–1.53) | 1.1 (0.7–1.6) | 0.436 |
| nPNA | 0.83 (0.75–1.01) | 1.03 (0.85–1.19) | 0.013 |
| Fructose (mg/d) | 23.5 (16.1–40.8) | 21.3 (14.1–33.9) | 0.340 |
BMI—body mass index; AC—arm circumference; TSF—triceps skinfold; FM—fat mass; FFM—fat-free mass; BIA—bioelectrical impedance analysis; R/H—resistance over height: Xc/H—reactance over height; PA—phase angle; kcals/d—kilocalories/day; kcals/AW/d—kilocalories/kilograms of actual weight/day; g/d—grams/day; g/kgAW/d—grams/kilograms of actual weight/day;; nPNA—normalized protein nitrogen appearance. Continuous variables are expressed as mean ± SD or median with interquartile range in case of non-normally distributed data, and categorical variables are expressed as a percentage. p < 0.05 was considered statistically significant.
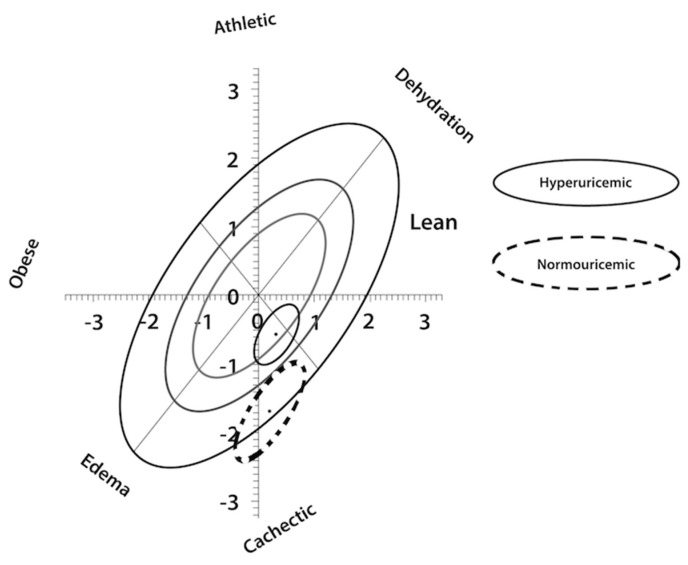
Regarding body composition and cachexia diagnosis by bioelectrical impedance vector analysis (BIVA), the subjects with hyperuricemia had better nutritional and hydration status than patients with normouricemia and diagnosis of cachexia and volume overload (Figure 1).
Figure 1.
Z Score of normouricemic and hyperuricemic patients by Bioelectrical Impedance Vector Analysis (BIVA). Continuous line represent hyperuricemic subjects and dotted line represent normouricemic subjects.
To evaluate the association between UA, nutritional status and antioxidant status, we performed logistic regressions analysis to determine which indicator of nutritional status was better explained by hyperuricemia. In the model adjusted for sex and URR, albumin ≥3.5 g/dL was independently associated with UA levels; p < 0.05; R2 = 0.541 (90.3% of the subjects). The model included other nutritional variables, such as kilocalories consumed per day, total kilocalories and grams of protein, kilocalories and grams of protein per kilogram of ideal weight, and handgrip strength measurements (Table 5).
Table 5.
Association between uric acid and nutritional status for albumin ≥3.5 g/dL in hemodialysis patients.
| Exp (B) | B | p | CI 95% | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Hyperuricemia | 11.792 | 2.467 | 0.032 | 1.24 | 112.1 |
| Cachexia diagnosis | 6.892 | 1.930 | 0.096 | 0.71 | 66.77 |
| Kcals/d | 1.045 | 0.044 | 0.001 | 1.02 | 1.074 |
| Kcals/kgIW/d | 0.084 | −2.476 | 0.001 | 0.02 | 0.39 |
| Proteins (g/d) | 0.258 | −1.355 | 0.001 | 0.12 | 0.58 |
| Proteins (g/kgIW/d) | 34.14 | 74.4 | 0.001 | 17.7 | 59.04 |
| Handgrip strength (kg) | 1.275 | 0.243 | 0.020 | 1.04 | 1.56 |
Kcals/d—kilocalories/day; Kcals/kg of ideal weight/day; g/d—grams/day; g/kg of ideal weight/day. CI—confidence interval. Model adjusted by sex and URR (urea reduction rate). p < 0.05 was considered statistically significant.
A risk analysis was carried out to identify variables that could be indicators of nutritional wasting along with hyperuricemia. The analysis showed that subjects with normouricemia were at higher risk of having a reactance to height (Xc/H) ratio less than 35 (OR 2.79; 95% CI, 1.1–7.017, p = 0.028); nPNA < 1.0 (OR 3.78; 95% CI, 1.4–10.2, p = 0.007), diagnosis of cachexia (OR 2.95; 95% CI, 1156–7.518, p = 0.021), potassium levels <5 (OR 2.97; 95% CI, 1.136–7.772, p = 0.023) and PA < 5.5° (OR 3.38; 95% CI, 1.309–8.749, p = 0.012 (Table 6).
Table 6.
Odds ratio for normouricemic patients to present malnutrition.
| OR | p | CI 95% | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Xc/H < 35 | 2.79 | 0.028 | 1.1 | 7.071 |
| nPNA < 1.0 | 3.78 | 0.007 | 1.4 | 10.208 |
| Cachexia (yes) | 2.95 | 0.021 | 1.156 | 7.518 |
| Cr < 10 mg/dL | 2.33 | 0.074 | 0.910 | 5.982 |
| K < 5 mg/dL | 2.97 | 0.023 | 1.136 | 7.772 |
| PA < 5.5° | 3.38 | 0.012 | 1.309 | 8.749 |
| PEW (MIS) | 0.95 | 0.913 | 0.367 | 2.452 |
| Albumin ≥ 3.5 g/dL | 1.2 | 0.363 | 0.815 | 2.197 |
OR—odds ratio; CI—confidence interval; Xc/H—reactance/height; nPNA—normalized protein nitrogen appearance; Cr—creatinine; K—potassium; PA—phase angle; PEW (MIS) —protein energy wasting diagnosed by malnutrition inflammation score; p < 0.05 was considered statistically significant.
4. Discussion
In numerous aspects, serum UA appears to be a two-faced marker in health and disease [34]. In the early stages of renal disease, UA is a risk factor marker for mortality, whereas during peritoneal dialysis treatment, it is a prognostic factor [35]. However, its role in hemodialysis patients is not very clear; similar than a reverse epidemiology phenomenon (paradoxical relationships between nutritional markers such as increased weight or high serum cholesterol levels that are protective in dialysis patients), could be associated with better satisfactory outcomes [36,37]. Regarding general mortality, in a study of 7333 HD patients, hyperuricemia correlated with lower mortality in the multivariate models analysis. Furthermore, UA concentrations correlated positively with nutritional markers such as BMI, nitrogen protein catabolic rate (nPCR) and phosphorus levels [38]. The latter finding has been attributed to a higher antioxidant capacity in patients with hyperuricemia, since it has been observed that UA protects cell membranes against lipid peroxidation and act like a free radical scavenger [34]. In children, UA has been proposed as a diagnostic tool for CKD progression, since its levels increased as do the antioxidant capacity, measured in saliva by ferric ion reducing antioxidant (FRAP) assay, according to the severity of CKD [39]. However, it is worth mentioning that the interplay between hyperuricemia, oxidative damage and nutritional status has not yet been explored.
To our best knowledge, this is the first study that explores the association between UA, nutritional status and antioxidant capacity and oxidative damage in HD patients. Since antioxidants inactivate free radicals through two mechanisms—SET (single electron transfer) or HAT (hydrogen atom transfer)—the antioxidant capacity was evaluated by the DPPH● scavenging and ORAC assays. ORAC is a HAT-based assay and DPPH radical scavenging is a SET-based assay. We are tempted to speculate that the differences in both assays may explain the fact that only the DPPH● scavenging ability was significant between normouricemic and hyperuricemic patients. Patients with hyperuricemia had higher antioxidant capacity, as measured by DPPH● scavenging activity, and less oxidative damage, as measured by MDA. Uric acid plays a role as an antioxidant or oxidant in different clinical scenarios. Although fruits and vegetables have abundant amounts of dietary antioxidants, they cannot adequately represent the overall ability and interactions of antioxidants in the whole diet. For example, in our study we analyzed the consumption of food groups, and the consumption of fruits and vegetables that could have a greater association with antioxidant capacity was only 1.3 servings of fruits (0.7 to 1.9 in hyperuricemic, and 0.8 to 2.2 in normouricemic) and 2 servings of vegetables (1.3 to 3.4 in hyperuricemic, and 1.2 to 2.4 in normouricemic). Thus, the dietary concept which takes the whole dietary antioxidants into account has become more prominent. Dietary total antioxidant capacity (DTAC) is a novel indicator of diet quality [40], which is used to estimate the cumulative power of antioxidants in the whole diet [41]. Recently, DTAC is described as an effective tool for determining the health outcomes in populations [42,43]. This new approach could serve for future lines of research to understand the behavior and association of uric acid, antioxidant status and diet in these patients [44].
In respect to our secondary outcomes, Beberashvili et al. [18,19] studied geriatric patients to find an association between UA and nutritional status. In contrast, we studied a relatively young population (median age was 40 (29–52) years) and found similar results; in our country, a dialysis registry is lacking but the mean age of people with CKD is 44.8 ± 17.2 years old [45,46]. In our population, the main etiology of CKD was unknown, contrary to that described in the studies by Abbas et al. [47] and Hsu et al. [48], in which the main causes were diabetic nephropathy and arterial hypertension. We found a prevalence of PEW (score MIS ≥6) of 35%, a low prevalence compared to those usually found in HD patients worldwide, which ranges from 50% [49] according to MIS, to 52.5% [50] and 70% [51] according to ISRNM criteria.
One of the main findings of our study was the existence of clinical and statistically significant differences between hyperuricemia and normouricemia, in the variables of nutritional status according to the logistic regression model between UA and albumin, which appropriately classified 90.3% of the study subjects. No independence was found between UA and other nutritional variables, likely because the nutritional status is a construct made up of different variables (the logistic regression data are not shown).
All patients had similar dialytic characteristics, but patients with hyperuricemia had higher creatinine, predialysis BUN and potassium levels. In patients with normouricemia, the risk of having potassium levels lower than 5 mg/dL was 1.97 times higher than in patients with hyperuricemia. Regarding the biochemical variables of nutritional status, patients with normouricemia had higher concentrations of creatinine and potassium. In the study by Beberashvili et al. [18], creatinine levels were higher in the third tertile than in the first (8.43 ± 2.2 vs. 6.51 ± 2 mg/dL); the same authors found that albumin levels were also higher in the last tertile (3.9 ± 0.3 vs. 3.7 ± 0.4, p < 0.001), although no significant differences were found in our study.
Some variables (biochemical parameters such as phosphorus, albumin, hemoglobin, transferrin levels and anthropometric parameters such as BMI, arm circumference, triceps skinfold and percentage of body fat) were similar between both study groups, which could explain why no differences were observed in the final score or in the diagnosis of PEW between patients. In the cohort of dialysis patients studied by Bae et al. [22], UA levels <5.5. mg/dL were associated with overall mortality (adjusted HR of 1.720; 95% CI, 1007–2937, p = 0.047); there was also a positive correlation between UA levels <5.5 mg/dL and nutritional factors such as BMI (regression coefficient [B] = 0.042; p = 0.091), Subjective Global Assessment (SGA) (B = −0.062, p = 0.014), serum phosphorus (B = 0.107, p < 0.001) and albumin (B = 0.127, p < 0.001). Among patients with low levels of UA (<5.5 mg/dL), there was a higher proportion of malnourished subjects, according to SGA score, lower BMI and lower concentrations of total proteins, phosphorus and serum albumin. In the study by Beberashvili et al. [18], mean MIS score was lower in the hyperuricemia group (5.06 ± 3.1 mg/dL) than in the normouricemia group (7.44 ± 3.5 mg/dL) (p < 0.001). In contrast, in our study we did not find differences between the scores of the groups classified according to UA levels. Although hyperuricemia was not statistically significant in the logistic regression models used in our study for some markers of nutritional status, including it in the model did not decrease the value of R2 or the percentage of patients it was able to accurately classify (the model explained 48.6% of the PEW and accurately classified 80.6% of the population).
In our study, handgrip strength values were very similar between both groups of subjects, which could be associated with the fairly similar prevalence of PEW among the population. Other studies found that low muscle strength, as measured by dynamometry, was associated with PEW and was considered a nutritional marker associated with muscle loss [52]. Some studies have even found a positive correlation (r = 0.26; p < 0.001) between handgrip strength and UA levels [18].
In contrast, the analysis of body composition by BIVA revealed differences in dielectric properties; patients with hyperuricemia had higher values of R/H and Xc/H than patients with normouricemia, indicating better body cell mass and lower volume overload. The risk that patients with normouricemia would have a ratio of Xc/H lower than 35 Ω/m (worse body cell mass) was 1.8 times greater than that for patients with hyperuricemia. Few studies have analyzed the BIVA values of patients on HD and their relationship with UA; most have analyzed only total body water, extracellular water, fat mass and fat-free mass values. One of the indicators of BIVA with the highest prognostic value in patients on HD is the PA, which has been demonstrated to have an inverse relationship with mortality (the greater the PA is, the lower the risk of mortality); for example, studying a cohort of 91 patients on HD, Beberashvili et al. [53] reported that for each 1° increase in PA, the crude and adjusted mortality HR were 0.6 (95% CI 0.54–0.71) and 0.61 (95% CI 0.53–0.71), respectively. In the present study, PA values were higher in patients with hyperuricemia than in those with normouricemia.
Furthermore, cachexia (the most severe form of malnutrition in dialysis patients) was lower in patients with hyperuricemia. In our study the risk that patients with normouricemia would have a PA lower than 5.5° was 2.28 times higher than that for patients with hyperuricemia; the risk of having cachexia, diagnosed by bioelectrical impedance vector analysis, according to Piccoli et al. [32] was 1.9 times higher. Figure 1 shows that the ellipse of patients with normouricemia is within the range of cachexia and volume overload, while patients with hyperuricemia had better nutritional and hydration status. There are no studies that use impedance vectors as variables of nutritional and hydration status and analyze their association with UA levels.
In addition to the changes induced by CKD in the metabolism of UA, dietary and pharmacological interventions, as well as the nature and extent of dialysis treatments, greatly modify the concentration of UA in the population with CKD. The intake of kilocalories consumed per day was the same in both groups, both in kilocalories/kg of actual and ideal weight. For proteins, contrary to what the dietary restrictions in the intake of purines for patients with hyperuricemia have led us to expect, patients had a higher protein intake as measured by grams of protein/kg of ideal weight and intake of protein of animal origin.
It has been widely described in the literature that the caloric and protein intake of HD patients is below the recommendations of the KDIGO guidelines (30 to 35 kcals/kg/d and 1.2 g/d of calories and proteins, respectively). The reported average caloric intake has ranged from 23.9 ± 7.01 to 24.8 ± 7.5 kcals/d, while the average protein intake has ranged from 0.98 ± 0.30 to 1.1 ± 0.4 g/d. Luis et al. [6] reported that only 11% and 41% of patients on HD meet the caloric and protein intake requirements, respectively. The nPNA, a more specific indicator of protein intake, was higher in patients with hyperuricemia. Beberashvili et al. [18] obtained similar results: 1.10 ± 0.27 vs. 1.00 ± 0.20 in the third and first tertile of UA of their study population, respectively. In our study, the risk that patients with normouricemia would have a nPNA below 1.0 was 2.8 times higher than for patients with hyperuricemia. In contrast with what is true for the general population, low UA levels (but not high UA) are associated with higher mortality from all causes for HD patients, especially when they have a low protein intake. In fact, protein-rich diets tend to contain large amounts of purines, so that the higher concentrations of UA could indicate a better nutritional status in the population with ESRD [20].
In general, the diet of patients on HD is very low in fruits, vegetables, legumes and dairy products (foods rich in antioxidants in the first two and in proteins in the latter one). In our study, this was true for both groups of patients. Fructose levels, a variable that has been implicated in the increase in UA levels, were not different between groups (data not shown), likely because patients tend to underreport the intake of sugar and high-fructose foods in the food record.
The most important strength of our study is that it includes measurements of antioxidant capacity and oxidative damage. Furthermore, it is possible that a low concentration of UA results in a lower total antioxidant capacity in patients on dialysis, although further research is needed to clarify these mechanisms.
Cohort studies have described a phenomenon of reverse epidemiology on HD patients, where high concentrations of UA are a protective factor against mortality and morbidity. Those studies have separately measured some variables of nutritional status and concluded that high UA levels are an indicator of better nutritional status, as evidenced by the positive association between UA levels and nutritional status variables. Furthermore, low concentrations of UA are considered a consequence of poor protein intake and the presence of malnutrition. Hsu et al. [48] found a positive correlation (r = 0.518, p < 0.001) between UA and predialysis BUN (indicator of protein intake), a similar result to that obtained in the present study, where the corresponding values were r = 0.479 and p < 0.001. This phenomenon of reverse epidemiology has been used to explain an improved antioxidant capacity of UA in vivo and in vitro, where it has contributed up to 60% of the elimination of free radicals in blood serum.
The results obtained in this study should be supported by measuring other markers of oxidative damage (more specific for proteins and DNA) to achieve a better understanding of the underlying mechanisms that promote antioxidant capacity in subjects with hyperuricemia on HD. This would also support the associations and correlations that have been found between hyperuricemia, some parameters of nutritional status and mortality, which have suggested the existence of a phenomenon of reverse epidemiology in HD patients with respect to the role of UA (similar to creatinine, potassium and BMI values in dialysis patients) [37]. Rethinking UA as a laboratory marker of nutritional status would require changing the dietary guidelines for subjects with hyperuricemia in order to prevent PEW. Establishing the existence of a phenomenon of reverse epidemiology with respect to UA levels in HD patients would require further studies with larger samples.
Limitations: We did not evaluate dietary supplements and drugs consumption except for allopurinol so we cannot analyze the effect of drugs (such as diuretics, angiotensin-converting enzyme inhibitors, statins and fibrates) on UA; however, we did a sub-analysis without including subjects with an allopurinol prescription and the results were similar. Furthermore, we did not register inflammatory markers such as white blood cells and C reactive protein. Finally, it is important to mention that all hemodialysis patients in this study had nutritional advice (AEC trained both dietitians); however, to our best knowledge, the same is not true for all hemodialysis units in our country and could be considered a bias factor in the study.
This research exposes how UA can be an indicator of the nutritional status associated with a greater antioxidant capacity. UA could be used as a marker of nutritional status, together with serum albumin, in environments where there are not enough nutritional resources to identify patients with nutritional risk in an inexpensive, easy and simple way.
5. Conclusions
In summary, patients with hyperuricemia had higher antioxidant capacity and less oxidative damage, and they also had better nutritional status in general, mainly according to impedance vectors. Furthermore, a positive association was found between hyperuricemia and albumin levels >3.5 g/dL. More studies are needed to stablish a cut point of UA levels in the renal population, similar to the URRAH project [13] in the hypertensive subject, and to confirm and clarify whether elevated uric acid could be an indicator of increased antioxidant capacity or better nutritional status in this population.
Acknowledgments
We want thank to Cinthya Islas-Olvera for her support in carrying out the nutritional status measurement in the Centro de Atención Renal hemodialysis unit.
Author Contributions
Conceptualization, E.D.-Z., A.L.L.-S., and A.E.-C.; data curation, E.D.-Z. and C.C.-R.; methodology, E.D.-Z., J.P.-C., A.L.L.-S., O.N.M.-C., C.C.-R., F.B.-H., and A.E.-C.; software, O.N.M.-C. and C.C.-R.; formal analysis, E.D.-Z., J.P.-C., A.L.L.-S., O.N.M.-C., and A.E.-C.; investigation, E.D.-Z., J.P.-C., A.L.L.-S., and A.E.-C.; resources, J.P.-C., C.C.-R., F.B.-H., and A.E.-C.; data curation, E.D.-Z. and A.E.-C.; writing—original draft preparation, E.D.-Z., J.P.-C., and A.E.-C.; writing—review and editing, E.D.-Z., J.P.-C., O.N.M.-C., F.B.-H., and A.E.-C.; supervision, J.P.-C., F.B.-H., and A.E.-C.; project administration, A.E.-C. All authors have read and agreed to the published version of the manuscript.
Funding
The National Council of Science and Technology (CONACYT) provided a graduate scholarship for the principal investigator (Grant 708801). The CONACYT took no part in design, conduct of the study, collection, management, analysis, interpretation of the data, preparation, review, and approval of the manuscript.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Bikbov B., Purcell C.A., Levey A.S., Smith M., Abdoli A., Abebe M., Adebayo O.M., Afarideh M., Agarwal S.K., Agudelo-Botero M., et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill N.R., Fatoba S.T., Oke J.L., Hirst J.A., O’Callaghan C.A., Lasserson D.S., Hobbs F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tirado-Gómez L.L., Durán-Arenas J.L., Rojas-Russell M.E., Venado-Estrada A., Pacheco-Domínguez R.L., López-Cervantes M. Las unidades de hemodiálisis en México: Una evaluación de sus características, procesos y resultados. Salud Pública de México. 2011;53:491–498. doi: 10.1590/S0036-36342011001000013. [DOI] [PubMed] [Google Scholar]
- 4.Carrero J.-J., Stenvinkel P., Cuppari L., Ikizler T.A., Kalantar-Zadeh K., Kaysen G., Mitch W.E., Price S.R., Wanner C., Wang A.Y., et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement from the International Society of Renal Nutrition and Metabolism (ISRNM) J. Ren. Nutr. 2013;23:77–90. doi: 10.1053/j.jrn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Espinosa-Cuevas M., Cobo G., Carrero J.J. Nutrición en diálisis peritoneal. El síndrome de desgaste proteico-energético: Etiología, diagnóstico y tratamiento. In: Montenegro-Martínez J., Correa-Rotter R., Riella M.C., editors. Tratado de Diálisis Peritoneal. 2nd ed. Elsevier España S.L.U.; Barcelona, Spain: 2006. pp. 81–87. [Google Scholar]
- 6.Luis-Rodríguez D., Zlatkis K., Comenge B., García Z., Navarro J.F., Lorenzo V., Carrero J.-J. Dietary Quality and Adherence to Dietary Recommendations in Patients Undergoing Hemodialysis. J. Ren. Nutr. 2016;26:190–195. doi: 10.1053/j.jrn.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Şanlier N., Demircioğlu Y., Şanlier N. Correlation of Dietary Intakes and Biochemical Determinates of Nutrition in Hemodialysis Patients. Ren. Fail. 2007;29:213–218. doi: 10.1080/08860220601098904. [DOI] [PubMed] [Google Scholar]
- 8.Choi H.K., Liu S., Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;52:283–289. doi: 10.1002/art.20761. [DOI] [PubMed] [Google Scholar]
- 9.Madero M., Sarnak M.J., Wang X., Greene T., Beck G.J., Kusek J.W., Collins A.J., Levey A.S., Menon V. Uric Acid and Long-term Outcomes in CKD. Am. J. Kidney Dis. 2009;53:796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fouque D., Kalantar-Zadeh K., Kopple J., Cano N., Chauveau P., Cuppari L., Lindholm B., Massy Z., Mitch W., Pineda E., et al. A proposed nomenclature and diagnostic criteria for protein—Energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 11.Álvarez-Lario B., Macarrón-Vicente J. Uric acid and evolution. Rheumatology. 2010;49:2010–2015. doi: 10.1093/rheumatology/keq204. [DOI] [PubMed] [Google Scholar]
- 12.De Giorgi A., Fabbian F., Pala M., Tiseo R., Parisi C., Misurati E., Manfredini R. Uric acid: Friend or foe? Uric acid and cognitive function “Gout kills more wise men than simple”. Eur. Rev. Med. Pharmacol. Sci. 2015;19:640–646. [PubMed] [Google Scholar]
- 13.Maloberti A., Giannattasio C., Bombelli M., Desideri G., Cicero A.F.G., Muiesan M.L., Rosei E.A., Salvetti M., Ungar A. Hyperuricemia and Risk of Cardiovascular Outcomes: The Experience of the URRAH (Uric Acid Right for Heart Health) Project. High. Blood Press. Cardiovasc. Prev. 2020;27:121–128. doi: 10.1007/s40292-020-00368-z. [DOI] [PubMed] [Google Scholar]
- 14.Zhu P., Liu Y., Han L., Xu G., Ran J.-M. Serum Uric Acid Is Associated with Incident Chronic Kidney Disease in Middle-Aged Populations: A Meta-Analysis of 15 Cohort Studies. PLoS ONE. 2014;9:e100801. doi: 10.1371/journal.pone.0100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson R.J., Kang D.-H., Feig D.I., Kivlighn S., Kanellis J., Watanabe S., Tuttle K.R., Rodriguez-Iturbe B., Herrera-Acosta J., Mazzali M. Is There a Pathogenetic Role for Uric Acid in Hypertension and Cardiovascular and Renal Disease? Hypertension. 2003;41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 16.Miric D., Kisic B., Stolic R., Miric B., Mitić R., Janicijevic-Hudomal S. The Role of Xanthine Oxidase in Hemodialysis-Induced Oxidative Injury: Relationship with Nutritional Status. Oxid. Med. Cell. Longev. 2013;2013:1–8. doi: 10.1155/2013/245253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang D.-H., Ha S.-K. Uric Acid Puzzle: Dual Role as Anti-oxidantand Pro-oxidant. Electrolytes Blood Press. 2014;12:1–6. doi: 10.5049/EBP.2014.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beberashvili I., Sinuani I., Azar A., Shapiro G., Feldman L., Stav K., Sandbank J., Averbukh Z. Serum uric acid as a clinically useful nutritional marker and predictor of outcome in maintenance hemodialysis patients. Nutrition. 2015;31:138–147. doi: 10.1016/j.nut.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Beberashvili I., Erlich A., Azar A., Sinuani I., Feldman L., Gorelik O., Stav K., Efrati S. Longitudinal Study of Serum Uric Acid, Nutritional Status, and Mortality in Maintenance Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2016;11:1015–1023. doi: 10.2215/CJN.10400915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park C., Obi Y., Streja E., Rhee C.M., Catabay C.J., Vaziri N.D., Kovesdy C.P., Kalantar-Zadeh K. Serum uric acid, protein intake and mortality in hemodialysis patients. Nephrol. Dial. Transplant. 2017;32:1750–1757. doi: 10.1093/ndt/gfw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beciragic A., Resic H., Prohic N., Karamehic J., Smajlovic A., Masnic F., Ajanovic S., Coric A. Correlation Between C-reactive Protein and Non-enzymatic Antioxidants (Albumin, Ferritin, Uric Acid and Bilirubin) in Hemodialysis Patients. Mater. Socio Medica. 2015;27:87–90. doi: 10.5455/msm.2015.27.87-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bae E., Cho H., Shin N., Kim S.M., Yang S.H., Kim D.K., Kim Y.-L., Kang S.-W., Yang C.W., Kim N.H., et al. Lower serum uric acid level predicts mortality in dialysis patients. Medicine. 2016;95:e3701. doi: 10.1097/MD.0000000000003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S.K., Lee A.L., Winters T.J., Tam E., Jaleel M., Stenvinkel P., Johnson R.J. Low serum uric acid level is a risk factor for death in incident hemodialysis patients. Am. J. Nephrol. 2008;29:79–85. doi: 10.1159/000151292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latif W., Karaboyas A., Tong L., Winchester J.F., Arrington C.J., Pisoni R.L., Marshall M.R., Kleophas W., Levin N.W., Sen A., et al. Uric acid levels and all-cause and cardiovascular mortality in the hemodialysis population. Clin. J. Am. Soc. Nephrol. 2011;6:2470–2477. doi: 10.2215/CJN.00670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bobulescu I.A., Moe O.W. Renal Transport of Uric Acid: Evolving Concepts and Uncertainties. Adv. Chronic Kidney Dis. 2012;19:358–371. doi: 10.1053/j.ackd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koren E., Kohen R., Ginsburg I. Polyphenols enhance total oxidant-scavenging capacities of human blood by binding to red blood cells. Exp. Biol. Med. 2010;235:689–699. doi: 10.1258/ebm.2010.009370. [DOI] [PubMed] [Google Scholar]
- 27.Huang D., Ou B., Hampsch-Woodill M., Flanagan J.A., Prior R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002;50:4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- 28.Gérard-Monnier D., Erdelmeier I., Régnard K., Moze-Henry N., Yadan J.-C., Chaudière J. Reactions of 1-Methyl-2-phenylindole with Malondialdehyde and 4-Hydroxyalkenals. Analytical Applications to a Colorimetric Assay of Lipid Peroxidation. Chem. Res. Toxicol. 1998;11:1176–1183. doi: 10.1021/tx9701790. [DOI] [PubMed] [Google Scholar]
- 29.Gracia-Iguacel C., González-Parra E., Barril-Cuadrado G., Sánchez R., Egido J., Ortiz-Arduán A., Carrero J.J. Definiendo el síndrome de desgaste proteico energético en la enfermedad renal crónica: Prevalencia e implicaciones clínicas. Nefrologia. 2014;34:507–519. doi: 10.3265/Nefrologia.pre2014.Apr.12522. [DOI] [PubMed] [Google Scholar]
- 30.Espinosa-Cuevas M.A., Rodriguez G.N., Martinez M.E.V., Carsi X.A., Alatriste P.M., Gutiérrez T.T., Correa-Rotter R. Body fluid volume and nutritional status in hemodialysis: Vector bioelectric impedance analysis. Clin. Nephrol. 2010;73:300–308. [PubMed] [Google Scholar]
- 31.Piccoli A., Nescolarde L.D., Rosell J. Análisis convencional y vectorial de bioimpedancia en la práctica clínica. Nefrología. 2002;22:228–238. [PubMed] [Google Scholar]
- 32.Piccoli A., Codognotto M., Piasentin P., Naso A. Combined evaluation of nutrition and hydration in dialysis patients with bioelectrical impedance vector analysis (BIVA) Clin. Nutr. 2014;33:673–677. doi: 10.1016/j.clnu.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Espinosa-Cuevas M., Rivas-Rodríguez L., González-Medina E.C., Atilano-Carsi X., Miranda-Alatriste P., Correa-Rotter R. Vectores de impedancia bioeléctrica para la composición corporal en población mexicana. Rev. Investig. Clínica. 2007;59:15–24. [PubMed] [Google Scholar]
- 34.Bellasi A., Raggi P. Among markers of risk, uric acid remains a two-faced Janus awaiting definitive framing. Atherosclerosis. 2018;272:219–221. doi: 10.1016/j.atherosclerosis.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava A., Kaze A.D., McMullan C.J., Isakova T., Waikar S.S. Uric Acid and the Risks of Kidney Failure and Death in Individuals with CKD. Am. J. Kidney Dis. 2018;71:362–370. doi: 10.1053/j.ajkd.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hur I., Choi S.J., Kalantar-Zadeh K. Serum uric acid and mortality risk among maintenance hemodialysis patients. Kidney Res. Clin. Pract. 2017;36:302–304. doi: 10.23876/j.krcp.2017.36.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalantar-Zadeh K., Fouque D., Kopple J.D. Outcome research, nutrition, and reverse epidemiology in maintenance dialysis patients. J. Ren. Nutr. 2004;14:64–71. doi: 10.1053/j.jrn.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Kim C.S., Jin D.-C., Yun Y.C., Bae E.H., Ma S.K., Kim S.W. Relationship between serum uric acid and mortality among hemodialysis patients: Retrospective analysis of Korean end-stage renal disease registry data. Kidney Res. Clin. Pract. 2017;36:368–376. doi: 10.23876/j.krcp.2017.36.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maciejczyk M., Szulimowska J., Taranta-Janusz K., Werbel K., Wasilewska A., Zalewska A. Salivary FRAP as A Marker of Chronic Kidney Disease Progression in Children. Antioxidants. 2019;8:409. doi: 10.3390/antiox8090409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abshirini M., Siassi F., Koohdani F., Qorbani M., Khosravi S., Hedayati M., Aslani Z., Soleymani M., Sotoudeh G. Dietary total antioxidant capacity is inversely related to menopausal symptoms: A cross-sectional study among Iranian postmenopausal women. Nutrition. 2018;56:161–167. doi: 10.1016/j.nut.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Puchau B., Zulet M.A., De Echávarri A.G., Hermsdorff H.H.M., Martínez J.A. Dietary total antioxidant capacity: A novel indicator of diet quality in healthy young adults. J. Am. Coll. Nutr. 2009;28:648–656. doi: 10.1080/07315724.2009.10719797. [DOI] [PubMed] [Google Scholar]
- 42.Nascimento-Souza M.A., Paiva P.G., Martino H.S.D., Ribeiro A.Q. Dietary total antioxidant capacity as a tool in health outcomes in middle-aged and older adults: A systematic review. Crit. Rev. Food Sci. Nutr. 2017;58:905–912. doi: 10.1080/10408398.2016.1230089. [DOI] [PubMed] [Google Scholar]
- 43.Mozaffari H., Daneshzad E., Surkan P.J., Azadbakht L. Dietary Total Antioxidant Capacity and Cardiovascular Disease Risk Factors: A Systematic Review of Observational Studies. J. Am. Coll. Nutr. 2018;37:533–545. doi: 10.1080/07315724.2018.1441079. [DOI] [PubMed] [Google Scholar]
- 44.Asghari G., Yuzbashian E., Shahemi S., Gaeini Z., Mirmiran P., Azizi F. Dietary total antioxidant capacity and incidence of chronic kidney disease in subjects with dysglycemia: Tehran Lipid and Glucose Study. Eur. J. Nutr. 2017;57:2377–2385. doi: 10.1007/s00394-017-1511-2. [DOI] [PubMed] [Google Scholar]
- 45.Valdez-Ortiz R., Navarro-Reynoso F., Olvera-Soto M.G., Martin-Alemañy G., Rodríguez-Matías A., Hernández-Arciniega C.R., Cortes-Pérez M., Chávez-López E., García-Villalobos G., Hinojosa-Heredia H., et al. Mortality in Patients with Chronic Renal Disease Without Health Insurance in Mexico: Opportunities for a National Renal Health Policy. Kidney Int. Rep. 2018;3:1171–1182. doi: 10.1016/j.ekir.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obrador G.T., De Arrigunaga S., Cuadra M., Villa A.R. Mismatch Between Kidney Disease Burden and Nephrology Workforce in Mexico. Kidney Int. Rep. 2020;5 doi: 10.1016/j.ekir.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abbas H.N., Rabbani M.A., Safdar N., Murtaza G., Maria Q., Ahamd A. Biochemical nutritional parameters and their impact on hemodialysis efficiency. Saudi J. Kidney Dis. Transplant. 2009;20:1105–1109. [PubMed] [Google Scholar]
- 48.Hsu S.-P., Pai M.F., Peng Y.S., Chiang C.K., Ho T.I., Hung K.Y. Serum uric acid levels show a ’J-shaped’ association with all-cause mortality in haemodialysis patients. Nephrol. Dial. Transplant. 2004;19:457–462. doi: 10.1093/ndt/gfg563. [DOI] [PubMed] [Google Scholar]
- 49.Kalantar-Zadeh K., Kopple J.D., Humphreys M.H., Block G. Comparing outcome predictability of markers of malnutrition—inflammation complex syndrome in haemodialysis patients. Nephrol. Dial. Transpl. 2004;19:1507–1519. doi: 10.1093/ndt/gfh143. [DOI] [PubMed] [Google Scholar]
- 50.Ruperto M., Barril G., Sánchez-Muniz F.J. Predictors of protein-energy wasting in haemodialysis patients: A cross-sectional study. J. Hum. Nutr. Diet. 2014;29:38–47. doi: 10.1111/jhn.12276. [DOI] [PubMed] [Google Scholar]
- 51.Foucan L., Merault H., Velayoudom-Cephise F., Larifla L., Alecu C., Ducros J. Impact of protein energy wasting status on survival among Afro-Caribbean hemodialysis patients: A 3-year prospective study. SpringerPlus. 2015;4:452. doi: 10.1186/s40064-015-1257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasheminejad N., Namdari M., Mahmoodi M.R., Bahrampour A., Azmandian J. Association of Handgrip Strength with Malnutrition-Inflammation Score as an Assessment of Nutritional Status in Hemodialysis Patients. Iran. J. Kidney Dis. 2016;10:30–35. [PubMed] [Google Scholar]
- 53.Beberashvili I., Azar A., Sinuani I., Kadoshi H., Shapiro G., Feldman L., Sandbank J., Averbukh Z. Longitudinal changes bioimpedance phase angle reflect inverse changes in serum IL-6 levels in maintenance hemodialysis patients. Nutrition. 2014;30:297–304. doi: 10.1016/j.nut.2013.08.017. [DOI] [PubMed] [Google Scholar]