This meta-analysis evaluates the difference in all-cause and cause-specific mortality in randomized clinical trials comparing percutaneous coronary intervention with coronary artery bypass grafting for the treatment of patients with coronary artery disease.
Key Points
Question
What is the difference in all-cause and cause-specific mortality in the randomized clinical trials that have compared percutaneous coronary intervention (PCI) with coronary artery bypass grafting?
Findings
In a pooled meta-analysis of 23 randomized clinical trials (13 260 unique patients) comparing PCI vs coronary artery bypass grafting, PCI was associated with a significantly higher rate of cardiac mortality, noncardiac mortality, and all-cause mortality.
Meaning
The significantly higher noncardiac mortality associated with PCI suggests that even noncardiac deaths after PCI may be procedure related and supports the use of all-cause mortality as the end point for myocardial revascularization trials.
Abstract
Importance
Mortality is a common outcome in trials comparing percutaneous coronary intervention (PCI) with coronary artery bypass grafting (CABG). Controversy exists regarding whether all-cause mortality or cardiac mortality is preferred as a study end point, because noncardiac mortality should be unrelated to the treatment.
Objective
To evaluate the difference in all-cause and cause-specific mortality in randomized clinical trials (RCTs) comparing PCI with CABG for the treatment of patients with coronary artery disease.
Data Sources
MEDLINE (1946 to the present), Embase (1974 to the present), and the Cochrane Library (1992 to the present) databases were searched on November 24, 2019. Reference lists of included articles were also searched, and additional studies were included if appropriate.
Study Selection
Articles were considered for inclusion if they were in English, were RCTs comparing PCI with drug-eluting or bare-metal stents and CABG for the treatment of coronary artery disease, and reported mortality and/or cause-specific mortality. Trials of PCI involving angioplasty without stenting were excluded. For each included trial, the publication with the longest follow-up duration for each outcome was selected.
Data Extraction and Synthesis
For data extraction, all studies were reviewed by 2 independent investigators, and disagreements were resolved by a third investigator in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guideline. Data were pooled using fixed- and random-effects models.
Main Outcomes and Measures
The primary outcomes were all-cause and cause-specific (cardiac vs noncardiac) mortality. Subgroup analyses were performed for PCI trials using drug-eluting vs bare-metal stents and for trials involving patients with left main disease.
Results
Twenty-three unique trials were included involving 13 620 unique patients (6829 undergoing PCI and 6791 undergoing CABG; men, 39.9%-99.0% of study populations; mean age range, 60.0-71.0 years). The weighted mean (SD) follow-up was 5.3 (3.6) years. Compared with CABG, PCI was associated with a higher rate of all-cause (incidence rate ratio, 1.17; 95% CI, 1.05-1.29) and cardiac (incidence rate ratio, 1.24; 95% CI, 1.05-1.45) mortality but also noncardiac mortality (incidence rate ratio, 1.19; 95% CI, 1.00-1.41).
Conclusions and Relevance
Percutaneous coronary intervention was associated with higher all-cause, cardiac, and noncardiac mortality compared with CABG at 5 years. The significantly higher noncardiac mortality associated with PCI suggests that even noncardiac deaths after PCI may be procedure related and supports the use of all-cause mortality as the end point for myocardial revascularization trials.
Introduction
During the course of the last 3 decades, several randomized clinical trials (RCTs)1,2,3,4,5 have compared the results of percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) in patients with stable ischemic heart disease and recent acute coronary syndromes. The individual trials were underpowered to detect differences in mortality, and all used a composite of major adverse cardiac or cardiovascular events as the primary outcome. Although mortality was included in the primary composite outcome of all the trials, some used all-cause mortality and others used cardiac mortality.
The use of all-cause mortality reduces the risk of adjudication bias due to incomplete, skewed, or inadequate supporting evidence,6 but it has the potential to dilute the treatment effect due to the inclusion of events unrelated to interventions for the coronary circulation.7 On the other hand, the use of cause-specific mortality reduces the event rate, is subject to bias, and can lead to underpowered comparisons.8
The controversy has been ignited by the recent publication of the 5-year results of the Evaluation of XIENCE vs Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trial.1 In the EXCEL trial at 5 years, PCI was associated with significantly higher all-cause mortality, but the difference between the 2 groups was not observed when considering cardiac mortality alone.1 To date, no systematic evaluation of cause-specific mortality in PCI vs CABG trials has been published. In this meta-analysis, we evaluate the difference in all-cause and cause-specific mortality in the RCTs that have compared PCI and CABG for the treatment of patients with coronary artery disease.
Methods
Search Strategy
Because no individual patient data are involved in the analysis, there was no need for ethical approval or individual patient consent according to the Weill Cornell Institutional Review Board. A medical librarian (M.D.) performed comprehensive searches to identify all RCTs comparing PCI vs CABG. Searches were run on November 24, 2019, on the following databases: Ovid MEDLINE (1946 to the present), Ovid Embase (1974 to the present), and the Cochrane Library (Wiley; 1992 to the present). The full search strategy for Ovid MEDLINE is available in eTable 1 in the Supplement. This review was registered with the PROSPERO register of systematic reviews (CRD42020165349) and followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Study Selection and Data Extraction
Searches retrieved 4916 results. After deduplication, 2 reviewers (I.H. and M. Rahouma) independently screened a total of 4411 citations. Discrepancies were resolved by a third author (M.G.). Titles and abstracts were reviewed against predefined inclusion and exclusion criteria. Articles were considered for inclusion if they were in English and were RCTs comparing PCI with bare-metal or drug-eluting stents and CABG for the treatment of coronary artery disease and reported mortality and/or cause-specific mortality. Trials of PCI involving angioplasty without stenting were excluded. For each included trial, the publication with the longest follow-up duration for each outcome was selected. Animal studies, case reports, conference presentations, editorials, expert opinions, and observational studies were excluded.
The full text was pulled for the selected studies for a second round of eligibility screening. Reference lists of articles were also searched to identify other relevant trials.
Two investigators (I.H. and M. Rahouma) performed data extraction independently, and the extracted data were verified by a third investigator (M.G.) for accuracy. The following variables were extracted: trial data, including number of enrolling centers, location, study period, number of patients randomized, and mean length of follow-up; patient demographics, including age, sex, body mass index, New York Heart Association Class, EuroSCORE (European System for Cardiac Operative Risk Evaluation), SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score, and comorbidities and/or past treatment (diabetes, insulin therapy, statin therapy, smoking, hypertension, hypercholesterolemia/hyperlipidemia, peripheral vascular disease, carotid artery disease, stroke, myocardial infarction, heart failure, previous PCI/CABG, stable or unstable angina pectoris, acute coronary syndrome); procedure-related factors, including number of stents, type of stent, total stent length, stent diameter, left main bifurcation stent technique, intravascular ultrasonongraphy, use of left or bilateral internal mammary arteries, off-pump CABG, number of arterial and venous grafts, use of epiaortic or transesophageal ultrasonography, and completeness of revascularization; details of medical therapy; and all-cause and cause-specific mortality in the CABG and PCI arms. The quality of the included studies was assessed using the Cochrane Collaboration’s tool for assessing risk of bias, version 2, in randomized trials.9
Outcomes and Effect Summary
The primary outcomes were all-cause and cause-specific (cardiac vs noncardiac) mortality. Subgroup analyses were performed for trials comparing PCI using bare-metal or drug-eluting stents vs CABG and for trials comparing PCI with CABG in patients with left main disease.
Meta-analysis
The generic inverse variance method was used to pool outcomes as natural logarithms of the incident rate ratios (IRRs) across studies to account for potentially different follow-up durations between the groups. Fixed- and random-effects inverse variance meta-analyses were performed using the metafor and meta packages10 in R, version 3.3.3 (R Project for Statistical Computing). Publication bias was assessed by funnel plot (using the trim and fill method)11 and Egger test. Heterogeneity was reported as low (I2 = 0%-25%), moderate (I2 = 26%-50%), or high (I2>50%). Because the I2 value was less than 50% in all primary comparisons, the fixed effect was considered as the primary model and the random effect as a sensitivity analysis. To better infer the predictive accuracy of the point estimates and minimize the selection bias, a cross-validation leave-one-out analysis was performed for the primary outcome. P value for interaction was used to ascertain subgroup differences. Statistical significance was set at the 2-tailed P = .05 level, without multiplicity adjustments.
Results
A total of 425 citations were evaluated, of which 23 trials in 24 studies1,2,5,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 met the eligibility criteria and were included in the final meta-analysis, the details of which are included in eTables 2 to 5 in the Supplement. Eighteen trials used all-cause mortality in their composite primary end point (eTable 6 in the Supplement). The details of the methods used for adjudication of the cause of death for each trial are summarized in eTable 7 in the Supplement. The full PRISMA flow diagram outlining the study selection process is available in eFigure 1 in the Supplement.33
A total of 13 620 patients were included (6829 undergoing PCI and 6791 undergoing CABG). The number of patients in the individual trials ranged from 44 to 1905. The mean follow-up duration of the individual studies was 4.5 years (range, 0.5-11.4 years). The mean age of patients ranged from 60.0 to 71.0 years. Women constituted 1.0% to 40.0% of the study populations (PCI, 1.0%-40.0%; CABG, 1.0%-30.5%); men, 39.9% to 99.0% (PCI, 60.0%-99.0%; CABG, 57.0%-99.0%). The prevalence of diabetes ranged from 6.0% to 100.0% (PCI, 8.0%-100.0%; CABG, 6.0%-100.0%). The details of patient characteristics are summarized in eTable 3 in the Supplement. The assessment of the quality of the individual studies and of the evidence is reported using the Cochrane risk of bias tool in the eMethods in the Supplement.
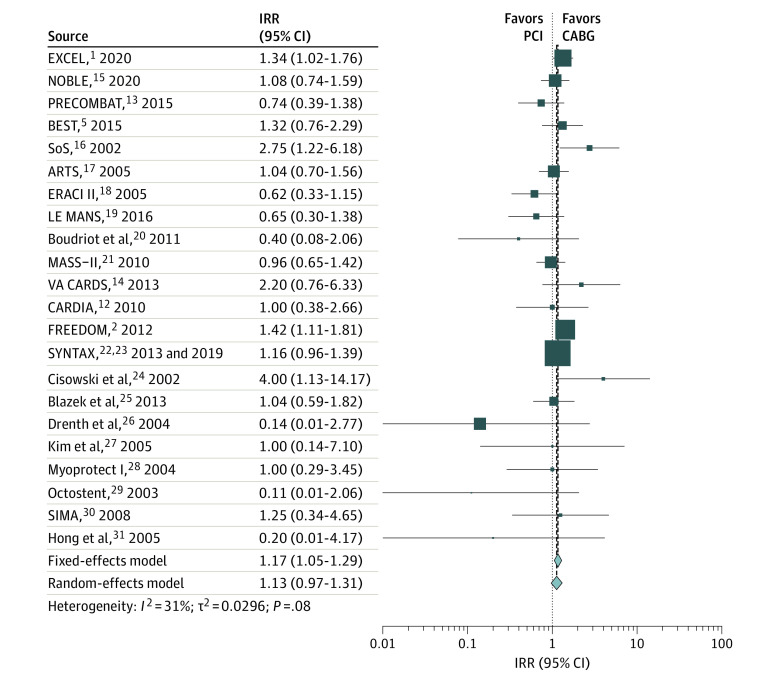
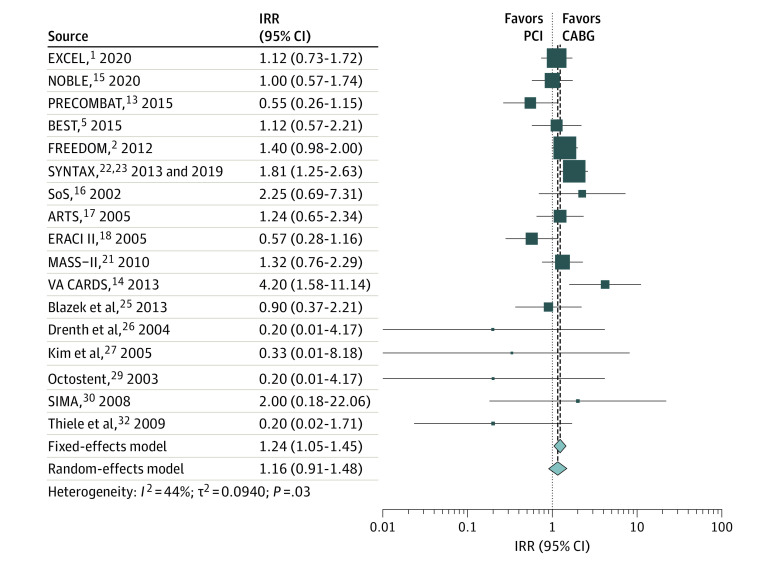
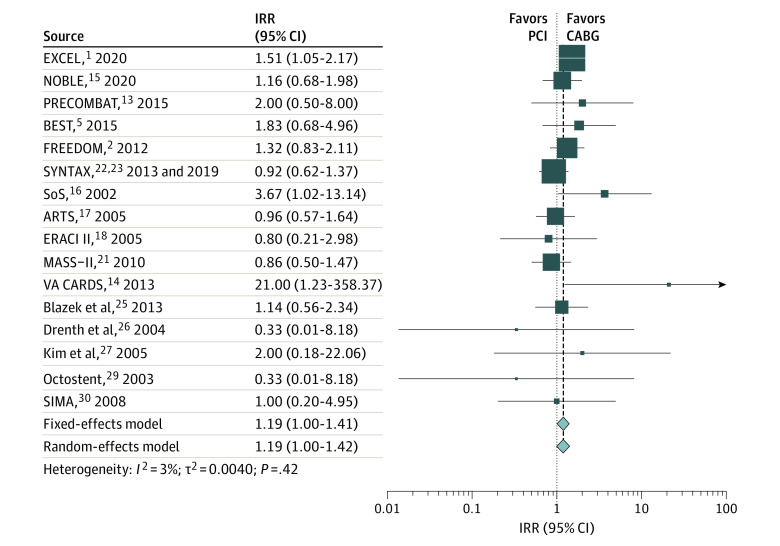
The weighted mean (SD) follow-up was 5.3 (3.6) years. Compared with CABG, PCI was associated with a higher rate of all-cause mortality (IRR, 1.17; 95% CI, 1.05-1.29) (Figure 1 and Table), cardiac mortality (IRR, 1.24; 95% CI, 1.05-1.45) (Figure 2 and Table), and noncardiac mortality (IRR, 1.19; 95% CI, 1.00-1.41) (Figure 3 and Table). Sensitivity analyses confirmed the solidity of the primary analysis (eFigures 2-7 in the Supplement). The causes of noncardiac mortality in each study are summarized in eTable 8 in the Supplement.
Figure 1. Pooled Incidence Rate Ratio (IRR) for All-Cause Mortality.
ARTS indicates Arterial Revascularization Therapies Study; BEST, Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease; CABG, coronary artery bypass grafting; CARDIA, Coronary Artery Revascularization in Diabetes; ERACI II, Coronary Angioplasty With Stenting vs Coronary Bypass Surgery in Patients With Multiple-Vessel Disease; EXCEL, Evaluation of XIENCE vs Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization; FREEDOM, Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease; LE MANS, Left Main Stenting Trial; MASS-II, Medicine, Angioplasty, or Surgery Study; NOBLE, Nordic–Baltic–British Left Main Revascularisation Study; PCI, percutaneous coronary intervention; PRECOMBAT, Premier of Randomized Comparison of Bypass Surgery vs Angioplasty Using Sirolimus-Eluting Stent in Patients With Left Main Coronary Artery Disease; SIMA, Stenting vs Internal Mammary Artery Grafting; SoS, Stent or Surgery; SYNTAX, Synergy Between PCI With Taxus and Cardiac Surgery; and VA CARDS, Veterans Affairs Coronary Artery Revascularization in Diabetes. Different size markers indicate 95% CIs.
Table. Summary of Outcomes for the Main Analysis Comparing PCI vs CABG and for the Subgroup Analysesa.
| Outcomes | No. of studies | No. of patients | Fixed-effect model | Random-effects model | Heterogeneity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR (95% CI) | P value | P value for interaction | IRR (95% CI) | P value | P value for interaction | I2, % | P value | ||||||
| All-cause mortality | 22 | 13 490 | 1.17 (1.05-1.29) | .003 | NA | 1.13 (0.97-1.31) | .12 | NA | 31.2 | .08 | |||
| PCI with drug-eluting stents | 9 | 8857 | 1.22 (1.09-1.38) | <.001 | .19 | 1.22 (1.06-1.41) | .006 | .51 | 15.8 | .30 | |||
| PCI with bare-metal stents | 11 | 4018 | 1.04 (0.84-1.29) | .70 | .19 | 1.08 (0.78-1.50) | .63 | .51 | 40.6 | .08 | |||
| Left main disease | 5 | 3995 | 1.11 (0.91-1.35) | .32 | .56 | 0.99 (0.73-1.36) | .97 | .37 | 41.5 | .15 | |||
| Other vessel disease | 17 | 9495 | 1.19 (1.06-1.33) | .004 | .56 | 1.17 (0.98-1.40) | .09 | .37 | 31.4 | .11 | |||
| Cardiac mortality | 17 | 12 471 | 1.24 (1.05-1.45) | .009 | NA | 1.16 (0.91-1.48) | .24 | NA | 43.8 | .03 | |||
| PCI with drug-eluting stents | 8 | 8597 | 1.31 (1.09-1.58) | .004 | .21 | 1.24 (0.89-1.73) | .21 | .46 | 62.1 | .01 | |||
| PCI with bare-metal stents | 9 | 3874 | 1.04 (0.76-1.43) | .79 | .21 | 1.04 (0.74-1.45) | .84 | .46 | 5.9 | .39 | |||
| Left main disease | 3 | 3689 | 0.96 (0.70-1.30) | .78 | .06 | 0.93 (0.64-1.35) | .71 | .20 | 27.4 | .25 | |||
| Other vessel disease | 14 | 8782 | 1.36 (1.13-1.64) | .001 | .06 | 1.27 (0.95-1.70) | .11 | .20 | 41.1 | .06 | |||
| Noncardiac mortality | 16 | 12 341 | 1.19 (1.00-1.41) | .046 | NA | 1.19 (1.00-1.42) | .05 | NA | 3.0 | .42 | |||
| PCI with drug-eluting stents | 7 | 8467 | 1.28 (1.04-1.57) | .02 | .22 | 1.30 (1.00-1.69) | .05 | .23 | 27.1 | .22 | |||
| PCI with bare-metal stents | 9 | 3874 | 1.02 (0.75-1.38) | .91 | .22 | 1.02 (0.75-1.38) | .91 | .23 | 0.0 | .68 | |||
| Left main disease | 3 | 3689 | 1.41 (1.05-1.89) | .02 | .15 | 1.41 (1.05-1.89) | .02 | .17 | 0.0 | .64 | |||
| Other vessel disease | 13 | 8652 | 1.09 (0.88-1.34) | .43 | .15 | 1.10 (0.88-1.36) | .42 | .17 | 4.2 | .40 | |||
Abbreviations: CABG, coronary artery bypass grafting; IRR, incidence rate ratio; NA, not applicable; PCI, percutaneous coronary intervention.
In all subgroup analyses, the reference is CABG. Differences were calculated using P for interaction.
Figure 2. Pooled Incidence Rate Ratio (IRR) for Cardiac Mortality.
ARTS indicates Arterial Revascularization Therapies Study; BEST, Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease; CABG, coronary artery bypass grafting; CARDIA, Coronary Artery Revascularization in Diabetes; ERACI II, Coronary Angioplasty With Stenting vs Coronary Bypass Surgery in Patients With Multiple-Vessel Disease; EXCEL, Evaluation of XIENCE vs Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization; FREEDOM, Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease; MASS-II, Medicine, Angioplasty, or Surgery Study; NOBLE, Nordic–Baltic–British Left Main Revascularisation Study; PCI, percutaneous coronary intervention; PRECOMBAT, Premier of Randomized Comparison of Bypass Surgery vs Angioplasty Using Sirolimus-Eluting Stent in Patients With Left Main Coronary Artery Disease; SIMA, Stenting vs Internal Mammary Artery Grafting; SoS, Stent or Surgery; SYNTAX, Synergy Between PCI With Taxus and Cardiac Surgery; and VA CARDS, Veterans Affairs Coronary Artery Revascularization in Diabetes. Different size markers indicate 95% CIs.
Figure 3. Pooled Incidence Rate Ratio (IRR) for Noncardiac Mortality.
ARTS indicates Arterial Revascularization Therapies Study; BEST, Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease; ERACI II, Coronary Angioplasty With Stenting vs Coronary Bypass Surgery in Patients With Multiple-Vessel Disease; EXCEL, Evaluation of XIENCE vs Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization; FREEDOM, Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease; MASS-II, Medicine, Angioplasty, or Surgery Study; NOBLE, Nordic–Baltic–British Left Main Revascularisation Study; PRECOMBAT, Premier of Randomized Comparison of Bypass Surgery vs Angioplasty Using Sirolimus-Eluting Stent in Patients With Left Main Coronary Artery Disease; SIMA, Stenting vs Internal Mammary Artery Grafting; SoS, Stent or Surgery; SYNTAX, Synergy Between PCI With Taxus and Cardiac Surgery; and VA CARDS, Veterans Affairs Coronary Artery Revascularization in Diabetes. Different size markers indicate 95% CIs.
Subgroup Analysis
The pooled IRR for all-cause mortality was 1.22 (95% CI, 1.09-1.38) for studies including drug-eluting stents vs 1.04 (95% CI, 0.84-1.29) for studies including bare-metal stents (P = .19 for subgroups) (Figure 4A). In the analysis by anatomical extent of coronary disease, the IRR was 1.11 (95% CI, 0.91-1.35) for studies including patients with left main disease vs 1.19 (95% CI, 1.06-1.33) for the others (P = .56 for subgroups) (Table and eFigure 8 in the Supplement).
Figure 4. Subgroup Analysis for All-Cause Mortality, Cardiac Mortality, and Noncardiac Mortality for Trials Using Drug-Eluting vs Bare-Metal Stents.
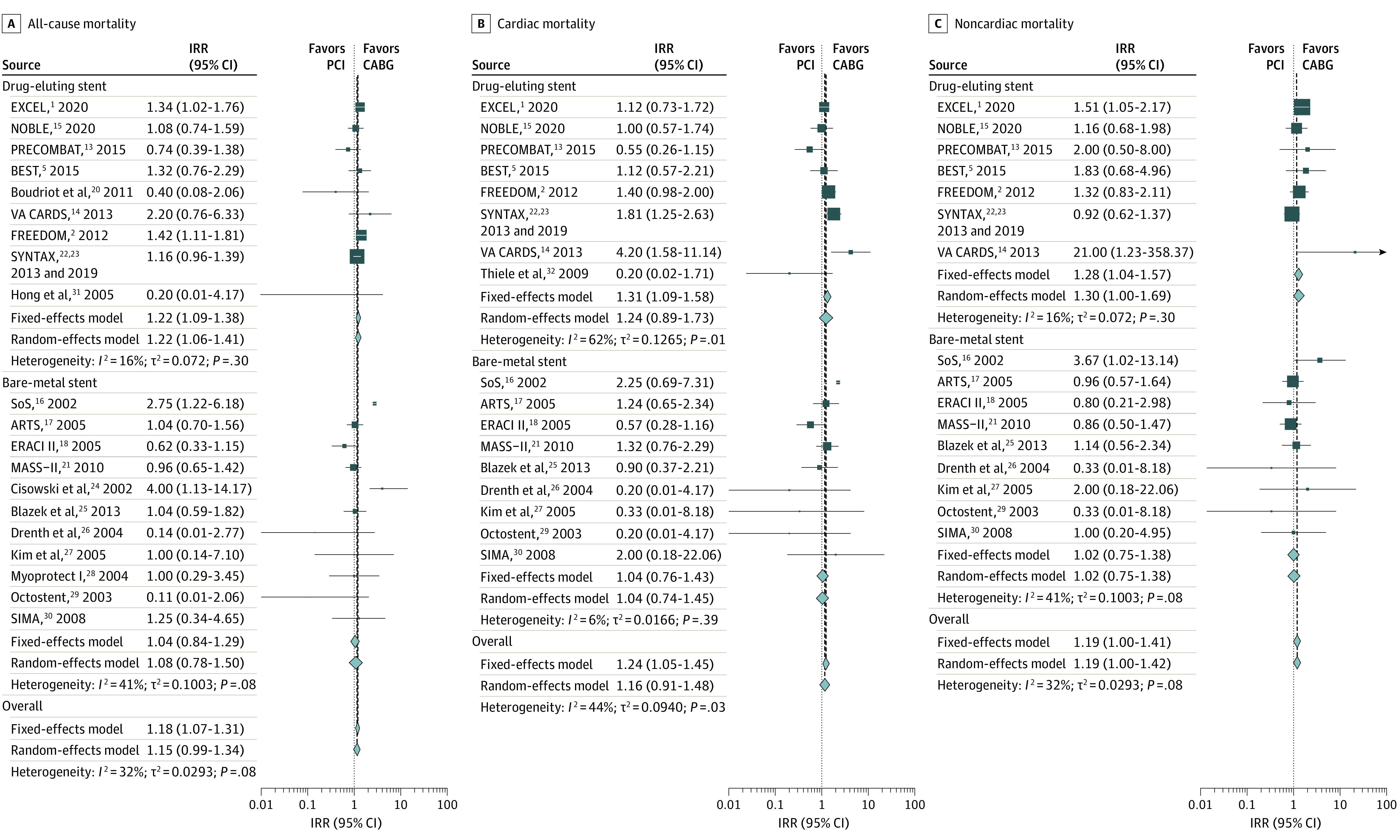
ARTS indicates Arterial Revascularization Therapies Study; BEST, Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease; CABG, coronary artery bypass grafting; ERACI II, Coronary Angioplasty With Stenting vs Coronary Bypass Surgery in Patients With Multiple-Vessel Disease; EXCEL, Evaluation of XIENCE vs Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization; FREEDOM, Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease; IRR, incidence rate ratio; MASS-II, Medicine, Angioplasty, or Surgery Study; NOBLE, Nordic–Baltic–British Left Main Revascularisation Study; PCI, percutaneous coronary intervention; PRECOMBAT, Premier of Randomized Comparison of Bypass Surgery vs Angioplasty Using Sirolimus-Eluting Stent in Patients With Left Main Coronary Artery Disease; SIMA, Stenting vs Internal Mammary Artery Grafting; SoS, Stent or Surgery; SYNTAX, Synergy Between PCI With Taxus and Cardiac Surgery; and VA CARDS, Veterans Affairs Coronary Artery Revascularization in Diabetes. Different size markers indicate 95% CIs.
The pooled IRR for cardiac mortality was 1.31 (95% CI, 1.09-1.58) for studies including drug-eluting stents vs 1.04 (95% CI, 0.76-1.43) for studies including bare-metal stents (P = .21 for subgroups) (Figure 4B). The pooled IRR was 0.96 (95% CI, 0.70-1.30) for studies including patients with left main disease vs 1.36 (95% CI, 1.13-1.64) for the others (P = .06 for subgroups) (Table and eFigure 9 in the Supplement).
The pooled IRR for noncardiac mortality was 1.28 (95% CI, 1.04-1.57) for studies including drug-eluting stents vs 1.02 (95% CI, 0.75-1.38) for studies including bare-metal stents (P = .22 for subgroups) (Figure 4C). The pooled IRR was 1.41 (95% CI, 1.05-1.89) for studies including patients with left main disease vs 1.09 (95% CI, 0.88-1.34) for the others (P = .15 for subgroups) (Table and eFigure 10 in the Supplement).
Discussion
The outcomes of PCI and CABG have been extensively evaluated, but comparative data on the cause of mortality after these revascularization procedures are limited. Our meta-analysis of 23 RCTs (13 620 patients) is the first, to our knowledge, to compare all-cause and cause-specific mortality between the 2 revascularization modalities. Among the included patients, compared with CABG, PCI was associated with higher all-cause, cardiac, and noncardiac mortality at a mean follow-up of 5.3 years. On subgroup analysis, PCI with drug-eluting stents was associated with higher all-cause, cardiac, and noncardiac mortality compared with bare-metal stents, although the test for interaction did not reach statistical significance.
Observational evidence shows that the causes of mortality after PCI and CABG are predominantly cardiac in the first year after the procedure and noncardiac in the following years.12,34,35,36,37 The common causes of cardiac mortality include cardiogenic shock, heart failure, stent thrombosis, bleeding, coronary dissection, malignant arrhythmia, and sudden death,12,34,38 whereas cancer, sepsis, bleeding, and vascular, pulmonary, and/or renal disease are among the most frequent causes of noncardiac mortality.12,34,36,37
Our finding of higher all-cause mortality with PCI is consistent with the most recent individual patient data meta-analysis of 11 RCTs and 11 518 patients.39 Compared with PCI, CABG offers additional protection against the evolution of lesions that were non-flow limiting at the time of the procedure, and this has been proposed as a potential mechanism for the observed survival benefit in the surgical arm.40
We found PCI to be also associated with significantly higher noncardiac mortality compared with CABG. All 6 revascularization RCTs during the last decade have shown PCI to be associated with an increase in the rate of noncardiac mortality compared with CABG.1,2,3,5,13,14 Large observational studies have shown an increased risk of noncardiac mortality in the late post-PCI period and independent of patients’ characteristics.41,42,43
This finding may have several explanations. Dual antiplatelet therapy has been linked to non-cardiac-related deaths in a large trial,44 but not in an individual data meta-analysis.45 Evidence suggests that longer duration of dual antiplatelet therapy is associated with an increased risk of noncardiac mortality.46 The reasons for these associations are not fully understood, but may include deaths due to major bleeding events (that are often coded as noncardiac) or a higher bleeding-related mortality in case of trauma or other acute events in patients receiving dual antiplatelet therapy.
Another explanation—perhaps the most likely—is that cardiac deaths were coded as noncardiac owing to bias or errors. The risk that insufficient or inadequate supporting data and/or assessors bias may lead to misclassification in the adjudication of cause-specific mortality is well known and has been described in detail previously.47,48 This may be particularly true in case of sudden cardiac death, a particularly frequent cause of death in patients who presented with acute coronary syndromes.38
In our analysis, PCI with drug-eluting stents was associated with higher all-cause and noncardiac mortality relative to CABG compared with PCI with bare-metal stents, although the difference did not reach statistical significance. This result is consistent with a previous meta-analysis of 17 RCTs (8221 patients)49 comparing first-generation drug-eluting vs bare-metal stents in which the use of sirolimus-eluting stents was significantly associated with higher noncardiac and, in particular, cancer-related mortality. Although the reasons for this association are unclear, it is most likely related to the differences in baseline characteristics and risk profile between patients with PCI in the era of bare-metal vs drug-eluting stents.
We also found that in patients with left main disease, the benefit of CABG over PCI for cardiac mortality was reduced, and the difference between the 2 revascularization modalities was mostly based on a higher rate of noncardiac deaths in the PCI arm. This finding is likely related to the reduced power of the left main disease subgroup analysis, to heterogeneity in the population with left main disease, or to differences in baseline characteristics between patients with left main disease compared with other types of coronary disease.
Our findings have implications for future RCTs comparing the 2 revascularization modalities. The use of all-cause mortality in myocardial revascularization trials remains controversial,6 as recently highlighted by the difference in all-cause, but not cause-specific, mortality in the EXCEL trial1 and from the fact that 5 of the 23 trials included in our analysis used cause-specific rather than all-cause mortality in their primary composite outcome. Based on our results, the use of cardiac mortality may exclude deaths that are in fact related to the procedure, either through noncardiac mechanisms or because of misclassification.
Limitations
Our results must be viewed in light of the limitations of this analysis. Differences in procedural aspects, postprocedural management, and follow-up protocol may have existed between the included trials. In addition, the exact causes of noncardiac mortality were not reported by several trials and could not be independently compared between PCI and CABG.
Conclusions
This meta-analysis found that percutaneous coronary intervention is associated with higher all-cause, cardiac, and noncardiac mortality compared with CABG. The significantly higher noncardiac mortality associated with PCI suggests that even noncardiac deaths after PCI may in fact be related to the procedure and/or subsequent management, and our data strongly support the use of all-cause mortality as the most comprehensive and unbiased end point for myocardial revascularization trials.
eTable 1. Full Search Strategy
eTable 2. Summary of the Included Randomized Clinical Trials
eTable 3. Details of Patient Characteristics
eTable 4. Procedural Characteristics
eTable 5. Details of Medical Therapy
eTable 6. Details of Trial End Points
eTable 7. Adjudication of Cause of Death in the Randomized Clinical Trials
eTable 8. Details of Noncardiac Mortality in Percutaneous Coronary Intervention (PCI) vs Coronary Artery Bypass Grafting (CABG) Randomized Clinical Trials
eFigure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flowchart of Our Analysis
eFigure 2. Funnel Plot for Studies Reporting All-Cause Mortality
eFigure 3. Funnel Plot for Studies Reporting Cardiac Mortality
eFigure 4. Funnel Plot for Studies Reporting Noncardiac Mortality
eFigure 5. Leave-One-Out Analysis for All-Cause Mortality for Fixed-Effects Model (A) and Random-Effects Model
eFigure 6. Leave-One-Out Analysis for Cardiac Mortality for Fixed-Effects Model (A) and Random-Effects Model (B)
eFigure 7. Leave-One-Out Analysis for Noncardiac Mortality for Fixed-Effects Model (A) and Random-Effects Model (B)
eFigure 8. Subgroup Analysis for All-Cause Mortality for Trials Including Patients With Left Main Disease vs Non-Left Main Disease
eFigure 9. Subgroup Analysis for Cardiac Mortality for Trials Including Patients With Left Main Disease vs Non-Left Main Disease
eFigure 10. Subgroup Analysis for Noncardiac Mortality for Trials Including Patients With Left Main Disease vs Non-Left Main Disease
eMethods. The Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2)
eReferences.
References
- 1.Stone GW, Kappetein AP, Sabik JF, et al. ; EXCEL Trial Investigators . Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381(19):1820-1830. doi: 10.1056/NEJMoa1909406 [DOI] [PubMed] [Google Scholar]
- 2.Farkouh ME, Domanski M, Sleeper LA, et al. ; FREEDOM Trial Investigators . Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375-2384. doi: 10.1056/NEJMoa1211585 [DOI] [PubMed] [Google Scholar]
- 3.Mäkikallio T, Holm NR, Lindsay M, et al. ; NOBLE Study Investigators . Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016;388(10061):2743-2752. doi: 10.1016/S0140-6736(16)32052-9 [DOI] [PubMed] [Google Scholar]
- 4.Serruys PW, Unger F, Sousa JE, et al. ; Arterial Revascularization Therapies Study Group . Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344(15):1117-1124. doi: 10.1056/NEJM200104123441502 [DOI] [PubMed] [Google Scholar]
- 5.Park S-J, Ahn J-M, Kim Y-H, et al. ; BEST Trial Investigators . Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med. 2015;372(13):1204-1212. doi: 10.1056/NEJMoa1415447 [DOI] [PubMed] [Google Scholar]
- 6.Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol. 1999;34(3):618-620. doi: 10.1016/S0735-1097(99)00250-8 [DOI] [PubMed] [Google Scholar]
- 7.Sasieni PD, Wald NJ. Should a reduction in all-cause mortality be the goal when assessing preventive medical therapies? Circulation. 2017;135(21):1985-1987. doi: 10.1161/CIRCULATIONAHA.116.023359 [DOI] [PubMed] [Google Scholar]
- 8.Zanolla L, Zardini P. Selection of endpoints for heart failure clinical trials. Eur J Heart Fail. 2003;5(6):717-723. doi: 10.1016/S1388-9842(03)00101-6 [DOI] [PubMed] [Google Scholar]
- 9.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 10.Viechtbauer W, Viechtbauer MW. Package “metafor”. Published March 19, 2020. Accessed April 20, 2020. https://cran.r-project.org/web/packages/metafor/metafor.pdf
- 11.Higgins JPT, Green S. 10.4.4.2 Trim and fill. Cochrane Handbook for Systematic Reviews of Interventions. Published March 2011. Accessed January 14, 2020. https://handbook-5-1.cochrane.org/chapter_10/10_4_4_2_trim_and_fill.htm
- 12.Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients: 1-year results of the CARDIA (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol. 2010;55(5):432-440. doi: 10.1016/j.jacc.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 13.Ahn J-M, Roh J-H, Kim Y-H, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease: 5-year outcomes of the PRECOMBAT Study. J Am Coll Cardiol. 2015;65(20):2198-2206. doi: 10.1016/j.jacc.2015.03.033 [DOI] [PubMed] [Google Scholar]
- 14.Kamalesh M, Sharp TG, Tang XC, et al. ; VA CARDS Investigators . Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol. 2013;61(8):808-816. doi: 10.1016/j.jacc.2012.11.044 [DOI] [PubMed] [Google Scholar]
- 15.Holm NR, Mäkikallio T, Lindsay MM, et al. ; NOBLE investigators . Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet. 2020;395(10219):191-199. doi: 10.1016/S0140-6736(19)32972-1 [DOI] [PubMed] [Google Scholar]
- 16.SoS Investigators . Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): a randomised controlled trial. Lancet. 2002;360(9338):965-970. doi: 10.1016/S0140-6736(02)11078-6 [DOI] [PubMed] [Google Scholar]
- 17.Serruys PW, Ong ATL, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol. 2005;46(4):575-581. doi: 10.1016/j.jacc.2004.12.082 [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez AE, Baldi J, Fernández Pereira C, et al. ; ERACI II Investigators . Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol. 2005;46(4):582-588. doi: 10.1016/j.jacc.2004.12.081 [DOI] [PubMed] [Google Scholar]
- 19.Buszman PE, Buszman PP, Banasiewicz-Szkróbka I, et al. Left main stenting in comparison with surgical revascularization: 10-Year Outcomes of the (Left Main Coronary Artery Stenting) LE MANS trial. JACC Cardiovasc Interv. 2016;9(4):318-327. doi: 10.1016/j.jcin.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 20.Boudriot E, Thiele H, Walther T, et al. Randomized comparison of percutaneous coronary intervention with sirolimus-eluting stents versus coronary artery bypass grafting in unprotected left main stem stenosis. J Am Coll Cardiol. 2011;57(5):538-545. doi: 10.1016/j.jacc.2010.09.038 [DOI] [PubMed] [Google Scholar]
- 21.Hueb W, Lopes N, Gersh BJ, et al. Ten-year follow-up survival of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2010;122(10):949-957. doi: 10.1161/CIRCULATIONAHA.109.911669 [DOI] [PubMed] [Google Scholar]
- 22.Mohr FW, Morice M-C, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381(9867):629-638. doi: 10.1016/S0140-6736(13)60141-5 [DOI] [PubMed] [Google Scholar]
- 23.Thuijs DJFM, Kappetein AP, Serruys PW, et al. ; SYNTAX Extended Survival Investigators . Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet. 2019;394(10206):1325-1334. doi: 10.1016/S0140-6736(19)31997-X [DOI] [PubMed] [Google Scholar]
- 24.Cisowski M, Drzewiecki J, Drzewiecka-Gerber A, et al. Primary stenting versus MIDCAB: preliminary report-comparision of two methods of revascularization in single left anterior descending coronary artery stenosis. Ann Thorac Surg. 2002;74(4):S1334-S1339. doi: 10.1016/S0003-4975(02)03971-1 [DOI] [PubMed] [Google Scholar]
- 25.Blazek S, Holzhey D, Jungert C, et al. Comparison of bare-metal stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery: 10-year follow-up of a randomized trial. JACC Cardiovasc Interv. 2013;6(1):20-26. doi: 10.1016/j.jcin.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 26.Drenth DJ, Veeger NJGM, Middel B, Zijlstra F, Boonstra PW. Comparison of late (four years) functional health status between percutaneous transluminal angioplasty intervention and off-pump left internal mammary artery bypass grafting for isolated high-grade narrowing of the proximal left anterior descending coronary artery. Am J Cardiol. 2004;94(11):1414-1417. doi: 10.1016/j.amjcard.2004.08.016 [DOI] [PubMed] [Google Scholar]
- 27.Kim JW, Lim DS, Sun K, Shim WJ, Rho YM. Stenting or MIDCAB using ministernotomy for revascularization of proximal left anterior descending artery? Int J Cardiol. 2005;99(3):437-441. doi: 10.1016/j.ijcard.2004.08.045 [DOI] [PubMed] [Google Scholar]
- 28.Pohl T, Giehrl W, Reichart B, et al. Retroinfusion-supported stenting in high-risk patients for percutaneous intervention and bypass surgery: results of the prospective randomized myoprotect I study. Catheter Cardiovasc Interv. 2004;62(3):323-330. doi: 10.1002/ccd.20060 [DOI] [PubMed] [Google Scholar]
- 29.Eefting F, Nathoe H, van Dijk D, et al. Randomized comparison between stenting and off-pump bypass surgery in patients referred for angioplasty. Circulation. 2003;108(23):2870-2876. doi: 10.1161/01.CIR.0000100723.50363.2C [DOI] [PubMed] [Google Scholar]
- 30.Goy J-J, Kaufmann U, Hurni M, et al. ; SIMA Investigators . 10-year follow-up of a prospective randomized trial comparing bare-metal stenting with internal mammary artery grafting for proximal, isolated de novo left anterior coronary artery stenosis the SIMA (Stenting versus Internal Mammary Artery grafting) trial. J Am Coll Cardiol. 2008;52(10):815-817. doi: 10.1016/j.jacc.2008.05.037 [DOI] [PubMed] [Google Scholar]
- 31.Hong SJ, Lim D-S, Seo HS, et al. Percutaneous coronary intervention with drug-eluting stent implantation vs. minimally invasive direct coronary artery bypass (MIDCAB) in patients with left anterior descending coronary artery stenosis. Catheter Cardiovasc Interv. 2005;64(1):75-81. doi: 10.1002/ccd.20238 [DOI] [PubMed] [Google Scholar]
- 32.Thiele H, Neumann-Schniedewind P, Jacobs S, et al. Randomized comparison of minimally invasive direct coronary artery bypass surgery versus sirolimus-eluting stenting in isolated proximal left anterior descending coronary artery stenosis. J Am Coll Cardiol. 2009;53(25):2324-2331. doi: 10.1016/j.jacc.2009.03.032 [DOI] [PubMed] [Google Scholar]
- 33.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal B, Ellis SG, Lincoff AM, et al. Cause of death within 30 days of percutaneous coronary intervention in an era of mandatory outcome reporting. J Am Coll Cardiol. 2013;62(5):409-415. doi: 10.1016/j.jacc.2013.03.071 [DOI] [PubMed] [Google Scholar]
- 35.Bricker RS, Valle JA, Plomondon ME, Armstrong EJ, Waldo SW. Causes of mortality after percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes. 2019;12(5):e005355. doi: 10.1161/CIRCOUTCOMES.118.005355 [DOI] [PubMed] [Google Scholar]
- 36.Butt JH, Sørensen R, Bäck C, et al. Short- and long-term cause of death in patients undergoing isolated coronary artery bypass grafting: a nationwide cohort study. J Thorac Cardiovasc Surg. 2018;156(1):54-60.e4. doi: 10.1016/j.jtcvs.2018.01.106 [DOI] [PubMed] [Google Scholar]
- 37.Herlitz J, Brandrup-Wognsen G, Caidahl K, et al. Cause of death during 13 years after coronary artery bypass grafting with emphasis on cardiac death. Scand Cardiovasc J. 2004;38(5):283-286. doi: 10.1080/14017430410021615 [DOI] [PubMed] [Google Scholar]
- 38.Berg DD, Wiviott SD, Braunwald E, et al. Modes and timing of death in 66 252 patients with non-ST-segment elevation acute coronary syndromes enrolled in 14 TIMI trials. Eur Heart J. 2018;39(42):3810-3820. doi: 10.1093/eurheartj/ehy556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Head SJ, Milojevic M, Daemen J, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet. 2018;391(10124):939-948. doi: 10.1016/S0140-6736(18)30423-9 [DOI] [PubMed] [Google Scholar]
- 40.Doenst T, Haverich A, Serruys P, et al. PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J Am Coll Cardiol. 2019;73(8):964-976. doi: 10.1016/j.jacc.2018.11.053 [DOI] [PubMed] [Google Scholar]
- 41.Pedersen F, Butrymovich V, Kelbæk H, et al. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol. 2014;64(20):2101-2108. doi: 10.1016/j.jacc.2014.08.037 [DOI] [PubMed] [Google Scholar]
- 42.Doost Hosseiny A, Moloi S, Chandrasekhar J, Farshid A. Mortality pattern and cause of death in a long-term follow-up of patients with STEMI treated with primary PCI. Open Heart. 2016;3(1):e000405. doi: 10.1136/openhrt-2016-000405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spoon DB, Psaltis PJ, Singh M, et al. Trends in cause of death after percutaneous coronary intervention. Circulation. 2014;129(12):1286-1294. doi: 10.1161/CIRCULATIONAHA.113.006518 [DOI] [PubMed] [Google Scholar]
- 44.Mauri L, Cox D, Hermiller J, et al. The PROXIMAL trial: proximal protection during saphenous vein graft intervention using the Proxis Embolic Protection System: a randomized, prospective, multicenter clinical trial. J Am Coll Cardiol. 2007;50(15):1442-1449. doi: 10.1016/j.jacc.2007.06.039 [DOI] [PubMed] [Google Scholar]
- 45.Palmerini T, Sangiorgi D, Valgimigli M, et al. Short- versus long-term dual antiplatelet therapy after drug-eluting stent implantation: an individual patient data pairwise and network meta-analysis. J Am Coll Cardiol. 2015;65(11):1092-1102. doi: 10.1016/j.jacc.2014.12.046 [DOI] [PubMed] [Google Scholar]
- 46.Palmerini T, Benedetto U, Bacchi-Reggiani L, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet. 2015;385(9985):2371-2382. doi: 10.1016/S0140-6736(15)60263-X [DOI] [PubMed] [Google Scholar]
- 47.Zumwalt RE, Ritter MR. Incorrect death certification: an invitation to obfuscation. Postgrad Med. 1987;81(8):245-247, 250, 253-254. doi: 10.1080/00325481.1987.11699876 [DOI] [PubMed] [Google Scholar]
- 48.Kircher T, Anderson RE. Cause of death: proper completion of the death certificate. JAMA. 1987;258(3):349-352. doi: 10.1001/jama.1987.03400030065033 [DOI] [PubMed] [Google Scholar]
- 49.Nordmann AJ, Briel M, Bucher HC. Mortality in randomized controlled trials comparing drug-eluting vs bare metal stents in coronary artery disease: a meta-analysis. Eur Heart J. 2006;27(23):2784-2814. doi: 10.1093/eurheartj/ehl282 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Full Search Strategy
eTable 2. Summary of the Included Randomized Clinical Trials
eTable 3. Details of Patient Characteristics
eTable 4. Procedural Characteristics
eTable 5. Details of Medical Therapy
eTable 6. Details of Trial End Points
eTable 7. Adjudication of Cause of Death in the Randomized Clinical Trials
eTable 8. Details of Noncardiac Mortality in Percutaneous Coronary Intervention (PCI) vs Coronary Artery Bypass Grafting (CABG) Randomized Clinical Trials
eFigure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flowchart of Our Analysis
eFigure 2. Funnel Plot for Studies Reporting All-Cause Mortality
eFigure 3. Funnel Plot for Studies Reporting Cardiac Mortality
eFigure 4. Funnel Plot for Studies Reporting Noncardiac Mortality
eFigure 5. Leave-One-Out Analysis for All-Cause Mortality for Fixed-Effects Model (A) and Random-Effects Model
eFigure 6. Leave-One-Out Analysis for Cardiac Mortality for Fixed-Effects Model (A) and Random-Effects Model (B)
eFigure 7. Leave-One-Out Analysis for Noncardiac Mortality for Fixed-Effects Model (A) and Random-Effects Model (B)
eFigure 8. Subgroup Analysis for All-Cause Mortality for Trials Including Patients With Left Main Disease vs Non-Left Main Disease
eFigure 9. Subgroup Analysis for Cardiac Mortality for Trials Including Patients With Left Main Disease vs Non-Left Main Disease
eFigure 10. Subgroup Analysis for Noncardiac Mortality for Trials Including Patients With Left Main Disease vs Non-Left Main Disease
eMethods. The Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2)
eReferences.