Abstract
Study Objectives
Disrupted nighttime sleep (DNS) is a core narcolepsy symptom of unconsolidated sleep resulting from hypocretin neuron loss. In this study, we define a DNS objective measure and evaluate its diagnostic utility for pediatric narcolepsy type 1 (NT1).
Methods
This was a retrospective, multisite, cross-sectional study of polysomnograms (PSGs) in 316 patients, ages 6–18 years (n = 150 NT1, n = 22 narcolepsy type 2, n = 27 idiopathic hypersomnia, and n = 117 subjectively sleepy subjects). We assessed sleep continuity PSG measures for (1) their associations with subjective and objective daytime sleepiness, daytime sleep onset REM periods (SOREMPs), self-reported disrupted nocturnal sleep and CSF hypocretin levels and (2) their predictive value for NT1 diagnosis. We then combined the best performing DNS measure with nocturnal SOREMP (nSOREMP) to assess the added value to the logistic regression model and the predictive accuracy for NT1 compared with nSOREMP alone.
Results
The Wake/N1 Index (the number of transitions from any sleep stage to wake or NREM stage 1 normalized by total sleep time) was associated with objective daytime sleepiness, daytime SOREMPs, self-reported disrupted sleep, and CSF hypocretin levels (p’s < 0.003) and held highest area under the receiver operator characteristic curves (AUC) for NT1 diagnosis. When combined with nSOREMP, the DNS index had greater accuracy for diagnosing NT1 (AUC = 0.91 [0.02]) than nSOREMP alone (AUC = 0.84 [0.02], likelihood ratio [LR] test p < 0.0001).
Conclusions
The Wake/N1 Index is an objective DNS measure that can quantify DNS severity in pediatric NT1. The Wake/N1 Index in combination with or without nSOREMP is a useful sleep biomarker that improves recognition of pediatric NT1 using only the nocturnal PSG.
Keywords: pediatric narcolepsy, NT1, disrupted nighttime sleep, DNS, diagnosis, validity, sleep
Statement of Significance.
Disrupted nighttime sleep (DNS) is a common narcolepsy symptom, and yet, there is no validated, objective measure of this symptom. Consequently, neurologists and sleep physicians have no way of systematically assessing DNS or studying its possible health effects. Furthermore, the diagnosis of narcolepsy in children is typically delayed 5–10 years as signs of narcolepsy are missed by their doctors, including those reading their sleep studies. In this study, we objectively define DNS, using polysomnogram (PSG) sleep quality measures that show content validity and diagnostic accuracy for pediatric narcolepsy type 1 (NT1). We identify sleep diagnostic biomarkers that will permit clinicians to diagnose pediatric NT1 using the PSG alone, enabling faster and more accurate diagnosis.
Introduction
Narcolepsy type 1 (NT1) is caused by near-complete loss of the hypocretin-producing neurons. Loss of these key neurons destabilizes wake and sleep states, with frequent lapses into sleep during the day and spontaneous awakenings at night, resulting in disrupted nighttime sleep (DNS) [1, 2]. NT1 usually begins before age 18 years, and about 48%–78% of pediatric NT1 patients and their caregivers report DNS based on the presence of frequent nocturnal wakings [3, 4]. While DNS is a common symptom in NT1, there is no validated, objective DNS measure.
On average, it takes about 10 years for pediatric patients to be correctly diagnosed with NT1 [5, 6] due to clinical providers’ lack of awareness about narcolepsy [7] and limited availability of sequential polysomnogram (PSG) and multiple sleep latency testing (MSLT) for pediatric patients. Thus, identification of new diagnostic biomarkers for pediatric NT1 is essential to reduce time to diagnosis and treatment [8].
A biomarker that has received increased attention is the nocturnal sleep onset REM period (nSOREMP). REM sleep normally occurs about 90 min after sleep onset, but REM sleep is dysregulated in NT1 and can occur as a nSOREMP (REM sleep within 15 min of sleep onset) [8–10]. The presence of a nSOREMP on the nocturnal PSG has high specificity (96%–97%), but it has disappointingly low sensitivity (47%–53%) [9, 11]. Researchers have also studied REM sleep without atonia, another physiological findings reflecting REM sleep dysregulation in NT1 [12–15] but this biomarker has similarly low sensitivity and high specificity as nSOREMP for NT1 diagnosis [16].
An objective measure of DNS would likely be another useful biomarker as NT1 patients commonly have increased awakenings, arousals, sleep stage transitions, and light NREM sleep (NREM stage 1, N1) compared with controls [14, 17, 18]. Thus, we investigated if an objective measure of DNS coupled with the nSOREMP could have strong sensitivity and specificity for diagnosing pediatric NT1 using the PSG alone.
The aims of this research were (1) to identify a DNS measure that is associated with daytime sleepiness and SOREMPs, self-reported sleep disturbance, reduced CSF hypocretin, and NT1 diagnosis and (2) determine if this DNS measure improves diagnostic accuracy in pediatric NT1 when coupled with the nSOREMP. If successful, these sleep biomarkers could hasten the diagnosis of pediatric NT1 [8] using the nocturnal PSG alone and reduce the psychological, academic, safety, and social burdens of untreated symptoms [6, 19].
Methods
Participants
We identified 331 participants ages 6–18 years from two sites (Boston Children’s Hospital and University of Bologna) who had PSG/MSLT testing between September 2015 and September 2019 and were either naive to drugs or weaned off potentially sedating, alerting or REM sleep-suppressing drugs for at least 2 weeks prior to sleep studies [20]. At the Bologna site, medications were not used at the time of PSG/MSLT assessment as all subjects were either drug-naive or evaluated after at least 3 weeks of drug withdrawal. At the Boston site, use of sedating or alerting medications or medications that would affect REM sleep were withdrawn 2 weeks or 5 half-lives of the drug prior to the PSG/MSLT night [20]. In Boston, additional urine comprehensive drug screening (Quest Diagnostics LLC, Marlborough, MA) was performed and subjects who tested positive for drugs of abuse (1 subjectively sleepy subject [SSS] and 1 narcolepsy type 2 [NT2] tested positive for marijuana) were excluded. Two participants who were positive for caffeine (1 NT2 and 1 NT1) were included in analysis. Additional exclusion criteria were: (1) presence of OSA (obstructive AHI ≥ 1/h) [8] based on current or prior PSG testing; (2) slept < 6 h on the PSG; (3) did not meet ICSD-3 diagnoses [8] of NT1, NT2, and idiopathic hypersomnia (IH). Patients with NT1, NT2, and IH were not excluded if they had co-morbid PSG findings of periodic limb movements of sleep or other medical and psychiatric conditions such as restless legs syndrome and mood disorders.
SSS were patients who reported daytime sleepiness but had normal PSG and MSLT results. The primary complaint of subjective sleepiness was assessed clinically, taking into account subjects’ sleeping habits, ruling out other sleep disorders, behavioral changes, and reports from teachers at school, and not necessarily confirmed by a high ESS score. In this SSS group, the underlying cause of subjective sleepiness was often unclear. One participant was excluded because their symptoms were consistent with Kleine Levin Syndrome [8], and 14 participants were excluded for total sleep time <6 h. In total, we included 316 participants (n = 150 NT1; n = 22 NT2; n = 27 IH, and n = 117 SSS).
Clinical and polysomnographic data
Subjects were clinically evaluated by a sleep medicine specialist and had PSG testing [21] run between 8:00 pm and 9:00 am followed by a fixed five nap MSLT. All PSG/MSLT studies were conducted as technician-attended sleep studies. In Boston, the sleep studies were conducted in-lab at the Boston Children’s Hospital Pediatric Sleep Center, and data were collected on Natus Sleep Works software (Natus Medical Inc., San Carlos, CA, USA). Dedicated BCH scoring technicians who successfully completed the American Academy of Sleep Medicine (AASM) Interscorer Reliability Program scored the sleep architecture, respiratory and movement events, and arousals using AASM criteria [21]. Board-certified sleep physicians at BCH reviewed and interpreted the recordings. At the Bologna site, sleep testing was conducted in hospital, and the MSLT was preceded by a 48 h continuous ambulatory PSG described elsewhere [22]. A board‐certified polysomnographic technician (S.V.) who was blind to the clinical diagnosis scored the PSG and the MSLT data. About 75% of the 197 Bologna subjects had lumbar puncture for CSF hypocretin-1 levels. Demographic data including adapted Epworth Sleepiness Scores (aESS) [23], self-reported sleep disturbance (categorical difficulty sustaining sleep or presence of nocturnal wakings), and medication status (drug-naive or not) were extracted from the patients’ medical charts or patient interview at both sites.
The study was approved by the local Institutional Review Boards at Boston Children’s Hospital and University of Bologna, and de-identified data use agreements were approved by participating institutions.
Sleep data
The first PSG night was scored in 30 s epochs based on AASM criteria [21]. Proposed DNS measures were derived from the literature [2, 17, 18, 22, 24, 25] and included sleep efficiency (SE) and percent N1 sleep (N1%). Arousal Index data were not available for the Bologna subjects so assessment of this measure was conducted with Boston site data only. To accurately identify sleep transitions, a template of participants’ states (wake, N1, N2, N3, and REM) every 30 s across the nocturnal PSG (lights out, light on) was created in Microsoft Excel for every participant. To extract nocturnal sleep stage and wake transitions, the Bologna site used an Excel Macro whereas the Boston site used a MATLAB script to collect transitions data. The translational indices included (1) Wake index (number of transition from any stage of sleep to Wake), (2) REM to Wake/N1 index (number of transitions from REM to Wake or N1), (3) NREM 2, 3 to Wake/N1 index (number of transitions from deep NREM sleep [N2 or N3] to Wake or N1), (4) REM/N2/N3 to Wake/N1 Index (number of transitions from REM, N2, or N3 sleep to Wake or N1), and (5) Wake/N1 index (number of transitions from sleep to wake and number of transitions from N2, N3, or REM to N1 sleep). An example of the Wake/N1 Index calculation is shown in Figure 1. Inter-rater scoring reliability between sites on the Wake/N1 Index was excellent (ICC = 0.97, 95% CI: 0.90–0.99, p < 0.0005) based on shared data from 11 randomly sampled subjects.
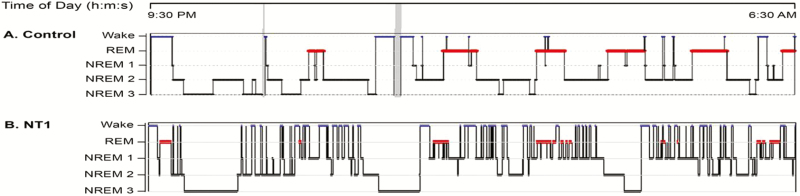
Figure 1.
(A) A 14-year-old male SSS and (B) a 14-year-old male with NT1. For the purposes of illustration, hypnograms are shown as 9 h of total sleep time. The number of Wake/N1 transitions is calculated based on the number of transitions from any sleep stage sleep to wake and number of transitions from N2, N3, or REM to N1 sleep. The SSS has 11 wake periods from sleep and 3 transitions from sleep to N1 (total of 14 Wake/N1 transitions for a Wake/N1 index of 1.6/h). The NT1 patient has 54 wake periods from sleep and 18 transitions to N1 from N2/N3/REM (total 72 Wake/N1 transitions for Wake/N1 Index of 8/h).
Statistical methods
Subject characteristics at baseline and sleep measures are summarized with descriptive statistics (Table 1). Correlations between sleep measures were assessed using Pearson correlation coefficients. Categorical and continuous variables are compared using Fishers’ exact test and independent t-test, respectively. We evaluated candidate DNS measures that had significant associations with at least four outcomes of interest. Logistic regression, adjusted for age, gender, race, and medication status (naive vs. weaned), was used to estimate the association between each DNS measures and NT1 diagnosis, and we estimated the area under the receiver operator characteristic curves (AUC). The likelihood ratio (LR) test was used to assess the added value of new DNS measures to the nSOREMP model. A bootstrap approach was used to correct for optimism because the accuracy was estimated from the same data that was used to fit the model. Resampling was stratified by site to account for potential differences in measured and unmeasured characteristics between sites. The optimal cut off point was the one with sensitivity and specificity close to 80%. Results were considered significant if p-values < 0.05. SAS 9.4 was used for analysis (Copyright 2013 SAS Institute; Cary, NC, USA).
Table 1.
Baseline demographic and clinical characteristics of subjects
| Baseline characteristics | NT1(n = 150) | NT2 (n = 22) | IH (n = 27) | SSS (n = 117) | P-value (NT1 vs. non-NT1) |
|---|---|---|---|---|---|
| Age mean years (SD) | 12 (3.6) | 14.3 (2.6) | 15.6 (2.2) | 13 (4.4) | <0.0005 |
| Gender, female, n (%) | 67 (44.6) | 9 (40.9) | 24 (88.9) | 45 (38.5) | 0.74 |
| Race, n (Caucasian%) | 122 (81.3) | 18 (81.8) | 22 (81.5) | 104 (88.9) | 0.28 |
| BMI | 24.5 (6.2) | 23.5 (4.1) | 22.4.8 (2.9) | 21.7 (5) | <0.0005 |
| Positive HLA DQB1*06:02 (%) | 139 (92.7) | 9 (40.9) | 5 (21.7) | 13 (17.3) | <0.0005 |
| Symptom duration (years) | 2.8 (2.5) | 4 (4.6) | 4 (3.9) | 3.5 (4.5) | NS |
| Drug-naive (yes %) | 148 (94.9) | 16 (72.7) | 21 (80.8) | 98 (81.7) | 0.001 |
| Site (Bologna %) | 107 (71.3) | 10 (45.5) | 10 (37) | 70 (59.8) | 0.002 |
| Epworth Sleepiness Scale score | 15.1 (3.6) | 13.3 (4.9) | 13.1 (5.4) | 11.7 (5) | <0.0005 |
| MSLT-mean sleep latency (min) | 3.6 (2.9) | 7.1 (4.7) | 9 (3.7) | 16.1 (3.2) | <0.0005 |
| MSLT-number of SOREMPs | 4.1 (1.1) | 2.3 (1.1) | 0.26 (0.4) | 0.14 (0.4) | <0.0005 |
| Self-reported sleep disturbance (yes %) | 80 (53.3) | 5 (22.7) | 1 (4) | 28 (27.7) | <0.0005 |
| PSG measures | |||||
| Total sleep time (h) | 8.7 (1.3) | 9.1 (1.3) | 9.5 (2.4) | 8.7 (1.3) | 0.34 |
| Sleep onset latency (min) | 9.4 (13.3) | 21.6 (19.3) | 27.4 (28.8) | 33.8 (30.1) | <0.0005 |
| nSOREMP (yes %) | 107 (71.3) | 4 (18.1) | 0 | 3 (2.6) | <0.0005 |
| SE (%) | 89.8 (6.6) | 93.2 (5.1) | 92.1 (5.8) | 90.4 (8.5) | 0.15 |
| Wake after sleep onset (min) | 57.2 (40.1) | 26.3 (17.5) | 29.8 (27.6) | 40.8 (44.1) | <0.0005 |
| NREM stage 1 (N1) % of TST | 10.7 (5.5) | 7.1 (3.7) | 5.9 (3.9) | 5.6 (3.6) | <0.0005 |
| NREM stage 2 (N2) % of TST | 40.1 (8.4) | 43.2 (8.5) | 50.4 (10.6) | 44 (10.4) | <0.0005 |
| NREM stage 3 (N3) % of TST | 24.3 (8.3) | 22.5 (11) | 18.7 (10.4) | 26.7 (10.3) | 0.67 |
| REM% of TST | 25 (6.2) | 27.2 (5.4) | 24.9 (4.2) | 23.2 (5.2) | 0.14 |
| Wake index | 3.4 (2) | 1.8 (1) | 1.7 (1.2) | 2 (1.3) | <0.0005 |
| Wake/N1 index | 5.6 (2.4) | 3.1 (1.4) | 3 (1.7) | 3.3 (1.6) | <0.0005 |
| N2/N3 to Wake/N1 Index | 2.8 (1.2) | 1.6 (0.9) | 1.6 (0.87) | 1.9 (1) | <0.0005 |
| REM to Wake/N1 Index | 1.8 (0.9) | 1 (0.6) | 0.95 (0.7) | 0.93 (0.67) | <0.0005 |
| REM/N2/N3 to Wake/N1 Index | 4.5 (1.7) | 2.6 (1.3) | 2.6 (1.3) | 2.8 (1.4) | <0.0005 |
| Bologna site only | NT1 (n = 96) | NT2 (n = 8) | IH (n = 7) | SSS (n = 36) | P-value (NT1 vs. non-NT1) |
| CSF hypocretin values (mean pg/ml) | 28.6 (52.6) | 342.2 (48.6) | 336.5 (32.2) | 342.3 (51.9) | <0.0005 |
| Boston site only | NT1 (n = 43) | NT2 (n = 12) | IH (n = 17) | SSS (n = 47) | P-value (NT1 vs. non-NT1) |
| Arousal index | 12.9 (5.7) | 10 (2.9) | 8.1 (4.6) | 8.8 (3.2) | <0.0005 |
NT1, narcolepsy type 1; NT2, narcolepsy type 2; IH, idiopathic hypersomnia; SSS, subjectively sleepy subjects; SOREMP, sleep onset REM periods (REM sleep stage ≤ 15 min from sleep onset during daytime naps); nSOREMP, nocturnal sleep onset REM period (REM sleep stage ≤15 min from sleep onset on a PSG). Index measures relative to total sleep time. Means presented with standard deviations.
Results
A STARD flow diagram of Index and Reference Testing is provided in Supplementary Materials (Figure S1).
Construct validity of DNS measures
Based on univariate screening, we identified four candidate DNS measures (N1%, Wake Index, Wake/N1 Index, and NREM 2/3 to Wake/N1 Index) that had significant associations with at least three outcomes of interest (Table 2).
Table 2.
PSG measures and their associations with sleepiness, sleep disturbance, and CSF hypocretin levels
| Adapted Epworth Sleepiness Score n = 316 | Mean sleep latency on MSLT n = 316 | #SOREMPs on MSLT n = 316 | Self-reported sleep disturbance n = 316 | CSF hypocretin* n = 147 | Candidate DNS biomarker | |
|---|---|---|---|---|---|---|
| SE | NS | NS | r = −0.14, p = 0.002 | t = 2.8, p = 0.005 | r = 0.18, p = 0.04 | |
| Arousal index† | r = 0.35, p = 0.005 | r = −0.38, p < 0.0005 | r = 0.23, p = 0.01 | t = −2.23, p = 0.03 | x | |
| N1% of TST | r = 0.21, p = 0.001 | r = −0.40, p < 0.0005 | r = 0.45, p < 0.0005 | t = −3.9, p < 0.0005 | r = −0.53, p < 0.0005 | x |
| Wake index | NS | r = −0.31, p < 0.0005 | r = 0.36, p < 0.0005 | t = 3.3, p = 0.002 | r = −0.35, p < 0.0005 | x |
| Wake/N1 Index | NS | r = −0.38, p < 0.0005 | r = 0.47, p < 0.0005 | t = 2.8, p = 0.006 | r = −0.51, p < 0.0005 | x |
| N2/N3 to Wake/ N1 Index | NS | r = −0.29, p < 0.0005 | r = 0.35, p < 0.0005 | t = 2.0, p = 0.04 | r = −0.34, p < 0.0005 | x |
| REM to Wake/ N1 Index | NS | r = −0.35, p < 0.0005 | r = 0.45, p < 0.0005 | NS | r = −0.47, p < 0.0005 | |
| REM/N2/N3 to Wake/N1 Index | NS | r = −0.38, p < 0.0005 | r = 0.46, p < 0.0005 | NS | r = −0.5, p < 0.0005 |
*CSF hypocretin available from Bologna site only.
†Arousal index available from Boston site only.
Candidate DNS Biomarkers are marked with “x” if they showed univariate associations with at least four outcome measures (Epworth Score, mean sleep latency on MSLT, self-reported sleep disturbance, and CSF Hypocretin). Spearman correlation values are reported for continuous variables and t-test. p-values are reported for self-reported sleep disturbance. NS signifies p ≥ 0.05.
Predictive value of DNS candidate measures
Of all candidate measures for the diagnosis of NT1, the Wake/N1 Index had the highest AUC. Specifically, the Wake/N1 Index had an AUC of 0.81 (0.02) with a 95% CI of 0.76 to 0.86 (p < 0.0005). In contrast, N1% had an AUC of 0.79 (0.03) and 95% CI of 0.74–0.84 (p < 0.0005); Wake Index had an AUC of 0.74 (0.03) and 95% CI of 0.68 to 0.79; and NREM 2/3 to Wake/N1 Index had an AUC of 0.73 (0.03) and 95% CI of 0.68–0.79 (p < 0.0005). In the Boston site, arousal index had an AUC of 0.74 (0.05) and 95% CI of 0.64–0.83 (p < 0.0005).
Predictive value of combined nocturnal PSG biomarkers for the diagnosis of NT1
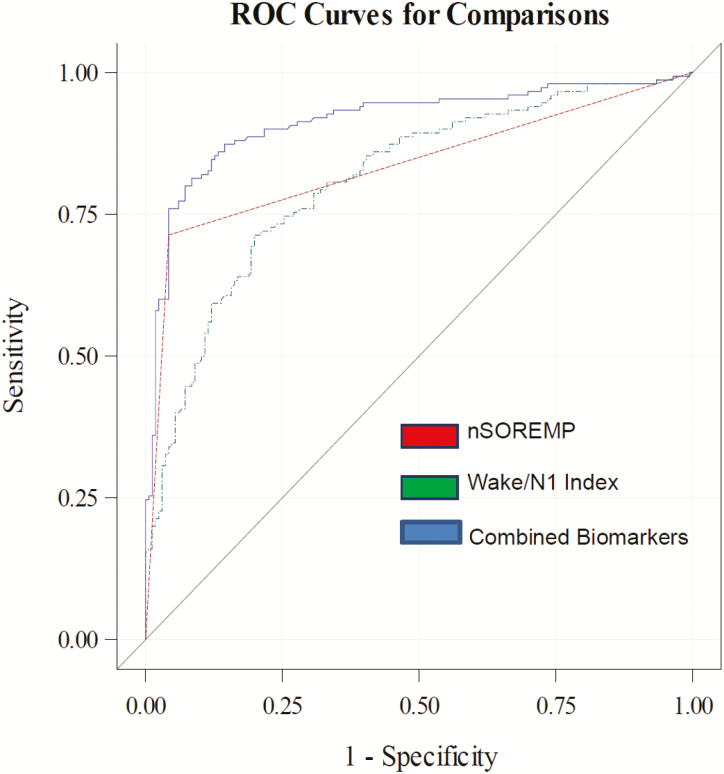
To correct for optimism, we used 1,000 bootstrap samples. A model with nSOREMP alone had an AUC of 0.84 (0.02) with 95% CI of 0.80 to 0.87 (p < 0.0005) and a sensitivity of 0.71 (0.04) and specificity of 0.96 (0.02). Combining the presence of nSOREMP with Wake/N1 index yielded an excellent AUC of 0.91 (0.02) with 95% CI of 0.88 to 0.95 (p < 0.0005; Figure 2) and resulted in improved fit to the model (LR test, p < 0.0001). This improvement in AUC was statistically significant (p < 0.0001), indicating superior predictive value. The optimal cutoff value for Wake/N1 Index was ≥ 5.5/h (0.77) for NT1, and when this cutoff was combined with the presence of a nSOREMP, the model sensitivity was 0.82 (0.03) and specificity was 0.88 (0.02). For diagnosing NT1, the combined biomarkers (presence of nSOREMP and/or DNS ≥ 5.5/h) had a positive predictive value of 0.87 (0.2) and negative predictive value of 0.85 (0.03). The results were unchanged after adjusting for age, race, gender, and medication status.
Figure 2.
Receiver operating characteristic curves for sleep biomarkers. Tested biomarkers include the nocturnal sleep onset REM period (nSOREMP), Wake/N1 Index (≥5.5/h), and the combination of the two biomarkers.
Discussion
While DNS has been studied in adult patients with CNS hypersomnias [2, 22], there are very limited data in pediatric cohorts. Between 48% and 78% of pediatric NT1 patients report subjective sleep disturbance [3, 4], yet it is unclear what objective DNS measures have good content validity. Among the many clinical sleep quality measures used in the literature to describe DNS [2, 17, 18, 22, 24, 25], we show that the Wake/N1 Index is an excellent objective measure of DNS based on its associations with objective daytime sleepiness, daytime SOREMPs, self-reported sleep disruption, CSF hypocretin levels, and NT1 diagnosis. In addition, combining the Wake/N1 Index with a nSOREMP is more accurate in diagnosing pediatric NT1 than a nSOREMP alone. The Wake/N1 Index can be calculated from routine PSGs, and it should be clinically useful in measuring DNS burden and screening for NT1 in children with a suspected hypersomnia disorder.
While DNS is a core symptom of NT1 [2], prior DNS research did not validate an objective measure of DNS. Dauvillers et al. and Roth et al. assessed the efficacy of sodium oxybate, a nighttime sedating narcolepsy medication, on DNS based on indices of transition from REM/N2/N3 to Wake/N1, N2/N3 to Wake/N1, and REM to Wake/N1 [17, 18]. In our study, these measures were not associated with self-reported sleep disturbance and thus hold less construct validity than the Wake/N1 Index. Possibly, patients’ perceptions of sleep disturbance are based on fragmentation of any sleep stage not just stable NREM and REM sleep. The Wake Index has also been used to assess DNS in NT1 [22], but in our study, this measure had less diagnostic accuracy than the Wake/N1 index. Since NT1 patients spend more time in N1 sleep than other groups [14, 22, 25], a DNS measure that includes N1 sleep transitions is important. Similarly, data from Boston site showed arousal index to be a good candidate measure of DNS but it too held less diagnostic accuracy for NT1 in comparison to the Wake/N1 Index. This indicates that arousals are a good measure of sleep fragmentation but less specific to NT1 diagnosis. Last, SE is a useful metric in patients with insomnia, but it is less helpful for assessing sleep quality in hypersomnias as it does not differentiate between hypersomnia groups and shows no relation to CSF hypocretin or objective sleepiness. Likely, DNS in NT1 reflects sleep/wake instability [1] which is distinct from the hyperarousal pathophysiology [26] that underlies insomnia.
The value of combining the nSOREMP and Wake/N1 Index is their enhanced sensitivity for NT1 diagnosis over the nSOREMP alone. The combined Wake/N1 index with cutoff ≥ 5.5/h and nSOREMP has a sensitivity of 82% and specificity of 88% (AUC 0.91 when corrected for optimism) compared with nSOREMP’s reported sensitivity of 47%–54% and specificity of 95%–97% (AUC’s 0.77–0.79) noted in the literature [11, 27]. Delayed diagnosis is a major clinical problem in narcolepsy with one meta-analysis reporting a mean delay of 15 years [5] for this debilitating but treatable condition. This diagnostic delay is nearly twice as likely to occur in people with symptom onset before age 18 years [6]. Based on unpublished data from the Boston site, about 20% of patients have a nocturnal PSG during their diagnostic journey for investigation of a primary sleep disorder such as obstructive sleep apnea. When the PSG proves negative for common abnormalities, patients are often re-directed to other non-sleep subspecialties for further evaluation. Thus, objective sleep biomarkers with improved sensitivity should hasten the recognition of patients with NT1 physiology. With relatively high sensitivity and specificity, the presence of nSOREMP, DNS Index ≥ 5.5/h or both biomarkers together are probably sufficient for pediatric NT1 diagnosis assuming the presence of [23] core symptoms including daytime sleepiness and cataplexy. Otherwise, clinicians could use these biomarkers to more efficiently direct patients to gold standard confirmatory tests, PSG and MSLT testing, or CSF hypocretin analysis [8, 28].
Strengths of this study include its large cohort of patients from different sites. The bootstrap approach of ROC analysis corrects for optimism to fit data (to prevent inflated AUC), promoting statistical rigor and reliability. Still, this study has some limitations. While all subjects met the minimum of 6 h of nocturnal sleep on the PSG night in order to conduct MSLT testing [20], only 6 h sleep is likely insufficient for children and adolescents [29]. It is unknown how much sleep deprivation in children/adolescents can result in nocturnal SOREMPs or affect SOREMPs present on the MSLT. The Bologna site did not collect arousal index, and the Boston site did not measure CSF hypocretin. The reliability of scoring arousals across multiple sites is complex as abnormalities in sleep microarchitecture such as cyclic alternating pattern exist in narcolepsy making agreement more challenging [30–32]. We believe that the wake state [21] is easier to recognize and makes scoring more reliable across sites. Furthermore, wake/sleep instability more accurately describes the pathophysiology of hypocretin deficiency across species [1, 25, 33]. Next, the generalizability of our findings may be limited because we did not include an OSA comparator group in this study. However, there is no evidence that OSA alters sleep stage transitions in pediatric cohorts [34]. The presence of a nSOREMP in our cohort (71%) is higher than reported in other studies (47%–53%) [9, 11], which may affect the generalizability of our findings. Last, we calculated sleep transitions using continuous 30 s stage logs extracted from PSGs and either MATLAB or Excel Macro programs to extract accurate transition measures. As the Wake/N1 Index is a new metric, this analysis may not be feasible in clinical practice, and counting transitions on hypnograms may be less accurate. Overall, we hope that future sleep scoring software will be adapted to calculate sleep stage transitions like the Wake/N1 Index for more accurate diagnosis and assessment of DNS symptom severity.
In conclusion, DNS is a distinct sleep pathology in pediatric NT1 patients that differs from the more stable sleep observed in CNS hypersomnia conditions and SSS. The Wake/N1 Index is an objective measure of DNS in pediatric NT1 patients that quantifies symptom severity and burden and holds diagnostic value. When coupled with the nSOREMP, the Wake/N1 Index has high sensitivity and specificity for diagnosis of pediatric NT1 using standard nocturnal PSGs alone. We believe this objective DNS measure should be included in future studies assessing the impact of DNS on daytime functioning, mood and medical co-morbidities of narcolepsy.
We urge practitioners to include validated questionnaires assessing daytime sleepiness (such as the aESS [23, 35]) in their clinical review of patients complaining of fatigue and/or sleepiness and to review PSG hypnogram data for the presence of a nSOREMP and Wake/N1 Index ≥ 5.5/h. Positive findings should prompt referral to sleep specialists for additional narcolepsy diagnostic testing as needed and disease management.
Supplementary Material
Funding
This study was supported by an investigator-initiated grant to K.M. from Jazz Pharmaceuticals, and in part from a National Institutes of Health grant 5K23NS104267-2 to K.M.
Conflict of interest statement. K.M. has received consulting fees from Jazz Pharmaceuticals, Harmony Biosciences, and Roche Pharmaceuticals. T.S. has received consulting fees from Accelerator, Alkermes, Avadel, Axsome, Harmony Biosciences, Jazz Pharmaceuticals, Merck, Suven, and Takeda. G.P. has participated on advisory boards for Jazz Pharmaceuticals, Inc., Bioprojet, and Idorsia. F.P., S.L., E.S., E.L., A.C., C.D.B., S.V., E.A., and E.W. report no disclosures. This study does not endorse any specific treatments for any disease entity.
Author Contributions
K.M., F.P., T.S., and G.P. contributed for study concept and design. Acquisition, analysis, or interpretation of data was done by K.M., F.P., E.S., E.L., S.V., E.A., E.W., S.L., C.D.B., A.C., G.P., and T.S. Drafting of the manuscript and/or figures was done by K.M., F.P., S.L., and T.S.
References
- 1. Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–181. [DOI] [PubMed] [Google Scholar]
- 2. Roth T, et al. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med. 2013;9(9):955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pizza F, et al. Childhood narcolepsy with cataplexy: comparison between post-H1N1 vaccination and sporadic cases. Sleep Med. 2014;15(2):262–265. [DOI] [PubMed] [Google Scholar]
- 4. Pizza F, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain. 2013;136(Pt 12):3787–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thorpy MJ, et al. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014;15(5):502–507. [DOI] [PubMed] [Google Scholar]
- 6. Maski K, et al. Listening to the patient voice in narcolepsy: diagnostic delay, disease burden, and treatment efficacy. J Clin Sleep Med. 2017;13(3):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carter LP, et al. Patients’ journeys to a narcolepsy diagnosis: a physician survey and retrospective chart review. Postgrad Med. 2014;126(3):216–224. [DOI] [PubMed] [Google Scholar]
- 8. American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition (ICSD-3). Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 9. Andlauer O, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol. 2013;70(7):891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Association; 2013: 372–378. [Google Scholar]
- 11. Reiter J, et al. Usefulness of a nocturnal SOREMP for diagnosing narcolepsy with cataplexy in a pediatric population. Sleep. 2015;38(6):859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DelRosso LM, et al. Characterization of REM sleep without atonia in patients with narcolepsy and idiopathic hypersomnia using AASM scoring manual criteria. J Clin Sleep Med. 2013;9(7):675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khalil A, et al. Loss of rapid eye movement sleep atonia in patients with REM sleep behavioral disorder, narcolepsy, and isolated loss of REM atonia. J Clin Sleep Med. 2013;9(10):1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antelmi E, et al. The spectrum of REM sleep-related episodes in children with type 1 narcolepsy. Brain. 2017;140(6):1669–1679. [DOI] [PubMed] [Google Scholar]
- 15. Dauvilliers Y, et al. REM sleep characteristics in narcolepsy and REM sleep behavior disorder. Sleep. 2007;30(7):844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bin-Hasan S, et al. Nocturnal REM sleep without atonia is a diagnostic biomarker of pediatric narcolepsy. J Clin Sleep Med. 2018;14(2):245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dauvilliers Y, et al. Effect of sodium oxybate, modafinil, and their combination on disrupted nighttime sleep in narcolepsy. Sleep Med. 2017;40:53–57. [DOI] [PubMed] [Google Scholar]
- 18. Roth T, et al. Effect of sodium oxybate on disrupted nighttime sleep in patients with narcolepsy. J Sleep Res. 2017;26(4):407–414. [DOI] [PubMed] [Google Scholar]
- 19. Flygare J, et al. Narcolepsy: let the patient’s voice awaken us! Am J Med. 2015;128(1):10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Littner MR, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28(1):113–121. [DOI] [PubMed] [Google Scholar]
- 21. Iber C, et al. The AASM Manual for the Scoring of Sleep and Associated Events. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 22. Pizza F, et al. Nocturnal sleep dynamics identify narcolepsy type 1. Sleep. 2015;38(8):1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janssen KC, et al. Validation of the epworth sleepiness scale for children and adolescents using rasch analysis. Sleep Med. 2017;33:30–35. [DOI] [PubMed] [Google Scholar]
- 24. Christensen JA, et al. Sleep-stage transitions during polysomnographic recordings as diagnostic features of type 1 narcolepsy. Sleep Med. 2015;16(12):1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sorensen GL, et al. Sleep transitions in hypocretin-deficient narcolepsy. Sleep. 2013;36(8):1173–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bonnet MH, et al. Hyperarousal and insomnia. Sleep Med Rev. 1997;1(2):97–108. [DOI] [PubMed] [Google Scholar]
- 27. Andlauer O, et al. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep. 2012;35(9):1247–155F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pizza F, et al. Validation of multiple sleep latency test for the diagnosis of pediatric narcolepsy type 1. Neurology. 2019;93(11):e1034–e1044. [DOI] [PubMed] [Google Scholar]
- 29. Paruthi S, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American academy of sleep medicine. J Clin Sleep Med. 2016;12(6):785–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferri R, et al. Sleep polygraphic study of children and adolescents with narcolepsy/cataplexy. Dev Neuropsychol. 2009;34(5):523–538. [DOI] [PubMed] [Google Scholar]
- 31. Terzano MG, et al. Cyclic alternating pattern (CAP) alterations in narcolepsy. Sleep Med. 2006;7(8):619–626. [DOI] [PubMed] [Google Scholar]
- 32. Poryazova R, et al. Cyclic alternating pattern in narcolepsy patients and healthy controls after partial and total sleep deprivation. Clin Neurophysiol. 2011;122(9):1788–1793. [DOI] [PubMed] [Google Scholar]
- 33. Branch AF, et al. Progressive loss of the orexin neurons reveals dual effects on wakefulness. Sleep. 2016;39(2):369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coussens S, et al. Movement distribution: a new measure of sleep fragmentation in children with upper airway obstruction. Sleep. 2014;37(12):2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murali H, et al. Off-label treatment of severe childhood narcolepsy-cataplexy with sodium oxybate. Sleep. 2006;29(8):1025–1029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.