Abstract
Study Objectives
Sleep deprivation and circadian disruptions impair brain function and cognitive performance, but few studies have examined the effect of sleep inconsistency. Here, we investigated how inconsistent sleep duration and sleep timing between weekends (WE) and weekdays (WD) correlated with changes in behavior and brain function during task and at rest in 56 (30 female) healthy human participants.
Methods
WE–WD differences in sleep duration and sleep midpoint were calculated using 1-week actigraphy data. All participants underwent 3 Tesla blood-oxygen-level-dependent functional Magnetic Resonance Imaging (fMRI) to measure brain activity during a visual attention task (VAT) and in resting-state condition.
Results
We found that WE–WD inconsistency of sleep duration and sleep midpoint were uncorrelated with each other (r = .08, p = .58) and influenced behavior and brain function differently. Our healthy participants showed relatively small WE–WD differences (WE–WD: 0.59 hours). Longer WE sleep duration (relative to WD sleep duration) was associated with better attentional performance (3-ball: β = .30, t = 2.35, p = .023; 4-ball: β = .30, t = 2.21, p = .032) and greater deactivation of the default mode network (DMN) during VAT (p < .05, cluster-corrected) and greater resting-state functional connectivity (RSFC) between anterior DMN and occipital cortex (p < .01, cluster-corrected). In contrast, later WE sleep timing (relative to WD sleep timing) (WE–WD: 1.11 hours) was associated with worse performance (4-ball: β = −.33, t = −2.42, p = .020) and with lower occipital activation during VAT and with lower RSFC within the DMN.
Conclusions
Our results document the importance of consistent sleep timing for brain function in particular of the DMN and provide evidence of the benefits of WE catch-up sleep in healthy adults.
Keywords: sleep inconsistency, weekend catch-up sleep, circadian misalignment, visual attention task, resting-state functional connectivity, default mode network, brain imaging
Statement of Significance.
Sleep inconsistency is highly prevalent in modern societies and usually related to work-induced sleep restriction and circadian misalignment on weekdays (WD) with subsequent compensatory sleep on weekends (WE). The current study sheds light on the changes in brain function associated with WD–WE inconsistent sleep duration and sleep timing in healthy adults. While WE catch-up sleep correlated with enhanced task-induced deactivation of default mode network (DMN) and DMN connectivity with occipital cortex at rest, inconsistent sleep timing, referred to as social jetlag, had detrimental effects on behavior and brain function during task and at rest (lower occipital activation during task and DMN resting-state functional connectivity). Our findings indicate a beneficial effect of consistent bedtime and catch-up sleep on WE.
Introduction
Sleep loss and circadian misalignment are common in modern societies and deteriorate various aspects of cognitive performance [1–3]. To compensate for work-induced sleep restriction and circadian misalignment, there is a tendency to sleep in and to align better with the inner circadian clock on weekends (WE), which leads to sleep inconsistency between WE and weekdays (WD). However, it is unclear whether WE catch-up sleep can help recover from WD sleep restriction and whether it is beneficial to switch back toward a better alignment with the internal circadian clock on WE.
So far, studies tackling these questions have focused on adolescents and young adults, who have delayed circadian rhythms due to their developmental stages. Mismatched school schedules cause chronic sleep loss and circadian disruptions in this population [4]. WE catch-up sleep (longer WE relative to WD sleep duration) was associated with poor attention [5] and academic performance [6] in adolescents and young adults. Larger WE–WD variation in sleep timing, which referred to as social jetlag and reflected circadian misalignment, was correlated with increased vulnerability to drug use in adolescents [4]. Few studies, however, have examined the effect of WE–WD sleep inconsistency in adults who experience work-related sleep loss and social jetlag of less severity than that seen in adolescents. One study showed that WE catch-up sleep lowered the risk for hypertension in Korean adults [7], indicating that sleeping in on WE might be beneficial in adults. Studies of how WE–WD sleep inconsistency affects brain function have been limited. To our knowledge, only one neuroimaging study has been reported and it showed that larger WE–WD sleep timing inconsistency was associated with decreased medial prefrontal cortex (MPFC) and striatal responses to reward in adolescents [8].
Two independent processes are believed to regulate sleep: circadian rhythmicity, which controls sleep timing, and homeostatic sleep pressure, which accumulates during wakefulness and declines with sleep [9]. These two processes distinctly modulate the brain during a sustained attention task: sleep debt had negative effects on higher-order association cortices that spanned frontal, parietal, insular, and cingulate regions, whereas circadian rhythmicity modulated subcortical areas and a few cortical areas including visual and sensorimotor cortices [10]. Here, we assessed in adults, how WE–WD inconsistency of sleep duration and sleep timing correlates with attentional performance and associated brain activation, which are known to be sensitive to sleep loss and circadian disruptions [11, 12] and whether differences in task-related activity were concurrent with differences in resting-state functional connectivity (RSFC) [13], which is also influenced by sleep loss and sleep patterns [14–16]. We hypothesized that (1) longer WE sleep duration (relative to WD) would correlate with better attentional performance and larger WE–WD differences in sleep timing would correlate with worse attentional performance; (2) greater WE–WD inconsistency of sleep duration would mainly affect cortical areas, whereas inconsistent sleep timing would mainly affect subcortical and visual areas; (3) longer WE sleep duration (relative to WD) would be associated with greater RSFC within the default mode network (DMN, referred to as task negative network), since the integration within the DMN [14–16], and its segregation with other networks was decreased by sleep deprivation [17], whereas habitual longer sleep duration increased them [18], and (4) inconsistent WE–WD sleep timing, which is thought to reflect circadian misalignment, would be associated with reduced RSFC within the DMN as evening-type individuals, who experience circadian disruption had lower DMN RSFC than morning-types [19].
Methods
Participants
Data were collected from 56 healthy participants (age: 43.95 ± 13.57; 30 female) who were free of psychiatric disorders, head injury, and substance use disorder (except for nicotine/caffeine). All participants were asked to arrive at the National Institutes of Health (NIH) at 09:00 am on the scan day and were scanned between 11:00 am and 01:00 pm. Participants were not allowed to consume any nicotine or caffeine after arriving at NIH (i.e. at least 2 hours before the scan). Participants were scanned on average 7 days after their 1-week actigraphy assessment. History of nicotine and caffeine use and the number of days between actigraphy assessments and scans are summarized in Table 1. Participants with a binge drinking history in the past 10 years were excluded. Written informed consent approved by the Institutional Review Board at the NIH was obtained from all participants.
Table 1.
Demographics, caffeine and cigarette consumption, chronotype, sleep behavior, and performance on the VAT
| Characteristic | Mean (SD) |
|---|---|
| Age (years) | 43.95 (13.57) median: 43, Range 21–73 |
| Gender | 30 female, 26 male |
| Caffeine use (mg/d) | 75 (117.63) |
| Cigarette use (pack/y) | 0.77 (3.52) median: 0, Range 0–21 |
| MEQ | 59% neither type, 27% moderate M type, 7% definite M type, 4% moderate E type |
| BDI | 1.24 (2.35) |
| Sleep duration per night (h) | 6.91 (1.24) |
| Sleep duration WE (h) | 7.31 (1.42) |
| Sleep duration WD (h) | 6.74 (1.46) |
| Sleep duration (WE–WD) (h) | 0.59 (1.66) |
| Bedtime per night (o’clock) | 11:30 pm (1.65 h) |
| Wake up time per night (o’clock) | 6:24 am (1.74 h) |
| Bedtime WE (o’clock) | 12:21 am (2.01 h) |
| Wake up time WE (o’clock) | 7:41 am (1.81 h) |
| Bedtime WD (o’clock) | 11:10 pm (1.73 h) |
| Wake up time WD (o’clock) | 5:54 am (2.04 h) |
| Sleep midpoint per night (o’clock) | 3:24 am (1.26 h) |
| Sleep midpoint WE (o’clock) | 4:00 am (1.78 h) |
| Sleep midpoint WD (o’clock) | 2:53 am (1.15 h) |
| Sleep midpoint (WE–WD) (h) | 1.11 (1.38) |
| VAT (2-ball) RT (s) | 0.93 (0.26) |
| VAT (3-ball) RT (s) | 0.90 (0.34) |
| VAT (4-ball) RT (s) | 0.96 (0.36) |
| VAT (2-ball) Hits% | 92.94 (11.88) median:100, Range: 46.67–100 |
| VAT (3-ball) Hits% | 88.05 (16.24) median: 93.33, Range: 26.67–100 |
| VAT (4-ball) Hits% | 80.13 (18.88) median 86.67, Range: 40.00–100 |
| Day of scans (% of participants) | Mon: 20%, Tue: 30%, Wed: 5%, Thurs: 43%, Fri:2% |
| Days (Actimetry-Scan) | 7.48 (16.03) |
MEQ, Morningness Eveningness Questionnaire; M type, morning type; E type, evening type; BDI, the Beck Depression Inventory; RT, reaction time; Days (Actimetry-Scan), days passed between actimetry assessment and scans (positive value: actimetry assessment preceded scans; negative value: actimetry assessment followed scans).
Sleep measurement
To record sleep behavior, participants were asked to wear a wrist-worn GENEActiv triaxial accelerometer monitor (Version 1.1; Activinsights Ltd., Cambridgeshire, UK) 24 hours continuously for 1 week. The monitor was placed on the nondominant wrist and participants were asked not to remove it. For analysis, we used the freely available R package GGIR (v1.8-1) to process the raw accelerometer data in a bin format. The algorithm implemented in GGIR allows us to detect sleep periods without the use of a sleep diary and its accuracy was independently validated with polysomnography [20]. Procedures for the identification of sleep period time-window (SPT-window) by GGIR were as previously described (van Hees et al. [20]). In brief, the algorithm uses median values of the absolute changes in z-angle across 5-minute rolling windows and detects the inactivity blocks for which the values are below a certain critical threshold. The longest block of inactivity (noon-noon) is defined as SPT-window. Sleep midpoint was then calculated as the timepoint between the onset and end of the SPT-window.
We computed inconsistent sleep duration and inconsistent sleep timing as two indexes of sleep inconsistency between WE (Friday and Saturday) and WD (from Sunday to Thursday). For each participant, inconsistent sleep duration was calculated as the difference between WE and WD sleep duration divided by the average sleep duration for all nights (including WE and WD). Inconsistent sleep timing was calculated as the difference between WE and WD sleep midpoint divided by the average sleep duration for all nights (including WE and WD). Of note, our study focused on the effects of WE–WD sleep inconsistency. To minimize the influence of interindividual differences of sleep duration on the measures of sleep inconsistency, we calculated the extent of sleep changes/shifting as the percentage of an individual’s sleep duration, i.e. divided WE–WD differences by the individual’s average sleep duration. By this means, we were able to compare the extent of sleep inconsistency across participants. Positive value of inconsistent sleep duration means longer WE sleep duration relative to WD and positive value of inconsistent sleep timing means delayed WE sleep timing relative to WD. Negative values indicate the opposite.
MRI acquisition and image preprocessing
All participants underwent Magnetic Resonance Imaging (MRI) on a 3.0T Magnetom Prisma scanner (Siemens Medical Solutions USA, Inc., Malvern, PA) with a 32-channel head coil. A multiplexed gradient echo-planar (EPI) pulse sequence [21] with multiband factor = 8, anterior-posterior phase encoding, Repetition time (TR)/Echo time (TE) = 720/37 ms, Flip angle (FA) = 52 deg, matrix = 104, and 72 slices were used to record blood-oxygen-level-dependent (BOLD) responses covering the whole brain with 2 mm isotropic voxels while participants remained with their eyes open. A liquid-crystal display screen (BOLD screen 32, Cambridge Research Systems, UK) was used for the presentation of stimuli during the visual attention task (VAT) and of a fixation cross during the resting-state scans (white cross, displayed at the center of the visual field on a black background for 15 minutes). Padding was used to minimize head motion.
T1-weighted 3D magnetization-prepared gradient-echo image [22] (MP-RAGE; TR/TE = 2400/2.24 ms, FA = 8 deg) and T2-weighted variable flip angle turbo spin-echo [23] (Siemens SPACE; TR/TE = 3,200/564 ms) pulse sequences were used to acquire high-resolution anatomical brain images with 0.7 mm isotropic voxels field-of-view (FOV) = 240 × 256 mm, matrix = 300 × 320, and 208 sagittal slices.
FreeSurfer version 5.3.0 (http://surfer.nmr.mgh.harvard.edu) was used to automatically segment the anatomical MRI scans into cortical and subcortical gray matter regions of interest [24]. We used the minimal preprocessing pipelines [25] of the Human Connectome Project (HCP) for motion correction and spatial normalization of the structural and functional scans to the stereotactic space of the Montreal Neurological Institute (MNI). The functional Magnetic Resonance Imaging (fMRI) data were then spatially smoothed with a 4-mm full width at half maximum (FWHM) using the Analysis of Functional NeuroImages (AFNI) program [26] 3dBlurToFWHM.
Visual attention task
Participants performed a blocked nonverbal VAT that alternated 1-minute-long “TRACK” epochs with 1-minute-long “DO NOT TRACK” epochs [27–30]. This task activates attention-related brain regions: prefrontal, parietal, occipital cortices, and subcortical regions, including thalamus and cerebellum [27, 29, 31, 32]. The brain responses to the VAT as well as performance are sensitive to sleep deprivation [12, 33].
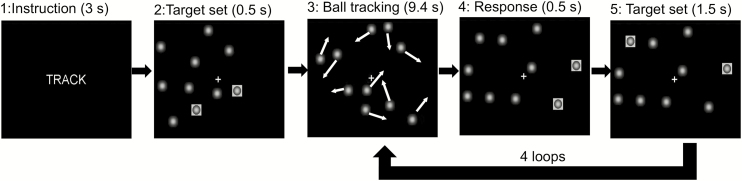
“TRACK” or “DO NOT TRACK” instructions were displayed at the beginning of each epoch for 3 seconds. TRACK epochs were subsequently followed by five 11.4-second-long periods. The target balls (2, 3, or 4 out of 10 balls) were initially highlighted for 0.5 seconds. Then, the highlights disappeared, and all balls started moving randomly across the screen with Brownian motion. Participants had to mentally track the moving targets for 9.4 seconds while fixating on a cross at the center of the visual field. Then, all the balls stopped moving, a set of targets was highlighted for 0.5 seconds, and participants pressed a button if these balls matched the original targets. After a 1-second response window, the original target set was re-highlighted for 0.5 seconds to refocus participants’ attention on the target balls and the balls began to move again (Figure 1). During the “DO NOT TRACK” epochs, the 10 balls moved and stopped in the same manner as the “TRACK” epochs, but no balls were highlighted; during this condition, participants were instructed to fixate on the center cross and ignore the moving balls. This condition was used to control for the confounding effect of visual input activation. All participants performed three runs of the VAT (2-, 3-, and 4-ball tracking), each one comprising three “TRACK” and three “DO NOT TRACK” epochs and lasting 6 minutes. The stimuli were synchronized with the MR acquisition using an MRI trigger pulse. Button press responses were recorded with the Lumina response pad LSC-400 (Cedrus Co., San Pedro, CA). The reaction time (RTs) and accuracy (Hits%) were recorded. Participants completed a training session of a shortened version of the VAT before scans to ensure task comprehension.
Figure 1.
Visual attention task (2-ball tracking). During TRACK epochs, participants tracked the target ball set, which was briefly highlighted (frame 2) after the instruction (frame 1), while all 10 balls randomly moved for 9.4 s (frame 3). Then, participants responded with a button press if the highlighted balls were the target set that they were tracking (frame 4). After a 1-s response window, the original target set was re-highlighted for 0.5 s to re-focus participants’ attention on the target balls and the balls began to move again.
Task performance analysis
Linear multiple regression models in SPSS 22 (IBM, Armonk, NY) were used to predict VAT performances (RTs and accuracy in 2-, 3-, and 4-ball tracking), with WE catch-up sleep and inconsistent sleep timing as predictors (regressors of interest) and age and gender as covariates. Distribution of the residuals from the models were tested and the linear model assumptions were satisfied.
Task activation analysis
AFNI software (Version 19.1.18) [26] was used for task-based fMRI analysis. For each participant, a general linear model (GLM) with Gamma distribution was used to estimate the hemodynamic response to ball-tracking using AFNI’s 3dDeconvolve. Twelve motion parameters (three rotational, three translational, and their derivatives) calculated from image realignment and three polynomials were included as regressors of no interest. Estimates of regression coefficients (β) and associated T-values were obtained for “TRACK” epochs for each of 2-, 3-, and 4-ball tracking condition in contrast to “DO NOT TRACK” epochs. The regression coefficients (β) were then used for second-level analysis.
For the second-level analysis, a linear mixed-effects (LME) modeling was conducted at a whole-brain level using AFNI’s 3dLME [34]. Task difficulty level (2-, 3-, or 4-ball tracking) was included as the within-subject effect, while inconsistent sleep duration, inconsistent sleep timing, age, and gender were included as between-subject effects. Additionally, interactions of “task difficulty*inconsistent sleep duration” and “task difficulty*inconsistent sleep timing” were modeled in LME.
All voxel-wise results were thresholded at a single voxel level of p < .001. Cluster size thresholds corrected for multiple comparisons (p < .05) were calculated through AFNI’s 3dClustSim (updated July 2016; Cox et al. [35]), which generates Monte Carlo simulations to determine appropriate cluster sizes and addresses the recent criticisms of the cluster method [36] by using a mixture of exponential functions to model more realistic noise distribution. Based on 3dClustSim, our whole-brain analyses required a cluster size larger than 20 voxels to be significant.
Resting-state analysis
We conducted RSFC using the CONN toolbox 18a (http://www.nitrc.org/projects/conn; Whitfield-Gabrieli and Nieto-Castanon, 2012) [37] in MATLAB. CONN implemented CompCor, a method for identifying principal components associated with segmented white matter and cerebrospinal fluid [38]. These components were entered as confounds into a first-level analysis. The global BOLD signal was not regressed out because it mathematically introduces negative correlations [39]. Twelve motion regressors calculated from image preprocessing were entered as nuisance covariates to further minimize the effects of head motion. None of the 56 participants showed excessive motion and none were excluded from the analysis (relative root mean square of motion regressors: .12 ± .05). The data were simultaneously band-pass filtered to .008–.09 Hz. Despiking was applied before regression to reduce the influence of potential outlier scans. To assess functional connectivity, we conducted seed-voxel correlation analysis. The MPFC and the posterior cingulate cortex (PCC) were a priori defined as seeds with a 10-mm radius sphere centered around MNI coordinates (1, 55, −3) and (1, −61, 38), respectively. These coordinates were provided by the toolbox and obtained from CONN’s Independent component analysis analyses of HCP dataset with 497 participants. We also conducted follow-up RSFC analyses with seeds from the task-based analysis to understand whether task-related effects reflect intrinsic connectivity changes in the resting state. According to the deactivation of DMN regions associated with sleep inconsistency during VAT (see Results), left and right superior frontal gyrus (SFG) and right angular gyrus (AG) obtained from the FSL Harvard–Oxford Atlas were additionally used as seeds. First-level connectivity maps computed Pearson’s correlation coefficients between the time-course of seeds and the time-courses of all other voxels in the brain. Correlation coefficients were then converted to normally distributed Z-scores using the Fisher transformation for the second-level analysis. At the group level, we examined the associations between WE–WD sleep inconsistency (inconsistent sleep duration and sleep midpoint) and RSFC using a GLM. To control for age and gender, we included them as regressors of no interest. Associations were considered significant if they exceeded a voxel-wise threshold level of p < .001, uncorrected, and a cluster-level threshold of p < .05, corrected for false discovery rate (FDR), two-tailed. A Bonferroni correction was then applied based on the number of seeds. Results were thus considered significant at the cluster-level threshold of p < .01 (.05/5) FDR.
Results
Demographics, timing of scans, and Beck’s depression inventory (BDI) scores are summarized in Table 1.
Sleep and sleep inconsistency
The results of sleep behaviors (sleep duration, bedtime, wake up time) measured by the accelerometer and the chronotype assessed by the Morningness-Eveningness Questionnaire are summarized in Table 1.
Inconsistent sleep duration (.10 ± .26) and inconsistent sleep timing (.17 ± .23) were uncorrelated with each other (r = .08, p = .58), which indicates that they are two independent indexes of sleep inconsistency. Neither inconsistent sleep duration nor inconsistent sleep timing were correlated with age, gender (all |r| < .26, all p > .05), or BDI (all |r| < .20, all p > .10).
VAT behavioral performances
The results of VAT performances (accuracy and RTs) are summarized in Table 1.
VAT accuracy decreased as the VA load increased (F(2,90) = 4.63, p < .001, η p2 = .258; post hoc t tests: all t > 3.25, Bonferroni-corrected all p < .01) and this effect was not significant for RT (F(2,90) = .74, p = .479, η p2 = .016).
Sleep inconsistency and VAT behavioral performances
VAT accuracy and RT were separately regressed on inconsistent sleep duration, inconsistent sleep timing, age, and gender. Of note, in our model, inconsistent sleep duration and timing are estimated as the percentage of an individual’s sleep duration. Positive value refers to longer WE sleep duration or delayed WE sleep timing relative to those on WD (please see Methods). This multiple regression model significantly predicted accuracy in the 3- and 4-ball conditions (3-ball: F(4,48) = 4.63, p = .003, adjusted R2 = .218; 4-ball: F(4,44) = 3.83, p = .009, adjusted R2 = .191) and predicted RTs in the 2-ball condition (F(4,46) = 3.28, p = .019, adjusted R2 =.154). Inconsistent sleep duration (longer WE relative to WD sleep duration) was positively associated with accuracy in the 3-ball (β = .30, t = 2.35, p = .023) and 4-ball conditions (β = .30, t = 2.21, p = .032), while inconsistent sleep timing (delayed sleep timing on WE) was negatively associated with accuracy in the 4-ball condition (β = −.33, t = −2.42, p = .020) and RTs in the 2-ball condition (β= .28, t = 2.06, p = .045).
Half of the participants were scanned at the beginning of the workweek (Monday and Tuesday) and the other half were scanned at the end of the workweek (Wednesday, Thursday, and Friday) (Table 1). To examine the effect of scan day, we conducted the same regression analyses separately for participants scanned in the first half of the workweek and participants scanned in the second half of the workweek. The beneficial effect of WE catch-up sleep (longer WE relative to WD sleep duration) on accuracy was only found in participants scanned at the beginning of the workweek (3-ball: F(4,22) = 6.04, p = .002, adjusted R2 = .436, β = .38, t = 2.21, p = .038) but not at the end of the workweek (3-ball: F(4,21) = 1.86, p = .115, adjusted R2 = .121, β = .34, t = 1.71, p = .102).
For the effects of age and gender on VAT performance, please refer to Supplementary Material.
There were n = 20 participants who showed shorter sleep duration on WE than WD and we compared them with those who had longer WE sleep duration than WD and examined the relationship between inconsistent sleep duration and behavioral performance in these two groups separately. We found a tendency toward a beneficial effect of longer WE sleep on behavioral performance even with the limited statistical power in these analyses: among participants who had longer WE sleep, larger sleep consistency (WE–WD) correlated with better behavioral performance. In contrast, among participants who have shorter WE sleep than WD, larger sleep inconsistency (WD–WE) correlated with worse behavioral performance (Supplementary Material shows results and related discussion). Few participants (n = 10) showed advanced WE sleep timing compared with WD, so we did not have statistical power to test its effects in performance or brain activation patterns.
Modeling sleep duration as an independent variable
Additionally, we modeled “average sleep duration” as a separate variable and used pure measures of sleep inconsistency “WE–WD differences in sleep midpoint” and “WE–WD differences in sleep duration” (rather than sleep inconsistency quantified as percentage of an individual’s sleep duration in the original model). The multiple regression model significantly predicted accuracy in the 3- and 4-ball conditions (3-ball: F(5,48) = 3.19, p = .015, adjusted R2 = .186; 4-ball: F(5,44) = 3.94, p = .005, adjusted R2 = .251) and predicted RTs in the 2-ball condition (F(5,47) = 3.68, p = .008, adjusted R2 = .222). Delayed WE sleep timing was negatively associated with accuracy in the 3-ball (β = −.32, t = −2.37, p = .023) condition, while longer WE sleep duration relative to WD was positively associated with accuracy in the 4-ball condition (β = .40, t = 2.85, p = .007) and negatively associated with RTs in the 2-ball condition (β = −.30, t = −2.18, p = .035). In sum, the results were roubust against different modeling.
Sleep inconsistency and VAT brain activation
VAT brain activation
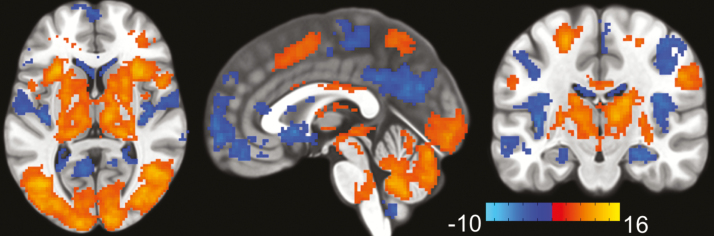
The VAT activated frontal, parietal and occipital cortices, cerebellum, and thalamus and deactivated the DMN [40] (Figure 2). The results were consistent with previous studies [12, 33].
Figure 2.
VAT brain activation. VAT brain activation and deactivation during “TRACK” in contrast to “DO NOT TRACK” epochs (t-map) averaged over 2-, 3-, and 4-ball conditions. The VAT activated frontal, parietal and occipital cortices, cerebellum, and thalamus (red) and deactivated the DMN (blue). The color bar shows the t-score.
Inconsistent sleep duration and VAT brain activation
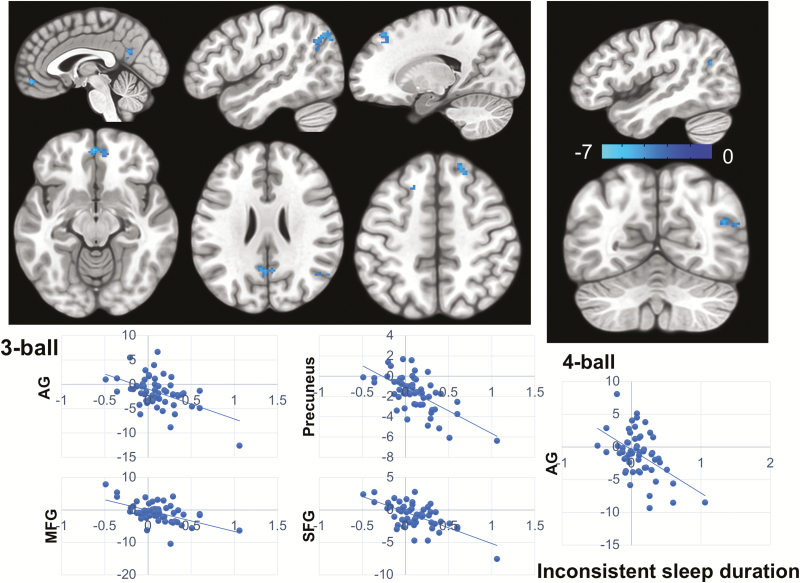
After controlling for age and gender, participants with longer WE relative to WD sleep duration showed greater deactivation of DMN, including precuneus, medial frontal gyrus (MFG), right AG, and bilateral SFG during the 3-ball tracking. In the 4-ball condition, this effect was only significant in the right AG (Table 2 and Figure 3).
Table 2.
Inconsistent sleep and brain activation during VAT
| Brain region | BA | Peak x y z (Talairach) | K | Z-value | ||
|---|---|---|---|---|---|---|
| Brain activation (3-ball tracking) associated with inconsistent sleep duration | ||||||
| AG (R) | 39 | +48 | −60 | +24 | 150 | −3.53 |
| Precuneus | 31 | −2 | −56 | +18 | 68 | −3.97 |
| MFG | 11 | 6 | +46 | −12 | 64 | −3.47 |
| SFG (R) | 8 | +18 | +34 | +46 | 53 | −3.35 |
| SFG (L) | 8 | −20 | +22 | +50 | 23 | −3.65 |
| Brain activation (4-ball tracking) associated with inconsistent sleep duration | ||||||
| AG (R) | 39 | +52 | −58 | +20 | 42 | −3.49 |
| Brain activation (4-ball tracking) associated with inconsistent sleep timing | ||||||
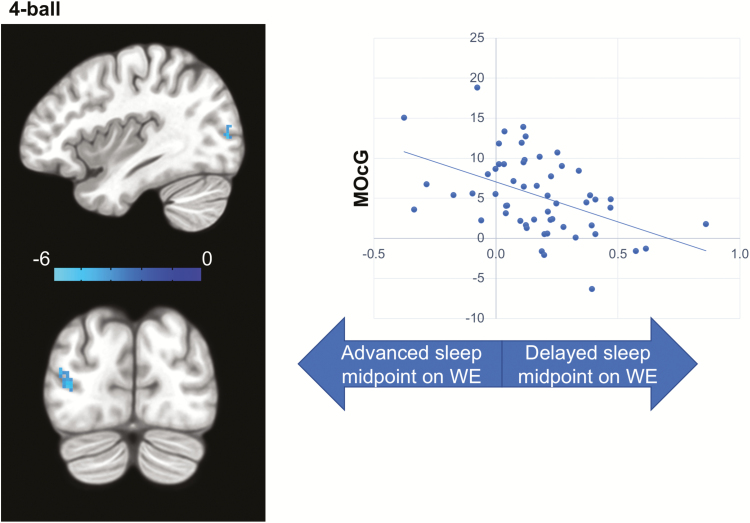
| MOcG (L) | 18 | −36 | −86 | +4 | 32 | −3.43 |
BA, Brodmann area; K, cluster size (voxels); L, left; R, right.
Figure 3.
Inconsistent sleep duration and VAT brain activation. Longer WE relative to WD sleep duration was associated with greater deactivation of DMN during VAT including right AG, MFG, precuneus, and bilateral SFG (z-map). For the 3-ball condition, longer WE relative to WD sleep duration was positively associated with greater deactivation of DMN regions (upper panel left), whereas for the 4-ball condition, it was associated with deactivation of only the right AG (upper panel right). The beneficial effect was not observed for the low VA-load condition (2-ball tracking). The color bar shows the z-score. The plots on the bottom are only for the visualization of the direction of effects and not for the inference (3-ball: lower panel left; 4-ball: lower panel right). The x-axis of the plots depicts inconsistent sleep duration calculated as WE–WD differences of sleep duration divided by an individual’s average sleep duration for all nights (see Methods). The y-axis depicts t-score for VAT brain activation/deactivation during “TRACK” in contrast to “DO NOT TRACK” epochs.
Inconsistent sleep timing and VAT brain activation
Participants with a greater delay of sleep midpoint on WE showed lower activation in the left middle occipital gyrus (MOcG) in the 4-ball condition (Table 2 and Figure 4).
Figure 4.
Inconsistent sleep timing and VAT brain activation. Delayed sleep timing on WE was associated with lower activation of left MOcG in the 4-ball condition (left), while no associations were observed in the 2-ball or 3-ball conditions (z-map). The color bar shows the z-score. The plot on the right is only for the visualization of the direction of effect and not for the inference. The x-axis of the plot depicts inconsistent sleep timing calculated as WE–WD differences of sleep midpoint divided by an individual’s average sleep duration for all nights (see methods). The y-axis depicts t-score for VAT brain activation/deactivation during “TRACK” in contrast to “DO NOT TRACK” epochs.
For the effects of age and gender on VAT brain activation, please refer to Supplementary Material.
Sleep inconsistency and RSFC
Inconsistent sleep duration and RSFC
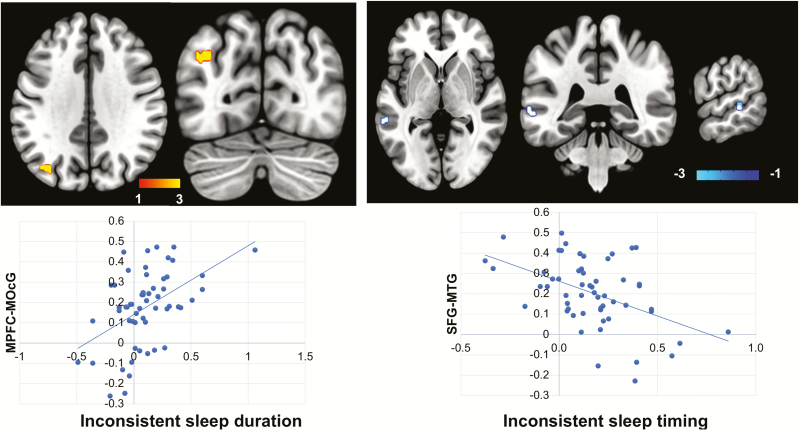
Participants with longer WE relative to WD sleep duration displayed higher RSFC between the MPFC and the left MOcG (Table 3 and Figure 5).
Table 3.
Inconsistent sleep and RSFC
| Seed | Target | BA | Peak x y z (MNI) | K | T | Cluster FDR (P-value) | ||
|---|---|---|---|---|---|---|---|---|
| RSFC associated with inconsistent sleep duration | ||||||||
| MPFC | MOcG (L) | 19 | −34 | −74 | 36 | 49 | 4.79 | .001 |
| RSFC associated with inconsistent sleep timing | ||||||||
| SFG(L) | MTG (L) | 21 | −64 | −38 | 2 | 34 | −4.81 | .008 |
BA, Brodmann area; MNI, Montreal Neurological Institute coordinates; K, cluster size (voxels); L, left; FDR, false discovery rate (correction for multiple comparisons).
Figure 5.
Sleep inconsistency and RSFC. Longer WE relative to WD sleep duration was associated with higher RSFC between seed MPFC and left MOcG (upper panel left), while delayed WE sleep timing was associated with lower RSFC between seed left SFG and left MTG (upper panel right). The color bar shows the t-score. The plots on the bottom are only for the visualization of the direction of effects and not for the inference. X-axis: inconsistent sleep duration (lower panel left) was calculated as WE–WD differences of sleep duration divided by an individual’s average sleep duration for all nights, while inconsistent sleep timing (lower panel right) was calculated as WE–WD differences of sleep midpoint divided by an individual’s average sleep duration for all nights (see methods). Y-axis: standard z-score for functional connectivity.
Inconsistent sleep timing and RSFC
Participants with a greater delay of sleep midpoint on WE showed lower RSFC between SFG and middle temporal gyrus (MTG) in the left hemisphere (Table 3 and Figure 5). There were no significant correlations between inconsistent sleep timing (greater delay of sleep midpoint in WE than WD) and RSFC when AG or PCC was used as seed.
For the effects of age and gender on RSFC, please refer to Supplementary Material.
Discussion
The current study examined the association between sleep inconsistency and attention performance, task-induced brain activation, and RSFC in healthy adults. The participants with longer WE sleep duration relative to WD displayed a better attentional performance that was associated with greater DMN deactivation and higher functional connectivity between the anterior DMN (MPFC) and the occipital cortex (left MOcG) at rest. Interestingly, the association between improvement in attention performance and related DMN deactivation with greater WE–WD sleep duration depended on the task difficulty. The beneficial effect was observed for the middle and high (3- and 4-ball) but not the low (2-ball) VA-load conditions and the extent of correlated DMN deactivation was smaller in the high than in the middle VA-load conditions. Independent of the effect of inconsistent sleep duration, inconsistent sleep timing correlated with worse attentional performance and with lower occipital activation and RSFC within the DMN (between left SFG and left MTG).
Consistent with our hypothesis, longer WE sleep duration relative to WD was associated with better attentional performance. This beneficial effect was only observed at the beginning but not at the end of the workweek reflecting a dissipation of the WE catch-up sleep benefit and the accumulation of work-induced sleep debt. In line with our previous study [41], better performance in VAT was related to greater DMN deactivation, especially deactivation of the inferior parietal lobe including the right AG, which is essential for visuospatial awareness [42]. The influence of sleep loss on the activity of DMN regions during cognitive tasks has been well-documented [43, 44]. Opposite to the reduced deactivation of DMN in sleep-deprived individuals [12], the greater DMN deactivation in participants with larger WE–WD sleep duration is consistent with recovery from WD sleep restriction by WE catch-up sleep in these individuals. Altered fronto-occipital RSFC could also contribute to attentional performance. Aberrant RSFC within fronto-temporo-occipital cortices was reported in Attention deficits hyperactivity disorder (ADHD) [45]. Enhanced RSFC between anterior DMN and occipital cortex related to longer WE–WD sleep duration could facilitate task-related visual activation. In sum, WE catch-up sleep might contribute to attention by enhancing task-induced deactivation of DMN and strengthening its connectivity with the occipital cortex at rest. Notably, longer WE sleep duration (relative to WD sleep duration) had opposite effect in adults compared with adolescents [5, 6]. Adults in the current study were less sleep deprived (WE–WD: 0.59 hours, average sleep duration 6.91 hours) than adolescents in previous studies (e.g. WE–WD: 2.70 hours in Kim et al. [5] and 2.13 hours in O’Brien et al. [46]). Thus, while larger WE–WD sleep duration might mainly reflect longer WE catch-up sleep in healthy adults, it might mainly relate to severe sleep debt accumulated over WD in adolescents. It is likely that WE catch-up sleep can help recover from mild but not severe sleep loss.
The next question is why the beneficial effect of WE catch-up sleep was not found when the task demand was low? A recent study reported that less DMN deactivation predicted greater task effort avoidance [47]. Lower DMN deactivation in sleep-deprived individuals [12] could reflect their reduced motivation to exert effort. Indeed, decreased attentional effort measured by pupillometry has been observed after sleep deprivation [48]. WE catch-up sleep enhancement of VAT induced DMN deactivation might have improved attentional performance by increasing the effort of participants. As effort makes the most difference when the task demand is moderate, it might explain the lack of benefits in the low VA-load condition as well as the smaller DMN deactivation in the high VA-load condition. Our finding is consistent with the finding of Gilbert et al. [49] that task-induced DMN deactivation was affected by task difficulty.
Delayed WE sleep timing (WE–WD: 1.11 hours) disrupted VAT performances. Unlike inconsistent sleep duration that affected frontal and parietal areas, inconsistent sleep timing reduced activation in the occipital cortex. Our findings are consistent with the two-process model of sleep regulation [9] and with the findings of Muto et al. [10] that these two processes distinctly affect the brain. However, different from Muto et al. [10], we did not find the effect of circadian misalignment in subcortical regions, which could be attributed to the lower sensitivity of multiband for detecting subcortical signals. As expected, participants with greater delays in WE sleep timing showed reduced RSFC between frontal and temporal regions of the DMN. Decreased DMN RSFC has been associated with inattention and impulsivity in ADHD [50]. Thus, reduced DMN RSFC associated with delayed WE sleep timing might underlie the attention impairments. Importantly, all participants were tested in the morning and most of them were morning chronotype, or neither morning nor evening chronotype (Table 1). Therefore, our results are unlikely to be confounded by diverse chronotypes or testing times and suggest that even mild social jetlag can impair performance during regular working hours when we are normally alert.
Our study revealed that the DMN was sensitive to inconsistency of both sleep duration and sleep timing. Accumulating evidence indicates that deactivation of the DMN during task and DMN RSFC are modulated by the dopaminergic (DA) system [51]. As sleep deprivation [52, 53] and disruption of circadian rhythms [54] strongly interfere with DA signaling, it is possible that alterations of the DA system might mediate the correlation between sleep inconsistency and DMN. Considering the independent effect of inconsistent sleep duration and sleep timing, future studies could evaluate if they differentially affect DA function. The cross-sectional design of our study does not allow us to determine the causality between sleep inconsistency and changes in brain function. There is evidence that DMN RSFC affects the sleep–wake cycle [55], so we can not rule out the possibility that altered DMN increases sleep inconsistency and leads to varied sleep duration and sleep timing.
The following are study limitations that should be considered when interpreting our findings. In our study, most participants had longer sleep duration in WE than in WD. Our findings of inconsistent sleep duration associated with improved performance and greater deactivation of DMN during the task might reflect the direction of the inconsistency toward greater WE catch-up sleep (please also refer to additional analyses in Supplementary Material). The effects of inconsistent sleep duration that results in shorter sleep during the WE than WD need to be determined. Similarly, most participants had delayed sleep timing on WE relative to WD, which could reflect the fact that circadian periods in humans are typically greater than 24 hours. However, as we had few participants (n = 10) who had advanced WE sleep timing (Figures 4 and 5), we were not able to compare participants with advanced vs. delayed WE sleep timing. Thus, it is unclear whether absolute sleep timing differences between WE and WD (irrespective of direction) or the WE sleep timing relative to WD matters. Additionally, our participants were healthy and showed relatively low sleep inconsistency. Thus, the observed changes in brain function could be larger in other populations. The effect of sleep inconsistency could vary among different chronotypes, which we could not test since most of our participants were morning or neither types. Evening types are more likely to accumulate a sleep debt during WD and to have greater sleep extension on WE [56, 57], so they might not be able to recover from sleeping in on WE and experience more severe consequences of circadian misalignment. Also, it remains unclear how sleep inconsistency affects more complex cognition, affective function, and risk-taking behavior [58, 59]. Our scans were conducted on average 7 days after the actigraphy assessment. The observed brain changes in the current study, therefore, might mainly reflect a chronic rather than an acute effect of sleep inconsistency. Comparing scans immediately before (on Friday) and after WE (on Monday) could be a better approach to assess the acute effect of WE catch-up sleep and delayed WE sleep timing in the future. Additionally, prolonged sleep assessments that expand over multiple weeks could provide more reliable measurements of acute vs. chronic effects of sleep inconsistency. Apart from this, though WE–WD differences in sleep timing have been proposed as an indirect measurement of circadian misalignment [60], it might not reflect the severity of circadian misalignment. Physiological assessments of the circadian phase (e.g. dim light melatonin onset) would provide a more precise quantification of circadian misalignment. A limitation of this study is that we do not have information on whether there were shift workers among our participants or whether they had recently traveled across time zones, which would have led to larger sleep inconsistencies. We did not restrict caffeine throughout the day of the scan which could be another limitation of this study as the half-life of caffeine is 5–6 hours. However, the beneficial effect of WE catch-up sleep occurred mainly in the first half of the week and it is doubtful that participants drank more caffeine at the beginning of the week, so it is unlikely that our findings were due to differences in caffeine intake on the day of study. Our study did not screen participants for nicotine or Tetrahydrocannabinol vaping, the use of which has been rising and thus future studies should control for this.
In summary, longer WE catch-up sleep appears to help adults recover from moderate sleep loss and might increase the effort of performing middle to high attention-demanding tasks by enhancing task-induced DMN deactivation and by enhancing its functional connectivity with occipital cortex at rest. In contrast, delayed WE sleep timing was associated with an impaired attentional performance by reducing task-induced occipital activation and intrinsic DMN functional connectivity at rest. This study shows the different effects of inconsistent sleep duration in the direction of longer WE than WD sleep duration (catch-up sleep) and sleep timing in the direction of delayed WE sleep on both behavior and brain activity especially of the DMN.
Supplementary Material
Acknowledgments
We thank Karen Torres, Minoo McFarland, Lori Talagala, and Christopher Wong for their contributions and our postbacs for helping with data collection.
Funding
This work was supported by National institute on alcohol abuse and alcoholism intramural research program (NIAAA IRP) (Y01AA3009). R.Z. received a research fellowship from the German Research Foundation (DFG).
Conflict of interest statement. Financial Disclosure: none. Non-financial Disclosure: none.
References
- 1. Rajaratnam SM, et al. Health in a 24-h society. Lancet. 2001;358(9286):999–1005. [DOI] [PubMed] [Google Scholar]
- 2. Lo JC, et al. Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS One. 2012;7(9):e45987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Selvi FF, et al. Effects of shift work on attention deficit, hyperactivity, and impulsivity, and their relationship with chronotype. Biol Rhythm Res. 2015;46:53–61. [Google Scholar]
- 4. Logan RW, et al. Impact of sleep and circadian rhythms on addiction vulnerability in adolescents. Biol Psychiatry. 2018;83(12):987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim SJ, et al. Relationship between weekend catch-up sleep and poor performance on attention tasks in Korean adolescents. Arch Pediatr Adolesc Med. 2011;165(9):806–812. [DOI] [PubMed] [Google Scholar]
- 6. Sun W, et al. Association between weekday-weekend sleep discrepancy and academic performance: systematic review and meta-analysis. Sleep Med. 2017;40:e318–e319. [Google Scholar]
- 7. Hwangbo Y, et al. Association between weekend catch-up sleep duration and hypertension in Korean adults. Sleep Med. 2013;14(6):549–554. [DOI] [PubMed] [Google Scholar]
- 8. Hasler BP, et al. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biol Psychol. 2012;91(3):334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borbély AA, et al. The two-process model of sleep regulation: a reappraisal. J Sleep Res. 2016;25(2):131–143. [DOI] [PubMed] [Google Scholar]
- 10. Muto V, et al. Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science. 2016;353(6300):687–690. [DOI] [PubMed] [Google Scholar]
- 11. Santhi N, et al. Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS One. 2007;2(11):e1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomasi D, et al. Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex. 2009;19(1):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tambini A, et al. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65(2):280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sämann PG, et al. Increased sleep pressure reduces resting state functional connectivity. MAGMA. 2010;23(5–6):375–389. [DOI] [PubMed] [Google Scholar]
- 15. De Havas JA, et al. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 2012;59(2):1745–1751. [DOI] [PubMed] [Google Scholar]
- 16. Yeo BT, et al. Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. Neuroimage. 2015;111:147–158. [DOI] [PubMed] [Google Scholar]
- 17. Chee MWL, et al. Functional connectivity and the sleep-deprived brain. Prog Brain Res. 2019;246:159–176. [DOI] [PubMed] [Google Scholar]
- 18. Khalsa S, et al. Variability in cumulative habitual sleep duration predicts waking functional connectivity. Sleep. 2016;39(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Facer-Childs ER, et al. Circadian phenotype impacts the brain’s resting-state functional connectivity, attentional performance, and sleepiness. Sleep. 2019;42(5). doi:10.1093/sleep/zsz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Hees VT, et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci Rep. 2018;8(1):12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moeller S, et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63(5):1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mugler JP 3rd, et al. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med. 1990;15(1):152–157. [DOI] [PubMed] [Google Scholar]
- 23. Mugler JP 3rd, et al. Optimized single-slab three-dimensional spin-echo MR imaging of the brain. Radiology. 2000;216(3):891–899. [DOI] [PubMed] [Google Scholar]
- 24. Fischl B, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. [DOI] [PubMed] [Google Scholar]
- 25. Glasser MF, et al. ; WU-Minn HCP Consortium The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. [DOI] [PubMed] [Google Scholar]
- 27. Culham JC, et al. Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol. 1998;80(5):2657–2670. [DOI] [PubMed] [Google Scholar]
- 28. Tomasi D, et al. Different activation patterns for working memory load and visual attention load. Brain Res. 2007;1132(1):158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tomasi D, et al. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27(8):694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tomasi D, et al. Practice-induced changes of brain function during visual attention: a parametric fMRI study at 4 Tesla. Neuroimage. 2004;23(4):1414–1421. [DOI] [PubMed] [Google Scholar]
- 31. Jovicich J, et al. Brain areas specific for attentional load in a motion-tracking task. J Cogn Neurosci. 2001;13(8):1048–1058. [DOI] [PubMed] [Google Scholar]
- 32. Zehra A, et al. Neural correlates of visual attention in alcohol use disorder. Drug Alcohol Depend. 2019;194:430–437. [DOI] [PubMed] [Google Scholar]
- 33. Tomasi D, et al. Association between striatal dopamine D2/D3 receptors and brain activation during visual attention: effects of sleep deprivation. Transl Psychiatry. 2016;6(5):e828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen G, et al. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage. 2013;73:176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cox RW, et al. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 2017;7(3):152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eklund A, et al. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113(28):7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whitfield-Gabrieli S, et al. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. [DOI] [PubMed] [Google Scholar]
- 38. Behzadi Y, et al. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murphy K, et al. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tomasi D, et al. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One. 2009;4(6):e6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mesulam M-M. Principles of Behavioral and Cognitive Neurology. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 43. Gujar N, et al. The unrested resting brain: sleep deprivation alters activity within the default-mode network. J Cogn Neurosci. 2010;22(8):1637–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krause AJ, et al. The sleep-deprived human brain. Nat Rev Neurosci. 2017;18(7):404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cocchi L, et al. Altered functional brain connectivity in a non-clinical sample of young adults with attention-deficit/hyperactivity disorder. J Neurosci. 2012;32(49):17753–17761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O’Brien EM, et al. Sleep and risk-taking behavior in adolescents. Behav Sleep Med. 2005;3(3):113–133. [DOI] [PubMed] [Google Scholar]
- 47. Sayalı C, et al. Neural systems of cognitive demand avoidance. Neuropsychologia. 2019;123:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Massar SAA, et al. Sleep deprivation increases the costs of attentional effort: performance, preference and pupil size. Neuropsychologia. 2019;123:169–177. [DOI] [PubMed] [Google Scholar]
- 49. Gilbert SJ, et al. Does “task difficulty” explain “task-induced deactivation?”. Front Psychol. 2012;3:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sudre G, et al. Estimating the heritability of structural and functional brain connectivity in families affected by attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang R, et al. Brain default-mode network dysfunction in addiction. Neuroimage. 2019;200:313–331. [DOI] [PubMed] [Google Scholar]
- 52. Volkow ND, et al. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J Neurosci. 2012;32(19):6711–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Volkow ND, et al. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28(34):8454–8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang J, et al. Circadian modulation of dopamine levels and dopaminergic neuron development contributes to attention deficiency and hyperactive behavior. J Neurosci. 2015;35(6):2572–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tagliazucchi E, et al. The large-scale functional connectivity correlates of consciousness and arousal during the healthy and pathological human sleep cycle. Neuroimage. 2017;160:55–72. [DOI] [PubMed] [Google Scholar]
- 56. Paine SJ, et al. Differences in circadian phase and weekday/weekend sleep patterns in a sample of middle-aged morning types and evening types. Chronobiol Int. 2016;33(8):1009–1017. [DOI] [PubMed] [Google Scholar]
- 57. Roenneberg T, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11(6):429–438. [DOI] [PubMed] [Google Scholar]
- 58. Emens J, et al. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168(3):259–261. [DOI] [PubMed] [Google Scholar]
- 59. Hasler BP, et al. Morningness-eveningness and depression: preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatry Res. 2010;176(2-3):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wittmann M, et al. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1–2):497–509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.