Abstract
Tessaria dodoneifolia [Asteraceae] is traditionally employed in Northwestern Argentina for fungal infections treatment. We report the antifungal activity guided isolation and identification of substances from aerial parts of this species, both individually and in combination with fluconazole (FLU), against Candida albicans strains. Two antifungal flavanones were identified as naringenin (NAR) and pinocembrin (PIN). These compounds could individually inhibit the growth of C. albicans strains. Combinations of NAR and PIN with FLU were synergistic against the FLU resistant and sensitive C. albicans strains. Genotoxic and cytotoxic evaluations were also performed. NAR, PIN and their combinations with FLU did not have a genotoxic effect on Bacillus subtilis rec strains. Finally, these compounds did not show cytotoxicity at concentrations below 80 μg/mL.
Keywords: Plant biology, Toxicology, Bioactive plant product, Mycology, Secondary metabolite, Flavonoid, Chromatography, Spectroscopy, Tessaria dodoneifolia, Antifungal activity, Candida albicans, Synergistic effect, Toxicity assays
Plant biology; Toxicology; Bioactive plant product; Mycology; Secondary metabolite; Flavonoid; Chromatography; Spectroscopy; Tessaria dodoneifolia; Antifungal activity; Candida albicans; Synergistic effect; Toxicity assays.
1. Introduction
Human's fungal infections caused by yeasts have increased in the last decades, generating infections at superficial levels, as skin or oral infections [1], or even at systemic levels [2]. Candida albicans is among the microbial agents most frequently involved in hospital infections around the world [3]. This situation, together with the restricted arsenal of therapeutic antifungal drugs and the continuous increase of resistance mechanisms, represents a major clinical challenge to the health system. In emerging countries, fluconazole (FLU) represents the most widely used antifungal drug [4], nonetheless the fungistatic action of this drug is a condition that favors the development of resistant strains [5]. Drug combinations have emerged as an interesting approach to avoid or minimize the development of resistance. The synergetic effect of combination therapies could improve the fungicidal activity, reducing at the same time the development of resistant strains [6]. Researchings related to antifungal therapies faces the task of discovering new drugs or associations between existing drugs that achieves antifungal effects [7].
Plants could be employed as sources of compounds that meet those needs [8]. Some reports on Argentinean Northwestern folk medicine have suggested various promising plant species traditionally employed as antifungals [9, 10]. Tessaria dodoneifolia (Hook & Arn.) Cabrera [Asteraceae] is a plant traditionally used as a natural sweetener in food and beverages in some countries (Paraguay, Argentina, Bolivia) where it is known as “chilca” [11], due to the sweet taste of a dihydroflavonol derivative identified as dihydroquercetin-3-acetate [12]. Many authors have reported the traditional use of this plant to treat fungal infections [9, 10, 11, 13], though there are no scientific studies validating those reports. The present article describes an antifungal activity study of T. dodoneifolia extracts, the isolation of active compounds, their chemical structures determination and the study of inhibitory activity against C. albicans strains exerted by those compounds (individually and mixed with FLU). Genotoxic and cytotoxic activities of purified substances were also tested.
2. Material and methods
2.1. Chemicals
HPLC and ACS solvents were from Sintorgan Labs (Buenos Aires, Argentina); penicillin, streptomycin, Sabouraud dextrose agar (SDA) and Mueller-Hinton Agar (MH) media from Britania Labs (Buenos Aires, Argentina); Fluconazole, histopaque 1077, 2H-tetrazolium-2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl] hydroxide salt (MTT), menadione, glucose, glutamine, methylene Blue and p-anisaldehyde, Na2HPO4 and NaH2PO4 from Sigma-Life Science (Saint Louis, MO, USA); KCl, Na3PO4 and NaCl from Biopack (Buenos Aires, Argentina); DMSO, glycerol, Silica Gel 60 (less than 0.063 mm) and Trypan Blue from Merck (Darmstadt, Hesse, Germany); sephadex LH20 from Amersham Biosciences, Uppsala, Sweden; RPMI 1640 from Microvet Labs (Buenos Aires, Argentina); CHROMagar® Candida from CHROMagar Microbiology (Paris, France) and silica gel 60 F254 plates from Macherey-Nagel GmbH & Co. KG (Düren, Germany).
2.2. Strains and media
Candida albicans (ATCC 10231) strain from American Type Culture Collection and C. albicans 12–99 clinical isolate strain resistant to FLU [14] were used. The strains were periodically refreshed from permanent cultures, as stated by the Clinical and Laboratory Standards Institute (CLSI) [15]. The strains sensibility to FLU was assessed by disk diffusion assay. CHROMagar® Candida medium was employed to periodically check viability and purity of cells cultures.
2.3. Plant material
We employed Tessaria dodoneifolia (Hook & Arn) Cabrera [Asteraceae] aerial parts. The specimens were from Tucumán, Northwest Argentinean, collected on August 2017 (latitude -26.228°, longitude -65.255°). The plant material was taxonomically classified. The voucher specimen was placed in the herbarium of Instituto de Estudios Farmacológicos – Facultad de Bioquímica, Química y Farmacia – Universidad Nacional de Tucumán (the number assigned was: IEF-2017-08-004-JRS) for future references. Plant material was dried in a ventilated chamber in the shade, pulverized and kept at -20 °C into dark glass bottles.
2.4. Extract preparation
Aqueous (Aq) and Ethanolic Extracts (EE) were prepared, according to the folk uses data. The extracts were obtained as stated in Farmacopea Argentina VIII Ed. [16], briefly: Aq extracts were obtained after mixing 10 g of vegetable coarse powder with 100 mL boiling water under agitation (38 cycles/min) for 20 min, afterward the extract was filtered through a Whatman No.1 filter paper and dried in a Virtis Liter freezer dryer, SL model (Virtis, Gardiner, NY, USA). The dried material obtained represented the extracted material (EM). EE was obtained by stirring at room temperature 10 g of vegetal powder using ethanol (96%) into a glass flask closed along four days. The extract was filtered as previously described, and dried under reduced pressure using rotary vacuum evaporator (Buchi model 011, Buchi Inc., Italy) at 40 °C to obtain the EM.
2.5. Activity-guided fractionation of T. dodoneifolia EE
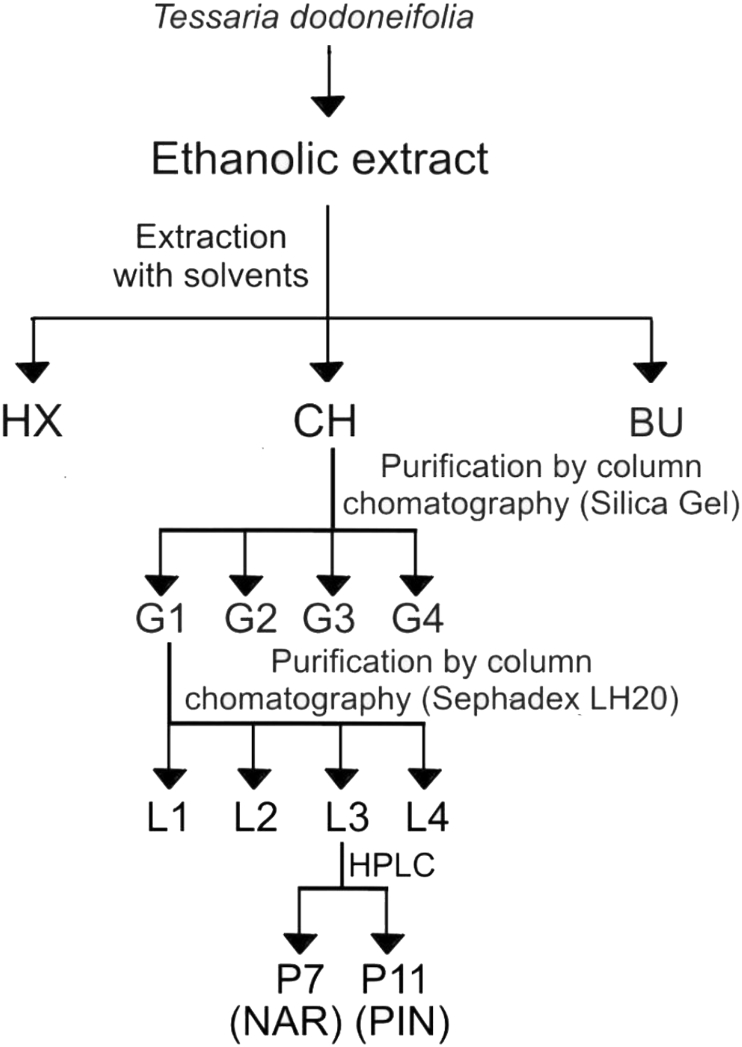
The EE was chosen for antifungal activity-guided fractionation procedures. Briefly: an ethanol:water solution (2:3 v:v) was added with 6 g of dried EE, afterwards a liquid-liquid extraction was carried out through successive solvent extraction in an increased polarity order with hexane, dichoromethane and n-butanol, to yield hexanic (HX), dichoromethanic (CH), n-butanolic (BU) fractions. The fractions were dried in rotary evaporator (at 38 °C) to produce EMs which were recovered in MeOH and stored at -20 °C. CH fraction was chosen for further purification steps. An aliquot of CH containing three grams of CH fraction was chromatographed on Silica Gel 60 (210 mL bed volume, particle size <0.063 mm) using CH2Cl2:MeOH mixtures in an increased polarity order (9:1, 7:3 and 1:1 v:v) as the mobile phase. The elution was aided with a compressor (Electrical RS290, Zhongshan city, Guandong, China). The 80 aliquots eluted (5 mL each one) were studied by TLC, grouped into four groups (G1-G4) taking into account their chemical compositions, and dried in rotary evaporator (at 38 °C). The EMs generated were recovered in MeOH. TLC experiments and disk diffusion assays were performed with samples from G1-G4 groups. G1 was selected for the next purification step. A sample containing 600 mg of EM from G1 was chromatographed on Sephadex LH 20 (182 mL bed volume) employing MeOH as solvent. Eighty aliquots (5 mL each one) were collected and analyzed by TLC, pooled into four groups (L1-L4) considering chemical profiles, dried in rotary evaporator (at 38 °C) to yield the EMs which were recovered in MeOH.
2.6. TLC experiments
Analytical TLC experiments were carried out on samples from EE, HX, CH, nBu, G1→G4 and L1→L4 on silica gel 60 F254 plates using toluene:ethyl acetate:formic acid mixture (5:4:1 v:v:v) as mobile phase. Dried developed plates were observed under visible and UV light (254 and 366 nm, UV Lamp Model UV 5L-58 Mineralight Lamp) prior and after either p-anisaldehyde⁄sulfuric acid reagent (for phenolics and terpenes detection) or natural products reagent (for flavonoids detection) staining [17].
2.7. HPLC experiments
HPLC methods were carried out with the L3 group. Analytical conditions were performed to define the running conditions on a Gilson HPLC (Villiers Le Bel, Val d’Oise, France) with an IB-SIL 5 C18 column (5 μm, 250 × 4.6 mm ID) from Phenomenex (Torrance, CA, USA), a 118 UV-Vis detector from Gilson and a Rheodyne injector fitted with a 50 μL loop. A gradient elution was performed with solvent A: 0.5 % (v⁄v) formic acid in water and solvent B: 0.5% formic acid in water and acetonitrile (15:85 v:v). The gradient was 0%–40% B, 3 min; 40%–45% B, 15 min; 45%–75% B, 3 min; 75%–75% B, 14 min and 0% B, 10 min. Compounds were detected at 254 nm at a flow rate of 1.2 mL min−1. HPLC semipreparative assays employed a Rheodyne injector fitted with a 500 μL loop, a LUNA PFP column (250 × 10 mm ID - 5 μm from Phenomenex), a flow rate of 2.6 mL/min, using the mobile phase and detector employed on analytical HPLC experiments. The collected compounds were first dried and the dissolved in MeOH for storage. HPLC semipreparative assays repeated as many times as necessary to obtain enough quantity of compounds for further assays. Compounds' purity was verified by analytical HPLC.
2.8. NMR spectra
Two compounds purified by HPLC (named P7 and P11) were studied by one-dimensional NMR methods (1H NMR, 13C NMR and 13C DEPT) and bidimensional NMR methods (1H–1H COSY, 1H–13C HSQC, 1H–13C HMBC and 1H-NOE). 10 mg of each compound were dissolved in 0.5 mL of CDOD3 to record NMR spectra. A Bruker Avance instrument (300 MHz for 1H NMR and 75.14 MHz for 13C NMR) was employed. Topspin software (Bruker) employed the solvent's non-deuterated impurities as internal standards to obtain the δ (ppm) values.
2.9. Polarimetric experiments
P7 and P11 were dissolved in ethanol and MeOH respectively to determine the optical rotations at 589 nm (0.1 dm cell, 25 °C) on Horiba Sepa-300 polarimeter (Horiba Ltd., Kyoto, Japan). The obtained [α]D25 values were compared with data reported previously [18, 19].
2.10. Disk diffusion assay
The antifungal activity of samples (i.e. plant extracts, fractions eluted and isolated substances) alone and in the presence of FLU (for synergy tests) were evaluated against C. albicans ATCC 10231 strain by disk diffusion assay. The experiments were carried out as described by Endo et al [20]. Briefly, yeast cells at exponential growth phase were collected from a 24 h culture on SDA, suspended in sterile distilled water until reach 103 colony forming units (CFU)/mL and afterward homogenously spread over MH-GMB agar plates surfaces. Synergy assays employed MH-GMB agar plates added with 6 μg/mL of FLU [20], referred to as “+FLU”. One hundred micrograms of samples were added on paper disks, and ten placed over agar plate surfaces. The growth inhibition halos were determined after incubation at 35 °C for 24 h. Experiments were performed at least three times.
2.11. Broth microdilution and cell viability assays
Microtiter 96 well plates were employed for growth microdilution assays as stated by CLSI [15] using RPMI-1640 (with glutamine, without bicarbonate) with 0.2 % Glucose. Both C. albicans strains previously described were used. Experiments were carried out with FLU, P7 and P11. Stock solutions of FLU, P7 and P11 were prepared in DMSO at a concentration of 1 mg/mL, and then serially diluted in sterile water. The final concentration of DMSO in the dilutions did not exceed 1%. FLU concentrations ranged from 0.125 to 32 μg/mL for experiments against C. albicans FLU sensible strain, and from 0.5 to 128 μg/mL for experiments against FLU resistant strain. P7 and P11 concentrations ranged from 1.25 to 80 μg/mL. The inoculum was adjusted to 103 CFU/mL in each well. Plates were incubated at 35 °C and the results were visually read after 48 h. Minimum inhibitory concentration (MIC) was stated as the minimum FLU concentration which caused 50% decrease in optical density (MIC-2) or 100% decrease in optical density for isolated compound (MIC-0 or optically clear wells). Optical densities were also recorded at 530 nm using a Bio-Rad 550 microplate reader (Bio-Rad Laboratories, CA, USA), according to EUCAST guidelines [21]. Cell viability assays were carried out as described elsewhere [8]. Briefly: 25 μL aliquots taken from each well were washed, diluted, plated onto SD agar plates and incubated for 48 h at 35 °C to count CFUs. Experiments were performed at least three times.
2.12. Checkerboard assays
The interactions between FLU + P7 or P11 against both C. albicans cells were evaluated employing checkerboard assays on microtiter plates as stated elsewhere [22]. Briefly, columns 3–11 of the “A” row wells were added with FLU. Column 12 (rows B–H) were added with either P7 or P11. The final concentrations of FLU, P7 and P11 were adjusted at the same values previously described. Column 1 was the growth control (i.e. growth medium + yeast strain) and column 2 was the sterility control (i.e. growth medium). The remaining wells contained a single combination of FLU and the compound under assay. All the wells (except sterility controls) were added with exponential growing C. albicans cells (suspended in RPMI 1640 medium) to obtain 103 CFU/mL as final concentration. All the wells were adjusted to 200 μL final volume. The plates were covered and incubated at 35 °C for 48 h [23]. CFU values were obtained through cell viability assays as previously described. A reduction in CFU/mL ≥ 2 log units exerted by the combination over the most active agent alone (i.e. the 2-log units criteria) was employed for determining the synergism of a combination [24]. Fractional Inhibitory Concentration (FIC) of each compound was calculated as the ratio between MIC of the compound in the combined experiments and the MIC of the same compound individually. The interactions between FLU and the isolated compounds were assessed through Fractional Inhibitory Concentration Index (FICI), i.e. the sum of FICs. FICI data interpretations were classified in three categories, according to Odds [25]: “synergy” (FICI ≤0.5), “antagonism” (FICI >4.0) and “no interaction” (FICI >0.5–4.0). The MIC values for FLU (alone) which were >4 μg/mL experimentally (against C. albicans ATCC 10231) and >128 μg/mL experimentally (against C. albicans 12–99), were considered to be 8 μg/mL and 256 μg/mL respectively (referred to as MIC-0FLU) for calculation purposes, a criterion reported by other authors [26]. Experiments were performed at least three times.
2.13. Fungicidal activity assays
Checkerboard assay carried out with 104 CFU/mL inocula [27], followed by viable cell count experiments were used to assess the fungicidal activity of P7 and P11 alone and in combination. To define the >99.9% cell viability lost of initial inocula the criterion of 3 log units decrease in CFU/mL compared to the growth control (above the 10 CFU/mL limit of detection) was employed. The minimal fungicidal concentration (MFC) was the minimal concentration of a sample which caused >99.9% growth inhibition. P7 and P11 concentrations ranged from 1.25 to 120 μg/mL, and the FLU concentrations used were the same as described in checkerboard assays. Experiments were repeated at least three times with both C. albicans strains.
2.14. Genotoxic activity
Bacillus subtilis rec assay was employed to assess the genotoxic effects of isolated compounds. The assay is based on the quantification of the differences in the growth curves (after Probit transformation) generated for B. subtilis rec+ (DNA repair proficient) and rec- (DNA repair deficient) strains exposed to samples [28]. Isolated compounds (i.e. P7 and P11) were evaluated at 0.93–60 μg/mL alone and in combination with FLU: P7 (20 μg/mL) + FLU (from 0.5-8 μg/mL) and P11 (30 μg/mL) + FLU (from 0.5-16 μg/mL). S-Probit values obtained were compared with those described elsewhere [29]. Reference drugs were K2Cr2O7 (genotoxic agent) and FLU (non-genotoxic agent).
2.15. Cytotoxicity assay
The cytotoxicity of isolated compounds was evaluated by MTT colorimetric assay on human lymphocytes. Human lymphocytes were isolated from fresh whole blood from healthy volunteers (25–35 years old) using Histopaque 1077. Anticoagulated blood (K2EDTA) was diluted with an equal volume of Ham F10 medium supplemented with glutamine, containing 10 % fetal calf serum (FCS), underlying it with Histopaque 1077 (2:1 ratio) and centrifuged at 200 g for 30 min. Mononuclear cells were separated as a white layer on the top of the Histopaque [30]. Cells were washed with Hank's Salt (HBSS) and centrifuged two times at 200 g for 5 min. Cells were rinsed with PBS, diluted to 5 × 106 cells/mL, and cultured in 10 mL Ham F10 supplemented with 10% FCS, 100 IU/mL of penicillin and 100 ng/mL of streptomycin (complete H–F10 medium) for 24 h. No adherent peripheral blood lymphocytes (PBL) were separated from adherent cells by aspiration, and the number of viable cells was determined by Trypan blue exclusion test on a hematological counter (Neubauer Chamber). Harvested lymphocytes were cultured in complete H–F10 medium (Ham F10 medium added with glutamine, 10 % FCS and Concanavalin A as mitogen agent), on 5% CO2 atmosphere for 24 h at 37 °C. The viable cells number was determined by Trypan blue exclusion test.
The MTT colorimetric assay was performed as follows: 100 μL aliquots of PBL suspension (adjusted up to 105 cells/100 μL) were added into 24-well flat-bottom plate (Nunclon Delta, Denmark), mixed with complete H–F10 medium plus lipopolysaccharide (2 μg/mL) and incubated into 5% CO2-air for 24 h at 37 °C to obtain the activated cells, which were washed with PBS buffer, and then incubated for 24 h in complete H–F10 medium containing the isolated compounds, to give the final concentrations of 1, 10, 50 and 100 μg/mL. Controls with DMSO (0.05–0.01 % v/v) were included. After incubation, the exposed cells were washed with HBSS and each well was added with 10 μL of 5 mg/mL MTT solution (dissolved in H–F10 without FCS), plates were further incubated in 5% CO2-air 4 h at 37 °C. Afterwards, each treatment was centrifuged at 1500 g for 5 min to precipitate cells containing formazan. The supernatant was removed from each well, and 175 μL of DMSO was added to dissolve the MTT formazan crystals. The plate was mixed on a microshaker for 10 min, and read on a microplate reader (Bio-Rad, California, USA) at 550 nm to attain the OD550 values. Data were mean values of two experiments performed in triplicate. The percent viability score (V%) was calculated with the formula: V (%) = (T - S)/(C - S) × 100; where T is OD550 value of the cell with samples after 24 h incubation, C is the OD550 value of the cell without samples and S is the OD550 value of the cell before sample addition. The concentration values of samples that crossed the V (%) were measured as the inhibitory concentration 50 values (IC50). The IC50 was the concentration of a sample necessary to inhibit the growth to 50% of the control.
2.16. Trypan blue exclusion test
Aliquots of each experiments including activated lymphocytes plus samples concentrations and controls were incubated 24 h, tenfold diluted, stained with 4 % Trypan Blue (TB) in PBS and counted. Mean values were analyzed and plotted respect to controls.
2.17. Statistical analysis
Data was analyzed by either Student's t-test or one-way ANOVA. Probability levels below 0.05 (p < 0.05) were considered as statistically significant.
3. Results and discussion
3.1. Activity-guided fractionation
The extraction yield obtained for Aq was 6.56 and for EE 9.89 g of EM/100 g of dry plant material. Only EE showed inhibitory halos against C. albicans by disk diffusion assay, with values of 8.3 ± 1.0 mm (without FLU) and 12.3 ± 0.5 mm (with FLU). Taking these results into consideration (higher extraction yield and antifungal activity), EE was selected as raw extract sample for activity-guided purification procedures. The flow chart of the process is shown in Figure 1. The EM and inhibition halos diameter values against C. albicans ATCC 10231 generated by active fractions are showed in Table 1.
Figure 1.
Flow chart of the purification procedure.
Table 1.
EM and inhibition halos diameter values against C. albicans ATCC 10231 generated by active fractions obtained though purification steps of T. dodoneifolia EE.
| Fraction | EM ± sd (g) | Inhibition halos diameters (mm) against C. albicans ATCC 10231 |
|
|---|---|---|---|
| - FLU | +FLU | ||
| HX | 0.81 ± 0.02 | 6.6 ± 0.60 | 8.0 ± 1.0 |
| CH | 2.99 ± 0.10 | 9.1 ± 0.30 | 13.5 ± 0.50 |
| G1 | 1.00 ± 0.05 | 8.8 ± 0.20 | 12.3 ± 0.40 |
| L3 | 0.332 ± 0.017 | 8.30 ± 0.60 | 11.3 ± 0.60 |
The EM and the inhibition halos diameters generated by CH fraction were higher than those for HX (p < 0.05), while BU did not exhibit C. albicans ATCC10231 strain growth inhibition (i.e. no inhibition halo observed). CH was selected for further purification steps.
CH was chromatographed on silica gel to yield only one group (G1) with antifungal activity against C. albicans ATCC10231 (+FLU and -FLU), determined by disk diffusion assay, therefore G1 was selected for further experiments.
G1 was chromatographed on Sephadex LH20 to yield two groups (L2 and L3) with antifungal activity against C. albicans ATCC10231 (+FLU and -FLU), determined by disk diffusion assay, however L3 yielded EM and diameters of the inhibition halos significant higher (p < 0.05) than those for L2 (data not shown). L3 was selected for HPLC experiments.
3.2. Chemical elucidation of the isolated compounds
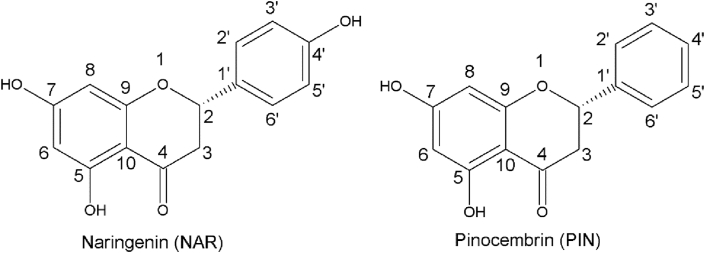
The L3 group was analyzed through HPLC experiments to yield two compounds (P7 and P11) with antifungal activities against C. albicans ATCC 10231. NMR and polarimetric measures allowed the elucidation of chemical structures, which were consistent with flavanones structures, P7: 4’,5,7-trihydroxiflavanone (naringenin) and P11: 5,7-dihydroxiflavanone (pinocembrin). The spectroscopic data of NMR experiments and polarimetric assays are showed in Tables 2, 3, and 4. The chemical structures of naringenin (NAR) and pinocembrin (PIN) are showed in Figure 2.
Table 2.
1H and 13C NMR data (δ in ppm) for naringenin (P7) in CD3OD.
| Asignation | δ13C | δ1H mult. (J in Hz) |
|---|---|---|
| C2 | 80.2 | 5.29 dd (1H, J1 = 12.0; J2 = 3.1 Hz) |
| C3 | 43.8 | 3.06 dd (1H, J1 = 18.0; J2 = 12.3 Hz) 2.68 d (1H, J1 = 18.0) |
| C4 | 197.5 | |
| C5 | 165.2 | |
| C6 | 95.8 | 5.88 d (J = 2.2 Hz) |
| C7 | 168.0 | |
| C8 | 96.0 | 5.90 s (J = 2.2 Hz) |
| C9 | 164.9 | |
| C10 | 103.2 | |
| C1′ | 131.8 | |
| C2′ | 128.9 | 7.28 d (1H, J = 9.9 Hz)a |
| C3′ | 116.4 | 6.81 d (1H, J = 8.26 Hz) |
| C4′ | 158.6 | |
| C5′ | 116.4 | 6.81 d (1H, J = 8.26 Hz) |
| C6′ | 128.9 | 7.28 d (1H, J = 9.9 Hz)a |
Deduced from 1H–13C HSQC.
Table 3.
1H and 13C NMR data (δ in ppm) for pinocembrin (P11) in CD3OD.
| Asignation | δ13C | δ 1H mult. (J in Hz) |
|---|---|---|
| C2 | 79.0 | 5.44 dd (1H, J1 = 12.6; J2 = 3.2 Hz) |
| C3 | 42.7 | 3.07 dd (1H, J1 = 17.0; J2 = 12.8 Hz) 2.76 dd (1H, J1 = 17.0 y J2 = 3.2) |
| C4 | 195.9 | |
| C5 | 164.1 | |
| C6 | 95.8 | 5.92 d (J = 2.2 Hz) |
| C7 | 163.2 | |
| C8 | 94.9 | 5.93 d (J = 2.2 Hz) |
| C9 | 167.2 | |
| C10 | 102.0 | |
| C1′ | 139.0 | |
| C2′ | 126.0 | 7.44 dd (2H, J = 9.9 Hz)a |
| C3′ | 128.2 | 7.35 m |
| C4′ | 128.3 | 7.35 m |
| C5′ | 128.2 | 7.35 m |
| C6′ | 126.0 | 7.44 dd (2H, J = 9.9 Hz)a |
Deduced from 1H–13C HSQC.
Table 4.
Polarimetric measures obtained for purified compounds.
Figure 2.
Chemical structures of NAR and PIN.
3.3. Broth microdilution and cell viability assays
Antifungal activities exerted by NAR and PIN against C. albicans strains (both FLU sensitive and FLU resistant), individually and in combination with FLU were studied by employing broth microdilution and checkerboard assays. The results are showed in Table 5.
Table 5.
MIC values for NAR and PIN against C. albicans ATCC 10231 and 12–99.
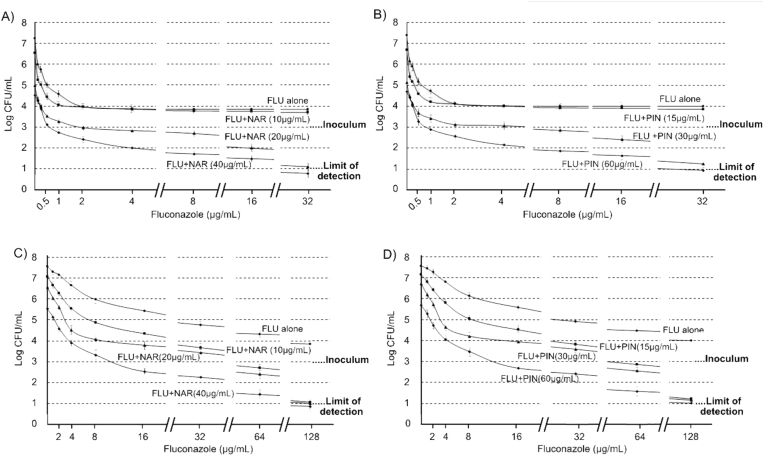
The most active isolated substance against both C. albicans strains was NAR. As showed in Figure 3A, a ≥2 log units decrease on the viability of C. albicans ATCC 10231 was obtained from checkerboard assays (initial inoculum of 103 CFU/mL) followed by viable cell count experiments with 10 μg/mL of NAR, from 6.52 log (experiments carried out with NAR alone) to 4.43 (experiments carried out with 10 μg NAR/mL + 0.5 μg FLU/mL), which suggests that there would be a synergistic combination between NAR and FLU (FICI = 0.258). These findings differ from those of a previous report, where NAR did not exert an inhibitory effect against C. albicans [31]. The assays carried out with C. albicans 12–99 showed identical results: there was a reduction in log units count from 7.06 (experiments carried out with 10 μg NAR/mL) to 4.87 (experiments carried out with 10 μg NAR/mL + 8 μg FLU/mL - line with squares in Figure 3C), which suggests that there would be a synergistic combination between NAR and FLU against C. albicans FLU resistant strain (FICI = 0.188).
Figure 3.
Viable cell count assays with 103 CFU/mL initial inoculums. A) Experiments carried out with NAR against C. albicans ATCC 10231. B) Experiments carried out with PIN against C. albicans ATCC 10231. C) Experiments carried out with NAR against C. albicans 12–99. D) Experiments carried out with PIN against C. albicans 12–99.
PIN showed lower antifungal activity than NAR against both strains, though our results showed a synergistic effect (FICI = 0.258) for PIN (15 μg/mL) + FLU (0.5 μg/mL) combination against C. albicans (ATCC10231) (Figure 3B), results that agree with those reported by [32] against the same strain. A synergistic effect was also observed for PIN (15 μg/mL) + FLU (8 μg/mL) combination against C. albicans 12–99 (FICI = 0.281) (Figure 3D).
PIN has been described to cause inhibition of trihydroxinaphtalen reductase, described as a key enzyme on modern fungicidal drugs development [33]. NAR has been described to cause proton-motive force alterations in some strains [34], a result which is in agreement with that obtained by Peng et al. [35], who found some flavanones (PIN included) affected the energetic homeostasis in pathogenic fungal strains. FLU is well known to cause alterations on ergosterol biosynthesis pathway [8]. It is accepted that a combination of antimicrobials which act over different cell targets may exert a synergistic effect on microbial cells [20]. Taking into consideration these reports, our results could match this statement. White et al. [14] suggested that the FLU resistance observed for C. albicans 12–99 is sustained on: alterations in the gene that codes for the ERG11 enzyme and the overexpression of the efflux pump genes MDR1, CDR1 and CDR2. Our results showed that those FLU resistance mechanisms did not affect the sensitivity of this yeast to either NAR of PIN, as each one of these substances showed the same MICs against both FLU sensitive (ATCC 10231) and FLU resistant (12–99) C. albicans strains.
3.4. Fungicidal activity assays
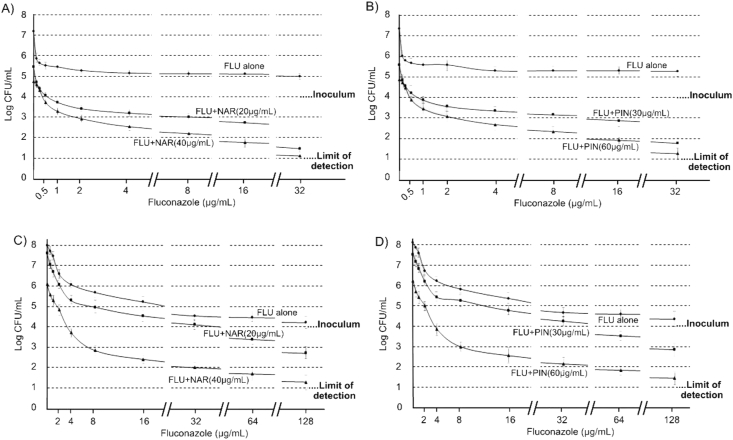
C. albicans FLU sensitive (ATCC 10231) and FLU resistant (12–99) strains were employed to assess fungicidal activity (i.e. using 104 CFU/mL inocula) and cell viability assays of NAR and PIN, both individually and in combination with FLU. No viable C. albicans FLU sensitive cells could be recovered from experiments performed with isolated substances individually (i.e. NAR and PIN), consequently the MFCs were 80 μg/mL and 120 μg/mL for NAR and PIN respectively. The same MFCs were obtained for these substances against C. albicans 12–99. No cell growth was observed for the assays carried out with NAR (20 μg/mL) + FLU (0.5 μg/mL) and PIN (30 μg/mL) + FLU (0.5 μg/mL) against C. albicans ATCC 10231 (Figure 4 A and B), suggesting >99.9% of cell viability lost from initial inoculum. No cell growth could be observed for assays performed with C. albicans 12–99 carried out with NAR (20 μg/mL) + FLU (8 μg/mL) and PIN (30 μg/mL) + FLU (16 μg/mL) (Figure 4 C and D).
Figure 4.
Viable cell count assays with 104 CFU/mL initial inoculums. A) Experiments carried out with NAR against C. albicans ATCC 10231. B) Experiments carried out with PIN against C. albicans ATCC 10231. C) Experiments carried out with NAR against C. albicans 12–99. D) Experiments carried out with PIN against C. albicans 12–99.
The isolated flavanones are common in plants, however, they have not been reported in this species before and the folk antifungal activity of this plant has not been attributed to any specific compound. Even though up-to-date only PIN antifungal activity against C. albicans has been reported [32], our results show that NAR + FLU and PIN + FLU mixtures are able to exert fungicidal action against the tested strains.
According to Rios & Recio [36], antimicrobial tests should be run with collection strains, and studies shall include isolated pathogens in the case of active compounds. We took this into consideration to asses NAR and PIN antifungal activities. These authors also suggest avoiding the claim “positive antimicrobial activity” for isolated compounds that exert the inhibition in concentrations above 100 μg/mL. Our experiments showed NAR and PIN exerts inhibitory antifungal activities when assayed between 60-80 μg/mL (individually) and 15–20 μg/mL in combinatory assays. NAR showed more antifungal potency than PIN against the assayed strains, which could be attributed to the hydroxylation on B ring in the chemical structure of NAR. Rondina et al. [10] described the folk use of T. dodoneifolia aqueous extract (i.e. infusion) as antifungal, but no chemical structure or group of chemical constituents were proposed as responsible for those actions. Our results showed T. dodoneifolia aqueous extract was not active against the tested strains, instead the ethanolic extract was the active one. The chemical nature of the antifungal compounds (i.e. flavanones) justifies these findings because of the poor water solubility exhibited for NAR and PIN, which are readily soluble in ethanol. NAR and PIN could not be found on T. dodoneifolia aqueous extract (data not shown).
3.5. Genotoxic activity
B. subtilis rec assay was employed to assess genotoxic activity of NAR, PIN and combinations of NAR + FLU and PIN + FLU. Table 6 shows S-probit values and results.
Table 6.
Genotoxic activity of isolated compounds, isolated compounds + FLU mixtures, and reference compounds.
| Sample | S-Probit | Result |
|---|---|---|
| NAR | 0.138 | Non-genotoxic (−) |
| PIN | 0.147 | Non-genotoxic (−) |
| NAR + FLU | 0.156 | Non-genotoxic (−) |
| PIN + FLU | 0.165 | Non-genotoxic (−) |
| K2Cr7O7 | 2.95 | Strong genotoxic (++) |
| FLU | 0.117 | Non-genotoxic (−) |
No genotoxic effect (i.e. −0.123 > S-Probit > 0.199) was observed for PIN, NAR and FLU against B. subtilis rec strains. As NAR and PIN combinations with FLU resulted in mixtures with synergistic antifungal effects against C. albicans strains, these mixtures were assayed against B. subtilis. NAR + FLU and PIN + FLU combinations resulted in non-genotoxic mixtures, while K2Cr2O7 exhibited a strong genotoxic effect (S-Probit > 0.593). These findings are in concordance with those previously reported on rats [12, 37] using T. dodoneifolia extracts. Moreover, NAR and PIN were recently reported as antigenotoxic molecules, linking the effect to the inhibition in the formation of pyrimidine cyclobutane (CPD) [38].
3.6. Cell viability
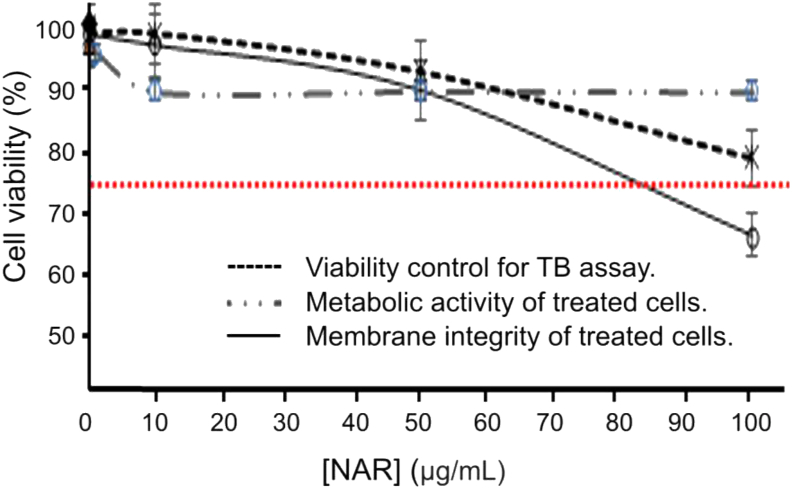
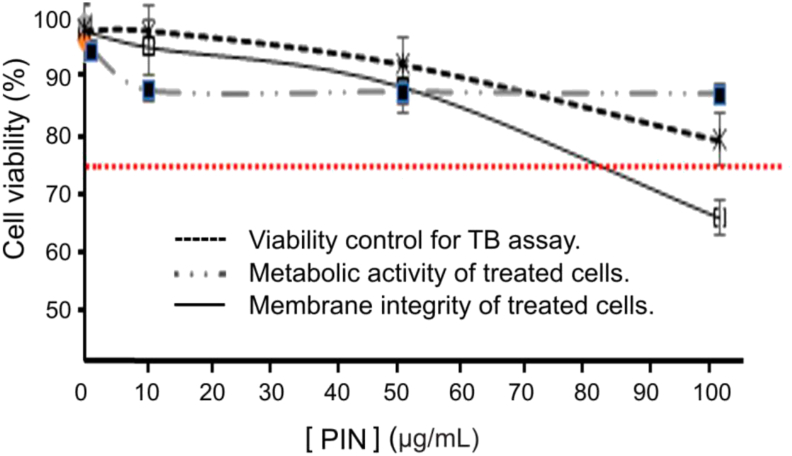
Percent of cell survival was assessed based on metabolic activity and cell membrane integrity, by the MTT reduction assay and TB staining, respectively, on PBL cultures. The activated lymphocytes treated with NAR showed decreased cell viability according to the metabolic activity observed, and up to 50 μg/mL could be correlated with the effect on membrane integrity. However, when the NAR concentration increased from 50 to 100 μg/mL, the metabolic activity showed a tendency toward loss of viability (greater than that due to DMSO), while the number of viable cells (according to evaluation of the membrane integrity) was maintained constant (Figure 5). Matching results were obtained in experiments performed with PIN on activated lymphocytes, where a decrease in cell viability was observed at the assayed concentrations (Figure 6), also correlated with membrane integrity decrease up to 50 μg/mL. PIN concentrations above 80 μg/mL exhibited a significant loss of metabolic activity. The number of viable cells (according to evaluation of the membrane integrity) was maintained constant. From these observations it could be concluded that concentrations of NAR and PIN lower than 80 μg/mL presented a 75% viability according to their metabolic activity level on the cytoxicity assay (considering the sensitivity of activated lymphocytes).
Figure 5.
Effects of NAR on the PBL viability (P < 0.05).
Figure 6.
Effects of PIN on the PBL viability (P < 0.05).
Others authors assessed the in vitro effect of NAR on cultured human normal cellsand found NAR possess low cytotoxicity (assessed by increased intracellular ROS levels). PIN was evaluated previously on cultured cell lines by MTT reduction assay, and showed cytotoxicity on a variety of cancer and normal cells [39]. Our results showed PIN cytotoxicity was identical to that of NAR. Moreover, when the cytotoxicity exerted by NAR was compared with that of other flavonoids, it could be observed that the most toxic flavonoids, such as luteolin and quercetin, share in common the hydroxyl group in position 3 (flavonol) and/or greater hydroxylation in A ring [40], a chemical structure absent in NAR and PIN.
4. Conclusion
The activity guided isolation of T. dodoneifolia metabolites allowed the identification of NAR and PIN as the main antifungal components with inhibitory activity against C. albicans FLU sensitive (ATCC 10231) and FLU resistant (12–99) strains. FLU combinations with NAR or PIN resulted in synergistic effects, with activities over both strains. As far as we know, this is the first report pointing to NAR and PIN as responsible compounds for anti-Candida activity of T. dodoneifolia ethanolic extract. NAR and PIN could only be found on ethanolic fraction, but not in aqueous extract, which could explain the lack of antifungal activity found the last one. No genotoxic effects were observed for NAR, PIN and combinations of these flavanones with FLU. A low cytoxicity exerted by NAR and PIN at the antifungal MIC give some safety information regarding the traditional use of T. dodoneifolia as a source of antifungals. NAR and PIN employed alone and in combination with FLU could potentially increase the antifungal effectiveness of the available therapies.
Declarations
Author contribution statement
José R. Soberón, Melina A. Sgariglia, Diego A. Sampietro, Julia Fernández de Luco, Guillermo R. Labadie: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
José A. Carabajal Torrez, Edgardo J.I. Peroe: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by grants from Secretaría de Ciencia, Arte e Innovación Tecnológica of Universidad Nacional de Tucumán [grants number 26D535 and D647/2], Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET; Buenos Aires, Argentina) [grant number PIP761], and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT; Buenos Aires, Argentina) [grant number PICT 2016 Nº3152].
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.García L.T., Luna L.J., Velasco T.K., Guerra B.E. A new multiplex PCR for species-specific diagnosis of human candidiasis. Biomedica. 2017;37(2):200–208. doi: 10.7705/biomedica.v37i2.3202. [DOI] [PubMed] [Google Scholar]
- 2.Prasad R., editor. Candida Albicans: Cellular and Molecular Biology. Springer; 2017. [Google Scholar]
- 3.Wisplinghoff H., Ebbers J., Geurtz L., Stefanik D., Major Y., Edmond M.B., Seifert H. Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features and antifungal susceptibilities. Int. J. Antimicrob. Agents. 2014;43:78–81. doi: 10.1016/j.ijantimicag.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Flynn S., Hollis A., Palmedo M. An economic justification for open access to essential medicine patents in developing countries. J. Law Med. Ethics. 2009;37:184–208. doi: 10.1111/j.1748-720X.2009.00365.x. [DOI] [PubMed] [Google Scholar]
- 5.Sanglard D., Ischer F., Marchetti O., Entenza J., Bille J. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 2003;48:959–976. doi: 10.1046/j.1365-2958.2003.03495.x. [DOI] [PubMed] [Google Scholar]
- 6.Wagner H., Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16:97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Thomas E., Vandebroek I., Van Damme P., Goetghebeur P., Douterlungne D., Sanca S., Arrazola S. The relation between accessibility, diversity and indigenous valuation of vegetation in the Bolivian Andes. J. Arid Environ. 2009;73:854–861. [Google Scholar]
- 8.Soberón J.R., Sgariglia M.A., Pastoriza A.C., Soruco E.M., Jäger S.N., Labadie G.R., Vattuone M.A. Antifungal activity and cytotoxicity of extracts and triterpenoid saponins obtained from the aerial parts of Anagallis arvensis L. J. Ethnopharmacol. 2017;203:233–240. doi: 10.1016/j.jep.2017.03.056. [DOI] [PubMed] [Google Scholar]
- 9.Hilgert N.I. Plants used in home medicine in the Zenta River basin, Northwest Argentina. J. Ethnopharmacol. 2001;76:11–34. doi: 10.1016/s0378-8741(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 10.Rondina R.V., Bandoni A.L., Coussio J.D. Argentine medicinal plants with potential antifungal activity. Dominguezia. 2010;26:31–39. [Google Scholar]
- 11.Soria N., Basualdo I., Ramoa L., López de Silva M.E. Descripción de Tessaria dodeneifolia (Hook. & Arn.) Cabrera, (Asteraceae), “la planta dulce” como endulzante natural. Bol. Latinoam. Caribe Plantas Med. Aromat. 2017;16:129–135. [Google Scholar]
- 12.Nanayakkara N.D., Hussain R.A., Pezzuto J.M., Soejarto D.D., Kinghorn A.D. Potential sweetening agents of plant origin. 13. An intensely sweet dihydroflavonol derivative based on a natural product lead compound. J. Med. Chem. 1988;31:1250–1253. doi: 10.1021/jm00401a030. [DOI] [PubMed] [Google Scholar]
- 13.Romio E., Gurni A.A. Estudio micrográfico preliminar de las estructuras foliares de dos especies palustres americanas con potencial actividad antiviral. Bol. Latinoam. Caribe Plantas Med. Aromat. 2007;6:219–220. [Google Scholar]
- 14.White T.C., Holleman S., Dy F., Mirels L.F., Stevens D.A. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 2002;46:1704–1713. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute (CLSI) 4th Informational Supplement. CLSI Document M27-S4. Wayne, PA., USA. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts. [Google Scholar]
- 16.Farmacopea Argentina – Medicamentarius Argentino. VIII Ed. Codex.; Buenos Aires, Argentina: 2011. [Google Scholar]
- 17.Wagner H., Bladt . 2a Ed. Springer-Verlag; Berlin, Heidelberg, Germany: 1996. Plant Drug Analysis. A Thin Layer Chromatography Atlas. [Google Scholar]
- 18.Olsen H.T., Stafford G.I., Van Staden J., Christensen S.B., Jäger A.K. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L. J. Ethnopharmacol. 2008;117:500–502. doi: 10.1016/j.jep.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Mu Q., Tang W.D., Liu R.Y., Li C.M., Lou L.G., Sun H.D., Hu C.Q. Constituents from the stems of Goniothalamus griffithii. Planta Med. 2003;69:826–830. doi: 10.1055/s-2003-43219. [DOI] [PubMed] [Google Scholar]
- 20.Endo E.H., Cortez D.A.G., Ueda-Nakamura T., Nakamura C.V., Dias Filho B.P. Potent antifungal activity of extracts and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Res. Microbiol. 2010;161:534–540. doi: 10.1016/j.resmic.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Arendrup M.C., Guinea J., Cuenca-Estrella M., Meletiadis J., Mouton J.W., Lagrou K., Howard S.J. Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). EUCAST definitive document E. DEF 7.3. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. 2015. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7_3_1_Yeast_testing__definitive.pdf
- 22.Martinez-Irujo J.J., Villahermosa M.L., Alberdi E., Santiago E. A checkerboard method to evaluate interactions between drugs. Biochem. Pharmacol. 1996;51:635–644. doi: 10.1016/s0006-2952(95)02230-9. [DOI] [PubMed] [Google Scholar]
- 23.Iten F., Saller R., Abel G., Reichling J. Additive antimicrobial effects of the active components of the essential oil of Thymus vulgaris–chemotype carvacrol. Planta Med. 2009;75:1231–1236. doi: 10.1055/s-0029-1185541. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller M.A., Sheehan D.J., Rex J.H. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 2004;17:268–280. doi: 10.1128/CMR.17.2.268-280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odds F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003;52 doi: 10.1093/jac/dkg301. 1-1. [DOI] [PubMed] [Google Scholar]
- 26.Fiori A., Van Dijck P. Potent synergistic effect of doxycycline with fluconazole against Candida albicans is mediated by interference with iron homeostasis. Antimicrob. Agents Chemother. 2012;56:3785–3796. doi: 10.1128/AAC.06017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantón E., Pemán J., Viudes A., Quindós G., Gobernado M., Espinel-Ingroff A. Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagn. Microbiol. Infect. Dis. 2003;45:203–206. doi: 10.1016/s0732-8893(02)00525-4. [DOI] [PubMed] [Google Scholar]
- 28.Matsui S. The Bacillus subtilis/microsome rec-assay for the detection of DNA-damaging substances in waters of a night soil treatment plant. Environ. Toxicol. 1988;3:173–193. [Google Scholar]
- 29.Takigami H., Matsui S., Matsuda T., Shimizu Y. The Bacillus subtilis rec-assay: a powerful tool for the detection of genotoxic substances in the water environment. Prospect for assessing potential impact of pollutants from stabilized wastes. Waste Manag. 2002;22:209–213. doi: 10.1016/s0956-053x(01)00071-x. [DOI] [PubMed] [Google Scholar]
- 30.Noroozi M., Angerson W.J., Lean M.E.J. Effects of flavonoids and vitamin C on oxidative DNA damage to human lymphocytes. Am. J. Clin. Nutr. 1998;67:1210–1218. doi: 10.1093/ajcn/67.6.1210. [DOI] [PubMed] [Google Scholar]
- 31.Salazar-Aranda R., Granados-Guzmán G., Pérez-Meseguer J., González G., de Torres N. Activity of polyphenolic compounds against Candida glabrata. Molecules. 2015;20:17903–17912. doi: 10.3390/molecules201017903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agüero M.B., Svetaz L., Baroni V., Lima B., Luna L., Zacchino S., Tapia A. Urban propolis from San Juan province (Argentina): ethnopharmacological uses and antifungal activity against Candida and dermatophytes. Ind. Crop. Prod. 2014;57:166–173. [Google Scholar]
- 33.Yang S., Zhou J., Li D., Shang C., Peng L., Pan S. The structure-antifungal activity relationship of 5, 7-dihydroxyflavonoids against Penicillium italicum. Food Chem. 2017;224:26–31. doi: 10.1016/j.foodchem.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Mirzoeva O.K., Grishanin R.N., Calder P.C. Antimicrobial action of propolis and some of its components: the effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997;152:239–246. doi: 10.1016/S0944-5013(97)80034-1. [DOI] [PubMed] [Google Scholar]
- 35.Peng L., Yang S., Cheng Y.J., Chen F., Pan S., Fan G. Antifungal activity and action mode of pinocembrin from propolis against Penicillium italicum. Food Sci. Biotechnol. 2012;21:1533–1539. [Google Scholar]
- 36.Ríos J.L., Recio M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 37.Ishizawa C., Shironoshita M., de Ugaz O.L. Más sobre las moléculas que endulzan. Revista de Química. 1995;9:145–157. [Google Scholar]
- 38.García Forero A., Villamizar Mantilla D.A., Núñez L.A., Ocazionez R.E., Stashenko E.E., Fuentes J.L. Photoprotective and antigenotoxic effects of the flavonoids Apigenin, naringenin and pinocembrin. Photochem. Photobiol. 2019;95:1010–1018. doi: 10.1111/php.13085. [DOI] [PubMed] [Google Scholar]
- 39.Suresh Kumar M.A., Mangalam N., Hema P.S., Mohan J., Santhoshkumar T.R. Pinocembrin triggers bax-dependent mitochondrial apoptosis in colon cancer cells. Mol. Carcinog. 2007;46:231–241. doi: 10.1002/mc.20272. [DOI] [PubMed] [Google Scholar]
- 40.Matsuo M., Sasaki N., Saga K., Kaneko T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol. Pharm. Bull. 2005;28:253–259. doi: 10.1248/bpb.28.253. [DOI] [PubMed] [Google Scholar]