Abstract
Accumulating evidence has demonstrated that aberrant microRNA (miRNA) expression is involved in hepatocellular carcinoma (HCC) progression. Previous findings suggested that miRNA (miR)-875-5p participates in the development of various types of cancer. However, the expression and function of miR-875-5p in HCC remains largely unclear. The analysis of clinical samples in the present study demonstrated that miR-875-5p expression was downregulated in HCC tissues compared to adjacent non-tumor tissues, which was associated with a large tumor size, venous infiltration, advanced tumor-node-metastasis stage and unfavorable overall survival. In vitro experiments revealed that ectopic expression of miR-875-5p suppressed, whereas inhibition of miR-875-5p promoted HCC cell proliferation, migration, invasion and epithelial-to-mesenchymal transition (EMT) progression. Overexpression of miR-875-5p restrained HCC tumor growth and metastasis in vivo. Mechanistically, eukaryotic translation initiation factor 3 subunit a (eIF3a) was identified as the downstream target of miR-875-5p in HCC. Further experiments demonstrated that the expression of eIF3a was upregulated and negatively correlated with that of miR-875-5p in HCC tissues. In addition, miR-875-5p negatively regulated the luciferase activity of wild-type, but not mutant 3′-untranslated region (3′UTR) of eIF3a mRNA. miR-875-5p suppressed eIF3a expression at the mRNA and protein level in HCC cells. Additionally, eIF3a exerted an oncogenic role, and knockdown of eIF3a inhibited the proliferation, motility and EMT of HCC cells. In addition, eIF3a overexpression abolished the inhibitory effects of miR-875-5p on the proliferation, motility and EMT in HCC cells. In conclusion, miR-875-5p, which was downregulated in HCC, may inhibit tumor growth and metastasis by eIF3a downregulation via targeting its 3′UTR and may be a promising prognostic and therapeutic strategy in HCC.
Keywords: hepatocellular carcinoma, tumor growth, tumor metastasis, microRNA-875-5p, eukaryotic translation initiation factor 3 subunit a
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors and the fourth leading cause of cancer-related death worldwide (1). Although progress has been achieved in the diagnosis and therapy over the last decades, the long-term survival rate is unsatisfactory due to the high recurrence and metastasis rates (2). Resection is the most widely used curative treatment for patients with early HCC; however, a number of patients diagnosed at an advanced stage are ineligible for surgery (3). Therefore, it is imperative to unravel the molecular mechanisms underlying the occurrence and development of HCC to identify novel therapeutic strategies for this malignancy.
As conserved single-stranded non-coding RNAs of ~22 nucleotides in length, microRNAs (miRNAs) serve pivotal regulatory roles by protein-coding gene cleavage or translation repression via interacting with the 3′-untranslated region (3′UTR) of target mRNAs with imperfect complementarity (4,5). Accumulating evidence has demonstrated that miRNAs capable of controlling cell proliferation, metabolism, invasion and angiogenesis serve crucial roles in the initiation, progression and metastasis of various types of cancer, including HCC (6,7). Recent findings have demonstrated that miRNA (miR)-1468, miR-876-5p, miR-532-3p, miR-3194-3p and miR-519c-3p regulate the growth and metastasis of HCC via different underlying molecular mechanisms (8–12). Previous results have suggested that miR-875-5p is dysregulated in various types of cancer, such as colorectal carcinoma, lung and prostate cancer (13–16). In those studies, miR-875-5p was demonstrated to function as a tumor suppressor or as an oncogenic factor involved in the proliferation, migration, invasion, apoptosis, chemotherapeutic sensitivity and radiation response of cancer cells. However, limited information is currently available about the role of miR-875-5p and its underlying molecular mechanisms in HCC.
Eukaryotic translation initiation factor 3, which serves a central role in translation initiation, comprises 13 subunits, among which eukaryotic translation initiation factor 3 subunit a (eIF3a) is the largest one (17). In humans, eIF3a appears to be ubiquitously expressed and involved in cellular processes such as translation initiation, cell cycle, and DNA synthesis and repair (18). Previous findings have revealed that eIF3a is abnormally expressed and involved in the tumorigenesis of lung cancer, ameloblastoma, pancreatic cancer and HCC (19–22). In HCC, eIF3a regulates the translation of hypoxia-inducible factor 1α (HIF-1α), mediating HIF-1α-dependent glycolytic metabolism in HCC (22). However, the exact functions of eIF3a on tumor growth and metastasis and the mechanisms underlying the aberrant expression of eIF3a in HCC remain unclear.
Current findings demonstrated that miR-875-5p was downregulated in HCC cells and tissues, which significantly correlated with short survival and progressed clinical features. The results of the loss- and gain-of-function experiments confirmed that miR-875-5p inhibited tumor growth and metastasis in vitro and in vivo. Mechanistically, miR-875-5p interacted with the 3′UTR of eIF3a mRNA, downregulating the expression of eIF3a, which exhibited oncogenic activities in HCC. Thus, the present study validated that miR-875-5p may inhibit tumor growth and metastasis by targeting eIF3a in HCC and may represent a potential target for HCC treatment.
Materials and methods
Tissue samples
HCC and corresponding adjacent non-tumor tissues were obtained from 90 patients in The First Affiliated Hospital of Xian Jiaotong University (Xian, China) after providing informed consent. The patients did not receive any therapy including radiotherapy, chemotherapy or radiofrequency ablation before the surgery. All procedures involving human participants were in accordance with the ethical standards of the Research Ethics Committee of The First Affiliated Hospital of Xian Jiaotong University and with the Declaration of Helsinki as revised in 2013. The clinicopathological and demographic information of the patients is presented in Table I.
Table I.
Clinical correlation of miR-875-5p expression in HCC (n=90).
| Expression level | ||||
|---|---|---|---|---|
| Clinical parameters | Cases (n) | miR-875-5phigh (n=45) | miR-875-5plow (n=45) | P-value |
| Age (years) | 0.499 | |||
| <50 | 29 | 16 | 13 | |
| ≥50 | 61 | 29 | 32 | |
| Sex | 0.561 | |||
| Male | 76 | 39 | 37 | |
| Female | 14 | 6 | 8 | |
| Tumor size (cm) | 0.006a | |||
| <5 | 47 | 30 | 17 | |
| ≥5 | 43 | 15 | 28 | |
| Tumor number | 0.267 | |||
| Solitary | 59 | 33 | 26 | |
| Multiple | 31 | 12 | 19 | |
| TNM stage | 0.016a | |||
| I+II | 72 | 41 | 31 | |
| III+IV | 18 | 4 | 14 | |
| Capsular infiltration | 0.255 | |||
| Present | 62 | 34 | 28 | |
| Absent | 28 | 11 | 17 | |
| Venous infiltration | 0.033a | |||
| Present | 38 | 14 | 24 | |
| Absent | 52 | 31 | 21 | |
| AFP (ng/ml) | 0.809 | |||
| <20 | 23 | 12 | 11 | |
| ≥20 | 67 | 33 | 34 | |
| HBsAg | 0.788 | |||
| Positive | 73 | 36 | 37 | |
| Negative | 17 | 9 | 8 | |
P<0.05.
Cell culture and transfection
The human HCC cell lines (Hep3B and HuH-7) and the immortalized human non-cancerous hepatic cell line THLE-3 were obtained from the Chinese Academy of Sciences (Shanghai, China). Human HCC cell lines (MHCC97-L, MHCC97-H and HCCLM3) were kindly provided by Dr Qing-An Jia (Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai, China). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; HyClone; Cytiva) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin (Sigma-Aldrich; Merck KGaA) in a humidified 5% CO2 incubator at 37°C.
Cell transfection was performed using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. miR-875-5p mimics (HmiR-SN0806), miR-875-5p inhibitors (HmiR-AN0806-SN-10), miRNA mimics negative control (CmiR-SN0001-SN), miRNA inhibitor negative control (CmiR-AN0001-SN) and miR-875-5p clones in lentiviral vectors (HmiR0504-MR04) were purchased from iGeneBio, Inc. Small interfering RNA (siRNA) used for eIF3a silencing and negative control (NC) siRNA were purchased from Biomics Biotechnologies Co., Ltd. Plasmids used for overexpressing eIF3a (M0274) and empty vectors (EV) were purchased from GeneCopoeia, Inc.
RNA extraction and reverse transcription-quantitative (RT-q) PCR
RNA was extracted from tissues and cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and Qiagen AllPrep DNA/RNA FFPE kit (cat. no. 80234) according to the manufacturer's instructions. For the detection of mRNA and miRNA expression, cDNA was synthesized using a cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). qPCR was performed with SYBR®-Green Premix PCR Master Mix (Roche Diagnostics GmbH). miR-875-5p primers (MQPS0002239-1-200) and eIF3a primers were purchased from Guangzhou RiboBio Co., Ltd. U6 snRNA and GAPDH were used as normalization controls. The primer sequences for eIF3a were: 5′-ACAGGCAGTGTTTGGACCTTC-3′ (forward) and 5′-CTTACGCGTGTATTGGAGGCA-3′ (reverse). The primer sequences for GAPDH were: 5′-GGTATGACAACGAATTTGGC-3′ (forward) and 5′-GAGCACAGGGTACTTTATTG-3′ (reverse).
Cell proliferation assays
Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.) assay was used to assess cell viability. Hep3B and HCCLM3 cells were seeded in a 96-well plate (3×103 cells/well); 10 µl CCK-8 solution was added to each well at 0, 24, 48 and 72 h and incubated at 37°C for 1 h. A microplate reader (Bio-Rad Laboratories, Inc.) was used to read the absorbance at 450 nm.
An EdU kit (cat. no. C10310-1; Guangzhou RioBio Co., Ltd.) was used to detect cell proliferation. HCC cells were seeded in a 24-well plate (5×104 cells/well). Following 4-h incubation with EdU solution, the nuclei were stained with DAPI. Images were captured under a Zeiss fluorescence photomicroscope (Carl Zeiss AG) in at least five random fields for quantification.
Transwell invasion and migration assays
Serum-free DMEM containing cells pre-starved for 12 h were added into the upper chambers of the Transwell inserts (5×104 cells/well) with or without pre-coating with Matrigel, and DMEM with 10% FBS was added into the lower chambers. Following 24-h incubation, the remaining cells in the upper chamber were removed, and the invaded or migrated cells were fixed with formalin and stained using crystal violet for 20 min. Cells from at least five random fields were counted under a light microscope with 100-fold magnification.
In vivo experiments
Male BALB/c nude mice were housed under pathogen-free conditions in the Centre of Laboratory Animals at The Medical College of Xian Jiaotong University. Animal experiments were performed according to the protocol approved by the Ethics Review Committee of Xian Jiaotong University. Humane endpoints included i) the body condition score was 1/5; ii) the body condition score was 2/5 and the mouse was profoundly lethargic; iii) the tumor affected the mouse's gait or normal posture, ability to eat, urinate, or defecate. No mice met these criteria and were euthanized before the end of the experiment.
Mice (4–6 weeks old) were randomly grouped for animal experiments (n=4 mice per group) for animal experiments. The mice were housed with filtered air, 12-h light/dark cycle, constant temperature (25°C) and had free access to food and water. A subcutaneous xenograft model was established for evaluating the tumor growth of HCC cells. HCCLM3 cells (3×106) transfected with miR-875-5p clones in lentiviral vectors or control vectors were suspended in 100 µl PBS and inoculated subcutaneously into the flank of the mice. Tumor volumes were determined every 3 days as length × width × width/2. The mice were sacrificed by cervical dislocation under 10% ether inhalant anesthesia at 3 weeks after implantation, and the xenograft tumor tissues were dissected for further examination. The pulmonary metastatic model was established to investigate the metastatic ability of HCC cells; transfected HCCLM3 cells (1×106) were injected into the tail vein. Mice were sacrificed at 10 weeks after injection, and the lung tissues were examined microscopically following hematoxylin and eosin (H&E) staining.
Bioinformatics analysis
The microRNA.org website (2010 version; http://www.microrna.org/microrna/home.do) and TargetScan Human 5.1 (http://www.targetscan.org/vert_72/) were used by entering ‘miR-875-5p’ into the search box to predict potential miRNA target genes and the binding sites.
Immunohistochemical (IHC) staining
IHC was performed as previously described (23). Briefly, formalin-fixed paraffin-embedded sections were dewaxed, dehydrated and rehydrated. Antibodies against Ki-67 (cat. no. ab92742; Abcam), which is the proliferation marker, were added to the sections and incubated at 4°C overnight, followed by the addition of the streptavidin peroxidase-conjugated secondary antibody (cat. no. SP-9001; OriGene Technologies, Inc.). The slides were counterstained with hematoxylin and inspected under a microscope.
Luciferase reporter assay
The 3′UTR sequence of eIF3a mRNA and the corresponding sequence with mutations in the predicted miR-875-5p target sites were synthesized and inserted into pGL3 vectors (Promega Corporation) to obtain eIF3a 3′UTR wild-type (wt) and eIF3a 3′UTR mutant (mt), respectively. Subsequently, Hep3B or HCCLM3 cells were seeded into a 24-well plate and transfected with different combinations of miR-875-5p mimics or inhibitors and eIF3a 3′UTR wt or mt plasmids followed by 48-h cultivation. The relative luciferase activities were quantified using the Dual-Luciferase Reporter Assay system (Promega Corporation) and normalized to Renilla luciferase activity.
Western blotting
Proteins were lysed from HCC cells using RIPA buffer (Beyotime Institute of Biotechnology) supplemented with PMSF and protease inhibitors (Beyotime Institute of Biotechnology). The protein concentration was determined by the BCA Protein assay kit (Beyotime Institute of Biotechnology) according to the manufacturer's instructions. Cell lysates with 15 µg protein per lane were separated by SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. Following blocking with 10% non-fat milk, the membranes were probed with primary antibodies overnight at 4°C and the corresponding secondary antibodies for 1 h at room temperature. Finally, the ECL reagent (Beyotime Institute of Biotechnology) was used for signal detection. The antibodies against eIF3a (cat. no. ab128996) were purchased from Abcam, Inc. The antibodies against E-cadherin (cat. no. 3195S), N-cadherin (cat. no. 13116S) and vimentin (cat. no. 5741S) were purchased from Cell Signaling Technology, Inc. The antibody against β-actin (cat. no. sc-47778) was purchased from Santa Cruz Biotechnology, Inc.
Statistical analysis
Data are presented as the mean ± SD. GraphPad Prism software version 8.0 (GraphPad Software, Inc.) was used for statistical analysis. Data were compared using a two-tailed Student's t-test and ANOVA with Dunnett-t test and Newman-Keuls test. The paired t-test was used to compare tumor and adjacent non-tumor samples of the same individuals. The overall survival (OS) between two groups was analyzed using Kaplan-Meier curves and log-rank analysis. The association between miR-875-5p expression and clinicopathological features was analyzed using the Chi-squared test and Fisher's exact test. Pearson's correlation analysis was performed to determine the correlation between miR-875-5p and eIF3a mRNA expression. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-875-5p is downregulated in HCC and associated with the progression and survival of HCC
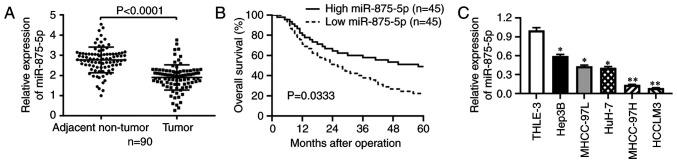
To determine whether miR-875-5p was dysregulated in HCC, RT-qPCR was performed in 90 pairs of tumor and adjacent non-tumor tissues. The results revealed that miR-875-5p expression was significantly downregulated in HCC tissues compared with that in the adjacent non-tumor tissues (P<0.001; Fig. 1A). Subsequently, the patients were divided into two groups according to the median value of miR-875-5p expression in HCC tissues, the high miR-875-5p group (n=45) and the low miR-875-5p group (n=45). The Chi-square test results verified that low expression of miR-875-5p was associated with a larger tumor size (P=0.006), venous infiltration (P=0.033) and advanced TNM stage (P=0.016) (Table I). Kaplan-Meier and log-rank analysis further revealed that patients with HCC with low miR-875-5p expression presented with unfavorable OS (P<0.05, Fig. 1B). Consistent with the results from the HCC tissue samples, RT-qPCR results demonstrated that miR-875-5p expression levels were downregulated in HCC cell lines compared with those in the immortalized hepatic cell line THLE-3 (P<0.05 or P<0.01, respectively; Fig. 1C). Thus, the above results suggested that miR-875-5p was downregulated in HCC tissues and cell lines, and that low miR-875-5p expression was associated with tumor progression and poor OS of patients with HCC.
Figure 1.
miR-875-5p is downregulated in HCC and correlates with HCC patient survival. (A) RT-qPCR was performed to determine miR-875-5p expression in 90 pairs of tumor tissues and matched adjacent non-tumor tissues. (B) HCC patients with lower miR-875-5p expression had poorer overall survival compared to those with higher miR-875-5p expression. (C) RT-qPCR was performed to explore miR-875-5p expression in HCC cell lines and the immortalized hepatic cell line THLE-3. *P<0.05, **P<0.01.
miR-875-5p suppresses HCC cell proliferation
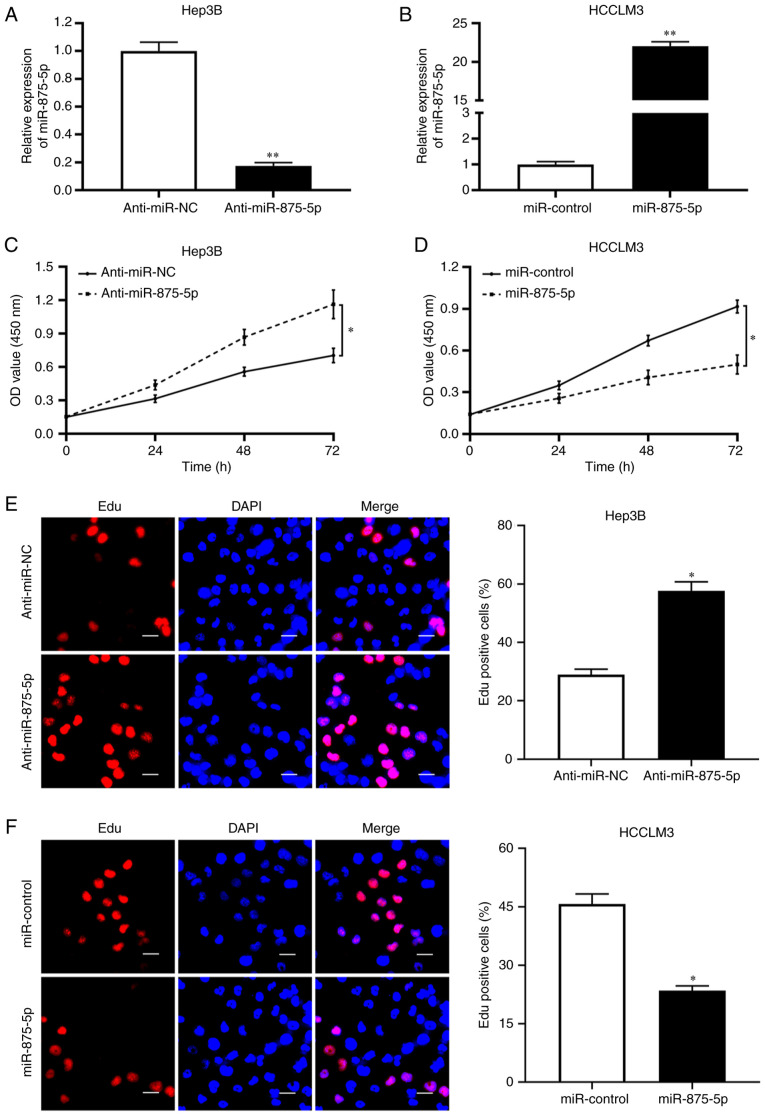
To determine the effects of miR-875-5p on HCC cell proliferation, we transfected miR-875-5p inhibitors in Hep3B cells which exhibited relatively high endogenous miR-875-5p level and overexpressed miR-875-5p in HCCLM3 cells which exhibited relatively low endogenous miR-875-5p to obtain satisfactory transfection efficiency and obvious biological effects. As determined by RT-qPCR, the expression of miR-875-5p was decreased in Hep3B cells (P<0.01; Fig. 2A) transfected with the miR-875-5p inhibitors compared with that in the negative control group and upregulated in HCCLM3 cells (P<0.01; Fig. 2B) transfected with the miR-875-5p mimics compared with that in the control group. CCK-8 assay results revealed that the miR-875-5p inhibitors enhanced the viability of Hep3B cells (P<0.05; Fig. 2C), whereas the viability of HCCLM3 cells transfected with the miR-875-5p mimics was significantly reduced (P<0.05; Fig. 2D). In addition, an EdU assay was performed, and the results demonstrated that the miR-875-5p inhibitors accelerated the proliferation of Hep3B cells (P<0.05; Fig. 2E), whereas the miR-875-5p mimics inhibited the proliferation of HCCLM3 cells (P<0.05; Fig. 2F). Thus, these results suggested that miR-875-5p suppressed HCC cell proliferation.
Figure 2.
miR-875-5p restrains proliferation in HCC cells. (A) miR-875-5p knockdown notably decreased the expression of miR-875-5p in Hep3B cells. (B) miR-875-5p overexpression significantly increased the expression of miR-875-5p in HCCLM3 cells. (C) CCK8 assay revealed that miR-875-5p knockdown increased the viability of Hep3B. (D) miR-875-5p overexpression restrained the viability of HCCLM3 as detected by CCK8 assay. (E) miR-875-5p knockdown enhanced the proliferation of Hep3B as detected by EdU assay. (F) EdU assay showed that miR-875-5p overexpression suppressed the proliferation of HCCLM3. Scale bars, 20 µm. *P<0.05, **P<0.01.
miR-875-5p inhibits HCC cell migration and invasion
To determine whether miR-875-5p served a role in the motility of HCC cells, Transwell assays were performed. The results demonstrated that Hep3B cells transfected with the miR-875-5p inhibitors exhibited increased migratory and invasive abilities (P<0.05; Fig. 3A), whereas the miR-875-5p mimics reduced the number of migrated and invasive HCCLM3 cells (P<0.05; Fig. 3B). As epithelial-mesenchymal transition (EMT) is a classical phenomenon of morphology change in HCC cells and serves a pivotal role on HCC cell migration and invasion, the present study further investigated whether miR-875-5p inhibited HCC cell motility via suppressing the EMT progression. Western blot analysis revealed that the miR-875-5p inhibitors decreased the expression levels of E-cadherin and increased those of vimentin and N-cadherin in Hep3B cells (P<0.05; Fig. 3C). By contrast, the miR-875-5p mimics increased the expression levels of E-cadherin and decreased those of vimentin and N-cadherin in HCCLM3 cells (P<0.05; Fig. 3D). Therefore, these results indicated that miR-875-5p inhibited HCC cell migration and invasion by suppressing the EMT.
Figure 3.
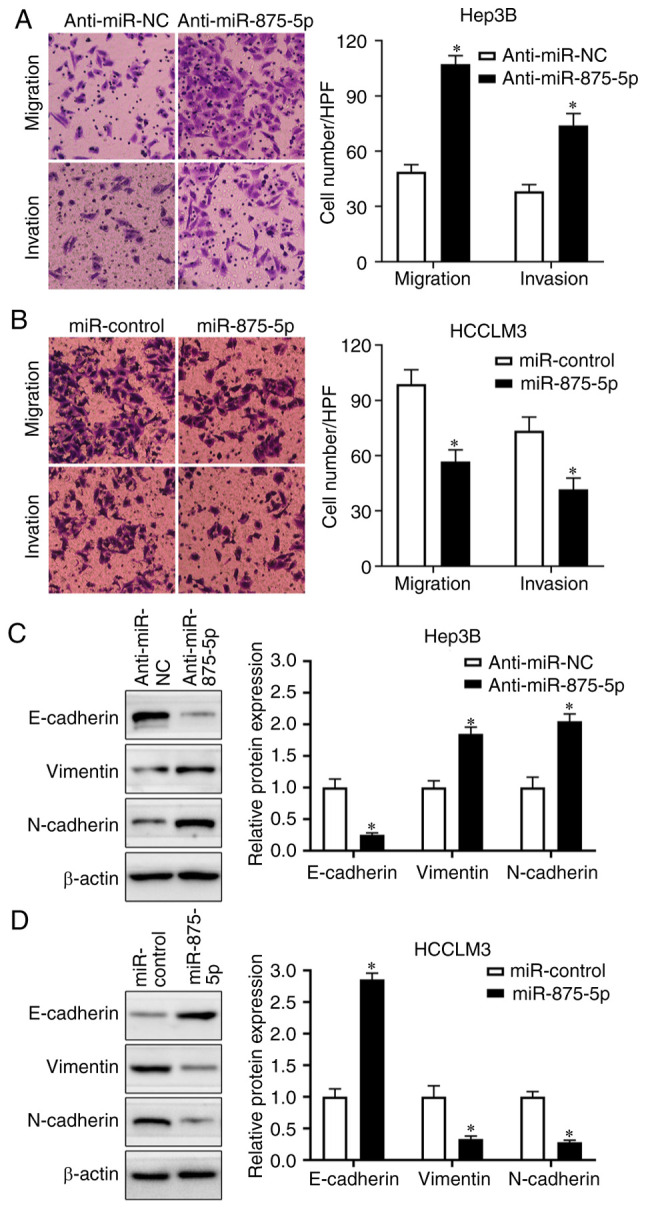
miR-875-5p inhibits migration and invasion of HCC cells. (A) miR-875-5p knockdown enhanced the migration and invasion of Hep3B cells. (B) The migration and invasion capacity of HCC cells was weakened by miR-875-5p overexpression in HCCLM3 cells by transwell assay. (C) The E-cadherin expression was reduced while the levels of vimentin and N-cadherin were increased by miR-875-5p knockdown in Hep3B. (D) miR-875-5p overexpression decreased the expression level of vimentin and N-cadherin accompanied by increased E-cadherin expression in HCCLM3 cells. *P<0.05.
miR-875-5p inhibits HCC tumor growth and metastasis in vivo
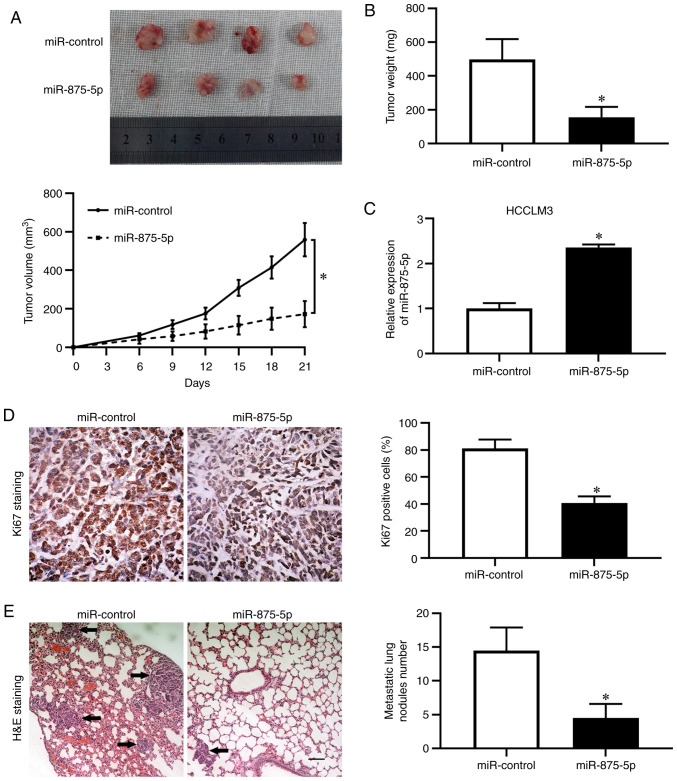
To further confirm the inhibitory effects of miR-875-5p on HCC in vivo, HCCLM3 cells stably overexpressing miR-875-5p were established and injected subcutaneously into nude mice. We found that the miR-875-5p had no detectable effect on the body weight in mice for xenograft model (Fig. S1A). The tumor growth curves revealed that miR-875-5p overexpression induced HCC growth restriction in mice (P<0.05; Fig. 4A). The weight of the tumors formed by miR-875-5p-overexpressing HCCLM3 cells was decreased (P<0.05; Fig. 4B). RT-qPCR results confirmed higher miR-875-5p expression in tumor tissues harvested from the miR-875-5p-overexpressing group compared with those from the control group (P<0.05; Fig. 4C). Immunohistochemistry results demonstrated that overexpression of miR-875-5p decreased the positive rate of the proliferation marker Ki-67 staining (P<0.05; Fig. 4D). Additionally, to determine the metastatic potential in vivo, a lung metastasis model was established by tail vein injection with HCCLM3 cells overexpressing miR-875-5p. The weight loss was lower in the mice injected with miR-875-5p-overexpressing cells compared with that with control, while the difference was not significant (Fig. S1B). RT-qPCR results validated that miR-875-5p expression level in HCCLM3 cells to be injected into the tail vein and in the metastatic nodules was higher in miR-875-5p-overexpressing group compared with that in control (P<0.05; Fig. S1C and D). The results revealed that the miR-875-5p overexpression group exhibited fewer and smaller foci in the lungs of nude mice compared with those in the control group (P<0.05; Fig. 4E). Together, these results suggested that miR-875-5p inhibited HCC growth and metastasis in mice.
Figure 4.
miR-875-5p inhibits HCC growth and metastasis in vivo. (A) HCCLM3 cells stably overexpressing miR-875-5p and control were subcutaneously injected into nude mice. Tumor volume was measured every 3 days from the 6th day after implantation. (B) The nude mice were sacrificed and tumors were harvested and weighed at the 21st day after subcutaneous injection. (C) Xenograft tissues were subjected to RT-qPCR for miR-875-5p expression. (D) Immunostaining of Ki-67 in xenograft tissues arising from miR-875-5p overexpression group and control group. (E) HCCLM3 cells stably overexpressing miR-875-5p and control were administered into mice via tail vein injections. Hematoxylin and eosin (H&E) staining was performed to identify the metastatic nodules from the mouse lung tissues. Black arrows indicate metastatic nodules. Scale bars, 100 µm. *P<0.05.
eIF3a is the downstream target of miR-875-5p in HCC
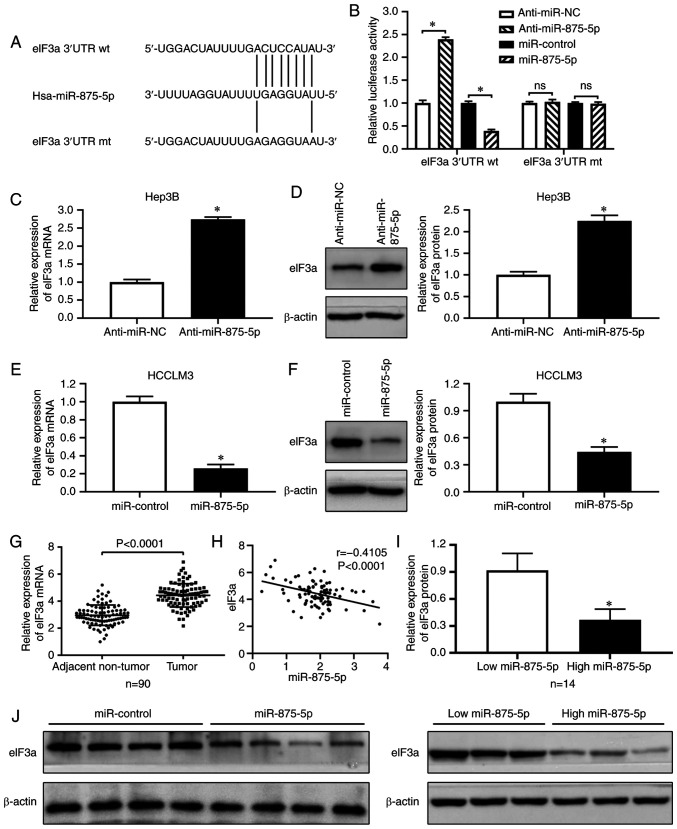
To investigate the exact mechanism underlying the inhibitory function of miR-875-5p in HCC, the candidate downstream targets of miR-875-5p were identified using bioinformatics tools (microRNA.org, TargetScan). Comprehensive analysis of a previous study identified eIF3a as an oncogenic molecule in HCC (22), and bioinformatics analysis predicted miR-875-5p binding sites in the eIF3a mRNA 3′UTR (Fig. 5A); thus, eIF3a was selected as the potential target of miR-875-5p. A luciferase reporter assay was performed, and the results demonstrated that the miR-875-5p inhibitors enhanced, whereas the mimics reduced the luciferase activities of plasmids carrying the wt, but not the mt eIF3a 3′UTR (Fig. 5B). In addition, RT-qPCR and western blotting results demonstrated that the miR-875-5p inhibitors increased the expression of eIF3a at the mRNA and protein level in Hep3B cells (P<0.05; Fig. 5C and D). By contrast, the miR-875-5p mimics decreased eIF3a mRNA and protein expression in HCCLM3 cells (P<0.05; Fig. 5E and F). Consistently, RT-qPCR results revealed that eIF3a mRNA expression was significantly upregulated in HCC tissues compared with that in adjacent non-tumor tissues (P<0.001; Fig. 5G), and eIF3a mRNA expression was significantly negatively correlated with miR-875-5p expression in HCC tissues (r=−0.4105, P<0.0001; Fig. 5H). Western blotting results demonstrated that eIF3a expression was lower in HCC tissues with high miR-875-5p expression compared with those with low miR-875-5p expression (P<0.05; Fig. 5I). Additionally, the protein expression of eIF3a was downregulated in miR-875-5p-overexpressing xenograft tumor tissues compared with that in the control group (P<0.05; Fig. 5J). Collectively, these results confirmed that miR-875-5p downregulated eIF3a expression by directly targeting the 3′UTR of eIF3a mRNA in HCC.
Figure 5.
eIF3a is a downstream target of miR-875-5p in HCC cells. (A) Results from TargetScan (http://www.targetscan.org) and miRanda (microRNA.org) showed the predicted miR-875-5p binding sites in 3′UTR of eIF3a mRNA. (B) Luciferase reporter assay indicated that alteration of miR-875-5p expression inversely regulated luciferase activities of wild-type (wt) but not mutant (mt) eIF3a 3′UTR plasmids. (C and D) RT-qPCR and western blotting were performed to identify the expression of eIF3a mRNA and protein in Hep3B cells transfected with miR-875-5p inhibitors. (E and F) HCCLM3 cells with miR-875-5p knockdown. (G) RT-qPCR was performed to detect eIF3a mRNA expression in 90 pairs of HCC tumor tissues and corresponding adjacent non-tumor tissues. (H) Pearson's correlation analysis disclosed the negatively correlation between eIF3a mRNA and miR-875-5p in HCC tissues. (I) Western blotting was performed to detect eIF3a expression of HCC tissues with low miR-875-5p expression and high miR-875-5p expression. (J) Western blotting was performed to determine eIF3a expression in mice xenograft tumor tissues. *P<0.05.
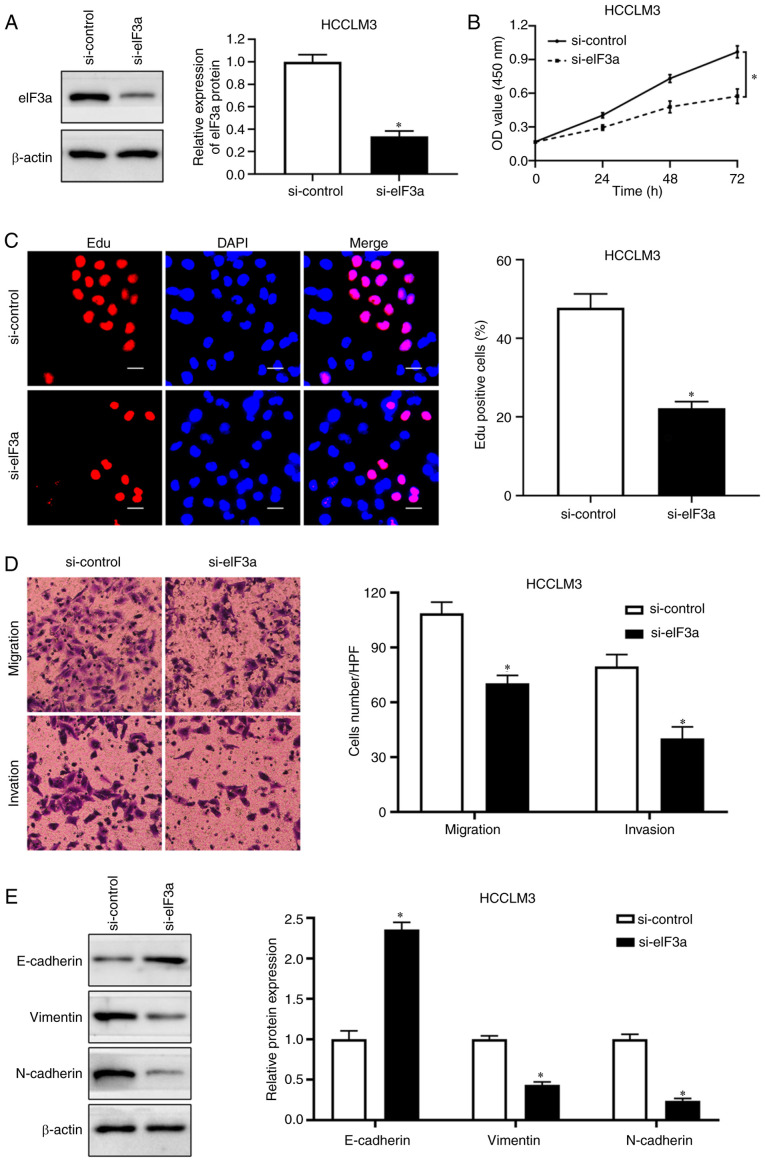
eIF3a-knockdown inhibits HCC cell proliferation, migration and invasion
To determine the role of eIF3a in the proliferation and motility of HCC cells, HCCLM3 cells were used to establish an eIF3a-knockdown cell line. Western blotting was performed to validate the transfection efficiency (P<0.05; Fig. 6A). CCK-8 assay results revealed that eIF3a-knockdown significantly decreased the viability of HCCLM3 cells (P<0.05; Fig. 6B). In addition, eIF3a-knockdown reduced the number of active proliferating HCC cells as determined by the EdU assay (P<0.05; Fig. 6C). To explore the effects of eIF3a on the migratory and invasive abilities of HCC cells, Transwell assays were performed, and the results demonstrated that knockdown of eIF3a decreased the migration and invasion of HCCLM3 cells (P<0.05; Fig. 6D). In addition, eIF3a-knockdown inhibited the EMT progression in HCCLM3 cells (P<0.05; Fig. 6E). Taken together, these results suggested that eIF3a promoted the proliferation and motility of HCC cells.
Figure 6.
eIF3a knockdown inhibits proliferation, migration and invasion in HCC cells. (A) Western blotting was performed to confirm the transfection efficiency. (B) CCK8 assay revealed that knockdown of eIF3a markedly retarded the proliferation of HCCLM3 cells. (C) HCCLM3 cells transfected with si-eIF3a exhibited restrained proliferation by Edu assay. (D) Transwell assay showed that eIF3a knockdown impaired migration and invasion in HCCLM3 cells. (E) The E-cadherin expression was increased while the expression of vimentin and N-cadherin was reduced by eIF3a knockdown. Scale bars, 20 µm. *P<0.05.
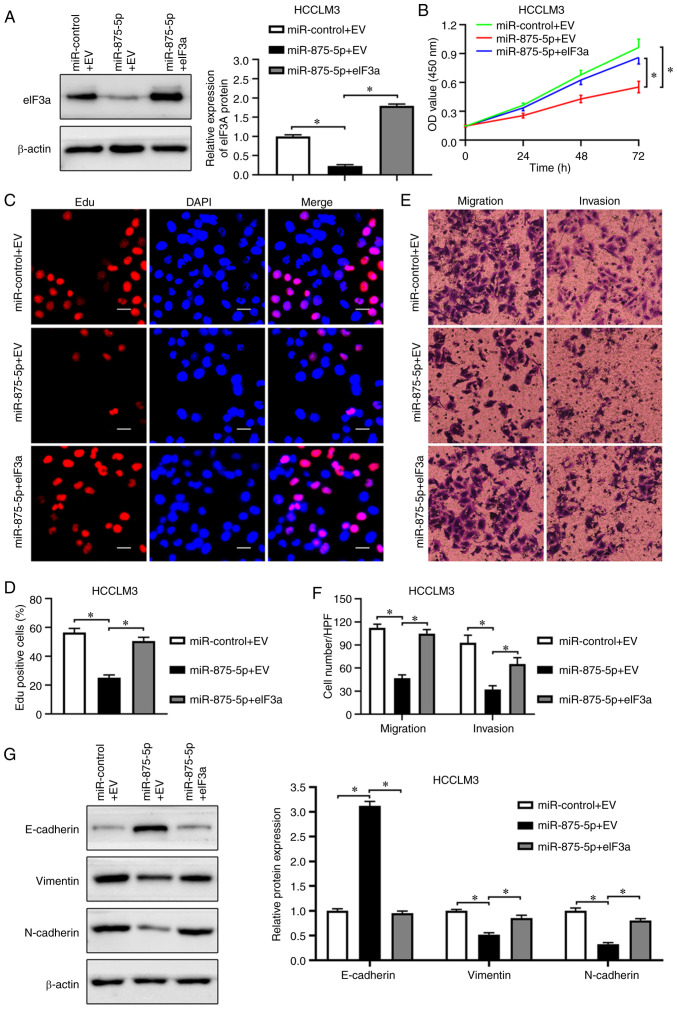
eIF3a mediates the inhibitory effects of miR-875-5p on HCC cell proliferation and mobility
Based on the functions of miR-875-5p and eIF3a, and the correlation between them, the present study further examined whether eIF3a may mediate the inhibitory functions of miR-875-5p in HCC. RT-qPCR results revealed that miR-875-5p expression was significantly upregulated by miR-875-5p mimics but not affected by eIF3a overexpression plasmid in HCCLM3 cells (P<0.05; Fig. S1E). Western blot analysis validated that eIF3a expression was significantly restored by an eIF3a overexpression plasmid in miR-875-5p-overexpressing HCCLM3 cells (P<0.05; Fig. 7A). Consistent with the promoting effects of eIF3a on HCC cell proliferation, eIF3a accelerated the proliferation of HCCLM3 cells inhibited by miR-875-5p, determined by the CCK-8 and EdU assays (P<0.05; Fig. 7B-D). The Transwell assay results demonstrated that eIF3a overexpression reversed the inhibitory effects of miR-875-5p on HCCLM3 cell migration and invasion (P<0.05; Fig. 7E and F). In addition, eIF3a overexpression rescued the miR-875-5p mimic-inhibited EMT progression of HCCLM3 cells (P<0.05; Fig. 7G). In summary, these results supported the role of eIF3a in mediating the tumor suppressor function of miR-875-5p in HCC cells.
Figure 7.
eIF3a restoration reverses the inhibitory effects of miR-875-5p on proliferation and motility of HCC cells. (A) miR-875-5p overexpressing HCCLM3 cells transfected with empty vector (EV) or eIF3a plasmid were subjected to western blotting for eIF3a. (B) CCK8 assay manifested that eIF3a overexpression reversed suppression functions of miR-875-5p on viability of HCCLM3 cells. (C and D) Edu assay showed that eIF3a overexpression rescued the proliferation of HCCLM3 cells inhibited by miR-875-5p. (E and F) Transwell assay revealed that miR-875-5p inhibits the migration and invasion of HCCLM3 cells while eIF3a overexpression abolished the effects. (G) Western blotting revealed that miR-875-5p increased E-cadherin and reduced N-cadherin and vimentin expression in HCCLM3 cells, while the overexpression of eIF3a blocked these effects. Scale bars, 20 µm. *P<0.05.
Discussion
miRNAs are small non-coding RNAs that serve pivotal roles in the majority of types of cancer, affecting numerous cancer-associated processes, such as cell proliferation, cell cycle, apoptosis, differentiation, migration and metabolism (24–25). Previous studies have observed the dysregulation of miR-875-5p in various types of cancer (13–15). For instance, miR-875-5p acts as a tumor suppressor by curbing the epidermal growth factor receptor (EGFR)-ZEB1 axis, repressing the EMT and increasing radiation response in prostate cancer (14). In addition, miR-875-5p promotes the proliferation and motility of non-small cell lung cancer cell lines by targeting SATB homeobox 2 (15). By contrast, miR-875-5p exerts a tumor suppressor role by inhibiting cell proliferation and metastasis and accelerating apoptosis via targeting EGFR in colorectal carcinoma (13). To determine the role of miR-875-5p in HCC, which remains largely elusive, the present study demonstrated that miR-875-5p was downregulated in HCC, and low expression of miR-875-5p was significantly associated with an unfavorable prognosis and clinical features including large tumor size, venous infiltration and an advanced TNM stage. Consistent with the clinical analysis, the results of the loss- and gain-of-function experiments further revealed that miR-875-5p suppressed the proliferation, migration and invasion of HCC cells. Additionally, in vivo experiments demonstrated that miR-875-5p overexpression inhibited tumor growth and metastasis. In a previous study, a hypoxic microenvironment was demonstrated to modulate the expression levels of miR-187-3p, miR-204, miR-1296, miR-671-5p (26–29) and long non-coding RNA AGAP2 antisense RNA 1 (30), which sponges miR-16-5p and promotes cell proliferation and metastasis in HCC. Therefore, whether hypoxia is responsive for the downregulation of miR-875-5p in HCC requires further investigation.
As the crucial component for translation initiation, eIF3a serves a vital role in various physiological and pathological processes, such as the cell cycle and DNA synthesis (31–33). Regarding its role in cancer, eIF3a has been reported to be involved in decreasing the expression of DNA repair proteins, resulting in enhanced chemotherapeutic sensitivity in lung cancer (16). Consistently, eIF3a negatively regulates the resistance to cisplatin via suppressing the cellular synthesis and activity of nucleotide excision repair proteins in nasopharyngeal carcinoma (34). In addition, eIF3a exerts an oncogenic role by accelerating cell proliferation and inhibiting apoptosis in ameloblastoma (21). Accumulating evidence has demonstrated that eIF3a participates in the development of ovarian, urinary bladder and pancreatic cancer, as well as HCC (20,22,35,36). eIF3a is upregulated in HCC and facilitates the translation of HIF-1α, which, in turn, regulates glycolytic metabolism (22). Additionally, a serum anti-eIF3a autoantibody has been identified as a potential diagnostic biomarker for HCC, further supporting the role of eIF3a in HCC (37). However, the mechanism underlying the upregulation of eIF3a and the functions of eIF3a on the proliferation and metastasis in HCC remains unclear. In the present study, eIF3a expression levels were upregulated and negatively correlated with those of miR-875-5p in HCC tissues. Knockdown of eIF3a inhibited the proliferation, migration and invasion of HCC cells. Additionally, the results of the present study demonstrated that eIF3a was a downstream target of miR-875-5p in HCC. Firstly, miR-875-5p negatively modulated the luciferase activity of reporter vectors carrying wild-type, but not mutant 3′UTR of eIF3a. Secondly, altering miR-875-5p expression negatively regulated the mRNA and protein expression of eIF3a in HCC cells. Lastly, overexpression of eIF3a reversed the suppressive effects of miR-875-5p on HCC cell proliferation and mobility. Therefore, the present study confirmed that miR-875-5p served a tumor suppressor role by downregulating eIF3a in HCC. The majority of HCC cases arise from liver fibrosis or cirrhosis (38). Of note, eIF3a has been reported to be involved in the fibrosis of various organs, including the lung, kidney, heart, skin and liver (39–43). The aforementioned studies demonstrated that eIF3a, the expression of which is upregulated by the transforming growth factor β1 (TGF-β1)/smad3 signaling pathway, mediated the TGF-β1-induced fibrosis. Thus, further studies are required to determine whether liver fibrosis participates in the eIF3a-induced tumorigenesis in HCC.
In summary, the downregulation of miR-875-5p serves a crucial role in tumor growth and metastasis in HCC and may be a valuable prognostic marker and potential therapeutic target for HCC.
Supplementary Material
Acknowledgements
We would like to thank Dr Qing-An Jia (Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai, P.R. China) for kindly providing Human HCC cell lines (MHCC-97L, MHCC-97H, HCCLM3).
Funding
This study was supported by grants from the National Natural Science Foundation of China (grant no. 81874069).
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Authors' contributions
TC contributed to writing the manuscript, study conception and design, and collection and analysis of data. LS, BY, and LW collected and interpreted data. YW, YN, RL, HM contributed to data analysis and interpretation and drafting the manuscript. ZL and KT contributed to study conception and revised the manuscript. QL contributed to study conception and design as well as revising and approving the final version of the manuscript. All authors have read and approved the final version of this manuscript.
Ethics approval and consent to participate
All procedures involving human participants were in accordance with the ethical standards of the Research Ethics Committee of The First Affiliated Hospital of Xian Jiaotong University and with the Declaration of Helsinki as revised in 2013. Written informed consent to participate in the study was obtained from patients with HCC prior to sample collection. Animal experiments were performed according to the protocol approved by the Ethics Review Committee of Xian Jiaotong University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 3.Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, Tada T, Chong CCN, Xiang BD, Li LQ, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69:1284–1293. doi: 10.1016/j.jhep.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giordano S, Columbano A. MicroRNAs: New tools for diagnosis, prognosis, and therapy in hepatocellular carcinoma? Hepatology. 2013;57:840–847. doi: 10.1002/hep.26095. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Mo H, Jiang Y, Wang Y, Sun L, Yao B, Chen T, Liu R, Li Q, Liu Q, Yin G. MicroRNA-519c-3p promotes tumor growth and metastasis of hepatocellular carcinoma by targeting BTG3. Biomed Pharmacother. 2019;118:109267. doi: 10.1016/j.biopha.2019.109267. [DOI] [PubMed] [Google Scholar]
- 9.Xu Q, Zhu Q, Zhou Z, Wang Y, Liu X, Yin G, Tong X, Tu K. MicroRNA-876-5p inhibits epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma by targeting BCL6 corepressor like 1. Biomed Pharmacother. 2018;103:645–652. doi: 10.1016/j.biopha.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Yang Z, Wang L, Sun L, Liu Z, Li Q, Yao B, Chen T, Wang C, Yang W, et al. MiR-532-3p promotes hepatocellular carcinoma progression by targeting PTPRT. Biomed Pharmacother. 2019;109:991–999. doi: 10.1016/j.biopha.2018.10.145. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Wang Y, Dou C, Sun L, Li Q, Wang L, Xu Q, Yang W, Liu Q, Tu K. MicroRNA-1468 promotes tumor progression by activating PPAR-ү-mediated AKT signaling in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37:49. doi: 10.1186/s13046-018-0717-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Yao B, Li Y, Wang L, Chen T, Niu Y, Liu Q, Liu Z. MicroRNA-3194-3p inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by decreasing Wnt/β-catenin signaling through targeting BCL9. Artif Cells Nanomed Biotechnol. 2019;47:3885–3895. doi: 10.1080/21691401.2019.1670190. [DOI] [PubMed] [Google Scholar]
- 13.Zhang T, Cai X, Li Q, Xue P, Chen Z, Dong X, Xue Y. Hsa-miR-875-5p exerts tumor suppressor function through down-regulation of EGFR in colorectal carcinoma (CRC) Oncotarget. 2016;7:42225–42240. doi: 10.18632/oncotarget.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Bezawy R, Cominetti D, Fenderico N, Zuco V, Beretta GL, Dugo M, Arrighetti N, Stucchi C, Rancati T, Valdagni R, et al. MiR-875-5p counteracts epithelial-to-mesenchymal transition and enhances radiation response in prostate cancer through repression of the EGFR-ZEB1 axis. Cancer Lett. 2017;395:53–62. doi: 10.1016/j.canlet.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Lu Y, Ding H, Gu T, Gong C, Sun J, Zhang Z, Zhao Y, Ma C. The miR-875-5p inhibits SATB2 to promote the invasion of lung cancer cells. Gene. 2018;644:13–19. doi: 10.1016/j.gene.2017.11.066. [DOI] [PubMed] [Google Scholar]
- 16.Yin JY, Shen J, Dong ZZ, Huang Q, Zhong MZ, Feng DY, Zhou HH, Zhang JT, Liu ZQ. Effect of eIF3a on response of lung cancer patients to platinum-based chemotherapy by regulating DNA repair. Clin Cancer Res. 2011;17:4600–4609. doi: 10.1158/1078-0432.CCR-10-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong Z, Zhang JT. Initiation factor eIF3 and regulation of mRNA translation, cell growth, and cancer. Crit Rev Oncol Hematol. 2006;59:169–180. doi: 10.1016/j.critrevonc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Yin JY, Zhang JT, Zhang W, Zhou HH, Liu ZQ. eIF3a: A new anticancer drug target in the eIF family. Cancer Lett. 2018;412:81–87. doi: 10.1016/j.canlet.2017.09.055. [DOI] [PubMed] [Google Scholar]
- 19.Fang C, Chen YX, Wu NY, Yin JY, Li XP, Huang HS, Zhang W, Zhou HH, Liu ZQ. MiR-488 inhibits proliferation and cisplatin sensibility in non-small-cell lung cancer (NSCLC) cells by activating the eIF3a-mediated NER signaling pathway. Sci Rep. 2017;7:40384. doi: 10.1038/srep40384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang SQ, Liu Y, Yao MY, Jin J. Eukaryotic translation initiation factor 3a (eIF3a) promotes cell proliferation and motility in pancreatic cancer. J Korean Med Sci. 2016;31:1586–1594. doi: 10.3346/jkms.2016.31.10.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Z, Liu J, Wang J, Huang B, Zhong M. Upregulation of eukaryotic translation initiation factor 3 subunit a promotes cell survival in ameloblastoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:146–153. doi: 10.1016/j.oooo.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Miao B, Wei C, Qiao Z, Han W, Chai X, Lu J, Gao C, Dong R, Gao D, Huang C, et al. eIF3a mediates HIF1α-dependent glycolytic metabolism in hepatocellular carcinoma cells through translational regulation. Am J Cancer Res. 2019;9:1079–1090. [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Dou C, Jia Y, Li Q, Zheng X, Yao Y, Liu Q, Song T. RIG-I suppresses the migration and invasion of hepatocellular carcinoma cells by regulating MMP9. Int J Oncol. 2015;46:1710–1720. doi: 10.3892/ijo.2015.2853. [DOI] [PubMed] [Google Scholar]
- 24.Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dou C, Zhou Z, Xu Q, Liu Z, Zeng Y, Wang Y, Li Q, Wang L, Yang W, Liu Q, Tu K. Hypoxia-induced TUFT1 promotes the growth and metastasis of hepatocellular carcinoma by activating the Ca2+/PI3K/AKT pathway. Oncogene. 2019;38:1239–1255. doi: 10.1038/s41388-018-0505-8. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Wang Y, Dou C, Xu M, Sun L, Wang L, Yao B, Li Q, Yang W, Tu K, Liu Q. Hypoxia-induced up-regulation of VASP promotes invasiveness and metastasis of hepatocellular carcinoma. Theranostics. 2018;8:4649–4663. doi: 10.7150/thno.26789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Q, Liu X, Liu Z, Zhou Z, Wang Y, Tu J, Li L, Bao H, Yang L, Tu K. MicroRNA-1296 inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting SRPK1-mediated PI3K/AKT pathway. Mol Cancer. 2017;16:103. doi: 10.1186/s12943-017-0675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dou C, Liu Z, Xu M, Jia Y, Wang Y, Li Q, Yang W, Zheng X, Tu K, Liu Q. MiR-187-3p inhibits the metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting S100A4. Cancer Lett. 2016;381:380–390. doi: 10.1016/j.canlet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Wang Y, Wang L, Yao B, Sun L, Liu R, Chen T, Niu Y, Tu K, Liu Q. Long non-coding RNA AGAP2-AS1, functioning as a competitive endogenous RNA, upregulates ANXA11 expression by sponging miR-16-5p and promotes proliferation and metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:194. doi: 10.1186/s13046-019-1188-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Dong Z, Zhang JT. EIF3 p170, a mediator of mimosine effect on protein synthesis and cell cycle progression. Mol Biol Cell. 2003;14:3942–3951. doi: 10.1091/mbc.e02-12-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu TR, Lu RF, Romano D, Pitt A, Houslay MD, Milligan G, Kolch W. Eukaryotic translation initiation factor 3, subunit a, regulates the extracellular signal-regulated kinase pathway. Mol Cell Biol. 2012;32:88–95. doi: 10.1128/MCB.05770-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong Z, Liu LH, Han B, Pincheira R, Zhang JT. Role of eIF3 p170 in controlling synthesis of ribonucleotide reductase M2 and cell growth. Oncogene. 2004;23:3790–3801. doi: 10.1038/sj.onc.1207465. [DOI] [PubMed] [Google Scholar]
- 34.Liu RY, Dong Z, Liu J, Yin JY, Zhou L, Wu X, Yang Y, Mo W, Huang W, Khoo SK, et al. Role of eIF3a in regulating cisplatin sensitivity and in translational control of nucleotide excision repair of nasopharyngeal carcinoma. Oncogene. 2011;30:4814–4823. doi: 10.1038/onc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Yu JJ, Tian Y, Li ZZ, Zhang CY, Zhang SF, Cao LQ, Zhang Y, Qian CY, Zhang W, et al. eIF3a improve cisplatin sensitivity in ovarian cancer by regulating XPC and p27Kip1 translation. Oncotarget. 2015;6:25441–25451. doi: 10.18632/oncotarget.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spilka R, Ernst C, Bergler H, Rainer J, Flechsig S, Vogetseder A, Lederer E, Benesch M, Brunner A, Geley S, et al. eIF3a is over-expressed in urinary bladder cancer and influences its phenotype independent of translation initiation. Cell Oncol (Dordr) 2014;37:253–267. doi: 10.1007/s13402-014-0181-9. [DOI] [PubMed] [Google Scholar]
- 37.Heo CK, Hwang HM, Lee HJ, Kwak SS, Yoo JS, Yu DY, Lim KJ, Lee S, Cho EW. Serum anti-EIF3A autoantibody as a potential diagnostic marker for hepatocellular carcinoma. Sci Rep. 2019;9:11059. doi: 10.1038/s41598-019-47365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 39.He P, Yu ZJ, Sun CY, Jiao SJ, Jiang HQ. Knockdown of eIF3a attenuates the pro-fibrogenic response of hepatic stellate cells induced by TGF-β1. Cell Mol Biol (Noisy-le-grand) 2016;62:107–111. [PubMed] [Google Scholar]
- 40.Zhang YF, Wang Q, Luo J, Yang S, Wang JL, Li HY. Knockdown of elF3a inhibits collagen synthesis in renal fibroblasts via Inhibition of transforming growth factor-β1/Smad signaling pathway. Int J Clin Exp Pathol. 2015;8:8983–8989. [PMC free article] [PubMed] [Google Scholar]
- 41.Li T, Zhao J. Knockdown of elF3a inhibits TGF-β1-induced extracellular matrix protein expression in keloid fibroblasts. Mol Med Rep. 2018;17:4057–4061. doi: 10.3892/mmr.2017.8365. [DOI] [PubMed] [Google Scholar]
- 42.Li XW, Wu YH, Li XH, Li D, Du J, Hu CP, Li YJ. Role of eukaryotic translation initiation factor 3a in bleomycin-induced pulmonary fibrosis. Eur J Pharmacol. 2015;749:89–97. doi: 10.1016/j.ejphar.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Li WQ, Li XH, Wu YH, Du J, Wang AP, Li D, Li YJ. Role of eukaryotic translation initiation factors 3a in hypoxia-induced right ventricular remodeling of rats. Life Sci. 2016;144:61–68. doi: 10.1016/j.lfs.2015.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.