Highlights
-
•
Brain-Computer Interfaces (BCI)-based therapy relies on timely sensory feedback.
-
•
Somatosensory loss is usually not reported for these therapies.
-
•
Yet, it influences motor imagery, neuroplasticity and motor rehabilitation.
-
•
And stroke-induced somatosensory impairments are frequent and diverse.
-
•
Thus, BCI-based motor therapy efficiency likely depends on somatosensory abilities.
Keywords: Stroke rehabilitation, Neurofeedback, Brain-computer interfaces, Motor recovery, Somatosensory impairments
Abstract
The neuronal loss resulting from stroke forces 80% of the patients to undergo motor rehabilitation, for which Brain-Computer Interfaces (BCIs) and NeuroFeedback (NF) can be used. During the rehabilitation, when patients attempt or imagine performing a movement, BCIs/NF provide them with a synchronized sensory (e.g., tactile) feedback based on their sensorimotor-related brain activity that aims at fostering brain plasticity and motor recovery. The co-activation of ascending (i.e., somatosensory) and descending (i.e., motor) networks indeed enables significant functional motor improvement, together with significant sensorimotor-related neurophysiological changes. Somatosensory abilities are essential for patients to perceive the feedback provided by the BCI system. Thus, somatosensory impairments may significantly alter the efficiency of BCI-based motor rehabilitation. In order to precisely understand and assess the impact of somatosensory impairments, we first review the literature on post-stroke BCI-based motor rehabilitation (14 randomized clinical trials). We show that despite the central role that somatosensory abilities play on BCI-based motor rehabilitation post-stroke, the latter are rarely reported and used as inclusion/exclusion criteria in the literature on the matter. We then argue that somatosensory abilities have repeatedly been shown to influence the motor rehabilitation outcome, in general. This stresses the importance of also considering them and reporting them in the literature in BCI-based rehabilitation after stroke, especially since half of post-stroke patients suffer from somatosensory impairments. We argue that somatosensory abilities should systematically be assessed, controlled and reported if we want to precisely assess the influence they have on BCI efficiency. Not doing so could result in the misinterpretation of reported results, while doing so could improve (1) our understanding of the mechanisms underlying motor recovery (2) our ability to adapt the therapy to the patients’ impairments and (3) our comprehension of the between-subject and between-study variability of therapeutic outcomes mentioned in the literature.
1. Introduction
Upper-limb paresis is a frequent consequence of stroke (Rathore et al., 2002). Despite spontaneous improvement of motor function, this impairment lingers at the chronic phase (3 months post-stroke onset), resulting in disabilities for around 40% of patients (Duncan et al., 2000).
Neuroplasticity, i.e., the ability of the brain to structurally adapt at the cellular, molecular and system levels in order to foster functional abilities, encompasses several mechanisms (Murphy and Corbett, 2009). It leads to a very plastic functional cortical representation which can favor the improvement of functional outcomes. Underlying mechanisms include the functional use of pre-existing synaptic networks as well as structural changes, with the creation of new networks (Murphy and Corbett, 2009). Hence, a crucial question for rehabilitation is how these mechanisms could be enhanced.
Post-stroke rehabilitation training procedures aim to improve recovery of functional abilities or to establish adaptive strategies in order to compensate for impaired body functions (Murphy and Corbett, 2009). Among the different rehabilitation procedures of the upper-limb, the ones providing patients with sensory feedback (e.g., visual feedback based on mirror therapy) or somatosensory stimulation (e.g., transcutaneous electrical stimulation) appear to be promising. On the one hand, mirror visual feedback1 induces changes from molecular to anatomical and physiological levels associated with functional recovery. Indeed, it is known to increase neurons’ excitability (Thieme et al., 2018), cortical reorganization in the primary motor cortex (M1) and to induce functional changes in somatosensory, premotor or higher-order visual areas (Fritzsch et al., 2014). On the other hand, somatosensory stimulation can enhance excitability of the motor cortex and motor function of post-stroke patients (Edwards et al., 2019, Conforto et al., 2018).
These therapies provide sensory feedback through afferent networks regardless of the voluntary activation of efferent sensorimotor networks. However, a co-occurrence of these synergistic networks seems to improve the outcome of the therapies (Biasiucci et al., 2018, Frolov et al., 2017, Mihara et al., 2013, Ramos-Murguialday et al., 2013).
Such co-occurrence is possible using Brain-Computer Interfaces (BCIs) (Clerc et al., 2016). These technologies record (e.g., using electroencephalography), process (using machine learning techniques) and translate patterns of brain activity into commands for different types of digital technologies. For example, BCIs enable paralyzed patients to drive a wheelchair by imagining right or left hand movements (Carlson and Millan, 2013). BCIs share multiple characteristics with neurofeedback (NF), which protocol aims to train people to self-regulate specific functional biomarkers, often associated with mental disorders (Batail et al., 2019, Sitaram et al., 2017). Actually, when used to provide feedback on sensorimotor activity for motor-rehabilitation, some authors even argue that BCIs and NF are two different names for the same concept (Perronnet et al., 2016).
BCIs are used to provide a time-matched sensory feedback depending on the sensorimotor cortex activity for post-stroke motor rehabilitation (Grosse-Wentrup et al., 2011). Motor imagery-based BCI therapies seem to be more efficient in improving motor functions than motor imagery alone (Pichiorri et al., 2015) or proprioceptive stimulation alone (Biasiucci et al., 2018). BCIs enable the online detection of the neuronal activity associated either with a motor imagery or attempted movement task (i.e., top-down processes) and then reward the patient by providing a sensory feedback (i.e., bottom-up processes) (Grosse-Wentrup et al., 2011). BCI-based motor rehabilitation post-stroke can thus be seen as a type of clinical neurofeedback (Sitaram et al., 2017). BCI-based training promotes the co-activation of sensorimotor neural networks associated with movements and induces Hebbian plasticity, which underlies functional improvement (Grosse-Wentrup et al., 2011).
The efficiency of therapies in general and of BCI-based therapies in particular greatly depends on patients’ ability to sense the feedback provided during therapy (Kessner et al., 2016). Thereby, it depends on the integrity of bottom-up afferent processes, i.e., somatosensory-related network. Somatosensory sensations encompass two types of information: exteroception, which represents the information arising from the skin, and proprioception, which encompasses information arising from the muscles and joint receptors (Kessner et al., 2016). Both may be impaired after a stroke (Carey, 2017, Kessner et al., 2016). More than half of the patients experience somatosensory loss, which crucially interferes with post-stroke motor recovery (Carey, 2017, Kessner et al., 2016, Pumpa et al., 2015). Indeed, somatosensory loss is known to have a negative effect on motor rehabilitation and daily use of the paretic arm (Kessner et al., 2016). Also, the prevalence of extremity paresis is significantly higher for patients with abnormal somatosensations (Andersen et al., 1995).
Given the essential role of somatosensory afferences in motor rehabilitation in general, it would seem logical that somatosensory impairments have an impact on BCI-based motor rehabilitation post-stroke. Thus, it raises the following questions: (1) Are somatosensory impairments assessed, controlled and reported in clinical studies carried out with BCI-based motor therapies? (2) What impact do these impairments have on motor rehabilitation? (3) How are somatosensory abilities impaired post-stroke? (4) Do patients recover from these impairments and how? (5) How to assess somatosensory impairments for post-stroke patients? (6) What could be the possible repercussion of assessing, controlling and reporting somatosensory-related measures on BCI-based therapies’ efficiency in future studies? This paper offers a theoretical analysis, based on the literature, to answer these questions. Because BCIs have proven promising for upper limb rehabilitation (Cervera et al., 2018, Bai et al., 2020), we chose to focus on strokes affecting motor abilities of the upper limb, e.g., hemiplegia or hemiparesis.
This paper is organized as follows. In Section 2 -BCI therapies for motor rehabilitation poststroke, we start by determining the role that somatosensory abilities play in BCI-based motor therapy after stroke. A literature-based study of the randomized control trials on the matter is presented afterward to weigh the importance that has been given to somatosensory abilities in these previous studies. Then, in Section 3 -The interconnection of somatosensory and motor abilities during recovery-, to further support our hypothesis that somatosensory abilities have an incidence on BCI-based motor rehabilitation post-stroke outcome, we introduce the results of previous research in other fields, indicating that somatosensory impairments influence neuroplasticity and motor recovery. This literature further support our hypothesis that somatosensory abilities have an incidence on BCI-based motor rehabilitation post-stroke outcome. Finally, in Section 4 -Somatosensory impairments, recovery and assessment post-stroke-, to evaluate to which extent somatosensory impairments could influence BCI-based motor therapies, we focus on the prevalence and specificity of these impairments post-stroke. In this same Section, we also provide information regarding the tools to assess these somatosensory impairments to promote their assessment by researchers and clinicians in future experiments. Throughout this article we discuss the main benefits that could arise from assessing, controlling and reporting patients’ somatosensory impairments and present some leads for future BCI research.
2. BCI therapies for motor rehabilitation post-stroke
Evidence of BCIs’ effectiveness for improving plasticity and motor rehabilitation post-stroke has only recently started to arise from the different research that have been led on the topic (Cervera et al., 2018, Bai et al., 2020). The literature indicates that using a BCI to provide visual feedback (e.g., a virtual representation of the patient’s hands movements) when motor imagery is detected, enables a significantly higher improvement of motor functions than motor imagery alone (Pichiorri et al., 2015). When providing somatosensory feedback (e.g., using an exoskeleton (Frolov et al., 2017, Ramos-Murguialday et al., 2013, Ang et al., 2009) or functional electrical stimulation (Biasiucci et al., 2018)) BCIs have proven more effective than proprioceptive stimulation alone (Biasiucci et al., 2018, Frolov et al., 2017, Ramos-Murguialday et al., 2013, Ang et al., 2009). Compared to traditional therapies, such as motor imagery or muscle and proprioceptive stimulation alone, BCIs allow the co-activation of both top-down processes (i.e., motor imagination or attempt) and bottom-up processes (i.e., coherent somatosensory afferences from visual or somatosensory stimulation of the affected limb). BCI efficiency is assumed to be the result of the timely somatosensory feedback in regards to motor imagination or attempt (Grosse-Wentrup et al., 2011).
Somatosensory impairments might impede the activation of somatosensory-related networks and thereby negatively affect the outcome of the therapy. Furthermore, somatosensory loss might interfere with motor imagery, which is the basis of various BCI studies. Indeed, severe somatosensory impairment affects the temporal aspects of motor imagery, i.e., the ability to estimate the time needed to perform a motor imagery task, even though spatial aspects such as the ability to visualize a 3D object do not seem to be altered (Liepert et al., 2016). Thus, somatosensory impairments most probably interfere with the use of BCI tools and the efficiency of BCI rehabilitation post-stroke. Hence, describing either inclusion/exclusion criteria that refer to somatosensory impairments or assessing these impairments a priori and taking them into account when analysing the results seems important.
Therefore, we investigated how somatosensory abilities were taken into account and reported in the literature on BCI-based motor rehabilitation post-stroke. We assessed the papers that were cited in reviews focusing on BCI-based motor rehabilitation of the upper-limb (Remsik et al., 2016, Monge-Pereira et al., 2017, Cervera et al., 2018, Carvalho et al., 2019, Bai et al., 2020). From the different papers cited we selected the 14 papers focusing on Randomized Clinical Trials (RCTs) of BCIs based on sensorimotor rhythms for post-stroke motor rehabilitation of the upper-limbs with different clinical trial registration number (Ang et al., 2009, Ang et al., 2010, Ang et al., 2014, Ang et al., 2015, Biasiucci et al., 2018, Frolov et al., 2017, Li et al., 2014, Mihara et al., 2013, Pichiorri et al., 2015, Ramos-Murguialday et al., 2013, Rayegani et al., 2014, Várkuti et al., 2013, Wada et al., 2019, Young et al., 2016).
The results of our review are summarized in two tables. Table 1 provides information regarding the procedure, the results and the interpretation of these studies. This table also provides insights on which underlying mechanisms the authors rely on to explain their results. Three articles explicitly state that the therapeutic outcome results from the co-activation of efferent and afferent sensorimotor networks (Biasiucci et al., 2018, Ramos-Murguialday et al., 2013, Várkuti et al., 2013). For instance, Biasiucci et al. state that “BCI[…] therapy can drive significant functional recovery and purposeful plasticity thanks to contingent activation of body natural efferent and afferent pathways” through “somatosensory input, in the form of peripheral nerve stimulation” (Biasiucci et al., 2018). Others authors argue that an increase in sensorimotor cortex (SMC) activation, premotor cortex activation, or sensorimotor rhythm contributes to the functional improvements observed (Biasiucci et al., 2018, Li et al., 2014, Mihara et al., 2013, Pichiorri et al., 2015, Ramos-Murguialday et al., 2013, Rayegani et al., 2014, Várkuti et al., 2013). For instance, Li et al. state that “BCI training may enhance the activation of the affected SMC to prime the motor functional reorganization” (Li et al., 2014). Based on these statements, sensorimotor-related inclusion/exclusion criteria might be expected to be used as sensorimotor-related neurophysiological activation, including somatosensory ones, mediate the therapeutic outcome.
Table 1.
Characteristics of the studies selected for this review. Main elements of the studies (columns 1 to 8), then mechanisms quoted by the authors to explain their results (column 9) and finally our analysis of the potential factors (intrinsic to the patients, e.g., somatosensory loss, or extrinsic to the patients, e.g., design issues) that could arise from the inclusion/exclusion criteria presented in Table 2 (column 10) are introduced. During the studies patients were either asked to perform motor-imagery (MI) or motor-attempt (MA) tasks. If they were reported in the article for both the experimental (exp) and control (contr) groups the number of patients included, chronicity and motor impairment of are reported in such order and separated by a slash. If not the average is provided. Chronicity is defined by the time between the stroke and the inclusion in the study such as: Acute= <1 month, Subacute= <3 months and Chronic= >3 months. Mean and standard deviation of time from stroke for each group distinctly or globally are provided either in days or months. When provided in the article minimal and maximal values are also reported, i.e., (min–max). Mean and standard deviation of motor impairment at inclusion is provided using the Fugl-Meyer Assessment of Upper Extremity (FMA-UE), Jebsen Hand Function Test (JHFT), Action Research Arm Test (ARAT) and Brunnstrom recovery stage. Indication regarding the motor capacity of the subjects are provided depending on the FMA-UE scores, i.e., no: 0–22, poor: 23–31, limited: 32–47, notable: 48–52, full:53–66, or on the ARAT scores, i.e., no: 0–10, poor: 11–21, limited: 22–42, notable: 43–54, full:55–57. Following is a list of the abbreviations used in the table and their signification: Classification Accuracy (CA), Blood Oxygen Level Dependent effect (BOLD), Diffusion Tensor Imaging (DTI), ElectroEncephaloGraphy (EEG), Electromyogram (EMG), European Stroke Scale (ESS), Event Related Desynchronization (ERD), functional Magnetic Resonance Imaging (fMRI), Goal Attainment Scale (GAS), Hand Grip Strength (HGS), Lateralization Index (LI), Medical Research Council (MRC), Modified Ashworth Scale (MAS), Motor Activity Log (MAL), Motor Evoked Potential (MEP), NASA Task Load Index (NASA-TLX), National Institute of Health Stroke Scale (NIHSS), Near InfraRed Spectroscopy (NIRS), Nine Hole Peg test (9-HPT), Power Spectral Density (PSD), Resting State Connectivity (RSC), revised Brain Symmetry Index (rBSI), SensoriMotor Cortex (SMC), Stroke Impact Scale (SIS), Transcranial Magnetic Stimulation (TMS).
| Study | Aim | Design of the study, Patients number (exp/contr), Chronicity (time to stroke) | Motor impairment at inclusion | BCI intervention | Control group | Outcome measures | Results | Mechanisms discussed supposedly underlying the results | Potential bias due to inclusion/exclusion criteria (detailed inTable 2) for mechanisms supposedly underlying the results |
|---|---|---|---|---|---|---|---|---|---|
| (Ang et al., 2009) | Compares the effect of MI-BCI with robotic feedback to standard robotic rehabilitation on functional improvement | Blindness not described, 18 (8/10), Subacute and chronic (Days, 385,5 ± 293,5 (57–1053)) | No capacity to full capacity (FMA-UE, 29,7 ± 17,7 (4–61)) | MI-BCI (EEG) to drive robotic orthosis to move the shoulder and elbow of the impaired arm with gamified visual feedback | Standard robotic rehabilitation | FMA-UE | Significant functional improvement post-rehabilitation and at the 2-months follow-up when groups are combined. Significant greater improvement in MI-BCI group for the 2 months follow-up after removal of the non-responders in both groups and correction for age and gender. | No hypothesis regarding underlying neurophysiological mechanisms. | Non-responders might have been due to abnormal abilities (visual, somatosensory, etc.) but not described. No prior assessment of somatosensory-related abilities for inclusion/exclusion criteria. Possible randomization bias. |
| (Ang et al., 2010) | Compares the effect of MI-BCI with robotic feedback to standard robotic rehabilitation on functional improvement | Blindness not described, 25 (11/14), Subacute and chronic (Days, 383 ± 291 (71–831)/ 250 ± 184 (37–668)) | No capacity to limited capacity (FMA-UE, 26,3 ± 10,3 (14–47)/ 26,6 ± 18,9 (4–57)) | MI-BCI (EEG) to drive robotic orthosis to move the shoulder and elbow of the impaired arm with gamified visual feedback | Standard robotic rehabilitation | FMA-UE | Significant functional improvement in both groups post-rehabilitation and at the 2-months follow-up. Slightly less functional improvement in the MI-BCI group but not significant. | “Ipsilesional motor cortex activation from motor imagery is effective in restoring upper extremities motor function in stroke.” | No prior assessment of somatosensory-related abilities for inclusion/exclusion criteria. Possible randomization bias. |
| (Ang et al., 2014) | Compares the effect of MI-BCI with robotic feedback to standard robotic rehabilitation and standard motor rehabilitation on functional improvement | Blinded assessment, 21 (6/8/7), Chronic (Days, 285,7 ± 64/ 398,2 ± 150,9/ 455,4 ± 109,6 (191–651)) | No capacity to notable capacity (FMA-UE, 33 ± 16,2/ 25,5 ± 11,5/ 23,4 ± 14,5 (10–50)) | MI-BCI (EEG) to drive robotic orthosis for fingers extension and wrist rotation with visual feedback | Standard robotic rehabilitation/ Standard Arm therapy | FMA-UE | Significant functional improvements in all groups 6 weeks post-rehabilitation still significant at 12 and 24 weeks follow-up for the MI-BCI and standard robotic groups. Significantly greater functional improvement for the MI-BCI group compared to the standard therapy group at 3, 12 and 14 weeks follow-ups. | “[…] performance of MI in the [experimental] group […] facilitated neuroplasticity” | No prior assessment of somatosensory-related abilities for inclusion/exclusion criteria except pain and spatial neglect. Possible randomization bias. |
| (Ang et al., 2015) | Compares the effect of MI-BCI with robotic feedback to standard robotic rehabilitation on functional and physiological improvement | Blinded assessment, 25 (11/14), Chronic (Days, 383 ± 290,8/ 234,7 ± 183,8) | No capacity to limited capacity (FMA-UE, 26,3 ± 10,3/ 26,5 ± 18,2 (4–40)) | MI-BCI (EEG) to drive robotic orthosis to move the shoulder and elbow of the impaired arm with gamified visual feedback | Standard robotic rehabilitation | FMA-UE, EEG (rBSI) | Significant functional improvement for both groups post-rehabilitation. Slightly less functional improvement in the BCI group close to significant post-training that could be caused by reduced arm exercise repetitions in BCI group. Negative correlation of rBSI over the sessions and functional improvement for the experimental group. Higher asymmetry in spectral power between the 2 cerebral hemispheres associated with less motor recovery in the BCI group. | “[…] possible role for BCI in long-term cortical plasticity.” | Non-responders might have been due to abnormal somatosensory abilities but not described. No prior assessment of somatosensory-related abilities for inclusion/exclusion criteria except pain and spatial neglect. Possible randomization bias. |
| (Biasiucci et al., 2018) | Compares the effect of MA-BCI with FES feedback to MA-BCI with sham FES feedback on functional and physiological improvement | Double blinded, 27 (14/13), Chronic (Months, 39,79 ± 45,9 (10–176)/ 33,46 ± 30,51 (11–121)) | No capacity to limited capacity (FMA-UE, 21,6 ± 10,8 (7–37)/ 19,9 ± 11,2 (4–40)) | MA-BCI (EEG) to trigger FES for fingers and wrist extension | Sham (random FES feedback) | FMA-UE, MRC, MAS, ESS | Significant functional recovery (FMA-UE, MRC) sustained at the 6 to 12 months follow-up correlated with significant increase in functional connectivity between motor areas in the affected hemisphere in favor of the BCI group | “BCI-FES therapy can drive significant functional recovery and purposeful plasticity thanks to contingent activation of body natural efferent and afferent pathways” “through” “somatosensory input, in the form of peripheral nerve stimulation” | No prior assessment of somatosensory-related abilities for inclusion/exclusion criteria except spatial neglect. Possible randomization bias. |
| (Frolov et al., 2017) | Compares the effect of MI-BCI with robotic feedback to sham robotic feedback on functional improvement | Blinded assessment, 74 (55/19), Subacute and chronic (Median Months, 8 [4–13]/ 8 [1–13]) | No capacity to limited capacity (Median FMA-UE, 24 [12–14]/ 12 [11–49]) | MI-BCI (EEG) to drive robotic orthosis for fingers extension and simple visual feedback | Sham without MI but with EEG (random robotic feedback) | FMA-UE, ARAT, MAS | Significant functional recovery (ARAT, FMA-UE) for both groups. More patients from the experimental group than the control group reached the MCID threashold (ARAT, FMA-UE). Correlation between CA and rehabilitation outcome (ARAT, FMA-UE). | “The kinesthetic imagination of both affected and unaffected limbs and even transition to the motor relaxation are related to motor functions and generally influence the mechanisms of neuroplasticity resulting in motor recovery.” | Worst motor impairments in the control group which could be due to greater somatosensory impairments. No prior assessment of somatosensory-related abilities for inclusion/exclusion criteria. Possible randomization bias. |
| (Li et al., 2014) | Compares the effect of MI-BCI with FES feedback to standard FES therapy on functional and physiological improvement | Blindness not described, 14 (7/7), Subacute and chronic (Months, 2,21 ± 1,8 (1–6)/ 2,79 ± 2 (1–6)) | No capacity to poor capacity (FMA-UE, 13,57 ± 4,72 (9–22)/ 11,71 ± 2,63 (9–16)) | MI-BCI (EEG) to trigger FES for wrist extension with gamified visual and auditory feedback | Standard FES therapy | FMA-UE, ARAT, EEG (ERD) | Significant functional improvement for both groups (ARAT, FMA-UE). Significant functional improvement (ARAT) of the experimental group compared to the control group at the 6 week follow-up. Significantly stronger ERD of the affected sensorimotor cortex for the experimental group post-training but not significantly different than the control group. Significant negative correlation of the ERD value and functional improvement (ARAT, FMA-UE). CA of the experimental group significantly improved and was significantly higher than the one from the control group. | “BCI training [using MI task and FES feedback] may enhance the activation of the affected SMC to prime the motor functional reorganization” | No prior assessment of somatosensory-related abilities for inclusion/exclusion criteria. Possible randomization bias. |
| (Mihara et al., 2013) | Compares the effect of MI-BCI with visual feedback to MI-BCI with sham visual feedback on functional and physiological improvement | Double blinded, 20 (10/10), Chronic (Days, 146,6 ± 36,2 (94–190)/ 123,4 ± 38,27 (89–194)) | No capacity to notable capacity (FMA-UE, 22,5 ± 14,14 (9–50)/ 24 ± 13,8 (4–50)) | MI-BCI (NIRS) to provide visual feedback | Sham (random visual feedback) | FMA-UE, ARAT, MAL, NIRS (BOLD) | Significant functional improvement for the experimental group (FMA-UE hand/finger subscale). Greater functional improvement for the experimental group associated with significantly greater motor imagery-related cortical activation (ipsilesional premotor area). | “[…] modulation of the excitability in the premotor area and related networks augments the functional recovery.” | No description of the precise sensory assessment limiting the reproductibility of the study. |
| (Pichiorri et al., 2015) | Compares the effect of MI to MI-BCI with realistic visual feedback on functional and physiological improvement | Double blinded, 28 (14/14), Subacute, (Months, 2,7 ± 1,7/ 2,5 ± 1,2) | ~No to limited capacity (FMA-UE, 23,4 ± 17,3/ 24,2 ± 18,2) | MI-BCI (EEG) to provide realistic visual feedback (finger extension of a virtual hand) | Standard MI therapy | FMA-UE, MRC, NIHSS, MAS, NASA-TLX, TMS (MEP), EEG (RSC, PSD) | Significant functional improvement for both groups (FMA-UE, MRC, NIHSS). Significantly higher functional improvement for the experimental group (FMA-UE, MRC, NIHSS) correlated with intrahemispheric connectivity increase at rest in the affected hemisphere (FMA-UE). Probability of reaching the MCID for FMA-UE significantly higher for the experimental group. Significantly higher working memory involvment for the BCI group (NASA-TLX). Significantly more robost desynchronisation for the experimental group than for the control group post-training. | “[…] it is plausible that the BCI promoted the activity of sensorimotor areas (the ipsilesional parietal area and mesial premotor and supplementary motor areas) other than the primary motor cortex that are stimulated during MI implying that the better clinical outcomes in the BCI group were mediated by compensatory changes rather than the restoration of primary motor cortex activity.” | No prior assessment of somatosensory-related abilities for inclusion/exclusion criteria except spatial neglect. Possible randomization bias. |
| (Ramos-Murguialday et al., 2013) | Compares the short term effect of MA-BCI with robotic feedback to MA-BCI with robotic sham feedback on functional and physiological improvement | Double blinded, 32 (16/16), Chronic (Months, 66 ± 45/ 71 ± 72) | ~No to poor capacity (cFMA-UE, 11,15 ± 6,92/ 13,28 ± 10,71) | MA-BCI (EEG) to drive robotic orthosis to move the upper limb forward and for finger extension | Sham (random robotic feedback) | FMA-UE, GAS, MAL, Ashworth scale, fMRI (LI), EMG | Significant functional improvement (FMA-UE, EMG) in the BCI group not present for the control group correlated with LI for patients with subcortical lesions (FMA-UE). Significant functional improvement (GAS, MAL) for both groups. Significant physiological improvement (LI) for the experimental group not found for the control group. | “BMI training, involving proprioceptive positive feedback and reward that is time-contingent upon control of ipsilesional sensorimotor brain oscillations, may prime and thus improve the beneficial effects of physiotherapy on motor function.” | No prior assessment of somatosensory-related abilities for inclusion/exclusion criteria except pain. Possible randomization bias. |
| (Rayegani et al., 2014) | Compares the effect of occupational therapy with MI-BCI using gamified feedback or with EMG biofeedback or alone on functional and physiological improvement | Blinded assessment and statistics, 30 (10/10/10), Chronic (Months, 8,5 ± 6/ 8,7 ± 10,8/ 8 ± 8,8) | N.A. (JHFT, 169 ± 66/ 167 ± 83/ 175 ± 78) | Occupational therapy and MI-BCI (EEG) to control a game (visual and auditory feedback) | Occupational therapy and EMG biofeedback, Occupational therapy alone | JHFT, EEG (PSD) | Similar funtional improvement in all the groups (JHFT). Significant increase of the PSD of the SMR band in the BCI group. Significant increase of mean and maximum contraction values of electrical activities of the paretic hand in the biofeedback group. Improved satisfaction for the biofeedback groups. | MI enables to increase or decrease SMR which was shown to be correlated with motor improvement post-stroke. | No description of the precise sensory assessment limiting the reproductibility of the study. |
| (Várkuti et al., 2013) | Compares the effect of MI-BCI with robotic feedback to standard robotic rehabilitation on physiological change | Blindness not described, 9 (6/3), Subacute and chronic (Months, 11,67 ± 13,51 (3,9–8,8)/ 6,8 ± 6,5 (3,2–35,1)) | Moderate to severe impairments (FMA-UE, 17,67 ± 16,28 (4–23)/ 14,67 ± 9,71 (4–39)) | MI-BCI (EEG) to drive robotic orthosis to move the shoulder and elbow of the impaired arm with gamified visual feedback | Standard robotic rehabilitation | fMRI (resting state), FMA-UE | Difference in resting state fMRI pre-post training are predictor of functional improvement (FMA-UE). | “MI-BCI training presumably strengthens the reassociation of neural representations of the paretic limb and the experienced afference, which could lead to better recovery.” | No prior assessment of somatosensory-related abilities for inclusion/exclusion criteria. Possible randomization bias. |
| (Wada et al., 2019) | Compares the effect of MI-BCI with robotic feedback to MI-BCI with sham robotic feedback on functional and physiological improvement | Crossover study, Blindness not described, 9, Subacute and chronic (Days, 104 ± 24) | N.A., Brunnstrom recovery stage from II to IV | MI-BCI (EEG) to drive robotic orthosis for finger extension and congruent visual feedback | Sham (random robotic feedback) | EEG (ERD), MAS, FMA-UE | Strong tendendy of increase of the ERD strength on the affected side and significant improvement of the spasticity (MAS) after BCI training none of which is observed in the control condition. Significant improvement of the spasticity after the BCI training which is not observed in the control condition. | “[…] promotion of remaining motor neurons on the affected hemisphere and the suppression of the hyperactivity of the unaffected hemisphere […] leading to better prognosis.” | No prior assessment of somatosensory-related abilities for inclusion/exclusion criteria reported. Possible randomization bias. |
| (Young et al., 2016) | Compares the effect of MA-BCI with multimodal feedback (Visual, FES and tongue stimulation) to customary care on functional and physiological improvement | Crossover study, Blindness not described, 19 (17/10), Subacute and chronic (Months, 34,53 ± 44,14 (2–168)) | No capacity to full capacity (ARAT, 30,06 ± 25,37 (0–57)/ 32,1 ± 24,96 (0–57)) | MA-BCI (EEG) to trigger visual feedback, FES for finger extension and tongue stimulation | Customary care | ARAT, SIS, 9-HPT, DTI | No significant neurophysiological difference between the control and experimental group (ARAT, SIS, 9-HPT, DTI). Fractional anisotropy values are significantly correlated to functional improvement (ARAT, SIS, 9-HPT). | N.A. | Non-responders might have been due to abnormal somatosensory abilities but not described. No prior assessment of somatosensory-related abilities for inclusion/exclusion criteria. Possible randomization bias. |
Thus, in Table 2, we report the sensorimotor-related inclusion/exclusion criteria that were used in these studies. All studies report using inclusion/exclusion criteria known to potentially influence motor rehabilitation outcomes (see Table 2). For example, the time since the stroke onset, that correlates with recovery (Kwakkel et al., 2006), has been used as an inclusion criterion by 71% of the studies included in this review. These criteria limit the bias that could arise from comparing subjects that do not have the same potential for recovery. Table 2 summarizes the information on somatosensory-related inclusion/exclusion criteria used in previous studies. Surprisingly, only 14% of these studies report checking the somatosensory impairments of the included patients. Rayegani et al. (Rayegani et al., 2014) and Mihara et al. (Mihara et al., 2013) respectively used sensory impairments as exclusion criteria. Mihara et al. (Mihara et al., 2013) were the only ones to provide information regarding the somatosensory impairments of their patients. Though, they did not report how they assessed these impairments and provided only subjective scales, i.e., ‘None’, ‘Mild’, ‘Moderate’, which limits the reliability and reproductibility between studies. Another exclusion criteria that involve somatosensory impairments is pain. Ang et al. (Ang et al., 2014, Ang et al., 2015) and Ramos-Murguialday et al. (Ramos-Murguialday et al., 2013) did not include patients that were respectively in pain or in severe pain.
Table 2.
Inclusion and exclusion criteria related to somatosensory impairments of the studies selected for this review. When stated by the authors the test or questionnaire associated with the criteria are stated in parentheses after the later. Following is a list of the abbreviations used in the table and their signification: Abbreviated Mental Test (AMT), Fugl-Meyer Assessment of Upper Extremity (FMA-UE), Intelligence quotient (IQ), Medical Research Council (MRC), Mini-Mental State Examination (MMSE), Modified Ashworth Scale (MAS), Montreal Cognitive Assessment (MoCA), Upper Extremity (UE), Visual Analogue Scale (VAS).
| Study | Instructions to use kinesthetic MI |
Inclusion/Exclusion criteria related to somatosensory assessment |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Time to stroke onset | Spasticity, dystonia, movement disorders | Visual abilities | Pain | Somato-sensory abilities | Cognitive Abilities | Language | Unilateral spatial neglect | ||
| (Ang et al., 2009) | |||||||||
| (Ang et al., 2010) | |||||||||
| (Ang et al., 2014) | Yes | >4 months | No capacity to notable capacity (FMA-UE10 and 50), Motor power controlled (MRC), No severe spasticity (MAS2) | No severe visual impairement | No pain (VAS4) | Able to understand simple instructions, No inattention, No severe depression, No psychiatric disorder | No severe aphasia | No hemispatial neglect | |
| (Ang et al., 2015) | Yes | >3 months | No capacity to limited capacity (FMA-UE45), No severe spasticity (MAS2), No fixed joint contractures | No severe visual impairement | No pain (VAS4) | Able to understand simple instructions (AMT > 6), No cognitive deficits, No severe depression | No severe aphasia | No hemispatial neglect | |
| (Biasiucci et al., 2018) | 10 months | No capacity to limited capacity (FMA-UE40), No severe dystonia/involontary movements, No other neurological disorders (e.g., Parkinson’s disease) | Good or corrected eyesight | Able to understand simple instructions, No cognitive deficits preventing to perform the rehabilitation task (Raven’s Test), No patients under heavy medication affecting the central nervous system (including vigilance) | No hemispatial neglect | ||||
| (Frolov et al., 2017) | Yes | 1 month | Hand paresis (MRC, mild to plegia), No spasticity (MAS < 4) | No severe vision impairment | No severe cognitive impairment (MoCA > 10) | No sensory aphasia, No severe motor aphasia | |||
| (Li et al., 2014) | Yes (with KVIQ assessment) | 1 to 6 months | Affected UE (Brunnstrom period level between I and III) | No cognitive impairment (MMSE > 27), Able to perform MI tasks evidenced by KVIQ, Able to understand the experimental commands | No speech disorders | ||||
| (Mihara et al., 2013) | Yes (with KVIQ assessment) | 12 weeks | Motor hemiparesis (FMA-UE50) | No hemianopia | No sensory loss | No cognitive impairment (MMSE23), No depression | No moderate to severe aphasia | No spatial neglect | |
| (Pichiorri et al., 2015) | Yes | 6 weeks to 6 months | Hemiplegia or hemiparesis, No spasticity (MAS < 4), No apraxia | No cognitive impairment (MMSE > 24) | No severe aphasia | No severe hemispatial neglect | |||
| (Ramos-Murguialday et al., 2013) | >10 months | Paresis of one hand, No active finger extension, No cerebellar lesion or bilateral motor deficit | No severe pain | Able to follow and understand instruction, No psychiatric or neurological condition other than stroke, No depression, IQ above 80 | No severe aphasia | ||||
| Rayegani et al., 2013 (Rayegani et al., 2014) | Yes | 3 to 12 months | Good trunk balance, Good motor recovery (stage 4 to 5 of Brunnstrom’s stage of motor recovery), Partial ability to grasp and release | No sensory impairment in the upper limbs | No cognitive disorders making communication difficult | ||||
| Varkuti et al., 2013 (Várkuti et al., 2013) | 1 month | No capacity to limited capacity (FMAleq45) | |||||||
| (Wada et al., 2019) (No criteria mentioned) (Wada et al., 2019) | Yes | ||||||||
| (Young et al., 2016) (Young et al., 2016) | Persistent UE motor impairment | No other known neurologic, psychiatric or developmental disabilities | |||||||
In Table 1, based on the central role that somatosensory abilities play in the perception of somatosensory feedback and the inclusion/exclusion criteria that the authors used, we hypothesize which potential somatosensory-related biases could arise. For instance, the presence of non-responders in several studies might have been related to abnormal somatosensory abilities (Ang et al., 2015, Young et al., 2016). Also, a potential randomization bias might be present in studies that did not report using somatosensory-related inclusion/exclusion criteria.
To summarize, our review of the literature indicates that somatosensory abilities may be assessed but are rarely reported in the literature. Though, we have reasons to believe that somatosensory impairments could impact BCI-based rehabilitation outcome. In future research we argue that it would be relevant to: (1) assess somatosensory impairments, (2) use the resulting somatosensory-related measures for inclusion/exclusion criteria and randomization and (3) report these measures. We argue that doing so might provide information regarding the mechanisms underlying the therapy, explain part of the between-subject and/or between-study variability, contribute to the improvement and adaptation of the therapy and offer leads for somatosensory rehabilitation. Each of these statements is developed in the following sections.
3. The interconnection of somatosensory and motor abilities during recovery
In the previous section, we focused on the specific influence of somatosensory abilities on BCI-based motor rehabilitation post-stroke. However, somatosensory abilities are interrelated with motor recovery in general. The influence of somatosensory abilities on motor rehabilitation therapies post-stroke was already demonstrated in other fields (Kessner et al., 2016, Edwards et al., 2019). To study the literature on the matter, we used the keywords “somatosensory”, “proprioceptive”, “kinesthetic”, “kinaesthetic”, “motor” and “stroke” (mostly in Medline and Scopus) and focused our research on human studies and models. We also analysed the references quoted in the relevant articles found and the articles quoting those articles.
Motor function is the main focus of sensorimotor assessment and rehabilitation, considering both clinical management and research (Kessner et al., 2016, Edwards et al., 2019). It is now acknowledged that spontaneous motor recovery reaches a plateau 3 months after stroke onset for most of the patients, due to the spontaneous cortical reorganization of the motor system which mostly occurs during this period of time (Kessner et al., 2016, Kwakkel et al., 2006). However, sensory and motor improvements are not specific but interrelated. An influence of motor rehabilitation on somatosensory network is to be expected in view of the role that somatosensory inputs play in motor rehabilitation (Edwards et al., 2019). Somatosensory impairment due to cortical lesion is almost always associated with motor impairment (Kessner et al., 2016, Sullivan and Hedman, 2008). Interestingly, somatosensory therapy seems to have an impact on motor function and vice versa (Byl et al., 2003). For instance, therapies based on repetitive electrical peripheral nerve stimulation have proven efficient to enhance excitability of the motor cortex and improve motor functions in daily activities (Conforto et al., 2018).
Motor skill learning is crucial for motor recovery and somatosensory inputs are involved in this learning (Edwards et al., 2019, Krakauer, 2006). Motor learning involves neuroplasticity, notably in the primary motor cortex (Stefan et al., 2000) which has dense connections with the primary somatosensory cortex. The conjoint activation of somatosensory afferences and motor cortical circuits affects the neural mechanisms of plasticity associated with skills learning (Edwards et al., 2019, Stefan et al., 2000). Hence, the primary somatosensory cortex is crucial in motor skill learning (Edwards et al., 2019). Ablation of the area dedicated to the hand in the primary sensory cortex of monkeys does not interfere with motor tasks learned before but impedes new learning (Pavlides et al., 1993). This influence of the somatosensory afferences on motor skill learning is also supported by post-stroke upper extremity motor rehabilitation studies.
First, somatosensory loss is associated with more paresis of the distal parts of the limbs, greater motor and functional impairments, as well as less independence in daily living in chronic stage (Edwards et al., 2019, Andersen et al., 1995, Carey et al., 2018). Also, somatosensory loss and especially proprioceptive loss, has a negative influence on the rehabilitation’s efficiency assessed through functional outcome, but also on the length of the rehabilitative treatment and on the participation in daily activities (Carey, 2017, Kessner et al., 2016). Abnormal SomatoSensory Evoked Potentials2 (SSEPs) are biomarkers of poor motor recovery. Zeman and Yiannikas (Zeman and Yiannikas, 1989) found that the pattern of the SSEPs, i.e., the amplitude of the negative and positive peaks, correlates with the functional rehabilitation outcome measured using the length of the stay at the rehabilitation center and the daily living abilities, e.g., the ability to dress. The authors also hypothesized that the correlation between SSEPs and motor recovery could be influenced by the location of the lesion. Abnormal SSEPs due to cortical lesions resulted in poorer motor outcomes than abnormal SSEPs due to subcortical lesions. The negative influence of somatosensory impairments on post-stroke motor rehabilitation could also originate from the non-use mechanism, which is the rarefied use of the plegic limb occurring in the absence of relevant proprioceptive and exteroceptive feedback (Kessner et al., 2016).
Second, the use of a constant sensory stimulation (mechanical vibration on the wrist) during motor rehabilitation has proven efficient in enhancing the motor function both at short and long terms and in increasing motor related brain activity (Fleming et al., 2015). Also, exteroceptive and proprioceptive stimulations seem to have a beneficial influence on motor function, even though further high quality studies are required (Yilmazer et al., 2019).
Finally, the rehabilitation of somatosensory perception requires taking into account motor abilities. For instance, somatosensory therapies could increase the daily use of the impacted limb, but only if the motor abilities are not too damaged (Turville et al., 2017). Motor therapies might have variable effects depending on the somatosensory impairments of the patients (Van der Lee et al., 1999). The feedback might also need to be adapted with regards to the somatosensory impairments of patients. For instance, post-stroke patients with pure somatosensory impairment can perform complex motor tasks with but not without visual feedback (Jeannerod et al., 1984). Also, somatosensory impairments sometimes include deficits in the integration of multimodal sensory information, e.g., visual and tactile, which could further impact feedback perception (Carey, 2017). Therefore, future research should provide more information about which therapies are the most beneficial depending not only on the motor impairments but also on the type of somatosensory loss (Sullivan and Hedman, 2008).
Based on these results, assessing, controlling and reporting somatosensory impairments could also provide some insight on the inter-subject and inter-study variability of BCI-based motor rehabilitation post-stroke outcome found in the literature (see Table 1). The somatosensory impairments might be relevant predictors of how patients would respond to BCI-based therapies. Taking into account these impairments might enable to better screen the patients that would most likely benefit from the therapy. Main elements of the therapy, such as the instructions, the feedback or the tasks to perform, might benefit from being adapted to the somatosensory impairments of the patients. For instance, the type of feedback, i.e., extrinsic (information originating from an external source, e.g., a screen or a person) or intrinsic (somatosensory sensations felt by the person during the training), could be adapted depending on the type and amount of somatosensory impairments. While both extrinsic and intrinsic feedback have proven efficient (Subramanian et al., 2010, Biasiucci et al., 2018), an irrelevant or inefficient proprioceptive feedback, e.g., altered due to somatosensory impairments, might impede the efficiency of the BCI therapy.
Also, the influence of the BCI therapy on the somatosensory rehabilitation should be explored. Interestingly enough, BCI-based motor rehabilitation might improve somatosensory abilities, along with motor ones. Sun et al. (Sun et al., 2011) mentioned the improvement of somatosensory abilities post BCI therapy in a non randomized clinical trial. This improvement could be related to the instruction given to the patients to perform kinesthetic motor imagery, i.e., focus on somatosensory sensations associated with the imagined movement. Asking the participants to perform kinesthetic motor imagery might participate to somatosensory rehabilitation. The vast majority of motor imagery BCI-based RCTs (73%) do report providing such instructions (see Table 1). Though, attempted movements BCI-based RCTs do not report asking their patients to focus on their sensations while trying to perform the movement. Mihara et al. reported the activation of the somatosensory associative cortex and the somatosensory primary cortex that could underly such somatosensory improvement (Mihara et al., 2013). Thus, the central role that sensory feedback plays in BCIs might be harnessed and beneficial for somatosensory rehabilitation. Such positive results were observed in previous experiments (Sun et al., 2011) and should be further investigated in future studies, for instance by recording somatosensory-related EEG activity using parietal electrodes.
4. Somatosensory impairments, recovery and assessment post-stroke
In the previous sections, we argued that somatosensory abilities play a central role in BCI-based motor rehabilitation post-stroke and for motor rehabilitation post-stroke in general. The following section provides more insights regarding the prevalence and characteristics of somatosensory impairments and recovery post-stroke. The aim is to better understand the extent of the influence that somatosensory abilities might have on BCI-based rehabilitation. We also present different methods to assess the somatosensory impairments post-stroke for experimenters and/or clinicians that wish to take into account and/or report their patients’ somatosensory impairments in their experimental protocol.
4.1. Impairments of somatosensory abilities post-stroke
Information regarding how stroke impacts somatosensory abilities is important to better understand to which extent somatosensory impairments could disrupt BCI-based motor therapy post-stroke. The prevalence of somatosensory impairments in stroke is still difficult to estimate because the studied population is heterogeneous and it remains subjective because it involves patient’s participation. Also, the assessment outcome depends on the time between the evaluation and the stroke onset, as well as on the spontaneous somatosensory recovery occurring in the first three months (Kwakkel et al., 2006). Finally, the prevalence is probably under-estimated because of the lack of standardized psychometric tools available to assess somatosensory impairments (see Section 4.3).
Despite these drawbacks, it has been estimated that more than half of strokes lead to somatosensory impairments (Carey, 2017, Kessner et al., 2016, Pumpa et al., 2015). Most often, i.e., 75% of the case, the sensory loss impacts the upper limbs (Rathore et al., 2002). Among the different types of somatosensory loss, exteroceptive impairments seem to be the most frequent. Indeed, most of the literature suggests that tactile impairments are for instance twice more frequent than proprioceptive impairments (Tyson et al., 2008) despite opposite findings (Connell et al., 2008). Impairments in proprioception and elementary sensory modalities, such as touch, pressure, pain, vibration and temperature, are equally reported for 53 to 64% of patients (Tyson et al., 2008, Connell et al., 2008). Moreover, discriminative sensations, such as stereognosis (i.e., ability to recognize objects using tactile sensations only), texture discrimination, position sense or two-point discrimination seem to be particularly affected (Klingner et al., 2012).
The following paragraphs present information regarding the neurophysiological correlates of these somatosensory impairments. Such anatomical aspects might be worth taking into account when selecting somatosensory-related inclusion/exclusion and randomization criteria for BCI-based motor rehabilitation studies.
The amount of sensory loss is correlated to the severity of the stroke as well as to the extent of the lesion (Tyson et al., 2008, Connell et al., 2008). Somatosensory submodalities can be differently affected in a given body part. For instance, at the level of the wrist, the light touch ability might not be as impacted as the proprioceptive one (Connell et al., 2008). Nonetheless, adjacent body parts are most likely to have similar amount of loss for a given somatosensory submodality, e.g., touch ability between wrist and hand are likely to be similar (Connell et al., 2008). Stroke lesions can also result in somatosensory loss (notably impairments in tactile discrimination and position senses) to the ipsilesional hand, even though the impairment seems less important than for the contralesional hand (Carey and Matyas, 2011). This phenomenon might be the consequence of damages in ipsilateral somatosensory pathways and bilateral networks processing somatosensory information (Connell et al., 2008). This result is of the utmost importance as it implies that the ipsilesional limb, i.e., the ’unimpacted limb’, cannot always be considered as a reference to evaluate the somatosensory impairments of the contralesional limb, i.e., the ’impacted limb’. This contributes to the difficulty of assessing somatosensory abilities.
Different types of strokes have been associated with different somatosensory losses (Carey, 2017, Kessner et al., 2016). Ischemic strokes are more likely to lead to sensory impairments than hemorrhagic strokes (Rathore et al., 2002). Also, right hemispheric strokes are more likely to be associated with somatosensory loss than left hemispheric strokes (Sullivan and Hedman, 2008). Though, spatial neglect3 is common for patients with right hemispheric strokes and could also explain this difference of somatosensory loss observed between right and left hemispheric strokes. Lesions affecting the thalamus, brainstem, lenticulocapsular or parietal regions are known to induce somatosensory symptoms (Klingner et al., 2012). The impairment of one somatosensory submodality or another (e.g., touch, pressure, pain, vibration and temperature) might be different depending on the lesion location (Carey, 2017, Kessner et al., 2016).
To summarize, somatosensory impairments are frequent and diverse post-stroke. Their influence on BCI-based motor rehabilitation post-stroke outcome is thus worth assessing.
4.2. Recovery of somatosensory abilities post-stroke
Beyond knowledge regarding somatosensory impairments post-stroke, the characteristics of the somatosensory recovery are important to describe. Indeed, a difference of somatosensory recovery between patients might lead to disparate somatosensory abilities among them and thereby to distinct BCI-based therapy outcomes.
While the time course of somatosensory recovery has not been as much studied as the motor one, this function also spontaneously improves after a stroke (Klingner et al., 2012, Zeman and Yiannikas, 1989). Somatosensory recovery occurs for a majority of patients within the first 3 months following the stroke (Kessner et al., 2016, Julkunen et al., 2005). Results indicate a functional and structural plasticity occurring in the primary and secondary somatosensory cortices after stroke regardless of the sensorimotor therapy followed (Schaechter et al., 2006). The recovery is highly variable between individuals and somatosensory functions can sometimes decrease and fluctuate over time (Schaechter et al., 2006, Julkunen et al., 2005). The somatosensory assessment on admission is a main predictor of recovery after 6 months (Connell et al., 2008). The amount of somatosensory recovery correlates positively with the severity of the stroke (Connell et al., 2008). Lesion location has an influence on the recovery from somatosensory impairment. One could hypothesize that cortical redundancy would lead to greater recovery of cortical lesions compared to subcortical ones (Sullivan and Hedman, 2008). Nonetheless, recent studies on proprioception have shown that persistent proprioceptive loss was associated with both subcortical and cortical lesions (Findlater et al., 2018).
Some research has been led to foster the recovery of somatosensory abilities. They focused on somatosensory discrimination tasks or on sensory stimulation involving tactile, electrical, thermal and magnetic stimulation. For an overview of the different somatosensory feedback investigated, see Sullivan and Hedman (Sullivan and Hedman, 2008). Influence of peripheral somatosensory stimulation has however been questioned by Grant et al. (Grant et al., 2018). Recent reviews (Conforto et al., 2018, Grant et al., 2018) concur on the need for further investigation with qualitative randomized controlled trials.
It is worth noting that it has been shown that Hebbian-like reinforcement occurs in the context of robot-assisted somatosensory rehabilitation (Ingemanson, 2017). Hebbian plasticity, i.e., the reinforcement of the synaptic connection induced by the conjoint activation of pre and post synaptic neurons, is also currently used to explain how BCIs foster plasticity and improve motor functions. BCI therapy often used robotic tools to improve motor control without assessing the impact on somatosensory abilities. Several authors have suggested that motor improvement observed using BCI therapy with robotic proprioceptive feedback might be due to the involvement of timely somatosensory afferences (see Table 1). However, these studies have not explored the possible biases that could arise from somatosensory abilities (see Table 2). We argue that assessing the influence of somatosensory impairments on BCI-based post-stroke motor rehabilitation outcome could lead to a better understanding of the underlying mechanisms of the therapy. The characteristics of the therapy should also be taken into account to assess the influence of somatosensory impairments. For instance, testing different types of modalities of feedback might be relevant, e.g., visual and somatosensory feedback. Indeed, depending on the somatosensory impairments, patients might benefit from one feedback more than another. If such results are found, they could be leveraged to then adapt the therapy to the patients’ profile.
Another approach might be to focus BCI-based rehabilitation on somatosensory abilities. Previous results of Yao et al. indicate that somatosensory imagination tasks can be recognized using EEG in healthy people (Yao et al., 2018). They have shown that imagining a tactile sensation of the right or left hand could be discriminated from one another with an accuracy comparable to the one of a motor imagery task, i.e., with 75.7% of online classification accuracy (percentage of mental tasks accurately recognized by the BCI). Using a BCI to provide somatosensory feedback when patients are imagining a somatosensory feeling might lead to improvements of somatosensory abilities and maybe motor ones as well (see Section 3) -The interconnection of somatosensory and motor abilities during recovery-.
4.3. Assessment of somatosensory abilities post-stroke
With the goal of fostering clinical trials assessing, controlling for and reporting somatosensory impairment, in the following paragraph we provide some information regarding somatosensory assessments tools.
Frequently, routine tests of patients after stroke consist in clinical tests and do not precisely assess all somatosensory submodalities (Kessner et al., 2016). They mostly focus on light touch and proprioception assessment but often fail to assess other submodalities, e.g., two-point discrimination or point localization (Pumpa et al., 2015). This limited scope in clinical somatosensory examination also contributes to the underestimation of the somatosensory loss (Sullivan and Hedman, 2008). Indeed, using standardized assessment of discriminative sensations, Kim and Choi-Kwon found that around 90% of patients who were thought to suffer from pure motor stroke had somatosensory impairments (Kim and Choi-Kwon, 1996). Clinical assessment can also be in contradiction with the results from standardized tests and patients identified as unimpaired using standardized tests can be identified as impaired using clinical assessment and vice versa (Carey et al., 2002).
Several standardized test protocols dedicated to the assessment of somatosensory loss have been identified in the literature (see Carey, 2017) for a review of the different tests assessing specific somatosensory abilities). Kessner et al. have summarized the different tools that assess different somatosensory modalities (Kessner et al., 2016). In their review, the authors recommended to use the “Erasmus-modified Nottingham Sensory Assessment” (EmNSA) (Stolk-Hornsveld et al., 2006) for clinical purpose because of its fair compromise between robustness and usability. For research purposes, they recommended the “Rivermead Assessment of Somatosensory Performance” (Winward et al., 2002) (RASP) because it is highly standardized and provides measures related to interval scales which are easier for statistical use. Though, the RASP is not produced by any company at the moment. Promising research was recently led using robotic technology to create more reliable proprioceptive, kinesthesic and motor assessments (Semrau et al., 2015). It is worth noting that cognitive impairments, e.g., aphasia or spatial neglect, might interfere with somatosensory assessment when assessed using clinical scales. For example, spatial neglect 3 could lead to an overestimation of somatosensory impairments of the left sided limbs and to a greater difference of somatosensory impairments between left and right sided limbs. Such cognitive impairments being frequent after a stroke, their influence on somatosensory tests should be assessed (Kessner et al., 2016). Inclusion criteria should take into account such impairments when assessing somatosensory impairments using non-physiological measures.
In order to avoid potential bias arising from cognitive impairment, specific biomarkers, such as somatosensory evoked potentials2 (SSEP), could be used in addition to standardized tools. Indeed, SSEP correlates with somatosensory impairments (Giblin, 1964). However, the relevance of such a biomarker remains unclear. Indeed, previous results found that two thirds of patients with abnormal SSEPs had somatosensory loss and four out of five patients with normal SSEP had normal sensations (Zeman and Yiannikas, 1989). Finally, other biomarkers, such as diffusion tensor imaging measures of fractional anisotropy seem to be well correlated with clinical symptoms (Yamada et al., 2003), but are still difficult to include in a clinical routine.
We have argued previously that the somatosensory impairments should be considered when selecting inclusion/exclusion and randomization criteria for BCI-based motor rehabilitation post-stroke studies. To summarize, from our review of the literature, we would suggest to use a dedicated standardized somatosensory test to assess, control and report the patients’ somatosensory impairments. The RASP was described as the most relevant test for research purpose. Though, at the moment, the test is not developed by any company. Therefore, the EmNSA, which can be passed fairly quickly (around 10 to 20 min) without specific equipment, might be used. Moreover, several factors that impact on somatosensory impairment should be taken into account. This includes the presence of cognitive impairments (e.g., aphasia, spatial neglect) and anatomical aspects (e.g., ischaemia, right hemispheric strokes, lesions to the thalamus, cortical lesions, etc., see Section 4.1, 4.2 for details). Assessing the somatosensory impairments using physiological measures such as SSEP or a diffusion tensor imaging measures of fractional anisotropy could be further informative even though it might be more difficult to include in a clinical routine.
5. Conclusion
BCI therapies have proven efficient to enhance motor functions post-stroke particularly post-intervention (Bai et al., 2020) and with effect sizes that are medium to large (Cervera et al., 2018). Further studies with larger sample sizes are necessary to validate these promising results. The therapy is based on the co-activation of top-down pathways, resulting from either motor imagery or attempted movements, and bottom-up pathways, resulting from visual and/or somatosensory feedback provided by the BCI. Based on the Hebbian theory, this co-activation should foster plasticity and improve motor abilities (Grosse-Wentrup et al., 2011). Hence, the integrity of the ascending sensory pathways, such as somatosensory ones, should be assessed. Our literature-based study of RCTs on BCI-based motor rehabilitation post-stroke indicates that somatosensory impairments may be assessed but usually are not used as inclusion/exclusion criteria and are not reported in papers. Somatosensory abilities have repeatedly been shown to influence the motor rehabilitation outcome. Yet, more than half of post-stroke patients suffer from somatosensory impairments. Based on these elements, we argue that somatosensory abilities should be systematically and rigorously reported in future experiments. This would allow us to improve our understanding of what makes BCI-based motor rehabilitation post-stroke successful. It might also enable us to optimize this rehabilitation approach possibly much further by adapting it to each patient. Depending on future experimental results, it might be worth including somatosensory-related measures as part of the control measures in the Consensus on the Reporting and Experimental Design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf) checklist (Ros et al., 2019).
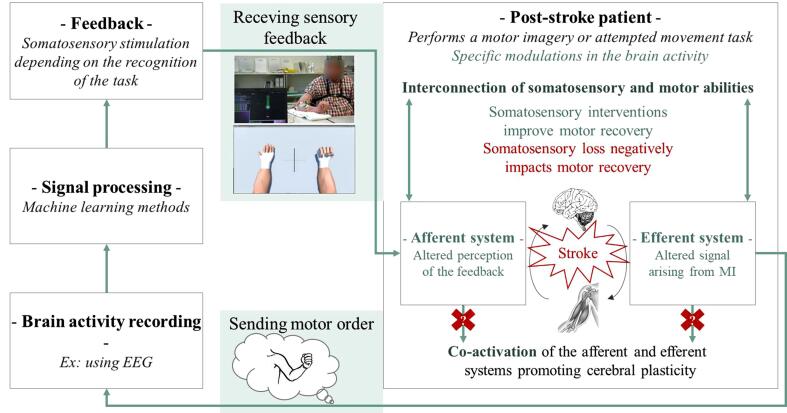
The theoretical and experimental results reviewed in the previous sections can be combined and synthesized into a global model of BCI-based post-stroke rehabilitation that explicitly takes into account somatosensory abilities. This model that we propose can be seen in Fig. 1. In short, as can be seen on this model and as discussed previously, a deficit in somatosensory abilities can negatively influence BCI-based stroke rehabilitation at two main levels: (1) at the feedback level, as it should lead to an impaired perception of the BCI feedback, that generally includes (and benefits from) somatosensory stimuli (e.g., tactile or proprioceptive feedback). Such impaired feedback perception was notably observed with various rehabilitation procedures other than BCI, as discussed previously; (2) at the BCI motor command production level, as it should lead to impaired motor imagery abilities, whereas motor imagery is a key paradigm used in BCI for stroke rehabilitation.
Fig. 1.
Schematic representation of BCI-based motor rehabilitation post-stroke taking into account the somatosensory abilities. The elements that might be impacted by the somatosensory impairments of the patients are colored in green. The photo of the person receiving sensory feedback during BCI-based motor rehabilitation is provided courtesy of ©EPFL/Alain Herzog.
From this model and from the studies reviewed above, we can also derive a number of guidelines to take into account somatosensory abilities during BCI-based stroke rehabilitation, as well as to improve our understanding of their influence. These guidelines are summarized in Table 3. Such guidelines notably include testing, measuring and reporting patients’ somatosensory abilities, as well as considering them in inclusion/exclusion criteria and for assessing the effectiveness of a given BCI feedback type.
Table 3.
Summary of the recommendations and ideas for future research for each of the main elements of BCI-based motor rehabilitation post-stroke presented in Fig. 1.
| Factors of BCI-based | Recommandations | Proposed research directions |
|---|---|---|
| Post-stroke patients | Assess somatosensory abilities using dedicated standardized tests and if possible physiological measures Take into account the somatosensory abilities of the patients in the inclusion/exclusion criteria and randomization Report the somatosensory abilities before and after the therapy |
Assess the influence of BCI-based motor therapy on somatosensory abilities and vice versa Explore BCI-based somatosensory rehabilitation Adapt the instruction and/or task that is performed to the somatosensory abilities Assess the influence of MI abilities, which can also be impacted by somatosensory abilities, on motor rehabilitation outcome Assess the influence of different types of kinestethic imagery on motor rehabilitation outcome |
| Brain activity recording | Record somatosensory-related activity | |
| Signal processing | Study the contribution of somatosensory-related activity on machine learning BCI models Study how this contribution evolves with the rehabilitation |
|
| Feedback | Take into account the somatosensory abilities when assessing the influence of a modality of feedback | Adapt the feedback, e.g., its modality, to the somatosensory abilities |
Similarly, from the model, we can identify a number of promising research directions, that would enable us to refine our understanding of BCI-based stroke rehabilitation, to hopefully improve its effectiveness as well as to derive more precise and specific guidelines. These new research directions are also summarized in Table 3. Such research directions include formally studying how patients’ somatosensory abilities and BCI-based motor rehabilitation post-stroke influence each other. Another promising lead is to explore somatosensory rehabilitation specifically, possibly using a BCI. Finally, future research should aim at finding how to adapt BCI-based stroke rehabilitation to the patients’ somatosensory abilities, notably by adapting the BCI design accordingly, e.g., the instructions, MI tasks, feedback or signal processing.
Finally, studies on healthy subjects have shown that the BCI ability to recognize the activation of top-down processes through brain activity patterns is modulated by numerous factors, such as the type of algorithm used to process the data (Lotte et al., 2018), the psychological profile of the subjects (Jeunet et al., 2016) or the characteristics of the feedback (e.g., modality of presentation, accuracy or latency) (Grosse-Wentrup et al., 2011, Lotte et al., 2013, Pillette, 2019). Though, the impact of these factors on BCI-based motor rehabilitation post-stroke outcome remains to be evaluated.
6. Funding information
This work was supported by the French National Research Agency (project REBEL, grant ANR-15-CE23-0013-01) and the European Research Council with the Brain-Conquest project (grant ERC-2016-STG-714567).
CRediT authorship contribution statement
Léa Pillette: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration. Fabien Lotte: Conceptualization, Methodology, Validation, Writing - review & editing, Supervision, Funding acquisition. Bernard N’Kaoua: Conceptualization, Methodology, Validation, Writing - review & editing. Pierre-Alain Joseph: Conceptualization, Methodology, Validation, Writing - review & editing. Camille Jeunet: Conceptualization, Methodology, Validation, Writing - review & editing. Bertrand Glize: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the French National Research Agency (project REBEL, grant ANR-15-CE23-0013-01) and the European Research Council with the Brain-Conquest project (grant ERC-2016-STG-714567).
Footnotes
Mirror visual feedback consists in positioning, with respect to a mirror, the patients’ arms in order for them to perceive their unimpaired limb in the position of the impaired limb, therefore providing the patients with the impression of two unimpaired arms.
Somatosensory evoked potentials are spontaneous electrical potentials from the nervous system following a tactile stimulation.
Spatial neglect corresponds to a deficit of attention dedicated to sensory information arising from one side of the body.
Contributor Information
Léa Pillette, Email: lea.pillette@ensc.fr.
Fabien Lotte, Email: fabien.lotte@inria.fr.
Bernard N’Kaoua, Email: bernard.nkaoua@u-bordeaux.fr.
Pierre-Alain Joseph, Email: pierre-alain.joseph@chu-bordeaux.fr.
Camille Jeunet, Email: camille.jeunet@univ-tlse2.fr.
Bertrand Glize, Email: bertrand.glize@chu-bordeaux.fr.
References
- Andersen G., Vestergaard K., Ingeman-Nielsen M., Jensen T.S. Incidence of central post-stroke pain. Pain. 1995;61:187–193. doi: 10.1016/0304-3959(94)00144-4. [DOI] [PubMed] [Google Scholar]
- Ang K.K., Guan C., Chua K.S.G., Ang B.T., Kuah C., Wang C., Phua K.S., Chin Z.Y., Zhang H. Engineering in Medicine and Biology Society. EMBC 2009. Annual International Conference of the IEEE. IEEE; 2009. A clinical study of motor imagery-based brain-computer interface for upper limb robotic rehabilitation; pp. 5981–5984. [DOI] [PubMed] [Google Scholar]
- Ang K.K., Guan C., Chua K.S.G., Ang B.T., Kuah C., Wang C., Phua K.S., Chin Z.Y., Zhang H. Engineering in Medicine and Biology Society (EMBC), 2010 Annual International Conference of the IEEE. IEEE; 2010. Clinical study of neurorehabilitation in stroke using EEG-based motor imagery brain-computer interface with robotic feedback; pp. 5549–5552. [DOI] [PubMed] [Google Scholar]
- Ang K.K., Guan C., Phua K.S., Wang C., Zhou L., Tang K.Y., Joseph E., Gopal J., Kuah C.W.K., Chua K.S.G. Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: results of a three-armed randomized controlled trial for chronic stroke. Front. Neuroeng. 2014;7:30. doi: 10.3389/fneng.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang K.K., Chua K.S.G., Phua K.S., Wang C., Chin Z.Y., Kuah C.W.K., Low W., Guan C. A randomized controlled trial of EEG-based motor imagery brain-computer interface robotic rehabilitation for stroke. Clinical EEG Neurosci. 2015;46:310–320. doi: 10.1177/1550059414522229. [DOI] [PubMed] [Google Scholar]
- Bai Z., Fong K.N., Zhang J.J., Chan J., Ting K. Immediate and long-term effects of BCI-based rehabilitation of the upper extremity after stroke: a systematic review and meta-analysis. J. NeuroEngineering Rehabilitation. 2020;17:1–20. doi: 10.1186/s12984-020-00686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batail J.-M., Bioulac S., Cabestaing F., Daudet C., Drapier D., Fouillen M., Fovet T., Hakoun A., Jardri R., Jeunet C., Lotte F., Maby E., Mattout J., Medani T., Micoulaud-Franchi J., Mladenovic J., Perronet L., Pillette L., Ros T., Vialatte F. EEG neurofeedback research: A fertile ground for psychiatry? L’Encéphale. 2019;45:245–255. doi: 10.1016/j.encep.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Biasiucci A., Leeb R., Iturrate I., Perdikis S., Al-Khodairy A., Corbet T., Schnider A., Schmidlin T., Zhang H., Bassolino M. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nature Commun. 2018;9:2421. doi: 10.1038/s41467-018-04673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byl N., Roderick J., Mohamed O., Hanny M., Kotler J., Smith A., Tang M., Abrams G. Effectiveness of sensory and motor rehabilitation of the upper limb following the principles of neuroplasticity: patients stable poststroke. Neurorehabilitation Neural Repair. 2003;17:176–191. doi: 10.1177/0888439003257137. [DOI] [PubMed] [Google Scholar]
- Carey L.M. Review on Somatosensory Loss after Stroke. Critical Rev. Phys. Rehabilitation Med. 2017;29 [Google Scholar]
- Carey L.M., Matyas T.A. Frequency of discriminative sensory loss in the hand after stroke in a rehabilitation setting. J. Rehabilitation Med. 2011;43:257–263. doi: 10.2340/16501977-0662. [DOI] [PubMed] [Google Scholar]
- Carey L.M., Matyas T.A., Oke L.E. Evaluation of impaired fingertip texture discrimination and wrist position sense in patients affected by stroke: comparison of clinical and new quantitative measures. J. Hand Ther. 2002;15:71–82. doi: 10.1053/hanthe.2002.v15.01571. [DOI] [PubMed] [Google Scholar]
- Carey L.M., Matyas T.A., Baum C. Effects of Somatosensory Impairment on Participation After Stroke. Am. J. Occupational Therapy. 2018;72 doi: 10.5014/ajot.2018.025114. 7203205100p1–7203205100p10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T. Carlson, J. d. R. Millan, Brain-controlled wheelchairs: a robotic architecture, IEEE Robotics & Automation Magazine 20 (2013) 65–73.
- Carvalho R., Dias N., Cerqueira J.J. Brain-machine interface of upper limb recovery in stroke patients rehabilitation: a systematic review. Physiotherapy Res. Int. 2019;24 doi: 10.1002/pri.1764. e1764. [DOI] [PubMed] [Google Scholar]
- Cervera M.A., Soekadar S.R., Ushiba J., Millán J.d.R., Liu M., Birbaumer N., Garipelli G. Brain-computer interfaces for post-stroke motor rehabilitation: a meta-analysis. Ann. Clin. Transl. Neurol. 2018;5:651–663. doi: 10.1002/acn3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc M., Bougrain L., Lotte F. Brain-computer interfaces. Wiley Online Library. 2016 [Google Scholar]
- Conforto A.B., dos Anjos S.M., Bernardo W.M., Silva A.A.D., Conti J., Machado A.G., Cohen L.G. Repetitive Peripheral Sensory Stimulation and Upper Limb Performance in Stroke: A Systematic Review and Meta-analysis. Neurorehabilitation Neural Repair. 2018;32:863–871. doi: 10.1177/1545968318798943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell L.A., Lincoln N., Radford K. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clinical Rehabilitation. 2008;22:758–767. doi: 10.1177/0269215508090674. [DOI] [PubMed] [Google Scholar]
- Duncan P.W., Lai S.M., Keighley J. Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology. 2000;39:835–841. doi: 10.1016/s0028-3908(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Edwards L.L., King E.M., Buetefisch C.M., Borich M.R. Putting the “Sensory” Into Sensorimotor Control: The Role of Sensorimotor Integration in Goal-Directed Hand Movements After Stroke. Front. Integrative Neurosci. 2019;13:16. doi: 10.3389/fnint.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlater S.E., Hawe R.L., Semrau J.A., Kenzie J.M., Amy Y.Y., Scott S.H., Dukelow S.P. Lesion locations associated with persistent proprioceptive impairment in the upper limbs after stroke. NeuroImage: Clinical. 2018;20:955–971. doi: 10.1016/j.nicl.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming M.K., Sorinola I.O., Roberts-Lewis S.F., Wolfe C.D., Wellwood I., Newham D.J. The effect of combined somatosensory stimulation and task-specific training on upper limb function in chronic stroke: a double-blind randomized controlled trial. Neurorehabilitation Neural Repair. 2015;29:143–152. doi: 10.1177/1545968314533613. [DOI] [PubMed] [Google Scholar]
- Fritzsch C., Wang J., dos Santos L.F., Mauritz K.-H., Brunetti M., Dohle C. Different effects of the mirror illusion on motor and somatosensory processing. Restorative Neurol. Neurosci. 2014;32:269–280. doi: 10.3233/RNN-130343. [DOI] [PubMed] [Google Scholar]
- Frolov A.A., Mokienko O., Lyukmanov R., Biryukova E., Kotov S., Turbina L., Nadareyshvily G., Bushkova Y. Post-stroke rehabilitation training with a motor-imagery-based brain-computer interface (BCI)-controlled hand exoskeleton: a randomized controlled multicenter trial. Front. Neurosci. 2017;11:400. doi: 10.3389/fnins.2017.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin D.R. Somatosensory evoked potentials in healthy subjects and in patients with lesions of the nervous system. Ann. N. Y. Acad. Sci. 1964;112:93–142. doi: 10.1111/j.1749-6632.1964.tb26744.x. [DOI] [PubMed] [Google Scholar]
- Grant V.M., Gibson A., Shields N. Somatosensory stimulation to improve hand and upper limb function after stroke—a systematic review with meta-analyses. Topics Stroke Rehabilitation. 2018;25:150–160. doi: 10.1080/10749357.2017.1389054. [DOI] [PubMed] [Google Scholar]
- Grosse-Wentrup M., Mattia D., Oweiss K. Using brain–computer interfaces to induce neural plasticity and restore function. J. Neural Eng. 2011;8 doi: 10.1088/1741-2560/8/2/025004. 025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingemanson, M.L., 2017. Proprioception and motor learning after stroke–insights from neuroimaging studies, Ph.D. thesis, UC Irvine.
- Jeannerod M., Michèl F., Prablanc C. The control of hand movements in a case of hemianaesthesia following a parietal lesion. Brain. 1984;107:899–920. doi: 10.1093/brain/107.3.899. [DOI] [PubMed] [Google Scholar]
- Jeunet, C., N’Kaoua, B., Lotte, F., 2016. Advances in user-training for mental-imagery-based BCI control: Psychological and cognitive factors and their neural correlates. In: Progress in brain research, volume 228, Elsevier, pp. 3–35. [DOI] [PubMed]
- Julkunen L., Tenovuo O.S., Jääskeläinen S.K., Hämäläinen H.A. Recovery of somatosensory deficits in acute stroke. Acta Neurologica Scandinavica. 2005;111:366–372. doi: 10.1111/j.1600-0404.2005.00393.x. [DOI] [PubMed] [Google Scholar]
- Kessner S.S., Bingel U., Thomalla G. Somatosensory deficits after stroke: a scoping review. Topics Stroke Rehabilitation. 2016;23:136–146. doi: 10.1080/10749357.2015.1116822. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Choi-Kwon S. Discriminative sensory dysfunction after unilateral stroke. Stroke. 1996;27:677–682. doi: 10.1161/01.str.27.4.677. [DOI] [PubMed] [Google Scholar]
- Klingner C.M., Witte O.W., Günther A. vol. 30. Karger Publishers; 2012. Sensory syndromes; pp. 4–8. (Manifestations of Stroke). [Google Scholar]
- Krakauer J.W. Motor learning: its relevance to stroke recovery and neurorehabilitation. Current Opinion Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- Kwakkel G., Kollen B., Twisk J. Impact of time on improvement of outcome after stroke. Stroke. 2006;37:2348–2353. doi: 10.1161/01.STR.0000238594.91938.1e. [DOI] [PubMed] [Google Scholar]
- Liepert J., Büsching I., Sehle A., Schoenfeld M.A. Mental chronometry and mental rotation abilities in stroke patients with different degrees of sensory deficit. Restorative Neurol. Neurosci. 2016;34:907–914. doi: 10.3233/RNN-160640. [DOI] [PubMed] [Google Scholar]
- Li M., Liu Y., Wu Y., Liu S., Jia J., Zhang L. Neurophysiological substrates of stroke patients with motor imagery-based brain-computer interface training. Int. J. Neurosci. 2014;124:403–415. doi: 10.3109/00207454.2013.850082. [DOI] [PubMed] [Google Scholar]
- Lotte F., Larrue F., Mühl C. Flaws in current human training protocols for spontaneous brain-computer interfaces: lessons learned from instructional design. Front. Human Neurosci. 2013;7:568. doi: 10.3389/fnhum.2013.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotte F., Bougrain L., Cichocki A., Clerc M., Congedo M., Rakotomamonjy A., Yger F. A review of classification algorithms for EEG-based brain–computer interfaces: a 10 year update. J. Neural Eng. 2018;15 doi: 10.1088/1741-2552/aab2f2. 031005. [DOI] [PubMed] [Google Scholar]
- Mihara M., Hattori N., Hatakenaka M., Yagura H., Kawano T., Hino T., Miyai I. Near-infrared spectroscopy–mediated neurofeedback enhances efficacy of motor imagery–based training in poststroke victims: a pilot study. Stroke. 2013;44:1091–1098. doi: 10.1161/STROKEAHA.111.674507. [DOI] [PubMed] [Google Scholar]
- Monge-Pereira E., Ibañez-Pereda J., Alguacil-Diego I.M., Serrano J.I., Spottorno-Rubio M.P., Molina-Rueda F. Use of electroencephalography brain-computer interface systems as a rehabilitative approach for upper limb function after a stroke: a systematic review. PM&R. 2017;9:918–932. doi: 10.1016/j.pmrj.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Murphy T.H., Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nature Rev. Neurosci. 2009;10:861. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Pavlides C., Miyashita E., Asanuma H. Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. J. Neurophysiol. 1993;70:733–741. doi: 10.1152/jn.1993.70.2.733. [DOI] [PubMed] [Google Scholar]
- Perronnet, L., Lécuyer, A., Lotte, F., Clerc, M., Barillot, C., 2016. Brain training with neurofeedback, Brain-Computer Interfaces 1: Foundations and Methods 271–292.
- Pichiorri F., Morone G., Petti M., Toppi J., Pisotta I., Molinari M., Paolucci S., Inghilleri M., Astolfi L., Cincotti F. Brain–computer interface boosts motor imagery practice during stroke recovery. Ann. Neurol. 2015;77:851–865. doi: 10.1002/ana.24390. [DOI] [PubMed] [Google Scholar]
- Pillette, L., 2019. Redefining and Adapting Feedback for Mental-Imagery based Brain-Computer Interface User Training to the Learners’ Traits and States, Ph.D. thesis, Université de Bordeaux.
- Pumpa L.U., Cahill L.S., Carey L.M. Somatosensory assessment and treatment after stroke: an evidence-practice gap. Australian Occupational Therapy J. 2015;62:93–104. doi: 10.1111/1440-1630.12170. [DOI] [PubMed] [Google Scholar]
- Ramos-Murguialday A., Broetz D., Rea M., Läer L., Yilmaz Ö., Brasil F.L., Liberati G., Curado M.R., Garcia-Cossio E., Vyziotis A. Brain–machine interface in chronic stroke rehabilitation: a controlled study. Ann. Neurol. 2013;74:100–108. doi: 10.1002/ana.23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore S.S., Hinn A.R., Cooper L.S., Tyroler H.A., Rosamond W.D. Characterization of incident stroke signs and symptoms: findings from the atherosclerosis risk in communities study. Stroke. 2002;33:2718–2721. doi: 10.1161/01.str.0000035286.87503.31. [DOI] [PubMed] [Google Scholar]
- Rayegani S.M., Raeissadat S.A., Sedighipour L., Rezazadeh I.M., Bahrami M.H., Eliaspour D., Khosrawi S. Effect of neurofeedback and electromyographic-biofeedback therapy on improving hand function in stroke patients. Top. Stroke Rehabilitation. 2014;21:137–151. doi: 10.1310/tsr2102-137. [DOI] [PubMed] [Google Scholar]
- Remsik A., Young B., Vermilyea R., Kiekhoefer L., Abrams J., Evander Elmore S., Schultz P., Nair V., Edwards D., Williams J. A review of the progression and future implications of brain-computer interface therapies for restoration of distal upper extremity motor function after stroke. Expert Rev. Med. Dev. 2016;13:445–454. doi: 10.1080/17434440.2016.1174572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros, T., Enriquez-Geppert, S., Zotev, V., Young, K.D., Wood, G., Whitfield-Gabrieli, S., Wan, F., Vuilleumier, P., Vialatte, F., Van De Ville, D., et al., 2019. Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist), Brain. [DOI] [PMC free article] [PubMed]
- Schaechter J.D., Moore C.I., Connell B.D., Rosen B.R., Dijkhuizen R.M. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain. 2006;129:2722–2733. doi: 10.1093/brain/awl214. [DOI] [PubMed] [Google Scholar]
- Semrau J.A., Herter T.M., Scott S.H., Dukelow S.P. Examining differences in patterns of sensory and motor recovery after stroke with robotics. Stroke. 2015;46:3459–3469. doi: 10.1161/STROKEAHA.115.010750. [DOI] [PubMed] [Google Scholar]
- Sitaram R., Ros T., Stoeckel L., Haller S., Scharnowski F., Lewis-Peacock J., Weiskopf N., Blefari M.L., Rana M., Oblak E. Closed-loop brain training: the science of neurofeedback. Nat. Rev. Neurosci. 2017;18:86. doi: 10.1038/nrn.2016.164. [DOI] [PubMed] [Google Scholar]
- Stefan K., Kunesch E., Cohen L.G., Benecke R., Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stolk-Hornsveld F., Crow J., Hendriks E., Van Der Baan R., Harmeling-van Der Wel B. The Erasmus MC modifications to the (revised) Nottingham Sensory Assessment: a reliable somatosensory assessment measure for patients with intracranial disorders. Clin. Rehabilitation. 2006;20:160–172. doi: 10.1191/0269215506cr932oa. [DOI] [PubMed] [Google Scholar]
- Subramanian S.K., Massie C.L., Malcolm M.P., Levin M.F. Does provision of extrinsic feedback result in improved motor learning in the upper limb poststroke? A systematic review of the evidence. Neurorehabilitation Neural Repair. 2010;24:113–124. doi: 10.1177/1545968309349941. [DOI] [PubMed] [Google Scholar]
- Sullivan J.E., Hedman L.D. Sensory dysfunction following stroke: incidence, significance, examination, and intervention. Topics Stroke Rehabil. 2008;15:200–217. doi: 10.1310/tsr1503-200. [DOI] [PubMed] [Google Scholar]
- Sun H., Xiang Y., Yang M. neurological rehabilitation of stroke patientsviamotor imaginary-based brain-computer interface technology. Neural Regeneration Res. 2011;6:2198–2202. [Google Scholar]
- Thieme H., Morkisch N., Mehrholz J., Pohl M., Behrens J., Borgetto B., Dohle C. Mirror therapy for improving motor function after stroke. Cochrane Database Systematic Rev. 2018 doi: 10.1002/14651858.CD008449.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turville M., Carey L.M., Matyas T.A., Blennerhassett J. Change in Functional Arm Use Is Associated With Somatosensory Skills After Sensory Retraining Poststroke. American Journal of Occupational Therapy. 2017;71 doi: 10.5014/ajot.2017.024950. 7103190070p1–7103190070p9. [DOI] [PubMed] [Google Scholar]
- Tyson S.F., Hanley M., Chillala J., Selley A.B., Tallis R.C. Sensory loss in hospital-admitted people with stroke: characteristics, associated factors, and relationship with function. Neurorehabilitation Neural Repair. 2008;22:166–172. doi: 10.1177/1545968307305523. [DOI] [PubMed] [Google Scholar]
- Van der Lee J.H., Wagenaar R.C., Lankhorst G.J., Vogelaar T.W., Devillé W.L., Bouter L.M. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke. 1999;30:2369–2375. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- Várkuti B., Guan C., Pan Y., Phua K.S., Ang K.K., Kuah C.W.K., Chua K., Ang B.T., Birbaumer N., Sitaram R. Resting state changes in functional connectivity correlate with movement recovery for BCI and robot-assisted upper-extremity training after stroke. Neurorehabilitation Neural Repair. 2013;27:53–62. doi: 10.1177/1545968312445910. [DOI] [PubMed] [Google Scholar]
- Wada K., Ono Y., Kurata M., Ito M.I., Minakuchi M.T., Kono M., Tominaga T. Development of a Brain-machine Interface for Stroke Rehabilitation Using Event-related Desynchronization and Proprioceptive Feedback. Adv. Biomed. Eng. 2019;8:53–59. [Google Scholar]
- Winward C.E., Halligan P.W., Wade D.T. The Rivermead Assessment of Somatosensory Performance (RASP): standardization and reliability data. Clinical Rehabilitation. 2002;16:523–533. doi: 10.1191/0269215502cr522oa. [DOI] [PubMed] [Google Scholar]
- Yamada K., Mori S., Nakamura H., Ito H., Kizu O., Shiga K., Yoshikawa K., Makino M., Yuen S., Kubota T. Fiber-tracking method reveals sensorimotor pathway involvement in stroke patients. Stroke. 2003;34:e159–e162. doi: 10.1161/01.STR.0000085827.54986.89. [DOI] [PubMed] [Google Scholar]
- Yao L., Mrachacz-Kersting N., Sheng X., Zhu X., Farina D., Jiang N. A Multi-class BCI based on Somatosensory Imagery. IEEE Trans. Neural Syst. Rehabil. Eng. 2018;26:1508–1515. doi: 10.1109/TNSRE.2018.2848883. [DOI] [PubMed] [Google Scholar]
- Yilmazer C., Boccuni L., Thijs L., Verheyden G. Effectiveness of somatosensory interventions on somatosensory, motor and functional outcomes in the upper limb post-stroke: A systematic review and meta-analysis. NeuroRehabilitation. 2019;44:459–477. doi: 10.3233/NRE-192687. [DOI] [PubMed] [Google Scholar]
- Young B.M., Stamm J.M., Song J., Remsik A.B., Nair V.A., Tyler M.E., Edwards D.F., Caldera K., Sattin J.A., Williams J.C. Brain–computer interface training after stroke affects patterns of brain–behavior relationships in corticospinal motor fibers. Front. Human Neurosci. 2016;10:457. doi: 10.3389/fnhum.2016.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman B.D., Yiannikas C. Functional prognosis in stroke: use of somatosensory evoked potentials. J. Neurol., Neurosurgery Psychiatry. 1989;52:242–247. doi: 10.1136/jnnp.52.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]