Abstract
Objective
Endovascular treatment is the mainstay therapy for brain aneurysms. About 15% of patients need re-treatment within six months due to early recanalization. In this study, we investigate risk factors associated with treatment failure.
Methods
This retrospective cohort study includes endovascularly treated aneurysm cases between July 2012 and December 2015 at the University of California Davis Medical Center with pre-treatment and early post-treatment imaging. Thin cut 3D aneurysm volume rendering was used for morphologic analyses. Univariate and bivariate analyses were conducted to evaluate differences between patients and clinical factors by treatment failure.
Results
Of the 50 patients who met the inclusion criteria, 41 (82.0%) were female, with an average age of 61 years. Most aneurysms were on the anterior communicating artery (40%) or posterior communicating artery (22.0%), and 34 (68%) aneurysms were ruptured. Early treatment failure was observed in 14 (28.0%) of endovascularly treated patients. Raymond-Roy class (RRC) was significantly associated with treatment failure (p = 0.0052), with 10 out of the 14 cases (71.4%) with early recanalization having an RRC of 3. Coil packing density did not associate with aneurysm recanalization (p = 0.61).
Conclusion
In our single institution series, patient characteristics, aneurysm characteristics, or coil packing density did not affect early aneurysm recanalization. RRC was the best predictor of early recanalization; however, further confirmation with additional studies are required. Although this study focused on early treatment failure, late recanalization has been shown with longer follow up. Further investigation into factors associated with late treatment failure will need further investigation. New intrasaccular devices and flow diverters will also likely play a role in reducing recurrence in the future as these treatments gain usage.
Keywords: Anatomy, Neurology, Neurosurgery, Radiology, Clinical research, Aneurysm coiling, Aneurysm recanalization, Brain aneurysm, Endovascular treatment failure
Anatomy; Neurology; Neurosurgery; Radiology; Clinical Research; aneurysm coiling; aneurysm recanalization; brain aneurysm; endovascular treatment failure.
1. Introduction
Brain aneurysms are the most common pathology in cerebrovascular surgery with an annual incidence of 50 per 100,000 patient-years [1]. About 3% of the population harbors a brain aneurysm [2]. Aneurysmal subarachnoid hemorrhage affects 8–10 per 100,000 patients with a mortality of 30–40% despite major advancements in our technology. Endovascular treatment of ruptured cerebrovascular aneurysms became the mainstay therapy after publication of the International Subarachnoid Aneurysm Trial (ISAT) [3, 4]. In a major shift over the last decade, ruptured aneurysms are currently coiled as frequently as they are clipped [5, 6]. At the same time, up to 70% of unruptured aneurysms are treated with coiling as the first line therapy [7].
At least 15% of patients who undergo endovascular therapy have remnant or recurrent aneurysms requiring re-treatment [8]. As a result, many institutions routinely perform diagnostic cerebral angiograms or 3D-Time of Flight (TOF) Magnetic Resonance Angiography (MRA) at 3–6 months to diagnose early recanalization [9]. Identifying risk factors associated with treatment failure is an important topic of evolving research. Recognizing such factors could help categorize patients based on the possibility of recanalization after aneurysm coiling. This would help guide the discussion on treatment options, individualize follow-up imaging intervals, and reduce costs. Several factors have been correlated with increased risk for treatment failure, namely aneurysm volume, residual uncoiled volume, coil packing density, and Raymond-Roy classification (RRC) [10, 11, 12]. These metrics rely on the accuracy of aneurysm volume estimation, which considering the complex and varied shapes of aneurysms, often requires careful assessment rather than a simple formulaic calculation [13]. Previous models have used aneurysm diameter or packing density calculated by a formula to assess recanalization [14, 15]. However, it is unknown how 3D volumetric measurements affect the risk of recanalization. In the current study, we incorporate a thin cut three-dimensional aneurysm volumetric measurement to investigate risk factors of recanalization. We consider both modifiable and non-modifiable risk factors for treatment failure. Identifying modifiable risk factors may help optimize patient selection, surgical planning, and decrease the healthcare utilization of these patients.
2. Methods
2.1. Patient selection criteria
We retrospectively reviewed patients that underwent endovascular treatment for aneurysm between July 1st, 2012 and December 31st, 2015. The UC Davis Institutional Review Board approved the study (IRBNet ID: 516055-3). We selected patients that had a preoperative computed tomography angiography (CTA) and follow up vascular imaging (diagnostic cerebral angiogram or MRA) between 3 and 6 months post-embolization. Variables selected for review were patient age, history of hypertension, smoking history (patients who quit more than 10 years earlier were considered ‘non-smokers’), aneurysm location and volume, rupture status, diameter of coils, and percentage of stent metal coverage area.
2.2. Aneurysm coiling and Raymond-Roy grading
Two senior authors performed treatments, supervised grading of the aneurysm coiling, and calculation of coil packing density. The coils used were Cerecyte (now Cerenovus, Johnson&Johnson, New Brunswick, NJ), Axium (Medtronic, Minneapolis, MN) and Stryker Neurovascular (Kalamazoo, MI). RRC grading was performed according to previous research [16]. We defined treatment failure as a Raymond-Roy class 3 aneurysmal remnant visualized on follow-up imaging after 3–6 months.
2.3. Measurement of aneurysm volume and coil packing density calculations
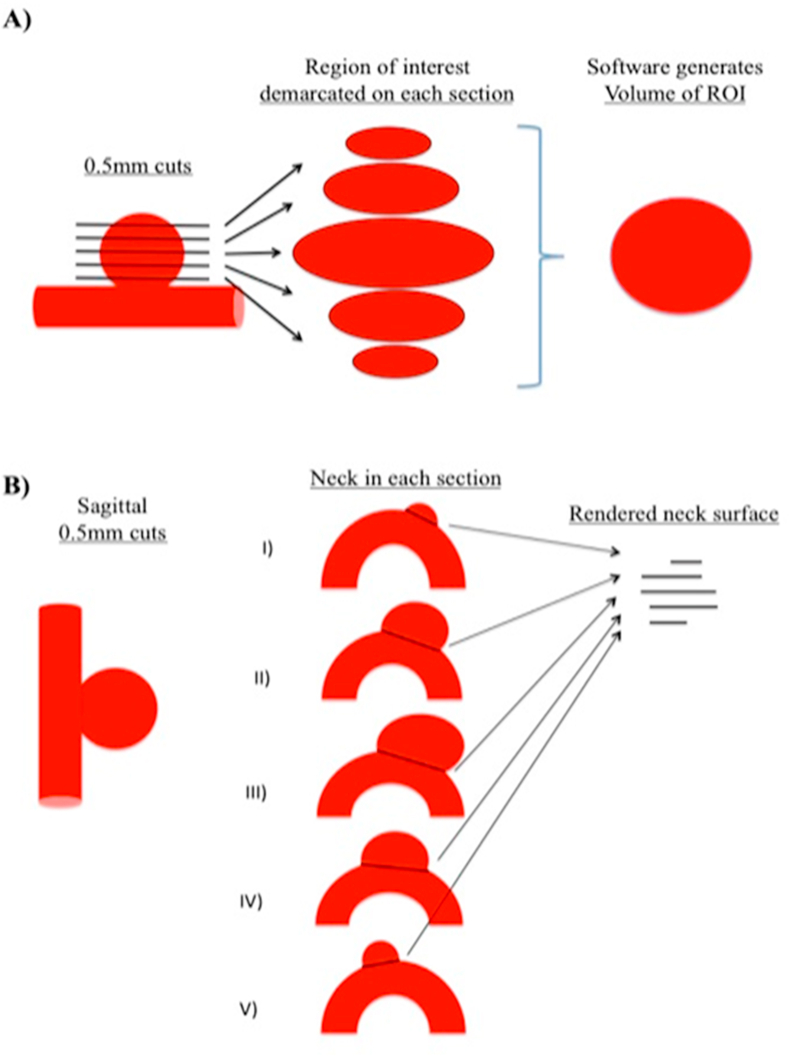
For the most accurate aneurysm volume measurement, a high-definition technique was employed as described previously [17]. Aneurysm measurements were captured on Digital Imaging and Communications in Medicine (DICOM) reformatted images uploaded to HOROS software (horosproject.com) as described in Figure 1A. For volume measurements, aneurysm walls were marked, and volume was generated as a region of interest and expressed in cubic millimeters (mm3). Neck surface area was calculated after measuring neck width in the best projection as described in Figure 1B. Coil packing density was calculated via AngioCalc based on the aneurysm volume generated as described above and the coils used in each procedure (http://www.angiocalc.com/percent_volume.php).
Figure 1.
Determining Volume and Neck Surface Area of the Aneurysm. For volume measurements aneurysm walls were marked and volume was generated as a region of interest and expressed in mm3 (Figure 1A). Neck surface area was calculated after measuring neck width in the best projection as described in Figure 1B.
2.4. Statistical analysis
Univariate and bivariate analyses were conducted to evaluate differences between patients who experienced treatment failure versus those that did not. Chi-square and Wilcoxon signed-rank tests were used to evaluate differences between the comparison groups for categorical and continuous variables, respectively. We calculated the Area Under the Curve (AUC) to evaluate the model performance for each individual variable considered in predicting the outcome of treatment failure. All tests were 2-sided and p-values <0.05 were concluded statistically significant. Analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Cohort
Between July 2012 and December 2015, 106 aneurysms in 101 patients were treated endovascularly at our institution. Patients without preoperative CTA or follow-up imaging within 3–6 months were excluded (Table 1). A final cohort of 50 patients were included in this study. The median age of our cohort was 61 years old (range 41–91 years). Most patients were females (82%) with ruptured aneurysms (68%). Aneurysms were located most frequently at the anterior-communicating artery complex (40%), followed by posterior communicating artery complex (22%) and basilar tip (18%). The remaining aneurysms were located as shown in Table 1.
Table 1.
Cohort characteristics.
| Variables | N (%) |
|---|---|
| Female | 41 (82) |
| Mean age (range) | 61 (41–91) |
| Aneurysm location | |
| Anterior communicating artery | 20 (40) |
| Posterior communicating artery | 11 (22) |
| Ophthalmic artery | 2 (4) |
| Superior Hypophyseal artery | 3 (6) |
| Basilar tip | 9 (18) |
| Other∗ | 5 (10) |
| More than one aneurysm | 2 (4) |
| Ruptured | 34 (68) |
| Smoking | 15 (30) |
| Hypertension | 34 (68) |
Superior Cerebellar (1), ICA terminal (1), PICA (2), Pericallosal (1).
3.2. Patient characteristics correlating with aneurysm treatment failure rates
Univariate analysis identified that age greater than 60 years is associated with a non-statistically significant lower chance of treatment failure. Non-failure patients had a mean age of 62 years old (SD ± 11.2) as compared to the treatment-failure group which had a mean age of 57 (SD ± 9.3), p = 0.051. Smoking history (p = 0.179), sex (p = 0.414), and history of hypertension (p = 0.746) were not associated with increased risk of treatment failure (Table 2).
Table 2.
Cohorts characteristics by treatment failure.
| All patients | Treatment Failure |
p value | ||
|---|---|---|---|---|
| Yes | No | |||
| Number of patients (%) | 50 | 14 (28.0) | 36 (72.0) | |
| Age at symptom onset, years | 0.051 | |||
| mean (SD) | 60.6 (11.0) | 56.1 (9.3) | 62.3 (11.2) | |
| median (IQR) | 61 [52–66] | 57 [50–61] | 62 [54–67] | |
| Male, N (%) | 9 (18.0) | 1 (7.1) | 8 (22.2) | 0.4138 |
| Smoking, N (%) | 15 (30.0) | 2 (14.3) | 13 (36.1) | 0.1787 |
| Hypertension, N (%) | 34 (68.0) | 9 (64.3) | 25 (69.4) | 0.7455 |
3.3. Aneurysm characteristics and treatment techniques effect on treatment failure rates
We strived for the most accurate quantification of aneurysm parameters. Thin-slice DICOM images were used for the best accuracy. Aneurysm volume was generated as a region of interest (ROI) after marking contrast enhancing areas in each slice and was expressed in mm3 (Figure 1A). Neck surface area was calculated after measuring neck width in the best projection as described in Figure 1B. Multiple aneurysm dimensions and post-treatment characteristics were studied in association with treatment failure rates (Table 3). RRC of the aneurysm was found to be the strongest predictive factor for aneurysm treatment failure (p = 0.005). None of the aneurysms with an RRC of 1 showed treatment failure on initial follow up imaging. Aneurysms with an RRC of 2 and 3 showed treatment failure rates of 28.6% and 71.4%, respectively. Aneurysm volume approached statistical significance in this analysis. Aneurysms that recanalized were 2.5 times the mean volume of non-recanalized aneurysms (289.5 vs. 117.9; p = 0.096). Other aneurysm characteristics, such as rupture status, height, largest diameter, presence of a major vessel originating at the neck of the aneurysm, neck surface area, and the volume to neck surface ratio were not significant (Table 3). Endovascular techniques, including packing density, use of 18-diameter framing coils, and stent-assisted techniques did not correlate with treatment failure. Coil packing density was 14.9% (SD ± 8.1) vs. 16.7% (SD ± 22.3) in the treatment to non-treatment failure groups respectively (p = 0.61). Stents or flow diverters were used in 28.6% vs. 25% in the treatment to non-treatment failure groups respectively (p = 0.3098). Aneurysmal neck metal coverage area percentage was based on previous studies on stents and was considered 0% if no stent was placed [39]. Metal coverage area was 4.3% vs 5.5% in the treatment to non-treatment failure groups respectively (p = 0.9443) (Table 3).
Table 3.
Aneurysm characteristics and treatment results.
| Variable | All Patients | Treatment Failure |
||
|---|---|---|---|---|
| yes | no | p value | ||
| Ruptured Aneurysm, N (%) | 34 (68.0) | 11 (78.6) | 23 (63.9) | 0.5012 |
| Maximum height (mm) | 0.2577 | |||
| mean (SD) | 6.2 (3.1) | 7.0 (3.7) | 5.9 (2.8) | |
| median (IQR) | 5.6 [4–7] | 6 [4–7] | 5 [4–7] | |
| Biggest diameter (m2) | 0.2362 | |||
| mean (SD) | 4.7 (3.4) | 5.4 (4.2) | 4.5 (3.0) | |
| median (IQR) | 4 [3–5] | 5 [4–5] | 4 [3–6] | |
| Neck surface (m2) | 0.2200 | |||
| mean (SD) | 6.1 (6.5) | 7.9 (7.8) | 5.5 (5.9) | |
| median (IQR) | 4 [2–7] | 5 [3–10] | 4 [2–7] | |
| Aneurysm volume (m3) | 0.0962 | |||
| mean (SD) | 165.9 (357.1) | 289.5 (601.5) | 117.9 (188.8) | |
| median (IQR) | 61 [37–130] | 79 [49–256] | 54 [29–123] | |
| Dome to neck volume/neck surface | 0.7882 | |||
| mean (SD) | 298.4 (1373.1) | 609.0 (2185.6) | 177.7 (901.0) | |
| median (IQR) | 12 [7–30] | 11 [8–24] | 14 [7–37] | |
| Coil packing density (%) | 0.6059 | |||
| mean (SD) | 16.2 (10.4) | 14.9 (8.1) | 16.7 (22.3) | |
| median (IQR) | 16 [11–22] | 15 [9–23] | 17 [12–22] | |
| Raymond scale, N (%) | 0.0052 | |||
| 1 | 13 (26.0) | 0 (0) | 13 (36.1) | |
| 2 | 17 (34.0) | 4 (28.6) | 13 (36.1) | |
| 3 | 20 (40.0) | 10 (71.4) | 10 (27.9) | |
| Terminal branch aneurysm dome, N (%) | 1.0000 | |||
| yes | 3 (6.0) | 1 (7.1) | 2 (5.6) | |
| no | 47 (94.0) | 13 (92.9) | 34 (94.4) | |
| Large coils, N (%) | 0.1857 | |||
| yes | 3 (6.0) | 2 (14.3) | 1 (2.8) | |
| no | 47 (94.0) | 12 (85.7) | 35 (97.2) | |
| Neck coverage stent percent#, N (%) | 0.9443 | |||
| mean (SD) | 5.2 (10.5) | 4.3 (8.8) | 5.5 (11.2) | |
| median (IQR) | 0 [0–6.5] | 0 [0–6.5] | 0 [0–3.3] | |
| Stent use, N (%) | 0.3098 | |||
| none | 37 (74.0) | 10 (71.4) | 27 (75.0) | |
| enterprise | 3 (6.0) | 1 (7.1) | 2 (5.6) | |
| LVIS | 1 (2.0) | 1 (7.1) | 0 (0) | |
| Neuroform EZ/EZ3 | 2 (4.0) | 1 (7.1) | 1 (2.8) | |
| Pipeline | 7 (14.0) | 1 (7.1) | 6 (16.7) | |
Metal coverage area comparison between the groups composed of both patients with stents and no stents. Patients with no stents were given a 0% coverage value.
3.4. Factors associated with treatment failure
We considered factors that differed (p ≤ 0.10) between the groups of patients with and without treatment failure to investigate the odds of treatment failure. These factors included age, RRC, and aneurysm volume (Table 4). We found that patients with RRC of 3 had an increased risk of treatment failure compared to RRC of 1 or 2 (OR 6.500, 95% CI: 1.753–28.455, p = 0.0074). Age (OR 0.941, 95% CI: 0.873–1.002, p = 0.0782) and aneurysm volume (OR 1.001, 95% CI: 1.000–1.004, p = 0.2153) were not significantly associated with treatment failure. As individual factors, RRC (AUC = 0.7183) seems to be the best predictor for treatment failure rather than age (0.6845) or aneurysm volume (AUC = 0.6567).
Table 4.
Aneurysm and patient characteristics role in recanalization.
| Variable | Odds Ratio (95% CI) | p value | ROC Analysis |
||
|---|---|---|---|---|---|
| AUC | AIC | SC | |||
| Age, year increment | 0.941 (0.873–1.002) | 0.0782 | 0.6845 | 59.687 | 63.511 |
| Raymond Scale III∗ | 6.500 (1.753–28.455) | 0.0074 | 0.7183 | 55.286 | 59.11 |
| Aneurysm volume (m3) | 1.001 (1.000, 1.004) | 0.2153 | 0.6567 | 61.151 | 64.975 |
Odds ratio (OR), 95% Confidence Interval (CI) of treatment failure, and Receiving Operating Characteristic Curve (ROC) analysis; Area Under the Curve (AUC), Akaike Information Criterion (AIC) and Schwarz Criterion (SC).
Reference: Raymond Class I/II.
4. Discussion
Aneurysm recurrence, or treatment failure, is typically defined as residual contrast filling in the body of the aneurysm. Several reports have investigated the rates of aneurysm recurrence following coiling, with rates estimated to range between 15 to 33% [18, 19, 20, 21, 22]. In our series we had a 28% failure rate. Previous studies have mainly focused on aneurysm and treatment specific risk factors for treatment failure and have identified suboptimal initial angiographic result, packing density, and aneurysm size as significant predictors [22, 23, 24, 25]. This study builds upon the previous literature and investigates patient, pathology, and treatment specific factors that may predict endovascular treatment failure. In a univariate analysis, RRC had the strongest correlation with treatment failure. Aneurysm properties such as size, volume, and coiling techniques showed poor correlation with treatment failure. Our findings agree with two other recent publications that show that packing density is not a predictor of treatment failure and initial RRC is a strong predictor of aneurysm recurrence [26, 27].
4.1. Patient characteristics
In our cohort, no patient characteristics correlated with treatment failure. Age was the only factor that was close to statistical significance (p = 0.051). Lanzino described age above 65 years to have half the rate of aneurysm recurrence [28]. However, it remains unclear how age affects the chances of recanalization. It has been proposed that lower metalloproteinase activity in the elderly may limit clot retraction and could prevent recurrences [29]. Smoking was found to not affect chances of early aneurysm recanalization (p = 0.18). However, Brinjikji et al. found that current smokers have a smaller chance of aneurysm recanalization (OR 0.44) after coiling [30]. Since endovascular coiling relies on intra-aneurysmal clot formation, it would be expected that a hypercoagulable condition such as smoking would increase its efficacy [31]. Nonetheless, this analysis was based on previous smoking history. Henceforth, smoking cessation should be advised to all patients given its known deleterious effects on aneurysmal growth and rupture on long-term follow up [32].
4.2. Aneurysm characteristics
Aneurysm characteristics have also been identified as potential predictors of recurrence. Different studies reported that ruptured aneurysms are 4–10 times as likely to recanalize after endovascular therapy [33, 34]. One factor that could contribute to higher risk of recanalization is the propensity of ruptured aneurysms to grow in size over time [35]. Aneurysm size may also influence failure rate, and aneurysm volume provides an additional parameter on which other variables such as coil packing density are based upon. In this study we employed a new strategy to quantify aneurysm volume and neck surface area (Figure 1). Quantification of aneurysm volume via a formula has been shown to be inaccurate and is inferior to 3D volume rendering [13]. We reconstructed aneurysm volume using thin cut CT DICOM images (Figure 1A). Although there is no generalized consensus, aneurysms larger than 10 mm in diameter or 0.4 mL in volume appear to have a higher chance of recurrence [10, 15]. In this study, recurrent aneurysms were non-significantly larger than the non-recurrent group (p = 0.096); however, both mean volumes fell short of 0.3 mL. Another factor to consider in treatment approach has been whether the aneurysm is located at the origin of an end-arterial branch such as a fetal posterior communicating artery, especially with the use of flow diverters. Some authors have recommended stent-assisted coiling in such cases to reduce the chances of recanalization [36].
4.3. Treatment characteristics
Endovascular techniques have received the most attention for their correlation with aneurysm treatment failure. RRC is a widely used tool that describes the result of the coiled aneurysm and predicts recanalization rate [16]. In this study, RRC was the best predictor of aneurysm recurrence with an OR of 6.5 for an RRC of 3 (Table 4). In our series, an RRC of 3 accounted for 40% of all cases, which is more than double the rate seen in previous series [24, 37]. Part of that increase could be the result of intentional partial dome protection for the ruptured aneurysm, which has been shown to be safe [38]. Low coil packing density has been considered as a risk factor for treatment failure; though this was not found to be a factor in our series (p = 0.6059). Mean packing density was relatively low at 16% and could have contributed to the results. Mascitelli et al. proposed that packing density of less than 31% of the aneurysm volume and an RRC of 3 were the highest predictors for recurrence [39]. Similar to the current study, Moret et al. found that coil packing density was no different between patients that had recurrence and those who did not [40]. Ogilvy's group also noted that after adjusting for confounding variables, the coil packing density was not related to protection against recurrence [41]. Lee et al. also noted volume packing density to have no correlation with recanalization [42].
Framing with bigger coils, such as size eighteen (0.018’) coils, has also been proposed to prevent endovascular treatment failure. Kaesmacher et al. reported that using volume vs. standard coils decreased coil compaction and aneurysm recurrence in lesions greater than 7mm [43]. Stiffer, or larger-bore framing coils may help in preventing aneurysmal recanalization. Framing coil percentage of total coil volume was shown to be a statistically significant predictor of recurrence in one study [44]. However, we did not see a significant impact of framing 18 coils on treatment failure in our univariate analysis. This study also assessed whether the percent metal coverage area of the stent or flow diverter could influence preventing treatment failure. No direct correlation between the percent stent neck metal coverage area and treatment failure was identified in the univariate analysis.
4.4. Strengths and limitations
A strength of this study is the use of thin-cut 3D volumetric measurements to determine aneurysm size as opposed to largest diameter or rough estimates based on formulas used by other studies.
The limitations of the study are the smaller sample size and single institution series, which may introduce regional bias. This study also primarily focused on early treatment failure at first imaging follow up at 3–6 months post-treatment. With longer follow up intervals, late treatment failure necessitating retreatment has been shown [24]. In their long-term follow up, 18 years, patients in the International Subarachnoid Aneurysm Trial (ISAT) had a 1.6% risk of aneurysm rebleeding after the first year [45]. Given the short follow up duration, this study is limited in assessing factors that may contribute to late treatment failure. It has also been found that there is an increased recurrence of endovascularly treated aneurysms treated in the acute phase after rupture [24]. Given that 68% of our patient cohort presented as ruptured, our early follow up may not fully capture all incidences of treatment failure. Therefore, our study is a preliminary study requiring confirmation of the results and the conclusion with further follow up and new studies. An ideal follow-up study would comprise a larger cohort and retrospective follow-up of more than 10 years.
In addition, follow-up imaging was not uniform since analysis of treatment failure was not only based on digital subtraction angiography but also on MRA. A previous study showed that 3D TOF MRA at this institution has a high sensitivity and specificity in detecting aneurysmal filling when compared to conventional digital subtraction angiography [46]. By using 3D TOF MRA to assess for treatment failure, a real-world scenario was replicated since treated aneurysms at many institutions are followed with non-invasive imaging.
However, given the limitations above, further confirmation with additional studies are required to confirm the preliminary results and conclusions found herein. Long term follow up, larger sample sizes, and multi-center involvement will likely be necessary to further investigate the myriad of factors that can contribute to treatment failure.
In the future, the importance of RRC or volume on aneurysm recurrence may become irrelevant with increased use of intra-saccular devices such as WEB (Woven EndoBridge) [47]. Intra-saccular devices are an alternative for wide necked aneurysms that are gaining increasing interest due to the advantage of not needing dual antiplatelet therapy after treatment. Recurrence rates and safety with WEB are similar to coiling [48]. Despite new technologies, coiling or stent-assisted coiling remains the first choice for most aneurysms.
5. Conclusion
Our study shows that early endovascular coiling results such as RRC grade are the best predictors of early aneurysm recurrence. These conclusions are preliminary given the limitations of the study and will require confirmation with additional studies. Further investigation into factors associated with late treatment failure will need further investigation. New intrasaccular devices and flow diverters will likely play a more important role in reducing recurrence in the future.
Declarations
Author contribution statement
B. Thaci: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
M. Nuño: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
K.l Varshneya and M. Kercher: Analyzed and interpreted the data; Wrote the paper.
C. Gerndt: Contributed reagents, materials, analysis tools or data; Wrote the paper.
B. Dahlin: Performed the experiments; Wrote the paper.
B. Waldau: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by National Institute of Neurological Disorders and Stroke.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Kim T., Lee H., Ahn S. Incidence and risk factors of intracranial aneurysm: a national cohort study in Korea. Int. J. Stroke. 2016;11(8):917–927. doi: 10.1177/1747493016660096. [DOI] [PubMed] [Google Scholar]
- 2.Vlak M.H., Algra A., Brandenburg R., Rinkel G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10(7):626–636. doi: 10.1016/S1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 3.Molyneux A.J., Kerr R.S., Yu L.M. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 4.Molyneux A., Kerr R., Stratton I. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 5.Lin N., Cahill K.S., Frerichs K.U., Friedlander R.M., Claus E.B. Treatment of ruptured and unruptured cerebral aneurysms in the USA: a paradigm shift. J. Neurointerventional Surg. 2012;4(3):182–189. doi: 10.1136/jnis.2011.004978. [DOI] [PubMed] [Google Scholar]
- 6.Sonig A., Shallwani H., Natarajan S.K. Better outcomes and reduced hospitalization cost are associated with ultra-early treatment of ruptured intracranial aneurysms: a US nationwide data sample study. Neurosurgery. 2018;82(4):497–505. doi: 10.1093/neuros/nyx241. [DOI] [PubMed] [Google Scholar]
- 7.Bekelis K., Gottlieb D., Su Y. Medicare expenditures for elderly patients undergoing surgical clipping or endovascular intervention for unruptured cerebral aneurysms. J. Neurointerventional Surg. 2017;9(3):324–328. doi: 10.1136/neurintsurg-2016-012313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Investigators C. Rates of delayed rebleeding from intracranial aneurysms are low after surgical and endovascular treatment. Stroke. 2006;37(6):1437–1442. doi: 10.1161/01.STR.0000221331.01830.ce. [DOI] [PubMed] [Google Scholar]
- 9.Gupta R., Griessenauer C.J., Adeeb N. Evaluating imaging follow-up strategies and costs of unruptured intracranial aneurysms treated with endovascular techniques: a survey of academic neurovascular centers in the United States. World Neurosurg. 2016;94:360–367. doi: 10.1016/j.wneu.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Sadato A., Hayakawa M., Adachi K., Nakahara I., Hirose Y. Large residual volume, not low packing density, is the most influential risk factor for recanalization after coil embolization of cerebral aneurysms. PloS One. 2016;11(5) doi: 10.1371/journal.pone.0155062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murias Quintana E., Gil Garcia A., Vega Valdes P. Anatomical results, rebleeding and factors that affect the degree of occlusion in ruptured cerebral aneurysms after endovascular therapy. J. Neurointerventional Surg. 2015;7(12):892–897. doi: 10.1136/neurintsurg-2014-011300. [DOI] [PubMed] [Google Scholar]
- 12.Darflinger R., Thompson L.A., Zhang Z., Chao K. Recurrence, retreatment, and rebleed rates of coiled aneurysms with respect to the Raymond-Roy scale: a meta-analysis. J. Neurointerventional Surg. 2016;8(5):507–511. doi: 10.1136/neurintsurg-2015-011668. [DOI] [PubMed] [Google Scholar]
- 13.Erhardt S., Marbacher S., Neuschmelting V., Coluccia D., Remonda L., Fandino J. Comparison between routine cylindrical cerebral aneurysm volume approximation and three-dimensional volume measurements in experimental aneurysms. Neurol. Res. 2014;36(8):739–745. doi: 10.1179/1743132813Y.0000000316. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen T.N., Hoh B.L., Amin-Hanjani S., Pryor J.C., Ogilvy C.S. Comparison of ruptured vs unruptured aneurysms in recanalization after coil embolization. Surg. Neurol. 2007;68(1):19–23. doi: 10.1016/j.surneu.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q., Jing L., Liu J. Predisposing factors for recanalization of cerebral aneurysms after endovascular embolization: a multivariate study. J. Neurointerventional Surg. 2018;10(3):252–257. doi: 10.1136/neurintsurg-2017-013041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy D., Milot G., Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32(9):1998–2004. doi: 10.1161/hs0901.095600. [DOI] [PubMed] [Google Scholar]
- 17.Waldau B., Domeshek L.F., Leigh F.A. Spontaneous resolution of a 13-mm Chiari malformation Type I in relation to differential growth of the posterior fossa volume. J. Neurosurg. Pediatr. 2009;3(2):110–114. doi: 10.3171/2008.10.PEDS08200. [DOI] [PubMed] [Google Scholar]
- 18.Thornton J., Debrun G.M., Aletich V.A., Bashir Q., Charbel F.T., Ausman J. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery. 2002;50(2):239–249. doi: 10.1097/00006123-200202000-00003. discussion 249-250. [DOI] [PubMed] [Google Scholar]
- 19.David C.A., Vishteh A.G., Spetzler R.F., Lemole M., Lawton M.T., Partovi S. Late angiographic follow-up review of surgically treated aneurysms. J. Neurosurg. 1999;91(3):396–401. doi: 10.3171/jns.1999.91.3.0396. [DOI] [PubMed] [Google Scholar]
- 20.Lin T., Fox A.J., Drake C.G. Regrowth of aneurysm sacs from residual neck following aneurysm clipping. J. Neurosurg. 1989;70(4):556–560. doi: 10.3171/jns.1989.70.4.0556. [DOI] [PubMed] [Google Scholar]
- 21.Manabe H., Fujita S., Hatayama T., Suzuki S., Yagihashi S. Rerupture of coil-embolized aneurysm during long-term observation. Case report. J. Neurosurg. 1998;88(6):1096–1098. doi: 10.3171/jns.1998.88.6.1096. [DOI] [PubMed] [Google Scholar]
- 22.Gallas S., Pasco A., Cottier J.P. A multicenter study of 705 ruptured intracranial aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol. 2005;26(7):1723–1731. [PMC free article] [PubMed] [Google Scholar]
- 23.Kawanabe Y., Sadato A., Taki W., Hashimoto N. Endovascular occlusion of intracranial aneurysms with Guglielmi detachable coils: correlation between coil packing density and coil compaction. Acta Neurochir. 2001;143(5):451–455. doi: 10.1007/s007010170073. [DOI] [PubMed] [Google Scholar]
- 24.Raymond J., Guilbert F., Weill A. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34(6):1398–1403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 25.Cognard C., Weill A., Spelle L. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999;212(2):348–356. doi: 10.1148/radiology.212.2.r99jl47348. [DOI] [PubMed] [Google Scholar]
- 26.Raymond J., Ghostine J., van Adel B.A. Does increasing packing density using larger caliber coils improve angiographic results of embolization of intracranial aneurysms at 1 Year: a randomized trial. AJNR Am J Neuroradiol. 2020;41(1):29–34. doi: 10.3174/ajnr.A6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greve T., Sukopp M., Wostrack M., Burian E., Zimmer C., Friedrich B. Initial raymond-roy occlusion classification but not packing density defines risk for recurrence after aneurysm coiling. Clin. Neuroradiol. 2020 doi: 10.1007/s00062-020-00926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinaldo L., Lanzino G. Increased age associated with reduced likelihood of recurrence after coiling of ruptured aneurysms. World Neurosurg. 2017;100:381–387. doi: 10.1016/j.wneu.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Fonfara S., Hetzel U., Hahn S., Kipar A. Age- and gender-dependent myocardial transcription patterns of cytokines and extracellular matrix remodelling enzymes in cats with non-cardiac diseases. Exp. Gerontol. 2015;72:117–123. doi: 10.1016/j.exger.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Brinjikji W., Lingineni R.K., Gu C.N. Smoking is not associated with recurrence and retreatment of intracranial aneurysms after endovascular coiling. J. Neurosurg. 2015;122(1):95–100. doi: 10.3171/2014.10.JNS141035. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen V.G., Hafner D.T., Steinbrenner E.B. Tobacco smoke-induced hypercoagulation in human plasma: role of carbon monoxide. Blood Coagul. Fibrinolysis. 2013;24(4):405–410. doi: 10.1097/MBC.0b013e32835d5458. [DOI] [PubMed] [Google Scholar]
- 32.Ortiz R., Stefanski M., Rosenwasser R., Veznedaroglu E. Cigarette smoking as a risk factor for recurrence of aneurysms treated by endosaccular occlusion. J. Neurosurg. 2008;108(4):672–675. doi: 10.3171/JNS/2008/108/4/0672. [DOI] [PubMed] [Google Scholar]
- 33.Abecassis I.J., Sen R.D., Barber J. Predictors of recurrence, progression, and retreatment in basilar tip aneurysms: a location-controlled analysis. Oper Neurosurg (Hagerstown) 2019;16(4):435–444. doi: 10.1093/ons/opy132. [DOI] [PubMed] [Google Scholar]
- 34.Ban S.P., Hwang G., Kim C.H. Risk factor analysis of recanalization and retreatment for patients with endovascular treatment of internal carotid artery bifurcation aneurysms. Neuroradiology. 2018;60(5):535–544. doi: 10.1007/s00234-018-2013-5. [DOI] [PubMed] [Google Scholar]
- 35.Cho Y.D., Jeon J.P., Yoo D.H. Growth-related major recanalization of coiled aneurysms: incidence and risk factors. Neurosurgery. 2018;82(2):185–191. doi: 10.1093/neuros/nyx176. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y.Q., Wang Q.J., Zheng T. Single-centre comparison of procedural complications, clinical outcome, and angiographic follow-up between coiling and stent-assisted coiling for posterior communicating artery aneurysms. J. Clin. Neurosci. 2014;21(12):2140–2144. doi: 10.1016/j.jocn.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Ries T., Siemonsen S., Thomalla G., Grzyska U., Zeumer H., Fiehler J. Long-term follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. AJNR Am J Neuroradiol. 2007;28(9):1755–1761. doi: 10.3174/ajnr.A0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waldau B., Reavey-Cantwell J.F., Lawson M.F. Intentional partial coiling dome protection of complex ruptured cerebral aneurysms prevents acute rebleeding and produces favorable clinical outcomes. Acta Neurochir. 2012;154(1):27–31. doi: 10.1007/s00701-011-1214-z. [DOI] [PubMed] [Google Scholar]
- 39.Mascitelli J.R., Oermann E.K., De Leacy R.A., Moyle H., Mocco J., Patel A.B. Predictors of treatment failure following coil embolization of intracranial aneurysms. J. Clin. Neurosci. 2015;22(8):1275–1281. doi: 10.1016/j.jocn.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Piotin M., Spelle L., Mounayer C. Intracranial aneurysms: treatment with bare platinum coils--aneurysm packing, complex coils, and angiographic recurrence. Radiology. 2007;243(2):500–508. doi: 10.1148/radiol.2431060006. [DOI] [PubMed] [Google Scholar]
- 41.Griessenauer C.J., Adeeb N., Foreman P.M. Impact of coil packing density and coiling technique on occlusion rates for aneurysms treated with stent-assisted coil embolization. World Neurosurg. 2016;94:157–166. doi: 10.1016/j.wneu.2016.06.127. [DOI] [PubMed] [Google Scholar]
- 42.Lee J.Y., Kwon B.J., Cho Y.D., Kang H.S., Han M.H. Reappraisal of anatomic outcome scales of coiled intracranial aneurysms in the prediction of recanalization. J Korean Neurosurg Soc. 2013;53(6):342–348. doi: 10.3340/jkns.2013.53.6.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaesmacher J., Muller-Leisse C., Huber T. Volume versus standard coils in the treatment of intracranial aneurysms. J. Neurointerventional Surg. 2016;8(10):1034–1040. doi: 10.1136/neurintsurg-2015-012014. [DOI] [PubMed] [Google Scholar]
- 44.Ishida W., Sato M., Amano T., Matsumaru Y. The significant impact of framing coils on long-term outcomes in endovascular coiling for intracranial aneurysms: how to select an appropriate framing coil. J. Neurosurg. 2016;125(3):705–712. doi: 10.3171/2015.7.JNS15238. [DOI] [PubMed] [Google Scholar]
- 45.Molyneux A.J., Birks J., Clarke A., Sneade M., Kerr R.S. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT) Lancet. 2015;385(9969):691–697. doi: 10.1016/S0140-6736(14)60975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Binyamin T.R., Dahlin B.C., Waldau B. Comparison of 3D TOF MR angiographic accuracy in predicting Raymond grade of flow-diverted versus coiled intracranial aneurysms. J. Clin. Neurosci. 2017;42:182–185. doi: 10.1016/j.jocn.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Shin D.S., Carroll C.P., Elghareeb M., Hoh B.L., Kim B.T. The evolution of flow-diverting stents for cerebral aneurysms; historical review, modern application, complications, and future direction. J Korean Neurosurg Soc. 2020;63(2):137–152. doi: 10.3340/jkns.2020.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pierot L., Szikora I., Barreau X. Aneurysm treatment with WEB in the cumulative population of two prospective, multicenter series: 3-year follow-up. J. Neurointerventional Surg. 2020 doi: 10.1136/neurintsurg-2020-016151. [DOI] [PMC free article] [PubMed] [Google Scholar]