Abstract
Aspilia pluriseta is associated with various bioactivities, although with limited scientific justification. In this study, we evaluated the antimicrobial activity, and characterized the phytochemicals of root extracts of A. pluriseta aimed at validating its therapeutic potential. We used BACTEC MGIT™ 960 system to test for antitubercular activity, disc-diffusion together with the microdilution method to evaluate antimicrobial activities and qualitative phytochemical tests together with gas chromatography-mass spectrometry (GC-MS) analysis to determine the phytochemicals that associated with A. pluriseta extracts activity. We show that methanolic crude extract (at 1 g/mL) had high Mycobacterium tuberculosis (MTB) inhibitory activity (0 growth unit) and considerable potency against Escherichia coli (11.7 mm), Staphylococcus aureus (9.0 mm), and Candida albicans (7.7 mm). All the extract fractions exerted remarkable antimycobacterial activities with minimum inhibitory activity of between 6.26 – 25 μg/mL. The highest antimicrobial activity of petroleum ether and dichloromethane fraction was against E. coli at inhibition zone diameters of 8.3 mm, and 8.0 mm, respectively, while ethyl acetate fraction was against S. aureus with an inhibition zone of 8.7 mm. Methanolic fraction exhibited broad-spectrum activity against 87.5% of the tested microbes (inhibition zones 6.3–8.3 mm). Furthermore, we qualitatively detected terpenoids, alkaloids, and phenolics such as flavonoids, and anthraquinones in extract fractions. GC-MS analysis detected an abundance of fatty acid esters, 2-hydroxy-1-(hydroxymethyl) ethyl ester-hexadecanoic acid, and 2,3-dihydroxy propyl ester-octadecanoic acid and four alkanes. Taken together, we show that A. pluriseta extract fractions (especially ethyl acetate and methanolic fractions) have strong selective antitubercular activity, and thus, we scientifically validate the use of A. pluriseta as a potential source for the discovery of novel antitubercular agents.
Keywords: Tuberculosis, Mycobacterium tuberculosis, Drug resistance, Phytochemicals, Fatty acid alkyl esters, Alkanes, Medicinal/herbal plants, Traditional/folk medicine, Microbiology, Pharmaceutical science, Biochemistry, Infectious disease, Pharmacology, Alternative medicine, Laboratory medicine
Tuberculosis; Mycobacterium tuberculosis; Drug resistance; Phytochemicals; Fatty acid alkyl esters; Alkanes; Medicinal/herbal plants; Traditional/folk medicine; Microbiology; Pharmaceutical science; Biochemistry; Infectious disease; Pharmacology; Alternative medicine; Laboratory medicine
1. Introduction
Infectious diseases are a primary cause of global human and animal mortality, which is further aggravated by frequent emergence and re-emergence of opportunistic infections [1]. However, one of the major global health challenges is attributed to tuberculosis. Tuberculosis (TB) is the leading cause of mortalities from a single infectious agent, which claimed the lives of over 1 million people, besides making an additional over 10 million people ill in 2018 [2,3]. In humans, TB is an airborne infection primarily caused by MTB [3, 4]. MTB thrives in the hostile environment of the human lungs, despite a sustained immunological onslaught of the host that prevents the growth of nearly all other bacteria [3, 5]. MTB effectively survives host defenses because of a highly impermeable cellular envelope that covers it. The mycobacterial cell envelope is a complex heteropolymer composed of peptidoglycan covalently attached to arabinogalactan terminated by mycolic acids, specific to mycobacteria. Also, MTB can manipulate the host immunological defense mechanisms to foster its survival in a harsh environment [5].
Effective management of MTB is hampered by a number of factors such as; (1) The widespread development of drug resistance (for example, multi-drug resistant TB (MDR-TB) which does not respond to isoniazid and rifampicin, and extensively drug-resistant TB (XDR-TB) which is resistant to isoniazid, rifampin, plus fluoroquinolones and one of the injectable second-line drugs such as amikacin, kanamycin, or capreomycin used in MDR-TB treatment regimens) [6,7], (2) The manifestation of asymptomatic latent TB infection in nearly a fourth of the global population, (3) Expensive and lengthy treatment regime, (4) Drug toxicity and associated adverse effects, and (5) Slow phase discovery of new antitubercular agents among other factors [3, 8, 9, 10]. Therefore, it is clear that further discovery and development of novel complementary treatment options are needed for improved TB treatment and control. This will significantly contribute to the World Health Organization (WHO) EndTB strategy [3, 8, 11].
Besides TB, there are other Gram-positive, Gram-negative, and fungal pathogens that have acquired noxious drug resistance. These pathogens are often responsible for the hospital- and community-acquired infections including and not limited to methicillin-resistant Staphylococcus aureus (MRSA), Klebsiella pneumoniae, fluoroquinolone-resistant Pseudomonas aeruginosa, Escherichia coli, among others [12]. Such emerging drug-resistant pathogenic strains are usually not sensitive to the first-line of antimicrobial therapy, thus forcing the use of a second- and third-line treatment option. Besides narrowing the available treatment options, the adoption of second- and third-line classes of drugs is often associated with severe adverse effects [13]. This further highlights the need for concerted effort in prospecting for novel formulations that are active against emerging, re-emerging, and drug-resistant pathogenic strain.
Although it is widely accepted that drug discovery is an expensive and time-consuming venture, exploration of certain plant species as an alternative source of pharmaceutical molecules, guided by traditional/indigenous knowledge has been very promising [14, 15, 16, 17, 18, 19]. The pharmacological activities of plant species are attributed to their phytochemicals such as terpenoids, saponins, flavonoids, glycosides, alkaloids, steroids, among others [19, 20]. The traditional/folk knowledge on medicinal plants has been heavily relied on since ancient times in search of new pharmaceutical molecules, antimicrobials, chemotherapeutics, as well as antitubercular agents [20, 21, 22, 23, 24]. Folk medicine has been applied to treat a wide range of diseases and infections, and reports are suggesting that plant-derived compounds exhibit fewer side effects, are less toxic, have low propensity to develop resistance, and are associated with improved efficacy [18, 20]. Herbal medicine is additionally prevalent in under-developed and developing countries where infectious diseases are common, compounded by poverty, poor hygiene and sanitation, and inaccessibility or high cost of good healthcare [25, 26]. In fact, it is estimated that 80% of the under-developed and developing world population rely on plant-derived remedies for alleviating various ailments [19, 27, 28, 29, 30, 31]. This fact is reinforced by the findings that plants are a rich source of bioactive molecules as efficacious as synthetic pharmaceutics or that can either be chemically modified to enhance their potency or act as templates for new pharmacophores [19, 32, 33, 34, 35]. Furthermore, plant extracts have multitargeting capacity since they contain multiple small biomolecules in low concentration, but showing synergistic and additive effects. In theory, plant extracts function in a 'polypharmacology' paradigm, entailing the use of a single product against multiple targets. This paradigm is touted as a game-changer against drug-resistant microbial strains, besides being associated with low toxicity and low side effects [3]. Therefore, the integration of traditional medicine into modern medicine is under serious consideration so as to ease drug resistance challenges and provide an alternative source for affordable, safe, and effective drugs [36, 37]. However, implementation of such herbal derived medicines requires in-depth scrutiny of their efficacy and identification of specific bioactive constituents [17, 30, 31, 38, 39]. This would provide rational scientific validation and justification for utilization and possible integration into conventional medicine, a measure that could strongly contribute to affordable, improved public health [19].
In this study, we used Aspilia pluriseta Schweinf. (Asteraceae) to investigate its potential antimicrobial activity and characterize the phytochemicals responsible for the bioactivity. A. pluriseta is locally known as Muuti (in Mbeere, Embu, and Kikuyu), Wuti (in Kamba), Ol-oiyabase (in Maasai), and Shilambila (in Luhya) communities of Kenya [40]. This plant is widely spread in East, Central, and Southern Africa, especially in open woodlands and grasslands [41, 42]. In East Africa (especially Kenya, Rwanda, and Uganda), the plant has been documented to manage and treat cough, stomach infections, burns, bruises, lacerations, wounds, pimples, ears-, eye-, nose infections, kwashiorkor, fever, worms, and diabetes mellitus with little or no scientific validation [40, 42, 43, 44, 45, 46, 47]. There are reports on antiviral activity [42], molluscicidal activity [48], complement modulating activity [49], antihelmintic activity [50], antimalarial, and hypoglycaemic activities [40, 47, 51] of A. pluriseta. However, the scientific evidence of its pharmacological activity against medically important bacteria, especially MTB, is at its early stages. As part of our continuous research efforts to discover novel, potent, antitubercular agents from Kenyans ethnobotanicals, in this study we describe the antitubercular activity of A. pluriseta solvent extract fractions. Our findings demonstrate that A. pluriseta extract fractions (especially ethyl acetate and methanolic fractions) have remarkable selective antitubercular activity, which is partly if not exclusively associated with phytochemicals such as terpenoids, phenolics, alkaloids, fatty acid alkyl esters detected in the extract fractions.
2. Methods
2.1. Plant material collection
We used the ethnopharmacological approach to identify the plant used in this study. The information on its herbal use and preparation among the Mbeere community of Embu County, Kenya, was gleaned from community herbal practitioners and further confirmed from documentation by Riley and Brokensha (1988) in The Mbeere in Kenya (ii), Botanical identity and use [52]. We collected the plant root materials in an open community field. The plant is not among the endangered species, and therefore no prior permission was sought before sample collection. The sampling was carried out within 0°46′27.0″S 37°40′54.9″E; -0.774156, 37.681908 of GPS co-ordinates. A botanist authenticated plant sample identity at Egerton University, Kenya, where voucher specimen number NSN2 was given and deposited.
2.2. Extraction and fractionation of active ingredients
The root samples were mechanically size-reduced, air-dried in the dark at 23 ± 2 °C to a constant weight, then ground into a fine powder using an electric miller (Retsch SR 200, Haan, Germany). In order to mimic the traditional preparation method, a portion of the sample powder (50 g) was subjected to cold extraction in distilled water with occasional shaking, after which the extract was lyophilized. A similar portion was macerated twice in 200 mL methanol (Sigma Aldrich, St. Louis, USA) for 48 h, pooled and filtered using Whatmann 1 filter paper. Excess methanol was evaporated from the filtrate using a rotor evaporator (Laborota 4000 efficient, Heidolph, Germany) and the extract stored at -20 °C until use. Fractionation of the A. pluriseta extract was performed using organic solvents of increasing polarity. Fifty grams of root powder was macerated in 200 mL of petro ether with intermittent shaking for 48 h. Subsequently, the material was filtered through Whatman number-1 filter paper. The residue was additionally re-extracted using the same fresh solvent for 48 h, and after that, the two filtrates pooled together. The resulting marc was air-dried and further extracted with dichloromethane solvent followed by ethyl acetate, and methanol solvent, using the same procedure carried out for petroleum ether. Organic crude extract and solvent fractions were concentrated and reconstituted into appropriate stock solution with 100% dimethyl sulfoxide (DMSO) but diluted appropriately so that the final DMSO in the test sample is 1% DMSO. Water crude extract was reconstituted in physiological saline, which served as its negative control.
2.3. Antimicrobial activity
2.3.1. Test microorganisms
All the test microorganisms were sourced from Kenya Medical Research Institute (KEMRI), Nairobi. These included; one acid-fast Mycobacterium tuberculosis strain H37Rv (ATCC 27294), one Gram-positive; Staphylococcus aureus (ATTC 25923) strain and Methicillin-resistant Staphylococcus aureus strain (clinical isolate), five Gram-negative bacteria; Escherichia coli (ATTC 25922), Pseudomonas aeruginosa (ATCC 27853), Klebsiella pneumoniae (clinical isolate), Salmonella typhi (clinical isolate) and Shigella sonnei (clinical isolate), and two fungi; Candida albicans (ATTC 90028), Cryptococcus neoformans (ATTC 66031).
2.3.2. Antimycobacterial activity
MTB was revived in Lowenstein Jensen slants under previously adopted standard conditions [53, 54] and later subjected to BACTEC MGIT 960 system (BD Biosciences, New York, USA) for antitubercular activity assays [55, 56]. BACTEC MGIT 960 system is a fully automated, high volume, a non-radiometric instrument that undertakes continuous monitoring of culture growth. Growth supplement (0.8 mL) containing a combination of oleic acid, dextrose, bovine albumin, and catalase was added to five 7 mL BBL™ MGIT™ tubes labeled GC (growth control), STR (streptomycin), INH (isoniazid), RIF (rifampicin), EMB (ethambutol) to provide essential substrates for the rapid growth of MTB. MTB suspension in 0.1 mL Middlebrook 7H9 broth adjusted to 0.5 McFarland standard with 10 mL sterile physiological saline was aseptically transferred into each BBL™ MGIT™ tube and incubated at 37 °C. One hundred microliters of BBL™ MGIT™ SIRE (streptomycin, isoniazid, rifampicin, ethambutol) prepared aseptically following the manufacturers' instructions were added into corresponding labeled BBL™ MGIT™ tube followed by addition of 0.5 mL of 1% MTB suspension. Streptomycin at 1.0 μg/mL, rifampicin at 1.0 μg/mL, ethambutol at 5.0 μg/mL, and isoniazid at 0.1 μg/mL were used as the positive controls whereas 1% DMSO (for solvent extract, and solvent extract fractions), and sterile physiological saline (for water extract) were used as negative controls. The protocol was repeated using crude extracts at 1.0 g/mL (for screening purposes) in place of SIRE and solvent fractions tested at concentrations ranging from 50 to 6.25 μg/mL for petroleum ether, dichloromethane, and methanol, and 25 to 3.125 μg/mL for ethyl acetate to determine the MTB minimum inhibitory concentration (MIC).
2.3.3. Disc diffusion test
To evaluate the general antimicrobial activity of A. pluriseta crude and solvent fractions extracts at various specified concentrations, we used the modified disc diffusion method [57, 58, 59, 60, 61]. A fresh microbial inoculum was made by suspending activated colonies in physiological saline. The bacterial and fungal suspensions were adjusted to 1.5 × 106 CFU/mL using 0.5 McFarland turbidity standard and aseptically inoculated onto Muller Hinton agar (MHA) and Sabouraud Dextrose agar (SDA) plates, respectively. Sterilized Whatmann 1 filter paper discs (diameter 6 mm) were impregnated with 10 μL of stock extract solutions (1.0 g/mL crude methanol and water extracts, 500 μg/mL for petroleum ether, dichloromethane, methanolic fractions, and 250 μg/mL for ethyl acetate fraction (inadequate amounts)). Three standard drugs were used as antibiotics positive controls; Oxacillin at 10 μg/disc (Oxoid Ltd, Tokyo, Japan) for Gram-positive bacteria, Gentamycin at 10 μg/disc (Oxoid Ltd, Tokyo, Japan) for Gram-negative bacteria, and Nystatin at 100 μg/disc (Oxoid Ltd, Tokyo, Japan) for all fungi. Whatmann filter paper discs loaded with 10 μL of 1% DMSO (for solvent extract, and solvent extract fractions), and 10 μL of sterile physiological saline (for water crude extract) served as negative controls. Air-dried discs were carefully placed on the agar plates at equidistance points using sterile forceps, including both positive antibiotic control and negative control discs into each plate. Subsequently, the plates were initially incubated at 4 °C for 2 h to allow pre-diffusion of extracts into media and incubated at 37 °C for 24 h. Antimicrobial activity was assessed in triplicates by measuring the size of the inhibition zone to the nearest mm. Fractions exhibiting strong antimicrobial inhibitory potential were considered for further MIC and minimum microbicidal concentration analysis (MMC) determination [62].
2.3.4. Determination of MIC and MMC
The MIC and MMC of A. pluriseta solvent fractions were analyzed as previously described [36, 57, 60, 61, 63]. Briefly, 50 μL of varying fraction concentrations (3.9–500 μg/mL petroleum ether, dichloromethane, and methanol; 1.95–250 μg/mL ethyl acetate) were added into 100 μL of nutrient broth held in a sterile 96-well plate followed by addition of 50 μL test organisms adjusted to 0.5 McFarland standard. All concentrations were tested in triplicates at 37 °C for 24 h. A negative control containing 1% DMSO in nutrient broth was included in column 11, while column 12 checked the capacity of the media to support the growth of the test organism. In order to evaluate the microbial growth in each well, 40 μL of 0.2 mg/mL p-iodonitrotetrazolium chloride (INT, Sigma) were added and incubated for 30 min. Formation of a pink-red color depicted growth while persistent clear coloration denoted growth inhibition. The lowest solvent fraction concentration that exhibited color change was considered as the MIC. MMC was determined by aseptically streaking a loopful of broth from wells that exhibited no color change onto sterile nutrient agar and SDA for bacteria and fungi, respectively, and thereafter incubated at 37 °C for 24 h. The lowest concentration that exhibited no growth was considered as the MMC [64].
2.4. Phytochemical analysis using GC-MS
We performed a preliminary screening for the presence of various phytochemicals such as alkaloids, terpenoids, phenolics such as flavonoids, and anthraquinones qualitatively as previously reported by us and others [4, 20, 36, 65]. Additionally, we undertook a GC-MS analysis of the methanolic extract fraction since it exhibited a broad-spectrum activity. An aliquot of the methanolic fraction (1.3 mg) was dissolved in 1 mL dichloromethane and analyzed with an Agilent Technologies 7890A gas chromatography coupled with a 5975C mass spectrometer in full scan mode (EI, 70 eV, Agilent, Palo Alto, CA). The system was equipped with an HP-5 MS low bleed capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness (J&W, Folsom, CA, USA)). An injection volume of 1 μL was subjected to a splitless mode during analysis, with helium used as the carrier medium at a constant flow rate of 1.25 mL/min. The oven temperature was maintained at 35 °C for 5 min, then programmed to increase at 10 °C/min to 280 °C and held at this temperature for 10.5 min. The obtained compound profiles were identified by comparing the corresponding reference retention indices and mass spectral in databases (NIST 05, NIST 08, Adams, and chemical).
2.5. Data analyses
The data was analyzed using Analysis of variance (ANOVA) using R (version 3.5.1) with Tukey HSD post-hoc. A p-value of less than 0.05 was considered statistically significance. Values were expressed as mean ± SEM of experimental replicates.
3. Results
3.1. Screening for general antimicrobial activities of A. pluriseta crude extracts
In order to mimic the traditional preparation of A. pluriseta herbal medicine, we initially assayed for the general bioactivity of the crude water and methanol extracts. Using a BACTEC MGIT™ 960 system (BD, New York, USA) to assay for antimycobacterial activity, we found that the water crude extract had no antituberculous activity (400 growth unit (GU)), equivalent to GU of the negative control. Interestingly, the methanolic crude extract exhibited high inhibitory activity against MTB similar to SIRE positive control (0 GU; Table 1).
Table 1.
Screening for the antimycobacterial activity of A. pluriseta crude extracts.
| Sample | Solvent | GU | NR/S |
|---|---|---|---|
| A. pluriseta | Water | 400 | NR |
| Methanol | 0 | S | |
| SIRE | 0 | S | |
| GC | 400 | NR |
Water and methanol crude extract at 1 g/mL; SIRE: Positive control of streptomycin at 1.0 μg/mL, isoniazid at 0.5 μg/mL, rifampicin at 1.0 μg/mL and ethambutol at 5.0 μg/mL; GC: Growth control acting as a negative control of media treated with 1% DMSO (methanolic crude extract) or physiological saline (water crude extract); NR: Non-responsive; S: Sensitive.
Further, we screened for general antimicrobial activity by disc diffusion method against representative Gram-positive bacteria (S. aureus), Gram-negative bacteria (E. coli), and fungi (C. albicans). Our results demonstrated a significant difference in antimicrobial activities of tested extracts against test microbes relative to controls (ANOVA, S. aureus; F(3,8) = 160.1, p < 0.001; E. coli, F(3,8) = 53.67, p < 0.001; C. albicans, F(3,8) = 72.67, p < 0.001). The water extract exhibited low general antimicrobial activity in all tested cases (zone of inhibition <10 mm), while the methanolic crude extract gave moderate but broad-spectrum activity, with the highest inhibition of 11.7 mm against E. coli (Table 2).
Table 2.
Screening for general antimicrobial activity of A. pluriseta crude extracts.
| Sample | Extract | The diameter of zone of inhibition (mm) |
||
|---|---|---|---|---|
| S. aureus | E. coli | C. albicans | ||
| A. pluriseta | Water | 6.7 ± 0.3ab | 7 ± 0.6 ab | 6.0 ± 0ab |
| Methanol | 9.0 ± 0.6 ab | 11.7 ± 0.3b | 7.7 ± 0.3 ab | |
| Positive control | 24.0 ± 1.3 | 22.0 ± 0 | 16.3 ± 0.9 | |
| Negative control | 0 | 0 | 0 | |
Water and methanol crude extract at 10 × 104 μg; Positive control (Oxacillin 10 μg/disc for Gram-positive, Gentamycin 10 μg/disc for Gram-negative bacteria and Nystatin 100 μg/disc for fungi); Negative control (Discs loaded with 10 μL of 1% DMSO (for methanol extract) or physiological saline (for water extract)); n = 3; Values = Mean ± SEM. Values followed by similar superscript letters are not significantly different from each other (ANOVA - Tukey's post-hoc multiple comparisons, P < 0.05).
Even though the crude extract concentrations were in the range of 104 times higher than the standard antibiotic controls, methanolic crude extract showed a remarkable antitubercular activity, that compared with the activity of the positive control. Since the active components in the crude extract could have comprised only a fraction of the total extract used, we reasoned that further purification would allow enrichment of active molecules in extract fractions. Therefore, we hypothesized that extract solvent fractionation would result in enhanced activity, and possibly at a lower concentration.
3.2. Antimycobacterial activities of A. pluriseta solvent extract fractions
To test whether fractionation of A. pluriseta could lead to improved antimycobacterial activity, as well as determine the MIC of different fractions, we used the BACTEC MGIT 960 system. If the GU of the extract fraction-containing tubes was greater than 100 when the GU of the growth control was 400, we defined the results as non-responsive. However, if the GU values of the extract fraction-containing tubes were ≤100, the results were considered susceptible, and the concentration of that tube was used to define the MIC [55, 56]. Our results revealed the most robust activity against MTB by more polar ethyl acetate (EA) fraction (MIC 6.25 μg/mL) followed by methanolic (MeOH) fraction (12.5 μg/mL). The less polar dichloromethane (DCM) and petroleum ether (PE) fractions had a MIC of 25 μg/mL, with DCM fraction inhibiting MTB growth in a dose-dependent manner (Table 3). For EA and MeOH fractions, concentrations ≥6.25 and 12.5 μg/mL, respectively, completely inhibited MTB growth, an observation comparable to a positive control (SIRE). These results confirmed our hypothesis that A. pluriseta fractionation would lead to robust antitubercular activity, and at a lower concentration. Further, the results are in agreement with other studies that have reported polar solvent extract fractions usually have higher activity than less polar fractions [66, 67, 68].
Table 3.
Antimycobacterial activity of A. pluriseta solvent extract fractions.
| Plant | Fraction | Concentration μg/mL | GU | NR/S | MIC (μg/mL) |
|---|---|---|---|---|---|
| A. pluriseta | PE | 50 | 0 | S | 25 |
| 25 | 0 | S | |||
| 12.5 | 400 | NR | |||
| GC | 400 | NR | |||
| SIRE | 0 | S | |||
| DCM | 50 | 0 | S | 25 | |
| 25 | 3 | S | |||
| 12.5 | 132 | S | |||
| GC | 400 | NR | |||
| SIRE | 0 | S | |||
| EA | 25 | 0 | S | 6.25 | |
| 12.5 | 0 | S | |||
| 6.25 | 0 | S | |||
| GC | 400 | NR | |||
| SIRE | 0 | S | |||
| MeOH | 50 | 0 | S | 12.5 | |
| 25 | 0 | S | |||
| 12.5 | 0 | S | |||
| GC | 400 | NR | |||
| SIRE | 0 | S |
PE: Petroleum ether fraction; DCM: Dichloromethane fraction; EA: Ethyl acetate fraction; MeOH: Methanol fraction; SIRE: Positive control of streptomycin at 1.0 μg/mL, isoniazid at 0.5 μg/mL, rifampicin at 1.0 μg/mL and ethambutol at 5.0 μg/ml; GU: Growth unit; GC: Growth control as the negative control of media treated with 1% DMSO; NR: Non-responsive; S: Sensitive.
3.3. General antimicrobial activities, MIC and MMC of A. pluriseta extract fractions
With crude solvent extract having demonstrated moderate broad-spectrum antimicrobial activity, we reasoned that solvent fractionation might yield improved antimicrobial activity. However, fractionation of root extract with solvents of increasing polarities resulted in attenuated but broad-spectrum mild antimicrobial activity, with all fractions giving inhibitory zones of less than 10 mm (Table 4). The best activity (though still a weak one) by petroleum ether fraction was against E. coli (zone of inhibition 8.3 mm), dichloromethane against E. coli (8.0 mm), ethyl acetate against S. aureus (8.7 mm) and methanolic against S. aureus (8.3 mm), C. albicans (8.3 mm) and against E. coli (8.0 mm) (Table 4). EA fraction showed a weak growth inhibition against C. neoformans (zone of inhibition 6.7 mm), while MeOH fraction weakly inhibited K. pneumonia (zone of inhibition 7.0 mm). Generally, the methanolic extract fraction exhibited significant broad spectrum activity against seven of the eight tested microorganisms (ANOVA; S. aureus, F(5,12) = 2267, p < 0.001; MRSA, F(4,10) = 129.7, p < 0.001; PA, F(5,12) = 2464, p < 0.001; E. coli, F(5,12) = 248.3, p < 0.001; KP, F(5,12) = 922.1, p < 0.001; S.S, F(5,12) = 507, p < 0.001; ST, F(5,12) = 143.7; p < 0.001; CN, F(3,8) = 1849; p < 0.001; CA, F(5,12) = 302.7; p < 0.001). Equally, relative to the negative controls, all the extract fractions significantly inhibited the growth of tested microorganisms (p < 0.001) but in a manner less comparable to the positive control.
Table 4.
Antimicrobial activity of A. pluriseta solvent extract fractions.
| Fractions | The diameter of zone of inhibition (mm) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gram-positive |
Gram-negative |
Fungi |
|||||||
| SA | MRSA | PA | EC | KP | SS | ST | CA | CN | |
| PE | 7.7 ± 0.3ab | NT | 0b | 8.3 ± 0.3ab | 0b | 0b | 0b | 7.7 ± 0.3ab | NT |
| DCM | 7.7 ± 0.3ab | 0b | 0b | 8.0±0ab | 0b | 7.7 ± 0.3ab | 0b | 7.3 ± 0.3ab | 0b |
| EA | 8.7 ± 0.3ab | 0b | 0b | 0b | 0b | 0b | 0b | 0b | 6.7 ± 0.3ab |
| MeOH | 8.3 ± 0.3ab | 6.3 ± 0.3ab | 0b | 8.0±0ab | 7.0±0ab | 8.0±0ab | 6.7 ± 0.3ab | 8.3 ± 0.3ab | NT |
| PC | 33.7 ± 0.3 | 24.3 ± 0.3 | 23.7 ± 0.6 | 15 ± 0 | 15.7 ± 0.3 | 19.7 ± 0.6 | 21.3 ± 0.3 | 16.3 ± 0.3 | 20.3 ± 0.3 |
| NC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
PE: Petroleum ether fraction at 5 μg/disc; DCM: Dichloromethane fraction at 5 μg/disc; EA: Ethyl acetate fraction at 2.5 μg/disc; MeOH: Methanol fraction at 5 μg/disc; PA: Pseudomonas aeruginosa; EC: Escherichia coli; SA: Staphylococcus aureus; KP: Klebsiella pneumoniae; MRSA: Methicillin Resistant Staphylococcus aureus; SS: Shigella sonnei; ST: Salmonella typhi; CA: Candida albicans; CN: Cryptococcus neoformans; PC: Positive control (Oxacillin 10 μg/disc and Gentamycin 10 μg/disc for Gram positive and Gram negative bacteria respectively. Nystatin 100 μg/disc for fungi); NC: Negative control (Discs loaded with 10 μl of 1% DMSO); n = 3; values = Mean ± SEM; Values followed by similar superscript letters are not significantly different from each other (ANOVA - Tukey's post-hoc multiple comparisons, P < 0.05).
Further, we tested for the MIC and MMC concentrations of the solvent extract fractions (Table 5), and we established that petroleum ether fraction against E. coli had a MIC of 250 μg/mL while methanolic fraction against S. aureus had a MIC of 125 μg/mL. In all other cases, the MIC was greater than 500 μg/mL indicating that the extract fractions were bacteriostatic in action.
Table 5.
MIC and MBC of A. pluriseta solvent extract fractions.
| Fractions | MIC (μg/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gram-positive |
Gram-negative |
Fungi |
|||||||
| SA | MRSA | PA | EC | KP | SS | ST | CA | CN | |
| PE | - | - | - | 250 | - | - | - | - | - |
| DCM | - | - | - | - | - | - | - | - | - |
| EA | - | - | - | - | - | - | - | - | - |
| MOH | 125 | - | - | - | - | - | - | - | - |
PE: Petroleum ether fraction; DCM: Dichloromethane fraction; EA: Ethyl acetate fraction; MOH: Methanol fraction; PA: Pseudomonas aeruginosa; EC: Escherichia coli; SA: Staphylococcus aureus; KP: Klebsiella pneumoniae; MRSA: Methicillin-resistant Staphylococcus aureus; SS: Shigella sonnei; ST: Salmonella typhi; CA: Candida albicans; CN: Cryptococcus neoformans; - indicates the MIC is >500 μg/mL; MMC in all cases were >500 μg/mL.
Therefore, the general antimicrobial activity results were counterintuitive, considering the fractions yielded robust antimycobacterial activity. This would suggest that the active principles in the extract fractions are, to some extent, specific to acid-fast bacteria. This would be very important for the discovery of selective antitubercular leads, as previously reported [69, 70].
3.4. Phytochemical analysis
The preliminary examination of A. pluriseta solvent extract fractions pointed to the presence of different phytoconstituents such as terpenoids, alkaloids, and phenolics such as flavonoids, and anthraquinones (Table 6). We speculate that these are the bioactive constituents that may be responsible for the bioactivity demonstrated by these extract fractions.
Table 6.
Phytochemical results of A. pluriseta solvent fraction extracts.
| Extract Fraction | V-Ts | A-F | MK-A | D-A | F-P |
|---|---|---|---|---|---|
| Petrol ether | +++ | - | - | + | - |
| Dichloromethane | +++ | + | + | ++ | ++ |
| Ethyl acetate | + | + | + | - | + |
| Methanol | +++ | - | - | - | + |
V-T, Vanillin test for terpenoids; A-F, Ammonia test for Flavonoids; MK-A, Methanolic Potassium hydroxide test for Anthraquinones; D-A, Dragendorff test for Alkaloids; F-P, Ferric Chloride test for Phenols; -, Absent phytochemicals; +, Low concentration of phytochemicals; ++, Medium concentration; +++, High concentration of phytochemicals.
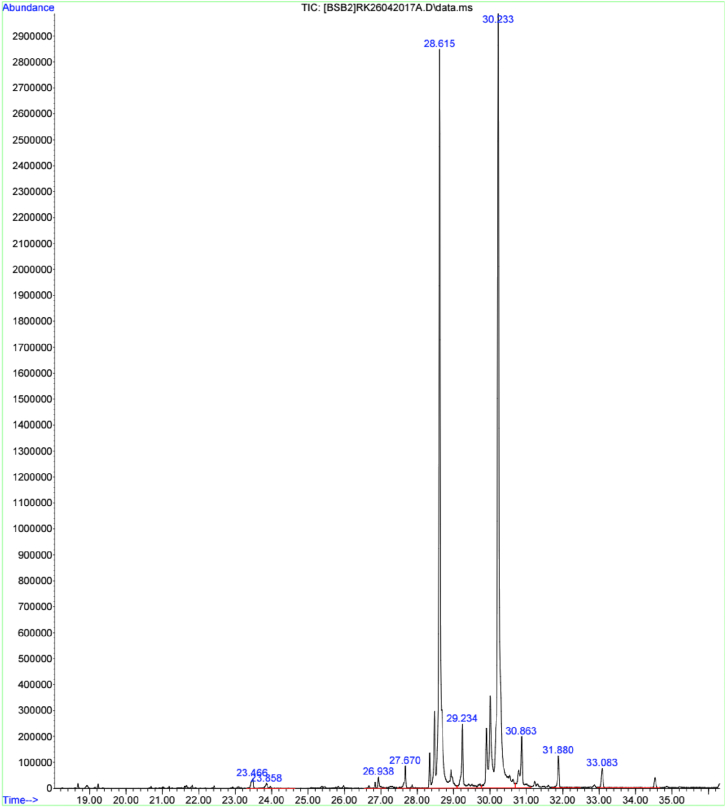
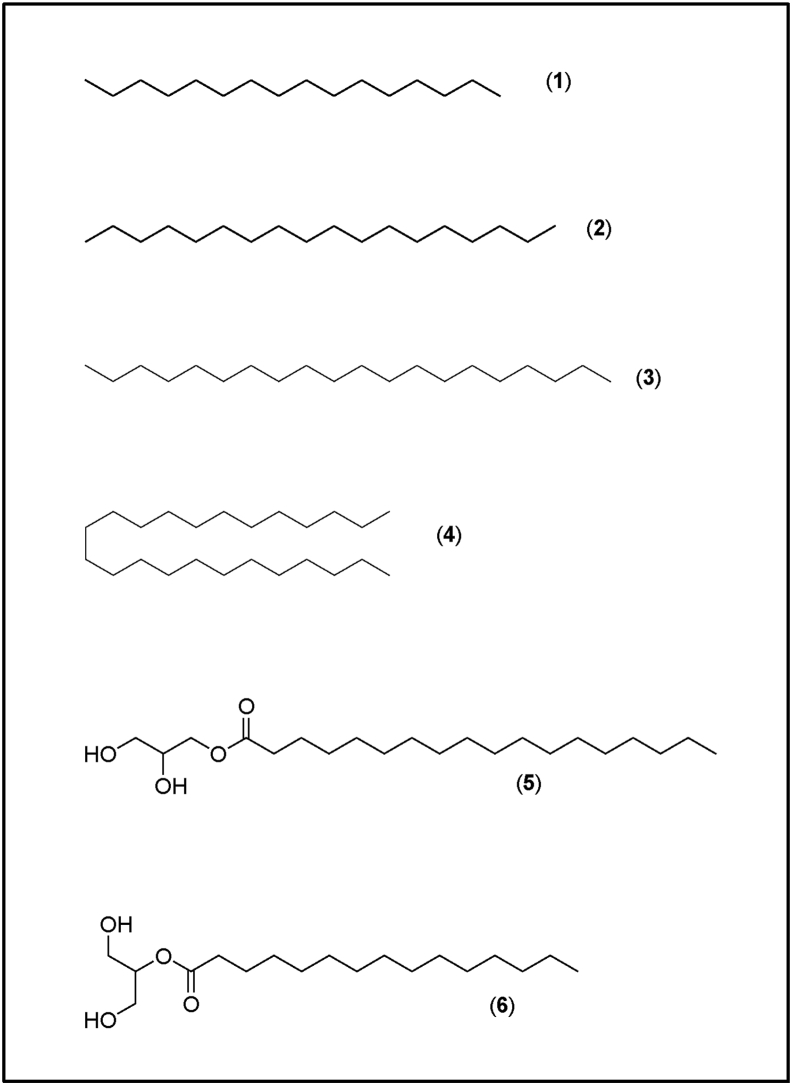
Methanolic extract fraction exhibited broad spectrum activity (inhibiting the growth of Gram-negative, Gram-positive, acid-fast bacteria, and fungi (Tables 3 and 4)). Additionally, it was easier to get more materials from this extract fraction for further analysis. Therefore, we subjected this fraction to GC-MS analysis to identify the specific bioactive compounds that could be partly associated with the activity of this fraction (Table 7; Figures 1 and 2). We detected six compounds from the extract fraction, four alkanes, and two fatty acid esters. Based on the mass spectral library databases, these compounds were tentatively identified as hexadecane, octadecane, eicosane, 2-hydroxy-1-(hydroxymethyl) ethyl ester-hexadecanoic acid, tetracosane, and 2,3-dihydroxy propyl ester-octadecanoic acid (Table 7; Figure 2). The fatty acid esters represented by peaks at retention times 28.61 min (2-hydroxy-1-(hydroxymethyl) ethyl ester-hexadecanoic acid) and 30.23 min (2,3-dihydroxy propyl ester-octadecanoic acid) were the most abundant compounds detected (Figure 1), and it is highly possible that on their own or in combination with other secondary metabolites contributed wholly or in part to the bioactivity of this extract fraction.
Table 7.
Compounds identified in A. pluriseta methanolic extract fraction using GC-MS.
| Peak no. | Rt (min) | Compound name | Class |
|---|---|---|---|
| 1 | 26. 84 | Hexadecane | Alkane |
| 2 | 27.67 | Octadecane | Alkane |
| 3 | 27.68 | Eicosane | Alkane |
| 4 | 28.61 | 2-hydroxy-1-(hydroxymethyl)ethyl ester-hexadecanoic acid | Fatty acid ester |
| 5 | 29.24 | Tetracosane | Alkane |
| 6 | 30.23 | 2,3-dihydroxypropyl ester-octadecanoic acid | Fatty acid ester |
Rt: Retention time in minutes; The data presented in the table above shows the peak number, corresponding retention time, compound identities, and their classification.
Figure 1.
GC-MS analysis spectrum of A. pluriseta methanolic extract fraction. The spectrum highlights the compound abundance and separation based on mass fragmentation and retention times.
Figure 2.
Compounds identified in A. pluriseta methanolic extract fraction using GC-MS. The chemical structures of the identified compounds (1) Hexadecane, (2) Octadecane, (3) Eicosane, (4) 2-hydroxy-1-(hydroxymethyl)ethyl ester-hexadecanoic acid, (5) Tetracosane, and (6) 2,3-dihydroxy propyl ester-octadecanoic acid.
4. Discussion
Increasing demand for effective antimicrobials to lessen antimicrobial drug resistance burden and accelerate prompt prevention and treatment of microbial infections necessitates the discovery of new pharmaceutical molecules. This calls for a collaborative approach involving the herbal practitioners and scientific community in search of pharmaceutical molecules from traditionally-claimed active plants and scientifically validating their bioactivity. This comes at a time when most pharmaceutical industries seem reluctant and/or slow to develop novel antimicrobial agents, prompting an overreliance on the limited available antibiotics. This has consequently led to the emergence of superbugs insensitive to available antibiotic regimes [71, 72]. Flashing back to before the advent of first-generation antibiotics in 1928, humans were using and are still using herbal preparations to manage, treat, and cure various ailments. Herbal medicine thus provides a solid foundation for the discovery of new agents against these health-threatening pathogens [73, 74].
The current study was motivated by an ethno-based claim by the Ambeere residents and herbalists from Embu county - Kenya that, A. pluriseta root extracts are used in the management of 'strong' coughs and complicated respiratory tract infections. We hypothesized that the alluded to 'strong' cough and complicated respiratory tract infection represented TB. We therefore sought to investigate the antitubercular and general antimicrobial activities of this plant, as well as identify the bioactive compounds therein that could be responsible for the bioactivity.
The traditional-based approach of preparing the extracts using water yielded very low antitubercular and antimicrobial activities, while the crude methanolic extract exhibited a broad-spectrum antimicrobial activity against acid-fast, Gram-positive, Gram-negative, and fungi. This could point to a possible implication of differential solubility of various plant compounds in solvents of different polarities. It is established that solvent polarity affects the qualitative and quantitative composition of bioactive compounds enriched in various solvents, and that methanol is a better and more powerful extractant compared to water [66, 67, 68]. With methanolic crude extract giving a robust antitubercular activity, the next question we asked ourselves was whether solvent fractionation would give us more fractions with potent activity, and at a lower concentration (from 1 g/mL to between 50-6.25 μg/mL; Tables 1 and 3). Compared to the positive control, the concentration of the crude methanolic extract was 104-fold higher. We reasoned that the active compound(s) in the crude methanolic extract comprised only a small fraction of the crude extract and if purified and isolated, might work at a lower concentration, or even more potently than the standard antibiotic used. To test this assumption, we fractionated the plant sample by organic solvents of increasing concentration. Interestingly, all solvent extract fractions had a strong antitubercular activity with MIC ranging between 25 and 6.25 μg/mL that yielded similar inhibitory capacity (0 GU) to the commercially available antimycobacterial drugs, streptomycin, isoniazid, rifampicin, and ethambutol (SIRE) (Table 3). These findings are consistent with other studies that have demonstrated that plants are an excellent potential source of active antitubercular compounds [18, 39, 51, 75]. However, solvent fractionation yielded relatively lower general antimicrobial activity against Gram-positive, Gram-negative, and fungi compared to the crude extract (Tables 2 and 4). This phenomenon suggests a possible synergistic and/or additive effect of the active molecules in the crude extract, an effect that was possibly lost by fractionation [76, 77, 78, 79]. Furthermore, the fact that fractions demonstrated a robust antitubercular activity, and low general activity against Gram-positive, Gram-negative, and fungi allude to a possible selective activity against MTB, which is an essential feature in search of novel selective antitubercular leads [69, 70].
Bioactivity of plant extracts is the work of secondary metabolites produced for purposes of normal plant defenses; to deter, stun, poison or kill threatening species, but inadvertently inhibiting various physiological targets/processes required for growth, biosynthesis of macromolecules, metabolism, and virulence of microbial systems [30, 80, 81, 82]. To characterize and identify the active compounds mediating activity against the tested organisms, we initially performed qualitative phytochemical screening in all extract solvent fractions (Table 6), and subsequent GC-MS analysis of the methanolic solvent extract fraction (Table 7 & Figures 1 and 2). We qualitatively identified terpenoids, phenolics such as flavonoids and anthraquinones, and alkaloids in A. pluriseta extracts, and thus speculated that they are the phytochemicals partly or wholly responsible of the antitubercular activity demonstrated by various extract fractions. Previous studies have also reported presence of terpenoids, alkaloid, flavonoids, anthraquinones and phenolic in A. pluriseta aqueous extract [40, 47, 83, 84]. Terpenes were enriched in all fractions tested, and a broad range of terpenes identified in other studies have been associated with antitubercular activity, partly due to their lipophilicity that makes it easier for them to penetrate through MTB wall [85, 86]. Alkaloids, terpenoids, and flavonoids have been speculated to induce antitubercular activity by interfering with the MTB efflux pump, thus modulating in non-specific manner membrane proteins and receptors as well as inhibiting natural methods for MTB resistance development [87].
Additionally, using GC-MS, we identified two abundant fatty acid alkyl esters in methanolic solvent extract fraction; 2-hydroxy-1-(hydroxymethyl) ethyl ester-hexadecanoic acid (2-palmitoylglycerol) and 2,3-dihydroxypropyl ester-octadecanoic acid (glyceryl monostearate); (Table 7 and Figures 1 and 2). We speculate that these fatty acid alkyl esters are associated with the observed bioactivity of this extract fraction. In fact, previous studies have reported antimicrobial and antimycobacterial activities of naturally occurring and synthetic fatty acid alkyl esters [88, 89, 90, 91]. Although the relationship between structure and antimicrobial activity of various fatty acids and their ester derivatives remains elusive, it is speculated that the inhibitory effect is greatly influenced by the number and presence of double bonds [90]. Mechanistically, fatty acids and their ester derivatives (FAED) act by interfering with the vital fatty acid synthase-I (FASI)- and FASIII-mediated fatty acid biosynthesis and degradation [92]. Furthermore, FAED and long-chain alkanes, by acting as exogenous mimics of microbial cell wall and membrane fatty acid derivatives, have been suggested to interfere with the cell membrane and wall biochemistry thus inducing an antimicrobial activity [93, 94, 95]. Damage to cell envelope leads to perturbation of ion homeostasis across the membrane, defective cell to cell adhesion, disturbed substrate attachment, and consequent inability to form biofilm, which is essential for tubercular pathogenicity [14]. However, it is necessary to isolate individual pure compounds and test their activity individually and/or in combination. Taken together, we show that A. pluriseta extract solvent fractions have robust selective antitubercular activity, and hence we provide a scientific rationalization and justification of the possible therapeutic use of A. pluriseta.
5. Conclusion
The findings from this work demonstrate that A. pluriseta root extract fractions have robust selective antitubercular activity. The extract fractions, especially the ethyl acetate and methanolic fraction, provide a potential source of novel, antitubercular lead candidate(s). GC-MS analysis revealed an abundance of fatty acid esters, which we strongly associated with demonstrated antitubercular activity. Further work is required to isolate pure compounds, test their specific molecular targets, with a view of deciphering the mode(s) of action.
Declarations
Author contribution statement
S.N. Njeru and J.M. Muema: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This study was supported by the International Foundation for Science, Stockholm, Sweden, through IFS grant No. F/5372-1 to Dr. Sospeter Ngoci, Njeru.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would wish to acknowledge KEMRI- especially Dr. Bii C., among others, Kisii University (Prof. Anakalo S.) and Egerton University, (Prof. Ngari S.M., Dr. Obonyo A.M, Prof. Matasyoh J.C., and Mr. Nyambati S.O.) for the support they gave to the work.
References
- 1.Njeru S.N., Obonyo M.A., Nyambati S.O., Ngari S.M. Bioactivity of Cissampelos pareira medicinal plant against Mycobacterium tuberculosis. J. Pharmacogn. Phytochem. 2015;3(6) [Google Scholar]
- 2.Scriba T.J., Mizrahi V. Renewing the fight against TB with an old vaccine. Cell. 2020;180(5):829–831. doi: 10.1016/j.cell.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Stelitano G., Sammartino C. 2020. Multitargeting Compounds : A Promising Strategy to Overcome Multi-Drug Resistant Tuberculosis; pp. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Njeru S.N., Obonyo M.A. Potency of extracts of selected plant species from Mbeere, Embu County-Kenya against Mycobacterium tuberculosis. J. Med. Plants Res. 2016;10(12):149–157. [Google Scholar]
- 5.Wang Q., Boshoff H.I.M., Harrison J.R., Ray P.C., Green S.R., Wyatt P.G., Barry C.E. PE/PPE proteins mediate nutrient transport across the outer membrane of Mycobacterium tuberculosis. Science. 2020;367(6482):1147–1151. doi: 10.1126/science.aav5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . World Health Organization; Geneva: 2019. Global Tuberculosis Report 2019; pp. 1–284.https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf. 2019 Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 7.Gupta V.K., Kaushik A., Chauhan D.S., Ahirwar R.K., Sharma S., Bisht D. Anti-mycobacterial activity of some medicinal plants used traditionally by tribes from Madhya Pradesh, India for treating tuberculosis related symptoms. J. Ethnopharmacol. 2018;227:113–120. doi: 10.1016/j.jep.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 8.van den Boogaard J., Slump E., Schimmel H.J., van der Hoek W., van den Hof S., de Vries G. High incidence of active tuberculosis in asylum seekers from Eritrea and Somalia in the first 5 years after arrival in The Netherlands. Emerg. Infect. Dis. 2020;26(4):675–681. doi: 10.3201/eid2604.190123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams R.J. Globalization of antimicrobial resistance: epidemiological challenges. Clin. Infect. Dis. : Off. Publ. Infect. Dis. Soc. Am. 2001;33(Suppl 3):S116–S117. doi: 10.1086/321835. [DOI] [PubMed] [Google Scholar]
- 10.Levy S.B., Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 11.Reid M.J.A., Goosby E. Improving quality is necessary to building a TB-free world: lancet Commission on Tuberculosis. J. Clin. Tuber. Other Mycobact. Dis. 2020;19:100156. doi: 10.1016/j.jctube.2020.100156. 100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bibi Y., Nisa S., Chaudhary F.M., Zia M. Antibacterial activity of some selected medicinal plants of Pakistan. BMC Compl. Alternative Med. 2011;11(1):52. doi: 10.1186/1472-6882-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Njeru S.N., Obonyo M., Nyambati S., Ngari S., Mwakubambanya R., Mavura H. Antimicrobial and cytotoxicity properties of the organic solvent fractions of Clerodendrum myricoides (Hochst.) R. Br. ex Vatke: Kenyan traditional medicinal plant. J. Intercult. Ethnopharmacol. 2016;5(3) doi: 10.5455/jice.20160416122003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherjee K., Tribedi P., Mukhopadhyay B., Sil A.K. Antibacterial activity of long-chain fatty alcohols against mycobacteria. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2013;338(2):177–183. doi: 10.1111/1574-6968.12043. [DOI] [PubMed] [Google Scholar]
- 15.Lewis K., Ausubel F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006;24(12):1504–1507. doi: 10.1038/nbt1206-1504. [DOI] [PubMed] [Google Scholar]
- 16.Abreu A.C., McBain A.J., Simoes M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012;29(9):1007–1021. doi: 10.1039/c2np20035j. [DOI] [PubMed] [Google Scholar]
- 17.Alian A.D., Nyegue M.A., Djova S.V., Etoa F.-X. Genotoxicity of Dissotis Multiflora (Sm) Triana (Melastomataceae) and Paullinia Pinnata Linn (Sapindaceae) J. Trop. Med. 2020:2020. doi: 10.1155/2020/5169847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mir M.A., Bashir N., Alfaify A., Oteef M.D.Y. GC-MS analysis of Myrtus communis extract and its antibacterial activity against Gram- positive bacteria. BMC Complement. Med. Ther. 2020;3:1–9. doi: 10.1186/s12906-020-2863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med. 2010:1479–1491. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- 20.Batiha G.E.-s., Beshbishy A.M., Alkazmi L., Adeyemi O.S., Nadwa E., Rashwan E., El-mleeh A., Igarashi I. Gas chromatography-mass spectrometry analysis , phytochemical screening and antiprotozoal effects of the methanolic Viola tricolor and acetonic Laurus nobilis extracts. BMC Compl. Med. Ther. 2020;2:1–14. doi: 10.1186/s12906-020-2848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cragg G.M., Newman D.J. Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta Gen. Subj. 2013;1830(6):3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed N., Mahmood A., Ashraf A., Bano A., Tahir S.S., Mahmood A. Ethnopharmacological relevance of indigenous medicinal plants from district Bahawalnagar, Punjab, Pakistan. J. Ethnopharmacol. 2015;175:109–123. doi: 10.1016/j.jep.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Ashraf A., Sarfraz R.A., Mahmood A., Din M.u. Chemical composition and in vitro antioxidant and antitumor activities of Eucalyptus camaldulensis Dehn. leaves. Ind. Crop. Prod. 2015;74:241–248. [Google Scholar]
- 24.Ashraf A., Sarfraz R.A., Rashid M.A., Mahmood A., Shahid M., Noor N. Chemical composition, antioxidant, antitumor, anticancer and cytotoxic effects of Psidium guajava leaf extracts. Pharmaceut. Biol. 2016;209(February):1–11. doi: 10.3109/13880209.2015.1137604. [DOI] [PubMed] [Google Scholar]
- 25.Beshbishy A.M., Batiha G.E.-s., Adeyemi O.S., Igarashi I. Inhibitory effects of methanolic Olea europaea and acetonic Acacia laeta on growth of Babesia and Theileria. Asian Pac. J. Trop. Med. 2019;12(9):425–434. [Google Scholar]
- 26.Kamatenesi-Mugisha M., Oryem-Origa H., Odyek O., Makawiti D.W. Medicinal plants used in the treatment of fungal and bacterial infections in and around Queen Elizabeth Biosphere Reserve, western Uganda. Afr. J. Ecol. 2008;46:90–97. [Google Scholar]
- 27.Ekor M. The growing use of herbal medicines : issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4(January):1–10. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qureshi R., Ghazanfar S.A., Obied H., Vasileva V., Tariq M.A. Ethnobotany: a living science for alleviating human suffering. Evid. base Compl. Alternative Med. 2016;2016:10–13. doi: 10.1155/2016/9641692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tugume P., Nyakoojo C. Ethno-pharmacological survey of herbal remedies used in the treatment of paediatric diseases in Buhunga parish, Rukungiri District, Uganda. BMC Compl. Alternative Med. 2019;19(1):1–10. doi: 10.1186/s12906-019-2763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makhafola T.J., McGaw L.J., Eloff J.N. In vitro cytotoxicity and genotoxicity of five Ochna species (Ochnaceae) with excellent antibacterial activity. South Afr. J. Bot. 2014;91:9–13. [Google Scholar]
- 31.Zirihi G.N., Mambu L., Bodo B., Grellier P. In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J. Ethnopharmacol. 2005;98:281–285. doi: 10.1016/j.jep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Stefanović O., Radojević I., Vasić S., Čomić L. The American Phytopathological Society; 2005. Antibacterial Activity of Naturally Occurring Compounds from Selected Plants; pp. 1–24. [Google Scholar]
- 33.Abdallah E.M. Plants: an alternative source for antimicrobials. J. Appl. Pharmaceut. Sci. 2011;1(6):16–20. [Google Scholar]
- 34.Khameneh B., Iranshahy M., Soheili V., Fazly Bazzaz B.S. Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob. Resist. Infect. Contr. 2019;8(1) doi: 10.1186/s13756-019-0559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vijayarathna S., Sasidharan S. Cytotoxicity of methanol extracts of Elaeis guineensis on MCF-7 and Vero cell lines. Asian Pac. J. Trop. Biomed. 2012;2(10):826–829. doi: 10.1016/S2221-1691(12)60237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Njeru S.N., Obonyo M.A., Nyambati S.O., Ngari S.M. Antimicrobial and cytotoxicity properties of the crude extracts and fractions of Premna resinosa (Hochst.) Schauer (Compositae): Kenyan traditional medicinal plant. BMC Compl. Alternative Med. 2015;15(January):295. doi: 10.1186/s12906-015-0811-4. 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sibanda T., Okoh A.I. The challenges of overcoming antibiotic resistance: plant extracts as potential sources of antimicrobial and resistance modifying agents. Afr. J. Biotechnol. 2007;6(25) [Google Scholar]
- 38.Cordell G.A., Colvard M.D. Natural products and traditional medicine: turning on a paradigm. J. Nat. Prod. 2012;75(3):514–525. doi: 10.1021/np200803m. [DOI] [PubMed] [Google Scholar]
- 39.Mongalo N.I., McGaw L.J., Finnie J.F., Van Staden J. Pharmacological properties of extracts from six South African medicinal plants used to treat sexually transmitted infections (STIs) and related infections. South Afr. J. Bot. 2017 [Google Scholar]
- 40.Yaouba S., Valkonen A., Coghi P., Gao J., Guantai E.M., Derese S., Wong V.K.W., Erdélyi M., Yenesew A. Crystal structures and cytotoxicity of ent-kaurane-type diterpenoids from two Aspilia species. Molecules. 2018;23(12):1–13. doi: 10.3390/molecules23123199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gachathi F.N. Kikuyu botanical dictionary: a guide to plant names, uses and cultural values. Trop. Bot. 2007 [Google Scholar]
- 42.Cos P., Hermans N., De Bruyne T., Apers S., Sindambiwe J.B., Witvrouw M., De Clercq E., Berghe D.V., Pieters L., Vlietinck A.J. Antiviral activity of Rwandan medicinal plants against human immunodeficiency virus type-1 (HIV-1) Phytomedicine. 2002;9(1):62–68. doi: 10.1078/0944-7113-00083. [DOI] [PubMed] [Google Scholar]
- 43.Njoroge G.N., Bussmann R.W. Traditional management of ear, nose and throat (ENT) diseases in Central Kenya. J. Ethnobiol. Ethnomed. 2006;2(1):1. doi: 10.1186/1746-4269-2-54. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Njoroge G.N., Bussmann R.W. Ethnotherapeautic management of skin diseases among the Kikuyus of Central Kenya. J. Ethnopharmacol. 2007;111(2):303–307. doi: 10.1016/j.jep.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 45.Piero N.M., Joan M.N., Cromwell K.M., Joseph N.J., Wilson N.M., Daniel M., Peter G.K., Eliud N.N. Hypoglycemic activity of some kenyan plants traditionally used to manage diabetes mellitus in Eastern Province. J. Diabetes Metabol. 2011;2(8) [Google Scholar]
- 46.Kuria J.M., Mbaria J.M., Gathumbi P.K., Kiama S.G. Influence of Aspilia pluriseta Schweinf ( Asteraceae ) on the healing of dermal excision wounds ( mouse model ) and skin sensitization activity ( Guinea pig model ) Afr. J. Pharmcol. Ther. 2015;4(3):112–117. [Google Scholar]
- 47.Sebisubi F.M., Odyek O., Anokbonggo W.W., Ogwal-Okeng J., Carcache-Blanco E.J., Ma C., Orjala J., Tan G.T. Antimalarial activity of Aspilia pruliseta, a medicinal plant from Uganda. Planta Med. 2010;76(16):1870–1873. doi: 10.1055/s-0030-1250028. [DOI] [PubMed] [Google Scholar]
- 48.Mwonga K.B., Mwaniki E., Dorcas Y.S., Piero N.M. Molluscicidal effects of aqueous extracts of selected medicinal plants. Pharm. Anal. Acta. 2015;6(11) [Google Scholar]
- 49.Cos P., Hermans N., Van Poel B., De Bruyne T., Apers S., Sindambiwe J.B., Vanden Berghe D., Pieters L., Vlietinck A.J. Complement modulating activity of Rwandan medicinal plants. Phytomedicine. 2002;9(1):56–61. doi: 10.1078/0944-7113-00085. [DOI] [PubMed] [Google Scholar]
- 50.Njonge F.K., Mutugi M., Kareru P.G., Githigia S.M., Waihenya R., Nyakundi W.O. Assessment of herbal anthelmintics used by the farmers in Kirinyaga County, Kenya, for the treatment of helminthiosis in cattle. Arf. J. Pharm. Pharmcol. 2013;7(29):2100–2104. [Google Scholar]
- 51.Munster W.B., Dusseldorf R.B., Franz G., Zurich O.S., Herz W., Zurich M.H., Lausanne K.H., Gif-sur-yvette P.P., Tyler Y.E., Lafayette W., Amsterdam P.a.V.Z., Cantreil C.L., Franzblau S.G., Fischer N.H. Antimycobacterial plant terpenoids. Planta Med. 2001;67:685–694. doi: 10.1055/s-2001-18365. [DOI] [PubMed] [Google Scholar]
- 52.Riley B.W., Brokensha D. University press of America; USA: 1988. The Mbeere in Kenya; Botanical Identity and Use Vol (Ii) [Google Scholar]
- 53.Mariita R., Ogol C., Oguge N., Okemo P. Antitubercular and phytochemical investigation of methanol extracts of medicinal plants used by the Samburu community in Kenya. Trop. J. Pharmaceut. Res. 2010;9(4) [Google Scholar]
- 54.Gupta V.K., Shukla C., Bisht G.R.S., Saikia D., Kumar S., Thakur R.L. Detection of anti-tuberculosis activity in some folklore plants by radiometric BACTEC assay. Lett. Appl. Microbiol. 2011;52(1):33–40. doi: 10.1111/j.1472-765X.2010.02963.x. [DOI] [PubMed] [Google Scholar]
- 55.Gallo J.F., Pinhata J.M.W., Saraceni C.P., de Oliveira R.S. Evaluation of the BACTEC MGIT 960 system and the resazurin microtiter assay for susceptibility testing of Mycobacterium tuberculosis to second-line drugs. J. Microbiol. Methods. 2017;139(May):168–171. doi: 10.1016/j.mimet.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 56.Adami A.G., Gallo J.F., Pinhata J.M.W., Martins M.C., Giampaglia C.M.S., de Oliveira R.S. Modified protocol for drug susceptibility testing of MGIT cultures of Mycobacterium tuberculosis by the MGIT 960. Diagn. Microbiol. Infect. Dis. 2017;87(2):108–111. doi: 10.1016/j.diagmicrobio.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 57.Yalcin D., Türk K.H., Özer T. Evaluation of phytotherapeutic activities and phytochemical content of Phormidium autumnale Gomont from natural freshwater sources. Environ. Monit. Assess. 2020;192 doi: 10.1007/s10661-020-8207-4. [DOI] [PubMed] [Google Scholar]
- 58.Wayne P.A. Vol. 17. Performance standards for antimicrobial susceptibility testing; 2007. (Clinical and Laboratory Standards institute). [Google Scholar]
- 59.Mbaveng A.T., Ngameni B., Kuete V., Simo I.K., Ambassa P., Roy R., Bezabih M., Etoa F.-X., Ngadjui B.T., Abegaz B.M. Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae) J. Ethnopharmacol. 2008;116(3):483–489. doi: 10.1016/j.jep.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 60.Naeim H., El-hawiet A., Rahman R.A.A., Hussein A., Demellawy M.A.E., Embaby A.M. Antibacterial activity of Centaurea pumilio L. root and aerial part extracts against some multidrug resistant bacteria. BMC Complement. Med. Ther. 2020:1–13. doi: 10.1186/s12906-020-2876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zawawi N.Z.M., Shaari R., Nordin M.L., Hamdan R.H., Peng T.L., Zalati C.W.S.C.W. Antibacterial and cytotoxic activity assessment of Channa striatus (Haruan) extract. Veter. World. 2020;13:508–514. doi: 10.14202/vetworld.2020.508-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mothana R.A.A., Abdo S.A.A., Hasson S., Althawab F., Alaghbari S.A.Z., Lindequist U. Antimicrobial, antioxidant and cytotoxic activities and phytochemical screening of some yemeni medicinal plants. Evid. base Compl. Alternative Med. 2010;7(3):323–330. doi: 10.1093/ecam/nen004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Photolo M.M., Mavumengwana V., Sitole L., Tlou M.G. Antimicrobial and antioxidant properties of a bacterial endophyte, methylobacterium radiotolerans MAMP 4754, isolated from Combretum erythrophyllum seeds. Int. J. Microbiol. 2020:2020. doi: 10.1155/2020/9483670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lai H.Y., Lim Y.Y., Kim K.H. Blechnum orientale Linn-a fern with potential as antioxidant, anticancer and antibacterial agent. BMC Compl. Alternative Med. 2010;10(1):15. doi: 10.1186/1472-6882-10-15. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laulloo S.J., Bhowon M.G., Soyfoo S., Chua L.S. Nutritional and biological evaluation of leaves of Mangifera indica from Mauritius. J. Chem. 2018:2018. [Google Scholar]
- 66.Tomczykowa M., Tomczyk M., Jakoniuk P., Tryniszewska E. Antimicrobial and antifungal activities of the extracts and essential oils of Bidens tripartita. Folia Histochemica et cytobiologica. 2008;46(3):389–393. doi: 10.2478/v10042-008-0082-8. [DOI] [PubMed] [Google Scholar]
- 67.Wojcikowski K., Stevenson L., Leach D., Wohlmuth H., Gobe G. Antioxidant capacity of 55 medicinal herbs traditionally used to treat the urinary system : a comparison using a sequential three-solvent extraction process. J. Alternative Compl. Med. 2007;13(1):103–109. doi: 10.1089/acm.2006.6122. [DOI] [PubMed] [Google Scholar]
- 68.Ngoci S.N., Matasyoh J.C., Mwaniki C.G., Mwendia C.M. Antibacterial activity of methanol root extract of Indigofera lupatana Baker F. E. J. Med. 2012;17(1):11–16. [Google Scholar]
- 69.Iwatsuki M., Uchida R., Takakusagi Y., Matsumoto A., Jiang C.L., Takahashi Y., Arai M., Kobayashi S., Matsumoto M., Inokoshi J., Tomoda H., Omura S. Lariatins, novel anti-mycobacterial peptides with a lasso structure, produced by Rhodococcus jostii K01-B0171. J. Antibiot. (Tokyo) 2007;60(6):357–363. doi: 10.1038/ja.2007.48. [DOI] [PubMed] [Google Scholar]
- 70.Koyama N., Kojima S., Nonaka K., Masuma R., Matsumoto M., Ōmura S., Tomoda H. Calpinactam, a new anti-mycobacterial agent, produced by Mortierella alpina FKI-4905. J. Antibiot. 2010;63(4):183–186. doi: 10.1038/ja.2010.14. [DOI] [PubMed] [Google Scholar]
- 71.Davies J., Davies D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010;74(3):417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nathan C. Antibiotics at the crossroads. Nature. 2004;431(7011):899–902. doi: 10.1038/431899a. [DOI] [PubMed] [Google Scholar]
- 73.Ashraf A., Sarfraz R.A., Rashid M.A., Shahid M. Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an important medicinal plant (Euphorbia royleana) from Pakistan. J. Food Drug Anal. 2015;23(1):109–115. doi: 10.1016/j.jfda.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12(4):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santhosh R.S., Suriyanarayanan B. Plants : a source for new antimycobacterial drugs. Planta Med. 2014;80(1):9–21. doi: 10.1055/s-0033-1350978. [DOI] [PubMed] [Google Scholar]
- 76.Caesar L.K., Cech N.B. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat. Prod. Rep. 2019;36(6):869–888. doi: 10.1039/c9np00011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Junio H.A., Sy-Cordero A.A., Ettefagh K.A., Burns J.T., Micko K.T., Graf T.N., Richter S.J., Cannon R.E., Oberlies N.H., Cech N.B. Synergy-directed fractionation of botanical medicines: a case study with goldenseal (hydrastis canadensis) J. Nat. Prod. 2011;74(7):1621–1629. doi: 10.1021/np200336g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stermitz F.R., Scriven L.N., Tegos G., Lewis K. Two flavonols from Artemisa annua which potentiate the activity of berberine and norfloxacin against a resistant strain of Staphylococcus aureus. Planta Med. 2002;68(12):1140–1141. doi: 10.1055/s-2002-36347. [DOI] [PubMed] [Google Scholar]
- 79.Wagner H., Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16(2-3):97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 80.Ayob M.K., Tan Y.N. Formatex; 2013. Natural Products-Current and Future Promising Source of Novel Drugs : A Review on Their Antimicrobial Mechanism of Actions; pp. 1196–1208. [Google Scholar]
- 81.Caillet S., Côté J., Sylvain J.-F., Lacroix M. Antimicrobial effects of fractions from cranberry products on the growth of seven pathogenic bacteria. Food Contr. 2012;23(2):419–428. [Google Scholar]
- 82.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol. Aspect. Med. 2006;27(1):1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Kareru P.G., Nyabola A.O., Madivoli E.S., Wanakai S.I., Maina E.G. Formation of silver nanoparticles via Aspilia pluriseta extracts their antimicrobial and catalytic activity. J. Inorg. Organomet. Polym. Mater. 2020;30:3493–3501. [Google Scholar]
- 84.Nyabola A.O., Kareru P.G., Madivoli E.S., Maina E.G., Wanakai I.S. Assessment of the anti-microbial action of zero valent iron nanoparticle synthesized by Aspilia pluriseta extracts. Chem. Sci. Int. J. 2018;25(3):1–10. [Google Scholar]
- 85.Cantrell C.L., Franzblau S.G., Fischer N.H. Review; Antimycobacterial Plant Terpenoids. Planta Med. 2001;67:685–694. doi: 10.1055/s-2001-18365. [DOI] [PubMed] [Google Scholar]
- 86.Higuchi C.T., Pavan F.R., Leite C.Q.F., Sannomiya M., Vilegas W., Leite S.R.D.A., Sacramento L.V.S., Sato D.N. Triterpenes and antitubercular activity of Byrsonima crassa. Quim. Nova. 2008;31(7):1719–1721. [Google Scholar]
- 87.Sieniawska E., Swatko-Ossor M., Sawicki R., Skalicka-Woźniak K., Ginalska G. Natural terpenes influence the activity of antibiotics against isolated Mycobacterium tuberculosis. Med. Princ. Pract. 2017;26(2):108–112. doi: 10.1159/000454680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chandrasekaran M., Kannathasan K., Venkatesalu V. Antimicrobial activity of fatty acid methyl esters of some members of chenopodiaceae. Zeitschrift fur Naturforschung - Section C Journal of Biosciences. 2008;63(5-6):7–12. doi: 10.1515/znc-2008-5-604. [DOI] [PubMed] [Google Scholar]
- 89.Chandrasekaran M., Senthilkumar a., Venkatesalu V. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharmacol. Sci. 2011;15(7):775–780. [PubMed] [Google Scholar]
- 90.Huang C.B., Altimova Y., Myers T.M., Ebersole J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011;56(7):650–654. doi: 10.1016/j.archoralbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin Q., Wang M., Li J., Shi W., Wang H., Zhao C. Analysis of fatty acids, aliphatic esters, and in vitro studies of antioxidant and antimicrobial activities for recineckea carnea and tupistra chinensis from the guizhou province. J. Med. Food. 2013;17(2):236–243. doi: 10.1089/jmf.2013.2855. [DOI] [PubMed] [Google Scholar]
- 92.Morbidoni H.R., Vilchèze C., Kremer L., Bittman R., Sacchettini J.C., Jacobs W.R. Dual inhibition of mycobacterial fatty acid biosynthesis and degradation by 2-alkynoic acids. Chem. Biol. 2006;13(3):297–307. doi: 10.1016/j.chembiol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 93.Chen S.C.a., Biswas C., Bartley R., Widmer F., Pantarat N., Obando D., Djordjevic J.T., Ellis D.H., Jolliffe K.a., Sorrell T.C. In vitro antifungal activities of bis(alkylpyridinium)alkane compounds against pathogenic yeasts and molds. Antimicrob. Agents Chemother. 2010;54(8):3233–3240. doi: 10.1128/AAC.00231-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lunde C.S., Hartouni S.R., Janc J.W., Mammen M., Humphrey P.P., Benton B.M. Telavancin disrupts the functional integrity of the bacterial membrane through targeted interaction with the cell wall precursor lipid II. Antimicrob. Agents Chemother. 2009;53(8):3375–3383. doi: 10.1128/AAC.01710-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martins C.d.M., do Nascimento E.A., de Morais S.A.L., de Oliveira A., Chang R., Cunha L.C.S., Martins M.M., Martins C.H.G., Moraes T.d.S., Rodrigues P.V., da Silva C.V., de Aquino F.J.T. Chemical constituents and evaluation of antimicrobial and cytotoxic activities of kielmeyera coriacea mart. & zucc. Essential oils. Evid. base Compl. Alternative Med. : eCAM. 2015;2015:842047. doi: 10.1155/2015/842047. 842047. [DOI] [PMC free article] [PubMed] [Google Scholar]