Abstract
Edible insects are an important protein rich natural resource that can contribute to resilient food security. Edible insects not only play an important role in traditional diets, but are also an excellent source of protein in traditional dishes in Africa. We systematically searched Web-of-Science and Google Scholar from year 2000–2019 for studies on the consumption of insects and their nutritional composition in Africa, resulting in 98 eligible papers, listing 212 edible insect species from eight orders. These insects were rich in protein, fats, and fibre. The highest protein content was reported for Lepidoptera (range: 20–80%). Coleoptera had the highest carbohydrate content (7–54%), while Lepidoptera had the highest fat content (10–50%). Considering the excellent source of nutrition, and potential socio-economic benefits, from edible insects, they can contribute strongly to improved food security, and rural development in developing countries. In addition, edible insects can be used as a sustainable food source to combat food shortages in the future, for example, providing resilience during times of drought or other climate stressors.
Keywords: entomophagy, Africa, edible insects, nutrition, food security
1. Introduction
Consumption of insects has recently received more attention because of their promising potential for contributing to livelihoods and mitigating food security problems around the world [1,2,3]. Food security problems are caused by an enormous increase in the global human population, which is estimated to increase to approximately 9 billion people by 2050 [1], resulting in a 70% increase in food demand, and an increase in food prices [1,4,5]. The increase in food prices will prompt the search for cheap alternative sustainable protein sources [1]. Entomophagy, which refers to the consumption of insects by humans, is an environmentally friendly approach to increasing food for consumption, and contributing to food security across the world [2,5,6,7].
Edible insects might be a solution to food shortages, owing to their promising potential in contributing to livelihoods and mitigating food security problems around the world [1,2,3]. Insects are consumed as food in Thailand [8,9], China [10,11], Mexico [12,13,14,15], Latin America [16], Japan [17], and Africa [18]. According to van Huis [1], approximately 2 billion people worldwide regularly consume insects as part of their diets. The consumption of insects is not a new phenomenon, as it dates back to before the development of agriculture when humans relied on gathering plants and hunting wild animals [4,11,19].
Edible insects have played a very important traditional role in nutritious diets in various countries in Africa [18,20]. In addition, edible insects are an important natural resource that is used as a coping strategy, particularly in months of food shortage [21,22,23]. Unfavourable climatic conditions experienced in Africa affect small scale animal husbandry and reduce animal protein production, so diets are then supplemented with edible insect protein [22]. Edible insects provide significant socio-economic and ecological benefits for developing countries [24,25]. Approximately 500 species of edible insects are consumed in Africa and form part of traditional diets [18]. Of these 500 species, 256 species were consumed in the Central African region, 164 in southern Africa, 100 species in eastern Africa, 91 in western Africa, and only eight species in northern Africa [18]. Insects are consumed among different African cultures because of their taste, cultural importance, and nutritive value, and as a supplementary food when staple food is limited [1,3,25,26,27].
Various studies in Africa have focused on studying the nutritional content of a single species, group, or genus [28,29,30,31,32]. Little is known about the diversity and nutritional content of various insects consumed in Africa. Therefore, the current study will review the existing literature on the diversity of insects, and their nutritional status in Africa, and, therefore, compile information on the nutrient composition of edible insects consumed in Africa. This will be done by asking the following questions: (1) What is the nutritional value of edible insects consumed in Africa, (2) what are the most consumed, and (3) the most studied insect species, in terms of nutrition, in Africa?
2. Materials and Methods
2.1. Search Strategy
To explore the diversity and nutritional status of edible insects in Africa, we followed the PRISMA guidelines for a systematic review. Peer-reviewed literature was obtained using the Thomson Reuters’ Web of Science database (https://apps.webofknowledge.com) and google scholar (https://scholar.google.co.za/) looking for publications that researched entomophagy in Africa, edible insects, diversity, nutrient content of edible insects, and consumption of insects. To source information, the following key words and phrases were used, “entomophagy”, “edible insects”, “diversity of edible insects”, “entomophagy in Africa”, “edible insects in eastern Africa”, “edible insects in north Africa”, “edible insects in western Africa”, “edible insects in Central Africa”, “edible insects in southern Africa” and “nutrient content of edible insects”. We also screened references included in selected articles in order to identify studies that might be relevant but did not appear in our search. We limited the search to literature published from 2000 to 2019. We started in the year 2000 because it was a starting point where most researchers began investigating the use of edible insects as a food source and as a solution to combat food insecurity problems [33,34].
2.2. Data Collection
Data from the selected articles were independently screened and extracted by a single author (Z.T.H). The search result was done by reading the title and abstract of the retrieved papers to determine if the article was relevant to the study. Once it was determined that the article was relevant, the full text of the selected articles was further analysed to extract relevant information. The information that was collected and extracted after full text reading from each article included year, study area and country, study insect species, reported nutrient composition of insects, consumption stage of an insect, main research findings, and conclusions. Collected articles were categorised by country and insect order.
2.3. Inclusion and Exclusion Criteria
2.3.1. Inclusion Criteria
Original research articles and review papers focusing on entomophagy, nutrient composition of single or multiple edible insect species.
Articles published in English.
Articles of work done in African countries.
Articles that reported nutrient composition of edible insects.
2.3.2. Exclusion Criteria
Conference papers, editorial material, book chapters
Articles on insect rearing and farming.
2.4. Data Quality
To evaluate the quality of studies included in this systematic review, we assessed quality based on the following criteria: (1) A clear food description (scientific name(s) of insects studied or genus), (2) a clear description on the part of the insects used for analysis, e.g., whole, head, abdomen, indication of geographic origin of the insects, and the country where it is used as food in Africa, (3) analytical method used, number of analytical samples, (4) clear indication of whether the nutritional composition was based on the dry weight. Studies were included if they meet all the above criteria.
2.5. Data Analysis
The methods and data sources used in the included studies were highly heterogeneous and a statistical meta-analysis was not possible. Instead, a more narrative synthesis approach was used, and data from each study were tabulated. We synthesised the results according to study species and mean values of all insect species belonging to the same insect order were calculated and represented in bold, the nutritional composition of consumed species were presented in the table, most consumed species in different countries were presented graphically.
3. Results and Discussion
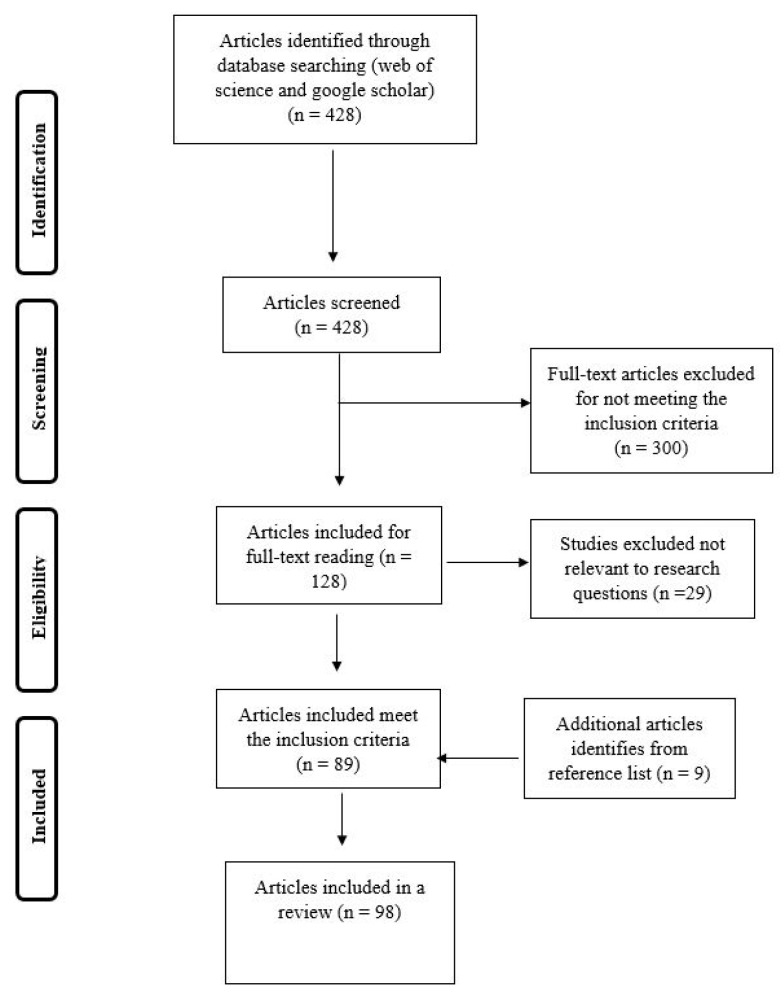
A total of 428 papers were identified for potential inclusion; after checking the title and abstract, 300 articles were excluded because they did not meet the inclusion criteria. From here, 128 articles were selected for full-text reading; from these, 29 articles were further excluded because they were not relevant or not conducted in Africa. After reading the full-text, 89 studies met all inclusion criteria, and a further nine articles were identified through screening references and confirming inclusion criteria were met. In total 98 articles were included in a systematic review (Figure 1).
Figure 1.
Flow chart of the study selection process for systematic review of the nutritional composition of edible insects.
3.1. Consumption of Insect Patterns in Africa
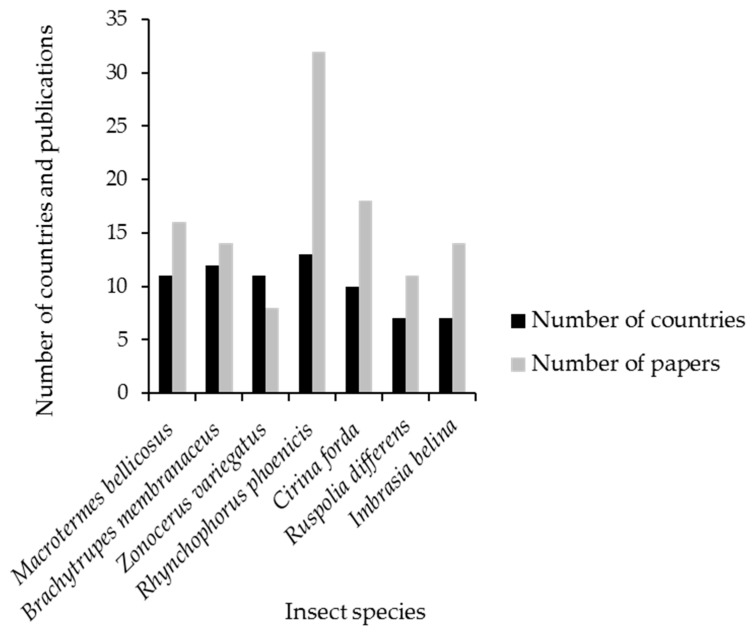
For the research articles published since 2000, a total of 212 edible insect species from nine orders were recorded and are potentially consumed in different African countries (Appendix A). Of these, 41% were Lepidoptera, 23% Orthoptera, 15% Coleoptera, 12% Blattodea (including both cockroaches and termites as recently classified), 4% Hemiptera, and Hymenoptera, Diptera, Blattodea, and Mantodea each contributed <1%. Rhynchophorus phoenicis (African palm weevil) and Cirina forda (Pallid emperor moth) were the most studied species in Africa, with 32 publications from 12 countries, and 18 publications from 10 countries, respectively (Figure 2). Most research has been done in the western African countries, particularly in Nigeria, mainly on Rhynchophorus phoenicis and Cirina forda, which are the most consumed species in West Africa. However, southern African countries (Zimbabwe, South Africa, and Bostwana) have the highest number of consumed species, but little research has been done on nutritional content and consumption patterns of edible insects.
Figure 2.
The number of countries with journal peer-reviewed articles published on the most consumed and economically important insects in Africa.
3.2. Nutrient Composition of Edible Insects
A compilation of nutrient composition of 54 edible insects based on the dry matter is presented in Table 1. Percentage of fat, protein, moisture, and ash content were calculated based on dry weight of the insect when ready for preparation to eat, noting that, in some cases, the insects had been processed since collecting. The highest protein was reported in Lepidoptera (range: 12–79%) and Orthoptera (12–73%), while the lowest protein content ranging from (0–39%) was reported for Blattodea.
Table 1.
Nutritional composition of edible insects, based on dry matter, from six orders consumed by people in Africa.
| Scientific Name | Stage of Consumption | Protein (%) | Crude Fibre (%) | Moisture (%) | Ash (%) | Carb (%) | Vitamin A (mg/100 g) | Vitamin B2 (mg/100 g) | Vitamin C (mg/100 g) | Fe (mg/100 g) | Ca (mg/100 g) | Zn (mg/100 g) | P (mg/100 g) | Mg (mg/100 g) | Fats (mg/100 g) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blattodea (termites and cockroaches) | 33.2 ± 14.5 | 4.7 ± 3.9 | 2.9 ± 0.1 | 5.2 ± 2.5 | 23.2 ± 0 | 2.7 ± 0.2 | 1.8 ± 0.2 | 3.2 ± 0.2 | 86 ± 96.8 | 54.1 ± 42.6 | 13.8 ± 3.5 | 125 ± 11 | 0.2 ± 0.1 | 22.2 ± 9.8 | ||
| Periplaneta Americana | Adult | 39.6 | 13.1 | 6.2 | [35] | |||||||||||
| Macrotermes nigeriensis | Adult | 35.9 | 5.5 | 5.8 | [35] | |||||||||||
| Macrotermes bellicosus | Adult | 20.4 | 2.7 | 2.8 | 11.3 | 23.2 | 2.9 | 2.0 | 3.4 | 27.0 | 21.0 | 136.0 | 0.2 | 36.1 | [36] | |
| Macrotermes natalensis | Adult | 22.1 | 2.2 | 3.0 | 4.1 | 2.6 | 1.5 | 3.0 | 29.0 | 18.0 | 114.0 | 0.3 | 21.4 | [36] | ||
| Pseudacathotermes spinige | Adult | 6.8 | 332.0 | 84.7 | 11.9 | [37] | ||||||||||
| Macrotermes spp. | Adult | 2.4 | 93.9 | 83.7 | 8.1 | [37] | ||||||||||
| Macrotermes herus | Adult | 6.8 | 161.0 | 132.0 | 14.3 | [37] | ||||||||||
| Macrotermes bellicosus | Adult | 40.7 | 5.7 | 42.7 | 16.9 | 8.4 | [38] | |||||||||
| Macrotermes bellicosus | Adult | 20.4 | 2.7 | 2.8 | 2.9 | 2.9 | 2.0 | 3.4 | 27.0 | 21.0 | 136.0 | 0.2 | [36] | |||
| Syntermes soldiers | Adult | 64.7 | 4.2 | 32.5 | 17.6 | 23.0 | [38] | |||||||||
| Macrotermes natalensis | Adult | 22.1 | 2.2 | 3.0 | 1.9 | 2.6 | 1.5 | 3.0 | 29.0 | 18.0 | 114.0 | 0.3 | [36] | |||
| Coleoptera (beetles) | 32.8 ± 11.5 | 6.2 ± 7.8 | 7.6 ± 15.7 | 4.7 ± 2.7 | 22.6 ± 13.2 | 11.2 ± 1.4 | 1.9 ± 0.9 | 5.4 ± 1.2 | 14.1 ± 8.9 | 43.6 ± 14.3 | 14.4 ± 12.1 | 109.6 ± 48.5 | 10.1 ± 4.2 | 29.1 ± 16.6 | ||
| Analeptes trifasciata | Larvae | 20.1 | 2.0 | 2.2 | 5.1 | 12.5 | 2.6 | 5.4 | 18.2 | 61.2 | 136.4 | 18.2 | [36] | |||
| Oryctes boas | Larvae | 26.0 | 1.5 | 1.9 | 1.5 | 2.3 | [6,36] | |||||||||
| Oryctes monoceros | Larvae | 26.4 | 4.7 | 7.8 | 51.6 | [39] | ||||||||||
| Aphodius rufipes | Larvae | 22.4 | 28.1 | 3.3 | 2.7 | 13.1 | 30.9 | 42.2 | 11.7 | 30.5 | [36] | |||||
| Rhynchophorus phoenicis | Larvae | 28.4 | 2.8 | 2.7 | 2.7 | 11.3 | 2.2 | 4.3 | 12.2 | 39.6 | 26.5 | 126.4 | 7.5 | 66.6 | [6] | |
| Oryctes rhinoceros | Larvae | 50.5 | 4.5 | 38.1 | [6] | |||||||||||
| Oryctes owariensis | Larvae | 50.6 | 8.4 | 7.7 | 14.3 | 18.9 | [40] | |||||||||
| Eulopida mashona | Larvae | 46.3 | 14.8 | 10.9 | 16.2 | 11.8 | [41] | |||||||||
| Heteroligus meles | Larvae | 38.1 | 3.0 | 1.0 | 5.8 | 20.1 | 32.0 | [42] | ||||||||
| Rhynchophorus phoenicis | Larvae | 50.0 | 2.6 | 1.2 | 4.9 | 20.2 | 21.1 | [42] | ||||||||
| Rhynchophorus phoenicis | Larvae | 28.4 | 2.8 | 2.7 | 2.7 | 11.3 | 2.2 | 4.3 | 12.2 | 39.6 | 126.4 | 7.5 | [36] | |||
| Analeptes trifasciata | Larvae | 29.6 | 2.0 | 2.2 | 4.2 | 12.5 | 2.6 | 5.4 | 18.2 | 61.3 | 136.4 | 6.1 | [36] | |||
| Oryctes boas | Larvae | 26.0 | 3.4 | 1.9 | 1.5 | 8.6 | 0.1 | 7.6 | 2.3 | 45.7 | 130.2 | 6.3 | [36] | |||
| Apomecyna parumpunctata | Larvae | 16.8 | 5.4 | 59.4 | 3.0 | 15.7 | 1.5 | 13.5 | 13.9 | [43] | ||||||
| Hemiptera (bugs) | 39.3 ± 4.0 | 5.3 ± 0 | 4.9 ± 0 | 1.7 ± 0 | 6.3 ± 1.3 | 0.2 ± 0 | 0.9 ± 0 | 20.2 ± 0 | 91.0 ± 0 | 46.0 ± 0 | 57 ± 0 | 109 ± 0 | ||||
| Encosternum delegorguei | 43.3 | 5.3 | 4.9 | 1.7 | 5.0 | 0.2 | 0.9 | 20.2 | 91.0 | 575.0 | 109.0 | 45.0 | [6] | |||
| Encosternum delegorguei | 35.2 | 4.9 | 1.7 | 7.6 | 20.2 | 91.0 | 46.0 | 109.0 | [28] | |||||||
| Hymenoptera (bees and ants) | 33.9 ± 9.2 | 7.7 ± 4.6 | 3.9 ± 0.1 | 4.1 ± 3.2 | 12.4 ± 0 | 3.2 ± 0 | 10.3 ± 0 | 17.8 ± 6.6 | 21.6 ± 6.3 | 7.5 ± 2.5 | 115.6 ± 9.6 | 7.8 ± 2.6 | 42.9 ± 4.7 | |||
| Apis mellifera | Adult | 21.0 | 2.0 | 3.8 | 2.2 | 12.4 | 3.2 | 10.3 | 25.2 | 15.4 | 125.5 | 5.2 | [6,36] | |||
| Carebara vidua | Adult | 42.5 | 9.1 | 8,6 | 10.4 | 22.3 | 5.7 | 106.0 | 10.4 | 38.2 | [6] | |||||
| Componotus spp. | Adult | 40.1 | 14.1 | 9.6 | [35] | |||||||||||
| Oecophylla longinoda | Adult | 37.8 | 12.3 | 7.3 | [35] | |||||||||||
| Crematogaster mimosa | Adult | 1.7 | 17.7 | 32.6 | 11.1 | [37] | ||||||||||
| Carebara vidua Smith | Adult | 40.8 | 6.9 | 3.9 | 1.6 | 10.7 | 22.2 | 5.7 | 106.0 | 10.4 | 47.5 | [44] | ||||
| Apis mellifera | Adult | 21.0 | 2.0 | 3.8 | 2.2 | 12.4 | 3.2 | 10.3 | 25.2 | 15.4 | 125.0 | 5.2 | [36] | |||
| Lepidoptera (caterpillars) | 46.3 ± 21.7 | 5.9 ± 5.4 | 29.3 ± 36.5 | 4.6 ± 2.2 | 18.0 ± 13.0 | 3.1 ± 0.2 | 1.7 ± 0.6 | 2.8 ± 1.0 | 15.4 ± 22.2 | 9.4 ± 2.3 | 10.6 ± 2.2 | 320.7 ± 367.9 | 18.9 ± 45.5 | 18.3 ± 14.8 | ||
| Anaphe venata | Larvae | 60.0 | 3.2 | 3.3 | 3.1 | 1.3 | 2.2 | 2.0 | 8.6 | 100.5 | 1.6 | [6] | ||||
| Anaphe infracta | Larvae | 20.0 | 2.4 | 2.7 | 3.0 | 2.0 | 4.5 | 1.8 | 8.6 | 113.3 | 1.0 | [6,36] | ||||
| Anaphe recticulata | Larvae | 23.0 | 3.1 | 3.2 | 3.4 | 2.0 | 2.2 | 2.2 | 10.5 | 102.4 | 2.6 | [6,36] | ||||
| Cirina forda | Larvae | 20.2 | 1.8 | 4.4 | 3.0 | 2.2 | 2.0 | 64.0 | 15.4 | 8.6 | 110.0 | 1.9 | [6,36] | |||
| Imbrasia epimethea | Larvae | 73.1 | 79.8 | 13.0 | 11.1 | 402.0 | 12.4 | [36] | ||||||||
| Imbrasia obscura | Larvae | 62.3 | 83.0 | 12.2 | [45] | |||||||||||
| Gonimbrasia (Nudaurelia) alopia | Larvae | 62.3 | 85.7 | 1.9 | [45] | |||||||||||
| Gonimbrasia (Nudaurelia) dione | Larvae | [45] | ||||||||||||||
| Pseudantheraea discrepans | Larvae | 48.9 | 72.2 | 21.3 | [45] | |||||||||||
| Anaphe panda | Larvae | 53.2 | 83.4 | 55.0 | [6,33] | |||||||||||
| Cirina butyrospermi | Larvae | 62.7 | 5.0 | 5.1 | 13.0 | [46] | ||||||||||
| Imbrasia belina | Larvae | 55.3 | 16.0 | 8.3 | 8.2 | 31.0 | 14.0 | 543.0 | 160.0 | [6,47] | ||||||
| Gynanisa maia | Larvae | 51.1 | 16.2 | 7.7 | 14.1 | 16.4 | [47] | |||||||||
| Loba leopardina | Larvae | 25.8 | 14.7 | 6.6 | 40.2 | 12.6 | [47] | |||||||||
| Imbrasia macrothyris | Larvae | 75.4 | [33] | |||||||||||||
| Nudaurelia macrothyrus | Larvae | 75.4 | [33] | |||||||||||||
| Gonimbrasia richelmanni | Larvae | 79.6 | [33] | |||||||||||||
| Cirina spp. | Larvae | 64.0 | 7.0 | 8.6 | 1090.0 | 32.4 | [48] | |||||||||
| Cirina butyrospermi | Larvae | 62.7 | 5.0 | 1160.0 | 14.3 | [46] | ||||||||||
| Hemijana variegata Rothschild, | Larvae | 8.3 | 5.9 | 5.2 | 9.5 | [49] | ||||||||||
| Anaphe infracta | Larvae | 20.0 | 2.4 | 2.7 | 1.6 | 3.0 | 2.0 | 4.5 | 1.8 | 8.6 | 111.3 | 1.0 | [36] | |||
| Anaphe recticulata | Larvae | 23.0 | 3.1 | 3.2 | 2.5 | 3.4 | 2.0 | 2.2 | 2.2 | 10.5 | 102.3 | 2.6 | [36] | |||
| Anaphe spp. | Larvae | 18.9 | 1.7 | 2.5 | 4.1 | 2.8 | 0.1 | 3.2 | 1.6 | 7.6 | 122.2 | 1.0 | [36] | |||
| Anaphe venata | Larvae | 25.7 | 2.3 | 3.3 | 3.2 | 3.1 | 1.3 | 2.2 | 2.0 | 8.6 | 100.5 | 1.6 | [36] | |||
| Orthoptera (grasshoppers, locust and crickets) | 39.8 ± 21.1 | 6.4 ± 4.8 | 3.5 ± 1.7 | 5.5 ± 4.0 | 26.8 ± 14.5 | 3.0 ± 3.5 | 0.2 ± 0.4 | 2.9 ± 4.0 | 120.1 ± 298.8 | 17.3 ± 15.8 | 91.1 ± 99.8 | 119.7 ± 12.7 | 2.8 ± 3.8 | 20.8 ± 18.9 | ||
| Brachytrupes membranaceus | Adult | 53.4 | 15.0 | 3.4 | 6.0 | 15.1 | 0.0 | 0.0 | 0.0 | 0.7 | 9.2 | 126.9 | 0.1 | 53.0 | [6,47] | |
| Cytacanthacris naeruginosus unicolor | Adult | 12.1 | 2.1 | 2.6 | 1.0 | 0.1 | 1.0 | 0.4 | 4.4 | 100.2 | 0.1 | [6,36] | ||||
| Zonocerus variegatus | Adult | 26.8 | 2.4 | 2.6 | 6.8 | 0.1 | 8.6 | 910.0 | 42.2 | 131.2 | 8.2 | [6,36] | ||||
| Gryllotalpa africana | Adult | 22.0 | 7.5 | 12.6 | 47.2 | 10.8 | [47] | |||||||||
| Henicus whellani | Adult | 53.6 | 10.6 | 14.0 | 4.3 | [50] | ||||||||||
| Cartarrtopsilus taeniolatus | Adult | 40.6 | 13.3 | 6.9 | [35] | |||||||||||
| Zulua cyanoptera | Adult | 33.7 | 13.3 | 6.6 | [51] | |||||||||||
| Ornithacris turbida | Adult | 42.7 | 2.0 | 4.5 | 18.2 | 2.0 | [47] | |||||||||
| Ruspolia differens | Adult | 72.7 | 6.3 | 4.6 | 1.2 | 0.1 | 13.0 | 24.5 | 12.4 | 121.0 | 33,1 | 46.2 | [6] | |||
| Anacridium melanorhodon melanorhodon (Walker) | Adult | 66.2 | 8.4 | 7.5 | 12.4 | [52] | ||||||||||
| Zonocerous variegatus | Adult | 62.7 | 3.6 | 1.2 | 8.9 | 0.1 | 9.8 | 2.0 | 29.0 | [6] | ||||||
| Brachytrypes membranaceus L | Adult | [53] | ||||||||||||||
| Zonocerous variegatus | Adult | 26.8 | 2.4 | 2.6 | 1.2 | 2.0 | 42.2 | 131.2 | 8.2 | [36] | ||||||
| Brachytrupes spp. | Adult | 65.4 | 4.9 | 33.6 | 232.0 | 16.9 | [38] | |||||||||
| Brachytrupes spp. | Adult | 6.3 | 1.0 | 3.4 | 1.8 | 0.0 | 0.0 | 0.0 | 0.7 | 9.2 | 126.9 | 0.1 | [36] | |||
| Cytacanthacris aeruginosus unicolor | Adult | 12.1 | 1.5 | 2.6 | 2.1 | 1.0 | 0.1 | 1.0 | 0.4 | 4.4 | 100.2 | 0.1 | [36] | |||
| * Recommended daily intakes (mg/day) for adults | 45.0 | 7.5–58.8 | 1300.0 | 3.0–14.0 | 700.0 | 220–260 | [37] |
Note the mineral abbreviations are Fe: Iron; Zn: Zinc; Ca: Calcium; P: Phosphorus; Mg: Magnesium. * Source [37]. Mean ± standard deviation of insects belonging to the same insect order are highlighted in bold and species names are in italics.
The crude fibre was reported to be higher in Coleoptera (2–28%) and Lepidoptera (2–16%), while the crude fibre content was reported to be lowest in Hemiptera (0–5%). Lepidoptera had the highest moisture content (3–86%), while Blattodea had the lowest moisture content (2.8–3%) (Table 1).
The highest carbohydrate content was recorded in Coleoptera (13–52%) and Orthoptera (15–47%), while the lowest carbohydrate content was recorded in Blattodea (0–32%). Fat content was the highest in Lepidoptera (2–55%) and lowest in Orthoptera (2–16%) (Table 1).
Orthoptera had the highest iron content (0.3–910 mg/100 g) followed by Blattodea (27–332 mg/100 g), while Hemiptera had the lowest iron content (0–20 mg/100 g). Calcium content was higher in Blattodea (18–132 mg/100 g) and lowest in Lepidoptera (8–15 mg/100 g). The highest Phosphorus was recorded in Lepidoptera (100–730 mg/100 g) and the lowest in Orthoptera (106–125 mg/100 g). Magnesium content was the highest in order Lepidoptera (1–160 mg/100 g), while Blattodea had the lowest magnesium content (0.1–0.3 mg/100 g) (Table 1).
Edible insects are widely consumed in Africa, and play an important role in nutritious diets. However, the preference and consumption of insects vary with species and orders. Lepidoptera caterpillars were the most consumed order, and they are the most preferred species because of their nutritional value, they are rich in protein, fats, and essential micronutrients [6,54]. In addition, several caterpillar species play an important role in income generation in rural areas in southern Africa, Uganda, and Nigeria [18,22,55].
Studies from western and Central Africa indicated that Rhynchophorus phoenic (palm weevil), and Cirina forda (pallid emperor moth) were the commonly consumed species [18,24,56]. The palm weevil and pallid emperor moth are a delicacy in western and Central Africa, and, in addition, these species were of economic importance in Nigeria, Cameroon, Benin, and Ghana [57]. In southern Africa, the literature indicates that the most consumed or preferred species were Imbrasia belina (mopane worm), Macrotermes natalensis, falciger, and bellicosus (termites) [28,50,58]. While in eastern Africa, the most consumed species were Ruspolia nitidula and differens (grasshoppers), [22,59,60,61]. Mopane worms, and termites are an important part of food culture in different ethnic groups in southern Africa [18,59]. Moreover, the trade of mopane worms and termites plays an important role in rural food security and income generation, as it provides rural people with household income [28,50,57,58].
Edible insects are a good source of protein content, which ranges from 12–79% of dry matter, which is consistent with studies from China, Germany, and Asia [6,10]. The protein content reported in edible insects is higher than protein found in chicken (43%) or beef (54%) [28,62]. The high protein content found in edible insects could help to combat protein deficiency in Africa. Protein deficiency is a major contributor to human malnutrition [63], and, in Africa, protein deficiency is the most common form of malnutrition, which needs to be addressed to halt starvation [64]. Therefore, including edible insects in daily diets might help reduce malnutrition rates.
Moisture content ranged from 1–7.5%, which is relatively low, such that most edible insects have longer preservation periods, and the risk of microbial deterioration and spoilage is minimal [29,42,65]. Unlike beef or chicken, which are prone to decay (unless refrigerated), edible insects can be stored for longer periods, especially during the dry season when food shortage is higher [42]. However, three caterpillars (Gonimbrasia (Nudaurelia) alopia, Anaphe panda, and Pseudontheraea discrepans) had higher moisture (>60%), meaning they are prone to spoilage and their preservation period is shorter unless processed in some manner. Siulapwa et al. [29] reported similar results, where caterpillars Imbrasia belina and Gynanisa maja had higher moisture content than other species. To increase shelf life, caterpillars are usually degutted, washed in boiling salt water, or roasted before drying in the sun, then packed in large sacks and containers [23,66].
Edible insects contain fat content ranging from 1–67%. The fat content of edible insects are higher in the larval stage. For example, a palm weevil, which is a beetle larva that is consumed as a delicacy in western Africa, contained the highest fat content of 67%. These results are consistent with Bukkens [67], who reported that Lepidopteran caterpillars and palm weevil larvae contain higher fat than any other insect species. Edible insects can be used to provide essential fatty acids required by the human body [10,68]. In addition, fat plays an important role in providing the human body with energy, which means that consuming insects such as Rhynchophorus phoenicis, Imbrasia belina, Anaphe panda, and Brachytrupes membranaceus, may help provide people with energy, thereby reducing malnutrition associated with energy deficiencies in developing countries [4,10,69].
Carbohydrates play a very important role in human nutrition as they are the primary source of energy. Carbohydrates found in edible insects varied from 5–51% [19,70]. Therefore, edible insects can be used as a source of carbohydrates, as they contain relatively high amounts of polysaccharides, which play an important role in enhancing the immune system of the human body [10]. In addition, carbohydrates are an essential nutritive element in the human body [29]. Species such as Oryctes monoceros and Gryllotalpa africana, reported in the current study, contained a high amount of carbohydrates; therefore, edible insects can be included in human diets to provide a good source of carbohydrates [29].
Excellent source of iron and zinc found in some edible insects indicate that edible insects could be used to combat malnutrition deficiencies such as zinc and iron deficiency anemia, which is prevalent in Africa [37]. Species such as Zonocerus variegatus, Pseudacathotermes spinige, and Macrotermes herus contained high iron content of 910, 332, and 161 mg/100 g respectively, which means that these species can be used as a good source of Iron. Zinc content was notably high in insects such as Zonocerous variegatus (29 mg/100 g) and Rhyncophorus phoenicis (26.5 mg/100 g) the Zinc content found in these insects exceed the daily recommended intake of 3.0–14 mg/100 g. Rumpold and Schluter [6] reported that Iron and Zinc content found in edible insects is generally higher than the Zinc and Iron content found in pork, beef, or chicken; therefore, edible insects might be a solution in fighting Iron and Zinc deficiency. Zinc and Iron deficiency are one of the health problems faced by many women of reproductive age and children in developing countries [37]. Therefore, consumption of edible insects might provide a solution to Iron deficiency health problems, such as anemia, reduced physical activity, and maternal mortality [37,71].
Edible insects reported in the current study contained a low amount of Vitamin A, B2, and C. The 100 g dry matter of edible insects reported in this study did not contain enough daily recommended Vitamin A (500–600 mg) or C (45 mg). As such, Chen et al. [10] reported that to meet the daily recommended amount of Vitamin C, insect tea derived from the excrement of insects is an option. This tea contains up to 15.04 mg of Vitamin C per 100 g, and the consumption of 300 mL of insect tea per day makes 45 mg of Vitamin C, which is the daily recommended amount of vitamin C for adults [10]. Contrary to findings reported in this study, Bukkens [67] reported that Vitamin B1, B2, and B3 content found in an edible house fly is richer than the Vitamin B1, B2, and B3 found in chicken, beef, or salmon. In addition, edible crickets contain twice more Vitamin B12 than the beef [69]. Igwe et al. [72] found that Microtermes nigeriensis contain a favourable high source of Niacin, Thiamine, Vitamin A, and C. Vitamins play an important role in human nutrition, as Vitamin C is important for human growth, development, and repair of various body tissues [73]. The excellent source of Vitamins found in some edible insects shows that insects have a great potential of being used as a healthy food supplement for malnourished people, or to prevent malnutrition [24].
There were several limitations to this review, which included studies reported in English only and excluded studies published in other languages used in Africa. There were significant gaps in data available on the nutritional composition of edible insects consumed in Africa. Most publications focused on a single macronutrient content, especially protein, carbohydrates, fats and fibre, and other nutrients, especially minerals, are not included in analyses. In addition, research focused on reporting the nutritional composition of economically important species such as Imbrasia belina, Macrotermes natalensis, bellicosus and falciger, Rhynchophorus phoenics, and Cirina forda. Strengths of this review incudes the robust approach to combine the nutritional composition of consumed insects in Africa, previous studies have focused on documenting the nutritional composition of single, or a group of, insects that are consumed in Africa.
This review reported combined nutritional data of consumed insects in Africa; this information can be useful to policy makers in the health and nutrition sector by including insects in food and nutrition policies. Health officials need to motivate people to include insects in their daily diets, particularly the most vulnerable groups such as elderly people, women, and children, with the aim to improve the quality of life for people. In addition, farming and rearing of insects by the agricultural sector need to be adopted to ensure that insects are easily accessible and available all year even when they are out of season in nature. Insects can be included as an ingredient in other food products such as bread, maize powder, chocolate, and biscuits to overcome discomfort and fear associated with eating whole insects in some groups of people. Future studies are required to research sustainable ways of farming and rearing insects in Africa and the implication that might have on the environment.
4. Conclusions
Meeting global food demand and halting poverty in Africa are among the greatest challenges, and these challenges are expected to continue if sustainable and innovative measures are not put into place. In 2017, approximately 256 million people were reported to be undernourished in Africa [74]. There is no doubt that Africa is far from achieving Sustainable Development Goal 2, which is to end hunger, achieve food security and improved nutrition, and promote sustainable agriculture by 2030. Edible insects are widely consumed in Africa, and they play an important socio-economic role for rural communities in Africa, by providing nutritious diets (this review), and income opportunities to traders and harvesters [22,75,76]. In addition, edible insects are a traditional delicacy, and are used as an emergency food source during times of food shortage [57]. They are rich in protein, carbohydrates, amino acids, and micronutrients such as Zinc and Iron. This implies that edible insects have a potential of contributing in sustainable diets, while assuring food security, and improving livelihoods of African people.
Acknowledgments
This study forms part of the Sustainable and Healthy Food Systems (SHEFs) supported by the Wellcome Trust’s Our Planet, our Health programme (grant number, 205200/Z/16/Z). We would like to thank Alan Dangour for providing constructive comments that helped improve this review. Lastly, we would like to thank the National Research Foundation (NRF) for financial support.
Appendix A
Table A1.
Edible insects consumed in different African countries.
| Order | Scientific Name/Morpho Species | Common Name | Country | Consumption Stage | References |
|---|---|---|---|---|---|
| Blattodea | Periplaneta americana | Common cockroach | Nigeria | Adult | [35] |
| Coleoptera | Analeptes trifasciata | Stem girdler | Nigeria | Larvae | [24,36,77] |
| Coleoptera | Oryctes boas Fabr | Rhinoceros beetle | Nigeria, Ivory Coast, Sierra Leone, Liberia, Democratic Republic of Congo, South Africa, Botswana, Namibia, Guinea Bissau | Larvae | [18,24,33,36,78,79] |
| Coleoptera | Oryctes monoceros | Rhinoceros beetle | Nigeria | Larvae | [24,33,36,39,56,79] |
| Coleoptera | Aphodius rufipes | Dung beetle | Nigeria | Larvae | [24,36,80] |
| Coleoptera | Rhynchophorus phoenicis | Palm weevil | Nigeria, Angola, Burkina Faso, Cameroon; Ghana, Cote D’ivioire, Democratic Republic of Congo, Liberia, Niger, Sao Tome, Togo, Benin, Guinea Bissau | Larvae, pupa and adult | [18,24,33,36,39,42,56,57,77,79,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98] |
| Coleoptera | Heteroligus meles | Yam beetle | Nigeria | Larvae, pupa, adult | [24,36,42,77,79,91,99,100] |
| Coleoptera | Eulepida mashona | Beetle | Zimbabwe | Larvae/adult | [51,58] |
| Coleoptera | Carbula marginella | Beetle | Burkina Faso | Adult | [98] |
| Coleoptera | oryctes sp. | Beetle | Burkina Faso | Larvae | [98] |
| Coleoptera | Oryctes rhinoceros larva | Beetle | Nigeria; Cote D’ivoire | Larvae | [79,81,99,101,102] |
| Coleoptera | Stenorcera orissa Buq | Giant jewel beetle | Botswana, Zimbabwe | Winged adult | [58,78] |
| Coleoptera | Eulepida anatine | Beetle | Zimbabwe | Larvae | [58] |
| Coleoptera | Eulepida nitidicollis | Beetle | Zimbabwe | Larvae | [58] |
| Coleoptera | Apomecyna parumpunctata | African longhorned beetle | Nigeria | Larvae | [43] |
| Coleoptera | Oryctes owariensis | Beetle | Cote D’ivioire, Democratic Republic of Congo, South Africa, Angola, Malawi, Botswana, Mozambique, Zambia, Zimbabwe Nigeria, Ivory Coast, Sierra Leona, Guinea, Ghana, Equatorial Guinea, Guinea Bissau | Adult | [18,33,40] |
| Coleoptera | Rhinoceros oryctes | Beetle | Nigeria | Larvae, pupa, adult | [91] |
| Coleoptera | Sitophilus oryzae | Rice weevil | Nigeria | Larvae, pupa, adult | [91] |
| Coleoptera | Callosobruchus maculatus | Bean beetle | Nigeria | Larvae, pupa, adult | [91] |
| Coleoptera | Dermestes maculatus | Beetle | Nigeria | Larvae, pupa, adult | [91] |
| Coleoptera | Cotinis nitida | Beetle | Nigeria | Adult/larvae | [79] |
| Coleoptera | Eulopida mashona | Beetle | Zimbabwe | Adult/larvae | [47] |
| Coleoptera | Sternocera funebris | Beetle | Zimbabwe | Adult/larvae | [47] |
| Coleoptera | Oryctes spp Oliver | Beetle | Nigeria | Larvae | [77] |
| Coleoptera | Augosoma centaurus | Beetle | Cameroon | Adult, larvae | [57] |
| Coleoptera | Phyllophaga nebulosa (Harris) | Beetle larvae | Ghana | Larvae | [94,103] |
| Coleoptera | Sitophilus zeamais | Beetle | Ghana | Larvae, adult | [104] |
| Coleoptera | Polycleis equestris | Weevil | South Africa | Adult | [33] |
| Coleoptera | Polycleis plumbeus | Weevil | South Africa | Adult | [33] |
| Coleoptera | Sipalus aloysii-sabaudiae | Beetle | South Africa | Larvae | [33] |
| Coleoptera | Teralobus flabellicornis | Beetle | South Africa | Larvae | [33] |
| Coleoptera | Sternocera orissa | Beetle | South Africa | Larvae | [33] |
| Diptera | Chaoborus edulis | Malawi | Adult | [33] | |
| Hemiptera | Nezara viridula | Southern green stink bug | Nigeria | Adult | [24,36,99] |
| Hemiptera | Encosternum delegorgui Spinola | Stink bug | South Africa, Zimbabwe, Swaziland, Malawi, Botswana, Namibia, Mozambique | Adult | [18,28,33,47,58,105] |
| Hemiptera | Monomatapa insingnis Distant | Cicada | Botswana | Adult | [78] |
| Hemiptera | Aspongubus viduatus | Melon bug | Sudan | Adult | [106] |
| Hemiptera | Agonoscelis pubescens | Sorghum bug | Sudan | Adult | [106] |
| Hemiptera | Rhynchophorus spp. | May bug | Nigeria, Cameroon | Larvae | [79,107] |
| Hemiptera | Brevisana brevis | African cicada | Zimbabwe | Ault | [47] |
| Hemiptera | Ugada limbalis | Cicada | Uganda | [108] | |
| Hemiptera | Pediculus capitata | Angola, Malawi, South Africa, Zambia, Zimbabwe, Mozambique, Namibia, Botswana | [33] | ||
| Hymenoptera | Apis mellifera | Honey bee | Nigeria, Botswana, Cote D’ivioire, Cameroon, Zambia, Zimbabwe, Botswana, Angola, Mozambique, Tanzania, Senegal, Ghana, Lesotho, Benin, South Africa | Egg, larva, pupa | [2,18,33,36,38,77,78,91,107,109,110] |
| Hymenoptera | Carebara vidua | African thief ant | Botswana, Zimbabwe; Kenya Burundi, South Africa, Malawi, Zambia, Sudan, Namibia, Mozambique | Winged adult | [18,33,44,47,58,78,81,108] |
| Hymenoptera | Plebeina hildebrandti Friese | Stingless bee | Botswana | Adult | [78] |
| Hymenoptera | Hypotrigona gribodoi Magretti | Stingless bee | Botswana | Adult | [78] |
| Hymenoptera | Cossus cossus | Capenter ant | Cote D’ivioire | Adult | [102] |
| Hymenoptera | Componotus spp. | Ant | Nigeria | Adult | [35] |
| Hymenoptera | Oecophylla longinoda | African weaver ant | Nigeria, Cameroon | Adult | [35,93,107] |
| Hymenoptera | Carebara lignata | Ant | Zambia, South Africa, Democratic Republic of Congo, Zimbabwe, Botswana, Mozambique, Namibia, Sudan | Adult | [18] |
| Blattodea | Macrotermes nigeriensis | Termite | Nigeria | Winged adult, queen | [24,33,35,36,72,111,112] |
| Blattodea | Macrotermes bellicosus | Termite | Nigeria, Kenya, Uganda, Democratic Republic of Congo, Cameroon, Cote D’ivioire, Sao Tome, Togo, Liberia, Burundi, Ghana, Zimbabwe, | Winged adult, queen | [18,24,33,36,59,77,79,82,94,109,111,113,114,115] |
| Blattodea | Macrotermes natalensis | Termite | Nigeria, South Africa, Zimbabwe, Cameroon, Democratic Republic of Congo, Burundi, Malawi | Winged adult, queen | [24,33,36,47,58,75,99] |
| Blattodea | Macrotermes falciger | Termite | Democratic Republic of Congo; South Africa, Zimbabwe, Burundi, Zambia, Burkina Faso, Benin | Winged adult | [18,33,58,75,108,115,116,117] |
| Blattodea | Macrotermes michaelseni | Termite | South Africa | Winged adult | [75] |
| Blattodea | Macrotermes subhyalinus | Termite | Burkina Faso Zimbabwe, Cote D’ivioire, Rwanda, Uganda, Angola, Togo, Kenya | Adult | [18,33,58,98,102,108,118] |
| Blattodea | Hodotermes mossambicus (Hagen) | Harvester termite | Botswana | Larvae | [78] |
| Blattodea | Macrotermes sp. | Termite | Nigeria, Uganda | Adult queen, soldiers | [35,38,91] |
| Blattodea | Syntermes soldiers | Termite | Uganda | Adult | [38] |
| Blattodea | Pseudacanthotermes militaris | Termite | Kenya, Uganda | Winged adult | [59,108,115] |
| Blattodea | Pseudacanthotermes spiniger | Termite | Kenya, Uganda, Burundi | Winged adult | [59,108,115] |
| Blattodea | Odontotermes kibarensis | Termite | Uganda | Winged adult | [108] |
| Blattodea | Pseudacanthotermes sp.1 | Termite | Uganda | Winged adult | [108] |
| Blattodea | Pseudacanthotermes sp.2 | Termite | Uganda | Winged adult | [108] |
| Blattodea | Odontotermes spp. | Termite | Uganda | Winged adult | [108] |
| Blattodea | Pseudacanthotermes sp.5 | Termite | Uganda | Winged adult | [108] |
| Blattodea | Pseudacanthotermes sp. 4 | Termite | Burundi | Adult | [108] |
| Blattodea | Macrotermes spp. | Termite | Rwanda, Cameroon | Winged adult | [93,107,108] |
| Blattodea | Macrotermes swaziae | Termite | Zimbabwe | [33] | |
| Blattodea | Microhodotermes viator | Termite | South Africa | [33] | |
| Blattodea | Termes badius | Termite | South Africa | Winged adult | [33] |
| Lepidoptera | Anaphe venata | African silkworm | Nigeria, Zambia, Cote D’ivioire, Sierra Leona, Guinea, Liberia, Guinea Bissau, Angola | Larvae | [18,24,33,36,77,96] |
| Lepidoptera | Anaphe infracta | African silkworm | Nigeria | Larvae | [24,33] |
| Lepidoptera | Anaphe recticulata | African silkworm | Nigeria | Larvae | [24,33,36] |
| Lepidoptera | Bunaea alcinoe | Emperor moth | Nigeria; Democratic Republic of Congo, Botswana, Zimbabwe, Cameroon, Zambia, South Africa, Democratic Republic of Congo, Tanzania | Larvae, pupa and adult | [18,24,36,58,77,78,79,87,93,99,117] |
| Lepidoptera | Lepidoptara litoralia | Caterpillar | Nigeria | Larvae | [24,36,119] |
| Lepidoptera | Cirina forda | Pallid emperor | Nigeria, Angola, Democratic Republic of Congo, Botswana, Zimbabwe; Togo, Zambia, Mozambique, Ghana, Namibia | Larvae | [18,24,30,31,33,36,48,56,58,78,96,112,117,120,121,122,123] |
| Lepidoptera | Imbrasia epimethea | Caterpillar | Angola, Democratic Republic of Congo | Larvae | [18,33,45] |
| Lepidoptera | Imbrasia obscura | Caterpillar | Angola | Larvae | [96] |
| Lepidoptera | Imbrasia truncata | Caterpillar | Angola | Larvae | [96] |
| Lepidoptera | Gonimbrasia (Nudaurelia) alopia | Caterpillar | Angola | Larvae | [96] |
| Lepidoptera | Gonimbrasia (Nudaurelia) dione | Caterpillar | Angola | Larvae | [96] |
| Lepidoptera | Pseudantheraea discrepans | Caterpillar | Angola | Larvae | [96] |
| Lepidoptera | Micragone cana | Caterpillar | Angola, Democratic Republic of Congo | Larvae | [33,96] |
| Lepidoptera | Anaphe panda | Bagnest moth | Angola, Zimbabwe, Zambia, Cameroon, Democratic Republic of Congo, Nigeria, Tanzania | Larvae | [18,33,47,58,124] |
| Lepidoptera | Notodontidae sp. 1 | Caterpillar | Angola | Larvae | [96] |
| Lepidoptera | Notodontidae sp. 2 | Caterpillar | Angola | Larvae | [96] |
| Lepidoptera | Notodontidae sp. 3 | Caterpillar | Angola | Larvae | [96] |
| Lepidoptera | Notodontidae sp. 4 | Caterpillar | Angola | Larvae | [96] |
| Lepidoptera | Gastroplakaeis rubroanalis | Caterpillar | Angola | Larvae | [96] |
| Lepidoptera | Sciatta inconcisa | Caterpillar | Angola | Larvae | [96] |
| Lepidoptera | Elaphrodes lactea Gaede | Caterpillar | Democratic Republic of Congo | Larvae | [33,117] |
| Lepidoptera | Lobobunaea saturnus Fabricius | Caterpillar | Democratic Republic of Congo, Zimbabwe | Larvae | [33,58,117] |
| Lepidoptera | Cinabra hyperbius (Westwood) | Caterpillar | Democratic Republic of Congo | Larvae | [33,117] |
| Lepidoptera | Gonimbrasia richelmanni Weymer | Caterpillar | Democratic Republic of Congo | Larvae | [33,117] |
| Lepidoptera | Antheua insignata | Caterpillar | Democratic Republic of Congo | Larvae | [33,117] |
| Lepidoptera | Imbrasia rubra | Caterpillar | Democratic Republic of Congo | Larvae | [117] |
| Lepidoptera | Athletes semialba (Sonthonnax) | Caterpillar | Democratic Republic of Congo, Zimbabwe, Zambia, South Africa, Namibia, Mozambique | Larvae | [33,58,117] |
| Lepidoptera | Cirina butyrospermi | Caterpillar | Burkina Faso, Cote D’ivioire, Zambia, Zimbabwe, South Africa, Nigeria, Mali, Ghana | Larvae | [18,46,102,103,118,125] |
| Lepidoptera | Hemijana variegata | Caterpillar | South Africa | Larvae | [49] |
| Lepidoptera | Imbrasia belina | Mopane worm | Nigeria; Botswana; Zimbabwe, Namibia, South Africa, Malawi, Zambia, Angola, Mozambique | Larvae | [18,33,34,47,58,76,78,91,109,126,127,128,129] |
| Lepidoptera | Isoberlina paniculata | Caterpillar | Zambia | Larvae | [127] |
| Lepidoptera | Urota sinope | Caterpillar | Zambia, Botswana | Larvae | [78,130] |
| Lepidoptera | Gonimbrasia zambesina | Caterpillar | Zambia; Zimbabwe, Democratic Republic of Congo | Larvae | [33,58,130] |
| Lepidoptera | Lophostethus dumolinii Angas | Arrow sphinx | Botswana | Larvae | [78] |
| Lepidoptera | Daphnis nerii L | Oleander hawk moth | Botswana | Larvae | [78] |
| Lepidoptera | Heniocha spp. | Marbled emperor moth | Botswana | Larvae | [78] |
| Lepidoptera | Imbrasia tyrrhea | Willow emperor moth | Botswana | Larvae | [78] |
| Lepidoptera | Sphingomorpha chlorea | Sundown emperor moth | Botswana | Larvae | [78] |
| Lepidoptera | Hippotion celerio L. | Silver striped hawk | Botswana | Adult | [78] |
| Lepidoptera | Agrius convolvuli L. | Convolvulus hawk moth. | Botswana, South Africa, Angola, Zimbabwe, Zambia, Malawi, Mozambique, Namibia | Larvae | [78] |
| Lepidoptera | Gonanisa maia | Caterpillar | Zimbabwe, Botswana, Malawi, Democratic Republic of Congo, South Africa | Larvae | [33,47,58] |
| Lepidoptera | Anthoaera zambezina | Caterpillar | Zimbabwe, Botswana, Malawi, Namibia, Zambia, South Africa, Mozambique, Angola | Larvae | [33,58] |
| Lepidoptera | Athletes gigas | Caterpillar | Zimbabwe, Botswana, Malawi, Namibia, Zambia, South Africa, Mozambique, Angola | Larvae | [33,58] |
| Lepidoptera | Bombycomorpha pallida | Moth | Zimbabwe, South Africa | Larvae | [33,58] |
| Lepidoptera | Bunaea caffra | Moth | Zimbabwe, Zambia, South Africa, Namibia, Botswana, Mozambique, Angola | Larvae | [33,58] |
| Lepidoptera | Bunaeopsis aurantica | Moth | Zimbabwe, Democratic Republic of Congo | Larvae | [33,58] |
| Lepidoptera | Gonometa postica | Moth | Zimbabwe, South Africa | Larvae | [33,58] |
| Lepidoptera | Heniocha dyops | Moth | Zimbabwe, South Africa, Botswana, Zambia, Malawi, Namibia, Mozambique, Angola | Larvae | [33,58] |
| Lepidoptera | Imbrasia epimethea | Moth | Zimbabwe, Democratic Republic of Congo | Larvae | [33,58] |
| Lepidoptera | Imbrasia ertli | Caterpillar | Zimbabwe, South Africa, Cameroon, Democratic Republic of Congo, Angola, Zimbabwe, Botswana, Angola | Larvae | [18,33,58] |
| Lepidoptera | Nudaurelia belina | Moth | Zimbabwe, Malawi, Botswana, Mozambique, Namibia, Zambia, South Africa | Larvae | [33,58] |
| Lepidoptera | Pseudobunaea irius | Moth | Zimbabwe, South Africa, Zambia, Angola, Malawi, Namibia, | Larvae | [33,58] |
| Lepidoptera | Loba leopardina | Moth | Zimbabwe | Larvae | [58] |
| Lepidoptera | Imbrasia oyemensis | Caterpillar | Cote D’ivioire | Adult | [102] |
| Lepidoptera | Imbrasia spp. | Caterpillar | Cameroon | Larvae | [93] |
| Lepidoptera | Eumeta spp. | Caterpillar | Cameroon | Larvae | [107] |
| Lepidoptera | Anaphe spp. | Caterpillar | Cameroon | Larvae | [107] |
| Lepidoptera | Dactyloceras spp. | Caterpillar | Cameroon | Larvae | [107] |
| Lepidoptera | Bunaea spp. | Caterpillar | Cameroon | Larvae | [107] |
| Lepidoptera | Dactyloceras lucina | Caterpillar | Democratic Republic of Congo, Zambia, South Africa, Cameroon, Angola, Gabon, Sierra Leone, Equatorial Guinea, Sao Tome, | Larvae | [18] |
| Lepidoptera | Platysphinx stigmatica | Caterpillar | Zambia, Democratic Republic of Congo, Sierra Leone, Rwanda, Burundi, Equatorial Guinea, Sao Tome, | Larvae | [18] |
| Lepidoptera | Epanaphe carteri | Caterpillar | Democratic Republic of Congo, Angola, Gabon, Sierra Leone, Sao Tome, Equatorial Guinea | Larvae | [18] |
| Lepidoptera | Gynanisa ata | Caterpillar | Democratic Republic of Congo, Zambia, Malawi, Sudan | Larvae | [18] |
| Lepidoptera | Eumeta cervina | Caterpillar | Democratic Republic of Congo, Cameroon, Angola, Gabon, Sierra Leone, Sao Tome, Equatorial Guinea, Rwanda, Burundi, Liberia | Larvae | [18] |
| Lepidoptera | Urota sinope | Caterpillar | Democratic Republic of Congo, South Africa, Zimbabwe, Zimbabwe, Botswana, Gabon, Mozambique, Namibia | Larvae | [18,33] |
| Lepidoptera | Anthoaera caffraria | Caterpillar | Angola, Malawi, South Africa, Zambia, Zimbabwe, Mozambique, Namibia, Botswana | Larvae | [33] |
| Lepidoptera | Anthoaera menippe | Caterpillar | Angola, Malawi, South Africa, Zambia, Zimbabwe, Mozambique, Namibia, Botswana | Larvae | [33] |
| Lepidoptera | Bunaea caffraria | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Drapetides uniformis | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Gonimbrasia hecate | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Goodia kuntzei | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Heniocha apollonia | Caterpillar | Angola, Malawi, South Africa, Zambia, Zimbabwe, Mozambique, Namibia, Botswana | Larvae | [33] |
| Lepidoptera | Heniocha marnois | Caterpillar | Angola, Malawi, South Africa, Zambia, Zimbabwe, Mozambique, Namibia, Botswana | Larvae | [33] |
| Lepidoptera | Herse convolvuli | Caterpillar | South Africa | Larvae | [33] |
| Lepidoptera | Imbrasia dione | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Imbrasia macrothyris | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Imbrasia rubra | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Lobobunaea phaedusa | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Melanocera parva | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Microgene cana | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Nudaurelia macrothyrus | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Nyodes prasinodes | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Rohaniella pygmaea | Caterpillar | Angola, Malawi, South Africa, Zambia, Zimbabwe, Mozambique, Namibia, Botswana | Larvae | [33] |
| Lepidoptera | Rheneae mediata | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Tagoropsis flavinata | Caterpillar | Democratic Republic of Congo | Larvae | [33] |
| Lepidoptera | Usta terpisichore | Caterpillar | Angola | Larvae | [33] |
| Lepidoptera | Usta wallengreni | Caterpillar | Angola, Malawi, South Africa, Zambia, Zimbabwe, Mozambique, Namibia, Botswana | Larvae | [33] |
| Mantodea | Mantis religiosa | African mantis | Nigeria, South Africa | Adult | [33,79] |
| Orthoptera | Brachytrupes membranaceus | Giant African cricket | Nigeria, Angola; Zimbabwe, Uganda; Cameroon, Democratic Republic of Congo, Burkina Faso, Tanzania, Angola, Togo, Benin; Malawi | Adult | [18,24,33,36,45,53,58,77,79,91,93,96,99,108,124] |
| Orthoptera | Gymnogryllus lucens | Cricket | Nigeria | Adult | [24,36,116] |
| Orthoptera | Cytacanthacris naeruginosus | Short horned grasshopper | Nigeria | Adult | [24,36] |
| Orthoptera | Zonocerus variegatus | Grasshopper | Nigeria, Cameroon, Uganda, Democratic Republic of Congo, Cote D’ivioire, Ghana, Guinea, Liberia, Sao Tome, Liberia, Guinea Bissau | Adult | [18,24,35,36,38,77,79,85,94,99,110,116,131] |
| Orthoptera | Gryllotalpa africana | Mole cricket | Nigeria; Zimbabwe; Malawi | Adult | [24,33,36,47,77,79,99,124] |
| Orthoptera | Ruspolia differens | Grasshopper | Kenya, Tanzania, Democratic Republic of Congo, Uganda, Zimbabwe, Rwanda, Cameroon, Uganda, Malawi, South Africa | Adult | [33,58,59,60,108,112,115,117,132] |
| Orthoptera | Melanoplus foedus | Grasshopper | Nigeria | Adult | [112] |
| Orthoptera | Gryllus assimilis | Cricket | Nigeria; Ghana | Adult | [94,112] |
| Orthoptera | Henicus whellani | Cricket | Zimbabwe | Adult | [50,51] |
| Orthoptera | Kraussaria ongulifera | Grasshopper | Burkina Faso | Adult | [98,118] |
| Orthoptera | Gryllus campestris | Field cricket | Burkina Faso; Cameroon, Malawi | Adult | [98,107,124] |
| Orthoptera | Ruspolia nitidula | Grasshopper | Uganda | Larvae and adult | [22,133] |
| Orthoptera | Normadacris septemfasciata | Red locust | Botswana; Uganda, Zambia, South Africa, Democratic Republic of Congo, Zimbabwe, Botswana, Nigeria, Uganda, Tanzania, Malawi, Mozambique | Adult | [18,78,108] |
| Orthoptera | Locustana pardalina | Brown locust | Botswana, South Africa, Zimbabwe, Malawi, Libya | Adult | [18,33,78] |
| Orthoptera | Schistocerca gregaria | Desert locust | Botswana, Zambia, South Africa, Cameroon, Democratic Republic of Congo, Zimbabwe, Burkina, Faso, Malawi, Mali, Niger, Togo, Benin | Adult | [18,78,116] |
| Orthoptera | Cyrtacanthacris tatarica L | Brown-spotted locust | Botswana | Adult | [78] |
| Orthoptera | Acrida acuminata | Common stick grasshopper | Botswana | Adult | [78] |
| Orthoptera | Zonocerus elegans | Elegant grasshopper | Botswana, South Africa | Adult | [33,78] |
| Orthoptera | Acrotylus spp. | Burrowing grasshopper | Botswana | Adult | [78] |
| Orthoptera | Homorocoryphus nitidulus | Cricket | Cameroon | Larvae | [85,107] |
| Orthoptera | Gynanisa maia | Cricket | Zimbabwe, Malawi, South Africa | Larvae | [33,58] |
| Orthoptera | Locusta migratoria | migratory locust | Zimbabwe, Cote D’ivioire; Nigeria; Sudan, Zambia, Democratic Republic of Congo, Sudan, Ghana | Adult | [18,33,79,94,102,134,135] |
| Orthoptera | Acheta domesticus | Cricket | Cote D’ivioire; Nigeria, Ghana | Adult | [79,94,102] |
| Orthoptera | Cartarrtopsilus taeniolatus | Grasshopper | Nigeria | Adult | [35] |
| Orthoptera | Zulua cyanoptera | Grasshopper | Nigeria | Adult | [35] |
| Orthoptera | Brachytrupes spp. | Cricket | Uganda, Cameroon | Adult | [107,110] |
| Orthoptera | Cyrtacanthacris aeruginosa unicolor | Grasshopper | Uganda | Adult | [110] |
| Orthoptera | Zonocerus sp. | Grasshopper | Nigeria | Adult | [91] |
| Orthoptera | Daraba (Sceloides) laisalis | Locust | Nigeria | Larvae, pupa, adult | [91] |
| Orthoptera | Ornithacris turbida | Grasshopper | Zimbabwe | Adult | [47] |
| Orthoptera | Acanthoplus discoidalis | Cricket | Zimbabwe | Adult | [47] |
| Orthoptera | Acanthacris ruficornis | Garden locust | Uganda, Zambia, South Africa, Cameroon, Democratic Republic of Congo, Zimbabwe, Burkina Faso, Malawi, Mali, Niger, Togo, Benin | Adult | [18,108] |
| Orthoptera | Schistocerca spp. | Grasshopper | Cameroon | Adult | [107] |
| Orthoptera | Acanthacris spp. | Grasshopper | Cameroon | Adult | [107] |
| Orthoptera | Gastrimargus spp. | Locust | Cameroon | Adult | [107] |
| Orthoptera | Phymateus spp. | Locust | Cameroon | Adult | [107] |
| Orthoptera | Anacridium spp. | Locust | Cameroon | Adult | [107] |
| Orthoptera | Pyrgomorpha spp. | Locust | Cameroon | Adult | [107] |
| Orthoptera | Gastrimargus africanus | Locust | Cameroon, Democratic Republic of Congo, Niger, Lesotho, Liberia | Adult | [18] |
| Orthoptera | Phymateus viridipes brunneri Bolivar | Locust | Zambia, South Africa, Democratic Republic of Congo, Zimbabwe, Botswana, Mozambique, Namibia | Adult | [18] |
| Orthoptera | Gryllus bimaculatus | Cricket | Togo, Nigeria, Guinea Bissau, Sierra Leone, Liberia, Benin, Democratic Republic of Congo, Kenya, Sudan, Zambia | Adult | [18] |
| Orthoptera | Anacridium melanorhodon melanorhodon | Cricket | Cameroon, Sudan, Niger | Adult | [18,52] |
| Orthoptera | Paracinema tricolor | Cricket | Cameroon, Malawi, Lesotho | Adult | [18] |
| Orthoptera | Acheta spp. | Cricket | Zambia, Zimbabwe, Kenya | Adult | [18] |
| Orthoptera | Scapteriscus vicinus | Field cricket | Ghana | Adult | [94,103] |
| Orthoptera | Gryllotalpa gryllotalpa | Mole cricket | Malawi | Adult | [124] |
| Orthoptera | Homorocoryphus vicinus | Cricket | Uganda | Adult | [33] |
| Orthoptera | Nomadacris septumfasciata | Cricket | South Africa | Adult | [33] |
| Orthoptera | Schistocerca gregaria | Cricket | Zimbabwe | Adult | [33] |
| Blattodea | Pseudacathotermes spinige | Termite | Kenya | Adult | [37] |
| Blattodea | Macrotermes spp. | Termite | Kenya | Adult | [37] |
| Blattodea | Macrotermes subhylanus | Termite | Kenya | Adult | [37] |
| Hymenoptera | Crematogaster mimosae | Ant | Kenya | Adult | [37] |
Author Contributions
Conceptualization of the study was done by Z.T.H., R.S. and T.C.M. methodology, Z.T.H.; data collection, Z.T.H; writing—original draft preparation, Z.T.H.; writing—review and editing, R.S., T.C.M., and Z.T.H.; supervision, R.S. and T.C.M.; funding acquisition, R.S., and T.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Sustainable and Healthy Food Systems (SHEFs) supported by the Wellcome Trust’s Our Planet, our Health programme (grant number, 205200/Z/16/Z), and the National Research Foundation (NRF) (grant number, 114416).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Van Huis A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013;58:563–583. doi: 10.1146/annurev-ento-120811-153704. [DOI] [PubMed] [Google Scholar]
- 2.Verbeke W. Profiling consumers who are ready to adopt insects as a meat substitute in a Western society. Food Qual. Prefer. 2015;39:147–155. doi: 10.1016/j.foodqual.2014.07.008. [DOI] [Google Scholar]
- 3.Nowak V., Persijn D., Rittenschober D., Charrondiere U.R. Review of food composition data for edible insects. Food Chem. 2016;193:39–46. doi: 10.1016/j.foodchem.2014.10.114. [DOI] [PubMed] [Google Scholar]
- 4.Van Huis A. Edible insects are the future? Proc. Nutr. Soc. 2016;75:294–305. doi: 10.1017/S0029665116000069. [DOI] [PubMed] [Google Scholar]
- 5.Myers G., Pettigrew S. A qualitative exploration of the factors underlying seniors’ receptiveness to entomophagy. Food Res. Int. 2017;103:163–169. doi: 10.1016/j.foodres.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Rumpold B.A., Schlüter O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013;57:802–823. doi: 10.1002/mnfr.201200735. [DOI] [PubMed] [Google Scholar]
- 7.Dobermann D., Swift J.A., Field L.M. Opportunities and hurdles of edible insects for food and feed. Nutr. Bull. 2017;42:293–308. doi: 10.1111/nbu.12291. [DOI] [Google Scholar]
- 8.Hanboonsong Y., Jamjanya T., Durst P.B. Six-legged Livestock: Edible Insect Farming, Collect on and Market in Thailand. Food and Agriculture Organization of the United Nations Regional Office for Asia and The Pacific; Bangkok, Thailand: 2013. pp. 1–57. [Google Scholar]
- 9.Halloran A., Roos N., Hanboonsong Y. Cricket farming as a livelihood strategy in Thailand. Geogr. J. 2017;183:112–124. doi: 10.1111/geoj.12184. [DOI] [Google Scholar]
- 10.Chen X., Feng Y., Chen Z. Common edible insects and their utilization in China: Invited review. Entomol. Res. 2009;39:299–303. doi: 10.1111/j.1748-5967.2009.00237.x. [DOI] [Google Scholar]
- 11.Feng Y., Chen X.M., Zhao M., He Z., Sun L., Wang C.Y., Ding W.F. Edible insects in China: Utilization and prospects. Insect Sci. 2018;25:184–198. doi: 10.1111/1744-7917.12449. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Elorduy J. Insects: A sustainable source of food? Ecol. Food Nutr. 1997;36:247–276. doi: 10.1080/03670244.1997.9991519. [DOI] [Google Scholar]
- 13.Ramos-Elorduy J., Moreno J.M.P. Edible insects of Chiapas, Mexico. Ecol. Food Nutr. 2002;41:271–299. doi: 10.1080/03670240214081. [DOI] [Google Scholar]
- 14.Ramos-Elorduy J., Moreno J.M.P., Prado E.E., Perez M.A., Otero J.L., De Guevara O.L. Nutritional value of edible insects from the state of Oaxaca, Mexico. J. Food Compos. Anal. 1997;10:142–157. doi: 10.1006/jfca.1997.0530. [DOI] [Google Scholar]
- 15.Acuña A.M., Caso L., Aliphat M.M., Vergara C.H. Edible insects as part of the traditional food system of the Popoloca Town of Los Reyes Metzontla, Mexico. J. Ethnobiol. 2011;31:150–169. doi: 10.2993/0278-0771-31.1.150. [DOI] [Google Scholar]
- 16.Costa-Neto E.M. Anthropo-entomophagy in Latin America: An overview of the importance of edible insects to local communities. J. Insects Food Feed. 2015;1:17–23. doi: 10.3920/JIFF2014.0015. [DOI] [Google Scholar]
- 17.Mitsuhashi J. Insects as traditional foods in Japan. Ecol. Food Nutr. 1997;36:187–199. doi: 10.1080/03670244.1997.9991514. [DOI] [Google Scholar]
- 18.Kelemu S., Niassy S., Torto B., Fiaboe K., Affognon H., Tonnang H., Maniania N.K., Ekesi S. African edible insects for food and feed: Inventory, diversity, commonalities and contribution to food security. J. Insects Food Feed. 2015;1:103–119. doi: 10.3920/JIFF2014.0016. [DOI] [Google Scholar]
- 19.Kouřimská L., Adámková A. Nutritional and sensory quality of edible insects. NFS J. 2016;4:22–26. doi: 10.1016/j.nfs.2016.07.001. [DOI] [Google Scholar]
- 20.Sogari G., Menozzi D., Mora C. Exploring young foodies’ knowledge and attitude regarding entomophagy: A qualitative study in Italy. Int. J. Gastron. Food Sci. 2017;7:16–19. doi: 10.1016/j.ijgfs.2016.12.002. [DOI] [Google Scholar]
- 21.De Foliart G.R. An overview of the role of edible insects in preserving biodiversity. Ecol. Food Nutr. 1997;36:109–132. doi: 10.1080/03670244.1997.9991510. [DOI] [Google Scholar]
- 22.Agea J., Biryomumaisho D., Buyinza M., Nabanoga G. Commercialization of Ruspolia nitidula (nsenene grasshoppers) in Central Uganda. Afr. J. Food Agric. Nutr. Dev. 2008;8:319–332. doi: 10.4314/ajfand.v8i3.19195. [DOI] [Google Scholar]
- 23.Mutungi C., Irungu F.G., Nduko J., Mutua F., Affognon H., Nakimbugwe D., Ekesi S., Fiaboe K.K.M. Postharvest processes of edible insects in Africa: A review of processing methods, and the implications for nutrition, safety and new products development. Crit. Rev. Food Sci. Nutr. 2019;59:276–298. doi: 10.1080/10408398.2017.1365330. [DOI] [PubMed] [Google Scholar]
- 24.Alamu O.T., Amao O.A., Nwokedi I.C., Oke A.O., Lawa O. Diversity and nutritional status of edible insects in Nigeria: A review. Int. J. Biodivers. Conserv. 2013;5:215–222. doi: 10.5897/IJBC12.121. [DOI] [Google Scholar]
- 25.Nonaka K. Feasting on insects. Entomol. Res. 2009;39:304–312. doi: 10.1111/j.1748-5967.2009.00240.x. [DOI] [Google Scholar]
- 26.Shockley M., Dossey A.T. Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens. Academic Press; Cambridge, MA, USA: 2014. Insects for Human Consumption; pp. 617–652. [DOI] [Google Scholar]
- 27.Nakimbugwe D., Ssepuuya G., Male D., Lutwama V., Mukisa I.M., Fiaboe K.K.M. Status of the regulatory environment for utilization of insects as food and feed in Sub-Saharan Africa-a review. Crit. Rev. Food Sci. Nutr. 2020:1–10. doi: 10.1080/10408398.2020.1756738. [DOI] [PubMed] [Google Scholar]
- 28.Teffo L.S., Toms R.B., Eloff J.N. Preliminary data on the nutritional composition of the edible stink-bug, Encosternum delegorguei Spinola, consumed in Limpopo province, South Africa. S. Afr. J. Sci. 2007;103:434–436. [Google Scholar]
- 29.Siulapwa N., Mwambungu A., Lungu E., Sichilima W. Nutritional Value of Four Common Edible Insects in Zambia. Int. J. Sci. Res. 2012;3:2319–7064. [Google Scholar]
- 30.Adepoju O.T., Daboh O.O. Nutrient composition of Cirina forda (westwood)-Enriched complementary foods. Ann. Nutr. Metab. 2013;63:139–144. doi: 10.1159/000353885. [DOI] [PubMed] [Google Scholar]
- 31.Badanaro F., Amevoin K., Lamboni C., Amouzou K. Edible Cirina forda (Westwood, 1849) (lepidoptera: Saturniidae) caterpillar among Moba people of the savannah region in North Togo: From collector to consumer. Asian J. Appl. Sci. Eng. 2014;3:13. doi: 10.15590/ajase/2014/v3i8/54479. [DOI] [Google Scholar]
- 32.Adepoju O.T., Ajayi K. Nutrient composition and adequacy of two locally formulated winged termite (Macrotermes bellicosus) enriched complementary Foods. J. Food Res. 2016;5:79. doi: 10.5539/jfr.v5n4p79. [DOI] [Google Scholar]
- 33.Illgner P., Nel E. The geography of edible insects in Sub-Saharan Africa: A study of the mopane caterpillar. Geogr. J. 2000;166:336–351. doi: 10.1111/j.1475-4959.2000.tb00035.x. [DOI] [Google Scholar]
- 34.Mpuchane S., Gashe B.A., Allotey J., Siame B., Teferra G., Ditlhogo M. Quality deterioration of phane, the edible caterpillar of an emperor moth Imbrasia belina. Food Control. 2000;11:453–458. doi: 10.1016/S0956-7135(00)00010-4. [DOI] [Google Scholar]
- 35.Mbah C., Elekima G.O. Nutrient composition of some terrestrial insects in Ahmadu Bello University, Samaru Zaria Nigeria. Sci. World J. 2010;2:17–20. doi: 10.4314/swj.v2i2.51728. [DOI] [Google Scholar]
- 36.Banjo A.D., Lawal O.A., Songonuga E.A. The nutritional value of fourteen species of edible insects in southwestern Nigeria. Afr. J. Biotechnol. 2006;5:298–301. doi: 10.5897/AJB05.250. [DOI] [Google Scholar]
- 37.Christensen D.L., Orech F.O., Mungai M.N., Larsen T., Friis H., Aagaard-Hansen J. Entomophagy among the Luo of Kenya: A potential mineral source? Int. J. Food Sci. Nutr. 2006;57:198–203. doi: 10.1080/09637480600738252. [DOI] [PubMed] [Google Scholar]
- 38.Akullo J., Agea J.G., Obaa B.B., Okwee-Acai J., Nakimbugwe D. Nutrient composition of commonly consumed edible insects in the Lango sub-region of northern Uganda. Int. Food Res. J. 2018;25:159–166. [Google Scholar]
- 39.Edijala J.K., Egbogbo O., Anigboro A.A. Proximate composition and cholesterol concentrations of Rhynchophorus phoenicis and Oryctes monoceros larvae subjected to different heat treatments. Afr. J. Biotechnol. 2009;8:2346–2348. doi: 10.5897/AJB09.113. [DOI] [Google Scholar]
- 40.Assielou B., Due E., Koffi M., Dabonne S., Kouame P. Oryctes owariensis Larvae as good alternative protein source: Nutritional and functional properties. Annu. Res. Rev. Biol. 2015;8:1–9. doi: 10.9734/ARRB/2015/19093. [DOI] [Google Scholar]
- 41.Manditsera F.A., Lakemond C.M.M., Fogliano V., Zvidzai C.J., Luning P.A. Consumption patterns of edible insects in rural and urban areas of Zimbabwe: Taste, nutritional value and availability are key elements for keeping the insect eating habit. Food Secur. 2018;10:561–570. doi: 10.1007/s12571-018-0801-8. [DOI] [Google Scholar]
- 42.Jonathan A.A. Proximate and anti-nutritional composition of two common edible insects: Yam beetle (Heteroligus meles) and palm weevil (Rhynchophorus phoenicis) Elixir Food Sci. 2012;49:9782–9786. [Google Scholar]
- 43.Thomas C.N. Nutritional Potentials of Edible Larvae of Longhorned Beetle (Apomecyna parumpunctata Chev) (Coleoptera:Cerambycidae) in Niger Delta, Nigeria. Int. J. Agric. Earth Sci. 2018;4:46–51. [Google Scholar]
- 44.Ayieko M.A., Kinyuru J.N., Ndong’a M.F., Kenji G.M. Nutritional value and consumption of black ants (Carebara vidua Smith) from the lake Victoria region in Kenya. Adv. J. Food Sci. Technol. 2012;4:39–45. [Google Scholar]
- 45.Lautenschläger T., Neinhuis C., Kikongo E., Henle T., Förster A. Impact of different preparations on the nutritional value of the edible caterpillar Imbrasia epimethea from northern Angola. Eur. Food Res. Technol. 2017;243:1–10. doi: 10.1007/s00217-016-2791-0. [DOI] [Google Scholar]
- 46.Anvo P.M., Toguyéni A., Otchoumou A.K., Zoungrana-Kaboré C.Y., Koumelan E.P. Nutritional qualities of edible caterpillars Cirina butyrospermi in southwestern of Burkina Faso. Int. J. Innov. Appl. Stud. 2016;18:639–645. [Google Scholar]
- 47.Musundire R., Zvidzai C.J., Chidewe C., Samende B.K., Chemura A. Habitats and nutritional composition of selected edible insects in Zimbabwe. J. Insects Food Feed. 2016;2:189–198. doi: 10.3920/JIFF2015.0083. [DOI] [Google Scholar]
- 48.Akinnawo O., Ketiku A.O. Chemical Composition and Fatty Acid Profile of Edible Larva of Cirina forda (Westwood) Afr. J. Biomed. Res. 2000;3:93–96. [Google Scholar]
- 49.Egan B.A., Toms R., Minter L.R., Addo-Bediako A., Masoko P., Mphosi M., Olivier P.A.S. Nutritional Significance of the Edible Insect, Hemijana variegata Rothschild (Lepidoptera: Eupterotidae), of the Blouberg Region, Limpopo, South Africa. Afr. Entomol. 2014;22:15–23. doi: 10.4001/003.022.0108. [DOI] [Google Scholar]
- 50.Musundire R., Zvidzai C.J., Chidewe C., Samende B.K., Manditsera F.A. Nutrient and anti-nutrient composition of Henicus whellani (Orthoptera: Stenopelmatidae), an edible ground cricket, in south-eastern Zimbabwe. Int. J. Trop. Insect Sci. 2014;34:223–231. doi: 10.1017/S1742758414000484. [DOI] [Google Scholar]
- 51.Manditsera F.A., Luning P.A., Fogliano V., Lakemond C.M.M. The contribution of wild harvested edible insects (Eulepida mashona and Henicus whellani) to nutrition security in Zimbabwe. J. Food Compos. Anal. 2019;75:17–25. doi: 10.1016/j.jfca.2018.09.013. [DOI] [Google Scholar]
- 52.El Hassan N.M., Hamed S.Y., Hassan A.B., Eltayeb M.M., Babiker E.E. Nutritional evaluation and physiochemical properties of boiled and fried tree locust. Pak. J. Nutr. 2008;7:325–329. doi: 10.3923/pjn.2008.325.329. [DOI] [Google Scholar]
- 53.Adeyeye E.I., Awokunmi E.E. Chemical composition of female and male giant African crickets, Brachytrypes membranaceus L. Int. J. Pharma Bio Sci. 2010;1:125–136. [Google Scholar]
- 54.Rumpold B.A., Schlüter O. Insect-based protein sources and their potential for human consumption: Nutritional composition and processing. Anim. Front. 2015;5:20–24. doi: 10.2527/af.2015-0015. [DOI] [Google Scholar]
- 55.Rahman A., Bordoloi S., Mazid S. Entomophagy practiced among the Tiwa community of Morigaon district. J. Entomol. Zool. Stud. 2018;6:484–486. [Google Scholar]
- 56.Agbidye F., Nongo N. Harvesting and processing techniques for the larvae of the pallid emperor moth. J. Res. For. Wildl. Enviro. 2009;1:123–132. [Google Scholar]
- 57.Muafor F.J., Levang P., Le Gall P. A crispy delicacy: Augosoma beetle as alternative source of protein in East Cameroon. Int. J. Biodivers. 2014;2014:1–7. doi: 10.1155/2014/214071. [DOI] [Google Scholar]
- 58.Dube S., Dlamini N.R., Mafunga A., Mukai M., Dhlamini Z. A survey on entomophagy prevalence in Zimbabwe. Afr. J. Food Agric. Nutr. Dev. 2013;13:7242–7253. doi: 10.18697/ajfand.56.10435. [DOI] [Google Scholar]
- 59.Kinyuru J.N., Konyole S.O., Roos N., Onyango C.A., Owino V.O., Owuor B.O., Estambale B.B., Friis H., Aagaard-Hansen J., Kenji G.M. Nutrient composition of four species of winged termites consumed in western kenya. J. Food Compos. Anal. 2013;30:120–124. doi: 10.1016/j.jfca.2013.02.008. [DOI] [Google Scholar]
- 60.Rutaro K., Malinga G.M., Lehtovaara V.J., Opoke R., Valtonen A., Kwetegyeka J., Nyeko P., Roininen H. The fatty acid composition of edible grasshopper Ruspolia differens (Serville) (Orthoptera: Tettigoniidae) feeding on diversifying diets of host plants. Entomol. Res. 2018;48:490–498. doi: 10.1111/1748-5967.12322. [DOI] [Google Scholar]
- 61.Malinga G.M., Valtonen A., Lehtovaara V.J., Rutaro K., Nyeko R.O.P., Roininen H. Diet acceptance and preference of the edible grasshopper Ruspolia differens (Orthoptera; Tettigonidae) Appl. Entomol. Zool. 2018;53:229–239. doi: 10.1007/s13355-018-0550-3. [DOI] [Google Scholar]
- 62.Blásquez J.R.E., Moreno J.M.P., Camacho V.H.M. Could grasshoppers be a nutritive meal? Food Nutr. Sci. 2012;3:164–175. doi: 10.4236/fns.2012.32025. [DOI] [Google Scholar]
- 63.Henley E.C., Taylor J.R.N., Obukosia S.D. The importance of dietary protein in human health: Combating protein deficiency in sub-Saharan Africa through transgenic biofortified sorghum. Adv. Nutr Food Sci. 2010;60:21–52. doi: 10.1016/S1043-4526(10)60002-2. [DOI] [PubMed] [Google Scholar]
- 64.De Onis M., Branca F. Childhood stunting: A global perspective. Matern. Child Nutr. 2016;12:12–26. doi: 10.1111/mcn.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhattacharyya B., Choudhury B., Das P., Dutta S.K., Bhagawati S., Pathak K. Nutritional composition of five soil-dwelling Scarab beetles (Coleoptera: Scarabaeidae) of Assam, India. Coleopt. Bull. 2018;72:339. doi: 10.1649/0010-065X-72.2.339. [DOI] [Google Scholar]
- 66.Nyangena D.N., Mutungi C., Imathiu S., Kinyuru J., Affognon H., Ekesi S., Nakimbugwe D., Fiaboe K.K.M. Effects of traditional processing techniques on the nutritional and microbiological quality of four edible insect species used for food and feed in East Africa. Foods. 2020;9:574. doi: 10.3390/foods9050574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bukkens S.G.F. The nutritional value of edible insects. Ecol. Food Nutr. 1997;36:287–319. doi: 10.1080/03670244.1997.9991521. [DOI] [Google Scholar]
- 68.Kewuyemi Y.O., Kesa H., Chinma C.E., Adebo O.A. Fermented edible insects for promoting food security in Africa. Insects. 2020;11:283. doi: 10.3390/insects11050283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akhtar Y., Isman M.B. Insects as an alternative protein Source. Food Sci. Nutr. 2018;2:263–288. doi: 10.1016/B978-0-08-100722-8.00011-5. [DOI] [Google Scholar]
- 70.Adámková A., Mlček J., Kouřimská L., Borkovcová M., Bušina T., Adámek M., Bednářová M., Krajsa J. Nutritional potential of selected insect species reared on the island of Sumatra. Int. J. Environ. Res. Public Health. 2017;14:521. doi: 10.3390/ijerph14050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mwangi M.N., Oonincx D.G.A.B., Stouten T., Veenenbos M., Melse-Boonstra A., Dicke M., Van Loon J.J.A. Insects as sources of iron and zinc in human nutrition. Nutr. Res. Rev. 2018;31:248–255. doi: 10.1017/S0954422418000094. [DOI] [PubMed] [Google Scholar]
- 72.Igwe C., Ujowundu C., Nwaogu L. chemical analysis of an edible African termite, Macrotermes nigeriensis; a potential antidote to food security problem. Biochem. Anal. Biochem. 2012;1:1–4. doi: 10.4172/2161-1009.1000105. [DOI] [Google Scholar]
- 73.Arigony A.L.V., De Oliveira I.M., Machado M., Bordin D.L., Bergter L., Pra D., Henriques J.A.P. The influence of micronutrients in cell culture: A reflection on viability and genomic stability. Biomed Res. Int. 2013;2013:1–22. doi: 10.1155/2013/597282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.FAO The State of Food Security and Nutrition in the World. [(accessed on 21 May 2019)];2019 Available online: http://www.fao.org/3/ca5162en/ca5162en.pdf/
- 75.Netshifhefhe S.R., Kunjeku E.C., Duncan F.D. Human uses and indigenous knowledge of edible termites in Vhembe District, Limpopo Province, South Africa. S. Afr. J. Sci. 2018;114:1–10. doi: 10.17159/sajs.2018/20170145. [DOI] [Google Scholar]
- 76.Baiyegunhi L.J.S., Oppong B.B., Senyolo M.G. Socio-economic factors influencing mopane worm (Imbrasia belina) harvesting in Limpopo Province, South Africa. J. For. Res. 2016;27:443–452. doi: 10.1007/s11676-015-0168-z. [DOI] [Google Scholar]
- 77.Adeoye O.T., Alebiosu B.I., Akinyemi O.D., Adeniran O.A. Socio economic analysis of forest edible insects species consumed and its role in the livelihood of people in Lagos State. J. Food Stud. 2014;3:104. doi: 10.5296/jfs.v3i1.6026. [DOI] [Google Scholar]
- 78.Obopile M., Seeletso T.G. Eat or not eat: An analysis of the status of entomophagy in Botswana. Food Secur. 2013;5:817–824. doi: 10.1007/s12571-013-0310-8. [DOI] [Google Scholar]
- 79.Ebenebe C.I., Amobi M.I., Udegbala C., Ufele A.N., Nweze B.O. Survey of edible insect consumption in south-eastern Nigeria. J. Insects Food Feed. 2017;3:241–252. doi: 10.3920/JIFF2017.0002. [DOI] [Google Scholar]
- 80.Paiko Y.B., Dauda B.E.N., Salau R.B., Jacob J.O. Preliminary data on the nutritional potential of the larvea of edible dung bettle consumed in Paikoro local government area of Niger state, Nigeria. Cont. J. Food Sci. Technol. 2012;6:3–42. [Google Scholar]
- 81.Onyeike E.N., Ayalogu E.O., Okaraonye C.C. Nutritive value of the larvae of raphia palm beetle (Oryctes rhinoceros) and weevil (Rhyncophorus pheonicis) J. Sci. Food Agric. 2005;85:1822–1828. doi: 10.1002/jsfa.2054. [DOI] [Google Scholar]
- 82.Ekpo K., Onigbinde A.O. Nutritional potentials of the larva of Rhynchophorus phoenicis. (F) Pak. J. Nutri. 2005;4:287–289. [Google Scholar]
- 83.Omotoso O.T., Adedire C.O. Nutrient composition, mineral content and the solubility of the proteins of palm weevil, Rhynchophorus phoenicis f. (Coleoptera: Curculionidae) J. Zhejiang Univ. Sci. B. 2007;8:318–322. doi: 10.1631/jzus.2007.B0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ekpo K.E., Onigbinde A.O., Asia I.O. Pharmaceutical potentials of the oils of some popular insects consumed in southern Nigeria. Afr. J. Pharm. Pharmacol. 2009;3:51–57. [Google Scholar]
- 85.Womeni H.M., Linder M., Tiencheu B., Mbiapo F.T., Villeneuve P., Fanni J., Parmentier M. Oils of insects and larvae consumed in Africa: Potential sources of polyunsaturated fatty acids. OCL Ol. Corps Gras Lipides. 2009;16:230–235. doi: 10.1051/ocl.2009.0279. [DOI] [Google Scholar]
- 86.Idolo I. Nutritional and quality attributes of wheat buns enriched with the larvae of Rhynchophorus phoenicis f. Pak. J. Nutr. 2010;9:1043–1046. doi: 10.3923/pjn.2010.1043.1046. [DOI] [Google Scholar]
- 87.Braide W., Nwaoguikpe R. Assessment of microbiological quality and nutritional values of a processed edible weevil caterpillar (Rhynchophorus phoenicis) in Port Harcourt, southern Nigeria. Int. J. Biol. Chem. Sci. 2011;5:410–418. doi: 10.4314/ijbcs.v5i2.72059. [DOI] [Google Scholar]
- 88.Ogbuagu M.N., Ohondu I., Nwigwe C. Fatty Acid and amino acid profiles of the larva of raffia palm weevil: Rhynchophorus phoenicis. Pac. J. Sci. Technol. 2011;12:392–400. [Google Scholar]
- 89.Elemo B.O., Elemo G.N., Makinde M., Erukainure O.L. Chemical evaluation of African palm weevil, Rhychophorus phoenicis, larvae as a food source. J. Insect Sci. 2011;11:1–6. doi: 10.1673/031.011.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Womeni H.M., Tiencheu B., Linder M., Nabayo E.M.C., Tenyang N., Mbiapo T.F., Parmentier M. Nutritional value and effect of cooking, drying and storage process on some functional properties of Rhynchophorus phoenicis. Int. J. Life Sci. Pharma Res. 2012;2:203–219. [Google Scholar]
- 91.Okore O., Avaoja D., Nwana I. Edible insects of the Niger Delta area in Nigeria. J. Nat. Sci. Res. 2014;4:1–9. [Google Scholar]
- 92.Muafor F.J., Gnetegha A.A.K., Levang P. Final Project Report Promoting the Farming of Edible Larvae of Palm Weevils (Rhyncophorus Spp.) as an Alternative to Sustain Rural Livelihoods and Community Based Conservation in Cameroon Humid Forest Regions. CIFOR LIFT IRD; Yaounde, Cameroon: 2016. [Google Scholar]
- 93.Meutchieye F., Tsafo K.E.C., Niassy S. Inventory of edible insects and their harvesting methods in the Cameroon centre region. J. Insects Food Feed. 2016;2:145–152. doi: 10.3920/JIFF2015.0082. [DOI] [Google Scholar]
- 94.Anankware J.P., Osekre E.A., Obeng-Ofori D., Khamala C. Identification and classification of common edible insects in Ghana. Int. J. Entomol. Res. 2016;1:2455–4758. [Google Scholar]
- 95.Laar A., Kotoh A., Parker M., Milani P., Tawiah C., Soor S., Anankware J.P., Kalra N., Manu G., Tandoh A., et al. An exploration of edible palm weevil larvae (Akokono) as a source of nutrition and livelihood: Perspectives from Ghanaian stakeholders. Food Nutr. Bull. 2017;38:455–467. doi: 10.1177/0379572117723396. [DOI] [PubMed] [Google Scholar]
- 96.Lautenschläger T., Neinhuis C., Monizi M., Mandombe J.L., Förster A., Henle T., Nuss M. Edible insects of Northern Angola. Afr. Invertebr. 2017;58:55–82. doi: 10.3897/afrinvertebr.58.21083. [DOI] [Google Scholar]
- 97.Quaye B., Charity C., Donkoh A. Nutritional potential and microbial status of African palm weevil nutritional potential and microbial status of African palm peevil (Rhynchophorus phoenicis) larvae raised on alternative feed resources. ARJETS. 2018;48:45–52. [Google Scholar]
- 98.Séré A., Bougma A., Ouilly J.T., Traoré M., Sangaré H., Lykke A.M., Ouédraogo A., Gnankiné O., Bassolé I.H.N. Traditional knowledge regarding edible insects in Burkina Faso. J. Ethnobiol. Ethnomed. 2018;14:1–12. doi: 10.1186/s13002-018-0258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agbidye F., Ofuya T., Akindela S. Some edible insect species consumed by the people of Benue State, Nigeria. Pak. J. Nutr. 2009;8:946–950. doi: 10.3923/pjn.2009.946.950. [DOI] [Google Scholar]
- 100.Tobih F. Occurrence and seasonal variation of Heteroligus meles billb (Coleoptera: Dynastidae) in upper Niger Delta, Nigeria. Agric. Biol. J. North Am. 2011;2:826–831. doi: 10.5251/abjna.2011.2.5.826.831. [DOI] [Google Scholar]
- 101.Okaraonye C.C., Ikewuchi J.C. Nutritional potential of Oryctes rhinoceros larva. Pak. J. Nutr. 2009;8:35–38. doi: 10.3923/pjn.2009.35.38. [DOI] [Google Scholar]
- 102.Ehounou G.P., Ouali-n’gorans W.M., Niassys W.M. Assessment of entomophagy in Abidjan (Cote Divoire, West Africa) Afr. J. Food Sci. 2018;12:6–14. doi: 10.5897/ajfs2017.1589. [DOI] [Google Scholar]
- 103.Anankware J.P., Osekre E.A., Fening K., Obeng-Ofori D. Insects as food and feed. Food Sci. Technol. 2015;32:22–25. doi: 10.1002/fsat.3201_7.x. [DOI] [Google Scholar]
- 104.Aydoğan Z., Gürol A., İncekara Ü., Tahidu O.D. Element content analysis of edible insect of Ghana (Curculionidae: Sitophilus zeamais) Using EDXRF Spectrometer. Erzincan Üniversitesi Fen Bilim. Enstitüsü Derg. 2016;9:86–94. doi: 10.18185/eufbed.77067. [DOI] [Google Scholar]
- 105.Dzerefos C.M., Witkowski E.T.F., Toms R. Life-history traits of the edible stinkbug, Encosternum delegorguei (Hem. Tessaratomidae), a traditional food in southern Africa. J. Appl. Entomol. 2009;133:749–759. doi: 10.1111/j.1439-0418.2009.01425.x. [DOI] [Google Scholar]
- 106.Mariod A.A., Abdel-Wahab S.I., Ain N.M. Proximate amino acid, fatty acid and mineral composition of two Sudanese edible pentatomid insects. Int. J. Trop. Insect Sci. 2011;31:145–153. doi: 10.1017/S1742758411000282. [DOI] [Google Scholar]
- 107.Tamesse J.L., Kekeunou S., Tchatchouang L.J., Ndegue O.L.M., Aissatou L.M., Tombouck D., Youssa B. Insects as food, traditional medicine and cultural rites in the west and south regions of Cameroon. J. Insects Food Feed. 2016;2:153–160. doi: 10.3920/JIFF2015.0088. [DOI] [Google Scholar]
- 108.Okia C.A., Odongo W., Nzabamwita P., Ndimubandi J., Nalika N., Nyeko P. Local knowledge and practices on use and management of edible insects in Lake Victoria basin, East Africa. J. Insects Food Feed. 2017;3:83–93. doi: 10.3920/JIFF2016.0051. [DOI] [Google Scholar]
- 109.Ekpo K., Onigbinde A.O. Characterization of lipids in winged reproductive of the termite Macrotermes bellicosus. Pak. J. Nutr. 2007;6:247–251. [Google Scholar]
- 110.Akullo J., Obaa B.B., Acai J.O., Nakimbugwe D., Agea J.G. Knowledge, attitudes and practices on edible insects in Lango sub-region, northern Uganda. J. Insects Food Feed. 2017;3:73–81. doi: 10.3920/JIFF2016.0033. [DOI] [Google Scholar]
- 111.Ntukuyoh A.I., Udiong D.S., Ikpe A.E., Akpakpan A.E. Evaluation of nutritional value of termites (Macrotermes bellicosus): Soldiers, workers, and queen in the Niger Delta Region of Nigeria. Int. J. Food Nutr. Saf. 2012;1:60–65. [Google Scholar]
- 112.Inje O.F., Olufunmilayo A.H., Audu J.A., Ndaman S.A., Chidi E.E. Food science and human wellness protein quality of four indigenous edible insect species in Nigeria. Food Sci. Hum. Wellness. 2018;7:175–183. doi: 10.1016/j.fshw.2018.05.003. [DOI] [Google Scholar]
- 113.Adepoju O.T., Omotayo O.A. Nutrient composition and potential contribution of winged termites (Marcrotermes bellicosus Smeathman) to micronutrient intake of consumers in Nigeria. Curr. J. Appl. Sci. Technol. 2014;4:1149–1158. doi: 10.9734/BJAST/2014/4420. [DOI] [Google Scholar]
- 114.Adepoju O.T. Assessment of fatty acid profile, protein and macronutrient bioavailability of winged termites (Macrotermes bellicosus) using albino rats. Malays. J. Nutr. 2016;22:153–161. [Google Scholar]
- 115.Odongo W., Okia C.A., Nalika N., Nzabamwita P.H., Ndimubandi J., Nyeko P. Marketing of edible insects in Lake Victoria basin: The case of Uganda and Burundi. J. Insects Food Feed. 2018;4:285–293. doi: 10.3920/JIFF2017.0071. [DOI] [Google Scholar]
- 116.Ajai A., Bankole M., Jacob J.O., Audu U.A. Determination of some essential minerals in selected edible insects. Afr. J. Pure Appl. Chem. 2013;7:194–197. doi: 10.5897/AJPAC2013.0504. [DOI] [Google Scholar]
- 117.Bomolo O., Niassy S., Chocha A., Longanza B., Bugeme D.M., Ekesi S., Tanga C.M. Ecological diversity of edible insects and their potential contribution to household food security in Haut-Katanga Province, Democratic Republic of Congo. Afr. J. Ecol. 2017;55:640–653. doi: 10.1111/aje.12400. [DOI] [Google Scholar]
- 118.Rémy D.A., Hervé B.B., Sylvain O.N. Study of some biological parameters of Cirina butyrospermi Vuillet (Lepidoptera, Attacidae), an edible insect and shea caterpillar (Butyrospermum paradoxum Gaertn. F.) in a context of climate change in Burkina Faso. Adv. Entomol. 2017;6:1–8. doi: 10.4236/ae.2018.61001. [DOI] [Google Scholar]
- 119.Solomon M., Prisca N. Nutritive value of Lepidoptara litoralia (edible caterpillar) found in Jos Nigeria: Implications and food security and poverty alleviation. AJFAND. 2012;12:6737–6747. [Google Scholar]
- 120.Ande A.T., Sciences B. The lipid profile of the pallid emperor moth Cirina forda Westwood (Lepidoptera: Saturniidae) caterpillar. Biokemistri. 2003;13:37–41. [Google Scholar]
- 121.Oyegoke O.O., Akintola A.J., Fasoranti J.O. Dietary potentials of the edible larvae of Cirina forda (westwood) as a poultry feed. Afr. J. Biotechnol. 2006;5:1799–1802. doi: 10.5897/AJB06.189. [DOI] [Google Scholar]
- 122.Omotoso O.T. Nutritional quality, functional properties and anti-nutrient compositions of the larva of Cirina forda (Westwood) (Lepidoptera: Saturniidae) J. Zhejiang Univ. Sci. B. 2006;7:51–55. doi: 10.1631/jzus.2006.B0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Osasona A.I., Olaofe O. Nutritional and functional properties of Cirina forda larva from Ado-Ekiti, Nigeria. Afr. J. Food Sci. 2010;4:775–777. [Google Scholar]
- 124.Morris B. Insects as food among hunter-gatherers. Anthropol. Today. 2008;24:6–8. doi: 10.1111/j.1467-8322.2008.00558.x. [DOI] [Google Scholar]
- 125.Bama H.B., Dabire R.A., Ouattara D., Niassy S., Ba M.N., Dakouo D. Diapause disruption in Cirina butyrospermi Vuillet (Lepidoptera, Attacidae), the shea caterpillar, in Burkina Faso. J. Insects Food Feed. 2018;4:239–245. doi: 10.3920/JIFF2017.0068. [DOI] [Google Scholar]
- 126.Madibela O.R., Seitiso T.K., Thema T.F., Letso M. Effect of traditional processing methods on chemical composition and in vitro true dry matter digestibility of the mophane worm (Imbrasia belina) J. Arid Environ. 2007;68:492–500. doi: 10.1016/j.jaridenv.2006.06.002. [DOI] [Google Scholar]
- 127.Ghally A.E. The use of insects as human food in Zambia. Online J. Biol Sci. 2009;9:93–104. doi: 10.3844/ojbsci.2009.93.104. [DOI] [Google Scholar]
- 128.Thomas B. Sustainable harvesting and trading of mopane worms (Imbrasia belina) in Northern Namibia: An experience from the Uukwaluudhi area. Int. J. Environ. Stud. 2013;70:494–502. doi: 10.1080/00207233.2013.829324. [DOI] [Google Scholar]
- 129.Pharithi M.T., Suping S.M., Yeboah S.O. Variations of the fatty acid composition in the oil from the larval stages of the emperor moth caterpillar, Imbrasia belina. Bull. Chem. Soc. Ethiop. 2004;18:67–72. doi: 10.4314/bcse.v18i1.61639. [DOI] [Google Scholar]
- 130.Mbata K.J., Chidumayo E.N., Lwatula C.M. Traditional regulation of edible caterpillar exploitation in the Kopa area of Mpika district in northern Zambia. J. Insect Conserv. 2002;6:115–130. doi: 10.1023/A:1020953030648. [DOI] [Google Scholar]
- 131.Adeyeye E.I. Amino acid composition of variegated grasshopper, Zonocerus variegatus. Trop. Sci. 2005;45:141–143. doi: 10.1002/ts.9. [DOI] [Google Scholar]
- 132.Mmari M.W., Kinyuru J.N., Laswai H.S., Okoth J.K. Traditions, beliefs and indigenous technologies in connection with the edible longhorn grasshopper Ruspolia differens (Serville 1838) in Tanzania. J. Ethnobiol. Ethnomed. 2017;13:1–11. doi: 10.1186/s13002-017-0191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ssepuuya G., Mukisa I.M., Nakimbugwe D. Nutritional composition, quality, and shelf stability of processed Ruspolia nitidula (edible grasshoppers) Food Sci. Nutr. 2017;5:103–112. doi: 10.1002/fsn3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mohamed E.H.A. Determination of nutritive value of the edible migratory locust Locusta migratoria, Linnaeus, 1758 (Orthoptera: Acrididae) Int. J. Adv. Pharm. Biol. Chem. 2015;4:144–148. [Google Scholar]
- 135.Mohamed E.H.A. Fatty acids contents of the edible migratory locust Locusta migratoria, Linnaeus, 1758 (Orthoptera: Acrididae) Int. J. Adv. Pharm. Biol. Chem. 2015;4:746–750. [Google Scholar]