Abstract
While adjustment of total energy and nutritional balance is critically important, meal sequence, a relatively simple method of correcting postprandial hyperglycemia, is becoming established as a practical dietary approach for prevention and management of diabetes and obesity. Meal sequence, i.e., consumption of protein and/or fat before carbohydrate, promotes secretion of glucagon-like peptide-1 (GLP-1) from the gut and ameliorates secretions of insulin and glucagon and delays gastric emptying, thereby improving postprandial glucose excursion. GLP-1 is known to suppress appetite by acting on the hypothalamus via the afferent vagus nerve. Thus, enhancement of GLP-1 secretion by meal sequence is expected to reduce body weight. Importantly, consumption of a diet rich in saturated fatty acids such as meat dishes before carbohydrate increases secretions of not only GLP-1 but also glucose-dependent insulinotropic polypeptide (GIP), which promotes energy storage in adipose tissue and may lead to weight gain in the long term. Dietary fiber intake before carbohydrate intake significantly reduces postprandial glucose elevation and may have a weight loss effect, but this dietary strategy does not enhance the secretion of GLP-1. Thus, it is suggested that their combination may have additive effects on postprandial glucose excursion and body weight. Indeed, results of some clinical research supports the idea that ingesting dietary fiber together with meal sequence of protein and/or fat before carbohydrate benefits metabolic conditions of individuals with diabetes and obesity.
Keywords: dietary therapy, meal sequence, diabetes, obesity, incretin
1. Introduction
Early metabolic changes in type 2 diabetes (T2D) typically show an increase in postprandial blood glucose [1]. Postprandial hyperglycemia is an independent risk factor for complications of T2D, including microvascular and macrovascular complications [2,3,4]. Furthermore, it has been shown that decreasing postprandial glucose elevation can reduce the incidence of T2D and that controlling postprandial blood glucose with dietary therapy may be effective in preventing diabetes [5,6]. Under these circumstances, there is much interest in meal sequence for the control of postprandial glucose elevation and body weight. Meal sequence, a relatively simple method of correcting postprandial hyperglycemia, is becoming established as a practical dietary treatment for diabetes and obesity. In this article, the mechanisms involved in the beneficial effects of meal sequence are discussed.
2. Secretion and Function of Glucagon-Like Peptide-1 (GLP-1)
GLP-1 is one of two incretins secreted from the gut in response to ingestion of the various nutrients (e.g., carbohydrate, protein, and fat), and stimulate insulin secretion from pancreatic β-cells glucose-dependently [7]. In addition, GLP-1 suppresses glucagon secretion from pancreatic α-cells and delays gastric emptying, thereby ameliorating postprandial glucose excursion [7]. GLP-1 has been attracting interest as an important therapeutic strategy for diabetes, and GLP-1 receptor agonists are now being used to manage glycemia in individuals with T2D globally. GLP-1 is also known to suppress appetite and reduce food intake; and GLP-1 receptor agonists reduce body weight substantially, making the drugs valuable for individuals with morbid obesity [7]. Studies in experimental animals revealed that effects of GLP-1 on secretions of insulin and glucagon, gastric emptying and appetite involve activation of the vagus nerve [8]. Studies using GLP-1 receptor- deficient mice revealed that GLP-1 exerts protective effects on heart, kidney, and nervous system, suggesting that GLP-1 may prevent diabetes-related complications [9,10,11]. Indeed, some GLP-1 receptor agonists have been shown to reduce incidence of cardiovascular death, non-fatal myocardial infarction, non-fatal cerebral infarction, and impaired renal function in high-risk T2D patients [12,13]. Taken together, it is conceivable that enhancement of GLP-1 secretion by nutrients should exert beneficial effects on prevention and progression of diabetes and obesity.
3. Preloading Protein and/or Fats before Carbohydrates
Various studies have been conducted to investigate the effects of amelioration of postprandial blood glucose levels by preloading protein and amino acids before carbohydrate intake. For example, intake of 55 g whey protein before potato soup enhances GLP-1 and insulin secretion, delays gastric emptying and ameliorates postprandial glucose elevation [14]. Intake of 40 g glutamine before mixed meal (37 g carbohydrate, 1.3 g fat, and 16 g protein) enhances GLP-1 and insulin secretion and ameliorates postprandial glucose elevation as well [15]. These effects have been observed in T2D and nondiabetic subjects [16]. It was reported that the glucose-lowering effect of protein preload was dose-dependent [17]. Several studies have been conducted to investigate the effects of preloading fats. Gentilcore D et al. reported that intake of 30 mL olive oil before mashed potato (61 g carbohydrate) enhances GLP-1, delays gastric emptying and ameliorates postprandial glucose elevation [18]. However, enhancement of insulin secretion was not found in the study. The effect of delay in gastric emptying appears more strongly when preloading olive oil than when preloading whey proteins. Thus, inhibition of gastric emptying may ameliorate glucose elevation more strongly than enhancement of insulin secretion by incretins.
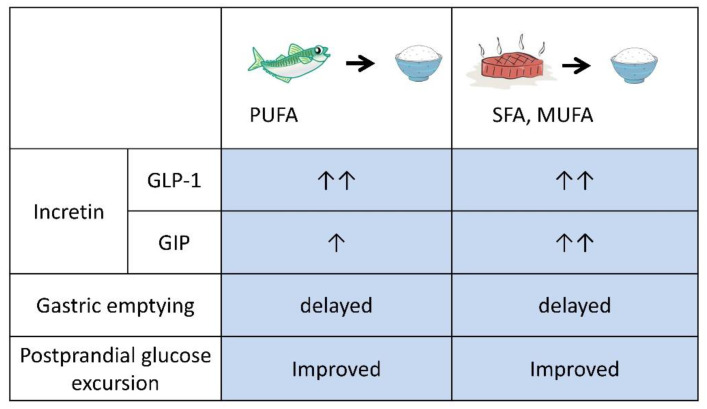
Preloading protein or fat before carbohydrate increased GLP-1 secretion and ameliorated postprandial hyperglycemia; however, intake of several tens of grams of whey protein, glutamine or olive oil at each meal, as performed in those protocols, is unrealistic. Thus, our group previously investigated preloading nutrients in daily meals. The effects of preloading fish (boiled mackerel: 15 g protein, 18 g fat, 0 g carbohydrate) and meat (grilled beef: 15 g protein, 18 g fat, 0 g carbohydrate) as a source of mixed protein and fat to be consumed before rice, as the source of carbohydrate, were evaluated in a crossover study comparing drug-naive T2D patients and healthy subjects (Figure 1) [19].
Figure 1.
Summary of preloading fish and meat before carbohydrate. Preload of fish and meat ameliorated postprandial glucose excursion by enhancing GLP-1 secretion and delaying gastric emptying. Preload of meat, which is rich in saturated fat (SFA) and monounsaturated fat (MUFA), enhances glucose-dependent insulinotropic polypeptide (GIP) secretion more than fish, which is rich in polyunsaturated fat (PUFA). As GIP promotes fat accumulation, frequent preload of meat before carbohydrate might well increase body weight.
When fish or meat dishes were consumed before rice, the postprandial glucose elevation was significantly reduced, and the secretion of GLP-1 was increased and gastric emptying time was prolonged. These findings are consistent with currently available data on preloading oil, protein, and their mixture with or without dietary fiber (Figure 2 and Table 1).
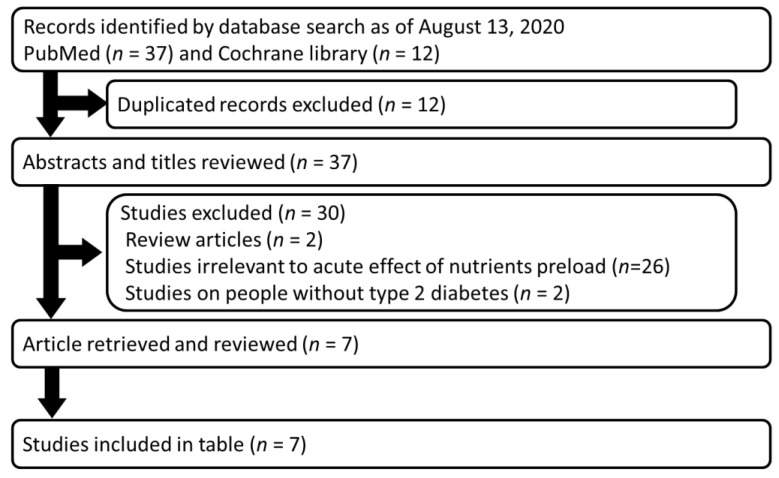
Figure 2.
Flow diagram of the systematic review. Relevant articles were searched in PubMed and Cochrane library. The search terms were (Fat OR Protein OR fiber) AND (Preload OR “meal sequence” OR “postprandial glycemia” OR “postprandial glucose” OR “glucose excursion”) AND (“type 2 diabetes”) AND (“glucose-dependent insulinotropic polypeptide”) AND (“glucagon-like peptide-1”) NOT (Review[pt]). The resulting 37 articles were inspected for their relevancy, and 7 manuscripts are listed in Table 1.
Table 1.
Preloading fat, protein, and their mixture with and without dietary fibers before carbohydrate in individuals with type 2 diabetes.
| Preload | Details of Main Meal | n | Outcomes | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Details | Timing | Glucose | Insulin | Glucagon | GLP-1 | GIP | CCK | GE | |||
| F | 30 ml olive oil | 30 min before C | 65 g mashed potato/20 g glucose (C 61 g) | 6 | Peak delayed | Peak delayed | ND | Enhanced | NC | ND | Delayed | [18] |
| P | 55 g whey protein | 30 min before C | 65 g mashed potato/20 g glucose (C 59.1 g; P 5.2 g; F 4.3 g) | 8 | Suppressed | Enhanced | ND | Enhanced | Enhanced | Enhanced | Delayed | [14] |
| P | 25 g whey protein | 30 min before C | 65 g mashed potato/20 g glucose/1 egg yolk | 22 | Suppressed | Enhanced | Enhanced | Enhanced | Enhanced | ND | Delayed | [20] |
| Mixed | 50 g cheese/one small-size boiled egg (C 2 g; P 23 g; F 17 g) | 30 min before C | 75 g glucose (C 75 g) | 10 | Suppressed | NC | Enhanced | Enhanced | Enhanced | ND | ND | [21] |
| Mixed | 100 g steamed mackerel (C 0g; P 15.1 g; F 17.7 g) | 15 min before C | 150 g rice (C 53.4 g; P 3.5 g; F 0.6g) | 12 | Suppressed | Enhanced | Enhanced | Enhanced | Enhanced | ND | Delayed | [19] |
| Mixed | 79 g grilled beef (C 0.2 g; P 16.4 g; F 17.1 g) | 15 min before C | 150 g rice (C 53.4 g; P 3.5 g; F 0.6g) | 12 | Suppressed | Enhanced | Enhanced | Enhanced | Enhanced* | ND | Delayed | [19] |
| Mixed | Protein enriched, dietary fiber fortified bar (C 0.4 g; P 10.7 g; F 0.3 g; Fiber 12.7 g) | 30 min before C | 286 g Bagle/70 g Cream cheese/95g Orange juice (C 79.5 g; P 15.5 g; F 50.5g) | 15 | Suppressed | Suppressed | ND | Enhanced | NC | ND | ND | [16] |
| Mixed | 50 g cheese/one small-size boiled egg (C 2 g; P 23 g; F 17 g) | 30 min before C | 75 g glucose (C 75 g) | 9 | Suppressed | NC | Enhanced | Enhanced | Enhanced | ND | ND | [22] |
Published results on preloading fat, protein, and their mixture with and without dietary fibers before carbohydrate in individuals with type 2 diabetes are summarized. C, carbohydrate; CCK, cholecystokinin; F, fat; GE, gastric emptying; GLP-1, glucagon-like polypeptide-1; GIP, glucose-dependent insulinotropic polypeptide; P, protein; NC, no change; ND, not determined. *, enhancement of GIP secretion was greater with preloading grilled meat compared to preloading steamed mackerel.
Interestingly, in a comparison between meat and fish dishes, a significant difference in secretion of another incretin, glucose-dependent insulinotropic polypeptide (GIP) was observed, although the energy content, nutrient ratio, and amino acid compositions were similar (Figure 1). Fish dishes contain more polyunsaturated fatty acids, eicosapentaenoic acid, and docosahexaenoic acid, while meat dishes contain more saturated fatty acids. The difference in fatty acids may underlie the difference in GIP secretion. It is known that saturated and monounsaturated fats can enhance GIP secretion in humans [23,24]. When a meal rich in saturated fatty acids is consumed, large amounts of GIP are secreted, which promotes energy storage in the adipose tissue [9]. Therefore, preloading meats, which are rich in saturated fatty acids, even if postprandial hyperglycemia is suppressed in the short term, may lead to weight gain in the long term.
4. Preloading Dietary Fiber before Carbohydrates
The effects of preloading dietary fiber before carbohydrate intake has also been discussed intensively. Dietary fiber is a generic term for components that are not digested and absorbed in the body. It has been shown that fiber has an effect on lifestyle-related diseases such as diabetes and obesity, through inhibition of carbohydrate and lipid absorption in the short term and through effects on gut microbiota in the long term [25]. It is believed that dietary fiber swells in the stomach, increasing the viscosity of the food mass and delaying the time of gastric emptying [26]; however, in our study, no obvious delay in gastric emptying time or increase in GLP-1 secretion when vegetables were ingested before rice was observed (S.K., H.K., and D.Y. unpublished observation). Sun et al. reports a similar effect under normal glucose tolerance, in which GLP-1 secretion shows no significant difference between eating vegetables before “meat and rice” and eating “vegetable, meat, and rice” together; however, glucose excursion was significantly ameliorated by eating vegetables before “meat and rice”. They found that ingesting vegetables first and meat afterward before rice was most ameliorative of postprandial glycemic excursion [27]. These results suggest that preloading protein or fat and dietary fiber before carbohydrate may have different mechanisms of effect on postprandial hyperglycemia and weight loss, and that the combination is expected to have additive effects. In addition, Jae Hyun Bae et al. reported the postprandial glucose-lowering effect of a premeal protein-enriched, dietary fiber-fortified bar containing a moderate amount of protein in individuals with T2D or normal glucose tolerance. The bar contained 0.4 g of carbohydrate, 9.3 g of whey protein, 1.4 g of soy protein, 0.3 g of fat, and 12.7 g of dietary fiber. The study showed a significantly decreased glucose elevation with a small amount of mixed protein and fiber [16].
5. Long-Term Effects of Preload-Based Dietary Strategies
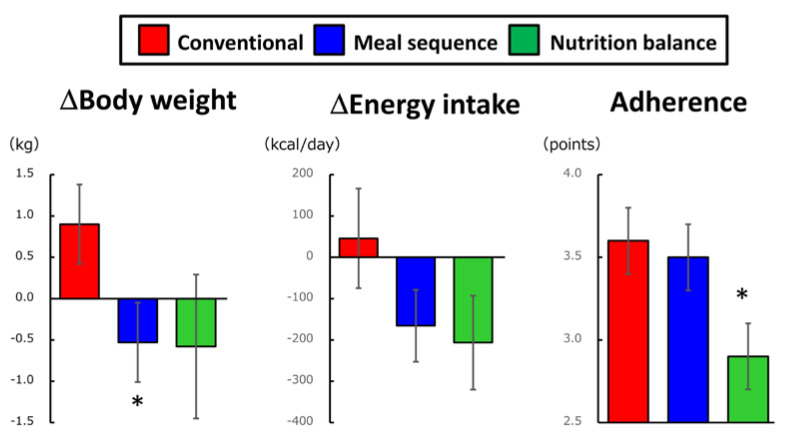
A few reports have examined the long-term effects of preload-based dietary strategies. Toriko et al. studied 17 patients with type 2 diabetes divided into two groups: those who consumed protein and fat before carbohydrate and those who did not sequence meal components for 8 weeks. A significant decrease in HbA1c in the group that consumed protein and fat before carbohydrate was noted [28]. Imai et al. reported that Japanese T2D patients who were instructed to consume fiber-rich vegetables before carbohydrate showed a significant improvement in HbA1c and a trend toward decreased BMI [29]. Recently, the effects of dietary instructions focusing on meal sequence versus nutritional balance in individuals with prediabetes in the Japanese national health check-up and guidance program were recently evaluated. In this study, effects of health guidance with dietary instructions focusing on meal sequence were compared to conventional health guidance and health guidance with dietary instructions focusing on nutritional balance (Figure 3). It was found that health guidance with dietary instructions focusing on meal sequence reduced body weight compared to that with dietary instructions focusing on nutritional balance [30].
Figure 3.
Dietary intervention focusing on meal sequence suppressed energy intake and reduced body weight in individuals with prediabetes. Effects of dietary instruction focusing on meal sequence (Meal sequence, n = 18) were compared to conventional dietary instruction (Conventional, n = 11) and dietary instruction focusing on nutritional balance (Nutrition balance, n = 13) using SMART Washoku® (Kao Corporation, Tokyo, Japan), which can help individuals consume a more nutritionally balanced diet, in an exploratory, cluster-randomized, prospective, open-label, clinical trial [30]. Each participant reported adherence to their goals for diet and healthy exercise every month using a scale of 1–5, where 1 indicated “seldom adhered” and 5 indicated “fully adhered”. The group receiving dietary instruction focusing on meal sequence exhibited similar adherence and greater reduction in body weight than the group receiving conventional health guidance, while the group receiving dietary instructions focusing on nutritional balance failed to show significant body weight reduction, partly due to poor adherence. Mean±SEM; *, p < 0.05 versus conventional and p < 0.05 versus meal sequence.
6. Conclusive Remarks and Future Perspectives
Many studies have found that preloading nutrients such as protein, fat, and fiber before carbohydrate can ameliorate postprandial glucose elevation. Preloading the various non-carbohydrate nutrients before carbohydrate intake engages distinct mechanisms but has a consistent effect on amelioration of elevated postprandial glucose. Interventions on the order of eating may be more readily followed than interventions on the nutritional balance of meals. Indeed, it has been reported that adherence to a meal sequence program is better than that to a nutritional balance program [30]. Interventions focusing on meal sequence facilitate secretion of GLP-1, which has the property of inhibiting appetite, suggesting that long-term interventions on meal sequence may lead to prevention or improvement of obesity. However, precise mechanisms underlying effects of meal sequence remains largely unknown. For example, it is unknown why consumption of protein and/fat before carbohydrate, but not after carbohydrate, enhances GLP-1 secretion. It is unknown whether other factors such as cholecystokinin and/or glucagon as well as the vagus nerve system that also regulates gastric emptying contribute to effects of gastric emptying. Experiments investigating mechanisms underlying meal sequence are underway. Meanwhile, currently available reports strongly support that meal sequence dietary therapy is beneficial in controlling postprandial glucose excursion and bodyweight to better prevent and manage diabetes and obesity.
Acknowledgments
The authors are very grateful to all researchers in the field who made substantial contributions to our understanding the effects of meal sequence.
Author Contributions
S.K., Y.L., K.I., H.K., Y.S. and D.Y. have made a substantial, direct and intellectual contribution to the work, and approved it for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Japan Society for the Promotion of Sciences (JSPS) KAKENHI Grant 17K09825 (to D.Y.), 20K11619 (H.K.) and 17K00850 (to K.I).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Monnier L., Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr. Pract. 2006;12(Suppl. 1):42–46. doi: 10.4158/EP.12.S1.42. [DOI] [PubMed] [Google Scholar]
- 2.Smith-Palmer J., Brandle M., Trevisan R., Orsini Federici M., Liabat S., Valentine W. Assessment of the association between glycemic variability and diabetes-related complications in type 1 and type 2 diabetes. Diabetes Res. Clin. Pract. 2014;105:273–284. doi: 10.1016/j.diabres.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Bhupathiraju S.N., Tobias D.K., Malik V.S., Pan A., Hruby A., Manson J.E., Willett W.C., Hu F.B. Glycemic index, glycemic load, and risk of type 2 diabetes: Results from 3 large US cohorts and an updated meta-analysis. Am. J. Clin. Nutr. 2014;100:218–232. doi: 10.3945/ajcn.113.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borch-Johnsen K., Neil A., Balkau B., Larsen S., Nissinen A., Pekkanen J., Keinanen-Kiukaanniemi S., Hiltunen L., Kivela S.L., Jousilahti P., et al. Glucose tolerance and mortality: Comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354:617–621. [PubMed] [Google Scholar]
- 5.Chiasson J.L., Josse R.G., Gomis R., Hanefeld M., Karasik A., Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 6.Raz I., Ceriello A., Wilson P.W., Battioui C., Su E.W., Kerr L., Jones C.A., Milicevic Z., Jacober S.J. Post hoc subgroup analysis of the HEART2D trial demonstrates lower cardiovascular risk in older patients targeting postprandial versus fasting/premeal glycemia. Diabetes Care. 2011;34:1511–1513. doi: 10.2337/dc10-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seino Y., Kuwata H., Yabe D. Incretin-based drugs for type 2 diabetes: Focus on East Asian perspectives. J. Diabetes Investig. 2016;7(Suppl. 1):102–109. doi: 10.1111/jdi.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yabe D., Seino Y., Seino Y. Incretin concept revised: The origin of the insulinotropic function of glucagon-like peptide-1—The gut, the islets or both? J. Diabetes Investig. 2018;9:21–24. doi: 10.1111/jdi.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seino Y., Yabe D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: Incretin actions beyond the pancreas. J. Diabetes Investig. 2013;4:108–130. doi: 10.1111/jdi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Kubota S., Iizuka K., Yabe D. Cardioprotective effects of GLP-1(28-36a): A degraded metabolite or GLP-1′s better half? J. Diabetes Investig. 2020 doi: 10.1111/jdi.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi M., Zochodne D.W. Diabetic neuropathy and the sensory neuron: New aspects of pathogenesis and their treatment implications. J. Diabetes Investig. 2018;9:1239–1254. doi: 10.1111/jdi.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yabe D., Seino Y. Cardiovascular safety trials of incretin-based drugs: What do they mean? J. Diabetes Investig. 2017;8:272–276. doi: 10.1111/jdi.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristensen S.L., Rorth R., Jhund P.S., Docherty K.F., Sattar N., Preiss D., Køber L., Petrie M.C., McMurray J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 14.Ma J., Stevens J.E., Cukier K., Maddox A.F., Wishart J.M., Jones K.L., Clifton P.M., Horowitz M., Rayner C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care. 2009;32:1600–1602. doi: 10.2337/dc09-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samocha-Bonet D., Wong O., Synnott E.L., Piyaratna N., Douglas A., Gribble F.M., Holst J.J., Chisholm D.J., Greenfield J.R. Glutamine reduces postprandial glycemia and augments the glucagon-like peptide-1 response in type 2 diabetes patients. J. Nutr. 2011;141:1233–1238. doi: 10.3945/jn.111.139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae J.H., Kim L.K., Min S.H., Ahn C.H., Cho Y.M. Postprandial glucose-lowering effect of premeal consumption of protein-enriched, dietary fiber-fortified bar in individuals with type 2 diabetes mellitus or normal glucose tolerance. J. Diabetes Investig. 2018;9:1110–1118. doi: 10.1111/jdi.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akhavan T., Luhovyy B.L., Brown P.H., Cho C.E., Anderson G.H. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am. J. Clin. Nutr. 2010;91:966–975. doi: 10.3945/ajcn.2009.28406. [DOI] [PubMed] [Google Scholar]
- 18.Gentilcore D., Chaikomin R., Jones K.L., Russo A., Feinle-Bisset C., Wishart J.M., Rayner C.K., Horowitz M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J. Clin. Endocrinol. Metab. 2006;91:2062–2067. doi: 10.1210/jc.2005-2644. [DOI] [PubMed] [Google Scholar]
- 19.Kuwata H., Iwasaki M., Shimizu S., Minami K., Maeda H., Seino S., Nakada K., Nosaka C., Murotani K., Kurose T. Meal sequence and glucose excursion, gastric emptying and incretin secretion in type 2 diabetes: A randomised, controlled crossover, exploratory trial. Diabetologia. 2016;59:453–461. doi: 10.1007/s00125-015-3841-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu T., Little T.J., Bound M.J., Borg M., Zhang X., Deacon C.F., Horowitz M., Jones K.L., Rayner C.K. A Protein Preload Enhances the Glucose-Lowering Efficacy of Vildagliptin in Type 2 Diabetes. Diabetes Care. 2016;39:511–517. doi: 10.2337/dc15-2298. [DOI] [PubMed] [Google Scholar]
- 21.Trico D., Baldi S., Tulipani A., Frascerra S., Macedo M.P., Mari A., Ferrannini E., Natali A. Mechanisms through which a small protein and lipid preload improves glucose tolerance. Diabetologia. 2015;58:2503–2512. doi: 10.1007/s00125-015-3710-9. [DOI] [PubMed] [Google Scholar]
- 22.Trico D., Nesti L., Frascerra S., Baldi S., Mengozzi A., Natali A. A Protein/Lipid Preload Attenuates Glucose-Induced Endothelial Dysfunction in Individuals with Abnormal Glucose Tolerance. Nutrients. 2020;12:2053. doi: 10.3390/nu12072053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itoh K., Moriguchi R., Yamada Y., Fujita M., Yamato T., Oumi M., Holst J.J., Seino Y. High saturated fatty acid intake induces insulin secretion by elevating gastric inhibitory polypeptide levels in healthy individuals. Nutr. Res. 2014;34:653–660. doi: 10.1016/j.nutres.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Lardinois C.K., Starich G.H., Mazzaferri E.L. The postprandial response of gastric inhibitory polypeptide to various dietary fats in man. J. Am. Coll. Nutr. 1988;7:241–247. doi: 10.1080/07315724.1988.10720241. [DOI] [PubMed] [Google Scholar]
- 25.InterAct C. Dietary fibre and incidence of type 2 diabetes in eight European countries: The EPIC-InterAct Study and a meta-analysis of prospective studies. Diabetologia. 2015;58:1394–1408. doi: 10.1007/s00125-015-3585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong J.M.W., Jenkins D.J.A. Carbohydrate Digestibility and Metabolic Effects. J. Nutr. 2007;137:2539S–2546S. doi: 10.1093/jn/137.11.2539S. [DOI] [PubMed] [Google Scholar]
- 27.Sun L., Goh H.J., Govindharajulu P., Leow M.K., Henry C.J. Postprandial glucose, insulin and incretin responses differ by test meal macronutrient ingestion sequence (PATTERN study) Clin. Nutr. 2020;39:950–957. doi: 10.1016/j.clnu.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Trico D., Filice E., Trifiro S., Natali A. Manipulating the sequence of food ingestion improves glycemic control in type 2 diabetic patients under free-living conditions. Nutr. Diabetes. 2016;6:e226. doi: 10.1038/nutd.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai S., Matsuda M., Hasegawa G., Fukui M., Obayashi H., Ozasa N., Kajiyama S. A simple meal plan of ‘eating vegetables before carbohydrate’ was more effective for achieving glycemic control than an exchange-based meal plan in Japanese patients with type 2 diabetes. Asia Pac. J. Clin. Nutr. 2011;20:161–168. [PubMed] [Google Scholar]
- 30.Yabe D., Kuwata H., Fujiwara Y., Sakaguchi M., Moyama S., Makabe N., Murotani K., Asano H., Ito S., Mishima H., et al. Dietary instructions focusing on meal-sequence and nutritional balance for prediabetes subjects: An exploratory, cluster-randomized, prospective, open-label, clinical trial. J. Diabetes Complicat. 2019;33:107450. doi: 10.1016/j.jdiacomp.2019.107450. [DOI] [PubMed] [Google Scholar]