Abstract
Background:
To develop a novel nomogram to improve the preoperative diagnosis of pathological grade of upper tract urothelial carcinoma (UTUC).
Methods:
Retrospective study was conducted with 245 patients with UTUC treated by radical nephroureterectomy from 2002 to 2016. Of the cohort, 57.6% received ureteroscopic (URS) biopsy and 35.9% received urine cytology examination. Preoperative clinical characteristics and examination results were collected. Final pathological grade was diagnosed by postoperative pathology. Univariable and multivariable binary logistic regressions were applied to establish a preoperative predictive model for tumor grade, and significant factors were included in the nomogram. The area under curve (AUC) was used to show the predictive efficacy, and the calibration plot was drawn for validation.
Results:
Of the 245 patients, 72.7% were diagnosed with pathological high-grade disease. Age (odds ratio [OR] = 1.03, P = .039), sessile (OR = 3.86, P = .021), positive urinary cytology (OR = 6.87, P = .035), and biopsy high-grade result (OR = 10.85, P < .001) were independent predictors for pathological high-grade disease. The predictive nomogram containing these factors achieved an AUC of 0.78, which was significantly better than URS biopsy alone (AUC = 0.62, P = .003) in the whole cohort. In the URS biopsy subgroup, the nomogram achieved an AUC of 0.79, better than biopsy alone (AUC = 0.76), but was not statistically significant (P = .431). When the cutoff value of the nomogram was set at 0.64, the sensitivity of detecting a high-grade lesion versus low-grade lesion was 80.3%, better than that of URS biopsy alone (sensitivity = 65.7%).
Conclusions:
Advanced age, sessile, positive urinary cytology, and biopsy high-grade were independent predictors of pathological high-grade disease in patients with UTUC. A nomogram containing these factors can improve diagnostic accuracy, potentially reducing the risk of “undergrading” by URS biopsy.
Keywords: Upper tract urothelial carcinoma, tumor grade, nomogram, predictive model, ureteroscopic biopsy
Introduction
Urothelial carcinoma comprises lower urinary tract urothelial carcinoma (including bladder cancer and uretheral cancer) and upper tract urothelial carcinoma (UTUC, including renal pelvic cancer and ureteral cancer). Of all urothelial carcinomas, 5% to 10% cases are UTUC, whereas bladder cancer accounts for the other 90% to 95%.1 The prevalence of UTUC has increased in recent years because of the increase in both diagnostic rate and survival rate.2,3 In western countries, the annual incidence of UTUC is about 2 of 100 0001 with more cases in men than those in women (2:1),2 and the average age of patients at the time of diagnosis is around 70.3 Incidence of renal pelvis cancer is about twice as high as that of ureteral cancer.3 At the time of diagnosis, about 60% of patients with renal pelvis cancer have muscle-invasive disease, which is more common than in bladder cancer (15%-25%),4 and about 7% of patients have metastatic diseases.3
Traditionally, radical nephroureterectomy (RNU) with bladder cuff excision was the standard treatment of UTUC, whereas kidney sparing surgery (KSS) was only suitable for patients with anatomically/functionally solitary kidneys or bilateral UTUC, or for those unsuitable for radical surgeries.5 However, the indications for KSS have expanded recently.6 The current 2019 European Association of Urology (EAU) guideline recommends KSS as an option for patients who meet all low-risk criteria, which includes (1) unifocal disease, (2) tumor size <2 cm, (3) low-grade cytology, (4) low-grade ureteroscope (URS) biopsy, (5) no invasive indication on computerized tomography urogram (CTU) or magnetic resonance urography (MRU), (6) no hydronephrosis, (7) no previous radical cystectomy or bladder cancer history, and (8) no variant histology, irrespective of contralateral kidney status.7 This risk-stratified strategy can preserve up to 15% estimated glomerular filtration rate (eGFR) compared with RNU and can potentially avoid long-term cardiovascular morbidity.6,8 However, the preoperative diagnosis of tumor grade is difficult. At present, urinary cytology and URS biopsy can both provide useful information for tumor grading, but their sensitivity of distinguishing high-grade disease from low-grade disease is low (about 60% for URS biopsy)9 with tremendous risk of “undergrade,” which requires frequent follow-up after KSS and has a potential to affect the oncological outcome.1 Thus, our aim is to establish a predictive nomogram containing comprehensive preoperative factors to improve the preoperative diagnostic accuracy of tumor grade and facilitate patient selection for KSS.
Patients and Methods
Study design and population
This study was conducted retrospectively with the approval of the ethics committee of Peking University Third Hospital (reference number 1RB00006761—M2019138). The case inclusion criteria included the following: (1) patients who received RNU + bladder cuff excision in our medical center from January 2002 to December 2016 and (2) postoperative pathology confirmed as UTUC. The exclusion criteria included the following: (1) history of previous UTUC, (2) history of renal transplantation, (3) harboring UTUC in both sides, (4) concomitant with other malignant tumors than bladder cancer, and (5) concomitant with distant metastases.
Definition of preoperative clinical factors and pathological grade
(1) The formula of eGFR was as follow: eGFR (mL/min/1.73 m2) = 186 × serum creatinine−1.154 × age−0.203 × 0.742 (if female).8 (2) Clean catch, catheterized, or in situ urine were used as urine specimens for urinary cytology. Detection of malignant urothelial cells or atypical cells highly suspected as urothelial carcinoma were defined as positive. Other results such as atypical hyperplasia, too few cells, or inability to judge were defined as negative. (3) Hydronephrosis was determined according to preoperative imaging examination (CTU, MRU, or B ultrasound). (4) Tumor, lymph node, and metastasis (TNM) stage was evaluated according to 2017 TNM staging system.1 (5) Morphology was determined by imaging and URS results as pedicle or sessile. (6) Multifocality was diagnosed by preoperative images (CTU, MRU, or B ultrasound) and URS results. Two or more lesions in the upper urinary tract were defined as multifocal. (7) Pathological grade of biopsy was evaluated according to the 2016 World Health Organization (WHO) histological grading system and was divided into high grade and low grade.1 (8) For multifocal tumors, tumor size was determined by the largest lesion, and tumor architecture was considered “sessile” if any sessile lesion was suspected.
Ureteroscopy and biopsy
After epidural or general anesthesia, the patient was placed in lithotomy position. The F21 cystoscope was placed into the urethra under direct vision. The bladder mucosa was observed and the bilateral ureteral orifices were identified. A guide wire was inserted through cystoscope into the ureteral orifice of the affected side, to the renal pelvis, and then cystoscope was withdrawn. A F8/9.8 or F4.5/6.5 rigid ureteroscope was inserted, entering the ureter along the guide wire for ureter and renal pelvis observation. If any suspicious lesion was found, a biopsy forceps or biopsy basket was used to collect tissue biopsy for pathology evaluation.
After 2010, flexible ureteroscope was used for some patients with renal pelvic cancer. The guide wire was inserted into the ureter in the same way mentioned above. The F11/13 or F12/14 ureteric soft sheath was inserted along the guide wire into the ureter, and then the flexible ureteroscope was inserted into the soft sheath all the way up for observation of renal pelvis and upper, middle, and lower calices lesions. A basket or biopsy forceps were used to collect tissue specimens. Then the sheath and flexible ureteroscope were removed slowly, and ureter were observed meticulously in case there was any other lesion.
Treatment modality
UTUC was diagnosed by image studies (CT urography, MR urography, or ultrasound), and some were confirmed by biopsy. All included patients received open or laparoscopic RNU. Adjuvant chemotherapy was instilled intravesically after 1 week. Follow-up plan was scheduled depending on the tumor risk stratification.
Statistical analysis
Continuous variables were reported as median (range). Categorical variables were reported as number (percentage). Univariate binary logistic regression analysis was used to analyze preoperative factors related to pathological grade, and statistically significant factors were included in multivariate binary logistic regression analysis to establish pathological grade prediction models. The above statistical analysis was completed by SPSS 24.0 software. R software (3.5.0) was used to construct the nomogram; calculate the Harrell’s concordance index (c-index), which is a measurement of the model’s discrimination ability; and draw the calibration curve to evaluate the model’s consistency (300 bootstrap resampling). Bilateral P < .05 was defined as statistically significant.
Results
Baseline clinical characteristics
A total of 245 patients fulfilled the inclusion criteria. Clinical features are listed in Table 1. There were 113 men and 132 women, with a median age of 69 years; 109 cases had renal pelvic cancer, 121 cases had ureteral cancer, and 15 cases had cancer in both locations. The median tumor size was 3 cm. Muscle-invasion (⩾pT2) was diagnosed in 66.0% cases, and pathological high grade in 72.7%.
Table 1.
Clinical features of patients with upper tract urothelial carcinoma.
| Clinical features | Median (range)/no. (%) (N = 245) |
|---|---|
| Gender (male/female) | 132/113 |
| Age,a y | 69 (31-88) |
| BMIa (n = 179), kg/m2 | 24.3 (15.6-34.0) |
| ASA score (n = 216), % | |
| 1 | 32 (14.8) |
| 2 | 146 (67.6) |
| 3 | 38 (17.6) |
| Surgery year, % | |
| 2002-2010 | 69 (28.2) |
| 2011-2016 | 176 (71.8) |
| Gross hematuria, % | 182 (74.3) |
| Concomitant bladder cancer, % | 28 (11.4) |
| History of bladder cancer, % | 10 (4.1) |
| Smoking history (n = 240), % | 29 (12.1) |
| eGFR (n = 243), mL/min/1.73 m2 | |
| ⩾90 | 20 (8.2) |
| 60-90 | 104 (42.4) |
| 30-60 | 96 (39.2) |
| <30 | 23 (9.4) |
| Side (left/right) | 109/136 |
| Location, % | |
| Renal pelvis | 109 (44.5) |
| Ureter | 121 (49.4) |
| Both | 15 (6.1) |
| Tumor sizea (n = 223), cm | 3.0 (0.5-11.0) |
| Hydronephrosis (n = 230), % | 132 (57.4) |
| Multifocality, % | 42 (17.1) |
| Sessile, % | 44 (18.0) |
| pT, % | |
| Ta | 31 (12.7) |
| 1 | 52 (21.2) |
| 2 | 78 (31.8) |
| 3 | 78 (31.8) |
| 4 | 6 (2.4) |
| pN+, % | 7 (2.9) |
| Urine cytology positive (n = 88), % | 27 (30.7) |
| Biopsy high-grade (n = 141), % | 48 (34.0) |
| Postop pathologic high grade, % | 178 (72.7) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Continuous variables were presented in median (range).
Diagnostic power of URS biopsy
Among these patients, 141 (57.6%) underwent URS biopsy, result of which is shown in Figure 1. In total, 98 (69.5%) specimens obtained a biopsy grade. The diagnostic specificity of high-grade disease was 87.1%, sensitivity was 65.7%, positive predictive value was 91.7%, negative predictive value was 54.0%, and overall accuracy was 72.4% (Table 2).
Figure 1.
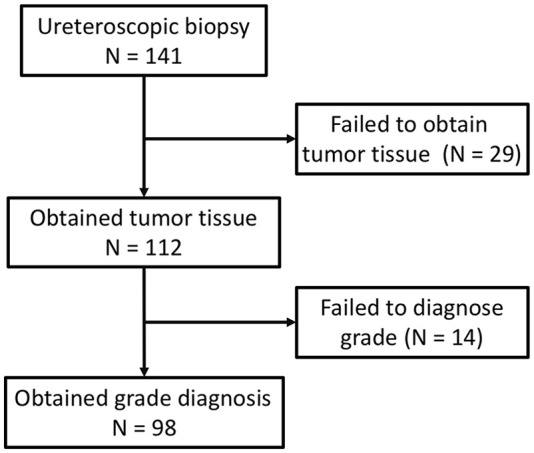
Information of ureteroscopic biopsy.
Table 2.
Ureteroscopic biopsy’s diagnostic power of tumor grade.
| Pathological high grade | Pathological low grade | ||
|---|---|---|---|
| Biopsy high grade |
44 | 4 | Positive predictive value 91.7% |
| Biopsy low grade |
23 | 27 | Negative predictive value 54.0% |
| Sensitivity 65.7% | Specificity 87.1% | Accuracy 72.4% |
Construction of tumor grade predictive nomogram
To improve the sensitivity of preoperative diagnosis of high-grade lesions, we analyzed preoperative clinical predictors of high-grade UTUC by univariate regression. As shown in Table 3, male (odds ratio [OR] = 0.51; 95% confidence interval [CI]: 0.29-0.90; P = .021), advanced age (OR = 1.03; 95% CI: 1.00-1.06; P = .024), sessile (OR = 4.57; 95% CI: 1.57-13.31; P = .005), positive urine cytology (OR = 4.20; 95% CI: 1.13-15.59; P = .032), and high-grade biopsy result (OR = 11.75; 95% CI: 4.04-34.15; P < .001) were significantly associated with pathological grade.
Table 3.
Univariable regression analysis of relevant predicters to tumor grade.
| Clinical features | Univariable |
||
|---|---|---|---|
| OR | 95% CI | P value | |
| Male vs female | 0.51 | 0.29-0.90 | .021 |
| Age, y | 1.03 | 1.00-1.06 | .024 |
| Right vs left | 1.42 | 0.81-2.49 | .228 |
| BMI, kg/m2 | 1.06 | 0.96-1.18 | .271 |
| Location | .468 | ||
| Renal pelvis | Ref. | Ref. | Ref. |
| Ureter | 0.00 | – | .998 |
| Both | 0.00 | – | .998 |
| Gross hematuria | 0.87 | 0.46-1.68 | .687 |
| Flank pain | 0.82 | 0.33-2.00 | .661 |
| Smoking history | 0.69 | 0.30-1.57 | .371 |
| Concomitant/history of bladder cancer | 1.38 | 0.59-3.19 | .457 |
| Hydronephrosis | 1.44 | 0.80-2.59 | .227 |
| eGFR, mL/min/1.73 m2 | 1.00 | 0.99-1.01 | .934 |
| Tumor size, cm | 1.02 | 0.88-1.20 | .773 |
| Multifocality | 2.10 | 0.88-4.99 | .093 |
| Sessile | 4.57 | 1.57-13.31 | .005 |
| Positive urine cytology | 4.20 | 1.13-15.59 | .032 |
| Biopsy high grade | 11.75 | 4.04-34.15 | <.001 |
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odd ratios; Ref., reference.
We then performed multivariate regression analysis by including the above significant clinical factors (Table 4). Advanced age (OR = 1.03; 95% CI: 1.00-1.07; P = .039), sessile (OR = 3.86; 95% CI: 1.22-12.21; P = .021), positive urine cytology (OR = 6.87; 95% CI: 1.59-29.77; P = .010), and high-grade biopsy finding (OR = 10.85; 95% CI: 3.58-32.91; P < .001) were independently associated with pathological grade. These factors were included in the final predictive nomogram, as shown in Figure 2.
Table 4.
Multivariable regression analysis of relevant predicters to tumor grade.
| Clinical features | Multivariable |
||
|---|---|---|---|
| OR | 95% CI | P value | |
| Male vs female | 0.57 | 0.30-1.08 | .085 |
| Age, y | 1.03 | 1.00-1.07 | .039 |
| Sessile | 3.86 | 1.22-12.21 | .021 |
| Urine cytology | .035 | ||
| Negative | Ref. | Ref. | Ref. |
| Positive | 6.87 | 1.59-29.77 | .010 |
| No results | 1.50 | 0.74-3.03 | .259 |
| Ureteroscopic | <.001 | ||
| Low grade | Ref. | Ref. | Ref. |
| High grade | 10.85 | 3.58-32.91 | <.001 |
| No results | 3.61 | 1.77-7.35 | <.001 |
Abbreviations: CI, confidence interval; OR, odd ratios; Ref., reference.
Figure 2.
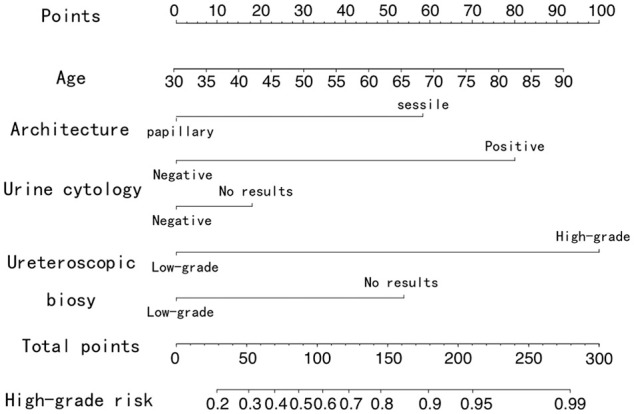
Diagnostic nomogram of tumor grade.
The optimal cutoff value for the predictive model was 0.73 (sensitivity + specificity maximum) with a sensitivity of 69.7% and a specificity of 74.6%. When we defined the cutoff value as 0.64, the diagnostic sensitivity achieved 80.3% and specificity 56.7%. The calibration plot of the model showed that the prediction was more accurate when high-grade probabilities ⩾70% (Figure 3).
Figure 3.
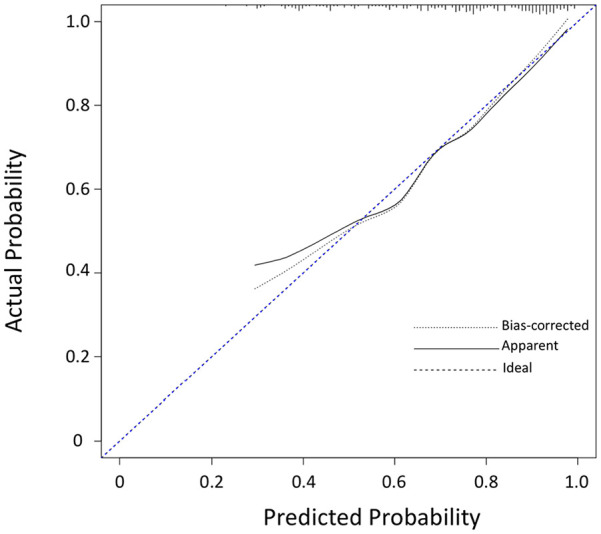
Calibration plot of the diagnostic nomogram.
Comparison between predictive nomogram and URS biopsy
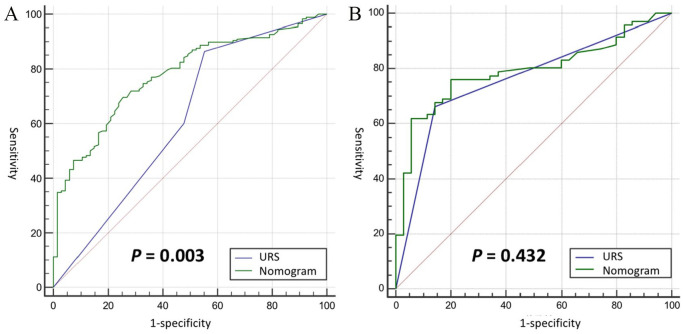
The nomogram model’s receiver operating characteristic (ROC) curve had an area under the curve (AUC) of 0.78, compared with that of 0.62 from URS biopsy (P < .001; Hanley & McNeil method), as shown in Figure 4A. The ROC curves of these 2 diagnostic methods were also compared in the subgroup with available URS biopsy grade. The AUC of the predictive model was 0.79, and the AUC of the URS biopsy was 0.76 (P = .431; Hanley & McNeil method), as shown in Figure 4B.
Figure 4.
Comparison between ureteroscopic (URS) biopsy and nomogram on diagnosing tumor grade. (A) Receiver operator characteristic (ROC) curve of URS biopsy and the nomogram in the whole cohort, which showed the nomogram performed significantly better than URS biopsy and (B) ROC of URS biopsy and the nomogram in the subgroup with available biopsy grade, which showed the nomogram slightly improved the area under the curve (AUC) but not statistically significant.
Discussion
Our study showed that advanced age, sessile, positive urine cytology, and high-grade biopsy finding were predictive factors of high-grade UTUC. A nomogram including these 4 factors improved the diagnostic power compared with URS biopsy alone.
Preoperative diagnosis of pathological grade is difficult in UTUC risk stratification. At present, the grade diagnosis depends on urine cytology or preoperative ureteroscopy. Urine cytology can provide useful information, but the overall sensitivity is only 34%,10 especially for low-grade lesions.11,12 Studies have reported that selective urine through catheterization, washing or brushing the cavities and lumen of the suspected renoureteral unit can improve the diagnosis,13 but its application in predicting tumor grade is still limited with the lack of reporting standard,14 the susceptibility of sampling technique,13 the influence of possible urinary infection,14 and the dependence on the cytopathologist’s experience.15
Our study confirmed the “undergrade” risk of URS biopsy and the potential of failure to obtain grade information. The accuracy of URS biopsy for pathological grade diagnosis is between 69% and 90%.13 As most high-grade biopsy results are consistent with final pathology, the “undergrade” rate of low-grade biopsy results can be as high as 30%.13 Clements et al9 reported that the sensitivity of diagnosing high-grade lesion by URS biopsy was 60%, with a negative predictive value of only 54%. Moreover, considering the chance of failure to diagnose tumor grade was 17.6%,16 now we eagerly need a better diagnostic solution for tumor grade.
In this study, we constructed a nomogram to improve preoperative diagnosis of tumor grade. The nomogram included patient age, tumor architecture, urine cytology result, and biopsy finding, which improved diagnostic performance compared with URS biopsy alone. The nomogram can be conveniently applied by adding the score of each risk factor and converting the total score to a predicted possibility.
The features included in the nomogram have all been widely mentioned in previous studies. Advanced age was reported to be related to high-grade UTUC both in a large Chinese cohort17 and in Surveillance, Epidemiology, and End Results (SEER) database18; sessile architecture was also a well-recognized feature associated with high-grade disease17,19; and urine cytology and URS biopsy, as we discussed previously, were most widely used methods to determine preoperative grade.13 We also included other previously reported predictors for pathologic grade in our analysis, such as smoking history,20,21 bladder cancer history,22 and hydronephrosis,17,23 and no statistically significant correlation was observed between these factors and high-grade disease in our cohort.
Our results showed that, compared with URS biopsy alone, the nomogram significantly improved AUC from 0.62 to 0.78 in the overall cohort, while slightly improved AUC from 0.76 to 0.78 in the subgroup with available biopsy grade information. This indicated that the nomogram can be used to provide additional information to biopsy results and can also risk stratify patients who have not undergone URS biopsy, or those who were unable to be diagnosed for tumor grade even with biopsy. The best cutoff value of the nomogram can be determined individually. For example, in case of reducing the possibility of “undergrade” before KSS, the cutoff value can be lowered to improve the sensitivity of high-grade diagnosis. If the cutoff value is set to 0.64, the model’s sensitivity of diagnosing high-grade disease reaches 0.80, significantly reducing the risk of “undergrade.”
According to our results, cases of younger patients with pedicle lesion, negative urine cytology finding, and low-grade biopsy result are most likely to be pathologically low-grade ones. In theory, such patients are also more suitable for kidney sparing treatment. Younger patients have a longer life expectancy, which means that kidney sparing may have a greater benefit (theoretic speculation, still needs to be confirmed); pedicle lesion tends to be low grade and noninvasive and usually harbors a better prognosis,19 which means less likely compromised oncological outcome following kidney sparing treatment and has been included in current risk stratification system1; urine cytology and URS biopsy are also necessary examinations to risk stratify preoperative patients.1 Thus, our grade-diagnostic nomogram is consistent with current understanding of indications for KSS and does not need any additional examinations.
There are several limitations in our study. First, 2 factors in our nomogram, urine cytology and URS biopsy findings, were still assigned a score when they had “no result,” which may cause instability of predictive performance due to the inherently inconsistent biopsy rate, urine cytology reporting system, and biopsy techniques across different medical centers. It suggests caution to extrapolate the conclusion of this study and external validation is needed in the future. However, the advantage is that the nomogram can be applied to patients who lack these examination results, for example, when patients do not undergo biopsy or fail to obtain biopsy grade. Second, with limited cohort size, this study may be under-powered when analyzing independent predictors for high-grade disease. Limited sample size restricts the number of factors that can be included in the nomogram, and may limit the diagnostic performance of the nomogram. Thus more cases are needed in the future.
Conclusions
In conclusion, our study confirmed that advanced age, sessile, positive urinary cytology, and biopsy high-grade were independent predictors of high-grade disease in patients with UTUC. A nomogram including these factors can improve diagnostic accuracy, potentially reducing the risk of “undergrading” before kidney-sparing treatment.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant from National key R&D program of China (2018YFC0115900).
Author Contributions: RM, JL and LM were responsible for concept and design. RM, HX, MQ were responsible for acquisition of data. ML was responsible for biopsy and pathology data. RM and LT were responsible for statistical analysis. RM and RH were responsible for analysis and interpretation of data and drafting of the manuscript. RM, JL and RH were responsible for critical revision of the manuscript for important intellectual content.
ORCID iD: Jian Lu  https://orcid.org/0000-0002-9144-7486
https://orcid.org/0000-0002-9144-7486
References
- 1. Rouprêt M, Babjuk M, Compérat E, et al. European Association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73:111-122. [DOI] [PubMed] [Google Scholar]
- 2. Shariat SF, Favaretto RL, Gupta A, et al. Gender differences in radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol. 2011;29:481-486. [DOI] [PubMed] [Google Scholar]
- 3. Soria F, Shariat SF, Lerner SP, et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J Urol. 2017;35:379-387. [DOI] [PubMed] [Google Scholar]
- 4. Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the upper tract urothelial carcinoma collaboration. Cancer. 2009;115:1224-1233. [DOI] [PubMed] [Google Scholar]
- 5. Seisen T, Peyronnet B, Dominguez-Escrig JL, et al. Oncologic outcomes of kidney-sparing surgery versus radical nephroureterectomy for upper tract urothelial carcinoma: a systematic review by the EAU Non-muscle Invasive Bladder Cancer Guidelines Panel. Eur Urol. 2016;70:1052-1068. [DOI] [PubMed] [Google Scholar]
- 6. Rouprêt M, Colin P, Yates DR. A new proposal to risk stratify urothelial carcinomas of the upper urinary tract (UTUCs) in a predefinitive treatment setting: low-risk versus high-risk UTUCs. Eur Urol. 2014;66:181-183. [DOI] [PubMed] [Google Scholar]
- 7. Rouprêt M, Babjuk M, Burger M, et al. EAU guidelines. Paper presented at: EAU Annual Congress Barcelona, 2019; March 15-19, 2019; Barcelona, Spain https://uroweb.org/guideline/upper-urinary-tract-urothelial-cell-carcinoma/. Accessed April 28, 2019. [Google Scholar]
- 8. Ma Y-C, Zuo L, Chen J-H, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937-2944. [DOI] [PubMed] [Google Scholar]
- 9. Clements T, Messer JC, Terrell JD, et al. High-grade ureteroscopic biopsy is associated with advanced pathology of upper-tract urothelial carcinoma tumors at definitive surgical resection. J Endourol. 2012;26:398-402. [DOI] [PubMed] [Google Scholar]
- 10. Messer J, Shariat SF, Brien JC, et al. Urinary cytology has a poor performance for predicting invasive or high-grade upper-tract urothelial carcinoma. BJU Int. 2011;108:701-705. [DOI] [PubMed] [Google Scholar]
- 11. Williams SK, Denton KJ, Minervini A, et al. Correlation of upper-tract cytology, retrograde pyelography, ureteroscopic appearance, and ureteroscopic biopsy with histologic examination of upper-tract transitional cell carcinoma. J Endourol. 2008;22:71-76. [DOI] [PubMed] [Google Scholar]
- 12. Tan WS, Sarpong R, Khetrapal P, et al. Does urinary cytology have a role in haematuria investigations? BJU Int. 2019;123:74-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baard J, de Bruin DM, Zondervan PJ, Kamphuis G, de la Rosette J, Laguna MP. Diagnostic dilemmas in patients with upper tract urothelial carcinoma. Nat Rev Urol. 2017;14:181-191. [DOI] [PubMed] [Google Scholar]
- 14. Owens CL, Vandenbussche CJ, Burroughs FH, Rosenthal DL. A review of reporting systems and terminology for urine cytology. Cancer Cytopathol. 2013;121:9-14. [DOI] [PubMed] [Google Scholar]
- 15. Karakiewicz PI, Benayoun S, Zippe C, et al. Institutional variability in the accuracy of urinary cytology for predicting recurrence of transitional cell carcinoma of the bladder. BJU Int. 2006;97:997-1001. [DOI] [PubMed] [Google Scholar]
- 16. Keeley FX, Kulp DA, Bibbo M, McCue PA, Bagley DH. Diagnostic accuracy of ureteroscopic biopsy in upper tract transitional cell carcinoma. J Urol. 1997;157:33-37. [PubMed] [Google Scholar]
- 17. Chen X-P, Xiong G-Y, Li X-S, et al. Predictive factors for worse pathological outcomes of upper tract urothelial carcinoma: experience from a nationwide high-volume centre in China. BJU Int. 2013;112:917-924. [DOI] [PubMed] [Google Scholar]
- 18. Yap SA, Schupp CW, Chamie K, Evans CP, Koppie TM. Effect of age on transitional cell carcinoma of the upper urinary tract: presentation, treatment, and outcomes. Urology. 2011;78:87-92. [DOI] [PubMed] [Google Scholar]
- 19. Remzi M, Haitel A, Margulis V, et al. Tumour architecture is an independent predictor of outcomes after nephroureterectomy: a multi-institutional analysis of 1363 patients. BJU Int. 2009;103:307-311. [DOI] [PubMed] [Google Scholar]
- 20. Rink M, Xylinas E, Margulis V, et al. Impact of smoking on oncologic outcomes of upper tract urothelial carcinoma after radical nephroureterectomy. Eur Urol. 2013;63:1082-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xylinas E, Kluth LA, Rieken M, et al. Impact of smoking status and cumulative exposure on intravesical recurrence of upper tract urothelial carcinoma after radical nephroureterectomy. BJU Int. 2014;114:56-61. [DOI] [PubMed] [Google Scholar]
- 22. Youssef RF, Shariat SF, Lotan Y, et al. Prognostic effect of urinary bladder carcinoma in situ on clinical outcome of subsequent upper tract urothelial carcinoma. Urology. 2011;77:861-866. [DOI] [PubMed] [Google Scholar]
- 23. Messer JC, Terrell JD, Herman MP, et al. Multi-institutional validation of the ability of preoperative hydronephrosis to predict advanced patho-logic tumor stage in upper-tract urothelial carcinoma. Urol Oncol. 2013;31:904-908. [DOI] [PubMed] [Google Scholar]