Abstract
Sea cucumbers (Holothuroidea; Echinodermata) are ecologically significant constituents of benthic marine habitats. We surveilled RNA viruses inhabiting eight species (representing four families) of holothurian collected from four geographically distinct locations by viral metagenomics, including a single specimen of Apostichopus californicus affected by a hitherto undocumented wasting disease. The RNA virome comprised genome fragments of both single-stranded positive sense and double stranded RNA viruses, including those assigned to the Picornavirales, Ghabrivirales, and Amarillovirales. We discovered an unconventional flavivirus genome fragment which was most similar to a shark virus. Ghabivirales-like genome fragments were most similar to fungal totiviruses in both genome architecture and homology and had likely infected mycobiome constituents. Picornavirales, which are commonly retrieved in host-associated viral metagenomes, were similar to invertebrate transcriptome-derived picorna-like viruses. The greatest number of viral genome fragments was recovered from the wasting A. californicus library compared to the asymptomatic A. californicus library. However, reads from the asymptomatic library recruited to nearly all recovered wasting genome fragments, suggesting that they were present but not well represented in the grossly normal specimen. These results expand the known host range of flaviviruses and suggest that fungi and their viruses may play a role in holothurian ecology.
Keywords: holothurian, Apostichopus, wasting, virus, flavivirus, totivirus
1. Introduction
Next generation DNA sequencing technology applied to viral metagenomics has enabled surveillance of viruses associated with invertebrate tissues. These studies, along with the mining of metazoan transcriptomes, have led to the discovery of novel viral lineages in aquatic invertebrates and broadened the host range of several viral families [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. While the majority of viral surveillance and discovery is focused on grossly normal individual specimens of aquatic metazoa, viral metagenomics has been used to examine the presence of potential pathogenic viruses through comparative asymptomatic/disease affected individuals [16]. Despite a growing appreciation of the diversity of aquatic metazoan-associated viruses, and of their potential roles in host disease, they remain largely under-sampled. Study of novel and/or highly divergent viral genomes across a wider range of aquatic invertebrates may provide clues to viral evolution and potential roles in host ecology.
Holothurians (Holothuroidea; Echinodermata) are ecologically important echinoderms [17,18] that can dominate benthic biomass, and contribute significantly to biogeochemical cycling in benthic compartments. On the abyssal plains, for example, holothurian biomass exceeds all other invertebrates [19]. Holothurians may be planktivorous, herbivorous or deposit feeders, and contribute to ecosystem function by regeneration of particulate organic matter into dissolved organic and inorganic constituents [18,20]. Feeding by holothurians on sediments stimulates bacterial production [20] and, potentially, bacterial diversity [21]. Holothurians produce pelagic larvae that as meroplankton contribute to herbivory [22,23,24]. Holothurians are also economically significant, since they are fished and aqua-cultured for human consumption [25]. In China, the world’s largest consumer of holothurians, more than 200 kT worth CNY 176M (US$26M) were produced in 2017 [26]. The economic, evolutionary and ecological significance of holothurians make these groups attractive to study for factors influencing their biology and population dynamics, including potentially pathogenic microorganisms.
Like all echinoderms, holothurians are deuterostomes and are therefore more closely related to chordates than other invertebrate groups. From a viral perspective, their similarity to chordates, and specifically the similarity of cell surface properties that mediate endocytosis and fusion, suggests that they may be infected by similar viral groups. For example, sialic acids, which are used by a variety of viral families to enter cells [27], became prominent late in evolution, especially in deuterostomes, where they play diverse physiological roles [28]. While echinoderms lack adaptive immunity, genome analyses of the echinoid Strongylocentrotus purpuratus revealed homologs of vertebrate immune factors [29,30]. Despite these similarities, few studies have assessed the composition of viruses in echinoderm tissues. DNA viruses, including Piccovirales [11,12,14] and Curlivirales [31,32] were discovered by viral metagenomic studies using amplified (ϕ29-mediated rolling circle replication) material. RNA viral surveys, which demand a different amplification protocol and handling from that of DNA viruses, have detected Baphyvirales, Picornavirales, Articulavirales and Tolivirales [13,33] in asteroids. However, RNA viruses in other echinoderm classes have not been investigated by viral metagenomic approaches.
In this study we surveyed RNA viruses in eight holothurian species collected from four distinct geographic locations (Heron Island, Moreton Bay, Salish Sea and Southeast Alaska) and representing four families of holothurians. We found that viruses associated with these tissues were dominated by Picornavirales (Marnaviridae) and Ghabrivirales (Totiviridae), but report on the presence of a deeply branched flavivirus in two holothurian species in the northeast Pacific Ocean. We also compared the composition of viruses associated with a grossly normal Apostichopus californicus and a specimen that was affected by sea cucumber wasting (a condition that is not extensively documented in the peer-reviewed literature) but found little difference in viral genome representation.
2. Materials and Methods
2.1. Specimen Collection
Holothurian specimens were collected at four geographic locations between 2015 and 2017 (Table 1). Specimens were collected by hand in the intertidal zone or by SCUBA Diver (with the exception of Cucumaria miniata, which was obtained by rock dredge). Specimens of Apostichopus californicus (renamed from Parastichopus californicus [34]) were collected by commercial fishers and retrieved by the Alaska Department of Fish and Game. The presence of a wasting-like condition was determined by gross disease signs on collection (see Section 3.4). Specimens were sub-sampled either immediately after collection, or by whole specimens being frozen on dry ice or liquid N2 before transport to the laboratory at Cornell University, where they were thawed and dissected prior to metavirome preparation.
Table 1.
Specimens collected for viral metagenomics survey and library characteristics.
| Species | Family | Sample Location | Latitude | Longitude | Collection Date | Sequence Library Size (reads) | # Contigs | N50 Contigs | Contigs Matching Viral Genomes E < 10−20 | Reads Mapped to Viral Contigs |
|---|---|---|---|---|---|---|---|---|---|---|
| Cucumaria miniata | Cucumariidae | Salish Sea | 48.2333 N | 122.7917 W | 7 January 2016 | 1,686,849 | 5394 | 811 | 3 | 247,494 |
| Apostichopus californicus * | Stichopodidae | Ketchikan | 55.4351 N | 131.9481 W | 26 October 2016 | 897,618 | 3431 | 804 | 11 | 195,230 |
| Apostichopus californicus | Stichopodidae | Ketchikan | 55.4351 N | 131.9481 W | 26 October 2016 | 1,019,071 | 3900 | 880 | 2 | 179,175 |
| Holothuria scabra | Holothuridae | Amity Banks | 27.4115 S | 153.4344 E | 10 December 2015 | 822,244 | 21,777 | 838 | 1 | 368 |
| Synaptula recta | Synaptidae | Dunwich | 27.4952 S | 153.3985 E | 10 December 2015 | 983,151 | 19,760 | 847 | 0 | 299 |
| Holothuria difficilis | Holothuridae | Amity Banks | 27.4115 S | 153.4344 E | 10 December 2015 | 794,579 | 5803 | 846 | 1 | 301 |
| Stichopus horrens | Stichopodidae | Amity Banks | 27.4115 S | 153.4346 E | 10 December 2015 | 1,577,532 | 21,194 | 817 | 2 | 423 |
| Holothuria atra | Holothuridae | Heron Island | 23.4441 S | 151.9113 E | 19 December 2015 | 875,840 | 18,866 | 793 | 1 | 345 |
| Holothuria pardalis | Holothuridae | Dunwich | 27.4952 S | 153.3985 E | 10 December 2015 | 1,467,795 | 7424 | 896 | 0 | 306 |
The * indicates that the specimen was affected by sea cucumber wasting (SCW).
2.2. Metavirome Preparation
Metaviromes were prepared from each specimen by first retrieving an 8 mm biopsy punch from body wall tissues, which was extruded into a sterile microcentrifuge tube. From here, viral metagenomes were prepared according to established protocols as described in [35] with modifications [36] and omitting the chloroform treatment step. Briefly, tissue samples were disrupted by bead beating (Zymo Bead Beaters) in 2 mL virus-free (i.e., 0.02 µm filtered) PBS, then centrifuged at 5000× g for 5 min to remove large particulate material. The resulting supernatant was then removed, filtered through 0.2 µm PES filters (to remove host cells and cell debris), and then treated with RNAseOne (50 U), DNAse I (5 U), and Benzonase nuclease (250 U) for 2 h at 37 °C. Nuclease activity was arrested by amendment with 50 µM EDTA. RNA was extracted from purified virus-sized material using the Zymo Viral RNA Kit. Extracted RNA was then amplified using the TransPlex WTA2 kit (Sigma Aldrich) applied to a 5 µL extract. Because we did not standardize template amounts, and because of uncertainties in bias introduced by TransPlex amplification, data presented in this manuscript are not quantitative and based solely on presence and absence of genome fragments [16]. Amplified DNA (the end product of the TransPlex protocol) was submitted to the Cornell University Biotechnology Resource Center, where it was prepared with the Nextera XT library preparation protocol and sequenced on an Illumina MiSeq 2 × 250 bp platform. Sequence libraries are available at National Center for Biotechnology Information (NCBI) under accession PRJNA417963.
2.3. Viral Metagenome Library Analyses
Sequence libraries were first trimmed for ambiguous bases, adapters, TransPlex primers, and poor quality (N < 2) sequences using the Trim Sequences function in the CLC Genomics Workbench 4.0 (Qiagen, Germany). Sequence libraries were then assembled using a minimum overlap of 0.2 and similarity of 0.9 resulting in contig spectra for each library. The resulting contig spectra was aligned against several boutique databases of RNA viruses: (1) all RNA viral library using tBLASTx [37] (genome sequences retrieved in September 2018 from NCBI using search term “RNA Virus”); (2) all Mononegavirus proteins (search term “mononegaviruses”) by BLASTx; Picornavirus ribosome-dependent RNA polymerase (RdRp) proteins (search term “RdRp AND picornavirus”) by BLASTx; invertebrate RNA viral proteins (search term “invertebrate AND RNA viruses”) by BLASTx; Flavivirus proteins (search term “flavivirus”) by BLASTx; Coronavirus proteins (search term “coronavirus”) by BLASTx; and nodavirus proteins (search term “nodavirus”) by BLASTx. Sequences were further vetted by comparing matches to viral proteins against the non-redundant database at NCBI by BLASTn, and matches (E < 10−30) to bacterial or eukaryotic genes were removed from further consideration. Finally, contigs were compared to the RefSeq and non-redundant protein database by BLASTx to retrieve closest matches from cultivated and uncultivated viruses. Viral contiguous sequences are available at NCBI under accessions MT949664–MT949685.
2.4. Viral Genome Architecture and Phylogeny
The open reading frames on vetted viral contigs were extracted with GetORF [38] and compared against the established genome architecture of viral families. If significant matches against proteins were detected, but open reading frame (ORF lengths) were shorter than expected based on reported genome architectures, the sequence was examined for internal frameshifts, which are common in viral ORFs. ORFs were then annotated by comparison against the RefSeq Viral Proteins Database (NCBI). ORFs and partial ORFs adjacent to annotated viral proteins were further compared against the non-redundant database at NCBI by tBLASTx [37] and, if no matches were found, then by the Phyre protein server [39]. Internal secondary RNA structures corresponding with published viral genomes were checked using the mFold server [40]. Additional features were identified by the conserved domain database (CDD) at NCBI [41]. α-helix and β-strand regions of ORFs were determined by PSIPRED [42]. Viral contig sequences and closest matches in NCBI were collected and aligned using the native alignment algorithm in CLC Sequence Viewer 8.0 (gap open cost 10.0; gap extension cost 1; end gap cost as any other), and trimmed to contiguous sequences within protein encoding regions. Phylogenetic representations were performed using both the CLC Sequence Viewer 8.0 and MEGA 10.1.8 [43].
2.5. Eukaryotic 18S and 28S rRNA Analyses
18S and 28S rRNAs were identified in viral metagenomes by comparison of contig spectra against the Silva database [44] by BLASTn (E < 10−25). Contigs matching this criterion were further annotated based on nearest match in NCBI by BLASTn, and classified based on taxonomic descriptions provided by the NCBI Taxonomy Database.
3. Results and Discussion
Twenty-two contiguous sequences met our criteria as matching viral proteins (from here referred to as “viral contigs”), which were primarily within the Picornavirales (Marnaviridae and Dicistroviridae, referred to from here as “picornavirus-like”), Ghabrivirales (Totiviridae, referred to from here as “totivirus-like”), and a single contig matching the Amarillovirales (Flaviviridae, referred to from here as “flavivirus-like”). The Apostichopus californicus wasting library yielded the most viral contigs (Table 1; n = 11), followed by Cucumaria miniata and Stichopus horrens (n = 3), and single viral contigs in the Holothuria atra, Holothuria scabra and Holothuria difficilis libraries. No viral contigs were retrieved from the Holothuria pardalis or Synaptula recta libraries. Read recruitment of all libraries against the 22 viral contigs revealed that some were specific to one library, but others were more cosmopolitan. Aside from a single picornavirus-like contig (708), all other picornavirus-like contigs were present in only the C. miniata and A. californicus libraries. Conversely, of the five totivirus-like contigs, only two were specific to a single library, and two (contigs 5835 and 4411) recruited from both Australian and North American holothurians. The flavivirus-like contig (91) recruited from only the North American holothurian libraries, in both C. miniata and A. californicus. Comparing the composition of wasting and grossly normal A. californicus, all contigs except one (picornavirus-like contig 1223) that were assembled in the wasting affected specimen also recruited reads from the grossly normal specimen library.
These results indicate that most RNA viruses discovered in this survey were restricted to a single or perhaps sympatric holothurian species, with few that spanned species or genera. Viral host tropism is circumscribed by inherent cell properties, including those affecting entry, replication and release/shedding of virions. Similar species may exhibit greater shared tropism for viruses, which may result in spillover events of pathogens [45]. While variation in factors affecting tropism may result in restricted host range [46], viruses may also infect across highly unrelated hosts, e.g., arboviruses that infect both insects and vertebrates. Amongst the 22 viral contigs retrieved in this survey, genome architecture and features suggest variable hosts, which may include non-holothurian microorganisms and metazoa. Hence, it is not possible to speculate on their host range. However, our data demonstrate that some viral genotypes may have geographically widespread distribution, since contigs recruited reads from libraries generated from Australian and North American specimens (Table 2).
Table 2.
Read recruitment to metagenome-assembled viral genome fragments across all libraries. + indicates that reads recruited from the metavirome library.
| Species of Metagenome | Contig # | Viral Order | Contig Length (nt) | Holothurian Species Recruited | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sh | Ha | Hs | Hp | Hd | Sr | Cm | Ac | Ac * | ||||
| Stichopus horrens | 17,482 | Ghabrivirales | 1070 | + | ||||||||
| Stichopus horrens | 5835 | Ghabrivirales | 2224 | + | + | + | + | + | + | + | ||
| Stichopus horrens | 10,295 | Ghabrivirales | 796 | + | + | + | + | + | ||||
| Holothuria atra | 17,799 | Picornavirales | 793 | + | ||||||||
| Holothuria scabra | 11,085 | Ghabrivirales | 679 | + | ||||||||
| Holothuria difficilis | 4411 | Ghabrivirales | 688 | + | + | + | + | |||||
| Cucumaria miniata | 4304 | Picornavirales | 636 | + | ||||||||
| Cucumaria miniata | 1913 | Picornavirales | 1826 | + | ||||||||
| Cucumaria miniata | 1781 | Picornavirales | 782 | + | ||||||||
| Apostichopus californicus | 91 | Amarillovirales | 8883 | + | + | + | ||||||
| Apostichopus californicus | 3426 | Picornavirales | 1158 | + | + | |||||||
| Apostichopus californicus * | 942 | Picornavirales | 1156 | + | + | + | ||||||
| Apostichopus californicus * | 792 | Picornavirales | 767 | + | + | |||||||
| Apostichopus californicus * | 791 | Picornavirales | 1491 | + | + | |||||||
| Apostichopus californicus * | 708 | Picornavirales | 1241 | + | + | + | + | + | + | + | + | + |
| Apostichopus californicus * | 557 | Picornavirales | 789 | + | + | |||||||
| Apostichopus californicus * | 45 | Picornavirales | 1368 | + | + | |||||||
| Apostichopus californicus * | 1580 | Picornavirales | 854 | + | + | |||||||
| Apostichopus californicus * | 1446 | Picornavirales | 597 | + | + | |||||||
| Apostichopus californicus * | 1223 | Picornavirales | 810 | + | ||||||||
| Apostichopus californicus * | 1120 | Picornavirales | 1132 | + | + | |||||||
| Apostichopus californicus * | 1020 | Picornavirales | 934 | + | + | |||||||
* indicates wasting-affected individual. Sh = Stichopus horrens; Ha = Holothuria atra; Hs = Holothuria scabra; Hp = Holothuria pardalis; Hd = Holothuria difficilis; Sr = Synaptula recta; Cm = Cucumaria miniata; Ac = Apostichopus californicus.
3.1. Flavivirus-Like Genome Fragment
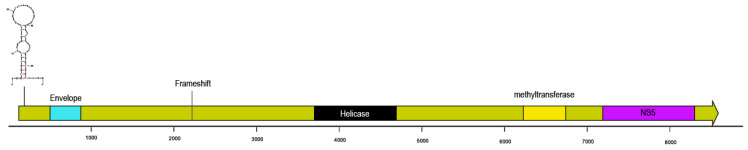
A single flavivirus-like contig (A. californicus contig 91) was retrieved based on homology with flaviviruses deposited at NCBI. This 8883 nt contig bore a single open reading frame (ORF; 8318 nt; flavivirus polyprotein by CDD search), with 391 nt untranslated region (UTR) at the 5′-end. Within the polyprotein ORF, a region corresponding to the NS5 of flavivirus RNA-dependent RNA polymerase (RdRp) was observed at the 3′ end. Preceding this is a conserved helicase domain (comprising DEAD N- and C-terminus motifs flanking a Walker motif) but at the 5′ end there was no similarity based on homology with any known flavivirus. However, analysis of this region by predicted peptide folding revealed a high confidence match to flavivirus envelope protein (nt positions ~400–700; confidence = 86.7; crystal structure of the envelope glycoprotein ectodomain from dengue2 virus serotype 4), suggesting it was distant from known representative flaviviruses. Within this region, there was a slippery sequence frameshift at position 2061 (5′-CUCCCUUUUUUAUC-3′) which is also observed in insect-specific flaviviruses [47]. A start codon (AUG)-flanked hairpin structure, which is characteristic of flaviviruses is at nt positions 232–274 [48,49] (Figure 1). There was no similarity to known capsid or membrane proteins at the 3′ end based on CDD search or predicted peptide folding. The first 1000 amino acids contained mostly β helices, whereas the region closest to the RdRp bore more α-helices, suggesting it may bear the structural region [50]. The genome arrangement of the A. californicus flavivirus-like genome fragment is similar to other insect-specific flaviviruses and distinct from flavivirus arboviruses. Reads recruited from the C. miniata library to this flavivirus-like genome, but not from other holothurian species (Table 2).
Figure 1.
Contig map of Apostichopus californicus flavivirus-like contig 91. The open reading frame matched a flavivirus polyprotein by BLASTx [37]. Methyltransferase, NS5 and helicase domains were identified by comparison against the conserved domain database (CDD) at NCBI [41]. The location of the envelope region was determined by protein folding comparison in Phyre [39]. The hairpin like structure preceding the Envelope region was determined by folding all sites between start (AUG) codons by mFold [40].
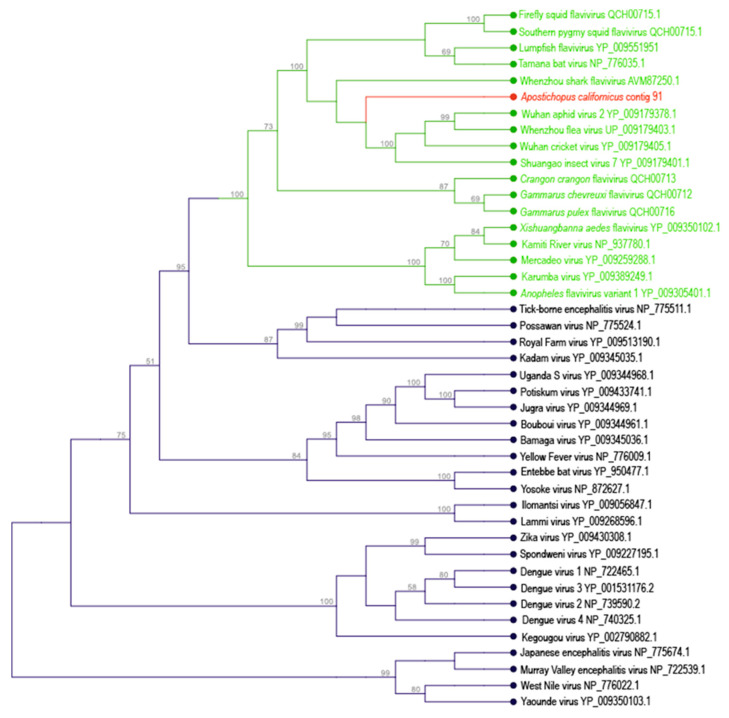
Alignment of the A. californicus flavivirus-like genome fragment against closest matches at NCBI revealed that it was firmly embedded within a clade of insect-specific flaviviruses [51], including flaviviruses recovered from cephalopods [52] and from invertebrate and vertebrate transcriptomes [53] (Figure 2). Enveloped RNA viruses are notable in marine ecosystems because they are not common constituents of virioplankton and generally experience high decay rates in seawater [54]. Flaviviruses (+ssRNA) represent important pathogens of mammals, including Dengue Fever, Yellow Fever, Hepatitis C Virus and Zika Virus. While most flaviviruses recovered to date infect mammals and represent arboviruses (arthropod-borne viruses) that cause pathology in mammals but do not cause disease in arthropod vectors, there is an expanding clade of flaviviruses that are found only in arthropods and never in vertebrates [55]. These viruses have been termed “insect-specific flaviviruses (iFVs)”. Despite this moniker, iFVs have also recently been observed in teleosts [56,57], crustaceans [52] and an elasmobranch [53]. Our discovery of a flavivirus genome fragment associated with a holothurian host expands the host range attributed to this group and suggests that these may be more widespread in invertebrate groups than currently understood. Parry and Asgari [52] inferred circulation of flaviviruses between vertebrates and invertebrates based on nucleotide frequencies and active interference RNA response to a shark flavivirus in crabs. The observation of vertebrate-invertebrate flavivirus associations in marine habitats and in terrestrial habitats may suggest they have arisen twice. Because flavivirus host range restriction occurs at multiple levels [48], vertebrate-invertebrate associations have evolved through complex interactions in order to overcome barriers to infection and replication. Our observation of a holothurian flavivirus that has greatest similarity to a shark flavivirus [53] suggests that either these are unique to deuterostomes, or that these may represent similar invertebrate-vertebrate viral associations.
Figure 2.
Phylogenetic representation of Apostichopus californicus flavivirus-like contig 91. The tree was constructed by performing an alignment of overlapping regions with best BLASTx matches at NCBI using the CLC Sequence Viewer 8.0 native alignment algorithm. The tree is based on a ~420 amino acid alignment by neighbor joining and based on Jukes-Cantor distance. Values above nodes indicate bootstrap statistics (>50%) based on 1000 iterations. The green branches indicate the emerging aquatic and invertebrate-associated flavivirus clade [52]. An additional phylogenetic representation based on maximum likelihood is provided in the Supplementary Materials.
3.2. Picornavirales-Like Genome Fragments
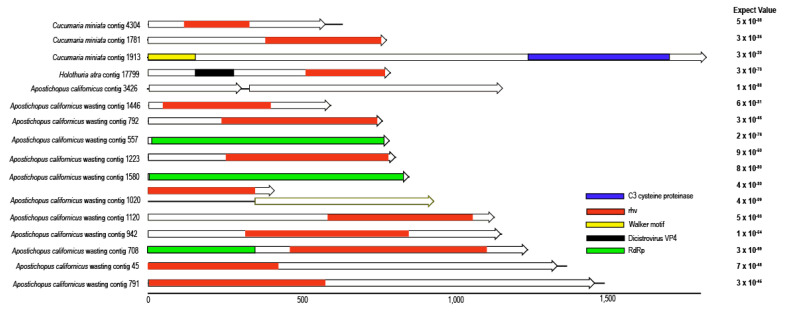
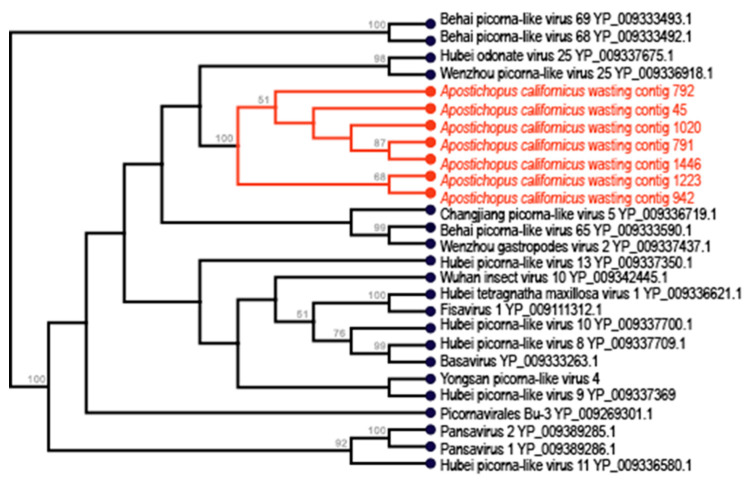
Sixteen contigs were most similar to Picornavirales (which comprises picornaviruses, dicistroviruses and iflaviruses) based upon sequence homology to representatives at NCBI (Figure 3). Of these, 11 were retrieved from the wasting-affected A. californicus library, while only one contig was retrieved from the grossly normal A. californicus library. In addition to A. californicus picornavirus-like genome fragments, 3 fragments were also retrieved from C. miniata. All contigs bore similarity to RdRp domains of picornavirus polyproteins. Apostichopus californicus contig 1020 bore 2 overlapping ORFs, which is uncharacteristic of picornaviruses and may indicate the presence of a frameshift in the overlapping region. Of the 17 picornavirus-like contigs, 11 bore similarity to rhinovirus capsid (rhv) regions, six bore similarity to reverse transcriptase (RT)-like RdRp domains (one contig, from wasting-affected A. californicus, contig 708, contained both rhv and RdRp domains), and one contig bore similarity to a Walker motif (P-loop-NTPase) and to the 3C cysteine protease (picornarin). Of particular note, Holothuria atra contig 17,799 bore a capsid protein motif most similar to VP4 of Cricket paralysis virus, suggesting that it may belong to the Dicistroviridae. Picornaviruses have conserved genome arrangement in the order rhv-helicase-protease-polymerase [58,59,60]. Interestingly, the one contig bearing both rhv and RdRp motifs was in the arrangement from 5′- to 3′- that was opposite to conserved picornaviruses and iflaviruses and more similar to dicistroviruses [60]. Phylogenetic analyses of detected Picornavirales based around the rhv domain demonstrate that they are similar to picorna-like viruses retrieved from transcriptomes of insects, suggesting an invertebrate host (Figure 4).
Figure 3.
Contig maps for Picornavirales-like genome fragments recovered from holothurians by viral metagenomics. The expect values of best matches by BLASTx against the non-redundant database at NCBI are indicated to the right of each contig. Solid lines indicate the total contig length, and arrows indicate open reading frames. Colored bars indicate shared homology between contigs based on reciprocal tBLASTx.
Figure 4.
Phylogenetic representation of holothurian-associated Picornavirales-like genome fragments. The tree was constructed by performing an alignment of an overlapping region (~100 amino acid) of the rhv domain with best BLASTx matches in the non-redundant database at NCBI. The tree was constructed by neighbor joining and based on Jukes-Cantor distance. Values above nodes indicate bootstrap statistics (>50%) based on 1000 iterations. An additional phylogenetic representation based on maximum likelihood is provided in the Supplementary Materials.
Picornavirus-like genomes are routinely recovered in invertebrate host-associated and environmental RNA viral metaviromes [12,33,61,62,63,64,65,66,67,68]. There have been no previous reports of picornaviruses associated with any disease in aquatic invertebrates, and their pattern of association between sea star wasting (SSW)-affected and grossly normal asteroid specimens does not suggest their involvement in disease process [33]. Picornavirales infect a wide range of hosts including metazoa and unicellular eukaryotes [69]. Hence, it is possible that some detected picornavirus-like viruses may infect microscopic eukaryotic constituents of the holothurian microbiome. Our observation of a dicistrovirus-like genome fragment in H. atra may suggest that this infects the holothurian. The known host range of dicistroviruses includes only arthropods and other invertebrates [62]. Similarly, the phylogenetic similarity of rhv domain-bearing contigs in this survey suggest an invertebrate host rather than unicellular eukaryotic hosts.
3.3. Totivirus-Like Genome Fragments
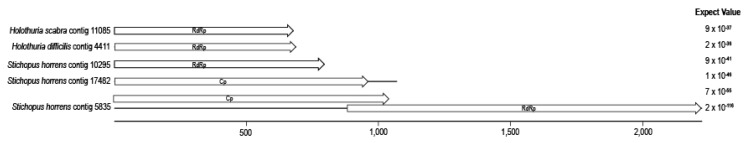
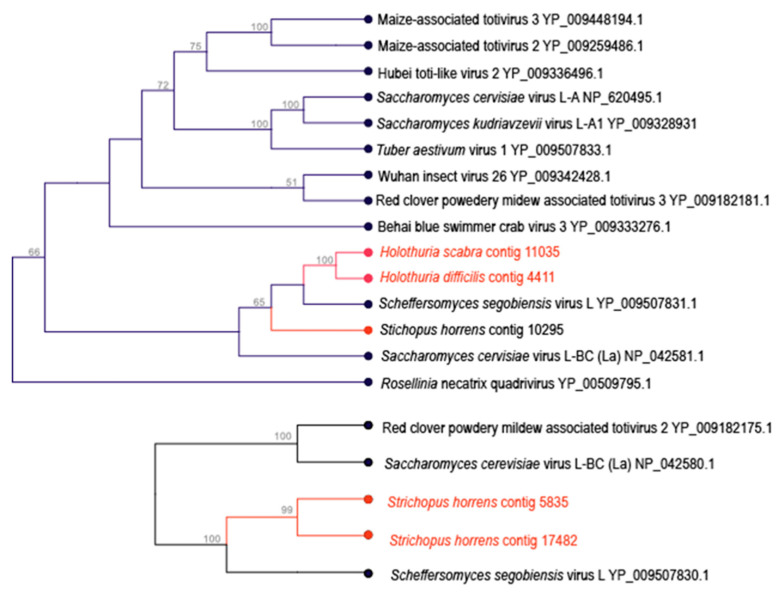
Five contiguous sequences matching totivirus genome fragments were recovered from three species of holothurian, with most coming from Stichopus horrens (Figure 5). These represented RdRps (n = 4) and capsid (Cp) proteins (n = 2), with one contig (S. horrens contig 5835) bearing an overlapping Cp-RdRp region, with a possible frameshift at position 882, characteristic of totivirus ORFs [70]. Predicted secondary structure (PSIPRED V4.0 [71]) of the S. horrens contig 5835 major capsid protein region revealed a 5′ α- and β-strand rich region, followed by a β-helix rich region at the 3′ end, which is most similar to fungal totiviruses and less similar to metazoan totiviruses, which have an α-helix rich 3′ region [72]. The Cp on S. horrens contig 17482, on the other hand, bears an α- and β-rich region at the 5′ end, followed by a β-rich region and terminating in an α-rich region, which suggests it may be more closely related to arthropod totiviruses [72]. Phylogenetically, the Cp regions on both S. horrens 5835 and 17,482 match more closely fungal totivirus Cp regions, as do all RdRp fragments recovered in this study (Figure 6). Hence, these are likely fungal viruses.
Figure 5.
Contig maps for totivirus-like genome fragments recovered from holothurians. The expected values of best matches by BLASTx [37] to the non-redundant database at NCBI are indicated to the right of the contigs. Solid lines indicate the total contig length, and arrows indicate open reading frames. RdRp = RNA-dependent RNA polymerase, Cp = capsid protein.
Figure 6.
Phylogenetic representations of holothurian-associated totivirus-like genome fragments. The trees were constructed by performing an alignment of an overlapping region of the RdRp (top) and Cp (bottom) domains with best BLASTx matches at NCBI. The trees are based on ~156 amino acid (for RdRp) and 87 amino acid (for Cp) alignments by neighbor joining and based on Jukes-Cantor distance. Values above nodes indicate bootstrap statistics (>50%) based on 1000 iterations. An additional phylogenetic representation based on maximum likelihood is provided in the Supplementary Materials.
Fungi do not comprise a large portion of free-living aquatic protistan communities, but they are frequently found in association with invertebrates [73] and are implicated in at least one invertebrate disease [74]. Fungi are easily cultivated from echinoderms, and especially holothurian tissues [75,76,77], and extracts of holothurian body wall bear antifungal substances [78,79]. To date there have been no cultivation-independent assessments of holothurian microbiome composition. Because viral metagenomes enrich particles <0.2 µm, which includes ribosome-sized particles, rRNAs can comprise a large fraction of metaviromic libraries, even after RNAse treatment to remove co-extracted host RNAs. We probed the phylogeny of 18S and 28S rRNAs recovered in RNA viromes and discovered that most bore signatures of basidiomycete and ascomycete rRNAs (Table 3). Hence, it is not surprising to retrieve fungal viruses in our survey. Nerva et al. [80] observed the diversity of viruses in a fungal collection prepared from Holothuria polii. They discovered that more than half were double-stranded RNA (dsRNA) viruses belonging to the Partitiviridae and Chrysoviridae, the latter of which belongs to the Ghabrivirales. Our observation of putatively fungal totiviruses in echinoderm viromes raises interesting questions about the role of fungi, and interactions between these and their viruses in host health and ecology.
Table 3.
Taxonomic assignment of contigs matching eukaryotic 18S/28S rRNAs. The number of contigs matching each group is indicated, but should be treated with caution because of un-defined biases in template amplification.
| Group | Taxonomic Assignment | Holothurian Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Holothuria pardalis | Holothuria difficilis | Holothuria scabra | Holothuria atra | Synaptula recta | Stichopus horrens | Cucumaria miniata | Apostichopus californicus | Apostichopus californicus * | ||
| Unicellular Eukaryotes | Alveolata | 1 | 2 | 6 | 8 | |||||
| Apusozoa | 2 | |||||||||
| Amoebozoa | 2 | |||||||||
| Cryptomonada | 1 | 9 | ||||||||
| Heterokonta | 1 | |||||||||
| Metamonada | 1 | |||||||||
| Viridiplantae | 2 | 2 | 2 | 63 | 11 | 18 | 6 | 1 | ||
| Fungi | Ascomycota | 17 | 14 | 6 | 7 | 8 | 17 | 14 | 2 | 21 |
| Basidiomycota | 11 | 8 | 6 | 9 | 8 | 13 | 14 | 12 | 16 | |
| Fungi intercae sedis | 1 | 1 | 2 | 2 | ||||||
| Metazoa | Annelida | 1 | ||||||||
| Arthropoda | 4 | 2 | 4 | 1 | 3 | 4 | 3 | 5 | 5 | |
| Asteroidea | 2 | |||||||||
| Chordata | 3 | 1 | 2 | 2 | 4 | 14 | 3 | 3 | ||
| Cnidaria | 1 | 1 | 1 | 2 | ||||||
| Echinoidea | 1 | |||||||||
| Holothuria | 1 | 1 | 1 | 3 | 1 | 1 | ||||
| Mollusca | 1 | 2 | 1 | 1 | ||||||
| Nematoda | 3 | |||||||||
| Phoronida | 1 | |||||||||
| Platyhelminthes | 1 | |||||||||
The * indicates that the specimen was affected by sea cucumber wasting (SCW).
3.4. Viral Diseases in Holothurians
A condition affecting A. californicus has been observed since at least 2014 in southeast Alaska and the Salish Sea. The ongoing sea star wasting (SSW) epidemic brought attention to the condition in sea cucumbers, which has not yet been reported in the peer-reviewed scientific literature. Because this species primarily inhabits subtidal habitats and is generally not as abundant as asteroids, the extent of sea cucumber wasting (SCW) and impacts on population decline is driven by wildlife manager and fisher observations (cf. extensive citizen science observations of SSW). Wasting was first anecdotally reported in Friday Harbor, WA, in February 2014, subsequently near Admiralty Island (AK) in August 2014, and Santa Catalina Island in September 2014. In April 2015 and again in November 2016, more extensive SCW was observed in the holothurian fishery in southeast Alaska (Tenakee Springs, Chicago Island) and in the central Salish Sea [81]. In February 2017, SCW was observed at an aquaculture facility in the southern Salish Sea (Manchester, WA) and again in the central Salish Sea and has since been reported more widely in the region (including in the Secheldt and Howe Sound, BC). Anecdotally reported disease signs based on gross observations include non-focal lesions and fissures across the body wall, some sloughing of epidermal tissues, and rapid liquefaction upon collection [81]. Anecdotally, the number of individuals recovered at fishing sites decreased from 2014 to the present day, suggesting that SCW may have affected overall holothurian population density. Wasting was mostly reported between September and January in 2014–2017, which corresponds with seasonal evisceration which is a response to more limited food availability and organ atrophy [82]. During seasonal self-evisceration, viscera are recycled by internal processes [83]. However, self-evisceration does not typically affect body wall tissues, so it is unclear whether SCW is an extension of normal self-evisceration processes.
We compared a single wasting A. californicus specimen virome to a single grossly normal A. californicus virome collected at the same site (Table 1). We expected to see differential representation of wasting-associated viruses in A. californicus which would provide targets for further pathology investigations. However, nearly all viral genome fragments discovered in the wasting-affected individual recruited reads from the asymptomatic specimen library, which is similar to metagenomic analyses performed on sea star wasting-affected individuals [12]. Because of uncertain amplification biases and variable amplification template amounts, it is not possible to quantitatively compare representation between tissue states [16]. Moreover, no challenge experiments with virus-sized material or isolated viruses were performed in this study. Hence, it would be highly speculative with the available information to attribute sea cucumber wasting to any virus.
Previous work has highlighted several virus-like particles associated with tissues of grossly diseased specimens of the aqua-cultured Apostichopus japonicus. A skin ulceration and evisceration-associated disease caused mass mortality in the Yellow Sea in 2004–2005. Occluded viral particles were observed by electron microscopy in muscles lining the water vascular system, alimentary canal, connective tissue and respiratory tree, although the virus was not sequenced [84]. In addition, exposure to virus-sized material yielded disease signs in naïve specimens. Separately, [85] reported microscopy-based assessments of acute peristome edema disease in the Yellow Sea in 2007, and described particles with consistent morphology to coronaviruses. Similarly, a spherical virus with a bilayer capsule was described in holothurians experiencing skin ulceration and peristome tumescence disease, and again this appeared to be transmissible [86]. Similar virus particles were also reported in A. japonicus larvae experiencing stomach atrophy, which were identical to virus particles observed in parent gonads [84].
The eight species of holothurian sampled in this study were broadly similar in ecology. Specimens of A. californicus, S. horrens, H. atra, H. scabra, H. difficilis, H. pardalis, and S. recta are deposit feeders that consume detritus and sediment-bound organisms. A. californicus inhabits subtidal waters in the northeastern Pacific, whereas other species inhabit primarily subtropical and tropical waters of the Indo-Pacific. C. miniata is a suspension feeder that occurs in rocky intertidal zones in the northeastern Pacific. In the context of virology, there were no expected differences due to behavior (which may affect contact rates between individuals or with fomites), except for perhaps between C. miniata and other species. However, our observation of read recruits between viral genome fragments detected in C. miniata and A. californicus suggests that geographic location may ultimately influence viral types present in species more strongly than feeding behavior.
4. Conclusions
To the best of our knowledge, this is the first viral metagenomic survey of holothurians, and our work expands on knowledge of viruses inhabiting echinoderms. Our work revealed the presence of a flavivirus-like genome fragment that falls within an expanding group of aquatic invertebrate flaviviruses, along with novel totivirus and picornavirus-like fragments. While we cannot associate pathology with any of these detected viral genotypes and our comparison is limited to a single individual of sea cucumber wasting, we did not observe a viral genotype that was unique to SCW. It is recommended that future studies focus on the entry, replication and shedding of novel flaviviruses and the role of fungi and their associated viruses in holothurian ecology.
Acknowledgments
The authors are grateful to Elliot Jackson and Kalia Bistolas for support of specimen collection and bioinformatic processing. The authors thank Mike Donnellan (Alaska Department of Fish and Game) and Mike Erickson (Alaska Glacier Seafoods) for providing samples of A. californicus from Ketchikan. The Alaska Department of Fish and Game is the permit issuing authority for non-federally managed species in the State waters so the collection A. californicus falls under their existing authority. The authors also thank the Moreton Bay Research Station and Heron Island Research Station for assistance with sample collection. Samples from Moreton Bay were collected under Queensland Parks and Wildlife Service permit it QS2015/MAN316, and from Heron Island under the Great Barrier Reef Marine Park Authority permit G15/37877.1.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4915/12/9/1057/s1.
Author Contributions
Conceptualization, I.H. and I.R.T.; Methodology, I.H., M.R.J. and I.R.T.; Formal Analysis, I.H. and M.R.J.; Resources, I.H. and I.R.T.; Data Curation, I.H. and M.R.J.; Writing—Original Draft Preparation, I.H.; Writing—Review & Editing, I.H., M.R.J. and I.R.T.; Funding Acquisition, I.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by US National Science Foundation grants OCE-1537111 and OCE-1737127 awarded to IH and an award from the Mario Einaudi Center for International Studies at Cornell University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Thurber R.L.V., Barott K.L., Hall D., Liu H., Rodriguez-Mueller B., Desnues C., Edwards R.A., Haynes M., Angly F.E., Wegley L., et al. Metagenomic analysis indicates that stressors induce production of herpes-like viruses in the coral Porites compressa. Proc. Natl. Acad. Sci. USA. 2008;105:18413–18418. doi: 10.1073/pnas.0808985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Oppen M.J.H., Leong J.A., Gates R.D. Coral-virus interactions: A double-edged sword? Symbiosis. 2009;47:1–8. doi: 10.1007/BF03179964. [DOI] [Google Scholar]
- 3.Thurber R.L.V., Correa A.M.S. Viruses of reef-building scleractinian corals. J. Exp. Mar. Biol. Ecol. 2011;408:102–113. doi: 10.1016/j.jembe.2011.07.030. [DOI] [Google Scholar]
- 4.Correa A.M.S., Welsh R.M., Thurber R.L.V. Unique nucleocytoplasmic dsDNA and +ssRNA viruses are associated with the dinoflagellate endosymbionts of corals. ISME J. 2013;7:13–27. doi: 10.1038/ismej.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soffer N., Brandt M.E., Correa A.M.S., Smith T.B., Thurber R.V. Potential role of viruses in white plague coral disease. ISME J. 2014;8:271–283. doi: 10.1038/ismej.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin R.A., Voolstra C.R., Weynberg K.D., van Oppen M.J.H. Evidence for a role of viruses in the thermal sensitivity of coral photosymbionts. ISME J. 2017;11:808–812. doi: 10.1038/ismej.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitbart M., Benner B.E., Jernigan P.E., Rosario K., Birsa L.M., Harbeitner R.C., Fulford S., Graham C., Walters A., Goldsmith D.B., et al. Discovery, prevalence, and persistence of novel circular single-stranded DNA viruses in the ctenophores Mnemiopsis leidyi and Beroe ovata. Front. Microbiol. 2015;6:1427. doi: 10.3389/fmicb.2015.01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bistolas K., Besemer R., Rudstam L., Hewson I. Distribution and inferred evolutionary characteristics of a chimeric ssDNA virus associated with intertidal marine isopods. Viruses. 2017;9:361. doi: 10.3390/v9120361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bistolas K.S.I., Rudstam L.G., Hewson I. Gene expression of benthic amphipods (genus: Diporeia) in relation to a circular ssDNA virus across two Laurentian Great Lakes. PeerJ. 2017;5:e3810. doi: 10.7717/peerj.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunlap D.S., Ng T.F.F., Rosario K., Barbosa J.G., Greco A.M., Breitbart M., Hewson I. Molecular and microscopic evidence of viruses in marine copepods. Proc. Natl. Acad. Sci. USA. 2013;110:1375–1380. doi: 10.1073/pnas.1216595110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gudenkauf B.M., Eaglesham J.B., Aragundi W.M., Hewson I. Discovery of urchin-associated densoviruses (Parvoviridae) in coastal waters of the Big Island, Hawaii. J. Gen. Virol. 2014;95:652–658. doi: 10.1099/vir.0.060780-0. [DOI] [PubMed] [Google Scholar]
- 12.Hewson I., Button J.B., Gudenkauf B.M., Miner B., Newton A.L., Gaydos J.K., Wynne J., Groves C.J., Hendler G., Murray M., et al. Densovirus associated with sea-star wasting disease and mass mortality. Proc. Natl. Acad. Sci. USA. 2014;111:17276–17283. doi: 10.1073/pnas.1416625111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gudenkauf B.M., Hewson I. Comparative metagenomics of viral assemblages inhabiting four phyla of marine invertebrates. Front. Mar. Sci. 2016;3:23. doi: 10.3389/fmars.2016.00023. [DOI] [Google Scholar]
- 14.Jackson E.W., Pepe-Ranney C., Johnson M.R., Distel D.L., Hewson I. A highly prevalent and pervasive densovirus discovered among sea stars from the north american Atlantic coast. Appl. Environ. Microbiol. 2020;86:e02723-19. doi: 10.1128/AEM.02723-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosario K., Marinov M., Stainton D., Kraberger S., Wiltshire E.J., Collings D.A., Walters M., Martin D.P., Breitbart M., Varsani A. Dragonfly cyclovirus, a novel single-stranded DNA virus discovered in dragonflies (Odonata: Anisoptera) J. Gen. Virol. 2011;92:1302–1308. doi: 10.1099/vir.0.030338-0. [DOI] [PubMed] [Google Scholar]
- 16.Hewson I. Technical pitfalls that bias comparative microbial community analyses of aquatic disease. Dis. Aquat. Organ. 2019;137:109–124. doi: 10.3354/dao03432. [DOI] [PubMed] [Google Scholar]
- 17.Paine R.T. Food web complexity and species diversity. Am. Nat. 1966;100:65. doi: 10.1086/282400. [DOI] [Google Scholar]
- 18.Uthicke S. Nutrient regeneration by abundant coral reef holothurians. J. Exp. Mar. Biol. Ecol. 2001;265:153–170. doi: 10.1016/S0022-0981(01)00329-X. [DOI] [Google Scholar]
- 19.Billett D.S.M., Bett B.J., Rice A.L., Thurston M.H., Galeron J., Sibuet M., Wolff G.A. Long-term change in the megabenthos of the Porcupine Abyssal Plain (NE Atlantic) Prog. Oceanogr. 2001;50:325–348. doi: 10.1016/S0079-6611(01)00060-X. [DOI] [Google Scholar]
- 20.Moriarty D.J.W., Pollard P.C., Hunt W.G., Moriarty C.M., Wassenberg T.G. Productivity of bacteria and microalgae and the effect of grazing by holothurians in sediments on a coral reef flat. Mar. Biol. 1985;85:293–300. doi: 10.1007/BF00393250. [DOI] [Google Scholar]
- 21.Hewson I., Fuhrman J.A. Spatial and vertical biogeography of coral reef sediment bacterial and diazotroph communities. Mar. Ecol. Prog. Ser. 2006;306:79–86. doi: 10.3354/meps306079. [DOI] [Google Scholar]
- 22.Pernet B. Larval Feeding: Mechanisms, Rates, and Performance in Nature. Oxford Univ Press; New York, NY, USA: 2018. pp. 87–102. [Google Scholar]
- 23.Ameneiro J., Lubian L.M., Sangra P., Vazquez E. Food-limited invertebrate larvae in the Southern Ocean: Testing a paradigm. Mar. Ecol. Prog. Ser. 2016;554:71–80. doi: 10.3354/meps11786. [DOI] [Google Scholar]
- 24.Almeda R., Messmer A.M., Sampedro N., Gosselin L.A. Feeding rates and abundance of marine invertebrate planktonic larvae under harmful algal bloom conditions off Vancouver Island. Harmful Algae. 2011;10:194–206. doi: 10.1016/j.hal.2010.09.007. [DOI] [Google Scholar]
- 25.Toral-Granda V., Lovatelli A., Vasconcellos M. Sea Cucumbers: A Global Review of Fisheries and Trade. Food and Agriculture Organization of the United Nations; Rome, Italy: 2008. p. 331. [Google Scholar]
- 26.Liu X. Chinese Sea Cucumber Farming. [(accessed on 24 December 2018)]; Available online: http://www.fao.org/in-action/globefish/fishery-information/resource-detail/en/c/1113389/
- 27.Matrosovich M., Herrler G., Klenk H.D. Sialic acid receptors of viruses. In: Gerardy-Schahn R., Delannoy P., von Itzstein M., editors. SialoGlyco Chemistry and Biology II: Tools and Techniques to Identify and Capture Sialoglycans. Springer International Publishing; Cham, Germany: 2015. pp. 1–28. [Google Scholar]
- 28.Varki A., Schnaar R.L., Schauer R. Sialic acids and other nonulosonic acids. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., Prestegard J.H., et al., editors. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2015. pp. 179–195. [PubMed] [Google Scholar]
- 29.Smith L.C., Ghosh J., Buckley K.M., Clow L.A., Dheilly N.M., Haug T., Henson J.H., Li C., Lun C.M., Majeske A.J., et al. Echinoderm immunity. Adv. Exp. Med. Biol. 2010;708:260–301. doi: 10.1007/978-1-4419-8059-5_14. [DOI] [PubMed] [Google Scholar]
- 30.Kondo M., Akasaka K. Current status of echinoderm genome analysis—What do we know? Curr. Genom. 2012;13:134–143. doi: 10.2174/138920212799860643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson E.W., Bistolas K.S.I., Button J.B., Hewson I. Novel circular single-stranded DNA viruses among an asteroid, echinoid and holothurian (Phylum: Echinodermata) PLoS ONE. 2016;11:e0166093. doi: 10.1371/journal.pone.0166093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fahsbender E., Hewson I., Rosario K., Tuttle A.D., Varsani A., Breitbart M. Discovery of a novel circular DNA virus in the Forbes sea star, Asterias forbesi. Arch. Virol. 2015;160:2349–2351. doi: 10.1007/s00705-015-2503-2. [DOI] [PubMed] [Google Scholar]
- 33.Hewson I., Bistolas K.S.I., Quijano Carde E.M., Button J.B., Foster P.J., Flanzenbaum J.M., Kocian J., Lewis C.K. Investigating the complex association between viral ecology, environment and Northeast Pacific Sea Star Wasting. Front. Mar. Sci. 2018;5:77. doi: 10.3389/fmars.2018.00077. [DOI] [Google Scholar]
- 34.WoRMS Apostichopus californicus (Stimpson, 1857) [(accessed on 14 August 2020)]; Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=529363.
- 35.Thurber R.V., Haynes M., Breitbart M., Wegley L., Rohwer F. Laboratory procedures to generate viral metagenomes. Nat. Protocol. 2009;4:470–483. doi: 10.1038/nprot.2009.10. [DOI] [PubMed] [Google Scholar]
- 36.Ng F.F.T., Wheeler E., Greig D., Waltzek T.B., Gulland F., Breitbart M. Metagenomic identification of a novel anellovirus in Pacific harbor seal (Phoca vitulina richardsii) lung samples and its detection in samples from multiple years. J. Gen. Virol. 2011;92:1318–1323. doi: 10.1099/vir.0.029678-0. [DOI] [PubMed] [Google Scholar]
- 37.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 38.Williams G. GetORF. EMBOSS Wellcome Trust Genome Campus; Cambridge, UK: 2000. [Google Scholar]
- 39.Kelley L.A., Sternberg M.J.E. Protein structure prediction on the Web: A case study using the Phyre server. Nat. Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 40.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu S., Wang J., Chitsaz F., Derbyshire M.K., Geer R.C., Gonzales N.R., Gwadz M., Hurwitz D.I., Marchler G.H., Song J.S., et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020;48:D265–D268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGuffin L.J., Bryson K., Jones D.T. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- 43.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parrish C.R., Holmes E.C., Morens D.M., Park E.-C., Burke D.S., Calisher C.H., Laughlin C.A., Saif L.J., Daszak P. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol. Mol. Biol. Rev. 2008;72:457–470. doi: 10.1128/MMBR.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Junglen S., Korries M., Grasse W., Wieseler J., Kopp A., Hermanns K., León-Juárez M., Drosten C., Kümmerer B.M. Host range restriction of insect-specific flaviviruses occurs at several levels of the viral life cycle. mSphere. 2017;2:e00375-16. doi: 10.1128/mSphere.00375-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Firth A.E., Blitvich B.J., Wills N.M., Miller C.L., Atkins J.F. Evidence for ribosomal frameshifting and a novel overlapping gene in the genomes of insect-specific flaviviruses. Virology. 2010;399:153–166. doi: 10.1016/j.virol.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clyde K., Harris E. RNA secondary structure in the coding region of dengue virus type 2 directs translation start codon selection and is required for viral replication. J. Virol. 2006;80:2170–2182. doi: 10.1128/JVI.80.5.2170-2182.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clyde K., Barrera J., Harris E. The capsid-coding region hairpin element (cHP) is a critical determinant of dengue virus and West Nile virus RNA synthesis. Virology. 2008;379:314–323. doi: 10.1016/j.virol.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones C.T., Ma L., Burgner J.W., Groesch T.D., Post C.B., Kuhn R.J. Flavivirus capsid is a dimeric alpha-helical protein. J. Virol. 2003;77:7143–7149. doi: 10.1128/JVI.77.12.7143-7149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kenney J.L., Solberg O.D., Langevin S.A., Brault A.C. Characterization of a novel insect-specific flavivirus from Brazil: Potential for inhibition of infection of arthropod cells with medically important flaviviruses. J. Gen. Virol. 2014;95 Pt 12:2796–2808. doi: 10.1099/vir.0.068031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parry R., Asgari S. Discovery of novel crustacean and cephalopod flaviviruses: Insights into the evolution and circulation of flaviviruses between marine invertebrate and vertebrate hosts. J. Virol. 2019;93:e00432-19. doi: 10.1128/JVI.00432-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi M., Lin X.-D., Vasilakis N., Tian J.-H., Li C.-X., Chen L.-J., Eastwood G., Diao X.-N., Chen M.-H., Chen X., et al. Divergent viruses discovered in arthropods and vertebrates revise the evolutionary history of the Flaviviridae and related viruses. J. Virol. 2016;90:659. doi: 10.1128/JVI.02036-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mordecai G.J., Hewson I. Coronaviruses in the sea. Front. Microbiol. 2020;11:1795. doi: 10.3389/fmicb.2020.01795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuno G. Host range specificity of flaviviruses: Correlation with in vitro replication. J. Med. Entomol. 2007;44:93–101. doi: 10.1093/jmedent/41.5.93. [DOI] [PubMed] [Google Scholar]
- 56.Skoge R.H., Brattespe J., Økland A.L., Plarre H., Nylund A. New virus of the family Flaviviridae detected in lumpfish (Cyclopterus lumpus) Arch. Virol. 2018;163:679–685. doi: 10.1007/s00705-017-3643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soto E., Camus A., Yun S., Kurobe T., Leary J.H., Rosser T.G., Dill-Okubo J.A., Nyaoke A.C., Adkison M., Renger A., et al. First isolation of a novel aquatic flavivirus from Chinook Salmon (Oncorhynchus tshawytscha) and its in vivo replication in a piscine animal model. J. Virol. 2020;94:e00337-20. doi: 10.1128/JVI.00337-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ICTV . Order—Picornavirales. In: King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Virus Taxonomy. Elsevier; San Diego, CA, USA: 2012. pp. 835–839. [Google Scholar]
- 59.Lau S.K.P., Woo P.C.Y., Lai K.K.Y., Huang Y., Yip C.C.Y., Shek C.-T., Lee P., Lam C.S.F., Chan K.-H., Yuen K.-Y. Complete genome analysis of three novel picornaviruses from diverse bat species. J. Virol. 2011;85:8819–8828. doi: 10.1128/JVI.02364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christian P.D., Scotti P.D. Dicistroviruses. Encycl. Virol. 2008:37–44. doi: 10.1016/B978-012374410-4.00608-7. [DOI] [Google Scholar]
- 61.Wolf Y.I., Silas S., Wang Y., Wu S., Bocek M., Kazlauskas D., Krupovic M., Fire A., Dolja V.V., Koonin E.V. Doubling of the known set of RNA viruses by metagenomic analysis of an aquatic virome. Nat. Microbiol. 2020 doi: 10.1038/s41564-020-0755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanborn M.A., Klein T.A., Kim H.-C., Fung C.K., Figueroa K.L., Yang Y., Asafo-adjei E.A., Jarman R.G., Hang J. Metagenomic analysis reveals three novel and prevalent mosquito viruses from a single pool of Aedes vexans nipponii collected in the Republic of Korea. Viruses. 2019;11:222. doi: 10.3390/v11030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hewson I., Bistolas K.S.I., Button J.B., Jackson E.W. Occurrence and seasonal dynamics of RNA viral genotypes in three contrasting temperate lakes. PLoS ONE. 2018;13:e0194419. doi: 10.1371/journal.pone.0194419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miranda J.A., Culley A.I., Schvarcz C.R., Steward G.F. RNA viruses as major contributors to Antarctic virioplankton. Environ. Microbiol. 2016;18:3714–3727. doi: 10.1111/1462-2920.13291. [DOI] [PubMed] [Google Scholar]
- 65.Culley A.I., Lang A.S., Suttle C.A. The complete genomes of three viruses assembled from shotgun libraries of marine RNA virus communities. Virol. J. 2007;4:69. doi: 10.1186/1743-422X-4-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Culley A.I., Steward G.F. New genera of RNA viruses in subtropical seawater, inferred from polymerase gene sequences. Appl. Environ. Microbiol. 2007;73:5937–5944. doi: 10.1128/AEM.01065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Culley A.I., Lang A.S., Suttle C.A. High diversity of unknown picorna-like viruses in the sea. Nature. 2003;424:1054–1057. doi: 10.1038/nature01886. [DOI] [PubMed] [Google Scholar]
- 68.Culley A.I., Lang A.S., Suttle C.A. Metagenomic analysis of coastal RNA virus communities. Science. 2006;312:1795–1798. doi: 10.1126/science.1127404. [DOI] [PubMed] [Google Scholar]
- 69.Le Gall O., Christian P., Fauquet C.M., King A.M.Q., Knowles N.J., Nakashima N., Stanway G., Gorbalenya A.E. Picornavirales, a proposed order of positive-sense single-stranded RNA viruses with a pseudo-T = 3 virion architecture. Arch. Virol. 2008;153:715. doi: 10.1007/s00705-008-0041-x. [DOI] [PubMed] [Google Scholar]
- 70.Ghabrial S.A. Totiviruses. In: Mahy B.W.J., Van Regenmortel M.H.V., editors. Encyclopedia of Virology. 3rd ed. Academic Press; Oxford, UK: 2008. pp. 163–174. [Google Scholar]
- 71.Jones D.T. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 1999;292:195–202. doi: 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- 72.Okamoto K., Miyazaki N., Larsson D.S.D., Kobayashi D., Svenda M., Mühlig K., Maia F.R.N.C., Gunn L.H., Isawa H., Kobayashi M., et al. The infectious particle of insect-borne totivirus-like Omono River virus has raised ridges and lacks fibre complexes. Sci. Rep. 2016;6:33170. doi: 10.1038/srep33170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yarden O. Fungal association with sessile marine invertebrates. Front. Microbiol. 2014;5:228. doi: 10.3389/fmicb.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alker A.P., Smith G.W., Kim K. Characterization of Aspergillus sydowii (Thom et Church), a fungal pathogen of Caribbean sea fan corals. Hydrobiologia. 2001;460:105–111. doi: 10.1023/A:1013145524136. [DOI] [Google Scholar]
- 75.Pivkin M.V. Filamentous fungi associated with holothurians from the sea of Japan, off the primorye coast of Russia. Biol. Bull. 2000;198:101–109. doi: 10.2307/1542808. [DOI] [PubMed] [Google Scholar]
- 76.Zhuravleva O.I., Antonov A.S., Oleinikova G.K., Khudyakova Y.V., Popov R.S., Denisenko V.A., Pislyagin E.A., Chingizova E.A., Afiyatullov S.S. Virescenosides from the holothurian-associated fungus Acremonium striatisporum Kmm 4401. Mar. Drugs. 2019;17:616. doi: 10.3390/md17110616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marchese P., Garzoli L., Gnavi G., O’Connell E., Bouraoui A., Mehiri M., Murphy J.M., Varese G.C. Diversity and bioactivity of fungi associated with the marine sea cucumber Holothuria poli: Disclosing the strains potential for biomedical applications. J. Appl. Microbiol. 2020;129:612–625. doi: 10.1111/jam.14659. [DOI] [PubMed] [Google Scholar]
- 78.Ismail H., Lemriss S., Ben Aoun Z., Mhadhebi L., Dellai A., Kacem Y., Boiron P., Bouraoui A. Antifungal activity of aqueous and methanolic extracts from the Mediterranean sea cucumber, Holothuria polii. J. Mycol. Méd. 2008;18:23–26. doi: 10.1016/j.mycmed.2008.01.002. [DOI] [Google Scholar]
- 79.Adibpour N., Nasr F., Nematpour F., Shakouri A., Ameri A. Antibacterial and antifungal activity of Holothuria leucospilota isolated from Persian Gulf and Oman Sea. Jundishapur J. Microbiol. 2014;7:e8708. doi: 10.5812/jjm.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nerva L., Forgia M., Ciuffo M., Chitarra W., Chiapello M., Vallino M., Varese G.C., Turina M. The mycovirome of a fungal collection from the sea cucumber Holothuria polii. Virus Res. 2019;273:197737. doi: 10.1016/j.virusres.2019.197737. [DOI] [PubMed] [Google Scholar]
- 81.Schroeder L. Wasting-like lesions occurring on California Sea Cucumbers. Dredg. 2017;57:3. [Google Scholar]
- 82.Fankboner P.V., Cameron J.L. Seasonal atrophy of the visceral organs in a sea cucumber. Can. J. Zool. 1985;63:2888–2892. doi: 10.1139/z85-432. [DOI] [Google Scholar]
- 83.García-Arrarás J.E., Greenberg M.J. Visceral regeneration in holothurians. Microsc. Res. Tech. 2001;55:438–451. doi: 10.1002/jemt.1189. [DOI] [PubMed] [Google Scholar]
- 84.Deng H., Zhou Z.-C., Wang N.-B., Liu C. The syndrome of sea cucumber (Apostichopus japonicus) infected by virus and bacteria. Virol. Sin. 2008;23:63–67. doi: 10.1007/s12250-008-2863-9. [DOI] [Google Scholar]
- 85.Wang P., Chang Y., Yu J., Li C., Xu G. Acute peristome edema disease in juvenile and adult sea cucumbers Apostichopus japonicus (Selenka) reared in North China. J. Invertebr. Pathol. 2007;96:11–17. doi: 10.1016/j.jip.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu H., Zheng F., Sun X., Hong X., Dong S., Wang B., Tang X., Wang Y. Identification of the pathogens associated with skin ulceration and peristome tumescence in cultured sea cucumbers Apostichopus japonicus (Selenka) J. Invertebr. Pathol. 2010;105:236–242. doi: 10.1016/j.jip.2010.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.