Abstract
Doctor shopping is the practice of visiting multiple physicians to obtain multiple prescriptions. Health information technology (HIT) allows healthcare providers and patients to leverage records or shared information to improve effective care. Our research objective was to determine how HIT is being leveraged to control for doctor shopping. We analyzed articles that covered a 10-year time period from four databases and reported using preferred reporting items for systematic reviews and meta-analysis (PRISMA). We compared intervention, study design, and bias, in addition to showing intervention interactions with facilitators, barriers, and medical outcomes. From 42 articles published from six countries, we identified seven interventions, five facilitator themes with two individual observations, three barrier themes with six individual observations, and two medical outcome themes with four individual observations. Multiple HIT mechanisms exist to control for doctor shopping. Some are associated with a decrease in overdose mortality, but access is not universal or compulsory, and data sharing is sporadic. Because shoppers travel hundreds of miles in pursuit of prescription drugs, data sharing should be an imperative. Research supports leveraging HIT to control doctor shopping, yet without robust data sharing agreements, the efforts of the system are limited to the efforts of the entity with the least number of barriers to their goal. Shoppers will seek out and exploit that organization that does not require participation or checking of prescription drug monitoring programs (PDMP), and the research shows that they will drive great distances to exploit this weakest link.
Keywords: prescription drug monitoring program, pharma cloud, doctor shopping, PDMP
1. Introduction
1.1. Rationale
The World Health Organization (WHO) encourages providers to remove impediments that prevent a patient’s voice from being heard [1,2,3]. However, the recent opioid epidemic serves as a reminder of systemic inefficiencies that push patients’ voice into unhealthy practices. Unchecked, these inefficiencies are deadly [4]. An example of this can be described by the practice of doctor shopping, which is the practice of individuals visiting several physicians to obtain multiple prescriptions or to attain a preferred medical diagnosis without distinct material gain [4]. It is also defined by imposing a threshold of 6 or more prescriptions from at least 6 different prescribers within 6 months’ time [4]. Such a specific description made our team wonder why health information technology (HIT) could not be leveraged to control for this practice. The simple answer may lie in the concept of interoperability or lack thereof because even countries with a national health system struggle with issues of interoperability [5]. In its simplest form, interoperability “enables the secure exchange of health information with, and use of electronic health information from, other health information technology without special effort on the part of the user” [6]. If physicians could quickly check to see if the patient had recently received the same prescription from another physician outside the parameters of proper medication use, the practice of doctor shopping could be curtailed. Our team of researchers wondered if HIT was currently being leveraged to control for doctor shopping, if there are public health benefits to doing so, and if other researchers had already identified this problem.
An internationally focused systematic review in 2019 examined the characteristics of doctor shoppers [7]. This review found 40% of 43 studies focused on opioids, antidepressants, or psychoactive drugs, and 60% surrounded chronic disease. Only 0.5% originated from the U.S., while 25% originated from Japan. Contributing behaviors to doctor shopping were comorbidities, active substance abuse, greater distance from healthcare facility, younger age, longer disease, and poor patient satisfaction. A U.S. focused review in 2018 examined the association between prescription drug monitoring programs (PDMP) and nonfatal and fatal drug overdoses [8]. It found 47% of the 17 articles implemented PDMP only, 12% implemented program features, and 41% implemented both. Low-strength evidence existed for an association between PDMP and fatal overdoses, and program features were strongly associated a decrease in overdose deaths. An EU focused review from 2012 examined misuse of medicines and mentions doctor shopping [9]. It listed opioids analgesics, methadone, buprenorphine, and z-drugs as the chief drugs misused. It also identified the international drug control system as a control for abuse.
The United Nations’ International Narcotics Control Board predicts that misuse of prescription drugs will exceed illicit drug use soon [10]. In 01 January 2013, Americans reported misuse or abuse of prescription painkillers and 17,000 American died of overdose from painkillers [11]. Prescription drug abuse in the U.S. is second only to marijuana use across all age groups and close to 1.4 million Germans are dependent on prescription drugs. Overdose deaths involving prescription opioids in the U.S. were five times higher in 2016 than in 1999 [12].
Our research investigates doctor shopping and the HIT tools available to address it. Properly designed, HIT enables healthcare providers and patients to electronically access systems, records, or shared information in order to improve quality, safety, and effective care [6]. An example is the prescription drug monitoring program (PDMP): an electronic database in the U.S. that tracks controlled substance prescriptions in a state [13]. Another example can be seen in a health information exchange (HIE): the mobilization of health care information electronically across organizations within a region, community, or hospital system [14]. Outside the U.S., PharmaCloud is a system that enables physicians at contracted medical services providers to search patients’ medication records over the previous three months to prevent drug shopping [15]. Lastly, smart cards (NHI-IC cards) carry information about a patient’s prescribed medications received from different hospitals nationwide. This system can address the problem of duplicate medications for outpatients visiting multiple hospitals [16].
In addition to the HIT solutions associated with doctor shopping, some key terms for this research include multiple provider episodes (MPE), which is the obtainment of controlled substances from some minimum number of prescribers and/or pharmacies in a given period of time [17]; nonmedical use of prescription medications (NMPM) is the use of medications without a prescription from a health care provider, use in a manner other than as directed [18]; and national provider identifier (NPI) is a unique 10-digit identification number issued to healthcare providers in the United States by the Centers for Medicare and Medicaid Services [19]. Our research investigates why doctor shopping is dangerous and how health outcomes affect patient populations. Identifying doctor shopper drug shopping patterns with the use of HIT should theoretically help providers better understand and control this phenomenon.
1.2. Objectives
The objective of this systematic review is to determine the prevalence of doctor shopping and how HIT can be useful in reducing it. Our research demonstrates that doctor shopping is an international concern where abuse and addiction of drugs is prevalent. The lack of an integrated health information exchange makes it difficult for providers to gain the needed information to address if an individual has already received a medication or service [14]. This review should help identify other means to control doctor shopping and their effectiveness through the examination of medical outcomes.
2. Materials and Methods
2.1. Protocol and Registration
This review followed the Kruse protocol published in 2019 [20]. It was reported in accordance with preferred reporting items for systematic reviews and meta-analysis (PRISMA) [21]. This review was registered with PROSPERO on 2 May 2020. In accordance with rules at PROSPERO, the registration was completed before analysis began.
2.2. Eligibility Criteria
Studies in this review were eligible if some form of HIT was implemented in the control of doctor shopping, they were published in quality journal (peer reviewed), and were published in the last 10 years. Preferably, these studies also reported medical outcomes, but that was not a requirement for selection. Moreover, 10 years was chosen as the time frame because in the realm of technology, 10 years is enough time to capture current trends without confounding the results with outdated technology, and because 10 years was used in the systematic reviews referenced in our introduction section. A quality assessment of articles was made with the Johns Hopkins nursing evidence-based practice rating scale (JHNEBP) [22].
2.3. Information Sources
Reviewers queried four databases: The Cumulative Index of Nursing and Allied Health Literature (CINAHL), PubMed (MEDLINE), Web of Science, and Embase (Science Direct). Databases were filtered for the last 10 years. Database searches occurred between 1–15 February 2020.
2.4. Search
Reviewers conducted a Google Scholar search using general terms about the topic. The 10 most recent articles were identified on the subject and reviewers collected the key terms from these studies to help form a Boolean search string. Using the PubMed Medical Subject Headings (MeSH), reviewers used the terms gathered from the 10 articles to examine how they were indexed and categorized. Once a Boolean search string was assembled, it was tested out several times in PubMed and customized for maximum, most effective yield. The final search string was (“doctor shopping” OR “drug shopping”) AND (“health information technology” OR “health information exchange” OR informatics). The same string was used for all four databases. Reviews were filtered out and other filters were used to help the search focus on quality articles on the subject.
2.5. Study Selection
Reviewers followed the Kruse Protocol for conducting a systematic review through the use of three consensus meetings [20]. Search results from the four databases were downloaded to a common Excel spreadsheet used as a literature matrix. This spreadsheet was used throughout the process to extract data and analyze results. This piloted form had several standard data fields to collect at each stage of the process. The group leader assigned workload so all abstracts were screened by at least two reviewers against the objective statement. Reviewers made independent recommendations to keep or discard using the following codes to document their work: D = Duplicate, NJ = non-journal, P = Protocol, B = Book, R = Review, M = Model, and NG = Not Germane. During the first consensus meeting, disagreement with recommendations was discussed. A tie was broken through a third reviewer’s assessment of the article’s applicability. By the end of the meeting, a final set of articles was identified to analyze. A kappa statistic was calculated from this process [23,24].
2.6. Data Collection Process
In preparation for the second consensus meeting, the group leader assigned workload to ensure all articles were analyzed by at least two reviewers. Reviewers read through articles twice: (1) to collect participants, intervention, comparison, outcome, and study design (PICOS) data and (2) to make observations relevant to the objective. During the second consensus meeting, reviewers discussed their observations. From the collective set of observations, a thematic analysis was performed to make sense of the data [25]. This served two purposes: (1) to make sense of the observations and (2) to find synergistic effects that result in “ah ha” moments when reviewers remember reading about a theme but did not record it fully. Reviewers then carefully read through the articles a third time to make more detailed observations relevant to the themes. During the third consensus meeting, inferences were made through observed interactions between the themes.
2.7. Data Items
Through the spreadsheet that served as a piloted form [20], standard data items were collected: participants, intervention (health information technology), study design, results compared to a control group (where applicable), facilitators and barriers to the use of health information technology, medical outcomes, sample size, bias within studies, effect size, country of origin, statistics used, a quality assessment from the JHNEBP [22], and general observations about the article that would help interpret the results.
2.8. Risk of Bias within and across Studies
Along the process of extracting data, general observations of bias and quality were made by each reviewer. Bias, such as selection bias, was discussed in the second consensus meeting. These were important to observe because bias can limit the external validity of the results from studies. Quality assessments from the JHNEBP were also discussed in the second consensus meeting. The JHNEBP has existed since 2007. It is comprised of five levels for strength of evidence and three levels for quality of evidence. The levels under strength of evidence are as follows: level 1 is an experimental study or randomized control trial (RCT); level 2 is quasi-experimental studies; level 3 is non-experimental, qualitative, or meta-synthesis studies; level 4 is opinion of nationally recognized experts based on research evidence or consensus panels; and level 5 is opinions of experts that is not based on research evidence. The levels under quality of evidence are as follows: A (high), B (good), or C (low quality or major flaws). Under each of these levels, specifics are defined for research, summative reviews, organizational, and expert opinion; e.g., research in level A must have consistent results with sufficient sample size, adequate control, and definitive conclusions; research in level B must have reasonably consistent results, sufficient sample size, some control, and definitive conclusions; research at level C has little evidence with inconsistent results, insufficient sample size, and conclusions that cannot be drawn from the data. Articles with a strength of evidence rating below Level 4 will be screened out. Quality of evidence below level B are highly suspect and must have full consensus of the group to be kept for analysis.
2.9. Summary Measures
The review analyzed studies with qualitative, quantitative, and mixed methods, so the summary measures sought were not consistent. The preferred summary statistic would be the risk ratio, but descriptive statistics, means’ comparisons (student-t) are also sufficient. Summary statistics were also discussed at the second consensus meeting.
2.10. Additional Analysis
At the second consensus meeting, a thematic analysis was performed to group observations into themes. These themes were measured across all articles analyzed and reported in summary statistics in a series of affinity matrices. The thematic analysis summarized themes for facilitators, barriers, and medical outcomes. These are reported in affinity matrices in the Results section.
3. Results
3.1. Study Selection
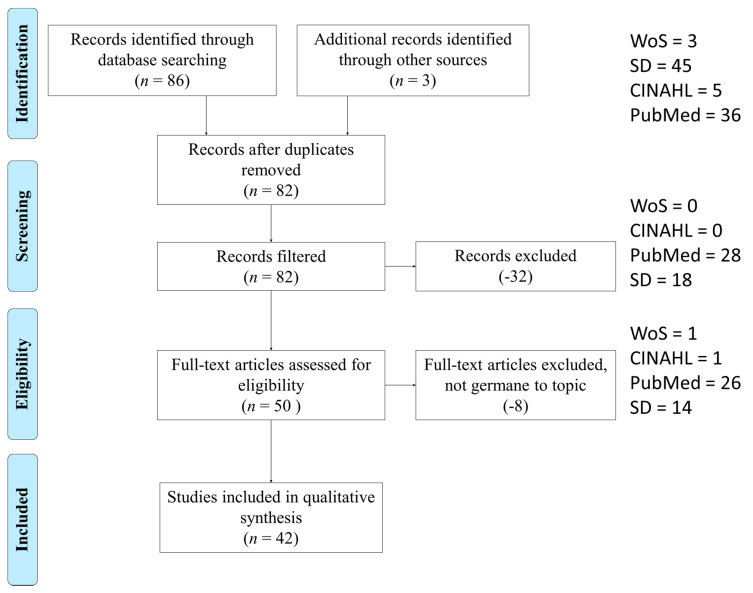
The study selection process performed is illustrated in Figure 1. A kappa statistic was calculated after the first consensus meeting (k = 0.95), which indicates near perfect agreement [23,24]. After screening, removing duplicates and assessing for eligibility, the 48 articles chosen for analysis came from CINAHL (1, 2%), Web of Science (1, 2%), PubMed (28, 58%), and Science Direct (18, 38%).
Figure 1.
Preferred reporting items for systematic reviews and meta-analysis (PRISMA) figure that demonstrates the study selection process.
3.2. Study Characteristics
Using the piloted form, reviewers collected several standard items used for summary, such as PICOS. A PICOS table is provided in Table 1. Additional items were collected for analysis, such as forms of assistive technology interventions, facilitators, and barriers to the use of assistive technologies, and the medical outcomes observed from those older adults using assistive technology solutions. These are presented in Table 2. Table 1 and Table 2 lists articles in reverse chronological order: 2020 (1) [26], 2019 (7) [27,28,29,30,31,32,33], 2018 (4) [34,35,36,37], 2017 (3) [38,39,40], 2016 (7) [17,41,42,43,44,45,46], 2015 (4) [15,47,48,49], 2014 (3) [11,14,50], 2013 (2) [51,52], 2012 (4) [53,54,55,56], 2011 (6) [13,16,57,58,59,60], 2010 (1) [61].
Table 1.
PICOS for all articles analyzed.
| Authors | Participants | Intervention | Results (Compared to Control Group) | Medical Outcomes Reported | Study Design |
|---|---|---|---|---|---|
| Pett RG et al. [26] | Pharmacists | PDMP | Frequent PDMP users were more likely to recommend naloxone. | Naloxone prescribed to shoppers | Explanatory, sequential 2-phase mixed-methods |
| Durand et al. [27] | Injured workers identified in worker’s compensation records | PDMP and worker’s compensation claims | No control group. Injured workers have a high prevalence of opioid use after injury, but prescribing patterns generally follow state guidelines. | Not reported | Retrospective cohort |
| Freeman et al. [28] | Pharmacists and providers | Interviews about PDMP | No control group. Both PCPs and pharmacists reported PDMPs are key tools to aid prescribing and dispensing. | Not reported | Qualitative |
| Nagarajan and Talbert [29] | Prescriber-prescriber networks | Computer model | Outliers were clearly identified in the model, which can help identify those prescribers contributing to the opioid epidemic. | Not reported | Computer model to detect prescriber outliers |
| Perry et al. [30] | Patients who submitted claims to commercial database in the Appalachian region of the U.S., 58.7% female | Computer model PageRank | Model clearly differentiates aberrant behavior identifying drug shoppers for both opioids and morphine milligram equivalents (MME). | Not reported | Computer model to detect prescriber outliers |
| Soffin et al. [31] | n/a | PDMP | n/a | Accessing real-time information about patients’ prescription opioid status using PDMP reduces opioid quantities prescribed | Opinion |
| Stopka et al. [32] | adults in Massachusetts prescribed opioids | Computer model | Hotspots were identified. | Not reported | Spatial epidemiological study |
| Wang et al. [33] | Patients in pharmacological database in Florida | Pharmacological database | Recipients of opioids, benzodiazepines, and carisoprodol in 2017 compared with 2012 were younger, more likely to be female, and geographically-localized. | Not reported | Retrospective, observational |
| Butler et al. [34] | n/a | PDMP, Lock-in programs | No control group. The lock-in program decreased doctor shopping. | Broader range of drug schedules and an increases frequency of updating the PDMP results in lower opioid-related mortality | Opinion |
| Lin et al. [35] | Patients from health insurance claim data | Medication record sharing program | The medication duplication rate was reduced 7.76 percentile, average medication overlap periods shortened 4.36 days. | Not reported | Retrospective pre-post test design |
| Ponte et al. [36] | Beneficiaries of the France public health system | SNIIRAM database | The strong opioid analgesics have the highest DSI (2.79%) versus 2.06% for BZD hypnotics. Flunitrazepam ranked first according to its DSI (13.2%), followed by morphine (4%), and zolpidem (2.2%). | Not reported | Retrospective, observational |
| Torrance et al. [37] | Beneficiaries of the Scotland National Health System (NHS) | NHS and Generation Scotland databases combined to identify trends | The number of strong opioid prescriptions more than doubled between 2003–2012. Patients in the most deprived areas were more likely to receive a strong opioid. | Not reported | Descriptive analysis |
| Ali et al. [38] | Respondents to the national survey of drug use and health, aged 12 and older, 48% male | Survey instrument to assess effectiveness of the PDMP | No control group. PDMP was effective at controlling for doctor shopping for opiate pain killers. | 10–20 fewer days of NMPR use | Qualitative |
| Rutkow et al. [39] | Prescribers in four states | PDMP | Prescribers need to work with law enforcement, law-enforcement need to share data with each other, data sharing between states needs to occur. | Not reported | Qualitative |
| Simeone [40] | Prescriptions | PDMP, education efforts, pharmacy panels that span the country | No control group. The number of prescriptions diverted fell from 4.3 million in 2008 to 3.37 million in 2012. | Decline in overdose mortality | Retrospective, observational |
| Chenaf et al. [17] | Adult patients with chronic non-cancer pain (CNCP) | French national health system | No control group. Shopping very low in drugs for these conditions. | Not reported | Retrospective cohort |
| Delorme et al. [42] | Patients treated by opioid substitution treatment over 8 years | French national health system | No control group. Shopping behavior was only found in high dosage buprenorphine patients, but still very low. | Not reported | Retrospective cohort |
| Kea et al. [43] | Emergency Department (ED) discharges | PDMP | Doctor shopping was not detected in ED survey. | Not reported | Qualitative |
Table 2.
Summary of analysis.
| Authors | Intervention Theme | Outcome Theme | Facilitator Theme | Barrier Theme |
|---|---|---|---|---|
| Pett et al. | PDMP | Shoppers prescribed treatment | Pharmacists support | Not reported |
| Durand et al. | Combination | Not reported | Government support | Data sharing |
| Freeman et al. | PDMP | Not reported | Government support | Access not mandatory |
| Pharmacists support | ||||
| Prescriber support | ||||
| Nagarajan and Talbert | Computer model | Not reported | Simple to implement | Not reported |
| Perry et al. | Computer model | Not reported | Not reported | Not reported |
| Soffin et al. | PDMP | Reduced opioids prescribed | Government support | Not reported |
| Prescriber support | ||||
| Stopka et al. | Computer model | Not reported | Simple to implement | Not reported |
| Wang et al. | Other | Not reported | Government support | Not reported |
| Butler et al. | Combination | Reduced mortality | Must use law | Access not mandatory |
| Does not treat addiction | ||||
| Lin et al. | Other | Not reported | Government support | Not reported |
| Ponte et al. | National health system database (DB) | Not reported | Government support | Not reported |
| Torrance et al. | National health system DB | Not reported | Government support | Not reported |
| Ali et al. | PDMP | Fewer days of use | Not reported | Not reported |
| Rutkow et al. | PDMP | Not reported | Government support | Data sharing |
| Simeone | Combination | Reduced mortality | Government support | Easily thwarted |
| Pharmacists support | ||||
| Chenaf et al. | National health system DB | Not reported | Government support | Not reported |
| Delorme et al. | National health system DB | Not reported | Government support | Not reported |
| Kea et al. | PDMP | Not reported | Government support | Not reported |
| National Council of State Boards of Nursing | PDMP | Not reported | Government support | Not reported |
| Okumura et al. | National health system DB | Not reported | Government support | Not reported |
| Ong et al. | Health insurance claims | Not reported | Government support | Data sharing |
| Prescriber support | ||||
| Takahashi et al. | Computer model | Not reported | Simple to implement | Not reported |
| Huang et al. | Other | Not reported | Cost savings | Participation not mandatory |
| Government support | Access not mandatory | |||
| Lin et al. | Health insurance claims | Not reported | Government support | Not reported |
| Lu et al. | Health insurance claims | Not reported | Government support | Not reported |
| Webster and Grabois | PDMP | Not reported | Government support | Access not mandatory |
| Han et al. | PDMP | Reduced shopping | Not reported | Not reported |
| Hypponen et al. | Health information exchange | Not reported | Government support | Not reported |
| Cost savings | ||||
| Increased efficiency | ||||
| Shepherd | Combination | Not reported | Not reported | Inadequate data collection |
| Ineffective data use | ||||
| Data sharing | ||||
| Constraints on enforcement | ||||
| Cash-only not captured | ||||
| Cepeda et al. | PDMP | Reduced shopping | Not reported | Data sharing |
| Modarai et al. | Other | Not reported | Government support | Not reported |
| Rouby et al. | National health system DB | Not reported | Government support | Not reported |
| Simoni-Wastila and Qian | PDMP | No decline in mortality | Government support | Not reported |
| Worley et al. | PDMP | Reduced shopping | Government support | Not reported |
| Prescriber support | ||||
| Worley et al. | PDMP | Not reported | Government support | Not reported |
| Prescriber support | ||||
| Fass and Hardigan | PDMP | Not reported | Government support | Not reported |
| Frauger et al. | Other | Not reported | Government support | Not reported |
| Hincapie et al. | Health information exchange | Not reported | Government support | Participation not mandatory |
| Hsu et al. | Combination | Not reported | Prescriber support | Participation not mandatory |
| Pauly et al. | Computer model | Not reported | Government support | Not reported |
| Wilsey et al. | PDMP | Not reported | Government support | Not reported |
| Pradel et al. | National health system DB | Not reported | Government support | Not reported |
3.3. Risk of Bias within Studies
Reviewers recorded observations of bias at the study level. The most common form of bias was convenience samples taken from one country only. This was common to every article in the review. This is logical since countries struggle enough with domestic interoperability. International interoperability may be too much to ask for in the near term. There was one instance of selection bias [40]. These examples of bias limit the external validity of the results.
3.4. Results of Individual Studies
Reviewers collected their observations of intervention and medical outcomes during the analysis phase. The narrative analysis of their observations identified themes. A summary of these themes is listed in Table 2. Repetition in the frame of a theme is due to multiple observations from the same article for that theme. For instance, the theme increased talking comprised observations of “increased utterances” and “increased sustained conversations.” A translation from observations to themes for interventions, medical outcomes, facilitators, and barriers are listed in Appendix A. Additional data items extracted are displayed in Appendix B: sample size, bias, country of origin, statistics, and quality assessments.
3.5. Synthesis of Results
This subsection addresses meta-analyses. This manuscript is a systematic review. This subsection will be deleted after the review process. It is included to reassure reviewers that we followed the PRISMA checklist.
3.6. Risk of Bias across Studies
Table 3 summarizes the quality indicators identified by the JHNEBP tool [22]. The most prevalent assessment in the strength of evidence (panel a) was level III, followed by IV, and II. For quality of evidence (panel b), the most frequently assessed level was level A, followed by B and C. It is certainly preferable for the strength of evidence be closer to level I, but that result did not materialize from the screening and selection process. This limitation will be addressed later.
Table 3.
Summary of quality assessments.
| Strength of Evidence | Frequency | Quality of Evidence | Frequency |
|---|---|---|---|
| III (Non-experimental, qualitative) | 28 (67%) | A (High quality) | 25 (60%) |
| IV (Opinion) | 10 (24%) | B (Good quality) | 11 (26%) |
| II (Quasi-experimental) | 4 (10%) | C (Low quality or major flaws) | 6 (14%) |
| I (Experimental study or RCT) | 0 (0%) | ||
| (a) | (b) | ||
3.7. Additional Analysis
3.7.1. Interventions of HIT
The results of consensus meeting three identified seven intervention themes that corresponded with utilizing HIT to control for doctor shopping. These are listed in Table 4. In the interest of brevity, only the top 90% are described. The intervention PDMP (state-run prescription drug monitoring program or interviews/surveys about PDMP) appeared in 15/42 articles (36%) [13,26,28,31,38,39,41,43,49,50,51,54,55,56,60]. These articles all originated from the United States because that is the only country that uses PDMP. The intervention national health system database (European, Scotland, French, Japan, Finland) appeared in 7/42 articles (17%) [17,36,37,42,44,53,61]. The intervention computer model (e.g., clustering models, PageRank, social network analysis) appeared in 5/42 articles (17%) [29,30,32,46,59]. Three of these articles originated from the United States, while the others were from Japan and France. The intervention combination (PDMP combined with worker’s compensation claims, PDMP and Lock-in programs, PDMP education efforts and pharmacist panels, PDMP and PBM, Computerized Provider Order Entry (CPOE) and NHI-IC cards) appeared in 5/42 articles (17%) [11,16,27,34,54]. Four of these articles originated in the United States, while the other was from Taiwan. The intervention other (pharmacological database, medication record sharing program, PharmaCloud, ACOS and DAWN, and CEIP) appeared in 5/42 articles (17%) [15,33,35,52,57]. Two of these articles originated from the United States, two from Taiwan, and one from France.
Table 4.
Affinity matrix of Health Information Technology (HIT) interventions to control for doctor shopping.
| Interventions | References | Occurrences (n = 42) | Frequency |
|---|---|---|---|
| PDMP | [13,26,28,31,38,39,41,43,49,50,51,54,55,56,60] | 15 | 36% |
| National health system DB | [17,36,37,42,44,53,61] | 7 | 17% |
| Computer model | [29,30,32,46,59] | 5 | 12% |
| Combination | [11,16,27,34,54] | 5 | 12% |
| Other | [15,33,35,52,57] | 5 | 12% |
| Health insurance claims | [45,47,48] | 3 | 7% |
| Health information exchange | [14,58] | 2 | 5% |
3.7.2. Facilitators of HIT
The results of consensus meeting three identified five themes and two individual observations that corresponded with facilitators of HIT to control for doctor shopping. These are listed in Table 5, which contains Table 5, Table 6 and Table 7 (facilitators, barriers, and medical outcomes). In the interest of brevity, only the first 75% will be listed (other than not reported). The facilitator theme was government support (state supports prescription monitoring or prescription monitoring programs) occurred in 31/52 occurrences (60%) [13,14,17,27,28,31,33,35,36,37,39,40,41,42,43,44,45,47,48,49,52,53,54,55,56,57,58,59,60,61]. In total, 17 of these articles originated from the United States, seven from France, three from Taiwan, while the others were from original originations in Finland and Scotland. The theme prescriber support (physician, nurse practitioner) occurred 6/52 times (12%) [28,31,45,55,56]. Four of these articles originated from the United States while the other was from Taiwan.
Table 5.
Affinity matrix of facilitators of Health Information Technology (HIT)to control for doctor shopping.
| Facilitators | References | Occurrences (n = 52) | Frequency |
|---|---|---|---|
| Government support | [13,14,17,27,28,31,33,35,36,37,39,40,41,42,43,44,45,47,48,49,52,53,54,55,56,57,58,59,60,61] | 31 | 60% |
| Prescriber support | [28,31,45,55,56] | 6 | 12% |
| Not reported | [11,30,38,50,51] | 5 | 10% |
| Simple to implement | [29,30,32,46] | 4 | 6% |
| Pharmacists support | [26,28,40] | 3 | 6% |
| Cost savings | [14,15] | 2 | 4% |
| Must use law | [34] | 1 | 2% |
| Increased efficiency | [14] | 1 | 2% |
Table 6.
Affinity matrix of barriers of Health Information Technology (HIT)to control for drug shopping.
| Barriers | References | Occurrences (n = 48) | Frequency |
|---|---|---|---|
| Not reported | [13,14,17,26,29,30,31,32,33,35,36,37,38,41,42,43,44,46,47,48,50,52,54,55,56,57,59,60,61] | 30 | 63% |
| Data sharing | [11,27,39,45,51] | 5 | 10% |
| Access not mandatory | [15,28,34,49] | 4 | 8% |
| Participation not mandatory | [15,16,58] | 3 | 6% |
| Easily thwarted | [40] | 1 | 2% |
| Inadequate data collection | [11] | 1 | 2% |
| Ineffective data use | [11] | 1 | 2% |
| Constraints on enforcement | [11] | 1 | 2% |
| Cash-only not captured | [11] | 1 | 2% |
| Does not treat addiction | [34] | 1 | 2% |
Table 7.
Affinity matrix of medical outcomes commensurate with Health Information Technology (HIT) as intervention.
| Medical Outcomes | References | Occurrences (n = 42) | Frequency |
|---|---|---|---|
| Not reported | [11,13,14,15,16,17,27,28,29,30,32,33,35,36,37,39,41,42,43,44,45,47,48,49,52,53,56,57,58,59,60,61] | 33 | 79% |
| Reduced shopping | [50,51,55] | 3 | 7% |
| Reduced mortality | [34,40] | 2 | 5% |
| Shoppers prescribed treatment | [26] | 1 | 2% |
| Fewer days of use | [38] | 1 | 2% |
| No decline in mortality | [54] | 1 | 2% |
| Reduced opioids prescribed | [31] | 1 | 2% |
3.7.3. Barriers of HIT
The results of the consensus meeting identified three themes and six individual observations that corresponded with barriers of HIT to control for doctor shopping. These are listed in Table 6. In the interest of brevity, only the themes will be discussed (other than not reported). The theme of data sharing (PDMP do not all share across state lines) occurred in 5/52 occurrences (10%) [11,27,39,45,51]. These articles all originated from the United States. The theme access not mandatory (access to PDMP or PharmaCloud not mandatory and the intervention is not widely implemented because of this fact) occurred in 4/52 occurrences (8%) [15,28,34,49]. Three of these articles originated from the United States while the other originated from Taiwan. The theme participation was not mandatory (not all states participate in health information exchanges, not all facilities participate in PharmaCloud) occurred in 3/52 occurrences (6%) [15,16,58]. Two of these articles originated in Taiwan, while the other was from the United States.
3.7.4. Medical Outcomes Commensurate with HIT as Intervention
The results of consensus meeting three identified two themes and four individual observations that corresponded with medical outcomes commensurate with HIT to control for doctor shopping. These are listed in Table 7. In the interest of brevity, only the themes will be listed (other than not reported). The theme reduced [doctor] shopping (decreased shopping and diversion) occurred in 3/42 articles (7%) [50,51,55]. These originated from the United States. The theme reduced mortality (decline in overdose mortality) occurred in 2/42 articles (5%) [34,40]. These originated from the United States.
3.8. Interactions between Observations
The intervention of the national health system database resulted in seven instances of government support [17,36,37,42,44,53,61]. The intervention of the computer model resulted in five instances of simple to implement [29,30,32,46,59]. The intervention PDMP resulted in two instances of access not mandatory [28,49] and two instances of data sharing [39,51].
4. Discussion
4.1. Summary of Evidence
Through the analysis of 42 articles, this review identified seven interventions, five facilitator themes with two individual observations, three barrier themes with six individual observations, and two medical outcome themes with four individual observations. Most of the articles analyzed were non-experimental (28/42, 67%) but of high quality (25/42, 60%). The facilitator themes mentioned most frequently were government support (31/52, 60%) [13,14,17,27,28,31,33,35,36,37,39,40,41,42,43,44,45,47,48,49,52,53,54,55,56,57,58,59,60,61] and prescriber support (5/52, 12%) [28,31,45,55,56]. The barrier themes mentioned most frequently were data sharing (5/48, 10%) [11,27,39,45,51] and access not mandatory (4/48, 8%) [15,28,34,49]. The medical outcome themes mentioned most frequently were reduced shopping (3/42, 7%) [50,51,55] and reduced mortality (2/42, 5%) [34,40]. A large majority originated from the United States (26/42, 62%), but also from France (7/42, 17%), Taiwan (5/42, 12%), Japan (2/42, 5%), and one each from Finland and Scotland.
The high level of government and prescriber support was reassuring to observe because it indicates that government health agencies recognize the problem of doctor shopping and are attempting to address it through regulation. It also indicates that governments and prescribers are communicating about the problem and collaborating for solutions. This result was expected because they had been observed in other research [7,8,9].
One interesting dichotomy was observed. In medical outcomes, 2/42 articles mentioned a reduction in overdose mortality commensurate with the use of HIT measures to control for doctor shopping [34,40], while one listed no decline in mortality [54]. A key point made by one article is that the control programs like PDMP do nothing to treat the disease of addiction driving the behavior [34]. This author mentioned that unless control programs institute treatment [26], doctor shoppers will find other means of finding their illicit drugs to misuse.
Policy makers should examine the barriers listed in this review. The barrier of data access predominantly spoke of the United States with state-run PDMPs. All but three states share data, but Florida, Georgia, and Nebraska do not allow data sharing [49]. Without nationwide data sharing agreements, shoppers just travel to the next state to do their shopping. Shoppers are documented to travel an average of 199.5 miles to shop, while non-shoppers do not travel at all [51]. Another key barrier is that participation is not mandatory. This occurred in the he U.S. with PDMP and Taiwan with PharmaCloud. If participation is not mandatory, universal enforcement cannot occur.
4.2. Limitations
The researchers analyzed articles from 2010 to 2020. The reviewers hoped to find higher strength studies from which to extract data and summarize results. However, there is a paucity of high-strength studies in this area. Future researchers should consider this high-need area to explore.
This review limited its search to four databases: PubMed, CINAHL, Science Direct, and Web of Science. It did not include broader sources such as Google Scholar. Even though 42 articles were analyzed, it is possible other sources may have yielded higher strength articles to include in the analysis. Only 67% were high quality articles, while the others (17) were of good or low quality. It is important to base conclusions on high quality articles, but often we are relegated to what is already published.
A team of reviewers determined the articles to be included in the study. This was also done to mitigate the risk of selection bias. The risk of this practice, however, is that the team may have differed in their selection process. To mitigate this risk, researchers held consensus meetings and identified the research objective, and had multiple reviews for each article. The limitation is that there may not have been enough consensus meetings. The kappa statistic shows the consensus meetings were effective, yet a stronger level of agreement is possible.
5. Conclusions
This research supports the use of HIT as a control for doctor shopping. Computer models are simple to implement and monitoring systems exist to help prescribers and dispensers’ control for doctor shopping behavior. Greater interaction, whether voluntary or mandatory, yields greater success as the behavior is identified. After identifying the behavior, treatment and help should follow. Otherwise, the patient will find other outlets for the behavior. Government intervention is evident in the research, and this enabler should be exploited. Robust data sharing and both participation and consultation of PDMP should be a standard in the industry to decrease doctor shopping and improve mortality.
Abbreviations
| CEIP | French Centers for Evaluation and Information on Pharmacodependence |
| CNCP | Chronic non-cancer pain |
| DSI | Drug Shopping Indicator |
| HIE | Health Information Exchange |
| NPI | National provider identifier |
| MME | morphine milligram equivalents |
| NMPR | Nonmedical prescription use |
| PBM | Pharmacy Benefit Managers |
| PDMR | Prescription Drug Monitoring Program |
| SNIIRAM | Global health insurance reimbursement database |
Appendix A
Table A1.
Map of Observations to Themes.
| Authors | Intervention | Intervention Theme | Medical Outcomes Reported | Outcome Theme | Facilitators | Facilitator Theme | Barriers | Barrier Theme |
|---|---|---|---|---|---|---|---|---|
| Pett RG, et al. | PDMP | PDMP | Naloxone prescribed to shoppers | Shoppers prescribed treatment | Pharmacists support PDMP | Pharmacists support | Not reported | Not reported |
| Durand Z, et al. | PDMP and worker’s compensation claims | Combination | Not reported | Not reported | Government support for PDMP | Government support | PDMP systems do not share data across state lines | Data sharing |
| Freeman PR, et al. | Interviews about PDMP | PDMP | Not reported | Not reported | Government support for PDMP, Pharmacists support for PDMP, PCP support for PDMP | Government support | Access not mandatory | Access not mandatory |
| Pharmacists support | ||||||||
| Prescriber support | ||||||||
| Nagarajan R and Talbert J | computer model | Computer model | Not reported | Not reported | Model only requires data and a statistician | Simple to implement | Not reported | Not reported |
| Perry BL, et al. | computer model PageRank | Computer model | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Soffin EM, et al. | PDMP | PDMP | Accessing real-time information about patients’ prescription opioid status using PDMP reduces opioid quantities prescribed | Reduced opioids prescribed | State supports PDMP, Providers support PDMP | Government support | Not reported | Not reported |
| Prescriber support | ||||||||
| Stopka TJ, et al. | computer model | Computer model | Not reported | Not reported | Computer model not difficult to implement (low cost) | Simple to implement | Not reported | Not reported |
| Wang Y, et al. | Pharmacological database | Other | Not reported | Not reported | State supports prescription monitoring | Government support | Not reported | Not reported |
| Butler JM, et al. | PDMP, Lock-in programs | Combination | Broader range of drug schedules and an increases frequency of updating the PDMP results in lower opioid-related mortality | Reduced mortality | A “must use” law (ten states) significantly decreased doctor shopping | Must use law | PDMP is not mandatory, Lock-in programs do not treat substance abuse (may just drive users to black-market) Only billing information is used (not cash) | Access not mandatory |
| Does not treat addiction | ||||||||
| Lin JH, et al. | Medication record sharing program | Other | Not reported | Not reported | Government support for the medication record sharing program | Government support | Not reported | Not reported |
| Ponte C, et al. | SNIIRAM database | National health system DB | Not reported | Not reported | Government support for prescription monitoring | Government support | Not reported | Not reported |
| Torrance N, et al. | NHS and Generation Scotland databases combined to identify trends | National health system DB | Not reported | Not reported | State supports prescription monitoring | Government support | Not reported | Not reported |
| Ali MM, et al. | Survey instrument to assess effectiveness of the PDMP | PDMP | 10–20 fewer days of NMPR use. | Fewer days of use | Not reported | Not reported | Not reported | Not reported |
| Rutkow L, et al. | PDMP | PDMP | Not reported | Not reported | Government support for prescription monitoring | Government support | PDMP systems do not share data across state lines | Data sharing |
| Simeone R | PDMP, education efforts, pharmacy panels that span the country | Combination | Decline in overdose mortality | Reduced mortality | Government support for PDMP, Pharmacy groups have designed a proprietary system to track prescriptions | Government support | Enterprising dealers can use more drug collection agents | Easily thwarted |
| Pharmacists support | ||||||||
| Chenaf C, et al. | French national health system | National health system DB | Not reported | Not reported | French national health system monitors drug use | Government support | Not reported | Not reported |
| Delorme J, et al. | French national health system | National health system DB | Not reported | Not reported | French national health system monitors drug use | Government support | Not reported | Not reported |
| Kea B, et al. | PDMP | PDMP | Not reported | Not reported | Government support for PDMP | Government support | Not reported | Not reported |
| National Council of State Boards of Nursing | PDMP | PDMP | Not reported | Not reported | Government support for PDMP | Government support | Not reported | Not reported |
| Okumura Y, et al. | Japan national health database | National health system DB | Not reported | Not reported | Government support for monitoring program | Government support | Not reported | Not reported |
| Ong MS, et al. | Provider patient-sharing networks | Health insurance claims | Not reported | Not reported | State supports PDMP, Providers support PDMP | Government support | State PDMPs cannot share data across state lines | Data sharing |
| Prescriber support | ||||||||
| Takahashi Y, et al. | Social network analysis | Computer model | Not reported | Not reported | Social network analysis is not difficult to implement (low cost) | Simple to implement | Not reported | Not reported |
| Huang SK, et al. | PharmaCloud | Other | Not reported | Not reported | The drug expense per person declined (2–5%), Government support for PharmaCloud | Cost savings | Intervention is not widely adopted and not mandatory | Participation not mandatory |
| Government support | Access not mandatory | |||||||
| Lin MH, et al. | Medication record sharing program | Health insurance claims | Not reported | Not reported | Government support for the medication record sharing program | Government support | Not reported | Not reported |
| Lu TH, et al. | Medication record sharing program | Health insurance claims | Not reported | Not reported | Government support for the medication record sharing program | Government support | Not reported | Not reported |
| Webster LR and Grabois M | PDMP | PDMP | Not reported | Not reported | State supports prescription monitoring | Government support | Access to PDMP not mandatory | Access not mandatory |
| Han H, et al. | PDMP | PDMP | prior to the PDMP in California, the prevalence of schedule II opioid users in California increased by 150%–280% and prevalence of doctor shoppers increased 111%–213% over 9 years. | Reduced shopping | Not reported | Not reported | Not reported | Not reported |
| Hypponen H, et al. | HIE | Health information exchange | Not reported | Not reported | Government support for the HIE, Decrease in healthcare costs through reduced duplicate testing, Increased efficiency of clinical information gathering | Government support | Not reported | Not reported |
| Cost savings | ||||||||
| Increased efficiency | ||||||||
| Shepherd J | PDMP and PBM | Combination | Not reported | Not reported | Not reported | Not reported | Inadequate data collection, PBM do not process all painkiller prescriptions, | Inadequate data collection |
| Ineffective utilization of data, | Ineffective data use | |||||||
| Insufficient interstate data sharing, | Data sharing | |||||||
| Constraints on data sharing with law enforcement and state agencies, | Constraints on enforcement | |||||||
| Prescription drugs purchased with cash (not insurance) not processed through PBM | Cash-only not captured | |||||||
| Cepeda MS, et al. | PDMP | PDMP | Shopping decreased within the same state, | Reduced shopping | Not reported | Not reported | PDMP systems do not share data across state lines | Data sharing |
| Modarai F, et al. | Automation of Reports and Consolidation Orders System (ARCOS), Drug Abuse Reporting Network (DAWN) |
Other | Not reported | Not reported | Government support for ARCOS and DAWN | Government support | Not reported | Not reported |
| Rouby F, et al. | Reimbursement database | National health system DB | Not reported | Not reported | Government support for prescription monitoring | Government support | Not reported | Not reported |
| Simoni-Wastila and Qian J | PDMP | PDMP | No decline in overdose mortality | No decline in mortality | State and federal government support for PDMP | Government support | Not reported | Not reported |
| Worley J, et al. | PDMP | PDMP | PDMPs decrease diversion and doctor shopping | Reduced shopping | State supports PDMP, | Government support | Not reported | Not reported |
| Providers support PDMP | Prescriber support | |||||||
| Worley J, et al. | PDMP | PDMP | Not reported | Not reported | State supports PDMP, | Government support | Not reported | Not reported |
| Providers support PDMP | Prescriber support | |||||||
| Fass JA and Hardigan PC | PDMP | PDMP | Not reported | Not reported | Government support for PDMP | Government support | Not reported | Not reported |
| Frauger E, et al. | CEIP | Other | Not reported | Not reported | Government support for CEIP | Government support | Not reported | Not reported |
| Hincapie AL, et al. | Interviews about HIE | Health information exchange | Not reported | Not reported | Government support for HIE | Government support | Not all participate in the HIE, therefore data cannot be universally exchanged | Participation not mandatory |
| Hsu MH, et al. | CPOE and NHI-IC cards | Combination | Not reported | Not reported | Physicians accept the intervention | Prescriber support | Participation is not universal or mandatory | Participation not mandatory |
| Pauly V, et al. | computer model | Computer model | Not reported | Not reported | Government supports prescription monitoring | Government support | Not reported | Not reported |
| Wilsey BL, et al. | PDMP | PDMP | Not reported | Not reported | State supports prescription monitoring | Government support | Not reported | Not reported |
| Pradel V, et al. | Prescription database | National health system DB | Not reported | Not reported | Government support for prescription monitoring | Government support | Not reported | Not reported |
Appendix B
Table A2.
Sample Size, Bias, Country of Origin, Statistics, and Quality Assessment.
| Authors | Sample Size | Bias within Study | Country of Origin | Statistics Used | JHNEBP | |
|---|---|---|---|---|---|---|
| Strength | Quality | |||||
| Pett RG, et al. | 967, average age not reported | United States only | United States | Logistic regression | II | A |
| Durand Z, et al. | 172,256, average age 41 | United States only | United States | t-test | III | A |
| Freeman PR, et al. | 48 PCP, 60 pharmacists, average age not reported | United States only | United States | n/a | III | B |
| Nagarajan R and Talbert J | 11,596 | United States only | United States | Tukey’s outlier detection and surrogate testing | III | B |
| Perry BL, et al. | 526,914 patients, 2,107,656 quarterly prescription entries | United States only | United States | Regression | III | A |
| Soffin EM, et al. | n/a | United States only | United States | n/a | IV | C |
| Stopka TJ, et al. | 3,143,817, average age not reported | United States only | United States | t-test | IV | C |
| Wang Y, et al. | 17,000, average age not reported | United States only | United States | t-test and descriptives | III | A |
| Butler JM, et al. | n/a | United States only | United States | n/a | IV | B |
| Lin JH, et al. | 106,508, average age not reported | Taiwan only | Taiwan | Regression | II | A |
| Ponte C, et al. | 11.7 million | France only | France | t-test | III | A |
| Torrance N, et al. | 1,036,446, average age not reported | Scotland only | Scotland | Chi squared | III | A |
| Ali MM, et al. | , average age not reported | United States only | United States | Logit regression | III | A |
| Rutkow L, et al. | 37, average age not reported | United States only | United States | language processing | III | B |
| Simeone R | 11 billion | United States Only, Selection bias because pharmacies involved in illicit activity are not going to provide data |
United States | t-test | III | A |
| Chenaf C, et al. | 3505, average age not reported | France only | France | Cox proportional hazard model | III | A |
| Delorme J, et al. | 2043 | France only | France | Cox proportional hazard model | III | A |
| Kea B, et al. | 139,256, average age not reported | United States only | United States | descriptives and natural language processing | III | B |
| National Council of State Boards of Nursing | n/a | United States only | United States | n/a | IV | B |
| Okumura Y, et al. | 1,178,361, average age not reported | Japan only | Japan | t-test | III | A |
| Ong MS, et al. | 5659 patients and 1448 provider pairs | United States only | United States | Logistic regression | III | A |
| Takahashi Y, et al. | 1.24 million, average age not reported | Japan only | Japan | regression | III | A |
| Huang SK, et al. | 30,000 physicians at 1898 facilities | Taiwan only | Taiwan | natural language processing | III | A |
| Lin MH, et al. | 32,813,217 visits, average age not reported | Taiwan only | Taiwan | descriptives and natural language processing | II | A |
| Lu TH, et al. | 6947, average age not reported | Taiwan only | Taiwan | descriptives and natural language processing | II | A |
| Webster LR and Grabois M | n/a | United States only | United States | n/a | IV | C |
| Han H, et al. | 3,260,824, average age not reported | United States only | United States | n/a | III | B |
| Hypponen H, et al. | 1693, average age not reported | Finland only | Finland | natural language processing | III | A |
| Shepherd J | n/a | United States only | United States | n/a | IV | A |
| Cepeda MS, et al. | 10,910,451, average age 45 | United States only | United States | t-test | III | A |
| Modarai F, et al. | United States only | United States | Regression, spatial cluster analysis | III | B | |
| Rouby F, et al. | 4.5 million | France only | France | t-test | III | A |
| Simoni-Wastila and Qian J | 2,175,012, average age not reported | United States only | United States | multinomial regressions | III | A |
| Worley J, et al. | n/a | United States only | United States | n/a | IV | C |
| Worley J, et al. | n/a | United States only | United States | n/a | IV | C |
| Fass JA and Hardigan PC | 836, average age not reported | United States only | United States | language processing | III | A |
| Frauger E, et al. | n/a | France only | France | n/a | IV | B |
| Hincapie AL, et al. | 34, average age not reported | United States only | United States | language processing | III | B |
| Hsu MH, et al. | 8, average age 47.9, 88% male | Taiwan only | Taiwan | Chi-squared and two-sided z-tests with Bonferroni adjustments | III | B |
| Pauly V, et al. | 4787 patients, average age 37.6 | France only | France | Clustering | III | A |
| Wilsey BL, et al. | 2,849,464 patients, average age of shoppers 50.7 | United States only | United States | Regression | IV | C |
| Pradel V, et al. | 128,000, average age not reported | France only | France | t-test | III | A |
Author Contributions
B.K. served as the project leader who analyzed 30% of the articles; G.G. served as the graphic artists who analyzed 30% of the articles; K.C. served as the literature matrix manager who analyzed 30% of the articles; S.B. served as the junior editor who analyzed 30% of the articles; C.S.K. served as the senior editor who analyzed 100% of the articles and repackaged the manuscript for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kieffer C.H. Citizen empowerment: A developmental perspective. Prev. Hum. Serv. 1984;3:9–36. doi: 10.1300/J293v03n02_03. [DOI] [PubMed] [Google Scholar]
- 2.Castro E.M., Van Regenmortel T., Vanhaecht K., Sermeus W., Van Hecke A. Patient empowerment, patient participation and patient-centeredness in hospital care: A concept analysis based on a literature review. Patient Educ. Couns. 2016;99:1923–1939. doi: 10.1016/j.pec.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Global Status Report on Noncommunicable Diseases 2014. World Health Organization; Geneva, Switzerland: 2014. [Google Scholar]
- 4.Schneberk T., Raffetto B., Friedman J., Wilson A., Kim D., Schriger D.L. Opioid prescription patterns among patients who doctor shop; Implications for providers. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0232533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balazote P.S., Sicilia M.-A. Interoperability in Healthcare Information Systems: Standards, Management, and Technology. IGI Global; Hershey, PA, USA: 2013. [Google Scholar]
- 6.What Is Interoperability. [(accessed on 11 July 2020)]; Available online: https://www.healthit.gov/topic/interoperability.
- 7.Biernikiewicz M., Taieb V., Toumi M. Characteristics of doctor-shoppers: A systematic literature review. J. Mark. Access Health Policy. 2019:7. doi: 10.1080/20016689.2019.1595953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fink D., Schleimer J., Sarvet A., Grover K., Delcher C., Castillo-Carniglia A., Kim J., Rivera-Aguirre A., Henry S., Martins S., et al. Association between prescription drug monitoring programs and nonfatal and fatal drug overdoses: A systematic review. Ann. Intern. Med. 2018;168:783–790. doi: 10.7326/M17-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casati A., Sedefov R., Pfeiffer-Gerschel T. Misuse of medicines in the European union: A systematic review of the literature. Eur. Addict. Res. 2012;18:228. doi: 10.1159/000337028. [DOI] [PubMed] [Google Scholar]
- 10.Report of the International Narcotics Control Board for 2007. [(accessed on 11 July 2020)]; Available online: https://www.incb.org/incb/en/publications/annual-reports/annual-report-2007.html.
- 11.Shepherd J. Combating the prescription painkiller epidemic: A national prescription drug reporting program. Am. J. Law Med. 2014;40:85–112. doi: 10.1177/009885881404000103. [DOI] [PubMed] [Google Scholar]
- 12.CDC Prescription Opiod Data. [(accessed on 11 July 2020)]; Available online: https://www.cdc.gov/drugoverdose/data/prescribing.html.
- 13.Fass J.A., Hardigan P.C. Attitudes of florida pharmacists toward implementing a state prescription drug monitoring program for controlled substances. J. Manag. Care Pharm. 2011;17:430–438. doi: 10.18553/jmcp.2011.17.6.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyppönen H., Reponen J., Lääveri T., Kaipio J. User experiences with different regional health information exchange systems in Finland. Int. J. Med. Inform. 2014;83:1–18. doi: 10.1016/j.ijmedinf.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Huang S.-K., Wang P.-J., Tseng W.-F., Syu F.-K., Lee M.-C., Shih R.-L., Sheen M.-T., Chen M.S. NHI-PharmaCloud in Taiwan—A preliminary evaluation using the RE-AIM framework and lessons learned. Int. J. Med. Inform. 2015;84:817–825. doi: 10.1016/j.ijmedinf.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Hsu M.-H., Yeh Y.-T., Chen C.-Y., Liu C.-H., Liu C.-T. Online detection of potential duplicate medications and changes of physician behavior for outpatients visiting multiple hospitals using national health insurance smart cards in Taiwan. Int. J. Med. Inform. 2011;80:181–189. doi: 10.1016/j.ijmedinf.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Chenaf C., Kabore J.L., Delorme J., Roche L., Eschalier A., Delage N., Authier N., Pereira B., Mulliez A. Incidence of tramadol shopping behavior in a retrospective cohort of chronic non-cancer pain patients in France. Pharmacoepidemiol. Drug Saf. 2016;25:1088–1098. doi: 10.1002/pds.4056. [DOI] [PubMed] [Google Scholar]
- 18.Popovici I., Hijazi B., Maclean J.C., Radakrishnan S. The effect of state laws designed to prevent nonmedical prescription opioid use on overdose deaths and treatment. Health Econ. 2018;27:294–305. doi: 10.1002/hec.3548. [DOI] [PubMed] [Google Scholar]
- 19.HHS . NPI: What You Need to Know. Centers for Medicare and Medicaid Services; Baltimore, MD, USA: 2016. p. 10. [Google Scholar]
- 20.Kruse C.S. Writing a systematic review for publication in a health-related degree program. JMIR Res. Protoc. 2019;8:e15490. doi: 10.2196/15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David M., Alessandro L., Jennifer T., Douglas G.A. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ Br. Med. J. 2009;339:332. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newhouse R., Dearbolt S., Poe S., Pugh L.C., White K. The Johns Hopkins Nursing Evidence-Based Practice Rating Scale. Centers for Medicare and Medicaid Services; Baltimore, MD, USA: 2005. JHNEBP evidence rating scales. [Google Scholar]
- 23.Light R.J. Measures of response agreement for qualitative data: Some generalizations and alternatives. Psychol. Bull. 1971;76:365. doi: 10.1037/h0031643. [DOI] [Google Scholar]
- 24.McHugh M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012;22:276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braun V., Clarke V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006;3:77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 26.Pett R.G., Mancl L., Revere D., Stergachis A. Prescription drug monitoring program use and utility by Washington State pharmacists: A mixed-methods study. J. Am. Pharm. Assoc. 2020;60:57–65. doi: 10.1016/j.japh.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Durand Z., Nechuta S., Krishnaswami S., Hurwitz E.L., McPheeters M. Prescription opioid use by injured workers in Tennessee: A descriptive study using linked statewide databases. Ann. Epidemiol. 2019;32:7–13. doi: 10.1016/j.annepidem.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Freeman P.R., Curran G.M., Drummond K.L., Martin B.C., Teeter B.S., Bradley K., Schoenberg N., Edlund M.J. Utilization of prescription drug monitoring programs for prescribing and dispensing decisions: Results from a multi-site qualitative study. Res. Soc. Adm. Pharm. 2019;15:754–760. doi: 10.1016/j.sapharm.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagarajan R., Talbert J. Network abstractions of prescription patterns in a medicaid population. Amia Jt. Summits Transl. Sci. Proc. 2019;2019:524–532. [PMC free article] [PubMed] [Google Scholar]
- 30.Perry B.L., Yang K.C., Kaminski P., Odabas M., Park J., Martel M., Oser C.B., Freeman P.R., Ahn Y.Y., Talbert J. Co-prescription network reveals social dynamics of opioid doctor shopping. PLoS ONE. 2019;14:e0223849. doi: 10.1371/journal.pone.0223849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soffin E.M., Lee B.H., Kumar K.K., Wu C.L. The prescription opioid crisis: Role of the anaesthesiologist in reducing opioid use and misuse. Br. J. Anaesth. 2019;122:e198–e208. doi: 10.1016/j.bja.2018.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stopka T.J., Amaravadi H., Kaplan A.R., Hoh R., Bernson D., Chui K.K.H., Land T., Walley A.Y., LaRochelle M.R., Rose A.J. Opioid overdose deaths and potentially inappropriate opioid prescribing practices (PIP): A spatial epidemiological study. Int. J. Drug Policy. 2019;68:37–45. doi: 10.1016/j.drugpo.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Delcher C., Li Y., Goldberger B.A., Reisfield G.M. Overlapping prescriptions of opioids, benzodiazepines, and carisoprodol: “Holy Trinity” prescribing in the state of Florida. Drug Alcohol Depend. 2019;205:107693. doi: 10.1016/j.drugalcdep.2019.107693. [DOI] [PubMed] [Google Scholar]
- 34.Butler J.M., Becker W.C., Humphreys K. Big data and the opioid crisis: Balancing patient privacy with public health. J. Law Med. Ethics. 2018;46:440–453. doi: 10.1177/1073110518782952. [DOI] [PubMed] [Google Scholar]
- 35.Lin J.-H., Cheng S.-H. The impact of a medication record sharing program among diabetes patients under a single-payer system: The role of inquiry rate. Int. J. Med. Inform. 2018;116:18–23. doi: 10.1016/j.ijmedinf.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Ponté C., Lepelley M., Boucherie Q., Mallaret M., Lapeyre Mestre M., Pradel V., Micallef J. Doctor shopping of opioid analgesics relative to benzodiazepines: A pharmacoepidemiological study among 11.7 million inhabitants in the French countries. Drug Alcohol Depend. 2018;187:88–94. doi: 10.1016/j.drugalcdep.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 37.Torrance N., Mansoor R., Wang H., Gilbert S., Macfarlane G.J., Serpell M., Baldacchino A., Hales T.G., Donnan P., Wyper G., et al. Association of opioid prescribing practices with chronic pain and benzodiazepine co-prescription: A primary care data linkage study. Br. J. Anaesth. 2018;120:1345–1355. doi: 10.1016/j.bja.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Ali M.M., Dowd W.N., Classen T., Mutter R., Novak S.P. Prescription drug monitoring programs, nonmedical use of prescription drugs, and heroin use: Evidence from the National Survey of Drug Use and Health. Addict. Behav. 2017;69:65–77. doi: 10.1016/j.addbeh.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Rutkow L., Smith K.C., Lai A.Y., Vernick J.S., Davis C.S., Alexander G.C. Prescription drug monitoring program design and function: A qualitative analysis. Drug Alcohol Depend. 2017;180:395–400. doi: 10.1016/j.drugalcdep.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 40.Simeone R. Doctor shopping behavior and the diversion of prescription opioids. Subst. Abus. Res. Treat. 2017;11:1–10. doi: 10.1177/1178221817696077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Council of State Boards of Nursing A changing environment: 2016 NCSBN environmental scan. J. Nurs. Regul. 2016;6:4–37. doi: 10.1016/S2155-8256(16)31007-9. [DOI] [Google Scholar]
- 42.Delorme J., Chenaf C., Kabore J.L., Pereira B., Mulliez A., Tremey A., Brousse G., Zenut M., Laporte C., Authier N. Incidence of high dosage buprenorphine and methadone shopping behavior in a retrospective cohort of opioid-maintained patients in France. Drug Alcohol Depend. 2016;162:99–106. doi: 10.1016/j.drugalcdep.2016.02.035. [DOI] [PubMed] [Google Scholar]
- 43.Kea B., Fu R., Lowe R.A., Sun B.C. Interpreting the national hospital ambulatory medical care survey: United States emergency department opioid prescribing, 2006–2010. Acad. Emerg. Med. 2016;23:159–165. doi: 10.1111/acem.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okumura Y., Shimizu S., Matsumoto T. Prevalence, prescribed quantities, and trajectory of multiple prescriber episodes for benzodiazepines: A 2-year cohort study. Drug Alcohol Depend. 2016;158:118–125. doi: 10.1016/j.drugalcdep.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Ong M.S., Olson K.L., Cami A., Liu C., Tian F., Selvam N., Mandl K.D. Provider patient-sharing networks and multiple-provider prescribing of benzodiazepines. J. Gen. Intern. Med. 2016;31:164–171. doi: 10.1007/s11606-015-3470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi Y., Ishizaki T., Nakayama T., Kawachi I. Social network analysis of duplicative prescriptions: One-month analysis of medical facilities in Japan. Health Policy. 2016;120:334–341. doi: 10.1016/j.healthpol.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 47.Lin M.H., Chang H.T., Tu C.Y., Chen T.J., Hwang S.J. Doctor-shopping behaviors among traditional chinese medicine users in Taiwan. Int. J. Environ. Res. Public Health. 2015;12:9237–9247. doi: 10.3390/ijerph120809237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu T.H., Lee Y.Y., Lee H.C., Lin Y.M. Doctor shopping behavior for zolpidem among insomnia patients in taiwan: A nationwide population-based study. Sleep. 2015;38:1039–1044. doi: 10.5665/sleep.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webster L.R., Grabois M. Current regulations related to opioid prescribing. PM&R. 2015;7:S236–S247. doi: 10.1016/j.pmrj.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Han H., Kass P.H., Wilsey B.L., Li C.S. Increasing trends in Schedule II opioid use and doctor shopping during 1999–2007 in California. Pharmacoepidemiol. Drug Saf. 2014;23:26–35. doi: 10.1002/pds.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cepeda M.S., Fife D., Yuan Y., Mastrogiovanni G. Distance traveled and frequency of interstate opioid dispensing in opioid shoppers and nonshoppers. J. Pain. 2013;14:1158–1161. doi: 10.1016/j.jpain.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Modarai F., Mack K., Hicks P., Benoit S., Park S., Jones C., Proescholdbell S., Ising A., Paulozzi L. Relationship of opioid prescription sales and overdoses, North Carolina. Drug Alcohol Depend. 2013;132:81–86. doi: 10.1016/j.drugalcdep.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Rouby F., Pradel V., Frauger E., Pauly V., Natali F., Reggio P., Thirion X., Micallef J. Assessment of abuse of tianeptine from a reimbursement database using ‘doctor-shopping’ as an indicator. Fundam. Clin. Pharm. 2012;26:286–294. doi: 10.1111/j.1472-8206.2010.00906.x. [DOI] [PubMed] [Google Scholar]
- 54.Simoni-Wastila L., Qian J. Influence of prescription monitoring programs on analgesic utilization by an insured retiree population. Pharmacoepidemiol. Drug Saf. 2012;21:1261–1268. doi: 10.1002/pds.3342. [DOI] [PubMed] [Google Scholar]
- 55.Worley J. Prescription drug monitoring programs, a response to doctor shopping: Purpose, effectiveness, and directions for future research. Issues Ment. Health Nurs. 2012;33:319–328. doi: 10.3109/01612840.2011.654046. [DOI] [PubMed] [Google Scholar]
- 56.Worley J. Psychiatric nursing’s role in preventing doctor shopping. J. Psychosoc. Nurs. Ment. Health Serv. 2012;50:4–5. doi: 10.3928/02793695-20120508-04. [DOI] [PubMed] [Google Scholar]
- 57.Frauger E., Pauly V., Pradel V., Rouby F., Arditti J., Thirion X., Lapeyre Mestre M., Micallef J. Evidence of clonazepam abuse liability: Results of the tools developed by the French Centers for Evaluation and Information on Pharmacodependence (CEIP) network. Fundam. Clin. Pharm. 2011;25:633–641. doi: 10.1111/j.1472-8206.2010.00882.x. [DOI] [PubMed] [Google Scholar]
- 58.Hincapie A.L., Warholak T.L., Murcko A.C., Slack M., Malone D.C. Physicians’ opinions of a health information exchange. J. Am. Med. Inf. Assoc. 2011;18:60–65. doi: 10.1136/jamia.2010.006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pauly V., Frauger E., Pradel V., Rouby F., Berbis J., Natali F., Reggio P., Coudert H., Micallef J., Thirion X. Which indicators can public health authorities use to monitor prescription drug abuse and evaluate the impact of regulatory measures? Controlling high dosage Buprenorphine abuse. Drug Alcohol Depend. 2011;113:29–36. doi: 10.1016/j.drugalcdep.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 60.Wilsey B.L., Fishman S.M., Gilson A.M., Casamalhuapa C., Baxi H., Lin T.C., Li C.S. An analysis of the number of multiple prescribers for opioids utilizing data from the California Prescription Monitoring Program. Pharmacoepidemiol. Drug Saf. 2011;20:1262–1268. doi: 10.1002/pds.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pradel V., Delga C., Rouby F., Micallef J., Lapeyre-Mestre M. Assessment of abuse potential of benzodiazepines from a prescription database using ‘doctor shopping’ as an indicator. CNS Drugs. 2010;24:611. doi: 10.2165/11531570-000000000-00000. [DOI] [PubMed] [Google Scholar]