Abstract
Dairy fat and its fatty acids (FAs) have been shown to possess pro-health properties that can support health maintenance and disease prevention. In particular, branched-chain FAs (BCFAs), comprising approximately 2% of dairy fat, have recently been proposed as bioactive molecules contributing to the positive health effects associated with the consumption of full-fat dairy products. This narrative review evaluates human trials assessing the relationship between BCFAs and metabolic risk factors, while potential underlying biological mechanisms of BCFAs are explored through discussion of studies in animals and cell lines. In addition, this review details the biosynthetic pathway of BCFAs as well as the content and composition of BCFAs in common retail dairy products. Research performed with in vitro models demonstrates the potent, structure-specific properties of BCFAs to protect against inflammation, cancers, and metabolic disorders. Yet, human trials assessing the effect of BCFAs on disease risk are surprisingly scarce, and to our knowledge, no research has investigated the specific role of dietary BCFAs. Thus, our review highlights the critical need for scientific inquiry regarding dairy-derived BCFAs, and the influence of this overlooked FA class on human health.
Keywords: anteiso, branched-chain amino acids, cancer, diabetes, inflammation, iso, metabolic diseases, milk, phytanic acid
1. Introduction
Milk and dairy products have been a staple of the human diet for thousands of years [1] and represent one of the most important agricultural commodities in regard to human nutrition. Dairy products have been instituted in most dietary guidelines around the world as they provide a large variety of essential nutrients and several key shortfall nutrients. Moreover, milk and dairy products hold a unique position among all foods as they are considered the largest single source of natural bioactive components [2]. In particular, dairy fat is the most complex and diverse dietary fat source in nature comprised of an impressive fatty acid (FA) repertoire (>400 different FAs and FA derivatives [3]) that accounts for the myriad of nutritional, organoleptic, and technological characteristics of milk and dairy products. Specifically, dairy fat contains a unique variety of bioactive FAs that are synthesized by rumen microbes (i.e., bacteria and protozoa) and the mammary gland. These FAs consist of short- and medium-chain FAs (4 to 13 carbons), positional and geometric isomers of octadecanoate (18:1), conjugated linoleic acids, odd-chain FAs (15:0 and 17:0), and branched-chain FAs (BCFAs). Of the repertoire of FAs in dairy fat, about 14% of them are these unique dairy-derived FAs [4] and several are known to impact normal mammalian physiology by functioning as bioactive molecules, exerting beneficial properties that support health and well-being [5,6,7]. To date, most research efforts in the arena of dairy FAs have been centered on the biological effects of conjugated linoleic acids, specifically rumenic acid (18:2 c9,t11), which was originally driven by the discovery of its demonstrated anticarcinogenic activity [8]. However, far less work has focused on other bioactive dairy-derived FAs.
BCFAs are an emerging group of bioactive FAs sparking growing research interest within the scientific community due to their biological effects and potential pro-health benefits. Because BCFAs are principally derived from rumen bacteria, milk and dairy products pose unique dietary sources of BCFAs. The objectives of this paper are to review and summarize the current knowledge on BCFAs and specifically to evaluate the possible role of dairy-derived BCFAs in human health.
2. Structure and Origin of BCFAs in Ruminants
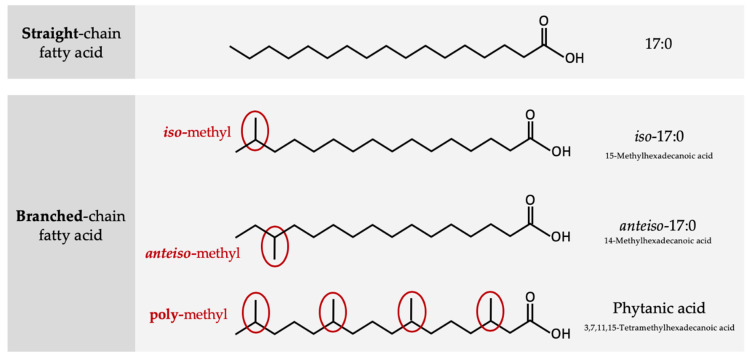
BCFAs are commonly saturated FAs substituted with one (mono-) or more (di-/poly-) methyl branch(es) on the carbon chain. Typically, BCFAs possess either an iso structure where the FA has the branch point on the penultimate carbon atom (i.e., one from the end) or an anteiso structure where the branch point is located on the antepenultimate carbon atom (i.e., two from the end; Figure 1). More than 50 BCFAs have been identified in ruminant-derived fats [9,10] but iso- and anteiso-mono-methyl BCFAs with chain lengths from 14 to 17 carbon atoms are quantitatively the most abundant BCFAs in milk fat [4,11,12,13,14,15,16].
Figure 1.
Structural differences between straight chain fatty acids and iso-, anteiso-, and multimethyl branched-chain fatty acids.
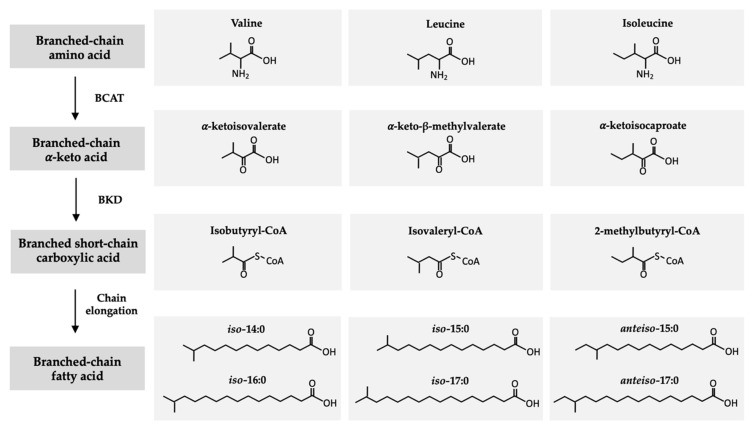
In ruminants, monomethyl BCFAs are synthesized by the microorganisms that reside within the rumen, specifically bacteria and protozoa [17], which utilize dietary branched-chain amino acids (BCAAs), i.e., valine, leucine, and isoleucine, to form BCFAs (Figure 2) [18]. Through a common biosynthetic pathway, BCAAs are first transformed into branched-chain α-ketoacids through the removal of the amino group by a BCAA transferase enzyme [19]. These α-ketoacid products, α-ketoisovalerate, α-keto-β-methylvalerate, and α-ketoisocaproate, are subsequently decarboxylated by branched-chain-α-ketoacid dehydrogenase producing the respective branched short-chain carboxylic acids isobutyral-CoA, isovaleryl-CoA, and 2-methylbutyral-CoA [20]. Finally, branched short-chain carboxylic acids are elongated by BCFA synthetase, with malonyl-CoA as the chain extender, to form iso- and anteiso-BCFAs [18]. The products of the BCFA biosynthetic pathway are iso-14:0 and iso-16:0, derived from valine, iso-15:0 and iso-17:0 from leucine, and anteiso-15:0 and anteiso-17:0 from isoleucine.
Figure 2.
Biosynthetic pathway of branched-chain fatty acids from branched-chain amino acids. BCAT: branched-chain amino acid transferase (BCAT) enzyme. BKD: branched-chain-α-ketoacid dehydrogenase.
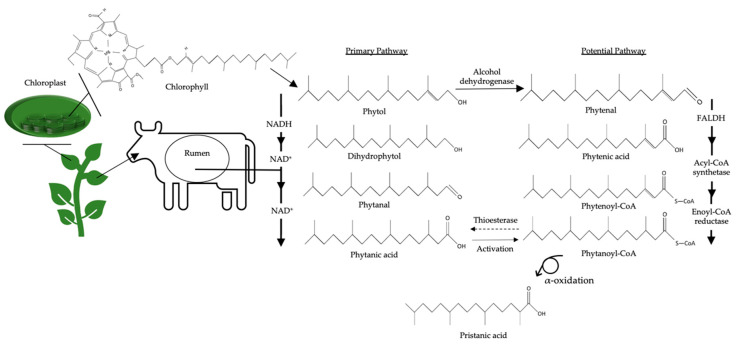
Phytanic acid (3,7,11,15-tetramethylhexadecanoic acid) and its metabolite pristanic acid (2,6,10,14-tetramethylpentadecanoic acid), two multimethyl BCFAs, are also predominantly synthesized in the rumen (Figure 3) [21,22]. Phytanic acid is derived from the phytol component of chlorophyll found in forages [21]. While mammals are unable to cleave the ring structure from the phytol moiety of chlorophyll, bacteria release phytol in the rumen [23]. The primary synthesis pathway of phytanic acid in ruminants is initiated by the biohydrogenation of phytol to produce dihydrophytol [21,24]. Dihydrophytol is subsequently converted to phytanal before it is synthesized into phytanic acid [21,24]. Notably, a potential secondary synthesis pathway has been described in non-ruminant tissues and marine bacteria [22,24,25] and may be applicable to ruminant animals as well, although there is only limited evidence [26]. In this case, phytol is first converted to phytenal by an alcohol dehydrogenase [22]. Fatty aldehyde dehydrogenase is used to transform phytenal to phytenic acid before it is modified into phytenoyl-CoA by an acyl-CoA synthetase. Subsequently, phytenoyl-CoA is converted by an enoyl-CoA reductase to form phytanoyl-CoA [24,25]. The final synthesis step of phytanoyl-CoA to phytanic acid is thought to occur through α-oxidation [25] due to the enzymatic activity of thioesterase, a protein involved in α-oxidation [24]. In order to form pristanic acid, phytanic acid is activated by phytanoyl-CoA synthetase to create phytanoyl-CoA, which can then undergo α-oxidation to produce pristanic acid.
Figure 3.
Synthesis of phytanic and pristanic acid via rumen microorganisms derived from chlorophyll within forages NAD: Nicotinamide adenine dinucleotide. NAD+: the oxidized form of NAD. NADH: the reduced form of NAD (protonated with a hydrogen).
BCFAs are key structural lipid constituents of the bacterial membranes [17] where they play an important regulatory role in membrane fluidity and permeability [27]. Importantly, bacterial membrane lipids make an important contribution to ruminant milk fat, as bacterial cells leaving the rumen pass to the duodenum where their membrane FAs are absorbed and subsequently incorporated into milk fat and other tissues [21].
3. Occurrence and Metabolism of BCFAs in Humans
BCFAs are common constituents of microbial lipids present in abundant quantities but have also been found in many other organisms (e.g., C. elegans, [28]) including mammals [29,30,31], although in much lower amounts. In humans, BCFAs have been detected in various tissues and fluids including vernix caseosa, the biofilm covering the skin of the fetus [32,33], colostrum and mature breast milk [34,35,36], adipose tissue [37], and serum [38]. Early work by Nicolaides and co-authors [39,40,41] established that the meibomian and sebaceous glands of the human skin produce BCFAs secreted into meibum and sebum, respectively. Subsequent research in vitro and in vivo using mouse models confirmed the endogenous synthesis of BCFAs from their respective BCAA precursors (i.e., valine, leucine, isoleucine) as a result of the BCAA catabolic pathway [30,31,42,43]. BCAA degradation in humans is comparable to that in bacteria (Figure 2). Despite the endogenous synthesis, dietary intake of BCFAs is presumably the principal source of BCFAs in the human body.
The intake of BCFAs depends on the type of food and the fat content of the food consumed. While ruminant-derived foods (i.e., milk and meat and their respective products) are the chief source of BCFAs, fish and non-dairy fermented foods (e.g., kimchi, sauerkraut, miso, tempeh) may also supply BCFAs, although only at very small quantities. The mean daily intake of BCFAs in the United States has been estimated to range between 220 mg/day (for milk only [44]) and 500 mg/day (from dairy and beef products combined [11]). The contribution of total dairy products to the BCFA intake has been calculated at 317 mg/day [11]. There is no requirement for BCFAs per se but it is conceivable that the daily dietary intake of BCFAs needs to be higher than the current estimates to achieve potential health benefits.
Very little is known about the fate and metabolism of dietary BCFAs in humans, thus representing an important area for future research. While information is scarce, it can be presumed that digestion, absorption, and transport of BCFAs in humans is comparable to that of other long-chain FAs. Our knowledge of BCFA uptake by cells is based largely on work in cell lines, specifically from the intestine (Caco-2), fetal small intestine (H4), and breast cancer (SKBR-3 and MCF-7), following exposure to one or a mixture of BCFAs [45,46,47,48,49]. From this limited perspective, it is thought that the cellular uptake of BCFAs is dependent on BCFA length and configuration. Using Caco-2 cells, Yan et al. [45] demonstrated that BCFAs can be taken up and further metabolized into their elongation or chain-shortened products. Other work indicates that in fetal intestinal cells incubated with identical concentrations of BCFAs (i.e., iso-16:0, anteiso-17:0, iso-18:0, and iso-20:0), anteiso-17:0 exhibited the greatest uptake efficiency, followed by iso-16:0, iso-18:0, then iso-20:0, with significant differences, and a specific hierarchy, between these BCFAs [47]. In contrast, MCF-7 human breast cancer cells accumulated greater amounts of iso-15:0 and iso-17:0 compared to their anteiso analogs [49]. Dietary supplementation of BCFAs to neonatal rat pups demonstrated that BCFAs incorporate into ileal phospholipids as well as liver tissue and serum [50]. Research conflicts as to whether BCFAs are preferentially incorporated into cellular triacylglycerols or phospholipids [47,48], and whether the FA structure plays a determinant role requires further investigation.
4. Occurrence of BCFAs in Dairy Products
4.1. Content and Composition of BCFAs in Milk
Within dairy foods, research to date has mainly focused on the content and composition of BCFAs derived from cow’s milk. From this work, studies established that cow’s milk is a significant source of BCFAs, typically comprising 1.7–3.4% BCFAs of total FAs (Table 1). When considered on a per serving basis, three servings of whole milk (3.25% milk fat) per day can thus provide 367–763 mg of BCFAs (Table 1). In milk fat, monomethyl BCFAs are the principal BCFAs present, with a chain length of 14–17 carbons and the methyl group occurring in either an iso or anteiso configuration. In general, total iso- and anteiso-BCFAs isomers occur in an approximate ratio of 1:1 (Table 1). Short-chain (4:0–6:0) and medium-chain (7:0–13:0) iso- and anteiso-BCFA isomers are also present in cow’s milk but in rather a minor abundance, accounting for approximately 0.01 and 0.12% of total BCFAs, respectively [51].
Table 1.
Branched-chain fatty acid composition and content of cow’s milk.
| FA 1 | Proportion (% of Total FAs) 2 |
Content (mg/Three Daily Servings) 3 |
|---|---|---|
| iso-13:0 | 0.02–0.03 [4,13,14,15] 4 | 5–7 [4,13,14,15] 4 |
| anteiso-13:0 | 0.07–0.09 [4,13] 4 | 15–19 [4,13] 4 |
| iso-14:0 | 0.08–0.22 [4,11,12,13,14,15] 4 | 18–48 [4,11,12,13,14,15] 4 |
| iso-15:0 | 0.13–0.44 [4,11,12,13,14,15] 4 | 29–97 [4,11,12,13,14,15] 4 |
| anteiso-15:0 | 0.37–0.93 [4,11,12,13,14,15] 4 | 81–206 [4,11,12,13,14,15] 4 |
| iso-16:0 | 0.17–0.45 [4,11,12,13,14,15] 4 | 38–100 [4,11,12,13,14,15] 4 |
| iso-17:0 | 0.26–0.56 [4,11,12,13,14,15] 4 | 58–123 [4,11,12,13,14,15] 4 |
| anteiso-17:0 | 0.11–0.76 [4,11,12,13,14,15] 4 | 24–169 [4,11,12,13,14,15] 4 |
| iso-18:0 | 0.01–0.09 [4,11,12,13,14,15] 4 | 2–20 [4,11,12,13,14,15] 4 |
| Σ iso-BCFAs | 0.87–1.75 [4,11,12,13,14,15] 4 | 193–387 [4,11,12,13,14,15] 4 |
| Σ anteiso-BCFAs | 0.67–1.69 [4,11,12,13,14,15] 4 | 149–375 [4,11,12,13,14,15] 4 |
| Σ BCFAs 5 | 1.66–3.44 [4,11,12,13,14,15] 4 | 367–763 [4,11,12,13,14,15] 4 |
1 FA = fatty acid. 2 Averages were estimated by calculating the median of reported FA values when appropriate. 3 Content of FAs per serving was calculated from FA values listed (% of total FAs) as described by Bainbridge et al. [13], assuming 3.25% fat per serving whole milk, then multiplied by three. 4 Raw data obtained from previously published work [4]. 5 BCFAs = branched-chain FAs; sum of iso and anteiso BCFA isomers (13:0–18:0).
Multimethyl BCFAs, namely phytanic and pristanic acid, are consistent constituents within milk fat, but are often overlooked or ignored, likely because of their very low content and analytical limitations. In cow’s milk, the content of phytanic acid typically ranges between 0.10 and 0.50% of total FAs, equivalent to 7–37 mg/serving whole milk [12,23,52,53,54,55,56]. The presence of phytanic acid in milk is specifically dependent on the feed type of the dairy cow, with higher amounts associated with the intake of grass-based rations rich in chlorophyll [23,52,53,55]. Pristanic acid, a metabolite of phytanic acid, occurs in very low amounts in milk fat, at approximately 0.04–0.06% (3–4 mg/serving whole milk) [23].
4.2. BCFAs in Milk across Ruminant Species
A survey of the recent literature regarding the BCFA composition of ruminant milk shows that milk from sheep and goats comprises approximately 1.8–3.1% and 1.2–2.4% BCFAs of total FAs, respectively, and is thus comparable to cow’s milk (1.7–3.4% BCFAs of total FAs; Table 1). Yet, the content of total and individual BCFAs in milk varies considerably based on the ruminant species of origin (Table 2). In particular, on a per serving basis, milk from sheep contains considerably more BCFAs per serving compared to milk from cows (204–502 mg/serving versus 123–254 mg/serving). In the U.S., cow’s milk is standardized to 3.25% milk fat (i.e., whole milk), however, this practice is less common for specialty milk such as goat and sheep milk. Differences in the BCFA content of ruminant-derived milks therefore appear to be chiefly reflective of milk fat standardization practices in retail milk, rather than species differences in BCFA occurrence.
Table 2.
Branched-chain fatty acid content (mg/serving) of common dairy products 1.
| FA 2 | Milk 3 | Cheese 4 | Yogurt 5 | Butter 6 | Sheep Milk 7 | Goat Milk 8 | ||
|---|---|---|---|---|---|---|---|---|
| Soft/Semi-Soft 9 | Semi-Hard/Hard 10 | Unknown 11 | ||||||
| iso-13:0 | 2 [4,13,14,15] 12 |
2 [65] |
8 [66] |
1 [67] |
||||
| anteiso-13:0 | 5–6 [4,13] 12 |
9 [65] |
5 [66] |
1–7 [67,68] |
||||
| iso-14:0 | 6–16 [4,11,12,13,14,15] 12 |
5–14 [11,69] |
6–20 [11,12,69] |
56–13 [11,69] |
9 [11] |
8–18 [11,65,70] |
8–24 [66,71,72] |
5–10 [67,68,73,74] |
| iso-15:0 | 10–32 [4,11,12,13,14,15] 12 |
6–33 [11,69] |
8–41 [11,12,69] |
12–33 [11,69] |
11 [11] |
1–22 [11,65,70] |
21–71 [66,71,72,75] |
13–36 [67,68,73,74,76] |
| anteiso-15:0 | 27–69 [4,11,12,13,14,15] 12 |
26–68 [11,69] |
33–87 [11,12,69] |
35–62 [11,69] |
47 [11] |
36–67 [11,65,70] |
45–122 [66,71,72,75] |
25–47 [67,68,73,74,76] |
| iso-16:0 | 13–33 [4,11,12,13,14,15] 12 |
12–30 [11,69] |
9–42 [11,12,69] |
11–26 [11,69] |
22 [11] |
20–36 [11,65,70] |
25–69 [66,71,72,75] |
13–22 [67,68,73,74] |
| iso-17:0 | 19–41 [4,11,12,13,14,15] 12 |
8–41 [11,69] |
8–52 [11,12,69] |
14–38 [11,69] |
19 [11] |
22–34 [11,65,70] |
34–115 [66,71,72,75] |
20–58 [67,68,73,74,76] |
| anteiso-17:0 | 8–56 [4,11,12,13,14,15] 12 |
23–71 [11,69] |
17–71 [11,12,69] |
25–61 [11,69] |
45 [11] |
34–42 [11,65,70] |
48–124 [66,71,72,75] |
25–57 [67,68,73,74,76] |
| iso-18:0 | 1–7 [4,11,12,13,14,15] 12 |
<1–8 [11,69] |
<1–8 [11,12,69] |
<1–7 [11,69] |
3 [11] |
<1–7 [11,65,70] |
17–20 [66,72] |
5 [67] |
| Σ iso-BCFAs | 64–129 [4,11,12,13,14,15] 12 |
32–127 [11,69] |
32–164 [11,12,69] |
53–116 [11,69] |
64 [11] |
68–106 [11,65,70] |
108–255 [66,71,72,75] |
53–96 [67,68,73,74,76] |
| Σ anteiso-BCFAs | 50–125 [4,11,12,13,14,15] 12 |
51–139 [11,69] |
50–158 [11,12,69] |
68–123 [11,69] |
91 [11] |
69–107 [11,65,70] |
96–247 [66,71,72,75] |
50–104 [67,68,73,74,76] |
| Σ BCFAs 13 | 123–254 [4,11,12,13,14,15] 12 |
83–264 [11,69] |
82–322 [11,12,69] |
123–239 [11,69] |
152 [11] |
137–204 [11,65,70] |
204–502 [66,71,72,75] |
109–180 [67,68,73,74,76] |
1 Averages were estimated by calculating the median of reported FA values when appropriate; content of FAs per serving was calculated from reported FA values (% of total FAs), assuming 93.3% of FAs in milk fat (correction for glycerol) [77]. 2 FA = fatty acid. 3 Cow-derived; based on the assumption of 3.25% fat, 244 g per serving [13]. 4 Cow-derived; based on reported % fat and serving sizes (g) listed by FoodData Central for each cheese variety [78,79,80,81,82,83,84,85,86,87,88,89] with the exception of Ricotta cheese (62 g/serving). 5 Cow-derived; based on reported % fat and serving size (245 g) listed by FoodData Central [90]. 6 Cow-derived; based on reported % fat (80%) and serving size (14.2 g) listed by FoodData Central [91]; when FA values were reported as μg/g butter [70], FAs per serving were calculated by assuming 14.2 g per serving [91] without correction factor for glycerol. 7 Based on reported % fat (median of values calculated when appropriate) or % fat listed by FoodData Central [92] if not reported; based on serving size (245 g) listed by FoodData Central [92]. 8 Based on reported % fat (median of values calculated when appropriate) or % fat listed by FoodData Central [93] if not reported; based on serving size (244 g) listed by FoodData Central [93]. 9 Includes Ricotta, Romadur, Cottage cheese, Camembert, Brie, Limburger, Feta, and Bavaria Blue. 10 Includes Provolone, low-moisture Mozzarella, Emmental, Cheddar, Montasio, Gouda, and Butter cheese (Butterkäse). 11 Includes Swiss, American, Alpine cheese, curd cheese, and Mozzarella (varieties without sufficient information reported to categorize). 12 Raw data underlying previously published work [4]. 13 BCFAs = branched-chain FAs; sum of iso and anteiso BCFA isomers (13:0–18:0).
4.3. Comparison of BCFAs among Dairy Products
The content of BCFAs in dairy also differs depending upon the type of dairy product (Table 2). For example, Ran Ressler et al. [11] found that yogurt contained an equivalent of 152 mg BCFAs/serving, while butter contained an equivalent of 195 mg/serving. Limited work assessing the BCFA content in cheese demonstrates that cheese type appears to be an important consideration as well (82–322 mg/serving depending upon cheese variety). One notable source of variation in the content of BCFAs per serving among cheese types is the differing fat content. For example, one serving (28.35 g) of low-moisture Mozzarella (22.1% fat) contains 82 mg BCFAs, whereas Cheddar (33.0% fat) contains 148 mg BCFAs [11]. One serving of butter (14.2 g), another dairy-derived product high in fat (<80%), can contain more than 200 mg of BCFAs (137–204 mg/serving; Table 2).
5. Human Trials Assessing Health Effects of BCFAs
To date, epidemiological research indicates that dairy fat consumption is neutral or protective against cardiometabolic diseases. However, the specific role of BCFAs is less understood, as these studies largely rely upon either food frequency questionnaires and/or the measurement of specific dairy FA biomarkers (i.e., 15:0, 17:0, and 16:1 t9) in blood or tissues [57,58,59,60], but not BCFAs.
5.1. Obesity
Epidemiological studies have noted that the intake of full-fat dairy products can be beneficial for long-term weight maintenance [61,62]. Other research which specifically focused on the consumption of BCFAs suggests that BCFAs have an important role in energy homeostasis. For example, one study found that total BCFAs in serum were higher in non-obese women than obese women and that iso-BCFAs were inversely associated with body mass index [38]. Similar results have been reported in a recent study by Pakiet et al. [63]. Another study found that the adipose tissue of lean subjects had higher proportions of total BCFAs and individual BCFAs than obese individuals [37].
One niche of scientific interest is the relationship between tissue concentrations of BCFAs and weight status in individuals post-gastric bypass surgery. In a longitudinal study, the BCFAs of adipose tissue in obese subjects was assessed at baseline and one year following Roux-en-Y gastric bypass surgery [37]. This study demonstrated that, after one year, the proportion of total BCFAs increased after surgery-induced weight loss. A follow-up study found similar results, showing that serum BCFAs increased in individuals after one-anastomosis gastric bypass surgery [63]. Of note, a similar study found no changes in serum BCFAs two weeks after one-anastomosis gastric bypass surgery, suggesting that a minimum time period may be needed to observe changes [64].
5.2. Insulin Sensitivity
Serum collected from fasted individuals showed a modest inverse correlation between serum BCFA concentrations and an index of insulin resistance (homeostatic model of assessment of insulin resistance), suggesting that BCFAs may promote insulin sensitivity [63]. Similarly, total BCFAs in adipose tissue have been associated with measurements of insulin sensitivity (i.e., insulin-stimulated glucose rate of disappearance) [37]. Moreover, Mika et al. [38] observed that two BCFAs, anteiso-15:0 and iso-17:0, were negatively correlated with fasted serum insulin levels but not with the homeostatic model assessment of insulin resistance.
5.3. Limitations of Current Evidence Available in Humans
Research suggests that BCFAs promote weight maintenance and metabolic health [37,38,63], however, more studies are needed to ascertain the specific impact of dietary BCFAs, and to what extent dietary BCFAs contribute to these outcome measures. Research assessing the metabolic effects of dairy-derived BCFAs is still lacking. Therefore, for this review, we considered studies evaluating the role of BCFAs on health, regardless of the source. While dietary patterns are the plausible source of variation in tissue BCFA occurrence in humans, BCFAs derived from an individual’s gut microbiota or metabolism from BCAAs cannot be discounted. Future work evaluating the relationship between diet and disease risk should consider the inclusion of BCFAs as biomarkers of dairy fat consumption.
6. Role of Dairy-Derived BCFAs in Health: Potential Mechanisms
6.1. Inflammation
Inflammation is an important underlying factor contributing to the pathogenesis of many metabolic diseases [94,95,96]. In particular, gastrointestinal health is critical for proper immune function and regulation of inflammation [97,98]. Research utilizing gastrointestinal cell lines has demonstrated the potent anti-inflammatory potential of dietary BCFAs. For example, exposure of Caco-2 cells to BCFAs reduced the lipopolysaccharide-induced gene expression of important proinflammatory mediators (i.e., IL-8, TLR-4, and NF-κB) [45]. In addition, certain shorter chain BCFAs (i.e., iso-14:0, iso-16:0, anteiso-13:0) improved the lipopolysaccharide-induced decrease in cell viability. Similar results were reported in a follow-up study [46] when Caco-2 cells were treated with monoacylglycerols or free FAs comprised of ~30% BCFAs isolated from the vernix.
Animal work is limited, but also supports that BCFAs attenuate inflammation and related diseases. Ran-Ressler et al. [50] examined the effect of a diet containing a mixture of BCFAs (iso-14:0, anteiso-15:0, iso-16:0, anteiso-17:0, iso-18:0, and iso-20:0) on necrotizing enterocolitis in neonatal Sprague–Dawley pups. BCFA-fed pups had a lower incidence of necrotizing enterocolitis and an enhanced gene expression of IL-10, an anti-inflammatory cytokine. Moreover, the cecal samples of pups fed the BCFA-enriched rat formula had a greater abundance of Bacillus subtilis and Pseudomonas aeruginosa compared to those fed standard rat formula, which is notable as both are known to contain BCFAs in their membranes [18,50,99]. Of note, an inverse association has been found in humans for serum iso-BCFAs (i.e., iso-15:0, iso-16:0, iso-17:0, and anteiso-15:0) and serum C-reactive protein [38], an important inflammatory marker for type 2 diabetes risk.
6.2. Anticarcinogenic Properties
One of the first beneficial effects attributed to BCFAs were their anticancer properties discovered 20 years ago. Specifically, iso-15:0 is known to inhibit the growth of T-cell non-Hodgkin lymphoma (Jurkat, Hut78, and EL4 cell lines [100]) and various types of carcinoma cell lines including, breast (MCF-7 and SKBR-3), prostate (DU145), lung (NCI-H1688), pancreas (BxPC-3), liver (SNU-423), bladder (T24, 5637, and UM-UC-3), leukemia (K-562), and gastric (NCI-SNU-1) and colorectal carcinoma (HCT 116) in a dose- and/or time-dependent manner by inhibiting proliferation and inducing apoptosis [48,49,101,102]. In a comparison of eight iso-BCFA species of varying carbon chain lengths (iso-12:0 through iso-20:0), Wongtangtintharn et al. [103] demonstrated that the anticarcinogenic activity of BCFAs are dependent on the chain length, with iso-16:0 exerting the highest cytotoxic activity. Furthermore, a more recent study showed that BCFAs with an iso structure (i.e., iso-15:0 and iso-17:0) were more potent than their anteiso counterparts (i.e., anteiso-15:0 and anteiso-17:0) [49]. Similarly, in vivo experiments have shown that BCFAs inhibit tumor growth in both mouse xenograft tumor models in which cancer cells were co-grafted [100,101] and in the orthotopic VX2 squamous cell carcinoma model in rabbits [104].
BCFAs may also induce beneficial, location-specific effects on angiogenesis. Treatment with iso-15:0 promoted angiogenesis post cerebral ischemia/reperfusion injury [105], while anteiso-15:0 suppressed angiogenesis to aid corneal recovery [106]. These effects appear to be structure specific, but studies in this area are still very limited, hence more research is needed. Moreover, the biological significance of the role of BCFAs on angiogenesis in the context of cancer growth is not yet established.
6.3. Energy and Glucose Homeostasis
Previous research has suggested that the body content, and specifically the liver content, of BCFAs reflects the ingestion of their respective BCAA precursors [30,107]. Furthermore, Brooks et al. [107] and Garcia-Caraballo et al. [30] observed that the body or hepatic content of BCFAs were inversely correlated with the body triacylglycerol content, as shown in C. elegans, or the liver triacylglycerol content, as shown in mice, respectively. Likewise, a recent in vitro study, using a human fatty liver cell line model (i.e., L02 cells), concluded that individual BCFAs (iso-15:0 and iso-18:0) reduced the cellular triacylglycerol content and were associated with an upregulation of multiple genes involved in lipid catabolism [108]. These observations are in accordance with human trials which showed that, in serum, total BCFAs [63] and specific iso-BCFAs [38] had an inverse relationship with triacylglycerols. Thus, the results from these studies indicate that BCFA may be directly or indirectly involved in the regulation of fat storage in the body.
As described above, BCFAs may favorably influence insulin sensitivity in humans, however, studies assessing potential mechanisms involved are scarce. One study performed with rat insulinoma INS-1 β-cells demonstrated that iso-17:0 may beneficially modulate β-cell function via the upregulation of Pdx1 and PPAR-γ transcription factors [109]. Yet, more in vivo research is necessary to confirm these results.
6.4. Biological Functions of Polymethyl BCFAs: Phytanic Acid
The health effects of dietary phytanic acid, both positive and negative, have been extensively examined in a recent review [110]. In particular, Roca-Saavedra et al. [110] summarize the biological complications that arise due to an excess of phytanic acid within the body, such as neurological injury, oxidative stress, and cancers. To that end, recent in vitro work continues to show that phytanic acid, when provided at concentrations exceeding normal physiological ranges, is neurotoxic [111,112]. Importantly, the nutritional relevance of such studies is called into question for the general population, as the accumulation of phytanic acid due to impaired lipid metabolism (e.g., Refsum disease) is thought to be rare in terms of prevalence [113]. Nakanishi et al. [114] demonstrated that when cells are treated with concentrations of phytanic acid comparable to that found in the plasma of healthy individuals, phytanic acid exerted beneficial immunomodulatory effects in a PPAR-α-dependent manner. Furthermore, there is no clinical evidence that the consumption of phytanic acid in naturally occurring amounts, e.g., phytanic acid derived from milk and dairy products, detrimentally impacts health in individuals with normal lipid metabolism.
Roca-Saavedra et al. [110] also describe favorable effects of phytanic acid on glucose and lipid metabolism, energy expenditure, as well as immune and anticancer functions. Indeed, efforts to ameliorate or prevent obesity have been gaining momentum, and phytanic acid has been attracting scientific interest as an active compound for potential novel therapeutic drugs. Emerging research identified phytanic acid as a potent agonist of PPAR-α [115], a transcription factor expressed in metabolically active tissues controlling cellular FA oxidation and regulating energy homeostasis [116]. An et al. [115] showed that phytol treatment in a high-fat diet-induced mouse model of obesity resulted in greater contents of phytanic acid in liver and brown adipose tissue as well as the activation of PPAR-α and its target genes. Additionally, a recent study reported that phytanic acid promotes beige adipogenesis in murine 3T3-L1 (white) adipocytes (also called browning) in a PPAR-α ligand-dependent manner [117]. Taken together, these novel findings add support to phytanic acid possibly playing a role in the regulation of energy homeostasis.
7. Conclusions
BCFAs are a class of saturated FAs that comprise a significant portion of total FAs in milk and dairy products. While humans can derive a limited amount of BCFAs from endogenous synthesis from BCAAs, dietary BCFAs remain the most important source of BCFAs within the body. Recent human trials indicate that BCFAs in tissues have a beneficial influence on metabolism. Mechanistic research validates these studies by demonstrating that BCFAs possess a wide range of structure-specific functions that may favorably influence health at the cellular and systemic level. Despite the promise of BCFAs as pro-health dietary constituents, our narrative review reveals a critical scarcity in the scientific understanding of the relationship between diet-derived BCFAs and disease risk. Of note, research has been slow to utilize these FAs as markers of dairy fat intake. Here we propose that BCFAs may be a useful and complimentary biomarker of dairy fat intake for future studies. It is clear that more research is needed to clarify the diverse biological roles of BCFAs in vivo, particularly through clinical and epidemiolocal studies that evaluate the relationship between BCFA consumption, food source, BCFA tissue concentrations, and disease.
Author Contributions
Conceptualization, J.K and M.T.-G.; Data curation, V.M.T., A.L.U., M.R.S., and J.K.; Writing—original draft preparation, V.M.T., A.L.U., M.R.S., and J.K.; Writing—review and editing, M.T.-G. and J.K.; Visualization, M.R.S. and J.K.; Supervision, J.K.; Project administration, M.T.-G. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
M.T.-G. is employee of National Dairy Council. J.K. has received research funding from National Dairy Council.
References
- 1.Cordain L., Eaton S.B., Sebastian A., Mann N., Lindeberg S., Watkins B.A., O’Keefe J.H., Brand-Miller J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 2.German J.B., Dillard C.J., Ward R.E. Bioactive components in milk. Curr. Opin. Clin. Nutr. Metab. Care. 2002;5:653–658. doi: 10.1097/00075197-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Jensen R.G. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002;85:295–350. doi: 10.3168/jds.S0022-0302(02)74079-4. [DOI] [PubMed] [Google Scholar]
- 4.Unger A.L., Bourne D.E., Walsh H., Kraft J. Fatty acid content of retail cow’s milk in the northeastern United States—What’s in it for the consumer? J. Agric. Food Chem. 2020;68:4268–4276. doi: 10.1021/acs.jafc.9b07390. [DOI] [PubMed] [Google Scholar]
- 5.Fuke G., Nornberg J.L. Systematic evaluation on the effectiveness of conjugated linoleic acid in human health. Crit. Rev. Food Sci. Nutr. 2017;57:1–7. doi: 10.1080/10408398.2012.716800. [DOI] [PubMed] [Google Scholar]
- 6.Shokryazdan P., Rajion M.A., Meng G.Y., Boo L.J., Ebrahimi M., Royan M., Sahebi M., Azizi P., Abiri R., Jahromi M.F. Conjugated linoleic acid: A potent fatty acid linked to animal and human health. Crit. Rev. Food Sci. Nutr. 2017;57:2737–2748. doi: 10.1080/10408398.2015.1060190. [DOI] [PubMed] [Google Scholar]
- 7.Den Hartigh L.J. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients. 2019;11:370. doi: 10.3390/nu11020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pariza M.W., Ha Y.L. Conjugated dienoic derivatives of linoleic acid: A new class of anticarcinogens. Med. Oncol. Tumor Pharmacother. 1990;7:169–171. doi: 10.1007/BF02988544. [DOI] [PubMed] [Google Scholar]
- 9.Kim Ha J., Lindsay R.C. Method for the quantitative analysis of volatile free and total branched-chain fatty acids in cheese and milk fat. J. Dairy Sci. 1990;73:1988–1999. doi: 10.3168/jds.S0022-0302(90)78877-7. [DOI] [Google Scholar]
- 10.Alonso L., Fontecha J., Lozada L., Fraga M.J., Juárez M. Fatty acid composition of caprine milk: Major, branched-chain, and trans fatty acids. J. Dairy Sci. 1999;82:878–884. doi: 10.3168/jds.S0022-0302(99)75306-3. [DOI] [PubMed] [Google Scholar]
- 11.Ran-Ressler R.R., Bae S., Lawrence P., Wang D.H., Thomas Brenna J. Branched-chain fatty acid content of foods and estimated intake in the USA. Br. J. Nutr. 2014;112:565–572. doi: 10.1017/S0007114514001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corazzin M., Romanzin A., Sepulcri A., Pinosa M., Piasentier E., Bovolenta S. Fatty acid profiles of cow’s milk and cheese as affected by mountain pasture type and concentrate supplementation. Animals. 2019;9:68. doi: 10.3390/ani9020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bainbridge M.L., Cersosimo L.M., Wright A.-D.G., Kraft J. Content and composition of branched-chain fatty acids in bovine milk are affected by lactation stage and breed of dairy cow. PLoS ONE. 2016;11:e0150386. doi: 10.1371/journal.pone.0150386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwendel B.H., Wester T.J., Morel P.C.H., Fong B., Tavendale M.H., Deadman C., Shadbolt N.M., Otter D.E. Pasture feeding conventional cows removes differences between organic and conventionally produced milk. Food Chem. 2017;229:805–813. doi: 10.1016/j.foodchem.2017.02.104. [DOI] [PubMed] [Google Scholar]
- 15.Schwendel B.H., Morel P.C.H., Wester T.J., Tavendale M.H., Deadman C., Fong B., Shadbolt N.M., Thatcher A., Otter D.E. Fatty acid profile differs between organic and conventionally produced cow milk independent of season or milking time. J. Dairy Sci. 2015;98:1411–1425. doi: 10.3168/jds.2014-8322. [DOI] [PubMed] [Google Scholar]
- 16.Yan Y., Wang Z., Wang X., Wang Y., Xiang J., Kothapalli K.S.D., Thomas Brenna J. Branched chain fatty acids positional distribution in human milk fat and common human food fats and uptake in human intestinal cells. J. Funct. Foods. 2017;29:172–177. doi: 10.1016/j.jff.2016.12.024. [DOI] [Google Scholar]
- 17.Or-Rashid M.M., Odongo N.E., McBride B.W. Fatty acid composition of ruminal bacteria and protozoa, with emphasis on conjugated linoleic acid, vaccenic acid, and odd-chain and branched-chain fatty acids. J. Anim. Sci. 2007;85:1228–1234. doi: 10.2527/jas.2006-385. [DOI] [PubMed] [Google Scholar]
- 18.Kaneda T. Iso-and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991;55:288–302. doi: 10.1128/MMBR.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlaeminck B., Fievez V., Cabrita A.R.J., Fonseca A.J.M., Dewhurst R.J. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed Sci. Technol. 2006;131:389–417. doi: 10.1016/j.anifeedsci.2006.06.017. [DOI] [Google Scholar]
- 20.Harper A.E., Miller R.H., Block K.P. Branched-chain amino acid metabolism. Ann. Rev. Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- 21.Harfoot C.G. Lipid metabolism in ruminant animals. In: Christie W.W., editor. Lipid Metabolism in Ruminant Animals. Pergamon Press Ltd; Oxford, UK: 1981. pp. 1–19. [Google Scholar]
- 22.Jansen G.A., Wanders R.J.A. Alpha-oxidation. Biochim. Biophys. Acta. 2006;1763:1403–1412. doi: 10.1016/j.bbamcr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Vetter W., Schröder M. Concentrations of phytanic acid and pristanic acid are higher in organic than in conventional dairy products from the German market. Food Chem. 2010;119:746–752. doi: 10.1016/j.foodchem.2009.07.027. [DOI] [Google Scholar]
- 24.Van Veldhoven P.P. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J. Lipid Res. 2010;51:2863–2895. doi: 10.1194/jlr.R005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gloerich J., Van Den Brink D.M., Ruiter J.P.N., Van Vlies N., Vaz F.M., Wanders R.J.A., Ferdinandusse S. Metabolism of phytol to phytanic acid in the mouse, and the role of PPARa in its regulation. J. Lipid Res. 2007;48:77–85. doi: 10.1194/jlr.M600050-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Lough A.K. The phytanic acid content of the lipids of bovine tissues and milk. Lipids. 1977;12:115–119. doi: 10.1007/BF02532982. [DOI] [PubMed] [Google Scholar]
- 27.Harfoot C., Hazelwood G. Lipid metabolism in the rumen. In: Hobson P., Stewart C., editors. The Rumen Microbial Ecosystem. Blackie Academic & Professional; London, UK: 1997. pp. 382–426. [Google Scholar]
- 28.Kniazeva M., Crawford Q.T., Seiber M., Wang C.Y., Han M. Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development. PLoS Biol. 2004;2:e257. doi: 10.1371/journal.pbio.0020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Caraballo S.C., Comhair T.M., Verheyen F., Gaemers I., Schaap F.G., Houten S.M., Hakvoort T.B.M., Dejong H.C., Lamers W.H., Koehler S.E. Prevention and reversal of hepatic steatosis with a high-protein diet in mice. Biochim. Biophys. Acta. 2013;1832:685–695. doi: 10.1016/j.bbadis.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Caraballo S.C., Comhair T.M., Houten S.M., Dejong H.C., Lamers W.H., Koehler S.E. High-protein diets prevent steatosis and induce hepatic accumulation of monomethyl branched-chain fatty acids. J. Nutr. Biochem. 2014;25:1263–1274. doi: 10.1016/j.jnutbio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Hirosuke O., Noriyasu Y., Junichi N., Isao C. Precursor role of branched-chain amino acids in the biosynthesis of iso and anteiso fatty acids in rat skin. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1994;1214:279–287. doi: 10.1016/0005-2760(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 32.Nicolaides N. The structures of the branched fatty acids in the wax esters of vernix caseosa. Lipids. 1971;6:901–905. doi: 10.1007/BF02531172. [DOI] [PubMed] [Google Scholar]
- 33.Ran-Ressler R., Devapatla S., Lawrence P., Brenna J.T. Comparison of BCFA types in vernix and meconium of healthy neonates. FASEB J. 2008;22 doi: 10.1096/fasebj.22.1_supplement.1091.1. [DOI] [Google Scholar]
- 34.Egge H., Murawski U., Ryhage R., György P., Chatranon W., Zilliken F. Minor constitutents of human milk IV: Analysis of the branched chain fatty acids. Chem. Phys. Lipids. 1972;8:42–55. doi: 10.1016/0009-3084(72)90042-4. [DOI] [PubMed] [Google Scholar]
- 35.Gibson R.A., Kneebone G.M. Fatty acid composition of human colostrum and mature breast milk. Am. J. Clin. Nutr. 1981;43:252–257. doi: 10.1093/ajcn/34.2.252. [DOI] [PubMed] [Google Scholar]
- 36.Dingess K.A., Valentine C.J., Ollberding N.J., Davidson B.S., Woo J.G., Summer S., Peng Y.M., Guerrero M.L., Ruiz-Palacios G.M., Ran-Ressler R.R., et al. Branched-chain fatty acid composition of human milk and the impact of maternal diet: The global exploration of human milk (GEHM) study. Am. J. Clin. Nutr. 2017;105:177–184. doi: 10.3945/ajcn.116.132464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su X., Magkos F., Zhou D., Eagon J.C., Fabbrini E., Okunade A.L., Klein S. Adipose tissue monomethyl branched-chain fatty acids and insulin sensitivity: Effects of obesity and weight loss. Obesity. 2015;23:329–334. doi: 10.1002/oby.20923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mika A., Stepnowski P., Kaska L., Proczko M., Wisniewski P., Sledzinski M., Sledzinski T. A comprehensive study of serum odd- and branched-chain fatty acids in patients with excess weight. Obesity. 2016;24:1669–1676. doi: 10.1002/oby.21560. [DOI] [PubMed] [Google Scholar]
- 39.Nicolaides N., Fu H.C., Ansari M.N.A., Rice G.R. The fatty acids of wax esters and sterol esters from vernix caseosa and from human skin surface lipid. Lipids. 1972;7:506–517. doi: 10.1007/BF02533016. [DOI] [PubMed] [Google Scholar]
- 40.Nicolaides N., Apon J.M.B. The saturated methyl branched fatty acids of adult human skin surface lipid. Biol. Mass Spectrom. 1977;4:337–347. doi: 10.1002/bms.1200040604. [DOI] [PubMed] [Google Scholar]
- 41.Nicolaides N., Kaitaranta J.K., Rawdah T.N., Macy J.I., Boswell F.M., Smith R.E. Meibomian gland studies: Comparison of steer and human lipids. Investig. Ophthalmol. Vis. Sci. 1981;20:522–536. [PubMed] [Google Scholar]
- 42.Horning M.G., Martin D.B., Karmen A., Vagelos P.R. Fatty Acid Synthesis in Adipose Tissue. II. Enzymatic Synthesis of Branched Chain and Odd-Numbered Fatty Acids. J. Biol. Chem. 1961;236:669–672. [PubMed] [Google Scholar]
- 43.Crown S.B., Marze N., Antoniewicz M.R. Catabolism of branched chain amino acids contributes significantly to synthesis of odd-chain and even-chain fatty acids in 3T3-L1 adipocytes. PLoS ONE. 2015;10:e0145850. doi: 10.1371/journal.pone.0145850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ran-Ressler R.R., Sim D., O’Donnell-Megaro A.M., Bauman D.E., Barbano D.M., Brenna J.T. Branched chain fatty acid content of United States retail cow’s milk and implications for dietary intake. Lipids. 2011;46:569–576. doi: 10.1007/s11745-011-3530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan Y., Wang Z., Greenwald J., Kothapalli K.S.D., Park H.G., Liu R., Mendralla E., Lawrence P., Wang X., Brenna J.T. BCFA suppresses LPS induced IL-8 mRNA expression in human intestinal epithelial cells. Prostaglandins Leukot. Essent. Fat. Acids. 2017;116:27–31. doi: 10.1016/j.plefa.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Yan Y., Wang Z., Wang D., Lawrence P., Wang X., Kothapalli K.S.D., Greenwald J., Liu R., Park H.G., Brenna J.T. BCFA-enriched vernix-monoacylglycerol reduces LPS-induced inflammatory markers in human enterocytes in vitro. Pediatric Res. 2018;83:874–879. doi: 10.1038/pr.2017.297. [DOI] [PubMed] [Google Scholar]
- 47.Liu L., Wang Z., Park H.G., Xu C., Lawrence P., Su X., Wijendran V., Walker W.A., Kothapalli K.S.D., Brenna J.T. Human fetal intestinal epithelial cells metabolize and incorporate branched chain fatty acids in a structure specific manner. Prostaglandins Leukot. Essent. Fat. Acids. 2017;116:32–39. doi: 10.1016/j.plefa.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wongtangtintharn S., Oku H., Iwasaki H., Inafuku M., Toda T., Yanagita T. Incorporation of branched-chain fatty acid into cellular lipids and caspase-independent apoptosis in human breast cancer cell line, SKBR-3. Lipids Health Dis. 2005;4:29. doi: 10.1186/1476-511X-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vahmani P., Salazar V., Rolland D.C., Gzyl K.E., Dugan M.E.R. Iso- but not anteiso-branched chain fatty acids exert growth-inhibiting and apoptosis-inducing effects in MCF-7 cells. J. Agric. Food Chem. 2019;67:10042–10047. doi: 10.1021/acs.jafc.9b03549. [DOI] [PubMed] [Google Scholar]
- 50.Ran-Ressler R.R., Khailova L., Arganbright K.M., Adkins-Rieck C.K., Jouni Z.E., Koren O., Ley R.E., Brenna J.T., Dvorak B. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS ONE. 2011;6:e29032. doi: 10.1371/journal.pone.0029032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shingfield K.J., Chilliard Y., Toivonen V., Kairenius P., Givens D.I. Trans fatty acids and bioactive lipids in ruminant milk. In: Bösze Z., editor. Bioactive Components in Milk. Springer; New York, NY, USA: 2008. pp. 3–66. [DOI] [PubMed] [Google Scholar]
- 52.Leiber F., Kreuzer M., Nigg D., Wettstein H.R., Scheeder M.R.L. A study on the causes for the elevated n-3 fatty acids in cows’ milk of alpine origin. Lipids. 2005;40:191–202. doi: 10.1007/s11745-005-1375-3. [DOI] [PubMed] [Google Scholar]
- 53.Schröder M., Larissa Lutz N., Chick Tangwan E., Hajazimi E., Vetter W. Phytanic acid concentrations and diastereomer ratios in milk fat during changes in the cow’s feed from concentrate to hay and back. Eur. Food Res. Technol. 2012;234:955–962. doi: 10.1007/s00217-012-1710-2. [DOI] [Google Scholar]
- 54.Che B.N., Kristensen T., Nebel C., Dalsgaard T.K., Hellgren L.I., Young J.F., Larsen M.K. Content and distribution of phytanic acid diastereomers in organic milk as affected by feed composition. J. Agric. Food Chem. 2013;61:225–230. doi: 10.1021/jf304079r. [DOI] [PubMed] [Google Scholar]
- 55.Baars T., Schröder M., Kusche D., Vetter W. Phytanic acid content and SRR/RRR diastereomer ratio in milk from organic and conventional farms at low and high level of fodder input. Org. Agric. 2012;2:13–21. doi: 10.1007/s13165-012-0021-z. [DOI] [Google Scholar]
- 56.Vetter W., Schröder M. Phytanic acid—A tetramethyl-branched fatty acid in food. Lipid Technol. 2011;23:175–178. doi: 10.1002/lite.201100127. [DOI] [Google Scholar]
- 57.Chen M., Li Y., Sun Q., Pan A., Manson J.E., Rexrode K.M., Willett W.C., Rimm E.B., Hu F.B. Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. Am. J. Clin. Nutr. 2016;104:1209–1217. doi: 10.3945/ajcn.116.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yakoob M.Y., Shi P., Willett W.C., Rexrode K.M., Campos H., Orav E.J., Hu F.B., Mozaffarian D. Circulating biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation. 2016;133:1645–1654. doi: 10.1161/CIRCULATIONAHA.115.018410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santaren I.D., Watkins S.M., Liese A.D., Wagenknecht L.E., Rewers M.J., Haffner S.M., Lorenzo C., Hanley A.J. Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am. J. Clin. Nutr. 2014;100:1532–1540. doi: 10.3945/ajcn.114.092544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mozaffarian D., De Oliveira Otto M.C., Lemaitre R.N., Fretts A.M., Hotamisligil G., Tsai M.Y., Siscovick D.S., Nettleton J.A. Trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: The multi-ethnic study of atherosclerosis (MESA) Am. J. Clin. Nutr. 2013;97:854–861. doi: 10.3945/ajcn.112.045468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rautiainen S., Wang L., Lee I.M., Manson J.E., Buring J.E., Sesso H.D. Dairy consumption in association with weight change and risk of becoming overweight or obese in middle-aged and older women: A prospective cohort study. Am. J. Clin. Nutr. 2016;103:979–988. doi: 10.3945/ajcn.115.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mozaffarian D., Hao T., Rimm E.B., Willett W.C., Hu F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pakiet A., Wilczynski M., Rostkowska O., Korczynska J., Jabłonska P., Kaska L., Proczko-Stepaniak M., Sobczak E., Stepnowski P., Magkos F., et al. The effect of one anastomosis gastric bypass on branched-chain fatty acid and branched-chain amino acid metabolism in subjects with morbid obesity. Obes. Surg. 2020;30:304–312. doi: 10.1007/s11695-019-04157-z. [DOI] [PubMed] [Google Scholar]
- 64.Mika A., Wilczynski M., Pakiet A., Kaska L., Proczko-stepaniak M., Stankiewicz M., Stepnowski P., Sledzinski T. Short-term effect of one-anastomosis gastric bypass on essential fatty acids in the serum of obese patients. Nutrients. 2020;12:187. doi: 10.3390/nu12010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Unger A.L., Eckstrom K., Jetton T.L., Kraft J. Colonic bacterial composition is sex-specific in aged CD-1 mice fed diets varying in fat quality. PLoS ONE. 2019;14:e0226635. doi: 10.1371/journal.pone.0226635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pellattiero E., Cecchinato A., Tagliapietra F., Schiavon S., Bittante G. The use of 2-dimensional gas chromatography to investigate the effect of rumen-protected conjugated linoleic acid, breed, and lactation stage on the fatty acid profile of sheep milk. J. Dairy Sci. 2015;98:2088–2102. doi: 10.3168/jds.2014-8395. [DOI] [PubMed] [Google Scholar]
- 67.Gómez-Cortés P., Cívico A., De La Fuente M.A., Núñez Sánchez N., Peña Blanco F., Martinez Marin A.L. Effects of dietary concentrate composition and linseed oil supplementation on the milk fatty acid profile of goats. Animal. 2018;12:2310–2317. doi: 10.1017/S1751731118000381. [DOI] [PubMed] [Google Scholar]
- 68.Cossignani L., Giua L., Urbani E., Simonetti M.S., Blasi F. Fatty acid composition and CLA content in goat milk and cheese samples from Umbrian market. Eur. Food Res. Technol. 2014;239:905–911. doi: 10.1007/s00217-014-2287-8. [DOI] [Google Scholar]
- 69.Hauff S., Vetter W. Quantification of branched chain fatty acids in polar and neutral lipids of cheese and fish samples. J. Agric. Food Chem. 2010;58:707–712. doi: 10.1021/jf9034805. [DOI] [PubMed] [Google Scholar]
- 70.Wang D.H., Wang Z., Brenna J.T. Gas chromatography chemical ionization mass spectrometry and tandem mass spectrometry for identification and straightforward quantification of branched chain fatty acids in foods. J. Agric. Food Chem. 2020;68:4973–4980. doi: 10.1021/acs.jafc.0c01075. [DOI] [PubMed] [Google Scholar]
- 71.Goudjil H., Fontecha J., Luna P., De La Fuente M.A., Alonso L., Juárez M. Quantitative characterization of unsaturated and trans fatty acids in ewe’s milk fat. Lait. 2004;84:473–482. doi: 10.1051/lait:2004017. [DOI] [Google Scholar]
- 72.Tzamaloukas O., Orford M., Miltiadou D., Papachristoforou C. Partial suckling of lambs reduced the linoleic and conjugated linoleic acid contents of marketable milk in Chios ewes. J. Dairy Sci. 2015;98:1739–1749. doi: 10.3168/jds.2014-8540. [DOI] [PubMed] [Google Scholar]
- 73.Lopez A., Vasconi M., Moretti V.M., Bellagamba F. Fatty acid profile in goat milk from high- and low-input conventional and organic systems. Animal. 2019;9:452. doi: 10.3390/ani9070452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Serment A., Schmidely P., Giger-Reverdin S., Chapoutot P., Sauvant D. Effects of the percentage of concentrate on rumen fermentation, nutrient digestibility, plasma metabolites, and milk composition in mid-lactation goats. J. Dairy Sci. 2011;94:3960–3972. doi: 10.3168/jds.2010-4041. [DOI] [PubMed] [Google Scholar]
- 75.Valenti B., Luciano G., Morbidini L., Rossetti U., Codini M., Avondo M., Priolo A., Bella M., Natalello A., Pauselli M. Dietary pomegranate pulp: Effect on ewe milk quality during late lactation. Animals. 2019;9:283. doi: 10.3390/ani9050283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Currò S., Manuelian C., De Marchi M., Claps S., Rufrano D., Neglia G. Effects of breed and stage of lactation on milk fatty acid composition of Italian goat breeds. Animals. 2019;9:764. doi: 10.3390/ani9100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glasser F., Doreau M., Ferlay A., Chilliard Y. Technical note: Estimation of milk fatty acid yield from milk fat data. J. Dairy Sci. 2007;90:2302–2304. doi: 10.3168/jds.2006-870. [DOI] [PubMed] [Google Scholar]
- 78.FoodData Central—Butterkase. [(accessed on 11 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/522677/nutrients.
- 79.FoodData Central—Cheese, Brie. [(accessed on 11 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/172177/nutrients.
- 80.FoodData Central—Cheese, Swiss. [(accessed on 7 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171251/nutrients.
- 81.FoodData Central—Ricotta Cheese, Ricotta. [(accessed on 14 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/678748/nutrients.
- 82.FoodData Central—Cheese, Camembert. [(accessed on 11 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/172178/nutrients.
- 83.FoodData Central—Cheese, Cottage, Creamed, Large or Small Curd. [(accessed on 7 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/172179/nutrients.
- 84.FoodData Central—Cheese, Gouda. [(accessed on 11 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171241/nutrients.
- 85.FoodData Central—Cheese, Limburger. [(accessed on 11 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/172175/nutrients.
- 86.FoodData Central—Cheese, Mozzarella, Whole Milk. [(accessed on 7 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170845/nutrients.
- 87.FoodData Central—Cheese, Pasteurized Process, American, Fortified with Vitamin D. [(accessed on 7 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170853/nutrients.
- 88.FoodData Central—Cheese, Provolone. [(accessed on 7 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170850/nutrients.
- 89.FoodData Central—Cheese, Ricotta, Whole Milk. [(accessed on 7 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170851/nutrients.
- 90.FoodData Central—Yogurt, Plain, Whole Milk. [(accessed on 4 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171284/nutrients.
- 91.FoodData Central—Butter, Salted. [(accessed on 4 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/173410/nutrients.
- 92.FoodData Central—Milk, Sheep, Fluid. [(accessed on 5 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170882/nutrients.
- 93.FoodData Central—Milk, Goat, Fluid, with Added Vitamin D. [(accessed on 5 May 2020)]; Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171278/nutrients.
- 94.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 95.Blackburn P., Després J.P., Lamarche B., Tremblay A., Bergeron J., Lemieux I., Couillard C. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity. 2006;14:1747–1754. doi: 10.1038/oby.2006.201. [DOI] [PubMed] [Google Scholar]
- 96.Skinner A.C., Steiner M.J., Henderson F.W., Perrin E.M. Multiple markers of inflammation and weight status: Cross-sectional analyses throughout childhood. Pediatrics. 2010;125:e801–e809. doi: 10.1542/peds.2009-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y., Chen F., Odle J., Lin X., Jacobi S.K., Zhu H., Wu Z., Hou Y. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 2012;142:2017–2024. doi: 10.3945/jn.112.164947. [DOI] [PubMed] [Google Scholar]
- 98.Lee S.I., Kang K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 2017;7:16530. doi: 10.1038/s41598-017-16561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chao J., Wolfaardt G.M., Arts M.T. Characterization of pseudomonas aeruginosa fatty acid profiles in biofilms and batch planktonic cultures. Can. J. Microbiol. 2010;56:1028–1039. doi: 10.1139/W10-093. [DOI] [PubMed] [Google Scholar]
- 100.Cai Q., Huang H., Qian D., Chen K., Luo J., Tian Y., Lin T., Lin T. 13-methyltetradecanoic acid exhibits anti-tumor activity on T-cell lymphomas in vitro and in vivo by down-regulating p-AKT and activating caspase-3. PLoS ONE. 2013;8:e65308. doi: 10.1371/journal.pone.0065308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang Z., Liu S., Chen X., Chen H., Huang M., Zheng J. Induction of apoptotic cell death and in vivo growth inhibition of human cancer cells by a saturated branched-chain fatty acid, 13-methyltetradecanoic acid. Cancer Res. 2000;60:505–509. [PubMed] [Google Scholar]
- 102.Lin T., Yin X.B., Cai Q., Fan X., Xu K., Huang L., Luo J., Zheng J., Huang J. 13-Methyltetradecanoic acid induces mitochondrial-mediated apoptosis in human bladder cancer cells. Urol. Oncol. Semin. Orig. Investig. 2012;30:339–345. doi: 10.1016/j.urolonc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 103.Wongtangtintharn S., Oku H., Iwasaki H., Toda T. Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells. J. Nutr. Sci. Vitaminol. 2004;50:137–143. doi: 10.3177/jnsv.50.137. [DOI] [PubMed] [Google Scholar]
- 104.Wright K.C., Yang P., Van Pelt C.S., Hicks M.E., Collin P., Newman R.A. Evaluation of targeted arterial delivery of the branched chain fatty acid 12-methyltetradecanoic acid as a novel therapy for solid tumors. J. Exp. Ther. Oncol. 2005;5:55–68. [PubMed] [Google Scholar]
- 105.Yu J., Yang L.N., Wu Y.Y., Li B.H., Weng S.M., Hu C.L., Han Y.L. 13-Methyltetradecanoic acid mitigates cerebral ischemia/reperfusion injury. Neural Regen. Res. 2016;11:1431–1437. doi: 10.4103/1673-5374.191216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cole N., Hume E.B., Jalbert I., Vijay A.K., Krishnan R., Willcox M.D. Effects of topical administration of 12-methyl tetradecanoic acid (12-MTA) on the development of corneal angiogenesis. Angiogenesis. 2007;10:47–54. doi: 10.1007/s10456-007-9063-3. [DOI] [PubMed] [Google Scholar]
- 107.Brooks K.K., Liang B., Watts J.L. The influence of bacterial diet on fat storage in C. elegans. PLoS ONE. 2009;4:e7545. doi: 10.1371/journal.pone.0007545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu L., Xiao D., Lei H., Peng T., Li J., Cheng T., He J. Branched-chain fatty acids lower triglyceride levels in a fatty liver model in vitro. FASEB J. 2017;31 doi: 10.1096/fasebj.31.1_supplement.969.4. [DOI] [Google Scholar]
- 109.Kraft J., Jetton T., Satish B., Gupta D. Dairy-derived bioactive fatty acids improve pancreatic ß-cell function. FASEB J. 2015;29 doi: 10.1096/fasebj.29.1_supplement.608.25. [DOI] [Google Scholar]
- 110.Roca-Saavedra P., Mariño-Lorenzo P., Miranda J.M., Porto-Arias J.J., Lamas A., Vazquez B.I., Franco C.M., Cepeda A. Phytanic acid consumption and human health, risks, benefits and future trends: A review. Food Chem. 2017;221:237–247. doi: 10.1016/j.foodchem.2016.10.074. [DOI] [PubMed] [Google Scholar]
- 111.Chaudhary S., Sahu U., Kar S., Parvez S. Phytanic acid-induced neurotoxicological manifestations and apoptosis ameliorated by mitochondria-mediated actions of melatonin. Mol. Neurobiol. 2017;54:6960–6969. doi: 10.1007/s12035-016-0209-4. [DOI] [PubMed] [Google Scholar]
- 112.Chaudhary S., Parvez S. Phytanic acid induced neurological alterations in rat brain synaptosomes and its attenuation by melatonin. Biomed. Pharmacother. 2017;95:37–46. doi: 10.1016/j.biopha.2017.07.156. [DOI] [PubMed] [Google Scholar]
- 113.Refsum Disease—Genetics Home Reference—NIH. [(accessed on 30 June 2020)]; Available online: https://ghr.nlm.nih.gov/condition/refsum-disease#statistics.
- 114.Nakanishi T., Motoba I., Anraku M., Suzuki R., Yamaguchi Y., Erickson L., Eto N., Sugamoto K., Matsushita Y., Kawahara S. Naturally occurring 3RS, 7R, 11R-phytanic acid suppresses in vitro T-cell production of interferon-gamma. Lipids Health Dis. 2018;17:147. doi: 10.1186/s12944-018-0793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.An J.-Y., Jheng H.-F., Nagai H., Sanada K., Takahashi H., Iwase M., Watanabe N., Kim Y.-I., Teraminami A., Takahashi N., et al. A Phytol-Enriched Diet Activates PPAR-α in the Liver and Brown Adipose Tissue to Ameliorate Obesity-Induced Metabolic Abnormalities. Mol. Nutr. Food Res. 2018;62:1700688. doi: 10.1002/mnfr.201700688. [DOI] [PubMed] [Google Scholar]
- 116.Lamichane S., Lamichane B.D., Kwon S.M. Pivotal roles of peroxisome proliferator-activated receptors (PPARs) and their signal cascade for cellular and whole-body energy homeostasis. Int. J. Mol. Sci. 2018;19:914. doi: 10.3390/ijms19040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang H., Mao X., Du M. Phytanic acid activates PPARα to promote beige adipogenic differentiation of preadipocytes. J. Nutr. Biochem. 2019;67:201–211. doi: 10.1016/j.jnutbio.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]