Abstract
Autophagy is an elegant and complex biological process that has recently attracted much attention from the scientific community. The compounds which are capable of control and modulation of this process have a promising potential as therapeutics for a number of pathological conditions, including cancer and neurodegenerative disorders. At the same time, due to the relatively young age of the field, there are still some pitfalls in the autophagy monitoring assays and interpretation of the experimental data. This critical review provides an overview of the marine natural compounds, which have been reported to affect autophagy. The time period from the beginning of 2016 to the middle of 2020 is covered. Additionally, the published data and conclusions based on the experimental results are re-analyzed with regard to the guidelines developed by Klionsky and colleagues (Autophagy. 2016; 12(1): 1–222), which are widely accepted by the autophagy research community. Remarkably and surprisingly, more than half of the compounds reported to be autophagy activators or inhibitors could not ultimately be assigned to either category. The experimental data reported for those substances could indicate both autophagy activation and inhibition, requiring further investigation. Thus, the reviewed molecules were divided into two groups: having validated and non-validated autophagy modulatory effects. This review gives an analysis of the recent updates in the field and raises an important problem of standardization in the experimental design and data interpretation.
Keywords: autophagy, macroautophagy, marine natural compounds, cancer, neurodegenerative disorders
1. Introduction
1.1. Autophagy as a Biological Process
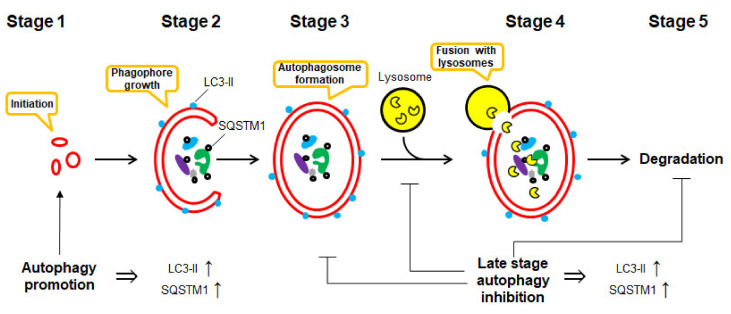
Macroautophagy (here referred to as autophagy) is defined as a regulated mechanism of the degradation of unnecessary or dysfunctional cellular components [1]. This “self-eating” process may lead to the degradation—either selective or non-selective—of organelles and proteins by the lysosome system. It is the basic cellular catabolic “self-eating” process that leads to non-selective bulk degradation of organelles and proteins by the lysosome system [2]. It can result from cellular stress, e.g., starvation and exposure to toxins [3], and includes the formation of double-membrane vesicles (autophagosomes), which later fuse with lysosomes, leading to degradation and recycling of sequestered contents [2]. Biochemically, autophagy is an elegant and beautiful process, which consists of several critical stages, including (Figure 1):
-
Stage 1
induction: autophagy begins with the stimuli-initiation event (starvation, radiation, drug treatment, etc.).
-
Stage 2
phagophore formation: LC3-I converts to LC3-II via conjugation with phosphatidylethanolamine (PE). This process is called “lipidation”. “Phagophores” (or isolation membranes) are the double-membrane structures, which are growing in the cytosol.
-
Stage 3
autophagosome formation: phagophore engulfs bulk cytoplasm nonspecifically, including entire organelles; or it targets organelle cargos specifically, therefore forming vesicles called “autophagosomes”.
-
Stage 4
docking and fusion with lysosomes: the autophagosome fuses with a lysosome leading to the formation of “autolysosome”.
-
Stage 5
vesicle breakdown and degradation: finally, the sequestered material and organelles are degraded inside the autolysosome by lysosome proteases and recycled for the synthesis of ATP and various macromolecules such as proteins [4].
Figure 1.
Autophagy flux.
In the past few years, autophagy has received considerable attention, especially at the end of 2016, after the Nobel Prize in Physiology or Medicine “For the discoveries of mechanisms of autophagy” was awarded to Prof. Yoshinori Ohsumi [5]. However, it should be noted that in comparison with, e.g., apoptosis, autophagy is still a much less studied process [6].
Autophagy is a complex biological phenomenon. Over the last few years, several new and previously known proteins have been reported to be involved in this process [7,8]. Additionally, its crosstalk and interaction with basically every single biological process in different living organisms have been reported and investigated [9]. Thus, autophagy contributes to a number of physiological conditions, both normal and pathological, and plays a significant role in mammalian cells’ death and survival. Due to particular importance in oncology and different neurodegenerative disorders (e.g., Parkinson’s, Alzheimer’s disease and others), it has drawn great attention and has been closely investigated by scientists across the world. In cancer, autophagy has emerged as one of the high-potential therapeutic targets, which in some cases might be cancer-cell-specific and therefore represent a chance to reduce the side effect of the therapy [10]. This is why the idea of searching and developing compounds that can control and modulate this process is so attractive.
1.2. Blue-Print Autophagy
Remarkably, the chemical structures and variety of natural compounds found in marine organisms differ significantly from terrestrial plants and animals. Specific environmental conditions have been identified as the main reasons for this phenomenon. Moreover, many marine organisms produce a large variety of unique small molecules, which are often used to protect themselves in a highly competitive marine environment. A significant proportion of these compounds exhibit potent biological activity, targeting one or several specific biological processes and, therefore, could be used for the therapy of human diseases. Some of them are already applied in clinics. The first marine-derived compound that was later developed into a clinically used drug was spongothymidine [11,12,13]. This molecule was discovered in 1951 and later became a lead compound for the cytarabine development [14]. Nowadays, thirteen marine-derived drugs are used clinically, mainly as therapeutics for the treatment of cancer and cancer-related conditions [14,15,16,17,18]. Two of these drugs were approved in 2019 and at least one in 2020 (as of August 2020). Many more are in all different phases of testing within clinical trials, and a plethora of substances have already been preclinically tested in vitro and in vivo [19,20,21]. Thus, marine-derived compounds are of particular interest to biomedical scientists across the globe.
The term “Blue-print autophagy” was introduced by Ruocco and colleagues in 2016 when the very first review article on autophagy-modulating marine-derived compounds was published in Marine Drugs [22]. That article covered around 20 marine compounds, which were described to possess a relevant activity [22]. Since then, the field of autophagy has grown notably. In particular, many new compounds isolated from marine sources have been described as autophagy modulators (both activators and inhibitors) [23,24]. Some of these compounds are of special interest to scientists not only as biochemical tools but also as potential therapeutics for various pathological conditions. A good example of these molecules is new macrolides belonging to the famous family of autophagy inhibitors bafilomycins, which were isolated from the marine-derived strain of Streptomyces spp. [25]. According to the PubMed database (U.S. National Library of Medicine NIH), the annual number of the scientific papers related to “autophagy” has increased more than 50 times over the past 20 years [26]. Despite the relative specificity of the “blue-print autophagy” topic, this area is also continually growing along with the “mother” autophagy research field. Thus, a search Pubmed database indicates its 25-fold growth over the same period, i.e., from only one related manuscript/year published in 1999, to 25 manuscripts/year in 2019 (and 19 manuscripts have been already published by August of 2020) [27].
The current review is intended to not only give an overview of the compounds but also to critically analyze all the autophagy modulators isolated from marine organisms and reported within the beginning of 2016 to the end of August 2020. The source organism and molecular type of the bioactive molecules as well as an effect on autophagy and related molecular targets are reported. Particular attention was paid to the validation of the suggested autophagy-modulating effects. The molecules that were proven to be the true autophagy modulators were differentiated from those requiring further detailed examination before they could be assigned to the group of either activators or inhibitors. The selection criteria are described in the following chapter.
1.3. Challenges in Autophagy Monitoring and Results Interpretation
The autophagy flux seems to be quite straightforward. However, the first impression of this biochemical process’ relative simplicity is deceptive. As was mentioned in the previous chapter, a number of molecules play important roles at the different stages of this complex process [7,28]. Additionally, autophagy crosstalks with a great number of biophysiological pathways [9]. One of the most important and well-studied molecules involved in autophagy is LC3 (microtubule-associated proteins 1A/1B light chain 3) [29]. During autophagy, LC3-I gets lipidated and converts to LC3-II, which can easily be monitored using, e.g., Western blotting [28,29]. The latter got further integrated into the phagophore double membrane (which later becomes a part of autophagosome and then autolysosome membrane). LC3-II is an essential protein for the formation of these structures. Therefore, LC3-II is the most often used marker of autophagosomes and autolysosomes [28,29].
Another well-known and often used protein to monitor the autophagy flux is a ubiquitin-binding protein sequestosome 1 (SQSTM1, or p62). SQSTM1 is a cargo protein that targets other proteins and delivers them to autophagosomes for selective autophagy (via binding with autophagosomal membrane LC3-II protein) [28,30].
Both LC3-II and SQSTM1 are upregulated when autophagy is activated and are degraded in late autolysosomes [7]. Thus, many authors, while detecting elevated LC3-II and SQSTM1 levels in the stimulated (e.g., drug-treated) cells, make false conclusions on the autophagy-activatory effect of the investigated stimuli. However, the inhibition of autophagy at the late stage (e.g., at the step of autophagosome fusion with lysosomes or at the step of autolysosomes degradation) also results in elevated LC3-II and SQSTM1 levels due to the disruption of autophagic turnover (Figure 1) [7,28,30,31]. Thus, autophagy inhibition may be giving a very similar, if not identical, picture as to when autophagy is activated [7,30]. Moreover, inhibition of autophagy at the early stages, on the contrary, may result in the downregulation of LC3-II and SQSTM1 expressional levels [7,30]. Finally, in some models, a time-dependent investigation of LC3-II protein level under the autophagy-inducing conditions has revealed its upregulation at the early time points following a further decrease. [7,30]. Therefore, monitoring of only LC3-II and SQSTM1 levels is certainly not enough to distinguish between such distinct effects as activation and inhibition of autophagy, especially in the models where this effect results from drug treatment [7,28,30,31].
The second challenge that the scientists working in the field of autophagy modulators face is an effect on cellular viability. In other words, the question to be answered―“does a particular autophagy promoter activates pro-survival or cytotoxic autophagy?” (or “does a particular autophagy inhibitor suppresses pro-survival or cytotoxic autophagy?”). This information is of critical importance for the further clinical application of the compound, especially for drugs, which are investigated as possible medications for cancer or neurodegenerative disorders. In the cellular context, autophagy’s biological effect can be either cytoprotective, cytotoxic, cytostatic, or even non-protective (no effect on viability or the sensitivity of cells to the drugs) [6]. Indeed, initially, autophagy has been described as a cytoprotective mechanism, which helps cells to survive the stress [1,3]. Note, autophagy induced in cancer cells exposed to the cytotoxic chemotherapeutics often exhibits cytoprotective characteristics [3,32]. However, excessive autophagy may lead to cellular death and is classified as a “programmed cell death type II” (apoptosis is a “programmed cell death type I”) [3]. Moreover, several natural compounds, also derived from the marine sources, have been reported to induce the autophagic cell death [22].
Since autophagy is a relatively young and sometimes still controversial topic, some standardization of the research methods, approaches, and interpretation of the results were needed. Thus, Prof. Daniel J. Klionsky and colleagues developed and published the very first “Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes” in 2008 [30]. Here, Klionsky et al. have collated and critically analyzed the methods of autophagy monitoring as well as gave a recommendation on the interpretation of the experimental results. These Guidelines were then updated in 2012 (2nd edition) [31] and 2016 (3rd edition) [7], and finally, the latest 4th edition is about to be published in 2020 [8,33]. These Guidelines may be referred to as the gold standard when planning and analyzing autophagy-modulating drugs’ activity. Thus, the current review is intended to critically analyze the data on the autophagy-related activity of the marine-derived compounds, which were reported in the scientific literature over the last four years (beginning of 2016–August 2020). Following the recommendation by Klionsky et al. [7,30,31], the activity (activation/inhibition) of the reported autophagy modulators was assumed to be validated if:
-
(a)
two or more independent methods were used to prove the suggested effect on autophagy;
-
(b)
these methods could clearly distinguish inhibition and activation of autophagy in the biological model used.
If at least one of these criteria is not met, the effect of the certain compound on autophagy, suggested by the authors of the corresponding article, was considered as non-validated (for a detailed description, please refer to [7,30,31]). Importantly, according to the Guidelines, there are no absolute criteria or a single universal assay to determine an autophagic status in every biological model [7,30,31]. In fact, an opposing activity has been observed depending on the cell type used and the stimuli examined (i.e., a type of the drug) [3]. Therefore, careful attention should always be paid to each specific case, and the biological backgrounds of the model and drug should always be considered [7].
2. Marine Compounds with a Validated Autophagy-Modulatory Effect
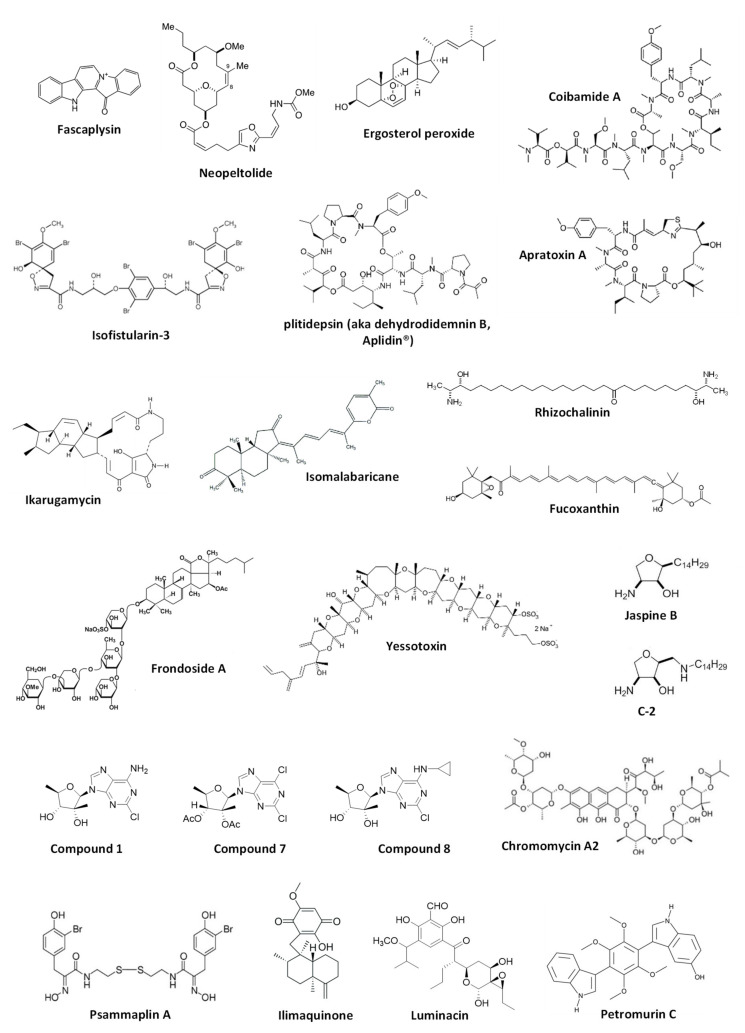
This chapter comprises marine-derived compounds reported to activate or inhibit autophagy in mammalian cells (Figure 2). For the compounds listed below, the effect has been validated according to the Guidelines by Klionsky et al. [7,8,30,31] and therefore, can be considered correct. The biological effect as well as autophagy-related targets, if any, are discussed. The data are summarized in Table 1.
Figure 2.
Marine-derived compounds with a validated autophagy-modulatory effect.
2.1. Alkaloids
Marine alkaloid fascaplysin was initially isolated from the marine sponge Fascaplysinopsis sp. in 1988 [34] and later from other sponge species [35]. This compound exhibited anticancer activity in several human cancer models [35]. Fascaplysin was reported to be a potent inducer of cancer cell apoptosis mainly exerted via cyclin-dependent kinase 4 (CDK4) inhibition and suppression of angiogenesis [36,37]. Meng et al. have reported the activation of cytoprotective autophagy in human vascular endothelial cells (HUVEC) treated with this drug, which was identified as a resistance mechanism [38]. Thus, fascaplysin-induced autophagy could decrease a pro-apoptotic as well as anti-angiogenetic effects of this alkaloid. Increased expression of ROS (reactive oxygen species) and p8 protein was reported to be a factor related to the autophagy induction in HUVEC cells by fascaplysin [38]. Finally, the authors suggested that combined treatment with autophagy inhibitors could increase the anticancer activity of the alkaloid [38]. In this research, the authors used several independent methods to demonstrate an effect on autophagy; hence, the evidence of the autophagy-inducing activity of fascaplysin is quite convincing. In 2016, the group of Diederich reported a new activity [39] of the previously known brominated alkaloid isofistularin-3, which has been found in several marine sponges, including Aplysina aerophoba, Verongia aerophoba and others [40,41]. Thus, isofistularin-3 exerted anticancer activity via DNA demethylation and induced autophagy in Raji cells (Burkitt’s lymphoma) [39]. The effect of this brominated natural alkaloid on autophagy was confirmed using several methods, including combination with autophagy inhibitors. However, the authors did not investigate the role of this process in the isofistularin-3-induced cancer cell death [39].
2.2. Macrocyclic Molecules
Coibamide A and apratoxin A are cytotoxic lariat depsipeptides isolated from marine cyanobacteria Leptolyngbya sp. [42] and Lyngbya majuscule [43], correspondently. The group of Ishmael has reported coibamide A to inhibit the tumor growth in vitro and in vivo in glioblastoma models [44]. They have also shown both coibamide A and apratoxin A to inhibit angiogenesis via suppression of VEGFR2 expression and induce autophagy in human non-cancer HUVEC cells. The latest finding was validated by the combinational experiments with bafilmycin A1 [44]. The effect of the induced autophagy on HUVEC cell viability is still to be investigated in the future. Using the mouse embryonic fibroblasts (MEFs) model two years later, the same group has shown that ATG5 is required for the cytotoxic activity of these depsipeptides, suggesting induction of cytotoxic autophagy [45]. Additionally, it was postulated that induced autophagy is not triggered by acute ER stress [45].
Another well-representative example of the discovery of autophagy-modulating marine natural compounds is the research performed by the group of Gao and White, who have developed a nanotechnology-enabled high-throughput screening system for the identification of transcription factor EB (TFEB) activators [46]. TFEB is known to be activated in starvation conditions and ultimately promotes autophagy. Thus, the authors have screened 15,000 natural and synthetic compounds and identified, among others, ikarugamycin as a potent agonist of TFEB. Ikarugamycin is a natural macrocyclic antibiotic initially isolated from marine-derived bacteria Streptomyces phaeochromogenes [47]. This compound was able to increase the concentration of cytosolic Ca2+, which resulted in the consequent activation of CaMKKβ and AMPK pathway, inhibition of mTORC1, activation of TFEB, and ultimately in promotion of autophagy [46]. Hence, it was shown that ikarugamycin could activate autophagy in the HeLa cell model in vitro, ameliorate metabolic syndrome in the mice model and extend C. elegans lifespan in vivo via the same mechanism [46].
The cyclic depsipeptide plitidepsin, also known as dehydrodidemnin B or as the anticancer drug Aplidin®, was initially extracted from the ascidian Aplidium albicans [48]. Losada et al. have explored the effect of plitidepsin in HeLa cells [49]. The authors could show and prove plitidepsin to induce ER stress and simultaneously inhibit proteasome- and autophagy-mediated degradation of misfolded proteins [49]. This results in an unfolded protein response, stipulated by the alternative XBP1 splicing, the proteolytic processing of ATF6 as well as phosphorylation of JNK and eIF2α. Of note, distinctly from the most autophagy inhibitors, plitidepsin was able to inhibit autophagy at the early stages, preventing LC3-I lipidation to LC3-II. Moreover, the authors showed that this effect was not due to the inhibition of Vps34 (PIK3C3) or activation of mTOR. Thus, it was suggested that the autophagy-inhibitory effect was eventually due to the direct binding of plitidepsin to eEF1A2, which, therefore, could be one of the molecular targets of this drug [49]. Another interesting research by Fuwa and Sato reports an anticancer activity of the synthetic analog of marine macrolide neopeltolide (isolated from a deep-water sponge Neopeltidae sp. [50])—8,9-dehydroneopeltolide (8,9-DNP)—in human pancreatic cancer PANC-1 cells [51]. The authors have shown 8,9-DNP to inhibit cytoprotective autophagy by preventing lipidation of LC3B-I to LC3B-II under starvation conditions. Even though the drug target was not identified, the generated results suggested that autophagy was inhibited at its early stages and that 8,9-DNP does not interfere with cellular energy-sensing signals [51].
2.3. Triterpenes
An examination of the anticancer activity of marine triterpene glycoside frondoside A, isolated from sea cucumber Cucumaria frondosa [52] using in vitro and in vivo models of human drug-resistant prostate cancer cells by Dyshlovoy et al. has revealed the time-dependent accumulation of autophagosomes [53]. Further examination of LC3B-II accumulation dynamic and combinational experiments suggested the inhibition of pro-survival autophagy in prostate cancer cells by frondoside A. The investigation of the biological activity in human bladder cancer [54], as well as Burkitt’s lymphoma models in vitro [55] by the same group, revealed the similar signs of autophagy inhibition. At the same time, as the autophagy-modulatory effect of the same drug may significantly differ depending on the experimental model used [7,8,30,31], autophagy inhibition by frondoside A in bladder cancer as well as in Burkitt’s lymphoma is still to be validated. Ergosterol peroxide was isolated from the marine fungus Phoma sp. and was described to be cytotoxic to the human lung adenocarcinoma cells [56]. On the other hand, this natural compound could induce autophagy in these cells, which was revealed to be cytoprotective. This cellular process attenuated the anticancer effects of ergosterol peroxide and was associated with ROS induction as well as with regulation of ERK, JNK and p38 MAPK, and other proteins [56]. The group of Kong has reported an isomalabaricane triterpene secondary metabolite of marine sponge Jaspis stellifera, stellettin B [57], to induce autophagy in human non-small cell lung cancer [58]. The inhibition of PI3K/Akt/mTOR pathway via suppression of PI3K-p110 has been suggested as a mechanism of autophagy induction in these cells. The authors speculated this process to contribute to the cytotoxic activity of stellettin B [58]. However, even though the autophagy-inducing effect was clearly proved, additional experiments are required to clarify the suggested cytotoxic role of the stellettin B-induced autophagy.
2.4. Other Molecules
Yessotoxin (YTX) is a polyether molecule produced by the dinoflagellates Protoceratium reticulatum and Gonyaulax grindleyi [59,60]. YTX has been reported to have a broad spectrum of biological activity while the mechanism of action may vary significantly depending on concentration, time of exposure and the model used. In mammalian cells, its cytotoxic action was reported to be associated with ER and ribotoxic stress [61,62]. In 2016, Korsnes et al. reported activation of autophagy by YTX in mouse brain tumor BC3H1 cells (which also possess some properties of smooth muscle cells) [63]. The authors used several methods, including electron microscopy, IHC and co-treatment with inhibitors, to prove autophagy activation by this natural compound. They speculated on the presence of the potential cross-talk between autophagy initiated by the drug-induced ribotoxic stress and cell death pathways. However, neither the autophagy associated molecular target of YTX, nor the precise effect of the observed autophagy on the viability of the tumor cells was identified [63]. Of note, in 2014 yessotoxin was reported to induce ER-stress-associated cytotoxic autophagy in human glioma cells [61]. Rhizochalinin is a semi-synthetic aglycon of an unusual marine two-headed sphingolipid rhizochalin, which was initially isolated from the marine sponge Rhizochalina incrustata [64,65]. This molecule possesses a wide range of biological activities [66,67,68,69]. Dyshlovoy et al. have reported the inhibitory effect of the rhizochalinin on cytoprotective autophagy in human drug-resistant prostate cancer cells [70]. This effect was confirmed using several methods. The authors postulated that the inhibition of autophagy by this lipid compound contributes to its anticancer effects in vitro and in vivo. It was also confirmed, that inhibition of voltage-gated potassium channels is a direct cellular target of rhizochalinin [70], however, its potential crosstalk with an effect on autophagy is still to be investigated. Later, the same group has reported the synthesis of 18-hydroxy- and 18-aminoderivatives of rhizochalin and rhizochalinin, for which the same effect on LC3-B-I/II expression in the same model was shown [69]. In their former research, the authors, however, have not confirmed the suggested inhibitory effect of the compounds. Nonetheless, it is highly likely that the synthesized derivatives exert the same autophagy inhibitory effect as the original rhizochalinin molecule [69]. The group of Koumbis has synthesized the derivatives of trachycladines, the naturally occurred bioactive nucleosides that were initially found in the marine sponge Trachycladus laevispirulifer [71] and Theonella sp. [72]. The authors have shown the synthesized compounds 1, 7 and 8 to bear anticancer properties and inhibit autophagy in human cervical carcinoma HeLa cells via inhibition of the fusion of autophagosome and lysosomes [73]. As this effect was demonstrated by several independent methods [73], the inhibitory effect trachycladines on autophagy may be considered verified [7,8,30,31].
Luminacins are secondary metabolites of the marine bacteria Streptomyces sp. [74]. Shin et al. have examined the anticancer effect of luminacin in vitro using the model of head and neck squamous cell carcinoma (HNSCC) as well as in vivo using the zebrafish model [75]. In this research, it was reported that luminacin induces autophagy, which was confirmed by several experiments with the compound alone as well as in combination with autophagy inhibitors. Simultaneously, the activation of p38 and JNK MAPK, as well as the inhibition of Akt, were observed. The authors postulated that the induced autophagy has a cytotoxic character and contributes to the compound-induced cell death [75]. However, this statement has not been validated experimentally and therefore, is still to be confirmed, especially as drug-induced autophagy induced in cancer cells may often have a pro-survival character [3,7,8,30,31]. Fucoxanthin was first isolated from the marine algae Fucus sp., Dictyota sp., and Laminaria sp. and later found in other Ochrophyta representatives, including brown algae (Phaeophyceae) and diatoms (Bacillariophyta) (reviewed in [76]). Feng at al. have shown that this marine algal carotenoid inhibits the proliferation of nasopharyngeal carcinoma (NPC) cells [77]. Their research also identified the induction of cytotoxic autophagy in NPC cells under the treatment, which was confirmed by the combined treatment with autophagy inhibitors [77]. This effect was ROS-mediated and no further molecular targets were identified. Another noteworthy report on fucoxanthin bioactivity was published by the group of Wang. The authors described its neuroprotective activity in the traumatic brain injury (TBI) model in vivo and ex vivo [78]. It was suggested that fucoxanthin executes its neuroprotective effect via the activation of Nrf2-ARE and Nrf2-autophagy pathways [78]. The conclusion on fucoxanthin-promoted autophagy in the model of TBI was made based on the formation of LC3 puncta, upregulation of Beclin-1 and LC3-II, and downregulation of p62. Whereas in Nrf2-/- mice, these effects could not be observed [78]. It should be noted that even though the observed signals, especially in in vivo models, are most likely attributed to the autophagy induction, additional experiments (e.g., the combination with late-stage autophagy inhibitors) would be useful to verify the suggested pro-survival nature of autophagy induced. The research by Liao et al. describes an anticancer in vitro and in vivo effects of phycocyanin, a pigment-protein complex belonging to the light-harvesting phycobiliprotein family, in the pancreatic cancer model [79]. Using a number of methods, including siRNA silencing of autophagy-related genes, immunohistochemistry, and DQ-BSA quenching assay, and authors have proved that the mechanism of action of phycocyanin includes induction of both apoptosis and cytotoxic autophagy. Moreover, it was shown that the mechanism of action is represented as a complex cross-talk between MAPK (p38, JNK and ERK), Akt/mTOR/p70S6K, and NF-κB pathways [79].
A good example of research on a marine-derived compound as possible autophagy modulators is the investigation of jaspine B and its 2-alkylaminomethyl derivatives performed by two different groups [80,81]. Jaspine B is an anhydrophytosphingosine that was first found in the marine sponge Pachastrissa sp. [82]. An extensive examination of the effect of this natural compound on gastric cancer cells was performed by Cingolani et al. [81]. The researchers have observed cytotoxic effects of jaspine B coupled with the cancer cell membrane vacuolization, which, however, could not be inhibited by the pan-caspase inhibitor zVAD nor by the autophagy inhibitor wortmannin. Moreover, the accumulated LC3-II-positive structures appeared to be the single-membrane vacuoles, and therefore, could not be identified as double-membrane autophagosomes. Consequently, the detected membrane vacuolization, which ultimately triggered a cytoplasmic disruption, was classified as a result of the autophagy-unrelated micropinocytosis [81]. The more recent research by the group of Jin, performed by Zhang et al., has reported a synthesis of several cytotoxic jaspine B derivatives and suggested these compounds to bear an autophagy-promoting activity in prostate cancer cells [83]. This speculation, however, requires further validation [83]. Nevertheless, in the following research by Yu et al. (Jin group), the authors have applied a number of diverse methods to prove the autophagy-inducing activity of the most promising jaspine B derivative called C-2 in human bladder cancer cells [80]. Thus, the authors successfully demonstrated the importance of JNK and Nrf2 pathways for the C-2-induced autophagy, which seems to be cytoprotective, in bladder cancer cells. Finally, the anticancer and autophagy-inducing activity as well as the mode of action of C-2 were validated in vivo using the nude mice xenografts [80]. The rather contradictive results of these two high-quality pieces of research performed by Cingolani et al. [81] and Yu et al. [80] could be explained by the different models used, namely, gastric [77] and bladder cancer cells [80], as well as by the chemical difference in the original natural jaspine B [81] and its synthetic derivative C-2 [80].
Ratovitski has reported an evaluation of the autophagy-modulatory activity of chromomycin A2 (from marine-derived bacteria Streptomyces sp. [84]), psammaplin A (from marine sponge Psammaplysilla sp. [85]), and ilimaquinone (from marine sponge Hippospongia metachromia [86]) in vitro using the models of human squamous cell carcinoma, glioblastoma, and colorectal carcinoma cells [87]. It was shown that these compounds exhibit cytotoxic activity in the cancer cells and induce autophagy, which seems to contribute to the observed anticancer effects. The induction of autophagy was validated using co-treatment with bafilomycin A1. It was also shown that the transcription of the autophagy-related genes is activated in the treated cells, and this process is regulated by the treatment-induced TP53 family members’ transcriptional activity [87]. Thus, the treatment with chromomycin A2, psammaplin A, and ilimaquinone induced the expression of TP53, TP63 and TP73 as well as phosphorylation of these proteins. At the same time, silencing of TP53, TP63 and TP73 expression resulted in the inhibition of the expression of several autophagy-related genes as well as in the reduction of the drug-induced expression of these genes [87]. He et al. have reported anticancer activity of petromurin C in the model of FLT3-ITD-positive AML in vitro using the cell lines MV4-11 and U937 as well as the toxicity of this compound in vivo in the zebrafish model [88]. Petromurin C is a secondary metabolite of a fungus Aspergillus candidus KUFA0062 (which was in turn isolated from the marine sponge Epipolasis sp. [89]). Initially, petromurin C was isolated from Petromyces muricatus [90]. The authors have detected the accumulation of autophagosome/autolysosome-like structures as well as LC3B-I/II in the treated cancer cells [88]. Combinational treatment with bafilomycin A1 could significantly increase the petromurin C-induced accumulation of LC3B-II suggesting the induction of autophagy by the second compound. On the contrary, the authors have reported bafilomycin A1 to significantly decrease the accumulation of vacuoles [88]. Being an inhibitor of the late autophagy stages, bafilomycin A1 usually leads to the accumulation of autophagosomes as a result of inhibition of their fusion to lysosomes (which, therefore, results in prevention of autolysosome formation and degradation) [91]. Thus, the nature of petromurin C-induced vacuoles and the effect of bafilomycin A1 on their formation should be further studied.
Table 1.
Marine compounds with a validated autophagy-modulatory effect 1.
| Name | Source Organism | Suggested Effect on Autophagy | Effect validated? 1 | Target 2 | Molecular Class | Model | Ref. |
|---|---|---|---|---|---|---|---|
| Alkaloids | |||||||
| Fascaplysin | Marine sponge Fascaplysinopsis sp., and others | Activation of cytoprotective autophagy | Yes | p8 protein; ROS | Alkaloid | Vascular endothelial cells (HUVEC cells) | [38] |
| Isofistularin-3 | Marine sponge Aplysina aerophoba | Activation | Yes | - | Alkaloid | Burkitt’s lymphoma (Raji cells) | [39] |
| Macrocyclic molecules | |||||||
| Coibamide A | Marine cyanobacteria Leptolyngbya sp. | Activation | Yes | VEGFR2 | Cyclic depsipeptide | Human umbilical vein endothelial cells (HUVEC) | [44] |
| Activation of cytotoxic autophagy | Yes | -(was shown that ATG5 is required) | Mouse embryonic fibroblasts (MEF cells) | [45] | |||
| Apratoxin A | Marine cyanobacteria Lyngbya majuscule | Activation | Yes | VEGFR2 | Cyclic depsipeptide | Human umbilical vein endothelial cells (HUVEC) | [44] |
| Activation of cytotoxic autophagy | Yes | -(ATG5 is required, whereas acute ER stress is not important) | Mouse embryonic fibroblasts (MEF cells) | [45] | |||
| Ikarugamycin | Marine bacteria Streptomyces phaeochromogenes | Activation | Yes | ER; CaMKKβ and AMPK pathways; mTORC1; TFEB | Macrocyclic antibiotic | Cervical carcinoma in vitro (HeLa cells); metabolic syndrome in vivo (mice); lifespan in vivo (C. elegans) | [46] |
| Plitidepsin (aka dehydrodidemnin B; Aplidin®) |
Ascidian Aplidium albicans | Inhibition | Yes | eEF1A2; ER stress | Cyclic depsipeptide | Cervical carcinoma (HeLa cells) | [49] |
| 8,9-Dehydroneopeltolide (8,9-DNP) | Marine sponge Neopeltidae sp. (synthetic derivative) | Inhibition of cytoprotective autophagy (at early stages) | Yes | - | Macrolide | Pancreatic cancer (PANC-1 cells) | [51] |
| Triterpenes | |||||||
| Frondoside A | Sea cucumber Cucumaria frondosa | Inhibition of cytoprotective autophagy | Yes | - | Triterpene glycoside | Prostate cancer | [53] |
| Inhibition | No | - | Bladder cancer | [54] | |||
| Inhibition | No | - | Burkitt’s lymphoma | [55] | |||
| Ergosterol peroxide | Marine fungus Phoma sp. | Activation of cytoprotective autophagy | Yes | ERK; JNK; p38; AKT; mTOR and others | Sterol | Lung adenocarcinoma cells (A549 cells) |
[56] |
| Stellettin B | Marine sponge Jaspis stellifera | Activation | Yes | PI3K-p110; PI3K/Akt/mTOR pathway | Isomalabaricane triterpene | Non-small cell lung cancer (A549 cells) |
[58] |
| Another molecules | |||||||
| Yessotoxin | Dinoflagellates Protoceratium reticulatum and Gonyaulax grindleyi |
Activation | Yes | ER- and ribotoxic stress | Polyether | Mouse brain tumor (BC3H1 cells) | [63] |
| Activation of cytotoxic autophagy | Yes | mTOR; BNIP3 | Glioma (SF295, SF539, and SNB75 cells) | [61] | |||
| Rhizochalinin and the derivatives | Marine sponge Rhizochalina incrustata (semisynthetic derivative) | Inhibition | Yes | - | Lipid | Prostate cancer (PC-3 cells) | [69,70] |
| Trachycladines derivatives (Compound 1, 7 and 8) | Marine sponges Trachycladus laevispirulifer and Theonella sp. (synthetic analogue) | Inhibition | Yes | - | Nucleoside | Cervical carcinoma (HeLa cells) | [73] |
| Luminacin | Marine bacteria Streptomyces sp. | Activation | Yes | p38; JNK; Akt | Secondary metabolite | Head and neck squamous cell carcinoma (HNSCC); zebrafish | [75] |
| Fucoxanthin | Various brown algae and diatoms | Activation of cytotoxic autophagy | Yes | ROS | Carotenoid | Nasopharyngeal carcinoma | [77] |
| Activation of cytoprotective autophagy | No | Nrf2 signaling | In vivo traumatic brain injury; primary cultured neuron | [78] | |||
| Phycocyanin | Cyanobacteria (Arthrospira sp. aka Spirulina) | Activation of cytotoxic autophagy | Yes | MAPK, Akt/mTOR/p70S6K and NF-κB pathways | Pigment-protein complex | Pancreatic cancer cells (PANC-1 cells) | [79] |
| Jaspine B | Marine sponge Pachastrissa sp. | No effect (autophagy-unrelated vacuolization of cytoplasm) | Yes | - | Cyclic anhydrophytosphingosine | Gastric Cancer (HGC-27 cells) |
[81] |
| C-2 (2-alkylaminomethyl derivatives of jaspine B) | Marine sponge Pachastrissa sp. (synthetic analogue) | Activation of cytoprotective autophagy | Yes | JNK; Nrf2 pathway | Cyclic anhydrophytosphingosine, 2-alkylaminomethyl derivative | Bladder cancer (BIU87, 5637 and EJ cells) | [80,83] |
| Cromomycin A2 | Marine bacterium Streptomyces sp. | Activation | Yes | TP53 family members (TP53, TP63 and TP73) | Anthraquinone antibiotic glycoside | Squamous cell carcinoma (SCC-11 cells) | [87] |
| Psammaplin A | Marine sponge Psammaplysilla sp. | Bromotyrosine-cystamine conjugate | Glioblastoma (U87-MG cells) | ||||
| Ilimaquinone | Marine sponge Hippospongia metachromia | Prenylquinone; monohydroxy-1,4-benzoquinones | Colon colorectal cancer (RKO cells) | ||||
| Petromurin C | Marine fungus Aspergillus candidus KUFA0062, and others | Activation | Yes | Mitochondrial stress; Mcl-1 | bis-Indolyl benzenoid | Acute myeloid leukemia (AML) (MV4-11 and U937 cells) | [88] |
3. Marine Compounds with a Non-Validated Autophagy-Modulatory Effect
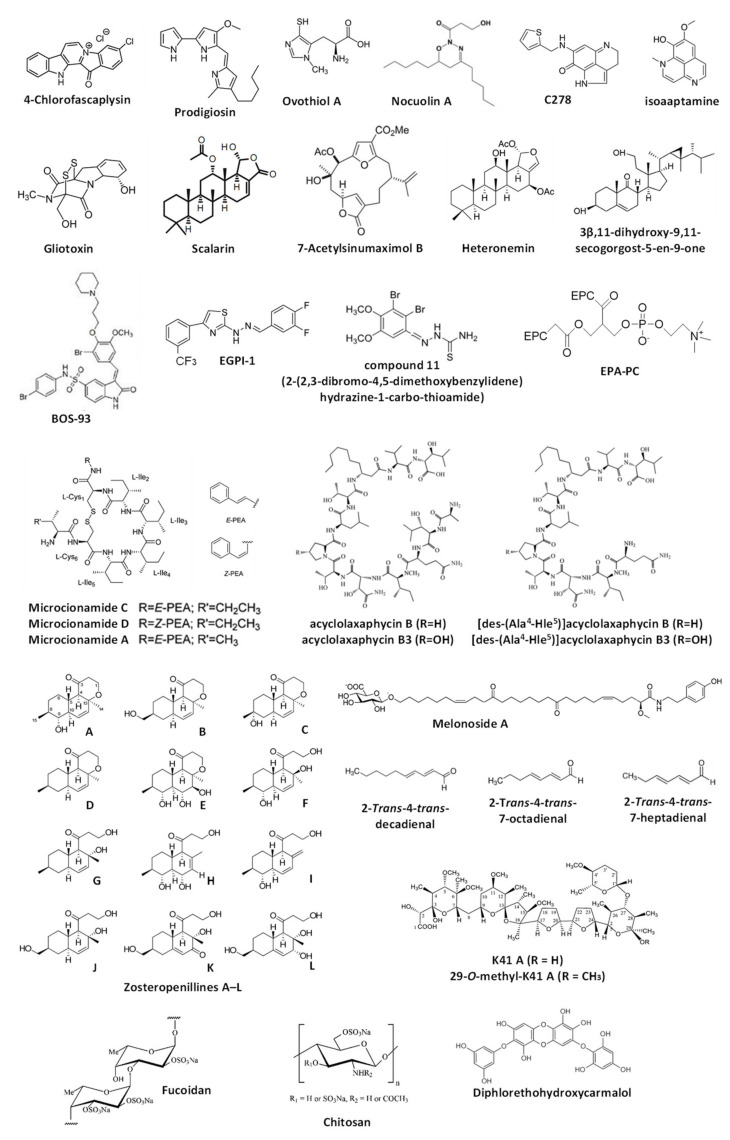
The current chapter overviews the molecules isolated from marine organisms that were reported to modulate autophagy but, according to the Guidelines by Klionsky et al., not yet validated as such [7,8,30,31] (Figure 3). Note, for all these compounds, the alterations of one or several autophagy markers are shown; therefore, these substances can be assumed as autophagy modulators. However, as the detected signals may be assigned to both promoted and suppressed autophagy, the suggested effect (i.e., either autophagy activation or inhibition) cannot be assumed as valid. For the compounds listed below, further experiments should be considered to clarify their effects on autophagy in certain models. The data are summarized in Table 2.
Figure 3.
Marine-derived compounds with non-validated autophagy-modulatory effect.
3.1. Alkaloids
In the previous chapter, the autophagy-inducing activity of the marine alkaloid fascaplysin was reported. Another piece of research by Sharma et al. describes the activity of 4-chlorofascaplysin in human breast cancer cells [92]. The compound could inhibit the PI3K/Akt/mTOR pathway in MDA-MB-231 cells. The authors have suggested induction of cytotoxic autophagy by the drug as an upregulated acridine orange staining as well as elevated LC3-II level were detected in the treated cells [92]. This speculation requires further confirmation since the observed signs could also be attributed to the autophagy inhibition. Moreover, the confirmation of the cytotoxic nature of the induced autophagy, if it exists, requires additional evidence.
Prodigiosin, a cytotoxic alkaloid with antimicrobial and anticancer activities, has been initially isolated from the red pigment of Gram-negative marine bacteria Serratia marcescens [93] and later from the marine Gram-negative γ-proteobacteria Vibrio sp. as well as other species [94,95]. Cheng at al. have described an anticancer activity of prodigiosin in human glioblastoma (GBM) cells and suggested this compound to activate autophagy in GBM cells via the activation of JNK and simultaneous inhibition of AKT/mTOR pathways, which was probably related to the prodigiosin-induced ER stress [96]. As the autophagy inhibitor 3-methyladenine was able to suppress the drug-induced cell death, the authors concluded that the investigated marine-derived compound induces cytotoxic autophagy in cancer cells [96]. At the same time, the observed upregulation of LC3-II and accumulation of LC3-II-positive organelles, which authors have provided as evidence for autophagy induction, may be observed during the autophagy inhibition too [7,8,30,31]. Therefore, additional experiments may be useful to confirm the autophagy-inducing activity of prodigiosin. The same research group has examined the anticancer activity of prodigiosin in the model of oral squamous cell carcinoma in vitro [97]. The authors reported that this compound may induce cytotoxic autophagy in these cells via inhibition of mTOR, Akt, and rpS6 phosphorylation, probably due to the cytotoxicity-induced degradation of the phosphor-forms of these proteins [97]. Moreover, the effect of autophagy inhibitor 3-MA on the cytotoxic activity of the alkaloid was controversial and dependent on its concentration. As no additional validation experiments were performed, and the observed results could indicate both induction and inhibition of autophagy (which could be either cytotoxic or cytoprotective), further examination is needed to confirm the suggested effects of prodigiosin on autophagy.
Ovothiol A is a 1-N-methyl-4-mercaptohistidine that has been isolated from eggs of the sea urchin Paracentrotus lividus as well as from other marine invertebrates (sea stars, cephalopods) [98]. Brancaccio et al. have reported this compound to inhibit the activity of γ-glutamyl transpeptidase in human cancer cells [99]. Based on the observed LC3-II upregulation and inhibitory effect of such autophagy inhibitors bafilomycin A1 and 3-methyladenin on the cytotoxic activity of ovothiol A, the authors concluded that this marine compound activates cytotoxic autophagy [99]. However, as the accumulation of LC3-II may also be observed during the inhibition of autophagy at the late stages, the conclusions made in this manuscript await additional confirmations. The group of Urbatzka reported an antiproliferative effect of marine natural oxadiazine nocuolin A (isolated from cyanobacteria Nodularia sp. LEGE 06071) on human colon cancer HCT116 cells [100]. Among others, the authors have reported mitochondria targeting, which results in mitochondrial oxidative phosphorylation (OxPhos) impairment. Additionally, the observed accumulation of LC3B-II and of autophagosome-like structures led to the preliminary conclusion on nocuolin A-induced autophagy induction. However, this should be confirmed by further experiments that will exclude the possible autophagy-inhibitory effect of nocuolin A, stipulated by the same signs. Natural pyrroloiminoquinone alkaloids makaluvamines were found in different marine sponges, i.e., Zyzzya cf. marsailis, Histodermella sp., Zyzzya fuliginosa, Smenospongia aurea and others (reviewed in [101]). Cowan et al. have reported the cytotoxic activity of synthetic makaluvamine derivative C278 in non-melanoma skin cancer SCC13 cells [102]. Moreover, the authors have reported C278 to induce the expression of Beclin-1 [102]. Based on this result, the authors have suggested this alkaloid to induce autophagy in cancer cells. As no other experiments have been performed, this suggestion awaits thorough verification. Wu et al. have studied the activity of isoaaptamine in human breast cancer T-47D cells [103]. This and related alkaloids were isolated from marine sponges mainly belonging to Aaptos genus [104,105,106]. The compound induces ER stress, ROS production and disruption of mitochondrial membrane potential (Δψm) [103]. Based on observed regulation of several autophagy-related proteins and accumulation of autophagosome-like structures, the authors suggested the activation of autophagy in the treated cells [103]. Thus, an upregulation of LC3B-II and p62/SQSTM1, as well as downregulation of total mTOR, was detected. At the same time, the authors did not report an alteration of phospho-mTOR. As these signs may be attributed to both activation and inhibition of late stages of autophagy, additional experiments would be required to clarify the effect of isoaaptamine on this basic cellular process. Park and colleagues have investigated the effect of gliotoxin, a secondary metabolite of marine fungus Aspergillus fumigatus (initially isolated from Trichoderrna lignosum [107]), in combination with paclitaxel [108]. The authors used paclitaxel-resistant ovarian cancer cells, which were consequently treated with gliotoxin and paclitaxel (GTX→PTX). This treatment led to the activation of DAPK1/TAp63 signaling [108]. Furthermore, the upregulation of LC3B-I/II levels together with increased apoptosis stipulated by the loss of mitochondria membrane potential (Δψm) were detected in both CaOV3/PTX_R and SKOV3/PTX_R cells exposed to GTX→PTX. Of note, these effects could be abolished by the pre-treatment with autophagy inhibitor 3-methyladenine. As a result, the authors have suggested that gliotoxin may enhance the cytotoxic autophagy in ovarian cancer cells. However, the speculation on autophagy activation by gliotoxin is still to be validated.
3.2. Terpenes and Similar Compounds
Scalarin is a bioactive metabolite initially isolated from the marine sponge Spongia nitens [109] and later from the sponge Euryspongia cf. rosea [110]. Guznam et al. have shown this compound to be active in human pancreatic cancer cells and able to inhibit the Receptor for Advanced Glycation End products (RAGE) [110]. The authors suggested that this effect leads to autophagy inhibition, as an accumulation of LC3-II in the scalarin-treated cells was detected. As no further evidence is presented, the experiments to validate the suggested effect are necessary. 7-acetylsinumaximol B is a cembranoid (belonging to diterpenes) that was isolated by Tsai et al. from the marine cultured soft coral Sinularia sandensis in 2015 [111]. Later, the same group examined the anti-cancer activity of this natural compound in vitro in the human gastric carcinoma model and reported the induction of apoptosis and autophagy in NCI-N87 cells [112]. The suggested mechanism was reported as the induction of mitochondria dysfunction and activation of the PERK/eIF2/ATF4/CHOP signaling [112]. However, the conclusion about autophagy induction was made solely based on the observed increased expression of LC3-I and LC3-II along with other proteins belonging to the Atg family. Therefore, the effect on autophagy (activation vs. inhibition) cannot be considered validated.
Lee et al. have studied the effect of sesterterpenoid metabolite heteronemin, isolated from marine sponge Hyrtios sp. [113], in human prostate cancer LNCaP cells [114]. Apart from the anticancer activity in vitro and in vivo, which was related to oxidative stress, ER-stress, topoisomerase II and Hsp90 inhibition, the researchers observed the upregulation of cellular LC3B-II under the treatment and suggested activation of cytoprotective autophagy by the drug [114]. Even though the co-treatment with autophagy inhibitors 3-methyladenine and chloroquine increased the cytotoxic effects of the heteronemin in the cells, future additional experiments are essential to confirm the autophagy-inducing activity of the studied sesterterpenoid. The group of Bai and Sheu reported the activation of PPARγ and promotion of autophagy in human breast cancer MCF-7 cells treated with 3β,11-dihydroxy-9,11-secogorgost-5-en-9-one [115], a stetol isolated from soft coral Klyxum flaccidum [116]. The authors showed a time-dependent upregulation of LC3B-II and p62/SQSTM1 in the treated cells (which could be inhibited by co-treatment with 3-methyladenine), accumulation of autophagosome-like structures, and a pro-survival effect of 3-methyladenine when applied together with the investigated stetol compound [115]. Despite the well-planned research, it should be noted that the detected signals may be attributed to either induction or inhibition of autophagy at its late stages. Consequently, additional experiments are required to clarify the effect of 3β,11-dihydroxy-9,11-secogorgost-5-en-9-one.
3.3. Bromphenols
Bromophenols are a small unique group of compounds mainly found in marine algae. They have also been detected in marine fungi, sponges, ascidians, and bryozoans. They possess various biological activities including the anti-diabetic, anticancer, antioxidant, antimicrobial, and others (reviewed in [117]). The group of Shi have designed and synthesized the novel bromophenol−thiosemicarbazone hybrid molecules, and shown its potent activity as selective PARP-1 inhibitors [118]. The authors indicated one of the synthesized compounds, 2-(2,3-dibromo-4,5-dimethoxybenzylidene)hydrazine-1-carbo-thioamide (compound 11), to induce autophagy in human ovarian cancer SK-OV-3 cells. Thus, the authors showed an accumulation of autophagosomes in the drug-treated cells using several appropriate methods [118]. However, the elevated number of autophagosomes/autolysosomes may indicate both the activation and inhibition of autophagy depending on the stimuli and model used [7,8,30,31]. For this reason, further elucidation would be required to confirm the suggestion [7,8,30,31]. The same group has also reported the design and synthesis of another bromophenol–thiazolylhydrazone hybrid molecule, which could inhibit the interaction of translation initiation factors eIF4E and eIF4G, upregulate ROS as well as inhibit the mitochondrial function via mTOR/4EBP1 pathway [119]. For the most promising molecule, namely EGPI-1 (where, in fact, all the bromine atoms were substituted), the authors reported an elevated level of LC-II along with LC3-positive structures in the lung carcinoma A549 cells exposed to this drug. Therefore, the activation of autophagy by EGPI-1 was postulated. At the same time, as has already been mentioned above, additional experiments would be required to prove that the activation of autophagy is indeed taking place. One more piece of research from the same authors reports the synthesis of the BOS-93 (3-(3-bromo-5-methoxy-4-(3-(piperidin-1-yl)propoxy)benzylidene)-N-(4-bromophenyl)-2-oxoindoline-5-sulfonamide), a novel bromophenol derivative [120]. Its anticancer activity was tested in vitro and in vivo in human lung cancer A549 cells [121]. The authors have observed an accumulation of LC3-positive structures in the BOS-93-treated cancer cells as well as LC3-II accumulation, which could be inhibited by co-treatment with 3-methylademine. Based on this, the authors suggested BOS-93 to induce autophagy of the cells [121]. In line with this, the inactivation of PI3K/Akt/mTOR pathway was observed [121]. It is rather likely that BOS-93, being a cytotoxic compound, induces the cytoprotective autophagy in cancer cells. However, it should be noted that similar signs (the accumulation of LC3-positive formations, which could be suppressed by the early-stage autophagy inhibitors) can also be observed under the autophagy-inhibitory conditions. Therefore, additional experiments would be of use to confirm the suggestions of Guo et al.
3.4. Peptides
The two new cyclic cystine-bridged peptides, namely, microcionamides C and D, and the previously known microcionamide A were isolated from the marine sponge Clathria basilana by Mokhlesi et al. [122]. The compounds bear an anticancer activity and similar to another macrolide bafilomycin A1 induce autophagosome accumulation in murine embryonic fibroblast (MEF) cells under starvation conditions in a flow-cytometry based experiment. Based on this, an autophagy-inhibitory activity has been suggested for these cyclic peptide molecules. On the other hand, the observed signs could result from both activation and inhibition of autophagy and therefore, require further detailed investigation [7,8,30,31]. The group of Auvray reported an anticancer activity of the peptides K092A (NFDTDEQALEDVFSKYG) and K092B (EAPPEAAEEDEW) [123] isolated from the dogfish Scyliorhinus canicula L. and pE-K092D—a pyroglutamate-modified peptide derived from S. canicula peptide K092D (QLTPEALADEEEMNALAAR) [124]. These molecules were active in human prostate cancer cells [123,124]. Thus, the treatment of the prostate cancer MDA-PCa-2b cells resulted in the decreased red fluorescence of the cells when stained with acridine orange. Based on this, the authors have concluded that the peptides inhibit autophagy in the cancer cells [123,124]. However, this suggestion requires thorough verification, as acridine orange stains acidic organelles (e.g., lysosomes, autolysosomes) and does not specifically stain autophagosomes [7,8,30,31].
Alvariño et al. have reported the isolation of four acyclic peptides—acyclolaxaphycin B and acyclolaxaphycin B3 (originally found in the marine cyanobacteria Anabaena torulosa [125]), as well as [des-(Ala4-Hle5)]acyclolaxaphycin B and [des-(Ala4-Hle5)]acyclolaxaphycin B3—from herbivorous gastropod Stylocheilus striatus [126]. The authors examined their activity in human neuroblastoma SH-SY5Y cells. It was shown that these acyclic peptides initiate the activation of AMPK, expression of Beclin-1, conversion of LC3-I into LC3-II, as well as the downregulation of p62, inactivation of mTOR, and partial inactivation of p70S6. Thus, the authors suggested that these four acyclic peptides may activate autophagy in human cancer cells. As no further investigation has been performed, this speculation awaits additional experiments. The group of Nam has examined the activity of the peptide PYP15 (DPKGKQQAIHVAPSF) isolated from the alga Pyropia yezoensis [127] using the mouse skeletal muscle atrophy model (C2C12 cells) [128]. Previously, it was shown that the extract of this alga is capable of inhibition of dexamethasone-induced lipidation of LC3B-I to LC3B-II in the same model (see Section 4) [129]. The authors reported a similar effect of PYP15 on LC3B-I/II and on cathepsin L expression [128]. As no other experiments have been performed in both studies, additional data confirming the autophagy-inhibitory activity of PYP15 are needed.
3.5. Lipids
Eicosapentaenoic acid (EPA) is one of the natural products commonly found in edible marine organisms, such as antarctic krill, sea cucumber, and fish oil [130]. It primarily exists in phosphatidylcholine-conjugated forms [130]. Wen et al. have examined an effect of eicosapentaenoic acid-enriched phosphatidylcholine (EPA-PC) on Aβ1-42-induced neurotoxicity in vivo using the Alzheimer’s disease rat model [131]. The authors suggested that EPA-PC enhances autophagy in neuronal cells, therefore, attenuating Aβ1-42-induced neurotoxicity. This conclusion was made solely based on the results of the Western blotting-based examination of autophagy-related protein expression in the rat hippocampus and, therefore, awaits further experimental validation [131]. However, it should be noted that earlier studies have reported the autophagy inducing activity of EPA in several human cancer and non-cancer cells, which was, in the majority of cases, reported to be cytoprotective [132,133,134,135]. In 2016, Guzii et al. announced the isolation of an ω-glycosylated fatty acid amide melonoside A from the marine sponge Melonanchora kobjakovae [136]. The authors reported the induction of autophagy in human cisplatin-resistant germinal tumor (GCT) NCCIT-R cells. This conclusion was made based on the observed downregulation of LC3B-II and SQSTM1/p62 proteins in the treated cells [136], which often reflects the acceleration of the autophagosome turnover, i.e., increased autophagy [7,8,30,31]. However, further experiments are expected to clarify the effect of melonoside A on autophagy in mammalian cells. Galasso and colleagues have analyzed the effect of polyunsaturated aldehydes (PUAs), produced by different diatoms (reviewed in [137,138]), on the activation of several autophagy-related genes in the embryos of the sea urchin Paracentrotus lividus, as well as human lung cancer A549 cells [139]. Thus, the effects of 2-trans-4-trans-decadienal, 2-trans-4-trans-7-octadienal, and 2-trans-4-trans-7-heptadienal were examined [139]. The authors identified the upregulation of the expression of three autophagy-related genes, namely ULK1/2, ULK3, and PINK, in both models under the treatment. However, this upregulation appeared to be significant only in the cells treated with 2-trans-4-trans-7-heptadienal [139]. The precise effect of these compounds on autophagy in current as well as in other models is to be further clarified.
3.6. Lectins
Li et al. have inserted the expression cassette the Ulva pertusa lectin 1 (UPL1) in the adenovirus genome, and delivered it to the liver cancer BEL-7404 and Huh7 cells via infection [140]. Consequently, an expressed UPL1 affected several signaling pathways in the cells, including autophagy. It was observed that the exogenous UPL1 could inhibit Beclin1 and induce LC3-II expression in the cells; therefore, the authors suggested UPL1 for the enhancement of starvation-induced autophagy [140]. This speculation, however, requires further examination as no extra experiments have been performed. Do Nascimento-Neto et al. have examined an anticancer activity of halilectin-3, a lectin isolated from the marine sponge Haliclona caerulea [141], in human breast cancer MCF7 cells [142]. Among others, they have also reported the halilectin-3-induced accumulation of the acidic vesicles (presumably, autophagosomes) and LC3-II, and thus suggested the activation of autophagy in MCF7 cells [142]. Yet, as the observed signs may also be assigned to autophagy inhibition, the conclusion made by the authors requires further validation [7,8,30,31].
3.7. Polysaccharides
It has been reported that 3,6-O-sulfated chitosan, which was derived from marine shrimp shells, is able to inhibit human papillomavirus (HPV) infection of HeLa cells via inhibition of PI3K/Akt/mTOR pathway [143]. Thus, Gao et al. suggested that 3,6-O-sulfated chitosan may activate autophagy (as some autophagy activators act via the same mechanism), which also contributes to the HPV infection suppression. However, no further experiments were performed. Hence, this speculation cannot be assumed to be validated [143]. Fucoidans are sulfated polysaccharides from brown algae containing mainly α-L-fucopyranose residues [144,145]. The group of Liu has investigated the hepatoprotective activity of this natural polymer isolated from Fucus vesiculosus in the in vivo model of CCl4- and bile duct ligation (BDL)-induced liver fibrosis [146]. The authors postulated that fucoidan executes its effect via TGF-β1/Smad pathway-mediated inhibition of cytotoxic autophagy and extracellular matrix formation. This conclusion was made based on the examination of LC3-I/II, SQSTM/p62 and Beclin-1 levels in the mice liver tissue [146]. Depending on the stimuli and model, the reported alteration of the protein levels could be attributed to both induction and inhibition of autophagy. Therefore, further experiments may be of use to clarify the observed modulatory effect of fucoidan on autophagy in the liver cells as well as the type of affected autophagy (cytoprotective/cytotoxic).
3.8. Other Metabolites
Khan et al. have reported the isolation of three polyether antibiotics from the marine actinobacterium Streptomyces cacao, namely, arenaric acid, K41 A, and 29-O-methyl-K41 A [147]. These compounds possess anticancer properties which were demonstrated using human cervical carcinoma (HeLa), human prostate cancer (PC-3), human lung adenocarcinoma (A549), and human colorectal adenocarcinoma (CaCo-2) cells [147]. The effect of two of the isolated compounds on autophagy in cancer cell lines was also examined. The authors postulated that the isolated polyether antibiotics can inhibit autophagy. At the same time, several contradictions were observed and reported in this research article, in particular, the treatment-induced degradation of GFP-LC3 [7,8,30,31]. This phenomenon may be a sign of the drug-promoted autophagy, or (taking in account the downregulation of other proteins in the cells exposed to the cytotoxic drug concentrations) an unspecific effect of the cell death-related processes [7,8,30,31]. Therefore, further experiments resolving the observed contradictions are needed. Afiyatullov, Leshchenko et al. have reported the isolation of new polyketides zosteropenillines A–L from the marine-derived fungus Penicillium thomii [148]. The compounds were reported to be non-cytotoxic towards cancer cells, however, based on the observed upregulated level of SQSTM1/p62 in the treated cells, the authors suggested that the compounds are capable of autophagy inhibition [148]. As no other experiments have been performed, this suggestion awaits further validation. The group of Hyun has shown the phlorotannin diphlorethohydroxycarmalol (DPHC), isolated from edible seaweed Ishige okamurae [149], to protect skin cells from the fine particulate matter (PM)-induced damage caused by the PM-mediated ROS generation, DNA damage, and ER-stress [150]. This research was performed using a human non-cancer keratinocyte HaCaT cell line. Additionally, it was shown that DPHC can reduce PM-induced red acridine orange staining and LC3B-II level in the cells. Therefore, the authors suggested that DPHC inhibits PM-induced autophagy, which was probably due to the antioxidant activity of this compound, leading to a general reduction of the ROS-related cellular stress [150]. However, these statements await further examination.
Table 2.
Marine compounds with non-validated autophagy-modulatory effect 1.
| Name | Source Organism | Suggested Effect on Autophagy | Effect Validated? 1 | Target 2 | Molecular Class | Model | Ref. |
|---|---|---|---|---|---|---|---|
| Alkaloids | |||||||
| 4-Chlorofascaplysin | Marine sponge Fascaplysinopsis sp., and others (synthetic derivatives) | Activation | No | PI3K/Akt/mTOR | Alkaloid | Breast cancer (MDA-MB-231 cells) | [92] |
| Prodigiosin | Marine bacteria Vibrio sp. | Activation | No | JNK; AKT/mTOR; CHOP; ER stress | Alkaloid | Glioblastoma (GBM) (U87MG and GBM8401 cells) | [96] |
| Bacteria Serratia marcescens | Activation of cytotoxic autophagy | No | mTOR, Akt, and rpS6 | Oral squamous carcinoma (SAS and OECM1 cells) | [97] | ||
| Ovothiol A | Sea urchin Paracentrotus lividus | Activation | No | γ-Glutamyl transpeptidase (GGT) | Alkaloid | Leukemia (HG3 cells) | [99] |
| Nocuolin A | Cyanobacteria Nodularia sp. LEGE 06071 | Activation | No | Mitochondria; Oxidative phosphorylation | Oxadiazine alkaloid | Colon cancer (HCT116 cells) | [100] |
| C278 (synthetic analog of makaluvamines) | Marine sponges Zyzzya spp. and others (synthetic derivative) | Activation | No | - | Pyrroloiminoquinone alkaloid | Non-melanoma skin cancer (SCC13 cells) | [102] |
| Isoaaptamine | Marine sponges Aaptos spp. and others | Activation | No | mTOR; ER stress; ROS; MMP | Alkaloid | Breast cancer (T-47D cells) | [103] |
| Gliotoxin | Marine fungus Aspergillus fumigatus | Activation of cytotoxic autophagy | No | DAPK1/TAp63 signaling | Alkaloid | Paclitaxel-resistant ovarian cancer (CaOV3/PTX_R and SKOV3/PTX_R cells) | [108] |
| Terpenes and similar compounds | |||||||
| Scalarin | Marine sponge Euryspongia cf. rosea and others | Inhibition | No | Receptor for advanced glycation end products (RAGE) | Sesterterpene | Pancreatic cancer (PANC-1 and MIA PaCa-2 cells) | [110] |
| 7-Acetylsinumaximol B | Soft coral Sinularia sandensis | Activation | No | Mitochondria dysfunction; PERK/eIF2/ATF4/CHOP signaling | Diterpene | Gastric cancer (NCI-N87 cells) | [112] |
| Heteronemin | Marine sponge Hyrtios sp. | Activation of cytoprotective autophagy | No | Oxidative and ER stress | Sesterterpenoid | Prostate cancer (LNCaP cells) | [114] |
| 3β,11-Dihydroxy-9,11-secogorgost-5-en-9-one | Soft coral Klyxum flaccidum | Activation of cytotoxic autophagy | No | PPARγ; ROS | Stetol | Breast cancer (MCF-7 cells) | [115] |
| Bromophenols | |||||||
| Bromophenol derivative (compound 11) | Various marine algae (synthetic analogue) | Activation | No | - | Bromophenol-thiosemicarbazone hybrid | Ovarian cancer (SK-OV-3 cells) | [118] |
| EGPI-1 | Various marine algae (synthetic analogue) | Activation | No | eIF4E/eIF4G; mTOR/4EBP1 pathway; ROS | Bromophenol-thiosemicarbazone hybrid | Lung carcinoma (A549 cells) | [119] |
| BOS-93 | Various marine algae (synthetic analogue) | Activation | No | PI3K/Akt/mTOR pathway; MAPK | Bromophenol derivative | Lung carcinoma (A549 cells) | [121] |
| Peptides | |||||||
| Microcionamide A | Marine sponge Clathria basilana | Inhibition | No | - | Cyclic peptide | Murine embryonic fibroblasts (MEF cells) | [122] |
| Microcionamide C | |||||||
| Microcionamide D | |||||||
| K092A and K092B | Dogfish Scyliorhinus canicula L. | Inhibition | No | - | Peptide | Prostate cancer (MDA-PCa 2b cells) | [123] |
| pE-K092D | Dogfish Scyliorhinus canicula L. (pyroglutamate modification of K092D peptide) | [124] | |||||
| acyclolaxaphycin B | Cyanobacteria Anabaena torulosa | Activation | No | Mitochondria; ROS; mTOR; AMPK; p70S6 | Peptide (acyclic B-type laxaphycins) |
Neuroblastoma (SH-SY5Y cells) | [126] |
| acyclolaxaphycin B3 | |||||||
| [des-(Ala4-Hle5)]acyclolaxaphycin B | Gastropod Stylocheilus striatus. | ||||||
| [des-(Ala4-Hle5)]acyclolaxaphycin B3 | |||||||
| PYP15 | Marine alga Pyropia yezoensis | Inhibition | No | IGF-IR; Akt/mTOR | peptide | Mouse skeletal muscle cells (C2C12 cells) | [128] |
| Lipids | |||||||
| Eicosapentaenoic acid-enriched phosphatidylcholine (EPA-PC) | Fish oil, antarctic krill, sea cucumbers | Activation | No | - | Lipid | Aβ1-42-induced neurotoxicity in vivo (rats) | [131] |
| Melonoside A | Marine sponge Melonanchora kobjakovae | Activation | No | - | ω-Glycosylated fatty acid amide | Germ cell tumor (GCT) (NCCIT-R cells) | [136] |
| 2-trans-4-trans-decadienal | Different diatoms | Activation | No | - | Polyunsaturated aldehydes | Sea urchin embrios Paracentrotus lividus; lung cancer (A549 cells) | [139] |
| 2-trans-4-trans-7-octadienal | |||||||
| 2-trans-4-trans-7-heptadienal | |||||||
| Lectins | |||||||
| Ulva pertusa lectin 1 (the expression cassette the lectin integrated in the adenovirus genome) | Marine alga Ulva pertusa | Activation | No | - | Lectin | Liver cancer (BEL-7404 and Huh7 cells) |
[140] |
| Halilectin-3 | Marine sponge Haliclona caerulea | Activation | No | - | Lectin | Breast cancer (MCF7 cells) | [142] |
| Polysaccharides | |||||||
| 3,6-O-sulfated chitosan | Marine shrimps | Inhibition | No | PI3K/Akt/mTOR pathway | Sulfated polysaccharide | Cervical carcinoma (HeLa cells) | [143] |
| Fucoidan | Fucus vesiculosus and other brown algae | Inhibition of cytotoxic CCl4-induced autophagy | No | TGF-β1/Smad pathway | Sulfated polysaccharide | In vivo CCl4- and BDL-induced liver fibrosis | [146] |
| Other metabolites | |||||||
| K41 A | Marine actinobacterium Streptomyces cacao | Inhibition (contradictive results are reported) | No (contradictive results are reported) | - | Polyether antibiotic | Cervical cancer (HeLa cells); prostate cancer (PC-3 cells); colorectal cancer (CaCo-2 cells) | [147] |
| 29-O-methyl-K41 A | - | ||||||
| Zosteropenillines A–L | Marine-derived fungus Penicillium thomii | Inhibition | No | - | Polyketide | Prostate cancer (PC-3 cells) | [148] |
| Diphlorethohydroxycarmalol (DPHC) | Marine alga Ishige okamurae | Inhibition of the particulate matter-induced autophagy | No | - | Polyphenol | Non-cancer keratinocytes (HaCaT cells) | [150] |
4. Autophagy-Modulatory Effect of the Compounds with an Undefined Structure, or of the Compounds Mixtures
This section reports an autophagy-modulatory effect of the marine-derived compounds with an undefined structure or of the compound mixtures. Specifically, the activity of extracts of the marine organisms, or of their fractions, is discussed. Note, none of the specific effects of the extracts and fractions on autophagy described below can be considered as validated (according to the Guidelines by Klionsky et al. [7,8,30,31]). Therefore, further investigation of the active molecules as well as their autophagy-modulating activity is required. The data are summarized in Table 3.
Choi et al. have suggested that the methanol/dichloromethane extract of marine sponge Lipastrotethya sp. contains compounds capable of autophagy induction in p53-deficient human colon cancer HCT116 p53 KO cells [151]. However, this suggestion was made exclusively based on the observed upregulation of LC3-II protein in the treated cells and, therefore, cannot be assumed as relevant evidence [7,8,30,31]. The group of Park has reported an extract of the marine sponge Agelas sp. to induce autophagy in human hepatocellular carcinoma cells [152]. This conclusion was made based solely on the altered expression of several autophagy-related proteins in the cells treated with the sponge extract. Hence, further validating experiments are required. Yu et al. have described a strain of marine bacteria Streptomyces sp. U3 isolated from mangrove [153]. The crude extract of this bacteria possesses an algicidal activity and could inhibit a growth of a harmful marine alga Heterosigma akashiwo. However, the active compounds were not isolated and identified. Based on electron microscopy data, the authors suggested that the compounds presented in the crude extract of Streptomyces sp. U3 could induce autophagy of the algal cells [153]. As no further validation experiments were performed, this speculation awaits further verification.
Castro-Carvalho et al. have reported the extracts of two marine-derived fungi, namely, Neosartorya tsunodae KUFC 9213 and Neosartorya laciniosa KUFC 7896 to induce autophagy in non-small-cell lung cancer cells A459 when combined with doxorubicin [154]. This conclusion was made exclusively based on the observation of an increased number of autophagosome-like vesicles in the treated cells and requires extensive validation and verification. No individual compounds responsible for the suggested activity of the extracts were identified. Leri et al. have described the ability of extract of the seagrass Posidonia oceanica (L.) Delile to modulate (induce) autophagy in human fibrosarcoma HT1080 cells [155]. This conclusion was made based on the increased autophagosome number in the treated cells. Hence, further careful examination of the effect on autophagy along with an active compound identification is required. The group of Nam investigated the effect of crude protein extract of an edible alga Pyropia yezoensis on the dexamethasone-induced skeletal muscle atrophy (myotube atrophy) using the model of mouse skeletal muscle C2C12 cells [129]. It was reported that the P. yezoensis crude protein extract can inhibit dexamethasone-induced conversion (lipidation) of LC3B-I into LC3B-II. No further validation experiments have been performed. Of note, later, the same group examined the activity of the individual peptide PYP15 isolated from this alga [127] (see Section 3.4.). Galasso and colleagues reported the isolation of a glycoprotein-containing fraction from the phenol-water extract of marine dinoflagellate Alexandrium minutum [156]. The isolated fraction was capable of selective killing of human lung adenocarcinoma A549 cells and induced the upregulation of several autophagy- and mitophagy-related genes. Thus, the authors suggested the activation of mitophagy in the treated cells [156]. However, further analysis of the relevant protein expression is required to validate the above-described speculation.
Table 3.
Autophagy-modulatory effect of the marine-derived compounds with undefined structure, or of the compounds mixtures 1.
| Name | Source organism | Suggested effect on autophagy | Effect validated? 1 | Target 2 | Molecular Class | Model | Ref. |
|---|---|---|---|---|---|---|---|
| Glycoprotein-containing fraction from Alexandrium minutum | Marine dinoflagellate Alexandrium minutum | Activation of mitophagy | No | - | Glycoprotein (?) | Lung adenocarcinoma (A549 cells) | [156] |
| Pyropia yezoensis crude protein extract | Marine alga Pyropia yezoensis | Inhibition | No | - | - | Mouse skeletal muscle cells (C2C12 cells) | [129] |
| Extract of Posidonia oceanica (L.) Delile | Seagrass Posidonia oceanica (L.) Delile | Activation | No | - | - | Fibrosarcoma (HT1080 cells) | [155] |
| Extract of Neosartorya tsunodae KUFC 9213 | Marine fungi Neosartorya tsunodae KUFC 9213 | Activation | No | - | - | Nonsmall cell lung cancer (A459 cells) | [154] |
| Extract of Neosartorya laciniosa KUFC 7896 | Marine fungi Neosartorya laciniosa KUFC 7896 | ||||||
| Extract of Streptomyces sp. U3 | Marine bacteria Streptomyces sp. U3/mangrove | Activation | No | - | - | Marine alga Heterosigma akashiwo | [153] |
| Extract of Agelas sp. | Marine sponge Agelas sp. | Activation | No | ER stress, ROS; IRE1α; CHOP; ATF4; JNK | - | Hepatocellular carcinoma (Hep3B cells) |
[152] |
| Extract of Lipastrotethya sp. | Marine sponge Lipastrotethya sp. | Activation | No | - | - | Colon cancer (HCT116 p53 KO cells) | [151] |
5. Concluding Remarks
The current review covers the scientific literature related to the Blue-print autophagy topic published between the beginning of 2016 and August 2020. Over this time, 62 marine-derived compounds have been reported to possess an autophagy-modulatory activity. It can be postulated that all these molecules indeed affect autophagy, as for the vast majority of them, an alteration of the established autophagy markers LC3-II and SQSTM1 was observed. However, for 59% of the substances (38/62), the suggested activity, i.e., activation or inhibition, has not been properly validated and, therefore, unfortunately, cannot be assumed. This is a highly important observation, which shall be considered as a warning and should promote a careful “double-check” of the already published data before they can be used as a basement for further hypotheses. It is very likely that a similar situation might appear in the adjacent fields of science, which not only deal with naturally derived substances but also with synthetic autophagy modulators.
Cancer cell lines are the model that was almost exclusively used in the studies reviewed in the current manuscript. Therefore, it is rather likely that a significant proportion of the molecules bearing cytotoxic properties and described above induces cytoprotective autophagy as a “side effect” of its cytotoxic action on cancer cells. Nevertheless, this speculation requires extensive validation in every single case. On the other hand, the ability to inhibit autophagy or to induce cytotoxic autophagy is also rather frequently reported—i.e., 25% (6/24) and 21% (5/24), correspondently, referring to the group of autophagy modulators with validated effects (Table 1).
Marine sponges were identified as the main source of the active compounds, reviewed in the current manuscript (29%, 18/62; Table 1 and Table 2). These animals are known to produce a variety of bioactive molecules often bearing unique chemical structure and possessing pronounced biological activities [14,157]. 16% (10/62) of the compounds were obtained from marine bacteria and 6.5% (4/62) from marine-derived fungi. Surprisingly, a significant proportion of the compounds, namely 14% (9/62), was obtained from alga. Chemically, the compounds could be assigned to the various chemical classes; most of them belong to alkaloids, macrocyclic molecules, triterpenes, and peptides.
Finally, it should be noted that, in recent years, it has become clear that autophagy does interact with basically every biochemical process in the living cells [9]. Therefore, one should expect that the drugs affecting any other biological targets, and especially cytotoxic molecules, will also directly or non-directly affect autophagy. Hence, it is highly likely that the “autophagy-modulating activity” can actually be assigned to the most (if not all) of biologically active natural and synthetic compounds. In other words, the only reason why the autophagy-modulatory activity has not been reported so far for the majority of bioactive molecules is that it has not been examined yet.
Acknowledgments
The author would like to thank Viktoriya Levitskaya (Queen Mary University of London) for English proofreading and editing.
Funding
This research was supported by RFBR grant number 20-04-00089.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Mizushima N., Komatsu M. Autophagy: Renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Yang Z.J., Chee C.E., Huang S., Sinicrope F.A. The role of autophagy in cancer: Therapeutic implications. Mol. Cancer Ther. 2011;10:1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathew R., Karantza-Wadsworth V., White E. Role of autophagy in cancer. Nat. Rev. Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Y., Ren X., Hait W.N., Yang J.M. Therapeutic targeting of autophagy in disease: Biology and pharmacology. Pharmacol. Rev. 2013;65:1162–1197. doi: 10.1124/pr.112.007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubinsztein D.C., Frake R.A. Yoshinori Ohsumi’s Nobel Prize for mechanisms of autophagy: From basic yeast biology to therapeutic potential. J. R. Coll. Physicians Edinb. 2016;46:228–233. doi: 10.4997/jrcpe.2016.403. [DOI] [PubMed] [Google Scholar]
- 6.Gewirtz D.A. The four faces of autophagy: Implications for cancer therapy. Cancer Res. 2014;74:647–651. doi: 10.1158/0008-5472.CAN-13-2966. [DOI] [PubMed] [Google Scholar]
- 7.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A., Adachi H., Adams C.M., Adams P.D., Adeli K., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klionsky D.J., Abdel-Aziz A.K., Abel S., Adamopoulos I.E., Adolph T., Agnello M., Agostinis P., Aits S., Aizawa S., Al-Abd A.M., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4rd edition) Autophagy. 2020 in press. [Google Scholar]
- 9.Klionsky D.J. Autophagy participates in, well, just about everything. Cell Death Differ. 2020;27:831–832. doi: 10.1038/s41418-020-0511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solitro A.R., MacKeigan J.P. Leaving the lysosome behind: Novel developments in autophagy inhibition. Future Med. Chem. 2016;8:73–86. doi: 10.4155/fmc.15.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergmann W., Burke D.C. Contributions to the Study of Marine Products. XL. The Nucleosides of Sponges.1 IV. Spongosine2. J. Org. Chem. 1956;21:226–228. doi: 10.1021/jo01108a020. [DOI] [Google Scholar]
- 12.Bergmann W., Feeney R.J. Contributions to the study of marine products. XXXII. The nucleosides of spongies. I. J. Org. Chem. 1951;16:981–987. doi: 10.1021/jo01146a023. [DOI] [Google Scholar]
- 13.Bergmann W., Stempien M.F. Contributions to the Study of Marine Products. XLIII. The Nucleosides of Sponges. V. The Synthesis of Spongosine1. J. Org. Chem. 1957;22:1575–1577. doi: 10.1021/jo01363a009. [DOI] [Google Scholar]
- 14.Stonik V. Marine natural products: A way to new drugs. Acta Nat. 2009;2:15–25. doi: 10.32607/20758251-2009-1-2-15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molinski T.F., Dalisay D.S., Lievens S.L., Saludes J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009;8:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 16.Newman D.J., Cragg G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004;67:1216–1238. doi: 10.1021/np040031y. [DOI] [PubMed] [Google Scholar]
- 17.Newman D.J., Cragg G.M. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs. 2014;12:255–278. doi: 10.3390/md12010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galmarini C.M., D’Incalci M., Allavena P. Trabectedin and plitidepsin: Drugs from the sea that strike the tumor microenvironment. Mar. Drugs. 2014;12:719–733. doi: 10.3390/md12020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyshlovoy S.A., Honecker F. Marine Compounds and Cancer: 2017 Updates. Mar. Drugs. 2018;16:41. doi: 10.3390/md16020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyshlovoy S.A., Honecker F. Marine Compounds and Cancer: The First Two Decades of XXI Century. Mar. Drugs. 2019:18. doi: 10.3390/md18010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyshlovoy S.A., Honecker F. Marine Compounds and Cancer: Where Do We Stand? Mar. Drugs. 2015;13:5657–5665. doi: 10.3390/md13095657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruocco N., Costantini S., Costantini M. Blue-Print Autophagy: Potential for Cancer Treatment. Mar. Drugs. 2016;14:138. doi: 10.3390/md14070138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyshlovoy A.S., Honecker F. Marine Compounds and Autophagy: Beginning of a New Era. Mar. Drugs. 2018;16:260. doi: 10.3390/md16080260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyshlovoy S.A., Honecker F. Marine Drugs Acting as Autophagy Modulators. Mar. Drugs. 2020;18:53. doi: 10.3390/md18010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carr G., Williams D.E., Díaz-Marrero A.R., Patrick B.O., Bottriell H., Balgi A.D., Donohue E., Roberge M., Andersen R.J. Bafilomycins Produced in Culture by Streptomyces spp. Isolated from Marine Habitats Are Potent Inhibitors of Autophagy. J. Nat. Prod. 2010;73:422–427. doi: 10.1021/np900632r. [DOI] [PubMed] [Google Scholar]
- 26.PubMed. U.S National Library of Medicine, National Institutes of Health. Search: Autophagy. [(accessed on 15 August 2020)]; Available online: https://www.ncbi.nlm.nih.gov/pubmed/?term=autophagy.
- 27.PubMed. U.S. National Library of Medicine, National Institutes of Health Search: “Autophagy” and “Marine”. [(accessed on 15 August 2020)]; Available online: https://pubmed.ncbi.nlm.nih.gov/?term=%28Autophagy%5BTitle%2FAbstract%5D%29+AND+%28marine%5BTitle%2FAbstract%5D%29&sort=date&size=50.
- 28.Yoshii S.R., Mizushima N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017:18. doi: 10.3390/ijms18091865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanida I., Ueno T., Kominami E. LC3 and Autophagy. Methods Mol. Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 30.Klionsky D.J., Abeliovich H., Agostinis P., Agrawal D.K., Aliev G., Askew D.S., Baba M., Baehrecke E.H., Bahr B.A., Ballabio A., et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klionsky D.J., Abdalla F.C., Abeliovich H., Abraham R.T., Acevedo-Arozena A., Adeli K., Agholme L., Agnello M., Agostinis P., Aguirre-Ghiso J.A., et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kung H.-J., Changou C., Nguyen H.G., Yang J.C., Evans C.P., Bold R.J., Chuang F. Autophagy and prostate cancer therapeutics. In: Tindal D.J., editor. Prostate Cancer. Volume 16. Springer; Berlin/Heidelberg, Germany: 2013. pp. 497–518. [Google Scholar]
- 33.Klionsky D.J. 2020 Is not that far away, which means it is time for the new guidelines. Autophagy. 2019;15:1129. doi: 10.1080/15548627.2019.1624415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roll D.M., Ireland C.M., Lu H.S.M., Clardy J. Fascaplysin, an unusual antimicrobial pigment from the marine sponge Fascaplysinopsis sp. J. Org. Chem. 1988;53:3276–3278. doi: 10.1021/jo00249a025. [DOI] [Google Scholar]
- 35.Bharate S.B., Manda S., Mupparapu N., Battini N., Vishwakarma R.A. Chemistry and biology of fascaplysin, a potent marine-derived CDK-4 inhibitor. Mini Rev. Med. Chem. 2012;12:650–664. doi: 10.2174/138955712800626719. [DOI] [PubMed] [Google Scholar]
- 36.Lin J., Yan X.J., Chen H.M. Fascaplysin, a selective CDK4 inhibitor, exhibit anti-angiogenic activity in vitro and in vivo. Cancer Chemother. Pharmacol. 2007;59:439–445. doi: 10.1007/s00280-006-0282-x. [DOI] [PubMed] [Google Scholar]
- 37.Oh T.I., Lee Y.M., Nam T.J., Ko Y.S., Mah S., Kim J., Kim Y., Reddy R.H., Kim Y.J., Hong S., et al. Fascaplysin Exerts Anti-Cancer Effects through the Downregulation of Survivin and HIF-1α and Inhibition of VEGFR2 and TRKA. Int. J. Mol. Sci. 2017;18:2074. doi: 10.3390/ijms18102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng N., Mu X., Lv X., Wang L., Li N., Gong Y. Autophagy represses fascaplysin-induced apoptosis and angiogenesis inhibition via ROS and p8 in vascular endothelia cells. Biomed. Pharmacother. 2019;114:108866. doi: 10.1016/j.biopha.2019.108866. [DOI] [PubMed] [Google Scholar]
- 39.Florean C., Schnekenburger M., Lee J.Y., Kim K.R., Mazumder A., Song S., Kim J.M., Grandjenette C., Kim J.G., Yoon A.Y., et al. Discovery and characterization of Isofistularin-3, a marine brominated alkaloid, as a new DNA demethylating agent inducing cell cycle arrest and sensitization to TRAIL in cancer cells. Oncotarget. 2016;7:24027–24049. doi: 10.18632/oncotarget.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bechmann N., Ehrlich H., Eisenhofer G., Ehrlich A., Meschke S., Ziegler C.G., Bornstein S.R. Anti-Tumorigenic and Anti-Metastatic Activity of the Sponge-Derived Marine Drugs Aeroplysinin-1 and Isofistularin-3 against Pheochromocytoma In Vitro. Mar. Drugs. 2018;16:172. doi: 10.3390/md16050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teeyapant R., Kreis P., Wray V., Witte L., Proksch P. Brominated Secondary Compounds from the Marine Sponge Verongia aerophoba and the Sponge Feeding Gastropod Tylodina perversa. Z. Für Nat. C. 1993;48:640. doi: 10.1515/znc-1993-7-818. [DOI] [PubMed] [Google Scholar]
- 42.Medina R.A., Goeger D.E., Hills P., Mooberry S.L., Huang N., Romero L.I., Ortega-Barría E., Gerwick W.H., McPhail K.L., Coibamide A. A, a potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium Leptolyngbya sp. J. Am. Chem. Soc. 2008;130:6324–6325. doi: 10.1021/ja801383f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luesch H., Yoshida W.Y., Moore R.E., Paul V.J., Corbett T.H. Total structure determination of apratoxin A, a potent novel cytotoxin from the marine cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 2001;123:5418–5423. doi: 10.1021/ja010453j. [DOI] [PubMed] [Google Scholar]
- 44.Serrill J.D., Wan X., Hau A.M., Jang H.S., Coleman D.J., Indra A.K., Alani A.W., McPhail K.L., Ishmael J.E. Coibamide A, a natural lariat depsipeptide, inhibits VEGFA/VEGFR2 expression and suppresses tumor growth in glioblastoma xenografts. Investig. New Drugs. 2016;34:24–40. doi: 10.1007/s10637-015-0303-x. [DOI] [PubMed] [Google Scholar]
- 45.Wan X., Serrill J.D., Humphreys I.R., Tan M., McPhail K.L., Ganley I.G., Ishmael J.E. ATG5 Promotes Death Signaling in Response to the Cyclic Depsipeptides Coibamide A and Apratoxin A. Mar. Drugs. 2018;16:77. doi: 10.3390/md16030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C., Niederstrasser H., Douglas P.M., Lin R., Jaramillo J., Li Y., Oswald N.W., Zhou A., McMillan E.A., Mendiratta S., et al. Small-molecule TFEB pathway agonists that ameliorate metabolic syndrome in mice and extend C. elegans lifespan. Nat. Commun. 2017;8:2270. doi: 10.1038/s41467-017-02332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jomon K., Kuroda Y., Ajisaka M., Sakai H. A new antibiotic, ikarugamycin. J. Antibiot. 1972;25:271–280. doi: 10.7164/antibiotics.25.271. [DOI] [PubMed] [Google Scholar]
- 48.Urdiales J., Morata P., De Castro I.N., Sánchez-Jiménez F. Antiproliferative effect of dehydrodidemnin B (DDB), a depsipeptide isolated from Mediterranean tunicates. Cancer Lett. 1996;102:31–37. doi: 10.1016/0304-3835(96)04151-1. [DOI] [PubMed] [Google Scholar]
- 49.Losada A., Berlanga J.J., Molina-Guijarro J.M., Jiménez-Ruiz A., Gago F., Avilés P., de Haro C., Martínez-Leal J.F. Generation of endoplasmic reticulum stress and inhibition of autophagy by plitidepsin induces proteotoxic apoptosis in cancer cells. Biochem. Pharmacol. 2020;172:113744. doi: 10.1016/j.bcp.2019.113744. [DOI] [PubMed] [Google Scholar]
- 50.Wright A.E., Botelho J.C., Guzmán E., Harmody D., Linley P., McCarthy P.J., Pitts T.P., Pomponi S.A., Reed J.K. Neopeltolide, a macrolide from a lithistid sponge of the family Neopeltidae. J. Nat. Prod. 2007;70:412–416. doi: 10.1021/np060597h. [DOI] [PubMed] [Google Scholar]
- 51.Fuwa H., Sato M. A Synthetic Analogue of Neopeltolide, 8,9-Dehydroneopeltolide, Is a Potent Anti-Austerity Agent against Starved Tumor Cells. Mar. Drugs. 2017;15:320. doi: 10.3390/md15100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Girard M., Bélanger J., ApSimon J.W., Garneau F.-X., Harvey C., Brisson J.-R., Frondoside A. A novel triterpene glycoside from the holothurian Cucumaria frondosa. Can. J. Chem. 1990;68:11–18. doi: 10.1139/v90-003. [DOI] [Google Scholar]
- 53.Dyshlovoy S.A., Menchinskaya E.S., Venz S., Rast S., Amann K., Hauschild J., Otte K., Kalinin V.I., Silchenko A.S., Avilov S.A., et al. The marine triterpene glycoside frondoside A exhibits activity in vitro and in vivo in prostate cancer. Int. J. Cancer. 2016;138:2450–2465. doi: 10.1002/ijc.29977. [DOI] [PubMed] [Google Scholar]
- 54.Dyshlovoy S.A., Madanchi R., Hauschild J., Otte K., Alsdorf W.H., Schumacher U., Kalinin V.I., Silchenko A.S., Avilov S.A., Honecker F., et al. The marine triterpene glycoside frondoside A induces p53-independent apoptosis and inhibits autophagy in urothelial carcinoma cells. BMC Cancer. 2017;17:1–10. doi: 10.1186/s12885-017-3085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dyshlovoy S., Rast S., Hauschild J., Otte K., Alsdorf W., Madanchi R., Kalinin V., Silchenko A., Avilov S., Dierlamm J., et al. Frondoside A induces AIF-associated caspase-independent apoptosis in Burkitt’s lymphoma cells. Leuk. Lymphoma. 2017;58:2905–2915. doi: 10.1080/10428194.2017.1317091. [DOI] [PubMed] [Google Scholar]
- 56.Wu H.-Y., Yang F.-L., Li L.-H., Rao Y.K., Ju T.-C., Wong W.-T., Hsieh C.-Y., Pivkin M.V., Hua K.-F., Wu S.-H. Ergosterol peroxide from marine fungus Phoma sp. induces ROS-dependent apoptosis and autophagy in human lung adenocarcinoma cells. Sci. Rep. 2018;8:17956. doi: 10.1038/s41598-018-36411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravi B.N., Wells R.J., Croft K.D. Malabaricane triterpenes from a Fijian collection of the sponge Jaspis stellifera. J. Org. Chem. 1981;46:1998–2001. doi: 10.1021/jo00323a006. [DOI] [Google Scholar]
- 58.Wang R., Zhang Q., Peng X., Zhou C., Zhong Y., Chen X., Qiu Y., Jin M., Gong M., Kong D. Stellettin B Induces G1 Arrest, Apoptosis and Autophagy in Human Non-small Cell Lung Cancer A549 Cells via Blocking PI3K/Akt/mTOR Pathway. Sci. Rep. 2016;6:27071. doi: 10.1038/srep27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satake M., MacKenzie L., Yasumoto T. Identification of Protoceratium reticulatum as the biogenetic origin of yessotoxin. Nat. Toxins. 1997;5:164–167. doi: 10.1002/19970504NT7. [DOI] [PubMed] [Google Scholar]
- 60.Draisci R., Ferretti E., Palleschi L., Marchiafava C., Poletti R., Milandri A., Ceredi A., Pompei M. High levels of yessotoxin in mussels and presence of yessotoxin and homoyessotoxin in dinoflagellates of the Adriatic Sea. Toxicon. 1999;37:1187–1193. doi: 10.1016/S0041-0101(98)00254-2. [DOI] [PubMed] [Google Scholar]
- 61.Rubiolo J.A., López-Alonso H., Martínez P., Millán A., Cagide E., Vieytes M.R., Vega F.V., Botana L.M. Yessotoxin induces ER-stress followed by autophagic cell death in glioma cells mediated by mTOR and BNIP3. Cell. Signal. 2014;26:419–432. doi: 10.1016/j.cellsig.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Korsnes M.S., Røed S.S., Tranulis M.A., Espenes A., Christophersen B. Yessotoxin triggers ribotoxic stress. Toxicol. Vitr. 2014;28:975–981. doi: 10.1016/j.tiv.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 63.Korsnes M.S., Kolstad H., Kleiveland C.R., Korsnes R., Ørmen E. Autophagic activity in BC3H1 cells exposed to yessotoxin. Toxicol Vitr. 2016;32:166–180. doi: 10.1016/j.tiv.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Molinski T.F., Makarieva T.N., Stonik V.A. (-)-Rhizochalin is a dimeric enantiomorphic (2R)-sphingolipid: Absolute configuration of pseudo-C(2v)-symmetric bis-2-amino-3-alkanols by CD. Angew. Chem. Int. Ed. Engl. 2000;39:4076–4079. doi: 10.1002/1521-3773(20001117)39:22<4076::AID-ANIE4076>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 65.Makarieva T.N., Denisenko V.A., Stonik V.A., Milgrom Y.M., Rashkes Y.V. Rhizochalin, a novel secondary metabolite of mixed biosynthesis from the sponge Rhizochalina Incrustata. Tetrahedron Lett. 1989;30:6581–6584. doi: 10.1016/S0040-4039(01)89027-4. [DOI] [Google Scholar]
- 66.Makarieva T.N., Zakharenko A.M., Denisenko V.A., Dmitrenok P.S., Guzii A.G., Shubina L.K., Kapustina I.I., Fedorov S.N. Rhizochalinin A, a new antileukemic two-headed sphingolipid from the sponge Rhizochalina incrustata. Chem. Nat. Compd. 2007;43:468–469. doi: 10.1007/s10600-007-0164-4. [DOI] [Google Scholar]
- 67.Fedorov S.N., Makarieva T.N., Guzii A.G., Shubina L.K., Kwak J.Y., Stonik V.A. Marine two-headed sphingolipid-like compound rhizochalin inhibits EGF-induced transformation of JB6 P+ Cl41 cells. Lipids. 2009;44:777–785. doi: 10.1007/s11745-009-3322-6. [DOI] [PubMed] [Google Scholar]
- 68.Khanal P., Kang B.S., Yun H.J., Cho H.G., Makarieva T.N., Choi H.S. Aglycon of rhizochalin from the Rhizochalina incrustata induces apoptosis via activation of AMP-activated protein kinase in HT-29 colon cancer cells. Biol. Pharm. Bull. 2011;34:1553–1558. doi: 10.1248/bpb.34.1553. [DOI] [PubMed] [Google Scholar]
- 69.Dyshlovoy S.A., Otte K., Tabakmakher K.M., Hauschild J., Makarieva T.N., Shubina L.K., Fedorov S.N., Bokemeyer C., Stonik V.A., von Amsberg G. Synthesis and anticancer activity of the derivatives of marine compound rhizochalin in castration resistant prostate cancer. Oncotarget. 2018;9:16962–16973. doi: 10.18632/oncotarget.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dyshlovoy S.A., Otte K., Alsdorf W.H., Hauschild J., Lange T., Venz S., Bauer C.K., Bahring R., Amann K., Mandanchi R., et al. Marine compound rhizochalinin shows high in vitro and in vivo efficacy in castration resistant prostate cancer. Oncotarget. 2016;7:69703–69717. doi: 10.18632/oncotarget.11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Searle P.A., Molinski T.F. Trachycladines A and B: 2’-C-methyl-5’-deoxyribofuranosyl nucleosides from the marine sponge Trachycladus laevispirulifer. J. Org. Chem. 1995;60:4296–4298. doi: 10.1021/jo00118a059. [DOI] [Google Scholar]
- 72.Ichiba T., Nakao Y., Scheuer P.J., Sata N.U., Kelly-Borges M. Kumusine, a chloroadenine riboside from a sponge, Theonella sp. Tetrahedron Lett. 1995;36:3977–3980. doi: 10.1016/0040-4039(95)00692-6. [DOI] [Google Scholar]
- 73.Peitsinis Z.V., Mitrakas A.G., Nakiou E.A., Melidou D.A., Kalamida D., Kakouratos C., Koukourakis M.I., Koumbis A.E. Trachycladines and Analogues: Synthesis and Evaluation of Anticancer Activity. ChemMedChem. 2017;12:448–455. doi: 10.1002/cmdc.201600620. [DOI] [PubMed] [Google Scholar]
- 74.Wakabayashi T., Kageyama-Kawase R., Naruse N., Funahashi Y., Yoshimatsu K. Luminacins: A family of capillary tube formation inhibitors from Streptomyces sp. II. Biological activities. J. Antibiot. 2000;53:591–596. doi: 10.7164/antibiotics.53.591. [DOI] [PubMed] [Google Scholar]
- 75.Shin Y.S., Cha H.Y., Lee B.-S., Kang S.U., Hwang H.S., Kwon H.C., Kim C.-H., Choi E.C. Anti-cancer Effect of Luminacin, a Marine Microbial Extract, in Head and Neck Squamous Cell Carcinoma Progression via Autophagic Cell Death. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2016;48:738–752. doi: 10.4143/crt.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng J., Yuan J.P., Wu C.F., Wang J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs. 2011;9:1806–1828. doi: 10.3390/md9101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Long Y., Cao X., Zhao R., Gong S., Jin L., Feng C. Fucoxanthin treatment inhibits nasopharyngeal carcinoma cell proliferation through induction of autophagy mechanism. Environ. Toxicol. 2020;35:1082–1090. doi: 10.1002/tox.22944. [DOI] [PubMed] [Google Scholar]
- 78.Zhang L., Wang H., Fan Y., Gao Y., Li X., Hu Z., Ding K., Wang Y., Wang X. Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE and Nrf2-autophagy pathways. Sci. Rep. 2017;7:46763. doi: 10.1038/srep46763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liao G., Gao B., Gao Y., Yang X., Cheng X., Ou Y. Phycocyanin Inhibits Tumorigenic Potential of Pancreatic Cancer Cells: Role of Apoptosis and Autophagy. Sci. Rep. 2016;6:34564. doi: 10.1038/srep34564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu H., Wu C.-L., Wang X., Ban Q., Quan C., Liu M., Dong H., Li J., Kim G.-Y., Choi Y.H., et al. SP600125 enhances C-2-induced cell death by the switch from autophagy to apoptosis in bladder cancer cells. J. Exp. Clin. Cancer Res. 2019;38:448. doi: 10.1186/s13046-019-1467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cingolani F., Simbari F., Abad J.L., Casasampere M., Fabrias G., Futerman A.H., Casas J. Jaspine B induces nonapoptotic cell death in gastric cancer cells independently of its inhibition of ceramide synthase. J. Lipid Res. 2017;58:1500–1513. doi: 10.1194/jlr.M072611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuroda I., Musman M., Ohtani I.I., Ichiba T., Tanaka J., Gravalos D.G., Higa T. Pachastrissamine, a Cytotoxic Anhydrophytosphingosine from a Marine Sponge, Pachastrissa sp. J. Nat. Prod. 2002;65:1505–1506. doi: 10.1021/np010659y. [DOI] [PubMed] [Google Scholar]
- 83.Zhang E., Wang S., Li L.-L., Hua Y.-G., Yue J.-F., Li J.-F., Jin C.-Y. Discovery of novel jaspine B analogues as autophagy inducer. Biorg. Med. Chem. Lett. 2018;28:497–502. doi: 10.1016/j.bmcl.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 84.Miyamoto M., Kawamatsu Y., Kawashima K., Shinohara M., Tanaka K., Tatsuoka S., Nakanishi K. Chromomycin A2, A3 and A4. Tetrahedron. 1967;23:421–437. doi: 10.1016/S0040-4020(01)83328-7. [DOI] [PubMed] [Google Scholar]
- 85.Quiñoà E., Crews P. Phenolic constituents of Psammaplysilla. Tetrahedron Lett. 1987;28:3229–3232. doi: 10.1016/S0040-4039(00)95478-9. [DOI] [Google Scholar]
- 86.Luibrand R.T., Erdman T.R., Vollmer J.J., Scheuer P.J., Finer J., Clardy J. Ilimaquinone, a sesquiterpenoid quinone from a marine sponge. Tetrahedron. 1979;35:609–612. doi: 10.1016/0040-4020(79)87004-0. [DOI] [Google Scholar]
- 87.Ratovitski E.A. Tumor Protein (TP)-p53 Members as Regulators of Autophagy in Tumor Cells upon Marine Drug Exposure. Mar. Drugs. 2016;14:154. doi: 10.3390/md14080154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ha Y.N., Song S., Orlikova-Boyer B., Cerella C., Christov C., Kijjoa A., Diederich M. Petromurin C Induces Protective Autophagy and Apoptosis in FLT3-ITD-Positive AML: Synergy with Gilteritinib. Mar. Drugs. 2020;18:57. doi: 10.3390/md18010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buttachon S., Ramos A.A., Inácio Â., Dethoup T., Gales L., Lee M., Costa P.M., Silva A.M.S., Sekeroglu N., Rocha E., et al. Bis-Indolyl Benzenoids, Hydroxypyrrolidine Derivatives and Other Constituents from Cultures of the Marine Sponge-Associated Fungus Aspergillus candidus KUFA0062. Mar. Drugs. 2018;16:119. doi: 10.3390/md16040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ooike M., Nozawa K., Kawai K.-I., Udagawa S.-i. Bisindolylbenzenoids from ascostromata of Petromycesmuricatus. Can. J. Chem. 1997;75:625–628. doi: 10.1139/v97-075. [DOI] [Google Scholar]
- 91.Mauvezin C., Neufeld T.P. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy. 2015;11:1437–1438. doi: 10.1080/15548627.2015.1066957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma S., Guru S.K., Manda S., Kumar A., Mintoo M.J., Prasad V.D., Sharma P.R., Mondhe D.M., Bharate S.B., Bhushan S. A marine sponge alkaloid derivative 4-chloro fascaplysin inhibits tumor growth and VEGF mediated angiogenesis by disrupting PI3K/Akt/mTOR signaling cascade. Chem. Biol. Interact. 2017;275:47–60. doi: 10.1016/j.cbi.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 93.Hubbard R., Rimington C. The biosynthesis of prodigiosin, the tripyrrylmethene pigment from Bacillus prodigiosus (Serratia marcescens) Biochem. J. 1950;46:220–225. doi: 10.1042/bj0460220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williamson N.R., Fineran P.C., Leeper F.J., Salmond G.P. The biosynthesis and regulation of bacterial prodiginines. Nat. Rev. Microbiol. 2006;4:887–899. doi: 10.1038/nrmicro1531. [DOI] [PubMed] [Google Scholar]
- 95.Bennett J.W., Bentley R. Seeing Red: The Story of Prodigiosin. Academic Press; Cambridge, MA, USA: 2000. Advances in Applied Microbiology. [DOI] [PubMed] [Google Scholar]
- 96.Cheng S.Y., Chen N.F., Kuo H.M., Yang S.N., Sung C.S., Sung P.J., Wen Z.H., Chen W.F. Prodigiosin stimulates endoplasmic reticulum stress and induces autophagic cell death in glioblastoma cells. Apoptosis. 2018;23:314–328. doi: 10.1007/s10495-018-1456-9. [DOI] [PubMed] [Google Scholar]
- 97.Cheng M.F., Lin C.S., Chen Y.H., Sung P.J., Lin S.R., Tong Y.W., Weng C.F. Inhibitory Growth of Oral Squamous Cell Carcinoma Cancer via Bacterial Prodigiosin. Mar. Drugs. 2017;15:224. doi: 10.3390/md15070224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Castellano I., Seebeck F.P. On ovothiol biosynthesis and biological roles: From life in the ocean to therapeutic potential. Nat. Prod. Rep. 2018;35:1241–1250. doi: 10.1039/C8NP00045J. [DOI] [PubMed] [Google Scholar]
- 99.Brancaccio M., Russo M., Masullo M., Palumbo A., Russo G.L., Castellano I. Sulfur-containing histidine compounds inhibit γ-glutamyl transpeptidase activity in human cancer cells. J. Biol. Chem. 2019;294:14603–14614. doi: 10.1074/jbc.RA119.009304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sousa M.L., Preto M., Vasconcelos V., Linder S., Urbatzka R. Antiproliferative Effects of the Natural Oxadiazine Nocuolin A Are Associated With Impairment of Mitochondrial Oxidative Phosphorylation. Front. Oncol. 2019;9:224. doi: 10.3389/fonc.2019.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kita Y., Fujioka H. Marine pyrroloiminoquinone alkaloids. Top. Curr. Chem. 2012;309:131–162. doi: 10.1007/128_2011_134. [DOI] [PubMed] [Google Scholar]
- 102.Cowan J., Shadab M., Nadkarni D.H., Kc K., Velu S.E., Yusuf N. A Novel Marine Natural Product Derived Pyrroloiminoquinone with Potent Activity against Skin Cancer Cells. Mar. Drugs. 2019;17:443. doi: 10.3390/md17080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu C.-F., Lee M.-G., El-Shazly M., Lai K.-H., Ke S.-C., Su C.-W., Shih S.-P., Sung P.-J., Hong M.-C., Wen Z.-H., et al. Isoaaptamine Induces T-47D Cells Apoptosis and Autophagy via Oxidative Stress. Mar. Drugs. 2018;16:18. doi: 10.3390/md16010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsukamoto S., Yamanokuchi R., Yoshitomi M., Sato K., Ikeda T., Rotinsulu H., Mangindaan R.E.P., de Voogd N.J., van Soest R.W.M., Yokosawa H. Aaptamine, an alkaloid from the sponge Aaptos suberitoides, functions as a proteasome inhibitor. Biorg. Med. Chem. Lett. 2010;20:3341–3343. doi: 10.1016/j.bmcl.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 105.Shubina L.K., Kalinovsky A.I., Fedorov S.N., Radchenko O.S., Denisenko V.A., Dmitrenok P.S., Dyshlovoy S.A., Krasokhin V.B., Stonik V.A. Aaptamine alkaloids from the vietnamese sponge Aaptos sp. Nat. Prod. Commun. 2009;4:1085–1088. doi: 10.1177/1934578X0900400813. [DOI] [PubMed] [Google Scholar]
- 106.Shubina L.K., Makarieva T.N., Dyshlovoy S.A., Fedorov S.N., Dmitrenok P.S., Stonik V.A. Three new aaptamines from the marine sponge Aaptos sp. and their proapoptotic properties. Nat. Prod. Commun. 2010;5:1881–1884. doi: 10.1177/1934578X1000501208. [DOI] [PubMed] [Google Scholar]
- 107.Weindling R. Trichoderma lignorum as a parasite of other soil fungi. Phytopathology. 1932;22:837–845. [Google Scholar]
- 108.Park G.-B., Jeong J.-Y., Kim D. Gliotoxin Enhances Autophagic Cell Death via the DAPK1-TAp63 Signaling Pathway in Paclitaxel-Resistant Ovarian Cancer Cells. Mar. Drugs. 2019;17:412. doi: 10.3390/md17070412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cimino G., De Stefano S., Minale L., Trivellone E. 12-epi-Scalarin and 12-epi-deoxoscalarin, sesterterpenes from the sponge Spongia nitens. J. Chem. Soc., Perkin Trans. 1977;1:1587–1593. doi: 10.1039/p19770001587. [DOI] [PubMed] [Google Scholar]
- 110.Guzmán E.A., Pitts T.P., Diaz M.C., Wright A.E. The marine natural product Scalarin inhibits the receptor for advanced glycation end products (RAGE) and autophagy in the PANC-1 and MIA PaCa-2 pancreatic cancer cell lines. Investig. New Drugs. 2019;37:262–270. doi: 10.1007/s10637-018-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsai T.C., Chen H.Y., Sheu J.H., Chiang M.Y., Wen Z.H., Dai C.F., Su J.H. Structural Elucidation and Structure-Anti-inflammatory Activity Relationships of Cembranoids from Cultured Soft Corals Sinularia sandensis and Sinularia flexibilis. J. Agric. Food Chem. 2015;63:7211–7218. doi: 10.1021/acs.jafc.5b01931. [DOI] [PubMed] [Google Scholar]
- 112.Tsai T.-C., Lai K.-H., Su J.-H., Wu Y.-J., Sheu J.-H. 7-Acetylsinumaximol B Induces Apoptosis and Autophagy in Human Gastric Carcinoma Cells through Mitochondria Dysfunction and Activation of the PERK/eIF2α/ATF4/CHOP Signaling Pathway. Mar. Drugs. 2018;16:104. doi: 10.3390/md16040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kobayashi M., Okamoto T., Hayashi K., Yokoyama N., Sasaki T., Kitagawa I. Marine natural products. XXXII. Absolute configurations of C-4 of the manoalide family, biologically active sesterterpenes from the marine sponge Hyrtios erecta. Chem. Pharm. Bull. 1994;42:265–270. doi: 10.1248/cpb.42.265. [DOI] [PubMed] [Google Scholar]
- 114.Lee M.-G., Liu Y.-C., Lee Y.-L., El-Shazly M., Lai K.-H., Shih S.-P., Ke S.-C., Hong M.-C., Du Y.-C., Yang J.-C., et al. Heteronemin, a Marine Sesterterpenoid-Type Metabolite, Induces Apoptosis in Prostate LNcap Cells via Oxidative and ER Stress Combined with the Inhibition of Topoisomerase II and Hsp90. Mar. Drugs. 2018;16:204. doi: 10.3390/md16060204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weng J.-R., Chiu C.-F., Hu J.-L., Feng C.-H., Huang C.-Y., Bai L.-Y., Sheu J.-H. A Sterol from Soft Coral Induces Apoptosis and Autophagy in MCF-7 Breast Cancer Cells. Mar. Drugs. 2018;16:238. doi: 10.3390/md16070238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tsai C.-R., Huang C.-Y., Chen B.-W., Tsai Y.-Y., Shih S.-P., Hwang T.-L., Dai C.-F., Wang S.-Y., Sheu J.-H. New bioactive steroids from the soft coral Klyxum flaccidum. RSC Adv. 2015;5:12546–12554. doi: 10.1039/C4RA13977A. [DOI] [Google Scholar]
- 117.Liu M., Hansen P.E., Lin X. Bromophenols in marine algae and their bioactivities. Mar. Drugs. 2011;9:1273–1292. doi: 10.3390/md9071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo C., Wang L., Li X., Wang S., Yu X., Xu K., Zhao Y., Luo J., Li X., Jiang B., et al. Discovery of Novel Bromophenol–Thiosemicarbazone Hybrids as Potent Selective Inhibitors of Poly(ADP-ribose) Polymerase-1 (PARP-1) for Use in Cancer. J. Med. Chem. 2019;62:3051–3067. doi: 10.1021/acs.jmedchem.8b01946. [DOI] [PubMed] [Google Scholar]
- 119.Wang L., Guo C., Li X., Yu X., Xu K., Jiang B., Jia X., Li C., Shi D. Design, synthesis and biological evaluation of bromophenol-thiazolylhydrazone hybrids inhibiting the interaction of translation initiation factors eIF4E/eIF4G as multifunctional agents for cancer treatment. Eur. J. Med. Chem. 2019;177:153–170. doi: 10.1016/j.ejmech.2019.05.044. [DOI] [PubMed] [Google Scholar]
- 120.Wang L.J., Guo C.L., Li X.Q., Wang S.Y., Jiang B., Zhao Y., Luo J., Xu K., Liu H., Guo S.J., et al. Discovery of Novel Bromophenol Hybrids as Potential Anticancer Agents through the Ros-Mediated Apoptotic Pathway: Design, Synthesis and Biological Evaluation. Mar. Drugs. 2017;15:343. doi: 10.3390/md15110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo C., Wang L., Zhao Y., Jiang B., Luo J., Shi D. BOS-93, a novel bromophenol derivative, induces apoptosis and autophagy in human A549 lung cancer cells via PI3K/Akt/mTOR and MAPK signaling pathway. Exp. Ther. Med. 2019;17:3848–3858. doi: 10.3892/etm.2019.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mokhlesi A., Stuhldreier F., Wex K.W., Berscheid A., Hartmann R., Rehberg N., Sureechatchaiyan P., Chaidir C., Kassack M.U., Kalscheuer R., et al. Cyclic Cystine-Bridged Peptides from the Marine Sponge Clathria basilana Induce Apoptosis in Tumor Cells and Depolarize the Bacterial Cytoplasmic Membrane. J. Nat. Prod. 2017;80:2941–2952. doi: 10.1021/acs.jnatprod.7b00477. [DOI] [PubMed] [Google Scholar]
- 123.Bosseboeuf A., Baron A., Duval E., Gautier A., Sourdaine P., Auvray P. K092A and K092B, Two Peptides Isolated from the Dogfish (Scyliorhinus canicula L.), with Potential Antineoplastic Activity Against Human Prostate and Breast Cancer Cells. Mar. Drugs. 2019;17:672. doi: 10.3390/md17120672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bosseboeuf A., Baron A., Duval E., Gautier A., Sourdaine P., Auvray P. A Potential Antineoplastic Peptide of Human Prostate Cancer Cells Derived from the Lesser Spotted Dogfish (Scyliorhinus canicula L.) Mar. Drugs. 2019;17:585. doi: 10.3390/md17100585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bonnard I., Rolland M., Salmon J.-M., Debiton E., Barthomeuf C., Banaigs B. Total Structure and Inhibition of Tumor Cell Proliferation of Laxaphycins. J. Med. Chem. 2007;50:1266–1279. doi: 10.1021/jm061307x. [DOI] [PubMed] [Google Scholar]
- 126.Alvariño R., Alonso E., Bornancin L., Bonnard I., Inguimbert N., Banaigs B., Botana L.M. Biological Activities of Cyclic and Acyclic B-Type Laxaphycins in SH-SY5Y Human Neuroblastoma Cells. Mar. Drugs. 2020;18:364. doi: 10.3390/md18070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choi Y.H., Yamaguchi K., Oda T., Nam T.J. Chemical and mass spectrometry characterization of the red alga Pyropia yezoensis chemoprotective protein (PYP): Protective activity of the N-terminal fragment of PYP1 against acetaminophen-induced cell death in Chang liver cells. Int. J. Mol. Med. 2015;35:271–276. doi: 10.3892/ijmm.2014.1992. [DOI] [PubMed] [Google Scholar]
- 128.Lee M.-K., Choi J.-W., Choi Y.H., Nam T.-J. Protective Effect of Pyropia yezoensis Peptide on Dexamethasone-Induced Myotube Atrophy in C2C12 Myotubes. Mar. Drugs. 2019;17:284. doi: 10.3390/md17050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee M.-K., Choi J.-W., Choi Y.H., Nam T.-J. Pyropia yezoensis Protein Prevents Dexamethasone-Induced Myotube Atrophy in C2C12 Myotubes. Mar. Drugs. 2018;16:497. doi: 10.3390/md16120497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burri L., Hoem N., Banni S., Berge K. Marine omega-3 phospholipids: Metabolism and biological activities. Int. J. Mol. Sci. 2012;13:15401–15419. doi: 10.3390/ijms131115401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wen M., Ding L., Zhang L., Zhang T., Teruyoshi Y., Wang Y., Xue C. Eicosapentaenoic Acid-Enriched Phosphatidylcholine Mitigated Aβ1-42-Induced Neurotoxicity via Autophagy-Inflammasome Pathway. J. Agric. Food Chem. 2019;67:13767–13774. doi: 10.1021/acs.jafc.9b05947. [DOI] [PubMed] [Google Scholar]
- 132.Bonofiglio D., Lanzino M., Giordano C., Catalano S., Andò S. Chapter 16-Omega-3 DHA and EPA Conjugates Trigger Autophagy Through PPARγ Activation in Human Breast Cancer Cells. In: Hayat M.A., editor. Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging. Academic Press; San Diego, CA, USA: 2016. pp. 291–305. [DOI] [Google Scholar]
- 133.Fukui M., Kang K.S., Okada K., Zhu B.T. EPA, an omega-3 fatty acid, induces apoptosis in human pancreatic cancer cells: Role of ROS accumulation, caspase-8 activation, and autophagy induction. J. Cell. Biochem. 2013;114:192–203. doi: 10.1002/jcb.24354. [DOI] [PubMed] [Google Scholar]
- 134.Gao B., Han Y.H., Wang L., Lin Y.J., Sun Z., Lu W.G., Hu Y.Q., Li J.Q., Lin X.S., Liu B.H., et al. Eicosapentaenoic acid attenuates dexamethasome-induced apoptosis by inducing adaptive autophagy via GPR120 in murine bone marrow-derived mesenchymal stem cells. Cell Death Dis. 2016;7:e2235. doi: 10.1038/cddis.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hsu H.C., Li S.J., Chen C.Y., Chen M.F. Eicosapentaenoic acid protects cardiomyoblasts from lipotoxicity in an autophagy-dependent manner. Cell Biol. Toxicol. 2018;34:177–189. doi: 10.1007/s10565-017-9406-9. [DOI] [PubMed] [Google Scholar]
- 136.Guzii A.G., Makarieva T.N., Denisenko V.A., Dmitrenok P.S., Kuzmich A.S., Dyshlovoy S.A., von Amsberg G., Krasokhin V.B., Stonik V.A. Melonoside A: An ω-Glycosylated Fatty Acid Amide from the Far Eastern Marine Sponge Melonanchora kobjakovae. Org. Lett. 2016;18:3478–3481. doi: 10.1021/acs.orglett.6b01678. [DOI] [PubMed] [Google Scholar]
- 137.Caldwell G.S. The influence of bioactive oxylipins from marine diatoms on invertebrate reproduction and development. Mar. Drugs. 2009;7:367–400. doi: 10.3390/md7030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leflaive J., Ten-Hage L. Chemical interactions in diatoms: Role of polyunsaturated aldehydes and precursors. New Phytol. 2009;184:794–805. doi: 10.1111/j.1469-8137.2009.03033.x. [DOI] [PubMed] [Google Scholar]
- 139.Galasso C., Celentano S., Costantini M., D’Aniello S., Ianora A., Sansone C., Romano G. Diatom-Derived Polyunsaturated Aldehydes Activate Similar Cell Death Genes in Two Different Systems: Sea Urchin Embryos and Human Cells. Int. J. Mol. Sci. 2020;21:5201. doi: 10.3390/ijms21155201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li G., Zhao Z., Wu B., Su Q., Wu L., Yang X., Chen J. Ulva pertusa lectin 1 delivery through adenovirus vector affects multiple signaling pathways in cancer cells. Glycoconj. J. 2017;34:489–498. doi: 10.1007/s10719-017-9767-6. [DOI] [PubMed] [Google Scholar]
- 141.Carneiro R.F., de Melo A.A., de Almeida A.S., Moura R.d.M., Chaves R.P., de Sousa B.L., do Nascimento K.S., Sampaio S.S., Lima J.P.M.S., Cavada B.S., et al. H-3, a new lectin from the marine sponge Haliclona caerulea: Purification and mass spectrometric characterization. Int. J. Biochem. Cell Biol. 2013;45:2864–2873. doi: 10.1016/j.biocel.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 142.do Nascimento-Neto L.G., Cabral M.G., Carneiro R.F., Silva Z., Arruda F.V.S., Nagano C.S., Fernandes A.R., Sampaio A.H., Teixeira E.H., Videira P.A. Halilectin-3, a Lectin from the Marine Sponge Haliclona caerulea, Induces Apoptosis and Autophagy in Human Breast Cancer MCF7 Cells Through Caspase-9 Pathway and LC3-II Protein Expression. Anticancer Agents Med. Chem. 2018;18:521–528. doi: 10.2174/1871520617666171114094847. [DOI] [PubMed] [Google Scholar]
- 143.Gao Y., Liu W., Wang W., Zhang X., Zhao X. The inhibitory effects and mechanisms of 3,6-O-sulfated chitosan against human papillomavirus infection. Carbohydr. Polym. 2018;198:329–338. doi: 10.1016/j.carbpol.2018.06.096. [DOI] [PubMed] [Google Scholar]
- 144.Usoltseva R.V., Shevchenko N.M., Malyarenko O.S., Anastyuk S.D., Kasprik A.E., Zvyagintsev N.V., Ermakova S.P. Fucoidans from brown algae Laminaria longipes and Saccharina cichorioides: Structural characteristics, anticancer and radiosensitizing activity in vitro. Carbohydr. Polym. 2019;221:157–165. doi: 10.1016/j.carbpol.2019.05.079. [DOI] [PubMed] [Google Scholar]
- 145.Lin Z., Tan X., Zhang Y., Li F., Luo P., Liu H. Molecular Targets and Related Biologic Activities of Fucoidan: A Review. Mar. Drugs. 2020;18:376. doi: 10.3390/md18080376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li J., Chen K., Li S., Feng J., Liu T., Wang F., Zhang R., Xu S., Zhou Y., Zhou S., et al. Protective effect of fucoidan from Fucus vesiculosus on liver fibrosis via the TGF-β1/Smad pathway-mediated inhibition of extracellular matrix and autophagy. Drug Des. Dev. Ther. 2016;10:619–630. doi: 10.2147/DDDT.S98740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Khan N., Yılmaz S., Aksoy S., Uzel A., Tosun Ç., Kirmizibayrak P.B., Bedir E. Polyethers isolated from the marine actinobacterium Streptomyces cacaoi inhibit autophagy and induce apoptosis in cancer cells. Chem. Biol. Interact. 2019;307:167–178. doi: 10.1016/j.cbi.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 148.Afiyatullov S., Leshchenko E., Berdyshev D., Sobolevskaya M., Antonov A., Denisenko V., Popov R., Pivkin M., Udovenko A., Pislyagin E., et al. Zosteropenillines: Polyketides from the MarineDerived Fungus Penicillium thomii. Mar. Drugs. 2017;15:46. doi: 10.3390/md15020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Heo S.J., Kim J.P., Jung W.K., Lee N.H., Kang H.S., Jun E.M., Park S.H., Kang S.M., Lee Y.J., Park P.J., et al. Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J. Microbiol. Biotechnol. 2008;18:676–681. [PubMed] [Google Scholar]
- 150.Zhen A.X., Piao M.J., Hyun Y.J., Kang K.A., Madushan Fernando P.D.S., Cho S.J., Ahn M.J., Hyun J.W. Diphlorethohydroxycarmalol Attenuates Fine Particulate Matter-Induced Subcellular Skin Dysfunction. Mar. Drugs. 2019;17:95. doi: 10.3390/md17020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Choi K., Lim H.K., Oh S.R., Chung W.-H., Jung J. Anticancer Effects of the Marine Sponge Lipastrotethya sp. Extract on Wild-Type and p53 Knockout HCT116 Cells. Evid.-Based Complement. Altern. Med. 2017;2017:7174858. doi: 10.1155/2017/7174858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Choi C., Son A., Lee H.-S., Lee Y.-J., Park H.C. Radiosensitization by Marine Sponge Agelas sp. Extracts in Hepatocellular Carcinoma Cells with Autophagy Induction. Sci. Rep. 2018;8:6317. doi: 10.1038/s41598-018-24745-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yu X., Cai G., Wang H., Hu Z., Zheng W., Lei X., Zhu X., Chen Y., Chen Q., Din H., et al. Fast-growing algicidal Streptomyces sp. U3 and its potential in harmful algal bloom controls. J. Hazard. Mater. 2018;341:138–149. doi: 10.1016/j.jhazmat.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 154.Castro-Carvalho B., Ramos A.A., Prata-Sena M., Malhão F., Moreira M., Gargiulo D., Dethoup T., Buttachon S., Kijjoa A., Rocha E. Marine-derived Fungi Extracts Enhance the Cytotoxic Activity of Doxorubicin in Nonsmall Cell Lung Cancer Cells A459. Pharmacogn. Res. 2017;9:S92–S98. doi: 10.4103/pr.pr_57_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Leri M., Ramazzotti M., Vasarri M., Peri S., Barletta E., Pretti C., Degl’Innocenti D. Bioactive Compounds from Posidonia oceanica (L.) Delile Impair Malignant Cell Migration through Autophagy Modulation. Mar. Drugs. 2018;16:137. doi: 10.3390/md16040137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Galasso C., Nuzzo G., Brunet C., Ianora A., Sardo A., Fontana A., Sansone C. The Marine Dinoflagellate Alexandrium minutum Activates a Mitophagic Pathway in Human Lung Cancer Cells. Mar. Drugs. 2018;16:502. doi: 10.3390/md16120502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]