Abstract
In addition to the risk of developing opioid use disorder (OUD), known side-effects of long-term opioid use include chronic inflammation and hyperalgesia, which may arise from immune responses induced following chronic opioid use. To investigate this hypothesis, blood samples were obtained from individuals with chronic back pain who were either chronically taking prescription opioids or had minimal recent opioid exposure. Patient samples were analyzed using an enzyme-linked immunosorbent assay (ELISA) against hydrocodone- or oxycodone-hapten conjugates to assess the levels of antibodies present in the samples. While no specific response was seen in opioid-naïve subjects, we observed varying levels of anti-opioid IgM antibodies in the exposed subjects. In these subjects, antibody formation was found to be weakly correlated with current reported daily opioid dose. Other drugs of abuse found to elicit an immune response have been shown to generate advanced glycation end-products (AGEs) through reaction with glucose and subsequent modification of self-proteins. Investigations into this potential mechanism of anti-opioid antibody production identified reduced the formation of reactive intermediate species upon norhydrocodone reaction with glucose in comparison with nornicotine, thus identifying potentially important differences in hapten processing to yield the observed adaptive immune response.
Keywords: opioid, amadori, glycation, antibody, haptenization, back pain
According to the Centers for Disease Control and Prevention (CDC), 68% of the drug overdose deaths in 2017 involved an opioid, resulting in the declaration of a national emergency.1,2 Additional surveys show that 48.5 million Americans have admitted to either using illicit opioids like heroin or misusing prescription opioids such as oxycodone and hydrocodone.3 In 2012, at the peak of the opioid epidemic, 255 million opioid prescriptions were dispensed in the United States, which corresponds to over 80 prescriptions filled per 100 persons, though that rate had decreased to an average of 51 per 100 persons in 2018.4,5 Despite the decrease in overall prescribing rate, some counties still had rates that were over 4-fold higher, and the prescribing of opioids in the United States remains substantially elevated in comparison to that in other countries.5 One study comparing the opioid prescribing habits of physicians in Japan and the United States (Oregon) found that Japanese physicians reported much lower rates of prescribing opioids for both acute (49.4 vs 97.0%) and chronic (63.7 vs 90.9%) pain.6 Among a cohort of patients with lower back pain in Oregon, 61% were prescribed opioids at least once in the year surrounding their visit to a doctor’s office, and 31% of patients who were prescribed opioids were categorized as long-term opioid users (>120 days or >90 days with 10 or more prescription fills), despite the lack of evidence supporting the efficacy of long-term use of opioids for chronic pain.7
In addition to the potential risk of developing opioid use disorder (OUD), known side-effects of long-term opioid use include chronic inflammation and hyperalgesia. Opioid-induced hyperalgesia is a complex process, thought to be at least partially mediated by central sensitization through potentiation of presynaptic N-methyl-d-aspartic acid receptors (NMDAR).8,9 Opioid-induced chronic inflammation is likely multifactorial as well, with immune responses arising secondary to chronic exposure and self-protein modification being one relatively unexplored contributor.10,11 This interpretation is preliminarily supported by data from factory workers occupationally exposed to airborne morphine in the course of manufacturing narcotics; this population had significantly elevated levels of anti-morphine immunoglobulin G (IgG) in their blood.12 After regulations were introduced to reduce exposure, the number of workers with significant levels of anti-morphine antibodies were significantly decreased.12 In a separate study of opioid-dependent individuals who primarily used crude preparations of the poppy plant, anti-morphine IgM antibodies were observed.13 These results are consistent with a rodent model experiment showing that it is possible to induce production of anti-morphine antibodies in mammals using chronic administration; interestingly, the route of administration and presence of additives may be important to this process, as subcutaneous implantation of a pellet containing morphine free base was more effective than subcutaneous injection of morphine sulfate in rabbits.14 Furthermore, other small molecules, such as cocaine, have also been shown to induce the production of antibodies after chronic exposure.10,15,16
Small molecules are usually unlikely to induce an antibody response alone in the absence of structural modification. For example, early research into the development of clinical allergies to penicillin revealed that penicillin itself was not responsible for allergic responses, but rather that the product of a ring-opening reaction between penicillin and a lysine from serum proteins subsequently induced IgE autoantibodies directed against these adducts.17,18 The process of conjugating small molecules to a protein to create an immunogenic species is called haptenization. Following the discovery of the process, haptenization has been used to create vaccines for various purposes, including vaccines against substances of abuse, such as nicotine, cocaine, and heroin.16,19−21
Research into other potential mechanisms by which inadvertent immunization occurs has been an intense area of study. One explanation for this phenomenon focuses on the generation of advanced glycation end-product (AGEs).10 AGEs have been implicated in chronic disease states such as atherosclerosis, cancer, and Alzheimer’s disease.10 The proposed mechanism for AGE formation begins with the formation of a Schiff base formed from the ring-opened form of a monosaccharide (such as glucose) and an amine, either from a small molecule or a protein (Figure 1). Amadori rearrangement of the Schiff base results in an α-ketoamine that can be further oxidized to a dideoxyosone. The dideoxyosone subsequently undergoes isomerization and oxidation, which can lead to cross-linking (in the context of AGEs, the nucleophile comes from an available lysine or arginine from self-proteins). These modified proteins can lead to the development of autoantibodies upon antigen processing. This mechanism has been validated with both methamphetamine and nicotine.10,15 Indeed, a synthesized methamphetamine–AGE–mouse-serum-albumin conjugate was prepared and used to immunize mice, which resulted in the development of antibodies reactive to the conjugate.15 The elucidated mechanism requires only a suitable nucleophile, such as the secondary amine found in hydrocodone and oxycodone’s primary metabolites norhydrocodone and noroxycodone, respectively. Thus, development of opioid–AGEs is a potential mechanism by which some of the negative side effects of chronic opioid use may occur. If validated, then the formation of anti-opioid antibodies in patients with lower back pain could lead to a reinforcing cycle, in which long-term opioid use leads to production of antibodies directed against opioid-modified self-proteins, causing chronic inflammation, which causes pain, which leads to increased use of opioids, which generates pro-inflammatory species, which causes pain, ad infinitum. Finally, there is evidence that patients with significant levels of self-reactive antibodies would be less likely to benefit from experimental vaccine-based therapies, as demonstrated in clinical trials studying an anti-cocaine vaccine in which patients with preexisting levels of anti-cocaine IgM antibodies (greater than 11 μg/mL) were unable to generate neutralizing levels of anti-cocaine IgG antibodies following vaccination.16 If an opioid vaccine were to come to market, then pre-existing anti-opioid antibodies would be an important biomarker to investigate for patients entering clinical trials. Therefore, the goal of this study was to assess the presence of opioid-directed immunoglobulins in long-term opioid users, identify modifying factors for antibody development, and characterize the formation of drug–protein conjugates formed in vitro.
Figure 1.
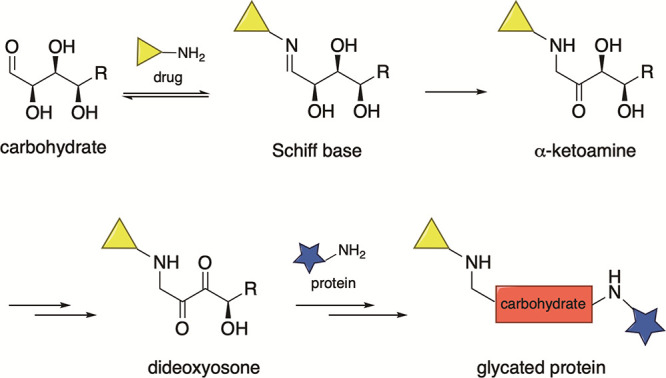
Proposed mechanism of AGE formation with a generic carbohydrate.
Materials and Methods
Approvals and Oversight
This study was approved by the TSRI and UW-Madison Institutional Review Boards, and all experiments were conducted in accordance with national and international guidelines on human subject research.
Chemicals and Reagents
Skim milk powder was obtained from US Biological Life Science. Goat anti-human IgG (31410), IgA (PA1–74395), and IgM (31415) secondary-antibody–horseradish-peroxidase (HRP) conjugates were obtained from Thermo Fisher. Goat anti-human IgE secondary antibody conjugated to HRP was obtained from Novex (A18793). bovine serum albumin (BSA) powder for in vitro AGE experiments was obtained from Sigma. d-Glucose was obtained from Sigma. The tetramethylbenzidine (TMB) substrate kit was obtained from BD OptEIA (Fisher Scientific). Pierce 20× phosphate-buffered saline (PBS) stock solution was used throughout. For competitive ELISAs, hydrocodone and oxycodone were obtained from Spectrum Chemical Manufacturing Group, while methyl nicotinate was obtained from Oakwood Laboratories.
Synthesis of N17-linked OxyBSA 1 has been previously reported in the literature.22 Syntheses of C6-linked OxyBSA 2 and N17-linked HydroBSA 3 were adapted from known precedents (see the Supporting Information).22−25
Subject Recruitment
The Scripps Health electronic medical records system was initially used to identify potentially eligible patients; inclusion criteria for the opioid-exposed arm included age between 20 and 65, history of lower back pain (>6 months), and use of hydrocodone or oxycodone on most days for >6 months (the opioid-naïve arm included the same patients who had used oxycodone or hydrocodone for 2 days or less over the last 6 months). Potentially eligible patients were contacted and asked to participate in the study. Additionally, a newspaper advertisement and later a radio advertisement were drafted and used to recruit patients. Interested participants were provided with a questionnaire in order to establish demographics, presence of inclusion and exclusion criteria, a brief pain inventory, opioid use history (current dose, length of use, etc.), and any nonpharmacologic methods of pain relief (see the Supporting Information, Appendix A). Patients were excluded if they had any use of illicit opioids or use of opioids considered structurally similar to hydrocodone or oxycodone (e.g., morphine, hydromorphone, oxymorphone) at least twice per month on average over a 6 month period, were hospitalized in the previous 12 weeks, were pregnant, or had Alzheimer’s disease, chronic kidney failure, or any religious or medical restrictions on the receipt of blood or blood-derived products. Patients were counseled on the long-term ramifications of opioid use and instructed on alternative methods of managing pain, such as changing diet, exercise, or massage, and were provided with a copy of the book “Less Pain, Fewer Pills: Avoid the Dangers of Prescription Opioids and Gain Control over Chronic Pain” and a pamphlet entitled “What YOU can do to reduce chronic pain” (see the Supporting Information, Appendix B). Blood was taken at week 0, and opioid-using patients were given the opportunity to participate in a follow-up visit to determine any changes in pain, medication use, lifestyle changes, and patient experience. Patients received monetary compensation of $50 for providing a blood sample.
Immunochemical Detection of Anti-Opioid Antibodies in Plasma via Indirect ELISA
Indirect ELISA was performed using previously established methods adapted for use with ELX405 automated plate washer/dispenser system (BioTek). Briefly, ELISA plates (Costar 3690 half-area-binding 96-well microtiter plates) were coated overnight at 37 °C with the antigen of choice (BSA or BSA conjugated to an opioid hapten at 1:200 dilutions of 1 mg/mL antigen). After plates were fixed with methanol, plates were blocked with Blotto (5% skim milk powder in PBS) for 30 min at room temperature. Plasma was added to plates in 2-fold serial dilutions beginning at a 1:40 dilution in Blotto. Plates were incubated for 1 h at 37 °C. After washing with PBS (20 wash cycles), secondary antibody (goat anti-human immunoglobulin/HRP conjugate, diluted 1:10 000 in Blotto) was added to the plate and incubated for 30 min. Following an additional washing step, plates were developed with TMB for 15 min. The reaction was stopped by the addition of 2 M H2SO4, and the optical density (O.D.) at 450 nm was determined using a microplate reader (Synergy H1). Antibody responses were assessed as a percent of the positive control (1:3 000 000 dilution of the secondary antibody in PBS).
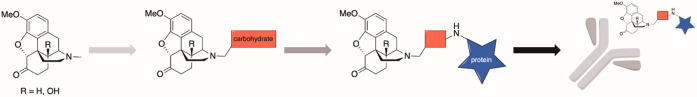
Two versions of the OxyBSA bioconjugate were synthesized, N17-linked OxyBSA (1) and C6-linked OxyBSA (2) (Figure 2). Differences in antibody recognition of this structurally distinct species were used to aid in determination of the likely reactive intermediate structure. These antigens were compared side-by-side using indirect ELISA on pooled opioid-exposed and pooled opioid-naïve samples.
Figure 2.
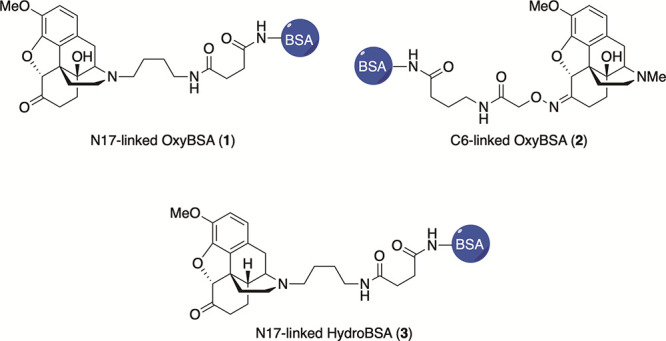
Structures of opioid antigens used to evaluate patient samples.
OxyBSA 1 and HydroBSA 3 (Figure 2) were used to conduct indirect ELISA on individual samples. Background antibody binding to BSA was subtracted from each sample to determine the quantity of opioid-specific antibodies. All measurements were made in triplicate.
Determination of Specificity of Antibody Binding via Competitive ELISA
For competitive ELISA, plasma samples were plated at a concentration of 1:60 and coincubated with drug (0–5 mM). Pooled samples (opioid-exposed or -naïve) were evaluated against both OxyBSA 1 and HydroBSA 3 and coincubated with hydrocodone, oxycodone, or methyl nicotinate (as a negative control) in a 6-point dose–response assay. Additionally, individual plasma samples were evaluated for specificity to opioids via coincubation with hydrocodone (0–5 mM) and either OxyBSA 1 or BSA as the antigen in a 12-point dose–response assay. Absorbance values were normalized to the highest absorbance value per sample against OxyBSA 1. All measurements were made in triplicate.
In Vitro Generation of Amadori Intermediates
Nornicotine or norhydrocodone (10 mM) were incubated with glucose (200 mM) in PBS at 37 °C for 4 weeks. At given time intervals, aliquots were removed (50 μL), condensed via rotary evaporator, diluted with methanol (1 mL), and analyzed by liquid chromatography/mass spectrometry (LC/MS) to detect the presence of the Amadori product and calculate the area under the curve (AUC) for the norhydrocodone–Amadori product (m/z: [M + 1]+ = 448). Nornicotine was utilized as a positive control in order to verify the procedure (m/z: [M + 1]+ = 311).
In Vitro Generation of Advanced Glycation End Products
Nornicotine or norhydrocodone (0.7 mg/mL) were incubated with BSA (1 mg/mL) in glucose-rich PBS (200 mM) for 4 weeks at 37 °C. At given time intervals, aliquots were removed, eluted through a ZipTip (EMD Millipore) to a final volume of 200 μL and analyzed by matrix-assisted laser desorption/ionization time-of-flight (MALDI/ToF) mass spectrometry to detect alterations in measured m/z of BSA.
Statistics
Statistical analysis was performed using GraphPad PRISM 8. All values are provided as mean ± standard error of the mean (SEM). This data was then fit using a log(inhibitor) vs normalized response—variable slope equation. To determine positive and negative samples, a Z-score was calculated for each sample; a Z-score above 1.65 as compared to the opioid-naïve sample average was considered significant. For questionnaire items measured on a Likert scale, responses were coded using evenly spaced ordinal numbering beginning with 1. Correlations between questionnaire response items were assessed using the coefficient of linear correlation R2, and the resulting fit was tested for deviation from zero slope. Comparison of N17-linked OxyBSA 1 and C6-linked OxyBSA 2 determined by one-way ANOVA. Outliers were identified using the ROUT method. A Student’s t-test was utilized to compare the difference between opioid-exposed and opioid-naïve response to the OxyBSA antigens. A type 1 error p ≤ 0.05 was considered statistically significant, and all tests were run using two-tailed analysis.
Results
Subject Enrollment and Demographics
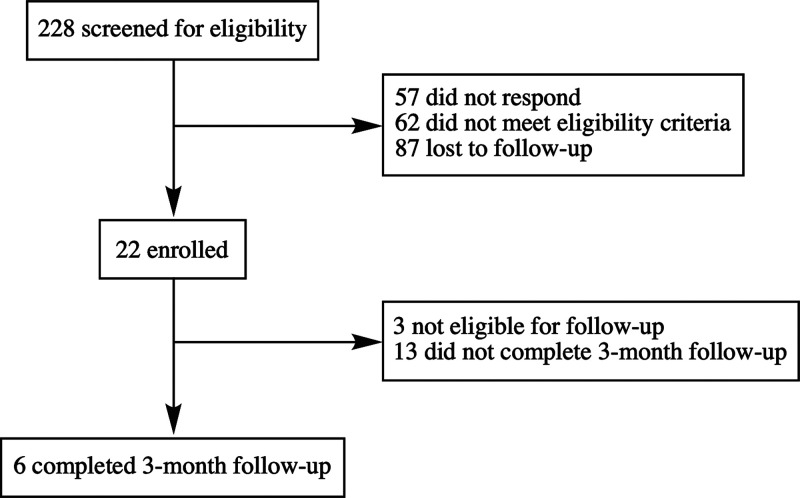
The screen of electronic medical records combined with print, radio, and Internet advertisements yielded 22 subjects with chronic lower back pain, comprised of long-term opioid users (n = 19) and opioid-naïve individuals (n = 3) (Figure 3). Of the recruited subjects, 21 of 22 were Caucasian, and 19 of 22 were male (Table 1). All three females were in the opioid-naïve cohort. Only 1 individual had experienced less than 24 months of severe back pain. There was no significant difference between opioid-exposed and -naïve cohorts in body mass index (BMI), average pain, current pain, number, and class of current medications, exercise, or number of nonpharmacological methods used to minimize pain. Reported medications were divided into 4 classes: analgesic, neuromodulatory, other prescription, and over the counter (OTC)/supplements. The nonpharmacological techniques used by the subjects were divided into 5 categories: diet, alternative medicines (supplements, chiropractic or osteopathic manipulation, massage, acupuncture, prolotherapy, energy medicine, traditional Chinese medicine, homeopathy, and naturopathy), mindfulness and relaxation (meditation, spiritual or religious practices, breathing exercises, biofeedback, guided imagery, and music therapy), movement therapy (physical exercise, yoga, dance therapy, or tai chi/qi gong), and devices (bracelets/bands or electric stimulation). There were no significant differences between the groups in the types of nonpharmacological techniques used. Six opioid-exposed individuals returned to complete the follow-up visit.
Figure 3.
Enrollment of subjects with chronic lower back pain.
Table 1. Subject Demographicsa.
| factor | exposed | naïve | p |
|---|---|---|---|
| Caucasian race (%)b | 100 | 66.6 | 0.13 |
| back pain >2 years (%)b | 94.7 | 100 | >0.99 |
| brief pain inventory | |||
| average pain (#, 1–100) | 61 ± 3.5 | 57 ± 9.0 | 0.65 |
| current pain (#, 1–100) | 50 ± 5.4 | 51 ± 15 | 0.92 |
| worst pain (#, 1–100) | 78 ± 3.7 | 64 ± 5.5 | 0.19 |
| least pain (#, 1–100) | 41 ± 5.8 | 42 ± 20 | 0.93 |
| pain interference (#, 1–100) | 62 ± 4.9 | 52 ± 1.1 | 0.44 |
| BMI (kg/m2)c | 28.4 ± 0.9 | 24.4 ± 1.2 | 0.12 |
| exercise (min/week)d | 303 ± 157 | 117 ± 7.3 | 0.26 |
| other medications (#)d | 8.5 ± 1.1 | 11.7 ± 2.1 | 0.52 |
| analgesic (#) | 0.53 ± 0.19 | 0.67 ± 0.33 | 0.74 |
| neuromodulatory (#) | 1.1 ± 0.32 | 0.33 ± 0.33 | 0.17 |
| other prescription (#) | 4.6 ± 0.73 | 3.7 ± 3.2 | 0.80 |
| OTC/supplement (#) | 1.3 ± 0.51 | 6 ± 1.5 | 0.079 |
| nonpharm techniques (#)d | 3.7 ± 0.7 | 5.3 ± 2.3 | 0.57 |
| diet (%) | 10.5 | 0 | >0.99 |
| alternative medicine (%) | 68.4 | 66.6 | >0.99 |
| mindfulness/relaxation (%) | 42.1 | 100 | 0.21 |
| movement (%) | 57.9 | 100 | 0.27 |
| wearables/devices (%) | 42.1 | 0 | 0.27 |
Continuous data shown as mean ± SEM.
Two-tailed Fisher’s exact test.
Two-tailed t-test.
Two-tailed Welch’s t-test.
Perception of Opioid Efficacy over Time
As long-term opioid use is associated with tolerance, chronic inflammation, and hyperalgesia, it was hypothesized that the analgesic efficacy of opioids would be decreased over time. Thus, subjects were questioned regarding their perception of the effect of their prescription opioid upon initiation of use and at the time of study enrollment, as well as the frequency of break-through pain (Table 2). Responses revealed that initially subjects saw decreased pain upon use of opioids, but at enrollment, the positive effect of the opioid was lessened (Δ −0.9 ± 0.3, p = 0.003, paired t-test). This decrease in efficacy was reported at time points ranging from 6 months to >20 years of opioid use. The relationship between chronic opioid use on frequency of breakthrough pain over time was not significant (Δ +0.4 ± 0.3, p = 0.5).
Table 2. Responses to Opioid Efficacy Due to Dose Change.
| question | response (n) |
|---|---|
| Previously, has dosage been decreased? | 5/19 |
| If yes, then did daily pain severity decrease? | 0/5 |
| If yes, then did pain daily severity increase? | 3/5 |
| If yes, then did breakthrough pain occurrence decrease? | 0/5 |
| If yes, then did breakthrough pain occurrence increase? | 1/5 |
| Desire a dose decrease following intake appointment? | 15/19 |
| Decreased dosage at follow-up? | 3/6 |
| If yes, then has daily pain severity decreased? | 2/3 |
| If yes, then has daily pain severity increased? | 1/3 |
| If yes, then has breakthrough pain occurrence decreased? | 1/3 |
| If yes, then has breakthrough pain occurrence increased? | 0/3 |
Subjects also provided information regarding changes to their opioid prescription over time (switching from hydrocodone to oxycodone or oxycodone to hydrocodone, or addition of a second opioid). Unsurprisingly, individuals that had their dose of opioid increased observed increased efficacy on treatment of average daily pain (Δ +2 ± 0.3) while subjects who had decreased their dosage observed decreased efficacy (Δ −0.6 ± 0.2); the effect of dose change on average daily pain was significant (p = 0.0005). The effect of dose increase on frequency of breakthrough pain was nonsignificant (Δ −0.8 ± 0.5 vs Δ +0.4 ± 0.4, p = 0.09).
At enrollment and following the counseling session, subjects were asked whether they desired to decrease their opioid dose in the next 3 months. The majority of subjects desired to decrease their dosage. At the 3 month follow-up, the 3 individuals who had seen no dose decrease had experienced worse pain compared to baseline, while 2 of 3 individuals who had decreased their dose at the time of the follow-up study reported that they were less severe daily pain than at baseline. Contrasting these results with the above-reported changes in pain when their dosages were previously decreased indicates that self-initiated tapering could reduce the perception of increased pain, as compared to prescriber-initiated tapers. This observed difference in reported pain outcome in these two circumstances is consistent with other observed positive outcomes seen through use of more patient-centered tapering approaches.26 Additionally, 2 of the subjects that completed the follow-up reported adding exercise for their pain management. One subject observed improvement in frequency of breakthrough pain but not average daily pain. The other subject had also decreased their opioid dose and experienced decreased frequency of breakthrough pain and less severe pain but attributed the improvement to the dose change.
Detection of Anti-Opioid Antibodies via Indirect ELISA
Indirect ELISA was utilized to ascertain the presence anti-opioid antibodies in the serum of opioid-exposed subjects; the presence of these antibodies could indicate that the immune system was being trained to detect opioids, which could have potentially negative consequences for patients chronically taking these drugs. The antigens utilized in the experiment consisted of oxycodone or hydrocodone covalently linked to BSA. Baseline signal for the ELISA with PBS was found to be 0.073 ± 0.0017 (n = 57) across all assay plates. The positive control signal used for normalization was 0.392 ± 0.011 (n = 57). Experiments were performed on individual samples as well as pooled opioid-exposed or pooled opioid-naïve samples.
In order to provide evidence regarding the identity of the opioid-protein antigen being recognized by the antibodies, two different oxycodone antigens were synthesized, which provided alternate faces for antibody recognition. Antibody binding was significantly higher when N17-linked OxyBSA 1 antigen was used compared to C6-linked OxyBSA 2 for both pooled opioid-exposed and pooled opioid-naïve samples (Figure 4A,B). Furthermore, while there was a significant difference between the opioid-exposed and opioid-naïve groups’ responses to OxyBSA 1 (p < 0.0001), there was no difference between the responses for OxyBSA 2 (p = 0.68) (Figure 4C). OxyBSA 1 was also more sensitive to opioid exposure in terms of detecting anti-opioid antibodies, as there was a significant difference in the Δ(exposed-naïve) between the two antigens (p = 0.001, Figure 4D). This difference in response suggests that the antibodies are more prone to recognize haptens with structural modifications occurring between the N17 amine and a reactive protein intermediate in vivo. Due to its superior response, OxyBSA 1 was used as the primary antigen for evaluating individual subject responses in subsequent experiments (see Figure S3). The signal from HydroBSA 3 was lower overall (see Figure S5) and not used for subsequent evaluation.
Figure 4.
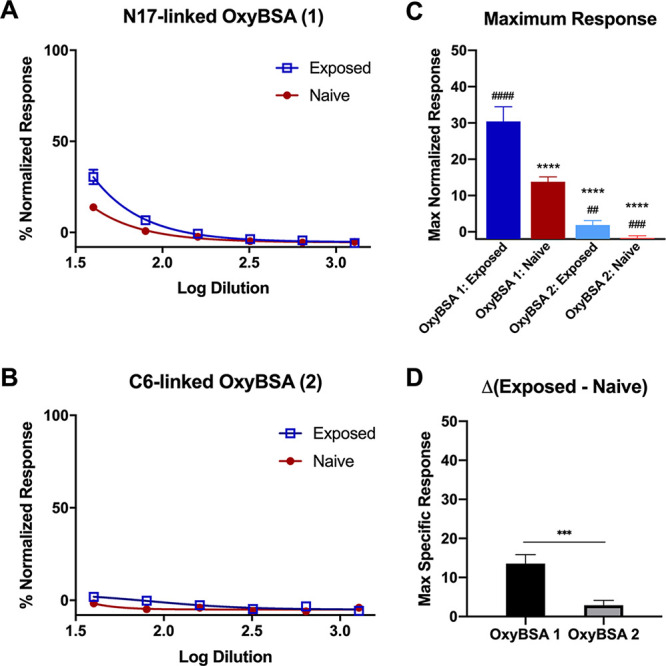
Determination of facial recognition of anti-opioid IgM antibody binding through comparison of response to two OxyBSA conjugates (1 and 2) via indirect ELISA. (A) Pooled opioid-exposed or opioid-naïve response to OxyBSA 1. (B) Pooled opioid-exposed or -naïve response to OxyBSA 2. (C) Comparison of the maximum response for opioid-exposed and -naïve patients against OxyBSA 1 and OxyBSA 2. One-way ANOVA. ****, p < 0.0001 compared to opioid-exposed response to OxyBSA 1. p < 0.0001 compared to opioid-naïve response to OxyBSA 1. ####, p < 0.0001; ###, p ≤ 0.001; ##, p < 0.01. (D) Quantification of the difference in opioid-exposed and opioid-naïve response at the maximum plasma concentration between OxyBSA 1 and OxyBSA 2 (Student’s t-test: p = 0.0009). All data shown as mean ± SEM from n = 3 replicates.
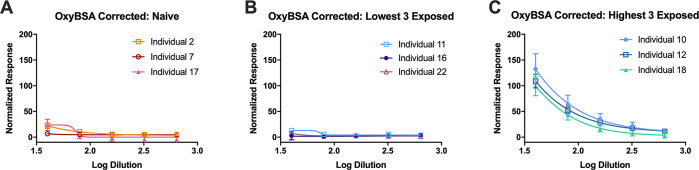
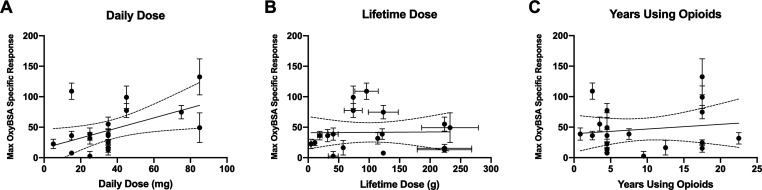
Evaluation of individual opioid-naïve samples revealed that the average maximum response to OxyBSA 1 following subtraction of background binding to BSA was 19.6% ± 10.5% of the positive control. Individual responses of the opioid-exposed subjects were far more variable, with considerable differences between the highest and lowest responders (Figure 5). IgM antibodies recognizing OxyBSA 1 were detected in 11 of the opioid-exposed subject samples (Z ≥ 1.65) (Figure 6). IgG anti-opioid responses were not detected (see Figure S6). Maximum IgM responses were plotted against the subjects’ opioid use to determine if there was a correlation between response and subject characteristics (Figure 7). We determined that there was no correlation between response and years taking the opioids (p = 0.55) or response and estimated lifetime dose (p = 0.53, one outlier removed); however, there was a correlation between daily dose and response (R2 = 0.26, p = 0.03). Considering that unlike with infectious diseases reactivation of immune response to drug-modified self-proteins requires ongoing exposure to the small molecule, this correlation with recent but not remote exposure is consistent with a haptenization mechanism for antibody formation.
Figure 5.
Detection of OxyBSA 1 specific response determined through subtraction of baseline BSA IgM response from OxyBSA 1 IgM response and then normalized to the 2° antibody-positive control response. (A) Antibody quantification using indirect ELISA using OxyBSA 1 antigen in opioid-naïve individuals. (B) Antibody quantification using indirect ELISA using OxyBSA 1 antigen in opioid-exposed individuals with the lowest responses. (C) Antibody quantification using indirect ELISA using OxyBSA 1 antigen in opioid-exposed individuals with the highest responses. All data shown as mean ± SEM from n = 3 replicates.
Figure 6.
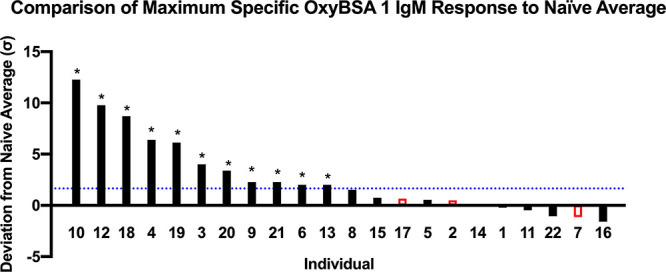
Comparison of results from indirect ELISA detection of anti-OxyBSA 1 IgM antibodies in each individual subject. Responses were compared to opioid-naïve individuals (shown in red) using Z scores. Dotted blue line delineates Z score of 1.65 as compared to opioid-naïve average. This line indicates a 95% confidence interval for positive anti-opioid IgM response. Z-test *, p < 0.05.
Figure 7.
Correlation of maximum anti-OxyBSA 1 IgM response vs opioid use. (A) Maximum specific OxyBSA 1 response vs daily dose. (B) Maximum specific OxyBSA 1 response vs estimated lifetime dose with assumed error of ±20%. One outlier removed following ROUT analysis; see Figure S4 for data with outlier included and sensitivity analysis to different error assumptions. (C) Maximum specific OxyBSA 1 response vs years of opioid use. Linear regression with 95% confidence bands are shown. All data provided as mean ± SEM from n = 3 replicates.
Determination of Selectivity of Anti-Opioid Antibodies via Competitive ELISA
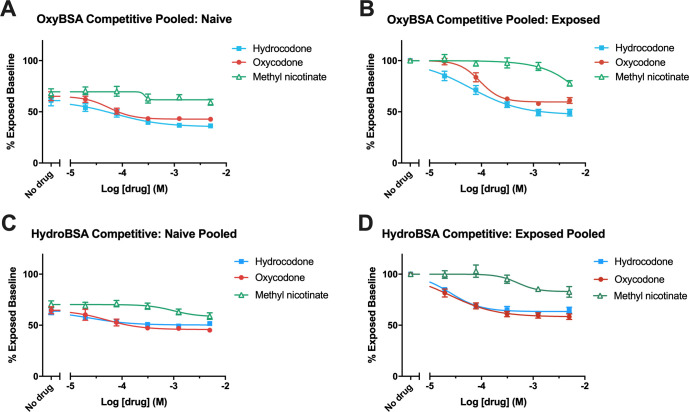
Competitive ELISA was utilized to establish the opioid-specific response by comparing the decrease in maximal signal to BSA–opioid conjugates following the addition of an opioid (hydrocodone or oxycodone) to the decrease in signal caused by a structurally unrelated compound (methyl nicotinate). Competitive ELISA performed on pooled samples (opioid-exposed or opioid-naïve), utilizing either of N17-linked antigens OxyBSA 1 or HydroBSA 3, revealed minimal response to methyl nicotinate, the negative control. The range of the pIC50 for the opioid-exposed subjects against antigen 1 was very broad and suggestive of weak overall binding. Responses to hydrocodone and oxycodone were very similar, which shows the response could be inhibited in a dose-dependent manner (Figure 8; Table S1).
Figure 8.
Determination of anti-opioid IgM antibody selectivity via competitive ELISA in pooled samples (opioid-exposed or -naïve) coincubated with hydrocodone, oxycodone, or methyl nicotinate, evaluated against opioid BSA conjugates. (A) Dose–response curves for pooled opioid-naïve samples vs OxyBSA 1. (B) Dose–response curves for pooled opioid-exposed samples vs OxyBSA 1. (C) Dose–response curves for pooled opioid-naïve samples HydroBSA 3. (D) Dose–response curves for pooled opioid-exposed samples vs HydroBSA 3. All data shown as mean ± SEM from n = 3 replicates.
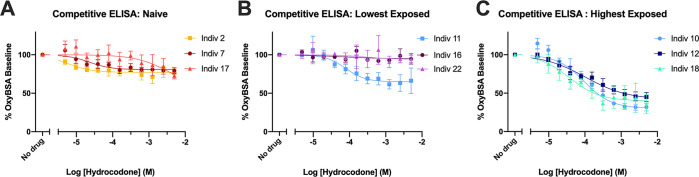
Competitive ELISA was also performed for each individual against OxyBSA 1 and evaluated using hydrocodone. Despite the low level of response in the opioid-naïve samples (individuals 2, 7, and 17 in Figure 9A) and individual 11 (Figure 9B), there was a slight dose response to hydrocodone remaining after correction for carrier protein binding. In contrast, three of the opioid-exposed subjects with the highest responses (individuals 10, 12, and 18) showed a robust dose-dependent decrease to increasing concentrations of hydrocodone (Figure 9C). Individual competitive ELISA results against OxyBSA 1 and BSA in response to hydrocodone for all subjects confirmed this high interindividual variability across all samples (see Figure S7). There was essentially no dose–response due to hydrocodone when BSA was utilized as the antigen.
Figure 9.
Determination of anti-opioid IgM antibody selectivity via competitive ELISA vs OxyBSA 1 against hydrocodone for individual subjects. (A) Dose–response curves for opioid-naïve subjects. (B) Dose response curves for lowest 3 opioid-exposed subjects. (C) Dose–response curves for highest 3 opioid-exposed subjects. All data shown as mean ± SEM from n = 3 replicates.
In Vitro Generation of Amadori Intermediates
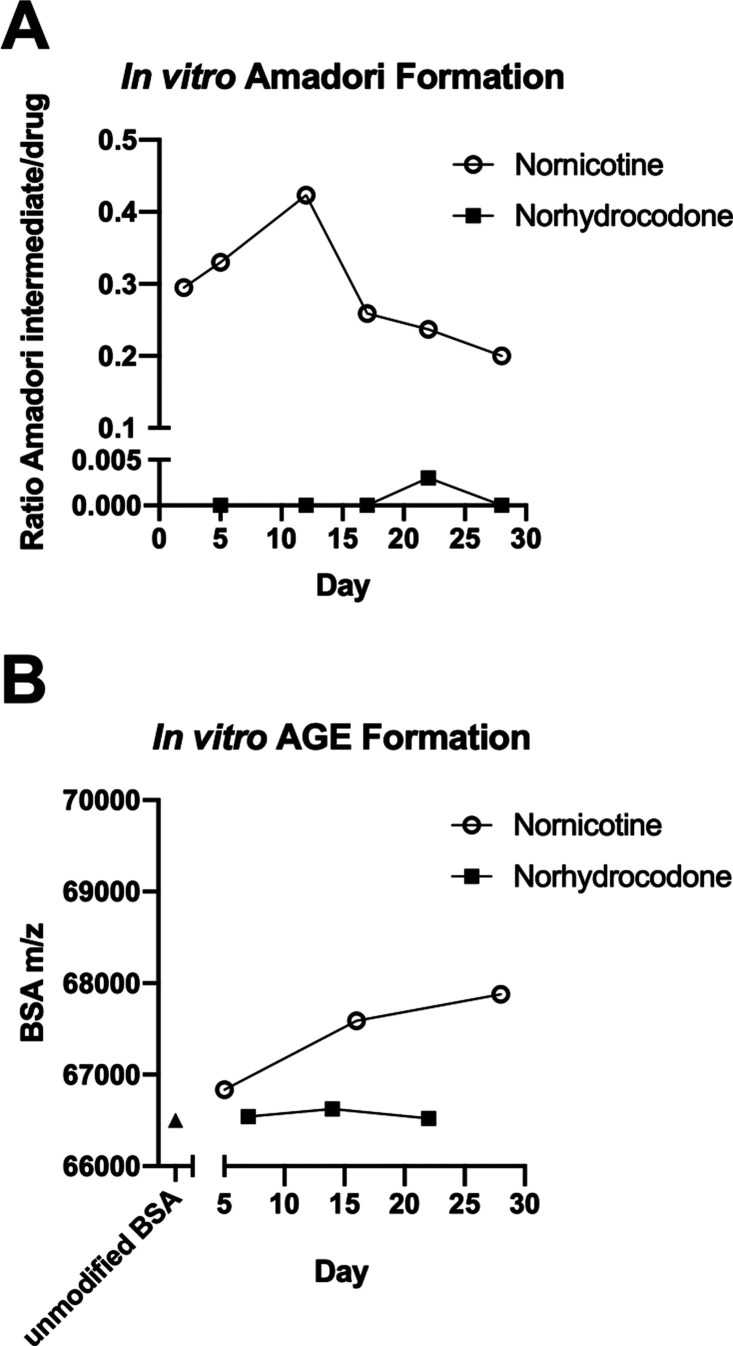
Previous research indicated that drugs containing an available amine could react with glucose to form a reactive species and subsequently modify proteins.10 Norhydrocodone’s ability to react with glucose to form the initial Amadori intermediate was compared with nornicotine, which had been previously shown to undergo this reaction.10 Thus, LC/MS was utilized to monitor the reaction of norhydrocodone or nornicotine with glucose in PBS at 37 °C. The ratio of area under the curves (AUCs) for nornicotine–Amadori product/nornicotine or norhydrocodone–Amadori product/norhydrocodone were determined and plotted over time (Figure 10A). The nornicotine–Amadori product, structurally confirmed by independent synthesis (Figure S8), was observed on day 2 of incubation, with the observed AUC being 30% of that for nornicotine, then slowly increasing to 42% on day 12, before gradually decreasing again thereafter. Surprisingly, the norhydrocodone Amadori intermediate was only observed as a small fraction of the norhydrocodone response on day 22 (0.3% of norhydrocodone).
Figure 10.
Evaluation of glycation as a pathway for antibody formation. (A) Comparison of rate of formation of Amadori intermediate between nornicotine and norhydrocodone through monitoring by LC/MS for the appearance of nornicotine or norhydrocone Amadori intermediates with m/z 311 or 448, respectively. (B) Comparison of rate of formation of BSA AGE over time for nornicotine and norhydrocodone determined by monitoring mass of BSA using MALDI-ToF. Unmodified BSA shown as black triangle.
In Vitro Generation of Advanced Glycation End Products
We determined the ability of norhydrocodone and nornicotine to modify proteins through the development of glycated intermediates by incubating norhydrocodone or nornicotine with BSA in glucose-enriched buffer at 37 °C and determining the approximate mass of BSA over time using MALDI-ToF. After 5 days, a small increase in the mass of BSA was detected on day 5 following incubation with nornicotine (correlating with 1.1 copies of the nornicotine hapten per BSA), an increase that continued through day 28 (correlating with 4.7 copies of the nornicotine hapten per BSA), suggesting that formation of the glycated product was occurring (Figure 10B). This time line is consistent with previous experiments with BSA and nornicotine in which the AGE was observed after 7 days.10 In contrast, no corresponding increase in mass was observed for norhydrocodone, which is consistent with the lack of Amadori intermediate seen in the previous experiment, indicating that alternative glycans and/or proteins may be required for haptenization of norhydrocodone.
Discussion
The low sample size, particularly with regard to the follow-up study, hindered our ability to determine the effect of dose change on frequency of breakthrough pain and severity of pain. Of the patients who did decrease their opioid dosage, the majority observed less severe average pain. More research is necessary to determine the correlation between patient-initiated dose decreases and pain. Additional research is also needed to determine the effect of lifestyle changes such as exercise on pain severity and breakthrough pain.
We discovered anti-opioid antibodies in the plasma of approximately half the individuals chronically taking prescription opioids for lower back pain. The levels of anti-opioid IgM response varied from subject to subject, with the only significant correlation found between daily dose and quantity of antibodies, although observation of potential correlations between response and lifetime dose or total time taking opioids were hampered by a small sample size overall. While no definitive general conclusions can be generally drawn in regard to antibody titer response and patient characteristics from this study, several interesting connections are observed in this specific sample. Two of the three subjects with the highest responses (individuals 10 and 12) had also been taking opioids that were structurally different from hydrocodone (buprenorphine or fentanyl). This elevation is particularly intriguing, given the lack of cross-reactivity previously observed between for compounds by hydrocodone/oxycodone-directed antibodies.22 Three of the subjects with the lowest responses (individuals 11, 15, and 16) had recently taken steroids or immunosuppressants. A final opioid-exposed subject with a low response (individual 22) was the only subject who reported having lower back pain for less than 2 years; however, this individual had reported taking opioids for 20 years overall. One additional note is that several subjects were taking drugs indicated for treatment of diabetes, a condition that not only increases the rate of AGE formation overall but also acts to suppress immune response.10,27 The outcomes in individuals taking these antidiabetic medications were highly variable. Our analysis was limited by the lack of opioid-naïve subjects that were recruited. Despite extensive recruitment efforts, we were unable to locate significant numbers of patients with chronic back pain with no opioid exposure. We cannot rule out the possibility that the lack of opioid response in this group is a result of the small sample size, given that the anti-opioid antibodies were only observed in approximately 50% of opioid-exposed individuals. We were, however, encouraged to observe a dose–response relationship between current daily dose and anti-opioid antibodies across all samples. We did not observe a similar relationship between response and lifetime dose. An important consideration for future work is that lifetime dose is a measure that may not be available for the general population, considering the intermittent and often temporally remote nature of opioid exposure. This likely complicates the ability of subjects to accurately recall total lifetime dosage, as exposure may have occurred even in utero.
Previous findings by Gamaleya et al. in other opioid-using populations indicated that a longer duration of opioid use is not associated with an increase in anti-opioid antibody titer, but it is associated with increased frequency of antibody presence in general.28 The data herein also reflect an overall lack of correlation between antibody amounts and duration of use, although this measure was sensitive to exclusion of a single outlier (see Figure S4). These findings support undertaking a broader study in order to determine the prevalence of these antibodies in wider populations as this study was not powered to determine the role of age, race, and gender on the development of anti-opioid antibodies, or their time to onset, which appears to be sooner than 6 months.
Furthermore, one of the exclusionary criteria in this study was previous use of illicit opioids. Separate study of this population is warranted, as illicit drugs are often adulterated with cutting agents such as levamisole, which has been associated with serious side effects, such as neutropenia, agranulocytosis, and arthralgia.29 These side effects are possibly due to the actions of a reactive metabolite of levamisole, such as aminorex, which also contains an available amine for bioconjugation.30 In addition to the presence of adulterants, illicit drugs are often utilized by other routes of administration, which may be associated with altered immune response; early research into penicillin haptenization showed that oral administration was less likely to cause allergic reactions compared to parenteral administration.18 Further expandsion of the pool of opioid-exposed subjects to include users of illicit opioids and gathering information regarding route of administration would therefore be likely to add additionally relevant information on the factors modifying the development of the observed anti-opioid antibodies. Inclusion of additional subjects with absent or relatively low opioid exposure will also be important to validate the modest dose–response correlation observed here.
Increasing our understanding of the conditions in which the form of these antibodies is important in the context of next-generation treatments for SUD; development of vaccines for various drugs of abuse is ongoing. Indeed, understanding the individual immunologic factors that can modify the efficacy of these vaccines could be key to effective patient selection, in order to proactively identify likely nonresponders.16
Regarding the antibodies that were detected, they exhibited enhanced recognition of N17-linked OxyBSA 1 versus C6-linked OxyBSA 2, suggesting that the N17 amine is more likely to be involved in the formation of the immunogenic species.31 Previous work has determined that nornicotine and methamphetamine react to form AGEs in serum, which subsequently lead to the development of an immune response. Our investigation suggests that the likelihood of norhydrocodone or noroxycodone reacting with glucose and then subsequently modifying serum albumin is unlikely, as the formation of the glucose-dependent Amadori product is much slower than the formation of the analogous nornicotine Amadori intermediate in vitro, and there was no subsequent conversion to the AGE observed for norhydrocodone. While seemingly at odds with the discovery of an immune response in some subjects, several explanations for this discrepancy are possible. In a study by Bunn and co-workers on reactivity of monosaccharides with hemoglobin, glucose reacted more slowly than did the majority of biologically relevant carbohydrates; thus, it is possible that norhydrocodone and noroxycodone react with an alternative sugar.32 Additionally, it is possible that as N-demethylation of oxycodone and hydrocodone occurs in the liver the AGE species could be occurring on a hepatic tissue protein not represented in the plasma sample. Indeed, previous human subject research on the link between inadvertent development of antinicotine antibodies and the presumed AGE responsible revealed that the protein involved in the process was a low molecular weight protein, not human serum albumin.10 Other studies have also implicated 1,5-anhydro-d-fructose (a metabolite of glycogen catabolism) in the formation of some AGEs, which also suggests the liver as a possible preferential site of AGE generation.33
We did find low levels of weakly opioid reactive antibodies even in the negative controls. There are several potential explanations for this phenomenon, which is consistent with previous research.28 First, due to the difficulty in locating subjects with chronic lower back pain who had never taken any opioids, the opioid-naïve cohort consisted of subjects who had not regularly taken opioids or taken opioids more than twice in the last 6 months and so may have had some previous exposure. Second, a study by Gamaleya found that there was cross-reactivity between a morphine antigen and beta-endorphin; thus, it is possible that the antibodies were generated in response to some other opioid-like structure instead of hydrocodone or oxycodone.28 Finally, the concentrations at which opioid-responsivity were seen were relatively high overall, and may represent nonspecific responding.
In summary, the studies described here demonstrate evidence that chronic use of opioids to treat lower back pain is associated with the presence of low concentrations of low-affinity anti-opioid IgM antibodies, particularly at higher opioid dosages. Future studies in this area are planned to isolate the key antigenic intermediate generating these antibodies and explore the use of anti-opioid IgM as a predictive biomarker for immunologic responses to experimental opioid vaccine approaches.
Acknowledgments
The authors thank Dr. Cameron Scarlett and Xiaolei Li at the Analytical Instrumentation Center at UW SOP for MALDI-ToF analysis. The authors thank Dr. Patrick Gentry for assistance with radio advertisement production.
Glossary
Abbreviations
- OUD
opioid use disorder
- ELISA
enzyme-linked immunosorbent assay
- AGE
advanced glycation end-product
- BSA
bovine serum albumin
- CDC
Centers for Disease Control and Prevention
- IgM
immunoglobulin M
- IgG
immunoglobulin
- NMDAR
N-methyl-d-aspartic acid receptor
- IgE
immunoglobulin E
- IgA
immunoglobulin A
- HRP
horseradish peroxidase
- TMB
tetramethylbenzidine
- PBS
phosphate-buffered saline
- O.D.
optical density
- LC/MS
liquid chromatography/mass spectrometry
- m/z
mass-to-charge ratio
- MALDI-ToF
matrix-assisted laser desorption/ionization time-of-flight
- SEM
standard error of the mean
- SD
standard deviation
- BMI
body mass index
- OTC
over the counter
- pIC50
–log(half-maximal inhibitory concentration)
- AUC
area under the curve
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.0c00057.
Additional methods, data, and materials supporting this publication(PDF)
Author Contributions
C.J.W. was responsible for study concept and experimental design, sample and data collection, and data analysis. J.L.K. was responsible for chemical synthesis, data collection, data analysis. M.M. was responsible for data collection. T.F.B. and H.P. were responsible for chemical synthesis. R.B. and K.D.J. were responsible for study concept. J.L.K., M.M., and C.J.W. were responsible for drafting the manuscript, and all authors edited and gave approval to the final version of the manuscript.
This work was generously funded by the ALSAM Foundation (to K.D.J.: 5–77693) as well as funds from the UW Madison School of Pharmacy and the UW Office of the Vice Chancellor for Research and Graduate Education.
The authors declare the following competing financial interest(s): K.D.J. has patents relating to the development and use of vaccine approaches to generate anti-opioid antibodies as a potential treatment for opioid use disorder, and he is a board member for Cessation Therapeutics but receives no compensation for his services.
Supplementary Material
References
- Scholl L.; Seth P.; Kariisa M.; Wilson N.; Baldwin G. (2018) Drug and Opioid-Involved Overdose Deaths — United States, 2013–2017. Morb. Mortal. Wkly. Rep. 67, 1419–1427. 10.15585/mmwr.mm6751521e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- President’s Commission on Combating Drug Addiction and the Opioid Crisis . Interim Report; Washington, DC, 2017.
- 2018 Annual Surveillance Drug-Related Risks and Outcomes -- United States; Centers for Disease Control and Prevention, 2018.
- Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings; NSDUH Series H-46, HHS Publication No. (SMA) 13–4795; Substance Abuse and Mental Health Services Administration: Rockville, MD, 2013.
- Centers for Disease Control and Prevention . U.S. Opioid Prescribing Rate Maps. https://www.cdc.gov/drugoverdose/maps/rxrate-maps.html (accessed May 4, 2020).
- Onishi E.; Kobayashi T.; Dexter E.; Marino M.; Maeno T.; Deyo R. A. (2017) Comparison of Opioid Prescribing Patterns in the United States and Japan: Primary Care Physicians’ Attitudes and Perceptions. J. Am. Board Fam. Med. 30, 248–254. 10.3122/jabfm.2017.02.160299. [DOI] [PubMed] [Google Scholar]
- Deyo R. A.; Smith D. H. M.; Johnson E. S.; Donovan M.; Tillotson C. J.; Yang X.; Petrik A. F.; Dobscha S. K. (2011) Opioids for Back Pain Patients: Primary Care Prescribing Patterns and Use of Services. J. Am. Board Fam. Med. 24, 717–727. 10.3122/jabfm.2011.06.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J.; Price D. D.; Mayer D. J. (1994) Thermal Hyperalgesia in Association with the Development of Morphine Tolerance in Rats: Roles of Excitatory Amino Acid Receptors and Protein Kinase C. J. Neurosci. 14, 2301–2312. 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. L.; Chen S.-R.; Chen H.; Pan H.-L. (2012) Chronic Opioid Potentiates Presynaptic but Impairs Postsynaptic N-Methyl-D-Aspartic Acid Receptor Activity in Spinal Cords: Implications for Opioid Hyperalgesia and Tolerance. J. Biol. Chem. 287, 25073–25085. 10.1074/jbc.M112.378737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson T. J.; Janda K. D. (2002) A Previously Undescribed Chemical Link between Smoking and Metabolic Disease. Proc. Natl. Acad. Sci. U. S. A. 99, 15084–15088. 10.1073/pnas.222561699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. L.; Lee S. Y.; Tao P. L.; Chang Y. H.; Chen S. H.; Chu C. H.; Chen P. S.; Lee I. H.; Yeh T. L.; Yang Y. K.; Hong J. S.; Lu R. B. (2012) Dextromethorphan Attenuated Inflammation and Combined Opioid Use in Humans Undergoing Methadone Maintenance Treatment. J. Neuroimmune Pharmacol. 7, 1025–1033. 10.1007/s11481-012-9400-1. [DOI] [PubMed] [Google Scholar]
- Biagini R. E.; Klincewicz S. L.; Henningsen G. M.; MacKenzie B. A.; Gallagher J. S.; Bernstein D. I.; Bernstein I. L. (1990) Antibodies To Morphine in Workers Exposed to Opiates at a Narcotics Manufacturing Facility and Evidence for Similar Antibodies in Heroin Abusers. Life Sci. 47, 897–908. 10.1016/0024-3205(90)90604-P. [DOI] [PubMed] [Google Scholar]
- Gamaleya N. B.; Parshin A. N.; Tronnikov S. I.; Yusupov D. V. (1993) Induction of Antibodies to Morphine during Chronic Morphine Treatment in Rodents and Opiate Addicts. Drug Alcohol Depend. 32, 59–64. 10.1016/0376-8716(93)90022-I. [DOI] [PubMed] [Google Scholar]
- Ringle D. A.; Herndon B. L. (1975) Immunologic Effects of Morphine Administration in Rabbits. J. Immunol. 115, 876–883. [PubMed] [Google Scholar]
- Dickerson T. J.; Yamamoto N.; Ruiz D. I.; Janda K. D. (2004) Immunological Consequences of Methamphetamine Protein Glycation. J. Am. Chem. Soc. 126, 11446–11447. 10.1021/ja047690h. [DOI] [PubMed] [Google Scholar]
- Orson F. M.; Rossen R. D.; Shen X.; Lopez A. Y.; Wu Y.; Kosten T. R. (2013) Spontaneous Development of IgM Anti-Cocaine Antibodies in Habitual Cocaine Users: Effect on IgG Antibody Responses to a Cocaine Cholera Toxin B Conjugate Vaccine. Am. J. Addict. 22, 169–174. 10.1111/j.1521-0391.2013.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B. B.; Ovary Z. (1961) Studies on the Mechanism of the Formation of the Penicillin Antigen. III. The N-(D-Alpha-Benzylpenicilloyl) Group as an Antigenic Determinant Responsible for Hypersensitivity to Penicillin G. J. Exp. Med. 114, 875–904. 10.1084/jem.114.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solensky R. (2004) Drug Desensitization. Immunol. Allergy Clin. North Am. 24, 425–443. 10.1016/j.iac.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Bremer P. T.; Schlosburg J. E.; Banks M. L.; Steele F. F.; Zhou B.; Poklis J. L.; Janda K. D. (2017) Development of a Clinically Viable Heroin Vaccine. J. Am. Chem. Soc. 139, 8601–8611. 10.1021/jacs.7b03334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockner J. W.; Lively J. M.; Collins K. C.; Vendruscolo J. C. M.; Azar M. R.; Janda K. D. (2015) A Conjugate Vaccine Using Enantiopure Hapten Imparts Superior Nicotine-Binding Capacity. J. Med. Chem. 58, 1005–1011. 10.1021/jm501625j. [DOI] [PubMed] [Google Scholar]
- Bremer P. T.; Janda K. D. (2017) Conjugate Vaccine Immunotherapy for Substance Use Disorder. Pharmacol. Rev. 69, 298–315. 10.1124/pr.117.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimishima A.; Wenthur C. J.; Zhou B.; Janda K. D. (2017) An Advance in Prescription Opioid Vaccines: Overdose Mortality Reduction and Extraordinary Alteration of Drug Half-Life. ACS Chem. Biol. 12, 36–40. 10.1021/acschembio.6b00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M.; Le Naour M.; Harmon T. M.; Tucker A. M.; Portoghese P. S.; Pentel P. R. (2012) An Oxycodone Conjugate Vaccine Elicits Drug-Specific Antibodies That Reduce Oxycodone Distribution to Brain and Hot-Plate Analgesia. J. Pharmacol. Exp. Ther. 341, 225–232. 10.1124/jpet.111.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopdekar V. M., Redkar S. N., and Schlek J. R.. Opioid Tannate Compositions. US 20040157784A1, 2004.
- Iijima I.; Minamikawa J.; Jacobson A. E.; Brossi A.; Rice K. C.; Klee W. A. (1978) Studies in the (+)-Morphinan Series. 5. Synthesis and Biological Properties of (+)-Naloxone. J. Med. Chem. 21, 398–400. 10.1021/jm00202a018. [DOI] [PubMed] [Google Scholar]
- Frank J. W.; Levy C.; Matlock D. D.; Calcaterra S. L.; Mueller S. R.; Koester S.; Binswanger I. A. (2016) Patients’ Perspectives on Tapering of Chronic Opioid Therapy: A Qualitative Study. Pain Med. (United States) 17, 1838–1847. 10.1093/pm/pnw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.; Meng G.; Liu F.; Zhang Q.; Liu L.; Wu H.; Du H.; Shi H.; Xia Y.; Liu X.; Li C.; Bao X.; Su Q.; Gu Y.; Fang L.; Yu F.; Yang H.; Yu B.; Sun S.; Wang X.; Zhou M.; Jia Q.; Chen X.; Huang G.; Song K.; Niu K. (2016) Serum Levels of Immunoglobulins in an Adult Population and Their Relationship with Type 2 Diabetes. Diabetes Res. Clin. Pract. 115, 76–82. 10.1016/j.diabres.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Gamaleya N. B. (1993) Antibodies to Drugs as Indicators of Chronic Drug Use. An Alternative to Toxicological Hair Analysis. Forensic Sci. Int. 63, 285–293. 10.1016/0379-0738(93)90282-F. [DOI] [PubMed] [Google Scholar]
- Lee K. C.; Ladizinski B.; Federman D. G. (2012) Complications Associated with Use of Levamisole-Contaminated Cocaine: An Emerging Public Health Challenge. Mayo Clin. Proc. 87, 581–586. 10.1016/j.mayocp.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan A. L.; Jen K. Y. (2015) Pathologic Manifestations of Levamisole-Adulterated Cocaine Exposure. Diagn. Pathol. 10, 4–9. 10.1186/s13000-015-0279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas G. R.; Rice K. C.; Cheng K.; Li F.; Antoline J. F. G.; Iyer M. R.; Jacobson A. E.; Mayorov A. V.; Beck Z.; Torres O.; Alving C. R. (2014) Facial Recognition of Heroin Vaccine Opiates: Type 1 Cross-Reactivities of Antibodies Induced by Hydrolytically Stable Haptenic Surrogates of Heroin, 6-Acetylmorphine, and Morphine. Vaccine 32, 1473–1479. 10.1016/j.vaccine.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F.; Higgins P. J. (1981) Reaction of Monosaccharides with Proteins: Possible Evolutionary Significance. Science 213, 222–224. 10.1126/science.12192669. [DOI] [PubMed] [Google Scholar]
- Sakasai-Sakai A.; Takata T.; Suzuki H.; Maruyama I.; Motomiya Y.; Takeuchi M. (2019) Immunological Evidence for in Vivo Production of Novel Advanced Glycation End-Products from 1,5-Anhydro-D-Fructose, a Glycogen Metabolite. Sci. Rep. 9, 10194. 10.1038/s41598-019-46333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.