Abstract
Background. To review currently available evidence on the effect of cow-milk proteins supplementation (CPS) on health in the elderly. Methods. Five electronic databases (Pubmed, Web of Science, Embase, Cochrane Library, ClinicalTrials.gov) were searched for studies about CPS among older people. All types of publications were included, with the exception of systematic reviews, meta-analyses, opinion letters, editorials, case reports, conference abstracts and comments. An additional search in Google Scholar and a manual review of the reference lists were performed. Results. Overall, 103 studies were included. Several studies explored the role of CPS in the preservation or improvement of muscle mass among healthy subjects (40 studies) and pre-frail, frail or sarcopenic patients (14), with evidence of beneficial effects. Other studies assessed the effect of CPS on bones (12), cardiovascular disease (8), inflamm-aging (7), chronic pulmonary disease (4), neurocognitive function (4), and vaccines (2), with weak evidence of positive effects. Seven studies in the field of protein metabolism investigated the role of CPS as an important contributor to nutritional needs. Other investigational areas are considered in the last five studies. Conclusions. The beneficial effects of CPS in achieving aged-related nutritional goals, in preserving muscle mass and in recovering after hospitalization may be particularly relevant in the elderly.
Keywords: whey protein, casein, older people, muscle mass, sarcopenia, nutrition
1. Introduction
The World Health Organization declared the years between 2020 and 2030 as the Decade of Healthy Ageing. By the end of 2020, the number of people aged over 60 years old will surpass the number of children under 5 years old. Elderly people will globally increase from 1 billion in 2019 to 1.4 billion in 2030 (about 34% increase rate). By 2050, the proportion of people aged 60 years among the population is expected to be one in five [1]. Considering this demographic transition, the preservation of wellbeing is a crucial issue of ‘adding years to life’ [2].
Since the beginning of 2000, ‘healthy ageing’ has been defined as “a lifelong process optimising opportunities for improving and preserving health and physical, social and mental wellness, independence, quality of life and enhancing successful life-course transitions” [3].
An emerging condition affecting healthy aging is sarcopenia. In 2009, the International Sarcopenia Consensus Conference Working Group defined sarcopenia as “an age-related loss of skeletal muscle mass, with or without an increase in fat mass” [4]. More recently, sarcopenia has been defined by a European Consensus as “a muscle disease (muscle failure) rooted in adverse muscle changes that accrue across a lifetime” [5]. According to this new consensus, the key characteristics of sarcopenia are low muscle strength and reduced physical performance. Optimal care is crucial in the prevention and treatment of sarcopenia, because this condition is related to increased risk of fractures, impaired ability to perform activities of daily living, cardiovascular and respiratory diseases, cognitive impairment, loss of independence, and eventually death.
Scientific evidence suggests a central role of protein intake in preserving lean mass and preventing sarcopenia, but the definition of the best quantity and quality of protein sources is still an open issue [6,7,8].
Ageing is a plastic process, and it may affect nutritional requirements [9,10]. For example, basic science studies demonstrated that protein metabolism in the elderly is characterized by a high splanchnic extraction and a declining anabolic response to ingested proteins [11]. Lifestyle factors, such as high-quality diet, physical activity, little or no alcohol consumption and smoking avoidance, can influence the quality of ageing, improving wellbeing throughout the life span [9]. Taking into account these findings, the European Union Geriatric Medicine Society (EUGMS), in cooperation with other scientific organizations, appointed an international study group to review dietary protein needs with aging (PROT-AGE Study Group) [11]. According to PROT-AGE position paper, recommendations for dietary protein intake in healthy older adults are as follows:
average protein intake for older people should range from 1.0 to 1.2 g/kg of body weight per day (while in young adults, the recommended intake is about 0.7–0.8 g/kg/day) [12];
it must be taken into account that the feeding-associated anabolic threshold for dietary protein is higher in the elderly than in younger subjects, with the amount of protein required to reach it from a variety of foods being in the order of 25–30 g of protein per meal;
dietary recommendations for protein intake in the elderly should consider, beyond quantity, also quality, protein source and timing of intake;
best protein sources are rich in leucine;
oral supplementation should be considered when dietary protein intake does not reach recommended goals [11].
Sources of animal proteins, such as meat, fish and poultry, are excellent for their essential amino-acid content, but their consumption may be impaired in the elderly, because of poor dentition, reduced appetite, or even anorexia, solid dysphagia, taste alteration, cost, and, when mobility is reduced, barriers in shopping and cooking [13,14]. Legumes and pulses are even good protein sources, but may enhance gastrointestinal functional disorders, such as slow gastric emptying, bloating, abdominal distention and diarrhea [15]. Dairy foods are rich in leucine, and they are available in many different forms, even soft and enriched with probiotics, but the weekly amount is usually restricted to 2–3 servings (excluding milk and yogurt), due to their fat content [16].
There is a large variety of protein oral supplements, mainly based on soy or cow-milk sources [17]. Among the latter, whey proteins (WP), a by-product of cheese making, should be regarded as one of the best sources for oral protein supplementation, for their high leucine content, fast digestibility and amino-acid availability (demonstrated in both young and old subjects) [18,19].
With regard to these considerations, we performed a systematic literature review, to investigate the role of cow-milk proteins (hence forward generally called “milk proteins”) supplementation in the elderly, exploring its effects on several health outcomes particularly relevant to older people (e.g., muscle and bone mass preservation, cognitive performance, cardiovascular risk factors). Our aim was to map the scientific evidence currently available, in order to evaluate which outcomes have already been widely targeted, and to identify those which still have to be studied in depth with new trials. We also aimed to assess the presence of knowledge gaps yet to be addressed. We expected to include studies with a high level of heterogeneity, so we considered it to be more appropriate to present the results of the systematic literature review as a narrative synthesis.
2. Materials and Methods
The systematic literature review started in April 2019, and it has been performed according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [20]. The review has been registered on PROSPERO—international prospective register of systematic reviews (CRD 42020137114).
2.1. Literature Search Strategy
Five electronic databases were systematically searched: PubMed, Web of Science, Embase, Cochrane Library, ClinicalTrials.gov. The strings used for the search in these databases were based on Medical Subject Heading (MeSH) terms, text keywords and Boolean operators, as follows:
PubMed: “Milk Proteins/administration and dosage” [MeSH] OR “Milk Proteins/adverse effects” [MeSH] OR “Milk Proteins/metabolism” [MeSH] OR “Milk Proteins/organization and administration” [MeSH] OR “Milk Proteins/pharmacokinetics” [MeSH] OR “Milk Proteins/pharmacology” [MeSH] OR “Milk Proteins/supply and distribution” [MeSH] OR “Milk Proteins/therapeutic use” [MeSH] OR “Milk Proteins/therapy” [MeSH] OR “Milk Proteins/toxicity” [MeSH]) AND “aged” [MeSH].
Web of Science: (“milk proteins” OR “whey proteins” OR “caseins”) AND (“older” OR “elderly”) in the categories Nutrition Dietetics, Endocrinology Metabolism, Medicine Research Experimental, Geriatrics Gerontology, Toxicology, Chemistry Medicinal, Gastroenterology Hepatology, Medicine General Internal, Pathology, Integrative Complementary Medicine, Gerontology, Rehabilitation.
Embase: (“milk proteins” OR “whey proteins” OR “caseins”) AND (“aged” OR “old” OR “elderly”) in titles, abstracts or keywords.
Cochrane Library: (“milk proteins” OR “whey proteins” OR “caseins”) AND (“aged” OR “old” OR “elderly”) in title, abstract or keywords.
ClinicalTrial.gov was checked for ongoing or unpublished trials: “ageing” as condition or disease, “milk proteins” as other terms.
A search in Google Scholar was also performed using the terms “milk proteins” AND “elderly”.
The whole literature search was limited to interventions on human beings, and the language restricted to English, whereas no restriction on publication year was applied.
The reference lists of the eligible studies were manually searched for additional articles.
2.2. Study Selection
Two investigators (B.Z. and M.Z.) independently selected the studies to be included in the review, on the basis of pre-defined eligibility criteria and screened titles and abstracts. One author (BZ) checked full texts for final inclusion. Any disagreement was resolved by discussion and re-examination of the studies by two investigators (B.Z. and M.Z.).
To be included in the review, studies had to consider: (i) subjects aged 60 years or more, or (ii) menopausal/postmenopausal women. We excluded studies whose target groups were children, adolescent, young or adults, or if an aged-based analysis in subjects over 60 years was not provided. In all included studies, at least one intervention arm had to include supplementation with cow-milk proteins, caseins, whey proteins or bioactive cow-milk peptides. We included randomized clinical trials (RCTs), observational, cross-sectional, cohort and case-control studies. We excluded opinion letters, editorials, case reports, conference abstracts, and comments. We also excluded systematic reviews and meta-analyses, after having manually searched their references lists to be sure that all relevant studies were already included in our review.
Studies are presented as a narrative synthesis, organized in sub-sections according to the different health outcomes. We reported the main features of each study, including information about participants, intervention and principal endpoints.
3. Results
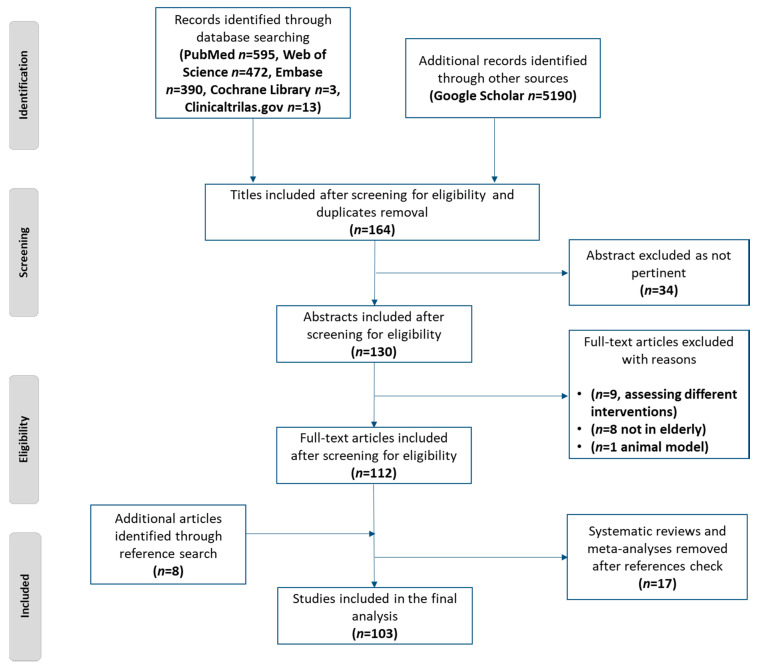
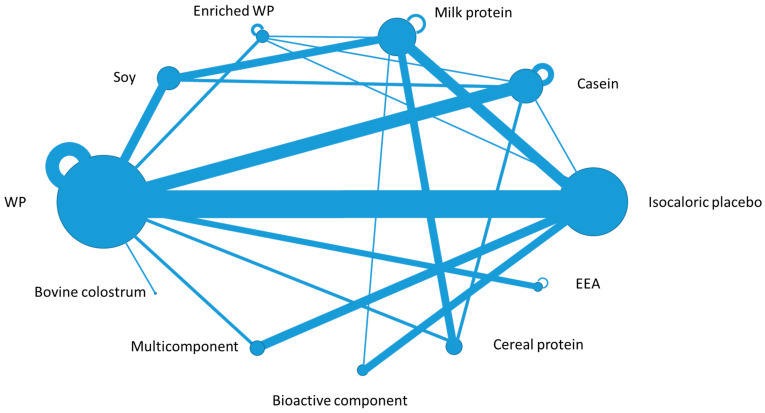
According to the predefined search strategy, 1473 studies were obtained from the 5 databases and 5190 studies from Google Scholar. The flow diagram of the screening process is reported in Figure 1. At the end of the screening process, 103 studies were included in the narrative synthesis, and out of them, 82 were RCTs. Figure 2 provides the network plot of the 11 intervention arms considered in the RCTs included in the review.
Figure 1.
Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) flow diagram of the literature screening process.
Figure 2.
The network plot of the 11 intervention arms included in the randomized clinical trials (82 studies). The intervention arms are coded in the plot as nodes: each node size is proportional to the number of direct comparisons involving each intervention. The 20 lines between nodes represent direct comparisons driven by the trials; the line thickness is proportional to the number of studies where the direct comparison was performed. The additional semicircles over five nodes represent comparisons of different dosages within the same intervention (WP: Whey Proteins, EEA: Essential Amino Acids).
3.1. Muscle Related Endpoints among Healthy Subjects (40 Studies)
In this section we considered only studies recruiting old healthy subjects, aged 60 years or more, not specifically addressed as ‘patients’. Subjects recruited in the studies of this section were not sarcopenic, hospitalized, frail or at risk for frailty). Ageing is commonly associated with a loss in skeletal muscle mass, and epidemiological research has assessed a strong association between muscle strength and risk for developing age-related diseases and mortality [21]. Protein intake with diet is crucial in stimulating muscle protein synthesis (MPS or anabolism), and in decreasing muscle protein breakdown (catabolism). Proteins in diet can affect the extent and the duration of muscle anabolism, both in young and elderly people [22].
In both elderly women and men, the leucine content of dietary protein seems to be the primary determinant of postprandial MPS [23,24]. Leucine is the most potent amino acid, because of its role as a signal in activation of protein anabolism. Leucine is a strong activator of protein synthesis, but its anabolic threshold is impaired in the elderly—a phenomenon called ‘anabolic resistance’ [25]. As a result, during ageing, a higher content of leucine intake may be necessary to reach an effective anabolic leucinemia level, correctly stimulating muscle anabolism [26,27]. Interestingly, leucinemia-mediated post prandial MPS is not affected by the previous habituation to a low or high protein content of diet in the elderly (0.7 g/kg vs. 1.5 g/kg) [28]. Moreover, a study among 12 healthy older and 12 young men confirmed a good MPS response in both groups, following the ingestion of a meal-like amount of dietary proteins (20 g casein) and carbohydrates (40 g) [29].
In Table 1, we gathered fifteen studies aiming to identify the best protein supplementation able to optimize digestion, absorption, post-prandial peak in leucinemia level, body protein balance, muscle protein synthesis and accretion [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. These studies mainly assessed the effect of a single dose administration of different protein supplementations. Despite several differences in intervention arms, these studies provide evidence of the superiority of WP, in comparison to casein, in stimulating MPS, with better results with leucine-enriched WP supplements and higher WP doses (35 g).
Table 1.
Studies aimed to identify the best protein supplementation in inducing muscle protein synthesis or accretion, after acute administration.
| Author, Year | Number Participants, Gender | Age (Mean or Range) | Type of Study: Intervention Arms | Main Endpoints | Results |
|---|---|---|---|---|---|
| Paddon-Jones, 2006 [30] | 14, 7 ♀/7 ♂ | 68 | RCT: 15 g WP vs. 15 g EAA | Muscle FSR for 3.5 h after ingestion | Both supplementations stimulated FSR, with greater increase in EAA arm |
| Katsanos, 2008 [31] | 15, 6 ♀/9 ♂ | 60–85 | RCT: 15 g WP vs. 6.72 g WP’s EAA vs. 7.57 WP’s Non-EAA | blood phenylalanine, insulin, glucose concentration, muscle biopsy | WP improves MP accrual through mechanisms beyond its EAA content |
| Koopman, 2009 [32] | 10, ♂ (cross over) | 64 | Case-control study: 35 g intact casein vs. 35 g hydrolyzed casein | blood phenylalanine concentration, muscle biopsy (FSR) | Hydrolysate accelerates protein digestion and absorption, increase AA availability and FSR |
| Pennings, 2011 [33] | 48, ♂ | 74 | RCT: 20 g WP vs. 20 g casein vs. 20 g casein hydrolysate | Postprandial Muscle FSR | MP accretion more effective in WP arm |
| Burd, 2012 [34] | 14, ♂ | 71 | RCT: 20 g micellar casein vs. 20 g WP | Rate of MPS at rest and after exercise | Greater rates of MPS in WP arm |
| Groen, 2012 [35] | 16, ♂ | 74 | RCT: intra-gastric administration during sleep of 400 mL of water with vs. without 40 g casein | BPB, MPS | Casein administration during sleep improves BPB and stimulates MPS |
| Pennings, 2012 [36] | 33, ♂ | 73 | RCT: 10 g vs. 20 g vs. 35 g WP | AA absorption, BPB, MPA | 35 g WP reaches best values in all endpoints |
| Wall, 2013 [37] | 24, ♂ | 74 | RCT: 20 g casein vs. 20 g casein + 2.5 g leucine | MPA | Leucine co-ingestion improves MPA |
| Luiking, 2014 [38] | 19, 10 ♀/9 ♂ | 69 | RCT: 20 g WP vs. 6 g milk protein, both arms after unilateral resistance exercise | MPS | Higher MPS with WP, without further enhance with exercise |
| Churchward-Venne, 2015 [39] | 32, ♂ | 71 | Parallel group study: 25 g casein in milk matrix vs. 25 g casein in water | Post-prandial MPS | Milk matrix delays casein digestion and absorption without affecting MPS |
| Borack, 2016 [40] | 20, ♂ | 55–75 | RCT: 30 g WP isolate vs. 30 g soy-dairy protein blend (25% soy, 25% WP and 50% casein); both arms after resistance exercise | Blood and muscle AA concentration; FSR | No differences in endpoints among arms |
| Gorissen, 2016 [41] | 60, ♂ | 71 | RCT: 35 g WhP vs. 35 g WhPH, vs. 35 g micellar casein vs. 35 g WP vs. 35 g WPH vs. 60 g WhP | Post-prandial AA concentration and MPS | Greater AA concentration after WP, greater MPS after micellar casein |
| Walrand, 2016 [42] | 31, ♂ | 72 | RCT: 10-day period of AP or HP diet followed by ingestion of 15 g or 30 g casein vs. 15 g or 30 g of soluble milk proteins | FSR | Greater increase in FSR after ingestion of soluble milk proteins only in the AP group |
| Kouw, 2017 [43] | 48, ♂ | 72 | RCT: before sleep administration of 40 g casein vs. 20 g casein vs. 20 g casein + 1.5 g leucine vs. placebo | MPS | Ingestion of 40 g casein increases MPS better than other arms |
| Hamarsland, 2019 [44] | 21, 8 ♀/13 ♂ | 74 | RCT: 20 g WP vs. 20 g native WP vs. milk (ingested after 2 h of resistance training) | Serum leucine concentration; FSR | Greater increase in serum leucine in native WP arm, but no difference with WP in FSR (only superior to milk) |
♀: females; ♂: males; RCT: Randomized Clinical Trial; WP: Whey Proteins; EAA; Essential Amino Acids; FSR: Fractional Synthetic Rate; MP: Muscle Protein; MPS: Muscle Protein Synthesis; BPB: Body Protein Balance, AA: Amino Acids; MPA: Muscle Protein Accretion; WhP: Wheat Protein; WhPH: Wheat Protein Hydrolysate; WPH: Whey Protein Hydrolysate; Adequate Protein; HP: High Protein.
In recent years, several RCTs investigated the effects of different multicomponent supplementation, consisting of WP, in association with other proteins, macro- or micro-nutrients:
co-ingestion of carbohydrates and fats with 21 g of leucine enriched WP did not affect the improvement of MPS rates, among 45 nonsarcopenic older men [45];
a high WP-leucine- and vitamin D-enriched supplement (21 g protein in 150 Kcal/serving, 10 servings per week) was effective in preserving muscle mass during intentional weight reduction in association with regular physical activity, among 80 obese older adults [46];
leucine-enriched WP (21 g) and vitamin D (800 IU) daily supplementation before breakfast enhanced post prandial MPS (acute effect) and muscle mass (long term effect) in 24 healthy elderly men in a ‘proof of principle’ trial [47];
a multi-ingredient supplementation consisting of 30 g WP, 2.5 g creatine, 500 IU vitamin D, 400 mg calcium and 1500 mg n-3 PUFA was tested with and without exercise versus placebo and was effective in increasing both muscle strength and mass among 49 older men [48];
co-ingestion of milk fat (26.7 g) did not affect the raise in plasma amino-acids and MPS after the ingestion of 20 g of casein, among 24 healthy older males [49].
A preliminary study on the effect of resistance exercise (RE), in combination with ingestion of a WP source (versus a control placebo group with RE alone) on myostatin and cell cycle related gene expression, demonstrated a positive effect of WP intake [50]. Another preliminary study concluded that a single session of neuromuscular electrical stimulation, prior to 20 g casein administration, was not effective in augmenting post-prandial MPS in older adults [51].
Nine RCTs further tested the effects of combination of different protein sources and RE in the elderly. The main findings of these studies are reported in Table 2 [52,53,54,55,56,57,58,59,60]. The overall results of these studies are concordant in providing evidence of the beneficial effects of RE and training, with some evidence encouraging a nutritional support with WP.
Table 2.
Randomized Clinical Trials testing the effects of different protein supplementation in combination with exercise training.
| Author, Year | Number Participants, Gender | Age (Mean or Range) | Duration | Intervention Arms | Main Endpoints | Results |
|---|---|---|---|---|---|---|
| Dideriksen 2011 [52] | 24, 9 ♀/15 ♂ | 68 | acute supplementation | RE + WP (0.45 g/kg) vs. RE + caseinate (0.45 g/kg) | MPS | Increase in MPS, no difference between arms |
| Yang 2012 [53] | 37 ♂ | 71 | acute supplementation | WP 0 g, 10 g, 20 g or 40 g vs. WP same doses + RE | MPS | RE increases MPS at all WP doses with greater extent with 40 g WP |
| Arnarson 2013 [54] | 161, 94 ♀/67 ♂ | 65–91 | 12 weeks | RE + WP (20 g) vs. RE + isocaloric CHO | Lean body mass, strength, physical function | Increase in all endpoints, no difference between arms |
| Gryson 2014 [55] | 48, ♂ | 61 | 16 weeks (sedentary) | MET + total milk proteins (10 g) vs. MET + soluble milk proteins rich in leucine (10 g) | Muscle mass and strength, time to task failure, index of muscle fatigue | Better results in all endpoints with soluble milk proteins + after MET |
| Karelis 2015 [56] | 99, 76 ♀/23 ♂ | 65–88 | 135 days | 20 g of cysteine enrich-WP vs. 20 g casein (both arms in combination with RT) | Body composition (DXA), muscle strength | Muscle strength increases in both arms, additional increasing WP arm |
| Weisgarber 2015 [57] | 12, ♀ | 57 | 10 weeks | RE (high volume) + WP (40 g) vs. RE + placebo | Lean tissue mass, muscle thickness, muscle strength | Increase in muscle thickness and strength, but no difference between arms |
| Thomson 2016 [58] | 179, 99 ♀/80 ♂ | 62 | 12 weeks | RE + high dairy protein (1.2 g/kg) vs. RE +high soy protein (1.2 g/kg) vs. RE + usual protein intake (<1.2 g/kg) | Muscle strength, body composition, physical function, quality of life | Increase in lean mass, physical function and mental health in all arms, increase in strength attenuated in soy arm |
| Mori 2018 [59] | 81, ♀ | 65–80 | 24 weeks | RE + WP (22.3 g/day) vs. RE alone vs. WP alone | Muscle mass, physical function | Higher improvement in all endpoints in RE + WP arm |
| Sugihara 2018 [60] | 31, ♀ | 67 | 12 weeks | RE + WP (35 g) vs. RE + placebo | Muscle strength, hypertrophy, muscle quality | Higher increase in muscle strength and hypertrophy in RE + WP |
♀: females; ♂: males; RE: Resistance Exercise; WP: Whey Protein; MPS: Muscle protein Synthesis; CHO: Carbohydrates; MET: multicomponent exercise training; RT: Resistance Training; DXA: Dual energy X-ray Absorptiometry.
Four RCTs tested the effects of a daily administration of different protein supplements among older people along different periods (from 15 days to 2 years) [61,62,63,64]. In the USA, 15-day consecutive administration of 0.4 g/kg of body weight of tryptophan-fortified collagen to 9 older women resulted in a better nitrogen balance and lean mass than the same dose of WP [61]. In Norway, a 12-week supplementation with a protein-enriched milk twice a day (for a total of 40 g protein/day), did not improved muscle mass and strength among 50 men and women aged over 70 years [62]. In China, a 6-month trial among 180 postmenopausal women revealed a mild favorable effect on body composition of 15 g soy protein daily supplementation, in comparison to 15 g WP + 100 mg isoflavones or 15 g milk proteins [63]. In Australia, a long term trial among well-nourished 229 postmenopausal women (196 included in the analysis) did not confirm the efficacy of adding 30 g of WP in improving muscle mass and function [64].
3.2. Muscle Related Endpoints among Patients (14 Studies)
In this section, we considered only studies recruiting older people, addressed as patients, in relation to a condition of sarcopenia, pre-frailty or frailty or hospitalization.
A basic science study demonstrated that a leucine-enriched WP supplementation (21 g) was able to stimulate post-prandial MPS in both sarcopenic and healthy older men [65]. Further studies among sarcopenic patients investigated the effect of different nutritional supplements, in association or not with physical activity. The main findings of these studies are the following:
during a six-month resistance training (RT) intervention among 80 mobility-limited older adults, 40 g of daily WP supplementation did not add benefit to exercise in improving lean mass, muscle strength and physical function [66];
a leucine-enriched WP supplement with vitamin D (20 g + 800 IU, twice a day for 13 weeks) was tested versus an iso-caloric dietary supplement, and was superior to placebo in improving muscle mass and lower-extremity function among a large cohort of 380 sarcopenic older adults, even in patients who were unable to exercise [67];
the association of physical activity with a daily supplementation consisting of WP (22 g), essential amino acids (10.9 g including 4 g of leucine) and vitamin D (100 IU) was more effective than physical activity plus placebo in increasing fat free mass and muscle strength, in improving quality of life and in decreasing inflammation index in 130 sarcopenic elderly people [68];
the combination of regular resistance muscle training with a nutrition therapy based on an oral supplement offered twice daily (containing 20 g WP, 9 g carbohydrates, 3 g fat, 800 IU vitamin D, and a mixture of vitamins, minerals, and fibers per serving) was superior to exercise alone in improving muscle mass and strength, in 34 elderly patients at high risk of sarcopenia [69];
the combination of RE with different isocaloric shakes containing 12 g of milk protein or 12 g of soy proteins versus placebo (rice milk, considered as non-protein control) had a positive effect on muscle mass, independently from the type of protein source (milk or soy), among 26 sarcopenic men. The same intervention study among 26 overweight sarcopenic men resulted in a decrease in fat mass only in the dairy supplemented group [70,71].
A double blind, randomized, multicenter study is taking place in Denmark to evaluate the efficacy of a ready-to-drink milk based, protein enriched supplement (27.5 g WP per day for 12 weeks), versus an iso-caloric placebo in acutely ill geriatric patients, at high risk of developing sarcopenia, recruiting 165 older adults [72]. The primary endpoint of this trial is lower extremity muscle strength and function. Up to date, no results of this trial have been published.
Two RCT assessed the effect of cow-milk protein supplements among frail patients in association or not with RT [73,74]. In the study by Dirks and colleagues, the supplementation of 15 g milk protein twice a day for 24 weeks, in association with RT, was superior to RT + placebo in augmenting muscle fiber [73]. In the study by Niccoli and colleagues, 47 hospitalized geriatric patients not able to participate in high intensity RT were randomly assigned to receive, or not, 24 g WP daily supplement, with a significant positive correlation between WP supplementation and both nutritional and rehabilitation outcome measures [74]. Similarly, in a 12-week intervention RCT among 120 frail and pre-frail old subjects at risk of malnutrition for a low baseline protein intake, participants received an individual adjusted amount of WP supplementation, to fulfil a total daily protein intake of 1.5 g/kg or 1.2 g/kg or 0.8 g/kg [75]. This study demonstrated that the total protein intake of 1.5 g/kg/day was statistically superior in improving muscle mass and physical performance without adverse effects, and suggested this protein intake goal as the most beneficial in the geriatric population.
In 2017, a Brazilian series of multicenter RCTs in pre-frail and frail elderly was launched and the rationale and protocol were published [76]. The main objective of these trials, called “Pro-Elderly Study”, is to assess the effect of 16-week intervention of different protein source supplementation, with or without RT, on the following end-points: muscle mass, strength and function, nutritional status, body composition, renal function and quality of life. The study design for pro-elderly was preceded by an exploratory trial, testing different dose combinations of WP, in association, or not, with creatine, together with RT. The preliminary trial did not support the addition of creatine in improving muscle function [77]. To date, no results for pro-elderly have been published.
In a Finnish RCT, among a cohort of 106 nursing home residents, mainly at risk of malnutrition, 20 g daily supplementation of WP vs. placebo was positively associated with maintenance of skeletal muscle mass, reduction of required assistance and improvement in general well-being [78].
3.3. Bones (12 Studies)
It is well known that bones deteriorate in composition, structure and function as a physiological effect of the ageing process, in both men and women [79]. The role of protein intake in this decline process is still under investigation. In our literature search, the majority of the studies addressing this health-related issue were gender specific. Although high-protein diets induce an increase in loss of calcium with urine, a pilot study among women, with a subgroup over 65 years of age, demonstrated that the calcium present in the urine is not of bone origin, and that high protein diets, up to 2.1 g/kg, are not detrimental for bone metabolism, at least in the short term [80]. Two cross-sectional large studies among 746 postmenopausal women (mean age 65 years) and 1016 older men (mean age 84 years), in Europe and USA respectively, were concordant in finding a positive association between the higher intake of animal-based and dairy protein (analyzed as a subgroup of animal-based sources) and outcomes of bone health (strength, microstructure and failure load). The studies did not find a significant association between plant-based proteins and outcomes of bone health [81,82].
Six RCTs investigated the effect of daily supplementation of different combination of protein sources, calcium, and fructans on several bone related endpoints [83,84,85,86,87,88]. The methodology and results of these trials are reported in Table 3. Despite heterogeneity in study design, milk-derived supplementation had some beneficial effects (increased insulin-like growth factor-1 (IGF-1) or reduced bone resorption markers) in 4 out of 6 RCTs.
Table 3.
RCTs investigating the effect of daily supplementation of different combination of protein sources, calcium and fructans on several bone related endpoints.
| Author, Year | Number Participants, Gender | Age Mean and/or Range | Duration | Intervention Arms | Main Endpoints | Results |
|---|---|---|---|---|---|---|
| Khalil 2002 [83] | 17, ♂ | 65–84 | 3 months | Daily supplementation of 40 g SP vs. 40 g MP | Bone specific ALP activity, urinary deoxypyridinoline excretion | No endpoint difference among arms |
| Holm 2008 [84] | 29, ♀ Postmenopausal | 55 | 24 weeks | 10 g WP + 31 g CHO + 1 g fat + 5 mcg vitamin D + 250 mg calcium vs. 6 g CHO + 12 mg calcium; both arms with ST | BMD with DXA, Osteocalcin, CTx | Increase in BMD and osteocalcin in WP multi-ingredient arm |
| Adolphi 2009 [85] | 85, ♀ postmenopausal | 59 (48–67) | 2 weeks | Bedtime consumption of 175 mL Fm vs. 175 mL Fm + 510 mg Calcium vs. Fm+ 510 mg calcium + 0.175 g CPP + 1.75 g ITF | Nocturnal bone resorption markers | Fm reduced bone resorption independently of further supplementation |
| Chevallley 2010 [86] | 45, ♀ (recent hip fracture) | 81 | 1 week | Daily supplementation of 20 g casein vs. 15 g WP vs. 5 g EAA | Elevation of circulating IGF-1 | Increase in IGF-1 in casein arm supplementation |
| Zhu 2011 [87] | 219, ♀ | 70–80 | 2 years | Daily supplementation of a drink with 30 g WP vs. placebo | BMD with DXA and QCT. IGF-1 level, urinary calcium excretion | Increase in IGF-1 at year 1 and 2 in WP arm, but no effect on bone mass or strength |
| Kerstetter 2015 [88] | 208, 178 ♀/30 ♂ | 70 | 18 months | Supplementation of 45 g WP vs. isocaloric placebo | BMD with DXA, fat free mass | No difference in BMD, better preservation of fat free mass in WP arm |
♂: males; ♀: females; SP: Soy Protein; MP: Milk Protein; ALP: Alkaline Phosphatase; WP: Whey Protein; CHO: Carbohydrates; ST: Strength Training; BMD: Bone Mineral Density; DXA: Dual energy X-ray Absorptiometry; CTx: C-Terminal telopeptide, Fm: Fermented milk; CPP: Casein Phospho-peptides; ITF: Inulin-Type Fructans; EAA; Essential Amino Acids; IGF-1: Insulin-like Growth Factor-1; QCT: Quantitative Computed Tomography.
Three RCTs among menopausal and postmenopausal women further investigated the effect of different isolated bioactive components of milk, such as lactoferrin, ribonuclease, milk basic protein fractions and caseinphosphopeptides [89,90,91]. With the exception of the caseinphosphopeptides (with neither positive nor negative demonstrated effect) [91], the supplementation of the other bioactive peptides had positive effects on osteoblastic bone formation and restoration of bone turnover, among 38 and 32 women, respectively, and for a relatively short period (6 months) [89,90].
3.4. Cardiovascular Diseases (Eight Studies)
Among elderly subjects, cardiovascular diseases are the worldwide leading cause of death [92]. Heart related morbidity and mortality are associated with the progressive decrease in endothelial function with ageing, possibly due to different serum level changes (in hormones, lipids, and inflammatory markers).
Three RCTs investigated the effect on vascular function of soy proteins supplementation versus milk proteins supplementation, regarded as control arm [93,94,95]. These studies tested different doses of soy protein supplementation (40, 28 and 40 g, respectively), versus different milk supplementations (casein, total milk protein and caseinate, respectively), for different study durations (3 months, 12 months and 4 weeks, respectively), and among different sample sizes of post-menopausal women (105, 202 and 18, respectively). Two studies out of three were concordant in not supporting a beneficial effect of soy on endothelial function, when compared to casein or milk protein control [93,94]. The third trial was conducted in association with a low fat/low cholesterol diet and suggested an improvement in endothelial function of isolated soy protein, compared to a caseinate supplement [95].
Two studies tested the effect of lactotripeptides, with and without regular aerobic exercise, on vascular endpoints, such as endothelium dependent dilatation and arterial compliance, among post-menopausal women [96,97]. These trials supported a beneficial effect of lactotripeptides when combined with regular physical activity.
One study tested the effect of a single dose of WP isolate on cardiovascular risk factor markers (total cholesterol, low density lipoproteins, Apolipoprotein B48, insulin levels) among 20 post-menopausal women, suggesting a possible decrease in arterial exposure to some lipoproteins [98].
A RCT tested the short-term effect of different protein-enriched supplementations among overweight postmenopausal women. Despite previous data on WP long-term supplementation showing a beneficial cardiovascular effect, the acute administration was not superior to casein or glucose on blood pressure, vascular function and inflammatory markers [99].
A long-term trial, investigating the effect on blood pressure of a two-year daily supplementation of a WP-based beverage (250 mL with 30 g proteins and 600 mg calcium) among women over 70 years, did not provide evidence of the hypotensive property of dairy proteins [100], as previously suggested in short term RCTs.
3.5. Protein Intake and Metabolism (Seven Studies)
A key issue in elderly nutrition is the role of dairy proteins and products as important contributors of nutrients. In 2002 and 2003, two studies assessed protein digestion and metabolism among young and old subjects. According to their results, fast proteins, WP in particular, may be beneficial in the elderly to limit protein losses during aging [101,102]. In a RCT comparing casein vs. WP supplementations among 31 elderly men, the authors outlined how postprandial protein retention was better improved by fast-digested WP [19]. In more recent years, an Australian research group explored the effect of WP supplementation in older people with malnutrition, in order to assess its effect on different endpoints: suppression of energy intake [103], gastric emptying and gut hormones response [104,105,106]. The main conclusions of these investigations were that WP supplementation in older adults increased overall protein intake without suppressing appetite, and positively affected gastric emptying and gut hormones response.
3.6. Inflammation Markers (Seven Studies)
The term “Inflamm-aging” was firstly used by the Italian researcher Claudio Franceschi in 2000, and it refers to the progressive and chronic development of a pro-inflammatory state with aging [107]. Increasing evidence indicates that inflamm-aging is associated with the risk of developing other diseases, such as Alzheimer’s disease, atherosclerosis, heart disease, type II diabetes, and cancer. According to this new area of research, serum markers of inflammation and the antioxidant capacity of plasma should be monitored, in order to act on the aging process.
A study tested the antioxidant capacity of a milk-based protein matrix, previously in vitro and then ex vivo, among healthy women aged 50–70 years, providing encouraging preliminary data [108].
Four RCTs evaluated the role of WP supplementation on inflammatory markers in the elderly [109,110,111,112]. Laviolette and colleagues explored the effect of 16-week daily WP supplementation vs. casein, in association with 8-week RT, among 22 patients with stable chronic obstructive pulmonary disease (COPD). They did not reported a positive effect on serum C reactive protein (CRP) or Interleukin-6 (IL-6) [109]. Among similar COPD patients, Sugawara and colleagues reported a decrease in CRP, Interleukin-8 (IL-8) and tumor necrosis factor-α (TNF α) supplemented with WP in association with exercise [110]. One trial among 31 elderly patients after acute ischemic stroke demonstrated a decrease in systemic inflammation markers with enteral formula, containing WP vs. a casein containing formula [111]. A further study among 40 community dwelling older adults, the supplementation of WP or bovine colostrum, in association with RT, did not change IGF-1 or CRP level [112].
Among 84 postmenopausal women, a multicenter 18-month supplementation trial reported a protective effect of WP on markers of inflammation, in comparison to a maltodextrin supplementation [113]. A multi-ingredient nutritional supplement was effective in progressively reducing the plasma level of TNF- α and IL-6 among healthy older men, in a two phase study with 6 weeks of supplementation alone, followed by 12 weeks of supplementation with physical exercise [114]. The multi ingredient supplement provided contained 30 g WP, 2.5 g creatine, 400 mg calcium, 500 IU vitamin D, and 1500 mg n-3 PUFA.
3.7. Chronic Obstructive Pulmonary Disease (Four Studies)
Within a few years, COPD is expected to become the 5th and 3rd leading cause of disability and mortality worldwide, respectively [115]. Older people with COPD, a syndrome consisting of chronic bronchitis, bronchiectasis, emphysema, and other reversible airway diseases, are at higher risk of developing complications and adverse outcomes [116].
Among eight elderly normal-weight patients with COPD, milk protein sip feeding (a drink with 8.1 g protein) was beneficial in stimulating an enhanced anabolic response, mediated by a reduction in multiple amino acids splanchnic extraction [117]. At present, available data are not conclusive about the superiority of bolus versus sip feeding of hydrolyzed milk protein mixture in stimulating the best anabolic response in older adults with COPD [118].
In a RCT among 59 elderly patients with COPD, the supplementation of 12 g of WP twice a day versus the same dose of casein reduced shortness of breath [119].
A more recent RCT with a cross-over design examined, among 23 old COPD patients, whether a free essential amino acids mixture with high level of leucine was superior in stimulating net protein gain than a similar mixture of balanced free essential and non-essential amino acid naturally present in WP [120].
3.8. Neurocognitive Function (Four Studies)
Age-related cognitive decline (ARCD) is one of the main increasing concerns in an ageing population, affecting to varying degrees about 40% of people above 60 years, even in physically healthy conditions [121]. Two RCTs evaluated the effect of milk protein and WP supplementation on neuro-cognitive endpoints in elderly subjects [122,123]. In the first RCT, 15 g of a milk protein concentrate supplementation was superior to placebo in improving reaction time among 65 old people, without improving other cognitive functions [122]. In the second study, 50 g of WP isolate (WPI) was tested, versus the same amount of soy protein isolate (SPI), in a cross-over intervention among 56 elderly subjects. The study revealed an improvement of vitamin B12 and folate status, without a direct effect on cognitive status in WPI phase, and a better reaction time and reasoning speed among female subjects (but not among males) during SPI phase [123].
In a randomized, placebo-controlled, double-blind clinical trial involving 15 healthy middle-aged and older adults, the supplementation for eight weeks of lactotripeptide, a milk protein-derived bioactive peptide, increased middle cerebral blood flow velocity, an outcome associated with lower cerebrovascular disease [124].
A still ongoing RCT, the Phospholipid Intervention for Cognitive Ageing Reversal (PLICAR) study, is investigating the effect of a phospholipid-rich milk protein concentrate among old people with an age-related memory impairment [125]. To date, no results of this trial have been published.
3.9. Response to Vaccines (Two Studies)
Immunosenescence is defined as a progressive and physiological decline of the immune system function associated with aging, and it may start as early as the age of 55 years [126]. The impaired functioning of both innate and acquired arms of the immune system leads to the increased incidence of infections as well as higher rates of complications and fatal events among elderly population [127]. The immunosenescence is furthermore responsible for a decreased immunogenicity following vaccination. For this reason, improving vaccine efficacy in the elderly has become a global public health issue [128]. Several investigations have been performed to find environmental factors, such as immune-stimulatory food in diet, which can help the ageing immune system to drive an appropriate response to infections and vaccination [129,130,131,132,133].
Two randomized, multicenter, double-blind, controlled clinical trials were performed among 86 and 222 elderly volunteers over 70 years of age, in a pilot and confirmatory study, respectively. The aim of these trials was to assess the effect on the immune response to influenza vaccination of a daily supplementation of a probiotic dairy drink for 7 and 13 weeks, respectively. Both trials demonstrated an increased relevant specific antibody response in the probiotic group in comparison with the control group, treated with a non-fermented dairy product [134].
The antibody immune response to Streptococcus pneumoniae (or pneumococcus) vaccine is a good marker of the immune function [135]. Based on this assumption, a randomized, controlled, double blind pilot study was conducted among 17 healthy subjects, aged over 60 years, to assess the efficacy in improving immune response to vaccine of an 8-week daily supplementation with 5 g of WP, versus 5 g of soy proteins in the control group [136]. After the administration of pneumococcal vaccine, subjects receiving WP supplementation had a higher serum response against 12 out of 14 pneumococcal types, especially for the four most virulent bacterial types.
A recent randomized, controlled, double blind pilot study was performed among 21 healthy subjects over 60 years of age. It compared 12 g of daily consumption of milk proteins and 12 g of soy proteins for an 8-week period, focusing on the response to Diphtheria, Tetanus, and Pertussis (DTaP) vaccine as the primary endpoint [137]. The study revealed a significant increase in tetanus antibodies in the dairy group compared to the soy group.
3.10. Miscellanea (5 Studies)
Preliminary reports have been published in other investigational areas, and the main findings are reported in Table 4 [138,139,140,141,142].
Table 4.
Preliminary reports have been published in other investigational areas.
| Author, Year | Study Type | Intervention | Main Endpoints | Results | Brief Comment |
|---|---|---|---|---|---|
| Numan 2007 [138] | Pilot study | Anti-Clostridium difficile WP concentrate | Prevention of relapse of Clostridium difficile infection | 10% relapse rate in comparison to 20–25% relapse rate in a control contemporary cohort | Waiting for confirmation in RCT |
| Coker 2012 [139] | RCT during caloric restriction | Meal replacement with WP and EAA vs. a standard meal replacement | Weight loss preserving lean tissue (muscle mass) | WP + EAA was effective in weight reduction promoting preferential reduction of adipose tissue | Small sample size (12 subjects) |
| Ooi 2015 [140] | RCT | 30 g WP supplementation vs. a high CHO energy match supplementation | Weight reduction and reduction of hepatic steatosis in women | No difference in weight reduction or hepatic steatosis | WP supplementation may reduce hepatic steatosis despite weight gain |
| Dhillon 2017 [141] | RCT crossover design | WP isolate (50 g) vs. soy protein isolate (50 g) | Bioavailability of folates and Vitamin B12 in elderly with subclinical deficiencies | WP isolate was superior to soy in improving active B12 and folate status | - |
| Song 2018 [142] | Blind sensory analysis | Rye bread and cream cheese enriched with:
|
Consumer acceptance | Better acceptance of WP hydrolysate in bread and of WP isolate in cheese | Developing protein enriched food may increase protein intake in elderly but innovation in protein enriched appealing food is challenging |
RCT: Randomized Control Trial; WP: Whey protein; EAA: Essential Amino Acids; CHO: Carbohydrates.
4. Discussion
To our knowledge, this is the first systematic literature review targeting the role of milk protein supplementation in the elderly. The large majority of the studies included in our review showed a beneficial effect of milk protein supplementation, whey proteins in particular, in promoting improved health outcomes in a wide range of body systems. The variety of outcomes reflects the multidimensionality of diet-based support therapies. This review clearly reveals the lack of long-term studies and the need for further research on the persistency over time of the beneficial effects of milk proteins supplementation, as well as on the late onset of possible side effects.
As stated in the guidelines on clinical nutrition and hydration in geriatrics by the European Society of Clinical Nutrition and Metabolism (ESPEN), nutrition is a key factor in preserving health and wellbeing in old people [143]. Dairy products provide not only proteins, but also a substantial amount of micronutrients (vitamins and minerals) relevant for healthy ageing, and according to some authors, reference national food patterns should consider their unique nutritional properties, especially for frail elderly people [144]. Several studies included in this review were concordant in identifying WP as good nutritional sources in ageing population, especially because they are fast digested and well absorbed, rich in essential amino-acid and in leucine, and able to stimulate muscular protein synthesis without suppressing overall energy intake or increasing fat mass. These characteristics are very relevant to older people, taking into account that muscle loss (in mass and in strength), anorexia and sarcopenia, with and without obesity, are frequent among this population group.
The main finding of this review is the evidence of the role of milk protein supplementation in the maintenance of skeletal muscle mass in ageing. Studies carried on up to 24 weeks showed that milk protein supplementation increased serum level of amino acids and leucine during the post-prandial period, resulting in leucine uptake by muscle cells, myofibrillary protein and mitochondria protein synthesis in both resting and active muscles. The increased MPS was demonstrated in the elderly, with and without adequate dietary protein intake. Most studies demonstrated the superiority of WP (fast proteins) to casein (slow proteins), soy or wheat proteins. It should be noted that the only long-period study (two years) included in this review, about this research area, did not show impact on muscle mass of WP supplementation in well-fed postmenopausal women [64]. Further long-term studies are mandatory, because there are still concerns about excessive protein load and related negative effects on kidney and ageing process [145].
Strictly linked to muscle loss, the role of milk protein in sarcopenia, “one of the most meaningful geriatric syndromes” [146], was addressed by several studies included in this review. There is consensus on three fundamental factors in the prevention of sarcopenia: exercise (‘use it or lose it!’), quantity of protein in the diet (20–30 g of proteins per main meal) and quality of proteins (preferring high leucine content, 4 g per meal) [147]. The ideal moment of protein intake could be after exercise, since physical exercise boosts blood circulation, which increases the absorption of leucine. Protein supplementation in patients with sarcopenia is strongly recommended in the absence of other medical contraindications, especially when reaching protein needs through diet modifications alone are unsuccessful [6]. There is an urgent need of evidence-based strategies aimed to improve recovery after hospital discharge in older adults. Exercise, probably the best way to recover muscle mass, is not always applicable, so efforts towards finding efficient nutritional strategies are expected. Preliminary findings [74] reported that 24 g of WP supplementation during a daily rehabilitation program have an impact in promoting better health outcomes. Future studies designed to incorporate longer-term intervention, or post-hospitalization lifestyle modifications, are warranted.
Most studies included in this review focused on the effects of milk protein supplementation on bone metabolism are gender specific, enrolling mainly postmenopausal women. In intervention studies, milk proteins have proven to be superior to soy proteins in reducing bone resorption. Promising results indicate that the increase in serum IGF-1 levels induced by milk protein supplements was accelerated, with a significant difference, by zinc addition [148].
The only long-term trial investigating the effect on blood pressure of two-year daily supplementation of a WP-based beverage among older women did not provide evidence of a hypotensive property of dairy proteins [100], as suggested by short term RCTs.
The protective role of milk protein supplementation on serum markers of inflammation is still under investigation: the RCTs included in this review are not concordant. According to a study by Ticinesi and colleagues, further large studies assessing the anti-inflammatory effect of combined dietary supplements, including n-3 PUFA, vitamin D and WP, are needed [149].
Milk protein supplementation for old COPD patients may have multiple positive therapeutic outcomes, thanks to their role in enhancing anabolic response [117] and attenuating perceived exertion during exercise [110]. Better results may be obtained, combining nutritional intervention with supervised exercise training, as part of a formal pulmonary rehabilitation program [150].
Results of milk protein supplementation to prevent or ameliorate ARCD and dementia are still contradictory. Limited evidence showed some improvements in reaction time [122], but no significant effects have been recorded on other cognitive functions. These results are probably due to the wide spectrum of neurocognitive manifestations with different underlying physiopathology mechanisms, which makes it difficult to standardize intervention protocols. As stated in a systematic review published in 2011, several research projects were involved in finding effective dietary interventions, which aimed to prevent or ameliorate ARCD [151]. The main conclusion of the review is that low-fat dairy products, together with an adequate diet, may play a beneficial role in neurocognitive function during aging.
Milk protein supplementation is a promising nutritional intervention to stimulate immune response [136,137]. Further studies are advocated, to investigate its role in counteracting immune-senescence, in reducing inflamm-aging and in stimulating response to infections and vaccination. The role of fermented dairy products, with and without milk protein supplementation, could represent an interesting starting point for further investigation, given the high compliance reported in this type of intervention [132,134].
One of the major strengths of this review is the wide overview on several health topics related to ageing. A narrative synthesis of 103 articles gave the opportunity to (i) discuss the evidence on key research areas as well as on poorly investigated ones, (ii) map different target population, mainly healthy subjects, but also frail, sarcopenic, hospitalized and chronically ill patients, (iii) include gender medicine studies, and (iv) underline some knowledge gap.
Other key strengths of our work are the robust methodology adopted according to PRISMA criteria, the approved registration on PROSPERO, the extensive research through five electronic databases, including Clinicaltrials.gov to overcome publication bias, and a further search in Google Scholar. Moreover, two independent investigators (BZ and MZ) screened titles and abstracts to select included studies.
We acknowledge some limitations. We did not provide any meta-analysis, the heterogeneity of the included studies was explored descriptively. We did not conduct an a priori quality assessment of the studies, because we preferred including all studies selected according to the pre-defined objective criteria, without introducing any subjective judgements.
5. Conclusions
Our systematic literature review supports the evidence of a beneficial effect of cow-milk protein supplementation among old people. Evidence of beneficial effects is stronger for whey proteins supplementation. Several health outcomes are reported with respect to milk proteins: achievement of nutritional aged-related goals, muscle mass preservation and functionality, prevention and treatment of sarcopenia, modulation of inflammation, response to vaccinations and rehabilitation after hospitalization.
Acknowledgments
We wish to acknowledge the help provided by Fabiana Raccagni in the preliminary phase of this work.
Author Contributions
Conceptualization, B.Z., A.S., G.G.; Supervision, B.Z., M.C., G.G.; Methodology, B.Z., M.Z.; Writing—Original Draft, B.Z., M.Z.; Writing—Review and Editing, B.Z., A.S., M.Z., M.C., G.G. All authors have read and agreed to this version of the manuscript.
Funding
This review is partially supported by AOP Latte Italia under the project “Evaluation of economic and development scenarios of a selection of semi-finished products derived from milk and other dairy products”.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Decade of Healthy Ageing (2020–2030) [(accessed on 13 May 2020)]; Available online: https://www.who.int/ageing/decade-of-healthy-ageing.
- 2.Cosco T.D., Howse K., Brayne C. Healthy ageing, resilience and wellbeing. Epidemiol. Psychiatr. Sci. 2017;26:579–583. doi: 10.1017/S2045796017000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peel N., Bartlett H., McClure R. Healthy ageing: How is it defined and measured? Australas. J. Ageing. 2004;23:115–119. doi: 10.1111/j.1741-6612.2004.00035.x. [DOI] [Google Scholar]
- 4.Fielding R.A., Vellas B., Evans W.J., Bhasin S., Morley J.E., Newman A.B., van Kan G.A., Andrieu S., Bauer J., Breuille D., et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beasley J.M., Shikany J.M., Thomson C.A. The Role of Dietary Protein Intake in the Prevention of Sarcopenia of Aging. Nutr. Clin. Pr. 2013;28:684–690. doi: 10.1177/0884533613507607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfe R.R. Update on protein intake: Importance of milk proteins for health status of the elderly. Nutr. Rev. 2015;73:41–47. doi: 10.1093/nutrit/nuv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walrand S., Boline Y. Optimizing protein intake in aging. Curr. Opin. Clin. Nutr. Metab. Care. 2005;8:89–94. doi: 10.1097/00075197-200501000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Malcomson F.C., Mathers J.C. Nutrition and Ageing. Subcell. Biochem. 2018;90:373–424. doi: 10.1007/978-981-13-2835-0_13. [DOI] [PubMed] [Google Scholar]
- 10.McGee M., Jensen G.L. Nutrition in the Elderly. J. Clin. Gastroenterol. 2000;30:372–380. doi: 10.1097/00004836-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Bauer J., Biolo G., Cederholm T., Cesari M., Cruz-Jentoft A.J., Morley J.E., Phillips S., Sieber C., Stehle P., Teta D., et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition (2002: Geneva, Switzerland) Food and Agriculture Organization of the United Nations. World Health Organization. United Nations University . Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation. World Health Organization; Geneva, Switzerland: 2007. [Google Scholar]
- 13.Hesselink M.K.C., Minnaard R., Schrauwen P. Eat the meat or feed the meat: Protein turnover in remodeling muscle. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:672–676. doi: 10.1097/01.mco.0000247471.64532.7d. [DOI] [PubMed] [Google Scholar]
- 14.Rémond D., Machebeuf M., Yven C., Buffière C., Mioche L., Mosoni L., Mirand P.P. Postprandial whole-body protein metabolism after a meat meal is influenced by chewing efficiency in elderly subjects. Am. J. Clin. Nutr. 2007;85:1286–1292. doi: 10.1093/ajcn/85.5.1286. [DOI] [PubMed] [Google Scholar]
- 15.Park S.-Y., Acosta A., Camilleri M., Burton D., Harmsen W.S., Fox J., Szarka L.A. Gastric Motor Dysfunction in Patients With Functional Gastroduodenal Symptoms. Am. J. Gastroenterol. 2017;112:1689–1699. doi: 10.1038/ajg.2017.264. [DOI] [PubMed] [Google Scholar]
- 16.Godos J., Tieri M., Ghelfi F., Titta L., Marventano S., Lafranconi A., Gambera A., Alonzo E., Sciacca S., Buscemi S., et al. Dairy foods and health: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2020;71:138–151. doi: 10.1080/09637486.2019.1625035. [DOI] [PubMed] [Google Scholar]
- 17.Mah J.Y., Choy S.W., Roberts M.A., Desai A.M., Corken M., Gwini S.M., McMahon L.P. Oral protein-based supplements versus placebo or no treatment for people with chronic kidney disease requiring dialysis. Cochrane Database Syst. Rev. 2020;5:CD012616. doi: 10.1002/14651858.CD012616.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson B., Taylor J., Favaloro E.J. Potential benefits of improved protein intake in older people. Nutr. Diet. 2008;65:151–156. doi: 10.1111/j.1747-0080.2008.00250.x. [DOI] [Google Scholar]
- 19.Gryson C., Walrand S., Giraudet C., Rousset P., Migne C., Bonhomme C., Le Ruyet P., Boirie Y. “Fast proteins” with a unique essential amino acid content as an optimal nutrition in the elderly: Growing evidence. Clin. Nutr. 2014;33:642–648. doi: 10.1016/j.clnu.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van den Borne J.J., Kudla U., Geurts J.M. Translating novel insights from age-related loss of skeletal muscle mass and phenotypic flexibility into diet and lifestyle recommendations for the elderly. Curr. Opin. Food Sci. 2016;10:60–67. doi: 10.1016/j.cofs.2016.08.006. [DOI] [Google Scholar]
- 22.Tang J.E., Phillips S.M. Maximizing muscle protein anabolism: The role of protein quality. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:66–71. doi: 10.1097/MCO.0b013e32831cef75. [DOI] [PubMed] [Google Scholar]
- 23.Devries M.C., McGlory C., Bolster D.R., Kamil A., Rahn M., Harkness L., Baker S.K., Phillips S.M. Protein leucine content is a determinant of shorter- and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: A randomized, controlled trial. Am. J. Clin. Nutr. 2018;107:217–226. doi: 10.1093/ajcn/nqx028. [DOI] [PubMed] [Google Scholar]
- 24.Bukhari S.S.I., Phillips B.E., Wilkinson D.J., Limb M.C., Rankin D., Mitchell W.K., Kobayashi H., Greenhaff P.L., Smith K., Atherton P.J., et al. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am. J. Physiol.-Endocrinol. Metab. 2015;308:E1056–E1065. doi: 10.1152/ajpendo.00481.2014. [DOI] [PubMed] [Google Scholar]
- 25.Dardevet D., Rémond D., Peyron M.-A., Papet I., Savary-Auzeloux I., Mosoni L. Muscle wasting and resistance of muscle anabolism: The “anabolic threshold concept” for adapted nutritional strategies during sarcopenia. Sci. World J. 2012;2012:269531. doi: 10.1100/2012/269531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devries M.C., McGlory C., Bolster D.R., Kamil A., Rahn M., Harkness L., Baker S.K., Phillips S.M. Leucine, Not Total Protein, Content of a Supplement Is the Primary Determinant of Muscle Protein Anabolic Responses in Healthy Older Women. J. Nutr. 2018;148:1088–1095. doi: 10.1093/jn/nxy091. [DOI] [PubMed] [Google Scholar]
- 27.Schnebelen-Berthier C., Baudry C., Clerc E., Jaruga A., Le Ruyet P., Lecerf J.-M. Effect of supplementing meals with soluble milk proteins on plasma leucine levels in healthy older people: A randomized pilot study. Nutr. Aging. 2015;3:139–146. doi: 10.3233/NUA-150056. [DOI] [Google Scholar]
- 28.Gorissen S.H., Horstman A.M., Franssen R., Kouw I.W., Wall B.T., Burd N.A., de Groot L.C., van Loon L.J. Habituation to low or high protein intake does not modulate basal or postprandial muscle protein synthesis rates: A randomized trial. Am. J. Clin. Nutr. 2017;105:332–342. doi: 10.3945/ajcn.115.129924. [DOI] [PubMed] [Google Scholar]
- 29.Kiskini A., Hamer H.M., Wall B.T., Groen B.B.L., de Lange A., Bakker J.A., Senden J.M.G., Verdijk L.B., van Loon L.J.C. The muscle protein synthetic response to the combined ingestion of protein and carbohydrate is not impaired in healthy older men. Age. 2013;35:2389–2398. doi: 10.1007/s11357-013-9522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paddon-Jones D., Sheffield-Moore M., Katsanos C.S., Zhang X.-J., Wolfe R.R. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp. Gerontol. 2006;41:215–219. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Katsanos C.S., Chinkes D.L., Paddon-Jones D., Zhang X., Aarsland A., Wolfe R.R. Whey protein ingestion in elderly persons results in greater muscle protein accrual than ingestion of its constituent essential amino acid content. Nutr. Res. 2008;28:651–658. doi: 10.1016/j.nutres.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koopman R., Crombach N., Gijsen A.P., Walrand S., Fauquant J., Kies A.K., Lemosquet S., Saris W.H.M., Boirie Y., van Loon L.J.C. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 2009;90:106–115. doi: 10.3945/ajcn.2009.27474. [DOI] [PubMed] [Google Scholar]
- 33.Pennings B., Boirie Y., Senden J.M.G., Gijsen A.P., Kuipers H., van Loon L.J.C. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011;93:997–1005. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- 34.Burd N.A., Yang Y., Moore D.R., Tang J.E., Tarnopolsky M.A., Phillips S.M. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br. J. Nutr. 2012;108:958–962. doi: 10.1017/S0007114511006271. [DOI] [PubMed] [Google Scholar]
- 35.Groen B.B.L., Res P.T., Pennings B., Hertle E., Senden J.M.G., Saris W.H.M., van Loon L.J.C. Intragastric protein administration stimulates overnight muscle protein synthesis in elderly men. Am. J. Physiol.-Endocrinol. Metab. 2012;302:E52–E60. doi: 10.1152/ajpendo.00321.2011. [DOI] [PubMed] [Google Scholar]
- 36.Pennings B., Groen B., de Lange A., Gijsen A.P., Zorenc A.H., Senden J.M.G., van Loon L.J.C. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am. J. Physiol.-Endocrinol. Metab. 2012;302:E992–E999. doi: 10.1152/ajpendo.00517.2011. [DOI] [PubMed] [Google Scholar]
- 37.Wall B.T., Hamer H.M., de Lange A., Kiskini A., Groen B.B.L., Senden J.M.G., Gijsen A.P., Verdijk L.B., van Loon L.J.C. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin. Nutr. 2013;32:412–419. doi: 10.1016/j.clnu.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Luiking Y.C., Deutz N.E.P., Memelink R.G., Verlaan S., Wolfe R.R. Postprandial muscle protein synthesis is higher after a high whey protein, leucine-enriched supplement than after a dairy-like product in healthy older people: A randomized controlled trial. Nutr. J. 2014;13:9. doi: 10.1186/1475-2891-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Churchward-Venne T.A., Snijders T., Linkens A.M.A., Hamer H.M., van Kranenburg J., van Loon L.J.C. Ingestion of Casein in a Milk Matrix Modulates Dietary Protein Digestion and Absorption Kinetics but Does Not Modulate Postprandial Muscle Protein Synthesis in Older Men. J. Nutr. 2015;145:1438–1445. doi: 10.3945/jn.115.213710. [DOI] [PubMed] [Google Scholar]
- 40.Borack M.S., Reidy P.T., Husaini S.H., Markofski M.M., Deer R.R., Richison A.B., Lambert B.S., Cope M.B., Mukherjea R., Jennings K., et al. Soy-Dairy Protein Blend or Whey Protein Isolate Ingestion Induces Similar Postexercise Muscle Mechanistic Target of Rapamycin Complex 1 Signaling and Protein Synthesis Responses in Older Men. J. Nutr. 2016;146:2468–2475. doi: 10.3945/jn.116.231159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorissen S.H., Horstman A.M., Franssen R., Crombag J.J., Langer H., Bierau J., Respondek F., van Loon L.J. Ingestion of Wheat Protein Increases In Vivo Muscle Protein Synthesis Rates in Healthy Older Men in a Randomized Trial. J. Nutr. 2016;146:1651–1659. doi: 10.3945/jn.116.231340. [DOI] [PubMed] [Google Scholar]
- 42.Walrand S., Gryson C., Salles J., Giraudet C., Migné C., Bonhomme C., Le Ruyet P., Boirie Y. Fast-digestive protein supplement for ten days overcomes muscle anabolic resistance in healthy elderly men. Clin. Nutr. 2016;35:660–668. doi: 10.1016/j.clnu.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 43.Kouw I.W.K., Holwerda A.M., Trommelen J., Kramer I.F., Bastiaanse J., Halson S.L., Wodzig W.K.W.H., Verdijk L.B., van Loon L.J.C. Protein Ingestion before Sleep Increases Overnight Muscle Protein Synthesis Rates in Healthy Older Men: A Randomized Controlled Trial. J. Nutr. 2017;147:2252–2261. doi: 10.3945/jn.117.254532. [DOI] [PubMed] [Google Scholar]
- 44.Hamarsland H., Aas S.N., Nordengen A.L., Holte K., Garthe I., Paulsen G., Cotter M., Borsheim E., Benestad H.B., Raastad T. Native Whey Induces Similar Post Exercise Muscle Anabolic Responses as Regular Whey, Despite Greater Leucinemia, in Elderly Individuals. J. Nutr. Health Aging. 2019;23:42–50. doi: 10.1007/s12603-018-1105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kramer I.F., Verdijk L.B., Hamer H.M., Verlaan S., Luiking Y., Kouw I.W.K., Senden J.M., van Kranenburg J., Gijsen A.P., Poeze M., et al. Impact of the Macronutrient Composition of a Nutritional Supplement on Muscle Protein Synthesis Rates in Older Men: A Randomized, Double Blind, Controlled Trial. J. Clin. Endocrinol. Metab. 2015;100:4124–4132. doi: 10.1210/jc.2015-2352. [DOI] [PubMed] [Google Scholar]
- 46.Verreijen A.M., Verlaan S., Engberink M.F., Swinkels S., de Vogel-van den Bosch J., Weijs P.J.M. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2015;101:279–286. doi: 10.3945/ajcn.114.090290. [DOI] [PubMed] [Google Scholar]
- 47.Chanet A., Verlaan S., Salles J., Giraudet C., Patrac V., Pidou V., Pouyet C., Hafnaoui N., Blot A., Cano N., et al. Supplementing Breakfast with a Vitamin D and Leucine—Enriched Whey Protein Medical Nutrition Drink Enhances Postprandial Muscle Protein Synthesis and Muscle Mass in Healthy Older Men. J. Nutr. 2017;147:2262–2271. doi: 10.3945/jn.117.252510. [DOI] [PubMed] [Google Scholar]
- 48.Bell K.E., Snijders T., Zulyniak M., Kumbhare D., Parise G., Chabowski A., Phillips S.M. A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: A randomized controlled trial. PLoS ONE. 2017;12:e0181387. doi: 10.1371/journal.pone.0181387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorissen S.H.M., Burd N.A., Kramer I.F., van Kranenburg J., Gijsen A.P., Rooyackers O., van Loon L.J.C. Co-ingesting milk fat with micellar casein does not affect postprandial protein handling in healthy older men. Clin. Nutr. 2017;36:429–437. doi: 10.1016/j.clnu.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Hulmi J.J., Kovanen V., Lisko I., Selänne H., Mero A.A. The effects of whey protein on myostatin and cell cycle-related gene expression responses to a single heavy resistance exercise bout in trained older men. Eur. J. Appl. Physiol. 2008;102:205–213. doi: 10.1007/s00421-007-0579-4. [DOI] [PubMed] [Google Scholar]
- 51.Dirks M.L., Wall B.T., Kramer I.F., Zorenc A.H., Goessens J.P.B., Gijsen A.P., van Loon L.J.C. A single session of neuromuscular electrical stimulation does not augment postprandial muscle protein accretion. Am. J. Physiol.-Endocrinol. Metab. 2016;311:E278–E285. doi: 10.1152/ajpendo.00085.2016. [DOI] [PubMed] [Google Scholar]
- 52.Dideriksen K.J., Reitelseder S., Petersen S.G., Hjort M., Helmark I.C., Kjaer M., Holm L. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scand. J. Med. Sci. Sports. 2011;21:e372–e383. doi: 10.1111/j.1600-0838.2011.01318.x. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y., Breen L., Burd N.A., Hector A.J., Churchward-Venne T.A., Josse A.R., Tarnopolsky M.A., Phillips S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012;108:1780–1788. doi: 10.1017/S0007114511007422. [DOI] [PubMed] [Google Scholar]
- 54.Arnarson A., Geirsdottir O.G., Ramel A., Briem K., Jonsson P.V., Thorsdottir I. Effects of whey proteins and carbohydrates on the efficacy of resistance training in elderly people: Double blind, randomised controlled trial. Eur. J. Clin. Nutr. 2013;67:821–826. doi: 10.1038/ejcn.2013.40. [DOI] [PubMed] [Google Scholar]
- 55.Gryson C., Ratel S., Rance M., Penando S., Bonhomme C., Le Ruyet P., Duclos M., Boirie Y., Walrand S. Four-Month Course of Soluble Milk Proteins Interacts With Exercise to Improve Muscle Strength and Delay Fatigue in Elderly Participants. J. Am. Med. Dir. Assoc. 2014;15:958.e1–958.e9. doi: 10.1016/j.jamda.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Karelis A.D., Messier V., Suppère C., Briand P., Rabasa-Lhoret R. Effect of cysteine-rich whey protein (immunocal®) supplementation in combination with resistance training on muscle strength and lean body mass in non-frail elderly subjects: A randomized, double-blind controlled study. J. Nutr. Health Aging. 2015;19:531–536. doi: 10.1007/s12603-015-0442-y. [DOI] [PubMed] [Google Scholar]
- 57.Weisgarber K.D., Candow D.G., Farthing J.P. Whey protein and high-volume resistance training in postmenopausal women. J. Nutr. Health Aging. 2015;19:511–517. doi: 10.1007/s12603-015-0454-7. [DOI] [PubMed] [Google Scholar]
- 58.Thomson R.L., Brinkworth G.D., Noakes M., Buckley J.D. Muscle strength gains during resistance exercise training are attenuated with soy compared with dairy or usual protein intake in older adults: A randomized controlled trial. Clin. Nutr. 2016;35:27–33. doi: 10.1016/j.clnu.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 59.Mori H., Tokuda Y. Effect of whey protein supplementation after resistance exercise on the muscle mass and physical function of healthy older women: A randomized controlled trial. Geriatr. Gerontol. Int. 2018;18:1398–1404. doi: 10.1111/ggi.13499. [DOI] [PubMed] [Google Scholar]
- 60.Junior P.S., Ribeiro A.S., Nabuco H.C.G., Fernandes R.R., Tomeleri C.M., Cunha P.M., Venturini D., Barbosa D.S., Schoenfeld B.J., Cyrino E.S. Effects of Whey Protein Supplementation Associated With Resistance Training on Muscular Strength, Hypertrophy, and Muscle Quality in Preconditioned Older Women. Int. J. Sport Nutr. Exerc. Metab. 2018;28:528–535. doi: 10.1123/ijsnem.2017-0253. [DOI] [PubMed] [Google Scholar]
- 61.Hays N.P., Kim H., Wells A.M., Kajkenova O., Evans W.J. Effects of whey and fortified collagen hydrolysate protein supplements on nitrogen balance and body composition in older women. J. Am. Diet. Assoc. 2009;109:1082–1087. doi: 10.1016/j.jada.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Ottestad I., Løvstad A.T., Gjevestad G.O., Hamarsland H., Benth J.Š., Andersen L.F., Bye A., Biong A.S., Retterstøl K., Iversen P.O., et al. Intake of a Protein-Enriched Milk and Effects on Muscle Mass and Strength. A 12-Week Randomized Placebo Controlled Trial among Community-Dwelling Older Adults. J. Nutr. Health Aging. 2017;21:1160–1169. doi: 10.1007/s12603-016-0856-1. [DOI] [PubMed] [Google Scholar]
- 63.Liu Z.M., Ho S.C., Chen Y.M., Ho Y.P. A mild favorable effect of soy protein with isoflavones on body composition--a 6-month double-blind randomized placebo-controlled trial among Chinese postmenopausal women. Int. J. Obes. 2010;34:309–318. doi: 10.1038/ijo.2009.236. [DOI] [PubMed] [Google Scholar]
- 64.Zhu K., Kerr D.A., Meng X., Devine A., Solah V., Binns C.W., Prince R.L. Two-Year Whey Protein Supplementation Did Not Enhance Muscle Mass and Physical Function in Well-Nourished Healthy Older Postmenopausal Women. J. Nutr. 2015;145:2520–2526. doi: 10.3945/jn.115.218297. [DOI] [PubMed] [Google Scholar]
- 65.Kramer I.F., Verdijk L.B., Hamer H.M., Verlaan S., Luiking Y.C., Kouw I.W.K., Senden J.M., van Kranenburg J., Gijsen A.P., Bierau J., et al. Both basal and post-prandial muscle protein synthesis rates, following the ingestion of a leucine-enriched whey protein supplement, are not impaired in sarcopenic older males. Clin. Nutr. 2017;36:1440–1449. doi: 10.1016/j.clnu.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 66.Chalé A., Cloutier G.J., Hau C., Phillips E.M., Dallal G.E., Fielding R.A. Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:682–690. doi: 10.1093/gerona/gls221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bauer J.M., Verlaan S., Bautmans I., Brandt K., Donini L.M., Maggio M., McMurdo M.E.T., Mets T., Seal C., Wijers S.L., et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015;16:740–747. doi: 10.1016/j.jamda.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 68.Rondanelli M., Klersy C., Terracol G., Talluri J., Maugeri R., Guido D., Faliva M.A., Solerte B.S., Fioravanti M., Lukaski H., et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016;103:830–840. doi: 10.3945/ajcn.115.113357. [DOI] [PubMed] [Google Scholar]
- 69.Molnár A., Sztruhár I.J., Csontos Á.A., Ferencz C., Várbíró S., Székács B. Special nutrition intervention is required for muscle protective efficacy of physical exercise in elderly people at highest risk of sarcopenia. Physiol. Int. 2016;103:368–376. doi: 10.1556/2060.103.2016.3.12. [DOI] [PubMed] [Google Scholar]
- 70.Maltais M.L., Perreault K., Courchesne-Loyer A., Lagacé J.C., Barsalani R., Dionne I.J. Effect of Resistance Training and Various Sources of Protein Supplementation on Body Fat Mass and Metabolic Profile in Sarcopenic Overweight Older Adult Men: A Pilot Study. Int. J. Sport Nutr. Exerc. Metab. 2016;26:71–77. doi: 10.1123/ijsnem.2015-0160. [DOI] [PubMed] [Google Scholar]
- 71.Maltais M.L., Ladouceur J.P., Dionne I.J. The Effect of Resistance Training and Different Sources of Postexercise Protein Supplementation on Muscle Mass and Physical Capacity in Sarcopenic Elderly Men. J. Strength Cond. Res. 2016;30:1680–1687. doi: 10.1519/JSC.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 72.Gade J., Beck A.M., Bitz C., Christensen B., Klausen T.W., Vinther A., Astrup A. Protein-enriched, milk-based supplement to counteract sarcopenia in acutely ill geriatric patients offered resistance exercise training during and after hospitalisation: Study protocol for a randomised, double-blind, multicentre trial. BMJ Open. 2018;8:e019210. doi: 10.1136/bmjopen-2017-019210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dirks M.L., Tieland M., Verdijk L.B., Losen M., Nilwik R., Mensink M., de Groot L.C.P.G.M., van Loon L.J.C. Protein Supplementation Augments Muscle Fiber Hypertrophy but Does Not Modulate Satellite Cell Content During Prolonged Resistance-Type Exercise Training in Frail Elderly. J. Am. Med. Dir. Assoc. 2017;18:608–615. doi: 10.1016/j.jamda.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 74.Niccoli S., Kolobov A., Bon T., Rafilovich S., Munro H., Tanner K., Pearson T., Lees S.J. Whey Protein Supplementation Improves Rehabilitation Outcomes in Hospitalized Geriatric Patients: A Double Blinded, Randomized Controlled Trial. J. Nutr. Gerontol. Geriatr. 2017;36:149–165. doi: 10.1080/21551197.2017.1391732. [DOI] [PubMed] [Google Scholar]
- 75.Park Y., Choi J.-E., Hwang H.-S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2018;108:1026–1033. doi: 10.1093/ajcn/nqy214. [DOI] [PubMed] [Google Scholar]
- 76.Fernandes A.L., Hayashi A.P., Jambassi-Filho J.C., de Capitani M.D., de Santana D.A., Gualano B., Roschel H. Different protein and derivatives supplementation strategies combined with resistance training in pre-frail and frail elderly: Rationale and protocol for the “Pro-Elderly” Study. Nutr. Health. 2017;23:251–260. doi: 10.1177/0260106017737465. [DOI] [PubMed] [Google Scholar]
- 77.Collins J., Longhurst G., Roschel H., Gualano B. Resistance Training and Co-supplementation with Creatine and Protein in Older Subjects with Frailty. J. Frailty Aging. 2016;5:126–134. doi: 10.14283/jfa.2016.85. [DOI] [PubMed] [Google Scholar]
- 78.Bjorkman M.P., Finne-Soveri H., Tilvis R.S. Whey protein supplementation in nursing home residents. A randomized controlled trial. Eur. Geriatr. Med. 2012;3:161–166. doi: 10.1016/j.eurger.2012.03.010. [DOI] [Google Scholar]
- 79.Demontiero O., Vidal C., Duque G. Aging and bone loss: New insights for the clinician. Ther. Adv. Musculoskelet. Dis. 2012;4:61–76. doi: 10.1177/1759720X11430858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kerstetter J.E., O’Brien K.O., Caseria D.M., Wall D.E., Insogna K.L. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J. Clin. Endocrinol. Metab. 2005;90:26–31. doi: 10.1210/jc.2004-0179. [DOI] [PubMed] [Google Scholar]
- 81.Durosier-Izart C., Biver E., Merminod F., van Rietbergen B., Chevalley T., Herrmann F.R., Ferrari S.L., Rizzoli R. Peripheral skeleton bone strength is positively correlated with total and dairy protein intakes in healthy postmenopausal women. Am. J. Clin. Nutr. 2017;105:513–525. doi: 10.3945/ajcn.116.134676. [DOI] [PubMed] [Google Scholar]
- 82.Langsetmo L., Shikany J.M., Burghardt A.J., Cawthon P.M., Orwoll E.S., Cauley J.A., Taylor B.C., Schousboe J.T., Bauer D.C., Vo T.N., et al. High dairy protein intake is associated with greater bone strength parameters at the distal radius and tibia in older men: A cross-sectional study. Osteoporos. Int. 2018;29:69–77. doi: 10.1007/s00198-017-4261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khalil D.A., Lucas E.A., Juma S., Smith B.J., Payton M.E., Arjmandi B.H. Soy protein supplementation increases serum insulin-like growth factor-I in young and old men but does not affect markers of bone metabolism. J. Nutr. 2002;132:2605–2608. doi: 10.1093/jn/132.9.2605. [DOI] [PubMed] [Google Scholar]
- 84.Holm L., Olesen J.L., Matsumoto K., Doi T., Mizuno M., Alsted T.J., Mackey A.L., Schwarz P., Kjaer M. Protein-containing nutrient supplementation following strength training enhances the effect on muscle mass, strength, and bone formation in postmenopausal women. J. Appl. Physiol. 2008;105:274–281. doi: 10.1152/japplphysiol.00935.2007. [DOI] [PubMed] [Google Scholar]
- 85.Adolphi B., Scholz-Ahrens K.E., de Vrese M., Acil Y., Laue C., Schrezenmeir J. Short-term effect of bedtime consumption of fermented milk supplemented with calcium, inulin-type fructans and caseinphosphopeptides on bone metabolism in healthy, postmenopausal women. Eur. J. Nutr. 2009;48:45–53. doi: 10.1007/s00394-008-0759-y. [DOI] [PubMed] [Google Scholar]
- 86.Chevalley T., Hoffmeyer P., Bonjour J.-P., Rizzoli R. Early serum IGF-I response to oral protein supplements in elderly women with a recent hip fracture. Clin. Nutr. 2010;29:78–83. doi: 10.1016/j.clnu.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 87.Zhu K., Meng X., Kerr D.A., Devine A., Solah V., Binns C.W., Prince R.L. The effects of a two-year randomized, controlled trial of whey protein supplementation on bone structure, IGF-1, and urinary calcium excretion in older postmenopausal women. J. Bone Miner. Res. 2011;26:2298–2306. doi: 10.1002/jbmr.429. [DOI] [PubMed] [Google Scholar]
- 88.Kerstetter J.E., Bihuniak J.D., Brindisi J., Sullivan R.R., Mangano K.M., Larocque S., Kotler B.M., Simpson C.A., Cusano A.M., Gaffney-Stomberg E., et al. The Effect of a Whey Protein Supplement on Bone Mass in Older Caucasian Adults. J. Clin. Endocrinol. Metab. 2015;100:2214–2222. doi: 10.1210/jc.2014-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bharadwaj S., Naidu A.G.T., Betageri G.V., Prasadarao N.V., Naidu A.S. Milk ribonuclease-enriched lactoferrin induces positive effects on bone turnover markers in postmenopausal women. Osteoporos. Int. 2009;20:1603–1611. doi: 10.1007/s00198-009-0839-8. [DOI] [PubMed] [Google Scholar]
- 90.Aoe S., Koyama T., Toba Y., Itabashi A., Takada Y. A controlled trial of the effect of milk basic protein (MBP) supplementation on bone metabolism in healthy menopausal women. Osteoporos. Int. 2005;16:2123–2128. doi: 10.1007/s00198-005-2012-3. [DOI] [PubMed] [Google Scholar]
- 91.Narva M., Kärkkäinen M., Poussa T., Lamberg-Allardt C., Korpela R. Caseinphosphopeptides in milk and fermented milk do not affect calcium metabolism acutely in postmenopausal women. J. Am. Coll. Nutr. 2003;22:88–93. doi: 10.1080/07315724.2003.10719280. [DOI] [PubMed] [Google Scholar]
- 92.Jackson C.F., Wenger N.K. Cardiovascular disease in the elderly. Rev. Esp. Cardiol. 2011;64:697–712. doi: 10.1016/j.recesp.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 93.Teede H.J., Dalais F.S., Kotsopoulos D., Liang Y.L., Davis S., McGrath B.P. Dietary soy has both beneficial and potentially adverse cardiovascular effects: A placebo-controlled study in men and postmenopausal women. J. Clin. Endocrinol. Metab. 2001;86:3053–3060. doi: 10.1210/jc.86.7.3053. [DOI] [PubMed] [Google Scholar]
- 94.Kreijkamp-Kaspers S., Kok L., Bots M.L., Grobbee D.E., Lampe J.W., van der Schouw Y.T. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am. J. Clin. Nutr. 2005;81:189–195. doi: 10.1093/ajcn/81.1.189. [DOI] [PubMed] [Google Scholar]
- 95.Cuevas A.M., Irribarra V.L., Castillo O.A., Yañez M.D., Germain A.M. Isolated soy protein improves endothelial function in postmenopausal hypercholesterolemic women. Eur. J. Clin. Nutr. 2003;57:889–894. doi: 10.1038/sj.ejcn.1601622. [DOI] [PubMed] [Google Scholar]
- 96.Yoshizawa M., Maeda S., Miyaki A., Misono M., Choi Y., Shimojo N., Ajisaka R., Tanaka H. Additive beneficial effects of lactotripeptides and aerobic exercise on arterial compliance in postmenopausal women. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1899–H1903. doi: 10.1152/ajpheart.00433.2009. [DOI] [PubMed] [Google Scholar]
- 97.Yoshizawa M., Maeda S., Miyaki A., Misono M., Choi Y., Shimojo N., Ajisaka R., Tanaka H. Additive beneficial effects of lactotripeptides intake with regular exercise on endothelium-dependent dilatation in postmenopausal women. Am. J. Hypertens. 2010;23:368–372. doi: 10.1038/ajh.2009.270. [DOI] [PubMed] [Google Scholar]
- 98.Pal S., Ellis V., Ho S. Acute effects of whey protein isolate on cardiovascular risk factors in overweight, post-menopausal women. Atherosclerosis. 2010;212:339–344. doi: 10.1016/j.atherosclerosis.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 99.Pal S., Ellis V. Acute effects of whey protein isolate on blood pressure, vascular function and inflammatory markers in overweight postmenopausal women. Br. J. Nutr. 2011;105:1512–1519. doi: 10.1017/S0007114510005313. [DOI] [PubMed] [Google Scholar]
- 100.Hodgson J.M., Zhu K., Lewis J.R., Kerr D., Meng X., Solah V., Devine A., Binns C.W., Woodman R.J., Prince R.L. Long-term effects of a protein-enriched diet on blood pressure in older women. Br. J. Nutr. 2012;107:1664–1672. doi: 10.1017/S0007114511004740. [DOI] [PubMed] [Google Scholar]
- 101.Dangin M., Boirie Y., Guillet C., Beaufrère B. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J. Nutr. 2002;132:3228S–3233S. doi: 10.1093/jn/131.10.3228S. [DOI] [PubMed] [Google Scholar]
- 102.Dangin M., Guillet C., Garcia-Rodenas C., Gachon P., Bouteloup-Demange C., Reiffers-Magnani K., Fauquant J., Ballèvre O., Beaufrère B. The rate of protein digestion affects protein gain differently during aging in humans. J. Physiol. 2003;549:635–644. doi: 10.1113/jphysiol.2002.036897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giezenaar C., Trahair L.G., Rigda R., Hutchison A.T., Feinle-Bisset C., Luscombe-Marsh N.D., Hausken T., Jones K.L., Horowitz M., Chapman I., et al. Lesser suppression of energy intake by orally ingested whey protein in healthy older men compared with young controls. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309:R845–R854. doi: 10.1152/ajpregu.00213.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giezenaar C., Hutchison A.T., Luscombe-Marsh N.D., Chapman I., Horowitz M., Soenen S. Effect of Age on Blood Glucose and Plasma Insulin, Glucagon, Ghrelin, CCK, GIP, and GLP-1 Responses to Whey Protein Ingestion. Nutrients. 2017;10:2. doi: 10.3390/nu10010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Giezenaar C., Luscombe-Marsh N.D., Hutchison A.T., Standfield S., Feinle-Bisset C., Horowitz M., Chapman I., Soenen S. Dose-Dependent Effects of Randomized Intraduodenal Whey-Protein Loads on Glucose, Gut Hormone, and Amino Acid Concentrations in Healthy Older and Younger Men. Nutrients. 2018;10:78. doi: 10.3390/nu10010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Giezenaar C., van der Burgh Y., Lange K., Hatzinikolas S., Hausken T., Jones K.L., Horowitz M., Chapman I., Soenen S. Effects of Substitution, and Adding of Carbohydrate and Fat to Whey-Protein on Energy Intake, Appetite, Gastric Emptying, Glucose, Insulin, Ghrelin, CCK and GLP-1 in Healthy Older Men-A Randomized Controlled Trial. Nutrients. 2018;10:113. doi: 10.3390/nu10020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xia S., Zhang X., Zheng S., Khanabdali R., Kalionis B., Wu J., Wan W., Tai X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016;2016:8426874. doi: 10.1155/2016/8426874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Power-Grant O., McCormack W.G., De Cap M.R., Amigo-Benavent M., Fitzgerald R.J., Jakeman P. Evaluation of the antioxidant capacity of a milk protein matrix in vitro and in vivo in women aged 50–70 years. Int. J. Food Sci. Nutr. 2016;67:325–334. doi: 10.3109/09637486.2016.1153607. [DOI] [PubMed] [Google Scholar]
- 109.Laviolette L., Lands L.C., Dauletbaev N., Saey D., Milot J., Provencher S., LeBlanc P., Maltais F. Combined effect of dietary supplementation with pressurized whey and exercise training in chronic obstructive pulmonary disease: A randomized, controlled, double-blind pilot study. J. Med. Food. 2010;13:589–598. doi: 10.1089/jmf.2009.0142. [DOI] [PubMed] [Google Scholar]
- 110.Sugawara K., Takahashi H., Kashiwagura T., Yamada K., Yanagida S., Homma M., Dairiki K., Sasaki H., Kawagoshi A., Satake M., et al. Effect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPD. Respir. Med. 2012;106:1526–1534. doi: 10.1016/j.rmed.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 111.De Aguilar-Nascimento J.E., Silveira B.R.P., Dock-Nascimento D.B. Early enteral nutrition with whey protein or casein in elderly patients with acute ischemic stroke: A double-blind randomized trial. Nutrition. 2011;27:440–444. doi: 10.1016/j.nut.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 112.Duff W.R.D., Chilibeck P.D., Rooke J.J., Kaviani M., Krentz J.R., Haines D.M. The effect of bovine colostrum supplementation in older adults during resistance training. Int. J. Sport Nutr. Exerc. Metab. 2014;24:276–285. doi: 10.1123/ijsnem.2013-0182. [DOI] [PubMed] [Google Scholar]
- 113.Stojkovic V., Simpson C.A., Sullivan R.R., Cusano A.M., Kerstetter J.E., Kenny A.M., Insogna K.L., Bihuniak J.D. The Effect of Dietary Glycemic Properties on Markers of Inflammation, Insulin Resistance, and Body Composition in Postmenopausal American Women: An Ancillary Study from a Multicenter Protein Supplementation Trial. Nutrients. 2017;9:484. doi: 10.3390/nu9050484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bell K.E., Snijders T., Zulyniak M.A., Kumbhare D., Parise G., Chabowski A., Phillips S.M. A multi-ingredient nutritional supplement enhances exercise training-related reductions in markers of systemic inflammation in healthy older men. Appl. Physiol. Nutr. Metab. 2018;43:299–302. doi: 10.1139/apnm-2017-0533. [DOI] [PubMed] [Google Scholar]
- 115.Vaz Fragoso C.A., Gill T.M. Defining Chronic Obstructive Pulmonary Disease in an Aging Population. J. Am. Geriatr. Soc. 2010;58:2224–2226. doi: 10.1111/j.1532-5415.2010.03128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Albertson T.E., Louie S., Chan A.L. The diagnosis and treatment of elderly patients with acute exacerbation of chronic obstructive pulmonary disease and chronic bronchitis. J. Am. Geriatr. Soc. 2010;58:570–579. doi: 10.1111/j.1532-5415.2010.02741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Engelen M.P.K.J., De Castro C.L.N., Rutten E.P.A., Wouters E.F.M., Schols A.M.W.J., Deutz N.E.P. Enhanced anabolic response to milk protein sip feeding in elderly subjects with COPD is associated with a reduced splanchnic extraction of multiple amino acids. Clin. Nutr. 2012;31:616–624. doi: 10.1016/j.clnu.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jonker R., Deutz N.E.P., Harrykissoon R., Zachria A.J., Veley E.A., Engelen M.P.K.J. A critical evaluation of the anabolic response after bolus or continuous feeding in COPD and healthy older adults. Clin. Sci. 2018;132:17–31. doi: 10.1042/CS20171068. [DOI] [PubMed] [Google Scholar]
- 119.Lum C., Lo R., Ng K., Woo J., Tang N., Fallows S. A study on whey protein supplement on physical performance and quality of life among elderly patients with chronic obstructive pulmonary disease. Australas. J. Ageing. 2007;26:168–172. doi: 10.1111/j.1741-6612.2007.00257.x. [DOI] [Google Scholar]
- 120.Jonker R., Deutz N.E., Erbland M.L., Anderson P.J., Engelen M.P. Effectiveness of essential amino acid supplementation in stimulating whole body net protein anabolism is comparable between COPD patients and healthy older adults. Metab. Clin. Exp. 2017;69:120–129. doi: 10.1016/j.metabol.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Konar A., Singh P., Thakur M.K. Age-associated Cognitive Decline: Insights into Molecular Switches and Recovery Avenues. Aging Dis. 2016;7:121–129. doi: 10.14336/AD.2015.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Van der Zwaluw N.L., van de Rest O., Tieland M., Adam J.J., Hiddink G.J., van Loon L.J.C., de Groot L.C.P.G.M. The impact of protein supplementation on cognitive performance in frail elderly. Eur. J. Nutr. 2014;53:803–812. doi: 10.1007/s00394-013-0584-9. [DOI] [PubMed] [Google Scholar]
- 123.Zajac I.T., Herreen D., Bastiaans K., Dhillon V.S., Fenech M. The Effect of Whey and Soy Protein Isolates on Cognitive Function in Older Australians with Low Vitamin B12: A Randomised Controlled Crossover Trial. Nutrients. 2018;11:19. doi: 10.3390/nu11010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Akazawa N., Hamasaki A., Tanahashi K., Kosaki K., Yoshikawa T., Myoenzono K., Maeda S. Lactotripeptide ingestion increases cerebral blood flow velocity in middle-aged and older adults. Nutr. Res. 2018;53:61–66. doi: 10.1016/j.nutres.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 125.Scholey A.B., Camfield D.A., Hughes M.E., Woods W.K., Stough C.K., White D.J., Gondalia S.V., Frederiksen P.D. A randomized controlled trial investigating the neurocognitive effects of Lacprodan® PL-20, a phospholipid-rich milk protein concentrate, in elderly participants with age-associated memory impairment: The Phospholipid Intervention for Cognitive Ageing Reversal (PLICAR): Study protocol for a randomized controlled trial. Trials. 2013;14:404. doi: 10.1186/1745-6215-14-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ginaldi L., Loreto M.F., Corsi M.P., Modesti M., De Martinis M. Immunosenescence and infectious diseases. Microbes Infect. 2001;3:851–857. doi: 10.1016/S1286-4579(01)01443-5. [DOI] [PubMed] [Google Scholar]
- 127.Akha A.A.S. Aging and the immune system: An overview. J. Immunol. Methods. 2018;463:21–26. doi: 10.1016/j.jim.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 128.Oh S.-J., Lee J.K., Shin O.S. Aging and the Immune System: The Impact of Immunosenescence on Viral Infection, Immunity and Vaccine Immunogenicity. Immune Netw. 2019;19:e37. doi: 10.4110/in.2019.19.e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gill H.S., Rutherfurd K.J., Cross M.L., Gopal P.K. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am. J. Clin. Nutr. 2001;74:833–839. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- 130.Gavazzi G., Krause K.-H. Ageing and infection. Lancet Infect. Dis. 2002;2:659–666. doi: 10.1016/S1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- 131.Guillemard E., Tondu F., Lacoin F., Schrezenmeir J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br. J. Nutr. 2010;103:58–68. doi: 10.1017/S0007114509991395. [DOI] [PubMed] [Google Scholar]
- 132.Makino S., Ikegami S., Kume A., Horiuchi H., Sasaki H., Orii N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010;104:998–1006. doi: 10.1017/S000711451000173X. [DOI] [PubMed] [Google Scholar]
- 133.Pawelec G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018;105:4–9. doi: 10.1016/j.exger.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 134.Boge T., Rémigy M., Vaudaine S., Tanguy J., Bourdet-Sicard R., van der Werf S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine. 2009;27:5677–5684. doi: 10.1016/j.vaccine.2009.06.094. [DOI] [PubMed] [Google Scholar]
- 135.Jackson L.A., Neuzil K.M., Yu O., Benson P., Barlow W.E., Adams A.L., Hanson C.A., Mahoney L.D., Shay D.K., Thompson W.W. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N. Engl. J. Med. 2003;348:1747–1755. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 136.Freeman S.L., Fisher L., German J.B., Leung P.S., Prince H., Selmi C., Naguwa S.M., Gershwin M.E. Dairy proteins and the response to pneumovax in senior citizens: A randomized, double-blind, placebo-controlled pilot study. Ann. N. Y. Acad. Sci. 2010;1190:97–103. doi: 10.1111/j.1749-6632.2009.05264.x. [DOI] [PubMed] [Google Scholar]
- 137.Schaefer S., Hettinga K.A., Cullor J., German J.B., Henrick B.M. Use of UV Treated Milk Powder to Increase Vaccine Efficacy in the Elderly. Front. Immunol. 2018;9:2254. doi: 10.3389/fimmu.2018.02254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Numan S.C., Veldkamp P., Kuijper E.J., van den Berg R.J., van Dissel J.T. Clostridium difficile-associated diarrhoea: Bovine anti-Clostridium difficile whey protein to help aid the prevention of relapses. Gut. 2007;56:888–889. doi: 10.1136/gut.2006.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coker R.H., Miller S., Schutzler S., Deutz N., Wolfe R.R. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr. J. 2012;11:105. doi: 10.1186/1475-2891-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ooi E.M., Adams L.A., Zhu K., Lewis J.R., Kerr D.A., Meng X., Solah V., Devine A., Binns C.W., Prince R.L. Consumption of a whey protein-enriched diet may prevent hepatic steatosis associated with weight gain in elderly women. Nutr. Metab. Cardiovasc. Dis. 2015;25:388–395. doi: 10.1016/j.numecd.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 141.Dhillon V.S., Zabaras D., Almond T., Cavuoto P., James-Martin G., Fenech M. Whey protein isolate improves vitamin B12 and folate status in elderly Australians with subclinical deficiency of vitamin B12. Mol. Nutr. Food Res. 2017;61:1600915. doi: 10.1002/mnfr.201600915. [DOI] [PubMed] [Google Scholar]
- 142.Song X., Perez-Cueto F.J.A., Bredie W.L.P. Sensory-Driven Development of Protein-Enriched Rye Bread and Cream Cheese for the Nutritional Demands of Older Adults. Nutrients. 2018;10:1006. doi: 10.3390/nu10081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Volkert D., Beck A.M., Cederholm T., Cruz-Jentoft A., Goisser S., Hooper L., Kiesswetter E., Maggio M., Raynaud-Simon A., Sieber C.C., et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019;38:10–47. doi: 10.1016/j.clnu.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 144.Van Staveren W.A., Steijns J.M., de Groot L.C.P.G.M. Dairy products as essential contributors of (micro-) nutrients in reference food patterns: An outline for elderly people. J. Am. Coll. Nutr. 2008;27:747S–754S. doi: 10.1080/07315724.2008.10719753. [DOI] [PubMed] [Google Scholar]
- 145.Pamplona R., Barja G. Mitochondrial oxidative stress, aging and caloric restriction: The protein and methionine connection. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006;1757:496–508. doi: 10.1016/j.bbabio.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 146.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.-P., Rolland Y., Schneider S.M., et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Marzetti E., Calvani R., Tosato M., Cesari M., Di Bari M., Cherubini A., Collamati A., D’Angelo E., Pahor M., Bernabei R., et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017;29:11–17. doi: 10.1007/s40520-016-0704-5. [DOI] [PubMed] [Google Scholar]
- 148.Rodondi A., Ammann P., Ghilardi-Beuret S., Rizzoli R. Zinc increases the effects of essential amino acids-whey protein supplements in frail elderly. J. Nutr. Health Aging. 2009;13:491–497. doi: 10.1007/s12603-009-0099-5. [DOI] [PubMed] [Google Scholar]
- 149.Ticinesi A., Meschi T., Lauretani F., Felis G., Franchi F., Pedrolli C., Barichella M., Benati G., Di Nuzzo S., Ceda G.P., et al. Nutrition and Inflammation in Older Individuals: Focus on Vitamin D, n-3 Polyunsaturated Fatty Acids and Whey Proteins. Nutrients. 2016;8:186. doi: 10.3390/nu8040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Van de Bool C., Rutten E.P.A., van Helvoort A., Franssen F.M.E., Wouters E.F.M., Schols A.M.W.J. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J. Cachexia Sarcopenia Muscle. 2017;8:748–758. doi: 10.1002/jcsm.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Camfield D.A., Owen L., Scholey A.B., Pipingas A., Stough C. Dairy constituents and neurocognitive health in ageing. Br. J. Nutr. 2011;106:159–174. doi: 10.1017/S0007114511000158. [DOI] [PubMed] [Google Scholar]