Abstract
The last seven years have seen the greatest surge of Ebola virus disease (EVD) cases in equatorial Africa, including the 2013–2016 epidemic in West Africa and the recent epidemics in the Democratic Republic of Congo (DRC). The vaccine clinical trials that took place in West Africa and the DRC, as well as follow-up studies in collaboration with EVD survivor communities, have for the first time allowed researchers to compare immune memory induced by natural infection and vaccination. These comparisons may be relevant to evaluate the putative effectiveness of vaccines and candidate medical countermeasures such as convalescent plasma transfer. In this study, we compared the long-term functionality of anti-EBOV glycoprotein (GP) antibodies from EVD survivors with that from volunteers who received the recombinant vesicular stomatitis virus vectored vaccine (rVSV-ZEBOV) during the Phase I clinical trial in Hamburg. Our study highlights important differences between EBOV vaccination and natural infection and provides a framework for comparison with other vaccine candidates.
Keywords: Ebola virus, VSV, vaccine, antibodies, immune memory
1. Introduction
Ebola virus disease (EVD) is a severe hemorrhagic fever caused by viruses of the genus Ebolavirus, more prominently by Ebola virus (species Zaire ebolavirus, EBOV). EBOV was the causative agent of the 2013–2016 EVD epidemic in West Africa as well as the recent epidemic in the Democratic Republic of Congo (DRC) [1]. The magnitude of the EVD epidemic in West Africa allowed for the first clinical studies, the establishment of clinical trials to test vaccines and therapeutics as well as investigations on post-Ebola syndrome. These research efforts have significantly improved our understanding of EVD pathophysiology and immunity. Regarding the latter, findings from several laboratories indicate that acute EVD is characterized by exacerbated and sustained adaptive immune responses [2,3,4] and that both arms of the adaptive immunity are critical for recovery [5,6,7].
Antibodies exert several complementary functions during the immune response to pathogen invasion. These functions rely on the structure of the antibody molecule, consisting of the variable region (Fab) which harbors a hypervariable antigen-binding site as well as a constant region (Fc). The latter can engage the complement system and Fc receptors (FcγR) in many immune cells, including natural killer (NK) cells as well as a variety of phagocytes. Thus, antibodies can trigger a wide range of immune responses to viruses including neutralization of the ability of the virus to enter or exit target cells, activation of infected cell phagocytosis, antibody-dependent cellular cytotoxicity (ADCC) and complement-mediated cytotoxicity (CMC) [8,9].
Since the characterization of the first monoclonal antibody against EBOV, KZ52 isolated from a survivor of the Kikwit outbreak [10], there has been substantial research devoted to the study of the functional properties of anti-EBOV antibodies isolated from EVD survivors. Perhaps surprisingly, these studies demonstrated that even though neutralizing antibodies (NAB) are maintained for years after recovery [11,12], neutralization of EBOV in cell culture may or may not translate into protection [13,14]. The maintenance of serum anti-ebolavirus antibodies with the capacity to bind to FcγR for up to a decade suggests that, in addition to neutralization, FcγR-dependent functions are also an important component of immune memory [12]. Indeed, monoclonal antibody therapy, which has shown promise as a successful post-exposure therapy, is based on the administration of antibody cocktails that include neutralizing and non-neutralizing antibodies. A recently developed human antibody cocktail with Fab- and FcγR-dependent biological functions has shown promising pan-Ebolavirus protection [15]. In agreement with the idea that multiple antibody functions are necessary to survive EVD, NAB are often not detected in survivors during the early weeks after discharge from the treatment center [16,17].
The magnitude of the 2013–2016 West African EVD outbreak led to the launch of clinical trials for EBOV vaccines that had shown strong efficacy in non-human primate models. Most notably, the recombinant vesicular stomatitis virus (rVSV)-vectored vaccine (rVSV-ZEBOV), in which the VSV envelope glycoprotein (GP) is replaced by EBOV GP, showed evidence of protection in ring vaccination-based clinical trials conducted in West Africa and the DRC [18], and it is now licensed for human use in the US [19]. Despite its success, our understanding of the host immunity that builds after rVSV-ZEBOV vaccination is incomplete, and, to the best of our knowledge, the study of the biological properties of the antibodies generated by rVSV-ZEBOV vaccination has been limited to neutralization [20,21,22]. However, other vaccine candidates have been shown to induce Fc-mediated antibody functions such as NK cell activation [23].
The purpose of this study was to explore immune memory induced by natural infection and vaccination against EVD. We compared the antibody functions of 10 rVSV-ZEBOV vaccinees and 25 EVD survivors 6 months after vaccination or approximately 12 months after discharge, respectively, for differences in anti-EBOV glycoprotein antibodies. An improved understanding of immune responses in disease and vaccination might ultimately aid in the development of novel therapeutics and vaccines.
2. Materials and Methods
2.1. Sample Preparation
Plasma samples were collected 180 days after a single vaccination with 2 × 107 plaque-forming units (PFUs) of rVSV-ZEBOV as described in the study protocol EudraCT No. 2014-003591-23, ClinicalTrials.gov No. NCT02287480 [24]. The mean age of vaccinees was 40 years of age. Plasma samples of long-term (~1 year) EVD survivors were collected in Guéckédou, Guinea, after receiving written consent. Samples were collected under Ethics protocols approved by the Guinean National Committee for Research and Health (33/CNERS/15) and the Medical Ethics Commission of the state of Hamburg (PV5309). For the survivor cohort, mean age was 32 years (see Table 1). Plasma samples were aliquoted and stored at −80 °C. All samples from Guéckédou were cooled and transported to Donka hospital in Conakry where they were stored at −80 °C. After that, survivor plasma samples were transported to Hamburg in dry ice.
Table 1.
Study cohort. Age, sex and ethnicity of rVSV-ZEBOV vaccinees at d0 and of EVD survivors on day of blood sampling are shown. Absolute numbers (%) are displayed unless otherwise specified.
| rVSV-ZEBOV Vaccinees (n = 10) |
EVD Survivors (n = 25) |
|
|---|---|---|
| Age, mean (SD) (y) | 40.4 (8.7) | 32.0 (12.9) |
| median (range) (y) | 40 (24–52) | 31 (18–58) |
| Sex | ||
| female | 3 (30%) | 11 (44%) |
| male | 7 (70%) | 14 (56%) |
| Ethnicity | ||
| white | 10 (100%) | 0 |
| Black or African American | 0 | 10 (100%) |
2.2. Plaque Reduction Neutralization Assay (PRNA)
PRNA was performed using donor plasma dilutions incubated with 100 focus-forming units (FFUs) of EBOV H.sapiens-tc/COD/1976/Yambuku-Mayinga strain for 1 h at room temperature (RT). These dilutions were then plaqued on 90 to 100% confluent Vero-E6 cell monolayers. Cells were overlaid with minimum essential medium (MEM) containing 0.64% agarose (Oxoid) and the corresponding plasma dilutions. After 4 days of incubation at 37 °C, the cells were fixed with 3.7% paraformaldehyde (PFA), and the plaques were stained using a polyclonal anti-EBOV mouse serum (1:1000), an anti-mouse secondary antibody conjugated to horseradish peroxidase (HRP) (Sigma-Aldrich, St. Louis, MO, USA) and Trueblue reagent (Sera Care, Milford, MA, USA). Plaques were counted in each plasma dilution, and the percent inhibition for each plasma sample and dilution was individually calculated based on a no-antibody control. All experiments with infectious EBOV were carried out in the biosafety level 4 laboratory of the Bernhard Nocht Institute for Tropical Medicine in Hamburg, Germany, according to institutional biosafety regulations. All personnel handling samples wore positive-pressure biosafety suits.
2.3. rVSV ZEBOV Vaccine and Recombinant Proteins
The vaccine was manufactured at IDT Biologika in Dessau, Germany, and stored in a manner consistent with good manufacturing practices. The stock of 1 × 108 plaque forming units (PFUs)/mL was further diluted as required. Recombinant Ebola virus glycoprotein (GP) minus the transmembrane region (EBOV rGPdTM) was purchased from IBT Bioservices (Rockville, MD, USA).
2.4. Generation of Antigen-Coupled Beads and Isotyping Assay
Magnetic carboxylated fluorescently labeled microbeads (Magplex beads, Luminex Corp., Austin, TX, USA) were coupled with Ebola-GP (EBOV rGPdTM by IBT Bioservices). The coupled beads were utilized in a multivariate Luminex assay for antibody class determination. Briefly, beads were diluted to a concentration of 50 beads/μL in Assay Buffer (PBS-1×, 0.1% BSA). Using a black, clear bottom 96-well plate (Greiner Bio One, Kremsmünster, Austria Cat. No. 655906), 50 μL of the working microsphere mixture (2500 beads/well), 40 μL of Assay Buffer and 10 μL of plasma sample diluted 1:10 (1 µL of plasma) were added to each well. The plate was covered and incubated overnight at 4 °C. A Bio-plex array reader (Bio-Plex 200, Bio-Plex Manager 5.0, Bio-Rad Laboratories, Inc., Hercules, CA, USA) detected the microspheres, and binding of PE detector antibody was measured to calculate a Median Fluorescence Intensity (MFI). Background signal, defined as the average MFI observed for each microsphere set when incubated with the PE detector antibody in the absence of a clinical antibody sample, was subtracted from the MFI for each sample. The human anti-EBOV-GP antibody KZ52 (IBT Bioservices Cat. No. 0260-001) was used as a positive control.
2.5. ADCC and Antibody-Dependent Cellular Phagocytosis (ADCP) Reporter Assays
ADCC was measured using the ADCC Reporter Bioassay kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. ADCP was measured using the FcyRIIa-H Reporter Kit (Promega). Luminescence was measured immediately using a Synergy Hybrid Reader (BioTek Instruments, Inc., Winooski, VT, USA).
2.6. NK Degranulation Assay
NK cell activation was measured by the ability of EBOV-GP specific antibodies to induce expression of CD107a on human NK cells. High-binding bottom ELISA plates (Greiner Bio One, Cat. No. 705073) were coated overnight with 300 ng/well EBOV-GP at 4 °C. Wells were washed twice with PBS, blocked overnight with PBS containing 5% fetal calf serum at 4 °C and washed twice again. Wells were then incubated with 100 µL of a 48-fold dilution of patient’s plasma for 2 h at 37 °C and washed again. Cryopreserved human PBMCs from one healthy individual were thawed, washed and picked up in RPMI 1640 medium containing low IgG Serum. A total of 3 × 105 PBMCs were added to each well and incubated overnight at 37 °C. Cells were then stained for CD3-AF700, CD45-Pacific Blue and CD107a-PE for 1 h at 4 °C, washed, picked up in 1% PFA and measured on a LSR-II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Degranulated NK cells were defined as CD107a+ CD3− CD56+ cells. Analysis was performed using FlowJo X 10 software (FlowJo LLC, Ashland, OR, USA).
2.7. Statistical Analysis
Statistical analysis was performed with Prism GraphPad version 7.03 (GraphPad Software, San Diego, CA, USA). Non-parametric statistics were applied throughout. For group comparisons we utilized Kruskal–Wallis analysis followed by Dunn’s post-test. To evaluate putative correlations, we utilized Spearman’s correlation analysis.
3. Results
3.1. EVD Survivors Show Enhanced Antibody Functions in Comparison with Vaccinees
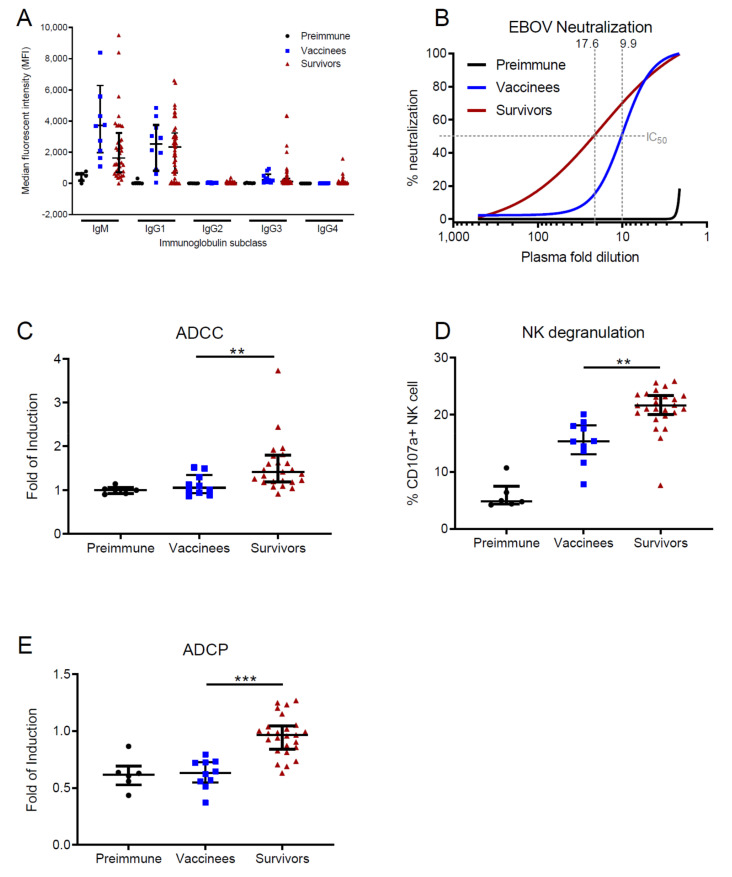
To characterize the presence of anti-EBOV antibodies in survivors and vaccine recipients in the long-term after recovery and vaccination, we first sought to determine the overall levels of EBOV-GP specific IgM and IgG isotypes as well as the four IgG subclasses, since the distribution of the latter may provide cues on the breadth of antibody functions in immune memory [25]. To this end, we set up a microsphere (“beads”)-based array allowing for on-bead affinity purification and subsequent classification of antigen-specific antibodies from clinical samples (see assay description in Figure S1). Surprisingly, both survivors and vaccinees showed significant levels of serum IgM 1 year and 180 days post-vaccination, respectively (vaccinees: median = 3712, Q1 = 1977, Q3 = 6279 Median Fluorescence Intensity (MFI); survivors: median = 1623, Q1 = 724, Q3 = 3241 MFI; Figure 1A). As expected, the most abundant IgG subclass in serum was IgG1 (median of IgG1, IgG2, IgG3 and IgG4 in vaccinees: 2521, 35, 241 and 16 MFI; survivors: 2339, 43, 122 and 21 MFI, respectively). Overall, there were no significant differences between survivors and vaccinees regarding the levels of circulating Ig subclasses, and the levels resembled those seen in survivors from other studies [6].
Figure 1.
Antibody quantity and functions in recombinant vesicular stomatitis virus vectored vaccine (rVSV-ZEBOV) vaccinees and Ebola virus disease (EVD) survivors. Plasma samples of individuals vaccinated with 2 × 107 PFUs of candidate vaccine rVSV ZEBOV 180 days after vaccination (vaccinees, n = 10, blue squares) were compared with plasma from EVD survivors (survivors, n = 25, red triangles). Median Ig levels of vaccinees before vaccination (preimmune, n = 6, black points) served as a control. Points show the individual measurements. Bars show the median as well as first and third quartile of the sample distribution. (A) Beads based ELISA for analysis of immunoglobulin (Ig) isotypes and subclasses. Shown is the Median Fluorescent Intensity (MFI) of the secondary antibody in logarithmic scale. (B) Plaque reduction (neutralization) assay using authentic EBOV (Mayinga variant). Lines indicate the nonlinear regression. IC50 values are indicated by light grey pointed lines. (C) Antibody-dependent cellular cytotoxicity (ADCC) reporter assay. (D) Natural killer (NK) cell degranulation assay. (E) Antibody-dependent cellular phagocytosis (ADCP) reporter assay. In all graphs, significance is indicated in lines. Significance levels are calculated using Mann–Whitney tests and indicated by asterisks, namely ** p ≤ 0.01; *** p ≤ 0.001. IC50: half maximal inhibitory concentration.
To analyze the quality of the humoral immune response in survivors and vaccinees, we performed four different quality assays. To test Fab-mediated Ab functions, we determined the NAB titers in survivor and vaccine recipient plasma samples using a plaque reduction neutralization assay (PRNA) with authentic EBOV. The plasma samples from survivors showed a higher neutralization capacity than that of vaccinees (Figure 1B and Figure S2) (median IC50 survivors: 17.6 (95%CI 0 to 37.03); median IC50 vaccinees: 9.9 (95%CI 7.979 to 12.42) fold dilution, respectively), which suggests that even when compared with high-dose vaccination, surviving EVD generates a broader pool of NAB. Next, we sought to determine the quality of FcγR-dependent functions in both cohorts. To this end, we performed two assays to quantify ADCC and one additional assay to determine antibody-dependent activation phagocytosis (ADCP). Both ADCC assays (see Methods Section) revealed a higher capacity to induce NK cell activation in survivors than in vaccinees (Figure 1C,D). Similarly, survivor plasma samples were more efficient at inducing ADCP (Figure 1E).
Taken together, these data indicate that natural infection leads to a more efficient antibody immunity than vaccination, which is reflected in a higher capacity of virus neutralization and activation of cellular responses.
3.2. Antibody Functions Are Associated with Viral Loads in Survivors
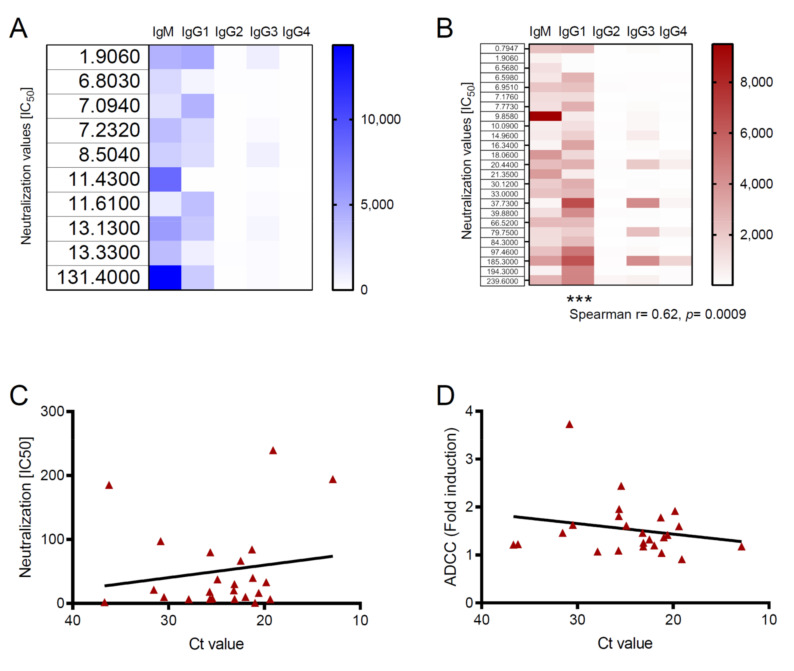
An interesting point in our study was the finding that, despite similar levels of circulating Ig, antibody functions were enhanced in survivors compared to vaccinees. These results suggested that specific Ig subclasses triggered more efficient immune responses in survivors than vaccine recipients. To investigate this, we performed multiple correlation analyses where we searched for associations between Ig subclasses and Ab functions. As shown in the heatmaps of Figure 2A,B, the levels of IgG1 correlated positively with virus neutralization in survivors but not in vaccinees. Indeed, neutralization was the only antibody function that showed a positive correlation with an Ig subclass; ADCC and ADCP did not (Figure S3 available online).
Figure 2.
Correlation analysis. Spearman correlation analysis between the plaque reduction neutralization assay (PRNA) values and Ig subclass in vaccine recipients (A) and EVD survivors (B). Significance is depicted as *** p ≤ 0.001. (C,D) Spearman correlation analysis between PCR Ct values and neutralization values (C) or ADCC fold induction (D) in individual EVD survivors. Lines represent linear regression.
Previous studies into other viral infections have found positive correlations between virus loads during the acute phase of infection and the subsequent neutralizing antibody activity [26,27]. Since natural EBOV infection presents different degrees of severity that are correlated with the viral loads [28,29], we next sought to assess the relationship between pathogen loads and virus neutralization in EVD. Linear regression analysis showed a positive association between virus loads (represented as PCR Ct values) and EBOV neutralization titers. However, this association did not reach statistical significance in the correlation analysis (Figure 2C). Interestingly, we observed the opposite trend when we evaluated the relationship between virus loads and ADCC. Although further research is needed to draw conclusions in this regard, this finding could indicate that Fc-dependent functions are more effective in survivors that showed low levels of viremia during the acute phase of EVD (Figure 2C).
4. Discussion
In this study we compared the levels of EBOV-specific antibodies and the antibody-mediated memory immune responses in EVD survivors and rVSV-ZEBOV vaccinees long-term after discharge (one year) and vaccination (6 months), respectively. Both survivors and vaccinees sustained similar levels of serum anti-EBOV Ig, but the antibody-mediated immune responses were superior in survivors. As shown for other infectious diseases [30,31], the levels of circulating antibodies do not necessarily correlate with the strength of the humoral response against EBOV. In fact, the correlates of protection (CoP) against EVD have not been fully established [32]. One reason for this is that immunity after EVD or vaccination can only be examined in an outbreak scenario. Another reason is that immunity after vaccination in animal models, such as rodents or nonhuman primates (NHPs), does not always correlate with findings in humans. As an example, while anti-EBOV IgG titers correlated with protection in rodents and NHPs [33], in humans the IgG level might be more of a surrogate parameter (i.e., non-mechanistic CoP) for the mechanistic CoP of neutralizing IgM antibodies [20].
While antibodies are considered an integral part of the immunity built against EBOV after disease and vaccination [34], cellular immunity, particularly CD8+ T cells, is also considered integral for protection [32]. Lastly, innate immune responses such as NK cell activity might offer another surrogate CoP [35].
Recently, some authors have suggested that different CoP might have to be utilized for different EBOV vaccines [36] depending, among other parameters, on the antigen used and the route of vaccination. Thus, we need to apply caution when using solely antibody titration to assess the effectiveness of putative vaccines, as this alone may not reflect immunity.
Our study shows that, in both survivors and vaccinees, EBOV-specific IgM is maintained long after exposure, which is consistent with previous findings in rVSV-ZEBOV vaccine recipients [20] and survivors [6]. In this previous rVSV-ZEBOV vaccine study, IgM was predominant even after the second vaccination and played an important role in virus neutralization. Long-lived IgM levels produced by germinal center-independent plasma cells have been previously described and demonstrated a contribution to protection against pathogen challenge [37,38,39]. In future studies it would be interesting to dissect the specific role of these IgM-producing plasma cells in EBOV immunity since, in some instances, they may be maintained for a lifetime [37].
The acute phase of EVD is associated with high levels of circulating virus, systemic dissemination of the pathogen and high levels of inflammation, which may result in dysregulation of cellular immunity [3], endothelial dysfunction [40] and secondary sepsis [41], among other complications. Some previous studies into animal models have indicated that EBOV infection causes severe lymphocytolysis in germinal centers which are necessary for B cell maturation [42]. Yet all the antibody functions were more effective in survivors than in vaccinees that received a controlled, vector-delivered dose of EBOV GP. These findings indicate that, despite all the immune disturbances associated with acute EVD, survivors were able to mount effective B cell-mediated immunity. Since most individuals are immunologically naïve prior to EBOV infection, these results also highlight the importance of making a successful transition between innate and adaptive immunity to survive EVD. To better understand how adaptive immune responses are initiated in response to infection and vaccination, it would be interesting for future studies to compare how antigen-presenting cells process antigens carried by VSV and authentic EBOV.
Previous studies have identified a positive correlation between virus loads and the strength of antibody-mediated neutralization [26,27], although this does not necessarily apply for all viral infections [43]. Antigen availability and the quality of CD4 T cell-mediated help are critical to induce B cell maturation [44], which suggests that survivors were able to maintain CD4 T cell immunity and successful rounds of B cell affinity maturation in the presence of high virus loads. Even though we do not have data on B cell clonality, we also speculate that, even in the presence of high loads of EBOV, the B cell response may be dominated by a few epitopes. This is because the availability of many epitopes reduces the affinity and diversity of the antibody repertoire [45].
The role of ADCC and other Fc-mediated antibody functions in EVD is poorly understood. It is becoming clear however that, in addition to neutralization, ADCC may significantly contribute to EBOV clearance [46,47]. The finding that the strength of ADCC is associated with low viremia is notable and may have different explanations. For example, ADCC may contribute significantly to control viremia during the acute phase of EVD; therefore, survivors show antibodies that retain ADCC function during the memory response. In addition, it is conceivable that the levels of antigen and the associated inflammation may polarize the antibody response towards Fab- or Fc-dependent functions. Finally, the breadth of the antibody repertoire and the epitope coverage may significantly influence antibody functions. In this regard, anti-EBOV GP antibodies exhibit a different capacity of FcγR function engagement which depends on the epitope to which they bind. Anti-GP mAbs targeting membrane proximal epitopes or the GP mucin domain do not rely on Fc–FcγR interactions to confer activity, whereas antibodies against the GP chalice bowl and the fusion loop require FcγR engagement for optimal in vivo antiviral activity [48]. Further experiments in adequate animal models would be very valuable in determining how acute EBOV infection shapes subsequent antibody responses.
The limitations of this study are the small sample sizes, especially for the vaccinee cohort, and differences in age, sex and ethnicity as reported in Table 1. The differences in timepoints between the two cohorts that were available for analysis (6 months post vaccination for vaccinees, approximately one year after recovery for EVD survivors) might also provide a confounding factor.
Our study highlights important immunological differences between vaccination and natural EBOV infection and provides evidence that antibody titers alone may not be sufficient to evaluate vaccination-induced antibody immunity. Comparisons between immune memory in survivors and vaccinees provide a unique opportunity to compare the immunogenicity of current vaccine candidates as well as future vaccines.
Acknowledgments
The authors would like to thank the Ebola survivor communities in Coyah, Guéckédou and Kissidougou for their inestimable support to this study.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4915/12/9/915/s1, Figure S1: Principle and quality control of the subclassing assay. Figure S2: Donor-specific EBOV neutralization curves. Figure S3: Analysis of correlation between Ig subclasses, ADCC and ADCP.
Author Contributions
Conceptualization T.K., M.M.A. and C.M.-F.; methodology, T.K. and C.M.-F.; investigation, T.K., M.R., P.R., S.G.-M., E.V.N., B.E.-P., M.P., M.L.L., F.R.K., J.A.B., N.M. and C.D.; resources, F.R.K., J.A.B. and M.M.A.; supervision, M.M.A., S.G., M.W.C. and C.M.-F.; writing—original draft preparation, C.M.-F.; writing—review and editing, T.K., S.G., M.W.C., M.M.A. and C.M.-F.; project administration, M.M.A. and C.M.-F.; funding acquisition, S.G., M.W.C. and C.M.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by German Research Foundation (DFG), grant number MU 3565/3-0 to C.M.-F. and GU 883/3-0 to S.G., T.K. was funded by a fellowship from the German Center for Infection Research (DZIF). This work was also partially funded by the DZIF grant TTU 01.702 to S.G., M.M.A. and C.M.-F.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Burki T. Ebola virus disease in DR Congo. Lancet Infect. Dis. 2020;20:418–419. doi: 10.1016/S1473-3099(20)30185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McElroy A.K., Akondy R.S., Davis C.W., Ellebedy A.H., Mehta A.K., Kraft C.S., Lyon G.M., Ribner B.S., Varkey J., Sidney J., et al. Human Ebola virus infection results in substantial immune activation. Proc. Natl. Acad. Sci. USA. 2015;112:4719–4724. doi: 10.1073/pnas.1502619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruibal P., Oestereich L., Lüdtke A., Becker-Ziaja B., Wozniak D.M., Kerber R., Korva M., Cabeza-Cabrerizo M., Bore J.A., Koundouno F.R., et al. Unique human immune signature of Ebola virus disease in Guinea. Nature. 2016;533:100–104. doi: 10.1038/nature17949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahlke C., Lunemann S., Kasonta R., Kreuels B., Schmiedel S., Ly M.L., Fehling S.K., Strecker T., Becker S., Altfeld M., et al. Comprehensive characterization of cellular immune responses following Ebola virus infection. J. Infect. Dis. 2016;215:287–292. doi: 10.1093/infdis/jiw508. [DOI] [PubMed] [Google Scholar]
- 5.Munoz-Fontela C., McElroy A.K. Ebola Virus Disease in Humans: Pathophysiology and Immunity. Curr. Top. Microbiol. Immunol. 2017;204:S796. doi: 10.1007/82_2017_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khurana S., Ravichandran S., Hahn M., Coyle E.M., Stonier S.W., Zak S.E., Kindrachuk J., Davey R.T., Dye J.M., Chertow D.S. Longitudinal Human Antibody Repertoire against Complete Viral Proteome from Ebola Virus Survivor Reveals Protective Sites for Vaccine Design. Cell Host Microbe. 2020;27:262–276.e4. doi: 10.1016/j.chom.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colavita F., Biava M., Castilletti C., Lanini S., Miccio R., Portella G., Vairo F., Ippolito G., Capobianchi M.R., Di Caro A., et al. Inflammatory and Humoral Immune Response during Ebola Virus Infection in Survivor and Fatal Cases Occurred in Sierra Leone during the 2014–2016 Outbreak in West Africa. Viruses. 2019;11:373. doi: 10.3390/v11040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowe J.E., Jr. Principles of Broad and Potent Antiviral Human Antibodies: Insights for Vaccine Design. Cell Host Microbe. 2017;22:193–206. doi: 10.1016/j.chom.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu L.L., Suscovich T.J., Fortune S.M., Alter G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018;18:46–61. doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maruyama T., Rodriguez L.L., Jahrling P.B., Sanchez A., Khan A.S., Nichol S.T., Peters C.J., Parren P.W., Burton D.R. Ebola virus can be effectively neutralized by antibody produced in natural human infection. J. Virol. 1999;73:6024–6030. doi: 10.1128/JVI.73.7.6024-6030.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimoin A.W., Lu K., Bramble M.S., Steffen I., Doshi R.H., Hoff N.A., Mukadi P., Nicholson B.P., Alfonso V.H., Olinger G., et al. Ebola Virus Neutralizing Antibodies Detectable in Survivors of the Yambuku, Zaire Outbreak 40 Years after Infection. J. Infect. Dis. 2017;217:223–231. doi: 10.1093/infdis/jix584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radinsky O., Edri A., Brusilovsky M., Fedida-Metula S., Sobarzo A., Gershoni-Yahalom O., Lutwama J., Dye J., Lobel L., Porgador A. Sudan ebolavirus long recovered survivors produce GP-specific Abs that are of the IgG1 subclass and preferentially bind FcγRI. Sci Rep. 2017;7:6054. doi: 10.1038/s41598-017-06226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oswald W.B., Geisbert T.W., Davis K.J., Geisbert J.B., Sullivan N.J., Jahrling P.B., Parren P.W.H.I., Burton D.R. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLoS Pathog. 2007;3:e9. doi: 10.1371/journal.ppat.0030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corti D., Misasi J., Mulangu S., Stanley D.A., Kanekiyo M., Wollen S., Ploquin A., Doria-Rose N.A., Staupe R.P., Bailey M., et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science. 2016;351:1339–1342. doi: 10.1126/science.aad5224. [DOI] [PubMed] [Google Scholar]
- 15.Wec A.Z., Bornholdt Z.A., He S., Herbert A.S., Goodwin E., Wirchnianski A.S., Gunn B.M., Zhang Z., Zhu W., Liu G., et al. Development of a Human Antibody Cocktail that Deploys Multiple Functions to Confer Pan-Ebolavirus Protection. Cell Host Microbe. 2019;25:39–48.e5. doi: 10.1016/j.chom.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luczkowiak J., Arribas J.R., Gómez S., Jiménez-Yuste V., la Calle de F., Viejo A., Delgado R. Specific neutralizing response in plasma from convalescent patients of Ebola Virus Disease against the West Africa Makona variant of Ebola virus. Virus Res. 2016;213:224–229. doi: 10.1016/j.virusres.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 17.Williamson L.E., Flyak A.I., Kose N., Bombardi R., Branchizio A., Reddy S., Davidson E., Doranz B.J., Fusco M.L., Saphire E.O., et al. Early Human B Cell Response to Ebola Virus in Four U.S. Survivors of Infection. J. Virol. 2019;93:392. doi: 10.1128/JVI.01439-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henao-Restrepo A.M., Camacho A., Longini I.M., Watson C.H., Edmunds W.J., Egger M., Carroll M.W., Dean N.E., Diatta I., Doumbia M., et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) Lancet. 2017;389:505–518. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Administration F.A.D. First FDA-Approved Vaccine for the Prevention of Ebola Virus Disease, Marking a Critical Milestone in Public Health Preparedness and Response. [(accessed on 18 August 2020)]; Available online: https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-ebola-virus-disease-marking-critical-milestone-public-health.
- 20.Khurana S., Fuentes S., Coyle E.M., Ravichandran S., Davey R.T., Beigel J.H. Human antibody repertoire after VSV-Ebola vaccination identifies novel targets and virus-neutralizing IgM antibodies. Nat. Med. 2016;22:1439–1447. doi: 10.1038/nm.4201. [DOI] [PubMed] [Google Scholar]
- 21.Cohen-Dvashi H., Zehner M., Ehrhardt S., Katz M., Elad N., Klein F., Diskin R. Structural Basis for a Convergent Immune Response against Ebola Virus. Cell Host Microbe. 2020;27:418–427.e4. doi: 10.1016/j.chom.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Ehrhardt S.A., Zehner M., Krähling V., Cohen-Dvashi H., Kreer C., Elad N., Gruell H., Ercanoglu M.S., Schommers P., Gieselmann L., et al. Polyclonal and convergent antibody response to Ebola virus vaccine rVSV-ZEBOV. Nat. Med. 2019;25:1589–1600. doi: 10.1038/s41591-019-0602-4. [DOI] [PubMed] [Google Scholar]
- 23.Wagstaffe H.R., Clutterbuck E.A., Bockstal V., Stoop J.N., Luhn K., Douoguih M., Shukarev G., Snape M.D., Pollard A.J., Riley E.M., et al. Antibody-Dependent Natural Killer Cell Activation after Ebola Vaccination. J. Infect. Dis. 2019;27:8. doi: 10.1093/infdis/jiz657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agnandji S.T., Huttner A., Zinser M.E., Njuguna P., Dahlke C., Fernandes J.F., Yerly S., Dayer J.-A., Kraehling V., Kasonta R., et al. Phase 1 Trials of rVSV Ebola Vaccine in Africa and Europe. N. Engl. J. Med. 2016;374:1647–1660. doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung A.W., Ghebremichael M., Robinson H., Brown E., Choi I., Lane S., Dugast A.-S., Schoen M.K., Rolland M., Suscovich T.J., et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci. Transl Med. 2014;6:228ra38. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 26.Gray E.S., Madiga M.C., Hermanus T., Moore P.L., Wibmer C.K., Tumba N.L., Werner L., Mlisana K., Sibeko S., Williamson C., et al. CAPRISA002 Study Team The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J. Virol. 2011;85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pastrana D.V., Wieland U., Silling S., Buck C.B., Pfister H. Positive correlation between Merkel cell polyomavirus viral load and capsid-specific antibody titer. Med. Microbiol. Immunol. 2012;201:17–23. doi: 10.1007/s00430-011-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanini S., Portella G., Vairo F., Kobinger G.P., Pesenti A., Langer M., Kabia S., Brogiato G., Amone J., Castilletti C., et al. Blood kinetics of Ebola virus in survivors and nonsurvivors. J. Clin. Investig. 2015;125:4692–4698. doi: 10.1172/JCI83111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebola viral load at diagnosis associates with patient outcome and outbreak evolution. J. Clin. Investig. 2015;125:4421–4428. doi: 10.1172/JCI83162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crone N.E., Reder A.T. Severe tetanus in immunized patients with high anti-tetanus titers. Neurology. 1992;42:761–764. doi: 10.1212/WNL.42.4.761. [DOI] [PubMed] [Google Scholar]
- 31.Maselle S.Y., Matre R., Mbise R., Hofstad T. Neonatal tetanus despite protective serum antitoxin concentration. FEMS Microbiol. Immunol. 1991;3:171–175. doi: 10.1111/j.1574-6968.1991.tb04211.x. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan N.J., Martin J.E., Graham B.S., Nabel G.J. Correlates of protective immunity for Ebola vaccines: Implications for regulatory approval by the animal rule. Nat. Rev. Microbiol. 2009;7:393–400. doi: 10.1038/nrmicro2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong G., Richardson J.S., Pillet S., Patel A., Qiu X., Alimonti J., Hogan J., Zhang Y., Takada A., Feldmann H., et al. Immune parameters correlate with protection against ebola virus infection in rodents and nonhuman primates. Sci. Transl. Med. 2012;4:158ra146. doi: 10.1126/scitranslmed.3004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medaglini D., Santoro F., Siegrist C.-A. Correlates of vaccine-induced protective immunity against Ebola virus disease. Semin. Immunol. 2018;39:65–72. doi: 10.1016/j.smim.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Rechtien A., Richert L., Lorenzo H., Martrus G., Hejblum B., Dahlke C., Kasonta R., Zinser M., Stubbe H., Matschl U. Systems Vaccinology Identifies an Early Innate Immune Signature as a Correlate of Antibody Responses to the Ebola Vaccine rVSV-ZEBOV. Cell Rep. 2017;20:2251–2261. doi: 10.1016/j.celrep.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer M., Malherbe D.C., Bukreyev A. Can Ebola Virus Vaccines Have Universal Immune Correlates of protection? Trends Microbiol. 2019;27:8–16. doi: 10.1016/j.tim.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long-lived antigen-induced IgM plasma cells demonstrate somatic mutations and contribute to long-term protection. Nat. Commun. 2016;7:11826. doi: 10.1038/ncomms11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foote J.B., Mahmoud T.I., Vale A.M., Kearney J.F. Long-term maintenance of polysaccharide-specific antibodies by IgM-secreting cells. J. Immunol. 2012;188:57–67. doi: 10.4049/jimmunol.1100783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Racine R., McLaughlin M., Jones D.D., Wittmer S.T., MacNamara K.C., Woodland D.L., Winslow G.M. IgM production by bone marrow plasmablasts contributes to long-term protection against intracellular bacterial infection. J. Immunol. 2011;186:1011–1021. doi: 10.4049/jimmunol.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McElroy A.K., Erickson B.R., Flietstra T.D., Rollin P.E., Nichol S.T., Towner J.S., Spiropoulou C.F. Ebola hemorrhagic fever: Novel biomarker correlates of clinical outcome. J. Infect. Dis. 2014;210:558–566. doi: 10.1093/infdis/jiu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Case of Severe Ebola Virus Infection Complicated by Gram-Negative Septicemia. N. Engl. J. Med. 2014;371:2394–2401. doi: 10.1056/NEJMoa1411677. [DOI] [PubMed] [Google Scholar]
- 42.Kozak R., He S., Kroeker A., La Vega de M.-A., Audet J., Wong G., Urfano C., Antonation K., Embury-Hyatt C., Kobinger G.P., et al. Ferrets infected with Bundibugyo virus or Ebola virus recapitulate important aspects of human filoviral disease. J. Virol. 2016;90:9209–9223. doi: 10.1128/JVI.01033-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sajadi M.M., Guan Y., DeVico A.L., Seaman M.S., Hossain M., Lewis G.K., Redfield R.R. Correlation between circulating HIV-1 RNA and broad HIV-1 neutralizing antibody activity. J. Acquir. Immune Defic. Syndr. 2011;57:9–15. doi: 10.1097/QAI.0b013e3182100c1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicholas K.J., Zern E.K., Barnett L., Smith R.M., Lorey S.L., Copeland C.A., Sadagopal S., Kalams S.A. B cell responses to HIV antigen are a potent correlate of viremia in HIV-1 infection and improve with PD-1 blockade. PLoS ONE. 2013;8:e84185. doi: 10.1371/journal.pone.0084185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Childs L.M., Baskerville E.B., Cobey S. Trade-offs in antibody repertoires to complex antigens. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20140245. doi: 10.1098/rstb.2014.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunn B.M., Roy V., Karim M.M., Hartnett J.N., Suscovich T.J., Goba A., Momoh M., Sandi J.D., Kanneh L., Andersen K.G., et al. Survivors of Ebola Virus Disease Develop Polyfunctional Antibody Responses. J. Infect. Dis. 2020;221:156–161. doi: 10.1093/infdis/jiz364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saphire E.O. A glimpse into immune responses evolving against Ebola virus. Nat. Med. 2019;25:1470–1471. doi: 10.1038/s41591-019-0607-z. [DOI] [PubMed] [Google Scholar]
- 48.Bournazos S., DiLillo D.J., Goff A.J., Glass P.J., Ravetch J.V. Differential requirements for FcγR engagement by protective antibodies against Ebola virus. Proc. Natl. Acad. Sci. USA. 2019;116:20054–20062. doi: 10.1073/pnas.1911842116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.