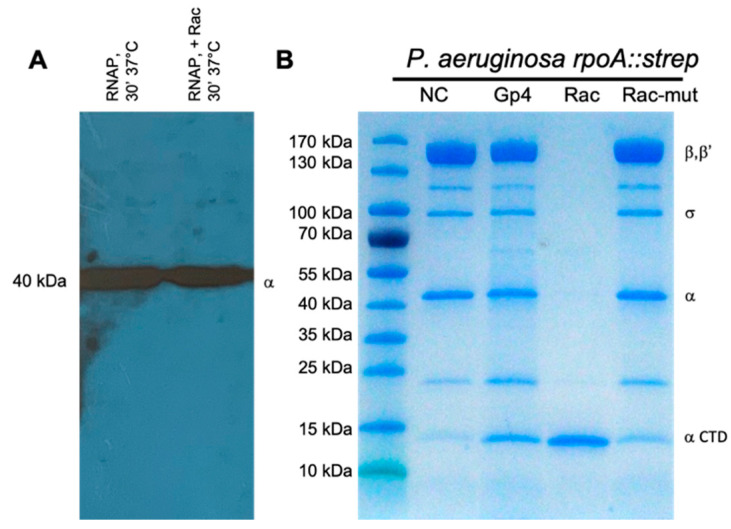
Figure A4.
Rac does not have protease activity and the predicted N-acetyl CoA binding pocket of Rac is required for cleavage. (A) Recombinant Rac was incubated at 37 °C for 30 min with P. aeruginosa RNAP purified using the RpoA-Strep tag. Western blot using antibody specific for the intact α RNAP subunit shows the α subunit is not cleaved. (B) The RpoA-Strep was purified from P. aeruginosa cultures 30 min after induction of Gp4 (control), wildtype Rac or mutated Rac (with an inactivated acetyl-CoA binding pocket). The wildtype rpoA::strep mutant was used as a negative control. After RpoA-Strep purification and separation on a 4–20% SDS-PAGE gel, it is clear that only the wildtype Rac provokes the α subunit cleavage.