Abstract
Ilheus virus is an arbovirus with the potential for central nervous system involvement. Accurate diagnosis is a challenge due to similar clinical symptoms and serologic cross-reactivity with other flaviviruses. Here, we describe the first documented case of a fatal outcome following the identification of Ilheus virus in the cerebrospinal fluid (CSF) of a patient with cerebral encephalitis in Brazil.
Keywords: Ilheus virus, cerebrospinal fluid, atypical manifestations, cerebrovascular event
1. Introduction
Ilheus virus (ILHV) is an arbovirus that was first described in 1944 and isolated from Aedes and Psorophora spp. mosquitoes during an epidemiological investigation of yellow fever in the city of Ilheus, Bahia State, Brazil [1,2]. ILHV belongs to the genus Flavivirus, family Flaviviridae in the Ntaya antigenic complex. ILHV is maintained in an enzootic transmission cycle between birds and arboreal mosquitoes belonging to eight genera (Aedes, Culex, Coquillettidia, Haemagogus, Sabethes, Trichoprosopon, Psorophora, and Ochlerotatus, with the last two being considered the primary vectors of transmission). Since its initial isolation, ILHV has been isolated or detected primarily in arboreal mosquitoes [1,3,4,5,6,7,8,9], birds [10,11], and humans [8,12,13,14,15,16,17,18,19] throughout Central America (Honduras, Guatemala, and Panama), the Caribbean (Trinidad and Tobago), and South America (Argentina, Bolivia, Brazil, Colombia, Ecuador, French Guyana, Peru, and Venezuela), suggesting a broad geographic range of transmission. Various serological surveys showed the presence of ILHV-neutralizing antibodies in rodents [20], coatis [10], birds [6,10,20,21], horses [22,23], sentinel [8] and wild monkeys [10,20,24,25,26,27], and humans [1,17,18,20,28,29].
Since 2006, we have established arbovirus surveillance for encephalitis-suggestive cases in the city of São José do Rio Preto (SJdRP), State of São Paulo, Brazil, located in a hyper-endemic area for dengue virus (DENV) [30,31,32,33,34,35,36,37,38,39], Saint Louis encephalitis [40,41], Zika virus (ZIKV) [34], and documented co-infection among various flaviviruses [30,41,42]. Herein, we report a case of cerebral encephalitis in a patient infected with ILHV that was observed by our surveillance team in São José do Rio Preto (SJdRP).
2. Materials and Methods
2.1. Ethics Statement
This study was submitted and approved by the Ethical Review Board (process number 15461513.5.0000.5415, 24 May 2019) of the School of Medicine of São José do Rio Preto (FAMERP), São Paulo, Brazil. Confidentiality was ensured by de-identifying all questionnaires and samples before the data entry and analysis.
2.2. Medical History and Sample Collection
Through an arbovirus surveillance program established in SJdRP, all dengue-suspected cases with warning signs (DwWS) or severe disease (SD) were monitored by our team from admission to discharge. The classification of warning signs includes abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleed, lethargy, restlessness, liver enlargement >2 cm, and an increase in the hematocrit concurrent with a rapid decrease in platelet count. Severe dengue disease is classified by the presence of severe plasma leakage (shock and fluid accumulation with respiratory distress), severe bleeding, and/or severe organ involvement (aspartate aminotransferase and/or alanine aminotransferase ≥1000 UI/L, impaired consciousness, and heart and other organ failure). This case was part of a hospital-based retrospective and descriptive study conducted with biobanked cerebrospinal fluids from patients with central nervous system (CNS) impairment observed between January 2016 and December 2017. During the same period, there were 16,898 laboratory-confirmed dengue cases in the city (DENV RT-PCR positive, NS1 antigen detection reagent, and/or anti-dengue immunoglobulin type M (IgM) reagent), of which, 287 were further screened and had clinical samples (notably, CSF) submitted retrospectively for further diagnostic tests for arbovirus detection at the Laboratório de Pesquisas em Virologia (LPV), located within FAMERP. Demographic, epidemiological (gender and age), and clinical data (symptoms and radiologic observations) were obtained from electronic records and reported to the medical team.
2.3. Diagnostic Analyses
Due to the limited quantity of the CSF samples available, we opted not to attempt virus isolation. Samples were submitted for virus RNA (vRNA) extraction using the Kit QIAmp® Viral RNA (QIAGEN®, Germantown, MD, USA) following the manufacturer’s recommendations. The Trioplex quantitative polymerase chain reaction (qPCR) assay was performed using a kit provided by the Centers for Disease Control and Prevention using primers and probes specifically designed for the detection of ZIKV, chikungunya virus (CHIKV), and all DENV serotypes [43]. A total of 10 µL of vRNA, 0.5 µM of each probe and primer mixed, 12.5 µL of the 2X PCR Master Mix, and 0.5 µL of Superscript III RT/Platinum Taq enzyme mix (SuperScript® III Platinum® One-Step qRT-PCR System, Invitrogen, Carlsbad, CA, USA) were applied to a 96-well plate using the QuantStudio™ Dx instrument (Thermo Fisher Scientific, Waltham, MA, USA) with the following conditions: 50 °C for 30 s, followed by 45 cycles of 95 °C for 15 s, and 60 °C for 1 min. The results were interpreted as being positive when the cycle threshold (Ct) values were less than 38.
The duplex-nested PCR assay targeting a conserved domain of the nonstructural protein 1 (nsp1) and 5 (NS5) genes that was designed for the detection and identification of Brazilian alphaviruses and flaviviruses, respectively [44], was also utilized. An amplicon that was 401 nucleotides long was obtained, purified, and sequenced via the Sanger method [45] using a Big Dye Terminator Kit v3.1 (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions, and deposited in GenBank (MK266240). The obtained sequence was analyzed using BLAST (www.ncbi.nlm.nih.gov/blast/Blast.cgi) software (v1.4.0) and the alignment to known GenBank sequences. To eliminate any chance of contamination, a strict processing protocol was established based on the unidirectional flow of processes, including separate rooms for pre-PCR sample preparation, RNA extraction, PCR assembly, and running steps, with intensive use of individual protection equipment. Additionally, a rigorous cleaning and decontamination protocol of all surfaces and equipment (20 min contact time with 70% ethyl alcohol and exposure to ultraviolet light) was performed throughout the process. For this study, the ILHV genetic material was not used at any time during this process as a positive control, further reducing the possibility of contamination.
2.4. Phylogenetic Analysis
The ILHV evolutionary history was inferred using the maximum likelihood method based on the general time-reversible model with a bootstrap of 1000 replicates [46] and using a dataset of 13 representative ILHV strains with a spatiotemporal span of 73 years (1944–2017) comprising 401-nucleotide-long sequence mapping on the NS5 gene. The tree with the highest log-likelihood (−1087.95) was found. Initial tree(s) for the heuristic search were obtained automatically by applying the Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances that were estimated using the maximum composite likelihood (MCL) approach and then selecting the topology with a superior log-likelihood value. A discrete gamma distribution was used to model the evolutionary rate differences between sites (five categories (+G, parameter = 0.8348)). The rate variation model allowed for some sites to be evolutionarily invariable ([+I], 52.27% sites). The tree was drawn to scale, with branch lengths measured in the number of substitutions per site. A homologous nucleotide sequence from Rocio virus (ROCV) was used as an outgroup to root the ILHV tree. All positions containing gaps and missing data were eliminated, leaving a total of 401 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 [47].
3. Results
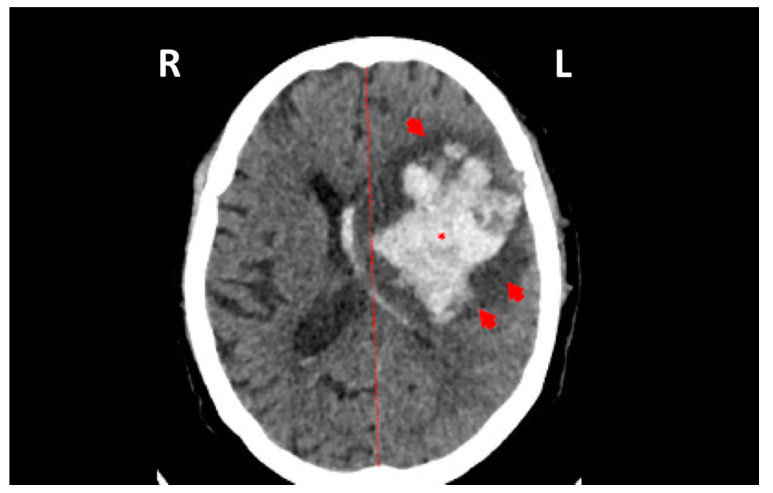
Between January 2016 and December 2017, 287 CSF samples were evaluated for possible arbovirus infection; in one of those, the genetic sequence of ILHV was detected by the highly specific PCR assay. The sample corresponds to a 68-year-old man who was admitted on 28 September 2017 with right hemiplegia, aphasia, dysarthria, and deviation of the left lip rhyme. He suffered from blood hypertension and diabetes. During hospitalization, brain computed tomography showed an intraparenchymal hemorrhage in the parietal lobe, surrounding brain edema, and a deviation from the cerebral middle line (Figure 1). He exhibited no symptoms of infection until admission and had no travel history. Cerebrospinal fluid was collected (leukocytes 840 cells/mm3 (83% lymphomonocytes), glucose 57 mg/dL, protein 338 mg/dL, and bacteria culture negative) and he underwent bleeding drainage. After 6 days of drainage, testing of the CSF showed leukocytes at 190 cells/mm3 (80% lymphomonocytes), glucose 102 mg/dL, protein 802 mg/dL, and the bacteria culture negative. Subsequently, the patient developed a urinary infection and pneumonia from multi-drug resistant bacteria (E. coli and Acinetobacter baumannii, respectively) and died 24 days following admission.
Figure 1.
Computed tomography imaging of the patient’s brain showing an intraparenchymal hemorrhage (asterisk) surrounded by a brain edema (red arrows), as well as deviation from the cerebral middle line (red line).
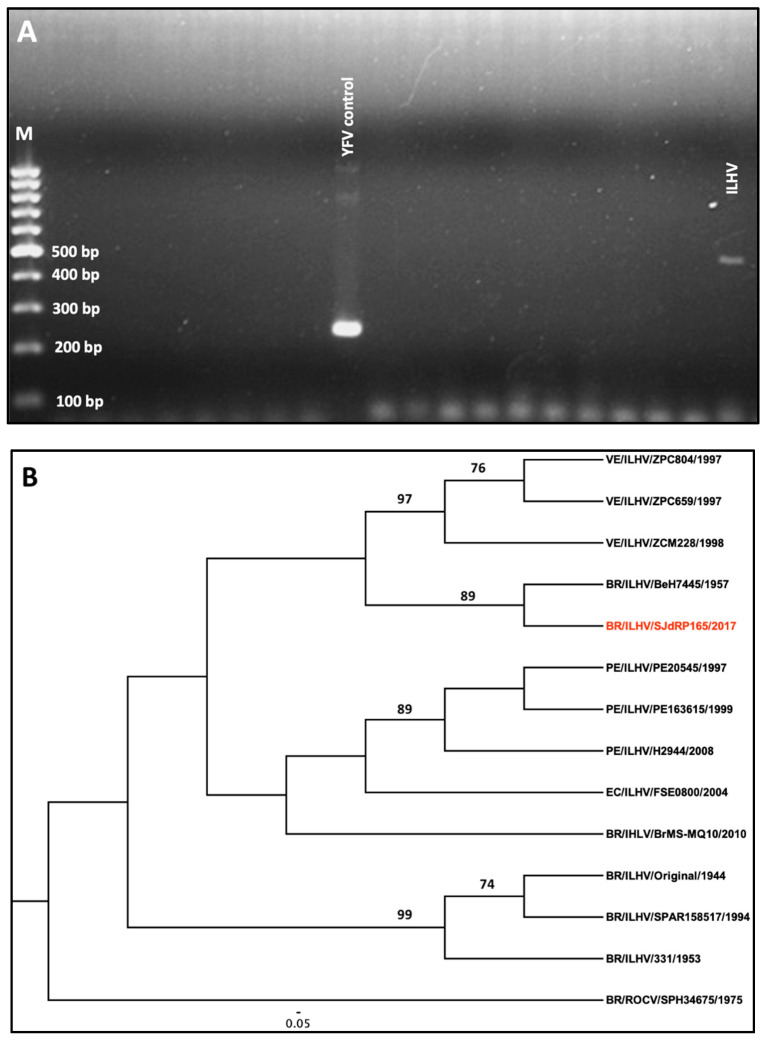
A CSF sample was also submitted for RNA extraction and tested for dengue, Zika, and chikungunya viruses using the Trioplex quantitative polymerase chain reaction (qPCR) [43] assay, as well as using an assay designed to detect several other arboviruses endemic to Brazil with primer sets that are specific for conserved domains of the gene encoding for the RNA-dependent RNA-polymerase (RdRp) [44]. The resulting amplicons that were 401 nucleotides long were sequenced and ILHV was the only arbovirus identified (Figure 2a). Phylogenetic analysis demonstrated clustering with isolates sampled in Venezuela in 1997, suggesting the widespread distribution of the virus throughout Latin America (Figure 2b).
Figure 2.
(A) Molecular detection of the Ilheus virus (ILHV) partial sequence of the NS5 gene on a 1.5% agarose gel using yellow fever virus (YFV) as a positive control. (B) Phylogeny of Ilheus virus inferred using the maximum likelihood method. The maximum likelihood tree was obtained from a sequence dataset of 11 isolates using a General Time Reversible (GTR) substitution model. Branches are labeled with bootstrap values that represent the percentage of 1000 replicates in which the members of a given clade were predicted to relate in the same topography. The scale shows a genetic distance of 0.05, or a 5% nucleotide sequence divergence. A homologous sequence from Rocio virus (ROCV) was used as an outgroup to root the ILHV tree. The sequence of the strain for this study is indicated in red. Abbreviations: VE—Venezuela; BR—Brazil; PE—Peru; EC—Ecuador.
4. Discussion
Current knowledge suggests that ILHV is not associated with any known epidemics, and human infection has been sporadically reported in Trinidad [15], Panama [16], Colombia [14], French Guyana [13], Brazil [8,48], Ecuador [12], and Bolivia [19]. The clinical spectrum of human infections ranges from asymptomatic to severe disease that is characterized by central nervous system involvement that is suggestive of encephalitis. Viremia lasts three to five days and most patients exhibit a mild febrile illness, accompanied by headache, myalgia, photophobia, and arthralgia, as well as non-specific symptoms that may suggest dengue fever, yellow fever, or influenza [8,15,49]. CNS involvement has been observed infrequently in the handful of documented human cases and may indicate progression to severe Ilheus disease, whose clinical presentation is suggestive of viral encephalitis ([15,48,50] and Table 1). Furthermore, the few clinically diagnosed cases documented to date (Table 1) contrast with the observed prevalence of ILHV antibodies in humans [1,17,18,20,28,29], suggesting that most infections are inapparent or misdiagnosed. Mild non-specific symptoms, short viremia, high levels of antibodies showing cross-reactivity with flaviviruses, and a lack of routine laboratory assays are some of the barriers that may complicate an accurate ILHV diagnosis [19].
Table 1.
Clinical symptoms that were observed in documented ILHV human cases.
| Country, Year | Number of Cases | Clinical Symptoms | Diagnostic Tests Performed | Reference |
|---|---|---|---|---|
| USA, 1950 | 19 |
|
Blood and serology testing (HI, CF, mouse neutralization test) | [50] |
| Brazil, 1957–1959 | 2 |
|
Blood and serology testing (HI, CF, mouse neutralization test) | [8] |
| Trinidad, 1955–1957 | 3 |
|
Blood and serology testing (HI, CF, mouse neutralization test) | [15] |
| Panama, 1964 | 1 |
|
Blood and serology testing (HI) | [16] |
| Colombia, 1966 | 1 |
|
Blood and serology testing (HI, CF, mouse neutralization test) | [14] |
| French Guiana, 1973 | 1 |
|
Blood and serology testing (HI, CF) | [13] |
| Brazil, 1995 | 5 * |
|
Blood and serology testing (HI, CF, mouse neutralization test) | [48] |
| Ecuador, 2004 | 1 |
|
Blood work | [12] |
| Bolivia, 2005 | 1 |
|
Blood, molecular (RT-PCR) and serology (IgM ELISA) testing | [19] |
| Brazil, 2017 | 1 |
|
Molecular testing of CSF (qPCR) | Present paper |
Abbreviations: HI—hemagglutination inhibition, CF—complement fixation, RT-PCR—reverse transcription polymerase chain reaction, IgM ELISA—immunoglobulin M enzyme-linked immunosorbent assay. (*) Although the virus was isolated from the serum of each patient, it was not sequenced for confirmation.
Given the shortage of data in the literature on neurological events in ILHV infections, it is reasonable to propose that physicians could leverage the acquired knowledge from other well-characterized flavivirus infections, such as DENV, Japanese encephalitis virus (JEV), and West Nile virus (WNV). Our observations may not conclusively demonstrate that ILHV infection led to the brain hemorrhage and death in a patient with an acute neurological syndrome and underlying conditions (diabetes and hypertension). Considering the increasing incidence of arboviruses with unusual manifestations ([51,52,53] and reviewed in [54,55,56]), and their potential tropism for the invasion of the CNS, leading to long-term neurofunctional sequelae, this report highlights the need for vigilance among physicians, healthcare providers, and researchers alike for arbovirus infections in patients presenting with meningoencephalitis and cerebrovascular events. Furthermore, this study demonstrates the importance of comprehensive arbovirus surveillance beyond urban arboviruses (e.g., DENV, CHIKV, and ZIKV), suggesting that urban and peri-urban populations may be at risk for ILHV infection and other emerging zoonotic arboviruses. Given the high level of antibody cross-reactivity with flaviviruses and the lack of routine laboratory serological assays, which complicates an accurate diagnosis of arboviruses, this poses an urgent call for employing new and affordable technologies [57,58] for the development of more accurate diagnostic assays
Author Contributions
Conceptualization: C.F.E., B.H.G.A.M., A.C.B.T., and M.L.N.; data curation: C.F.E., E.L., and V.M.S.B.; formal analysis: B.H.G.A.M., A.C.B.T., L.C.d.R., V.M.S.B, N.V., and M.L.N.; funding acquisition: M.L.N. and N.V.; investigation: B.H.G.A.M. and C.F.E.; methodology: B.H.G.A.M., A.C.B.T., N.V., and M.L.N.; supervision: C.F.E. and M.L.N.; writing—original draft: C.F.E., B.H.G.A.M., A.C.B.T., N.V., and M.L.N.; writing—review and editing: C.F.E., B.H.G.A.M., N.V., and M.L.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grant 2013/21719-3 from FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo) (to M.L.N.) and partly by grants U01AI115577 and U01AI151807 from the US National Institutes of Health (to N.V.). M.L.N. is a CNPq research fellow.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Laemmert H.W., Jr., Hughes T.P. The virus of Ilhéus encephalitis; isolation, serological specificity and transmission. J. Immunol. 1947;55:61–67. [PubMed] [Google Scholar]
- 2.Koprowski H., Hughes T.P. The virus of Ilhéus encephalitis; physical properties, pathogenicity and cultivation. J. Immunol. 1946;54:371–385. [PubMed] [Google Scholar]
- 3.Pauvolid-Corrêa A., Kenney J.L., Couto-Lima D., Campos Z.M., Schatzmayr H.G., Nogueira R.M., Brault A.C., Komar N. Ilheus virus isolation in the Pantanal, west-central Brazil. PLoS Negl. Trop. Dis. 2013;7:e2318. doi: 10.1371/journal.pntd.0002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Rodaniche E. Isolation of the virus of IIheus encephalitis from mosquitoes of the genus Psorophora captured in Honduras. Am. J. Trop. Med. Hyg. 1956;5:797–801. doi: 10.4269/ajtmh.1956.5.797. [DOI] [PubMed] [Google Scholar]
- 5.De Rodaniche E., Galindo P. Isolation of Ilhéus virus from sabethes chloropterus captured in Guatemala in 1956. Am. J. Trop. Med. Hyg. 1957;6:686–687. doi: 10.4269/ajtmh.1957.6.686. [DOI] [PubMed] [Google Scholar]
- 6.De Rodaniche E., Galindo P. Ecological observations on Ilhéus virus in the vicinity of Almirante, Republic of Panama. Am. J. Trop. Med. Hyg. 1963;12:924–928. doi: 10.4269/ajtmh.1963.12.924. [DOI] [PubMed] [Google Scholar]
- 7.Vieira C.J.D.S.P., De Andrade C.D., Kubiszeski J.R., Da Silva D.J.F., Barreto E.S., Massey A.L., Canale G.R., Bernardo C.S.S., Levi T., Peres C.A., et al. Detection of Ilheus virus in mosquitoes from southeast Amazon, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2019;113:424–427. doi: 10.1093/trstmh/trz031. [DOI] [PubMed] [Google Scholar]
- 8.Causey O.R., E Causey C., Maroja O.M., Macedo D.G. The isolation of arthropod-borne viruses, including members of two hitherto undescribed serological groups, in the Amazon Region of Brazil. Am. J. Trop. Med. Hyg. 1961;10:227–249. doi: 10.4269/ajtmh.1961.10.227. [DOI] [PubMed] [Google Scholar]
- 9.Aitken T.H.G., Anderson C.R., Downs W.G. The isolation of Ilhéus virus from wild caught forest mosquitoes in Trinidad 1. Am. J. Trop. Med. Hyg. 1956;5:621–625. doi: 10.4269/ajtmh.1956.5.621. [DOI] [PubMed] [Google Scholar]
- 10.Pereira L.E., Suzuki A., Coimbra T.L.M., Souza R.P.D., Chamelet E.L.B. Ilheus arbovirus in wild birds (Sporophila caerulescens and Molothrus bonariensis) Rev. Saude Publica. 2001;35:119–123. doi: 10.1590/S0034-89102001000200003. [DOI] [PubMed] [Google Scholar]
- 11.Galindo P., De Rodaniche E. Birds as hosts of Ilhéus encephalitis virus in Panama. Am. J. Trop. Med. Hyg. 1961;10:395–396. doi: 10.4269/ajtmh.1961.10.395. [DOI] [PubMed] [Google Scholar]
- 12.Johnson B.W., Cruz C., Felices V., Espinoza W.R., Manock S.R., Guevara C., Olson J.G., Kochel T.J. Ilheus virus isolate from a human, Ecuador. Emerg. Infect. Dis. 2007;13:956–958. doi: 10.3201/eid1306.070118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panon G., Fauran P., Digoutte J.P. Isolation of Ilheus virus in french Guyana. Bull. Soc. Pathol. Exot. Fil. 1979;72:315–318. [PubMed] [Google Scholar]
- 14.Prías-Landínez E., Bernal-Cubides C., Morales-Alarcón A. Isolation of Ilhéus virus from man in Colombia. Am. J. Trop. Med. Hyg. 1968;17:112–114. doi: 10.4269/ajtmh.1968.17.112. [DOI] [PubMed] [Google Scholar]
- 15.Spence L., Anderson C., Downs W. Isolation of Ilhéus virus from human beings in Trinidad, West Indies. Trans. R. Soc. Trop. Med. Hyg. 1962;56:504–509. doi: 10.1016/0035-9203(62)90074-3. [DOI] [PubMed] [Google Scholar]
- 16.Srihongse S., Johnson C.M. The isolation of Ilhéus virus from man in Panamá. Am. J. Trop. Med. Hyg. 1967;16:516–518. doi: 10.4269/ajtmh.1967.16.516. [DOI] [PubMed] [Google Scholar]
- 17.Anderson C.R., Downs W.G., Theiler M. Neutralizing antibodies against certain viruses in the sera of residents of Trinidad, B.W.I. Am. J. Trop. Med. Hyg. 1956;5:626–641. doi: 10.4269/ajtmh.1956.5.626. [DOI] [PubMed] [Google Scholar]
- 18.Causey O.R., Theiler M. Virus antibody survey on sera of residents of the Amazon valley in Brazil. Am. J. Trop. Med. Hyg. 1958;7:36–41. doi: 10.4269/ajtmh.1958.7.36. [DOI] [PubMed] [Google Scholar]
- 19.Venegas E.A., Aguilar P.V., Cruz C., Guevara C., Kochel T.J., Vargas J., Halsey E.S. Ilheus virus infection in human, Bolivia. Emerg. Infect. Dis. 2012;18:516–518. doi: 10.3201/eid1803.111486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degallier N., Travassos da Rosa A.P.A., Vasconcelos P.F.C., Herve J.P., Filho G.C., Travassos da Rosa F.S., Travassos da Rosa E.S., Rodrigues S.G. Modifications of arbovirus transmission in relation to construction of dams in Brazilian Amazonia. Cienc. Cult. 1992;44:124–135. [Google Scholar]
- 21.Ferreira I.B., Pereira L.E., Rocco I.M., Marti A.T., De Souza L.T.M., Iversson L.B. Surveillance of arbovirus infections in the atlantic forest region, State of São Paulo, Brazil: I detection of hemagglutination-inhibition antibodies in wild birds between 1978 and 1990. Rev. Inst. Med. Trop. São Paulo. 1994;36:265–274. doi: 10.1590/S0036-46651994000300011. [DOI] [PubMed] [Google Scholar]
- 22.Iversson L.B., Silva R.A.M., Da Rosa A.P.T., Barros V.L.R. Circulation of eastern equine encephalitis, western equine encephalitis, Ilhéus, Maguari and Tacaiuma viruses in equines of the Brazilian Pantanal, South America. Rev. Inst. Med. Trop. São Paulo. 1993;35:355–359. doi: 10.1590/S0036-46651993000400009. [DOI] [PubMed] [Google Scholar]
- 23.Mettler N.E., Fernández A.S., Santo Di M.I., Pardo D.A. Flavivirus: Serological survey in horses from the Tandil area. Rev. Argent. Microbiol. 1985;17:47–49. [PubMed] [Google Scholar]
- 24.De Almeida M.A.B., Dos Santos E., Cardoso J.D.C., Noll C.A., Lima M.D.M., Silva F.D.A.E., Ferreira M.S., Martins L.C., Vasconcelos P.F.D.C., Bicca-Marques J.C. Detection of antibodies against Icoaraci, Ilhéus, and Saint Louis Encephalitis arboviruses during yellow fever monitoring surveillance in non-human primates (Alouatta caraya) in southern Brazil. J. Med. Primatol. 2019;48:211–217. doi: 10.1111/jmp.12417. [DOI] [PubMed] [Google Scholar]
- 25.Catenacci L.S., Ferreira M., Martins L.C., De Vleeschouwer K.M., Cassano C.R., Oliveira L.D.C., Canale G., Deem S.L., Tello J.S., Parker P., et al. Surveillance of arboviruses in primates and sloths in the Atlantic Forest, Bahia, Brazil. EcoHealth. 2018;15:777–791. doi: 10.1007/s10393-018-1361-2. [DOI] [PubMed] [Google Scholar]
- 26.Laroque P.O., Valenca-Montenegro M.M., Ferreira D.R.A., Chiang J.O., Cordeiro M.T., Vasconcelos P.F.C., Silva J.C.R. Epidemiologic survey for arbovirus in galician capuchin monkeys (Cebus flavius) free living in Paraı’ba and captive capuchin monkey (Cebus libidinosus) from northeast Brazil. Pesqui. Vet. Bras. 2014;34:462–468. doi: 10.1590/S0100-736X2014000500013. [DOI] [Google Scholar]
- 27.Morales M.A., Fabbri C.M., Zunino G.E., Kowalewski M.M., Luppo V.C., Enria D.A., Levis S.C., Calderón G.E. Detection of the mosquito-borne flaviviruses, West Nile, Dengue, Saint Louis Encephalitis, Ilheus, Bussuquara, and Yellow Fever in free-ranging black howlers (Alouatta caraya) of Northeastern Argentina. PLoS Negl. Trop. Dis. 2017;11:e0005351. doi: 10.1371/journal.pntd.0005351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mettler N.E., Fernandez A.S., Schettino A.M., Di Santo M.I., Pardo D.A. Infecciones humanas por flavivirus en Tardil. Rev. Argent. Microbiol. 1983;96:105–107. [Google Scholar]
- 29.Tavares-Neto J., Travassos da Rosa A.P., Vasconcelos P.F., Costa J.M., Travassos da Rosa J.F., Marsden P.D. Research on antibodies to arbovirus in the serum of residents of the village of Corte de Pedra, Valencia, Bahia. Mem. Inst. Oswaldo Cruz. 1986;81:351–358. doi: 10.1590/S0074-02761986000400001. [DOI] [PubMed] [Google Scholar]
- 30.Terzian A.C.B., Mondini A., Bronzoni R.V.D.M., Drumond B.P., Ferro B.P., Cabrera E.M.S., Figueiredo L.T.M., Neto F.C., Nogueira M.L. Detection of Saint Louis encephalitis virus in dengue-suspected cases during a dengue 3 outbreak. Vector-Borne Zoonotic Dis. 2011;11:291–300. doi: 10.1089/vbz.2009.0200. [DOI] [PubMed] [Google Scholar]
- 31.Mondini A., Chiaravalloti Neto F., Gallo y Sanches M., Lopes J.C. Spatial analysis of dengue transmission in a medium-sized city in Brazil. Rev. Saude Publica. 2005;39:444–451. doi: 10.1590/S0034-89102005000300016. [DOI] [PubMed] [Google Scholar]
- 32.Colombo T.E., Vedovello D., Pacca-Mazaro C.C., Mondini A., Araújo J.C., Cabrera E.M.S., Lopes J.C., Dos Santos I.N.P., Reis A.F.N., Costa F.R., et al. Dengue virus surveillance: Detection of DENV-4 in the city of São José do Rio Preto, SP, Brazil. Acta Trop. 2016;164:84–89. doi: 10.1016/j.actatropica.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Mondini A., Bronzoni R.V.D.M., Nunes S.H.P., Neto F.C., Massad E., Alonso W.J., Lázzaro E.S.M., Ferraz A.A., Zanotto P.M.D.A., Nogueira M.L. Spatio-temporal tracking and phylodynamics of an urban dengue 3 outbreak in São Paulo, Brazil. PLoS Negl. Trop. Dis. 2009;3:e448. doi: 10.1371/journal.pntd.0000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Estofolete C.F., Terzian A.C.B., Parreira R., Esteves A., Hardman L., Greque G.V., Rahal P., Nogueira M.L. Clinical and laboratory profile of Zika virus infection in dengue suspected patients: A case series. J. Clin. Virol. 2016;81:25–30. doi: 10.1016/j.jcv.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Drumond B.P., Mondini A., Schmidt D.J., Bosch I., Nogueira M.L. Population dynamics of DENV-1 genotype V in Brazil is characterized by co-circulation and strain/lineage replacement. Arch. Virol. 2012;157:2061–2073. doi: 10.1007/s00705-012-1393-9. [DOI] [PubMed] [Google Scholar]
- 36.Drumond B.P., Mondini A., Schmidt D.J., Bronzoni R.V.D.M., Bosch I., Nogueira M.L. Circulation of different lineages of dengue virus 2, genotype American/Asian in Brazil: Dynamics and molecular and phylogenetic characterization. PLoS ONE. 2013;8:e59422. doi: 10.1371/journal.pone.0059422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mota M.T.D.O., Estofolete C.F., Zini N., Terzian A.C.B., Gongora D.V.N., Maia I.L., Nogueira M.L. Transverse myelitis as an unusual complication of dengue fever. Am. J. Trop. Med. Hyg. 2017;96:380–381. doi: 10.4269/ajtmh.16-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neto F.C., Pereira M., Fávaro E.A., Dibo M.R., Mondini A., Rodrigues-Júnior A.L., Chierotti A.P., Nogueira M.L. Assessment of the relationship between entomologic indicators of Aedes aegypti and the epidemic occurrence of dengue virus 3 in a susceptible population, São José do Rio Preto, São Paulo, Brazil. Acta Trop. 2015;142:167–177. doi: 10.1016/j.actatropica.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Villabona-Arenas C.J., Mondini A., Bosch I., Schimdt D.J., Schimitt D., Calzavara-Silva C.E., Zanotto P.M., Nogueira M.L. Dengue virus type 3 adaptive changes during epidemics in São Jose de Rio Preto, Brazil, 2006–2007. PLoS ONE. 2013;8:e63496. doi: 10.1371/annotation/5b2477b7-f68d-4800-a17e-fd85f5ad8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mondini A., Cardeal I.L.S., Lázaro E., Nunes S.H., Moreira C.C., Rahal P., Maia I.L., Franco C., Góngora D.V.N., Góngora-Rúbio F., et al. Saint Louis encephalitis virus, Brazil. Emerg. Infect. Dis. 2007;13:176–178. doi: 10.3201/eid1301.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mondini A., Bronzoni R.V.D.M., Cardeal I.L.S., Dos Santos T.M.I.L., Lázaro E., Nunes S.H.P., Silva G.C.D., Madrid M.C.F.S., Rahal P., Figueiredo L.T., et al. Simultaneous infection by DENV-3 and SLEV in Brazil. J. Clin. Virol. 2007;40:84–86. doi: 10.1016/j.jcv.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Estofolete C.F., Terzian A.C.B., E Colombo T., Guimarães G.D.F., Ferraz H.C., A Da Silva R., Greque G.V., Nogueira M.L. Co-infection between Zika and different Dengue serotypes during DENV outbreak in Brazil. J. Infect. Public Health. 2018;12:178–181. doi: 10.1016/j.jiph.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 43.CDC Trioplex Real-Time RT-PCR Assay Instruction for Use. [(accessed on 7 February 2018)]; Available online: https://www.cdc.gov/zika/pdfs/trioplex-real-time-rt-pcr-assay-instructions-for-use.pdf.
- 44.de Morais Bronzoni R.V., Baleotti F.G., Ribeiro Nogueira R.M., Nunes M., Moraes Figueiredo L.T. Duplex reverse transcription-PCR followed by nested PCR assays for detection and identification of Brazilian alphaviruses and flaviviruses. J. Clin. Microbiol. 2005;43:696–702. doi: 10.1128/JCM.43.2.696-702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanger F., Nicklen S., Coulson A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 47.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nassar E., Coimbra T., Rocco I., Pereira L., Ferreira I., De Souza L., De Souza D., Ueda-Ito M., Moura J., Bergo R., et al. Human disease caused by an arbovirus closely related to Ilheus virus: Report of five cases. Intervirology. 1997;40:247–252. doi: 10.1159/000150554. [DOI] [PubMed] [Google Scholar]
- 49.Figueiredo L.T.M. The Brazilian flaviviruses. Microbes Infect. 2000;2:1643–1649. doi: 10.1016/S1286-4579(00)01320-4. [DOI] [PubMed] [Google Scholar]
- 50.Southam C.M., Moore A.E. West nile, Ilheus, and bunyamwera virus infections in man 1,2,3. Am. J. Trop. Med. Hyg. 1951;31:724–741. doi: 10.4269/ajtmh.1951.s1-31.724. [DOI] [PubMed] [Google Scholar]
- 51.Marinho P.E., Alvarenga P.P., Crispim A.P., Candiani T.M., Alvarenga A.M., Bechler I.M., Alves P.A., Dornas F.P., De Oliveira D.B., Bentes A.A., et al. Wild-type yellow fever virus RNA in cerebrospinal fluid of child. Emerg. Infect. Dis. 2019;25:1567–1570. doi: 10.3201/eid2508.181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Araújo S.D.A., E Cordeiro T.M., Belisário A.R., Araújo R.F.D.A., Marinho P.E.S., Kroon E.G., De Oliveira D.B., Teixeira M.M., E Silva A.C.S. First report of collapsing variant of focal segmental glomerulosclerosis triggered by arbovirus: Dengue and Zika virus infection. Clin. Kidney J. 2019;12:355–361. doi: 10.1093/ckj/sfy104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parra B., Lizarazo J., Jiménez-Arango J.A., Zea-Vera A.F., González-Manrique G., Vargas J., Angarita J.A., Zuniga G., Lopez-Gonzalez R., Beltran C.L., et al. Guillain–barré syndrome associated with Zika virus infection in Colombia. N. Engl. J. Med. 2016;375:1513–1523. doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- 54.Estofolete C.F., Mota M.T.D.O., Terzian A.C.B., Milhim B.H.G.D.A., Ribeiro M.R., Nunes D.V., Mourão M.P., Rossi S.L., Nogueira M.L., Vasilakis N. Unusual clinical manifestations of dengue disease—Real or imagined? Acta Trop. 2019;199:105134. doi: 10.1016/j.actatropica.2019.105134. [DOI] [PubMed] [Google Scholar]
- 55.Marinho P.E.S., Kroon E.G. Flaviviruses as agents of childhood central nervous system infections in Brazil. New Microbes New Infect. 2019;31:100572. doi: 10.1016/j.nmni.2019.100572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anand K.S., Agrawal A.K., Garg J., Dhamija R.K., Mahajan R.K. Spectrum of neurological complications in chikungunya fever: Experience at a tertiary care centre and review of literature. Trop. Dr. 2019;49:79–84. doi: 10.1177/0049475518825219. [DOI] [PubMed] [Google Scholar]
- 57.De Puig H., Bosch I., Collins J.J., Gehrke L. Point-of-care devices to detect Zika and other emerging viruses. Annu. Rev. Biomed. Eng. 2020;22:371–386. doi: 10.1146/annurev-bioeng-060418-052240. [DOI] [PubMed] [Google Scholar]
- 58.Bosch I., De Puig H., Hiley M., Carré-Camps M., Perdomo-Celis F., Narváez C., Salgado D.M., Senthoor D., O’Grady M., Phillips E., et al. Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aan1589. [DOI] [PMC free article] [PubMed] [Google Scholar]