Abstract
To determine the risk of a wide range of second malignancy in patients with myeloproliferative neoplasms (MPNs), we conducted a large population-based study and compared the results to matched controls. From nationwide Swedish registers, 9 379 patients with MPNs diagnosed between 1973 and 2009, and 35 682 matched controls were identified as well as information on second malignancies, with follow-up until 2010. Hazard ratios (HRs) with 95 % confidence intervals (CIs) were calculated using Cox regression and a flexible parametric model. There was a significantly increased risk of all non-hematologic cancer with HR of 1.6 (95 % CI 1.5-1.7). The HRs for non-melanoma skin cancer was 2.8 (2.4-3.3), kidney cancer 2.8 (2.0-4.0), brain cancer 2.8 (1.9-4.2), endocrine cancers 2.5 (1.6-3.8), malignant melanoma 1.9 (1.4-2.7), pancreas cancer 1.8 (1.2-2.6), lung cancer 1.7 (1.4-2.2), and head and neck cancer 1.7 (1.2-2.6). The HR of second malignancy was similar across all MPN subtypes, sex, and calendar periods of MPN diagnosis. The risk of developing a hematologic malignancy was also significantly increased; the HR for acute myeloid leukemia was 46.0 (32.6-64.9) and for lymphoma 2.6 (2.0-3.3). In conclusion, our study provides robust population-based support of an increased cancer risk in MPN patients.
Keywords: Myelofibrosis, Polycythemia Vera, Essential Thrombocythemia, Myeloproliferative Neoplasms, Second malignancy
Introduction
Myeloproliferative neoplasms, MPNs, are a group of clonal malignancies characterized by excess hematopoiesis in myeloid cell lineages, and include polycythemia vera (PV), essential thrombocythemia (ET), primary myelofibrosis (PMF), and MPN unclassifiable (MPN-U).(1) Clinically they are characterized by a prolonged disease course that can be complicated by thromboembolic events, bleedings, progression to secondary myelofibrosis, and transformation to acute myeloid leukemia (AML). Treatment is aimed at improving symptoms, and reducing the risk of thromboembolic events, and includes cytoreductive drugs, i.e. hydroxyurea (HU), busulfan, interferon-α, and JAK-2 inhibitors.(2) Even though patients with MPN have a life expectancy that can span over decades, patients with PV and PMF have a reduced life expectancy compared to the general population.(3, 4) Patients with true ET according to the recent WHO criteria, on the other hand, likely have a survival that is similar to the general population.(5) Due to the relative indolence of MPN, patients will have long expected survival why other challenges may emerge, such as second malignancies.
Earlier studies have indicated that patients with MPN have a higher risk of developing non-hematologic malignancies and lymphomas, as well as transformation to more aggressive myeloid malignancies.(6-13) Frederiksen et al. performed a study in Denmark of 6 203 patients with ET and PV, where they found an increased risk of non-hematologic and hematologic malignancies.(10) The largest risk increase was observed for cancers of the skin, kidney, urinary tract, lung, esophagus, parathyroid and thyroid.(10, 12) Controversy regarding the subject of second malignancy in MPN patients still exist, with other authors reporting no increase in risk of second malignancies in MPN patients.(14) Not many of the existing studies include patients with PMF, and few studies are population-based. Such register and population-based studies have an important advantage when assessing disease complications in MPNs, to ensure a long follow-up time and a minimized loss-to-follow-up.
To further elucidate the risk of second malignancy in patients with MPN, we conducted a large cohort study in a population-based setting including all MPN subtypes, and used matched controls for comparison.
Subjects and Methods
Central Registries, patients and controls
Sweden, with a population of 10 million, offers publically provided and tax financed health care for the entire population. A unique personal identification number is used in all contacts with health care, enabling cross linking between different health registers. All diagnoses of malignant disease are registered in the nationwide Swedish Cancer Register since 1958 and since 1984 there is a double reporting routine, where both the clinician and the pathologist/cytologist are obliged by law to report all new cases of cancer. The Inpatient Register captures information on all hospital admissions and discharge diagnoses. Diagnoses of MPN were based on the Polycythemia Vera Study Group criteria from the mid1970s to the early 2000s when the WHO criteria were published.(15, 16) In 1993, MPN-U was included in the Swedish Cancer Register.
MPN patients were identified from the Swedish Cancer Register and the Inpatient Register from 1st of January 1973 to 31st of December 2009. Although the quality and degree of coverage of the Swedish Cancer Register is high, above 95%, there has likely been an underreporting of more indolent cancers such as MPNs.(17, 18) Therefore, patients were also included from the Inpatient Register. For each patient, four controls were randomly selected from the Register of Total Population, matched by age, sex, and region of residency. Controls had to be alive at the date of the corresponding patients’ MPN diagnosis. Exclusion criteria for both patients and controls were a previous or same-day diagnosis of another malignancy and age 17 or younger. Patients with a postmortem MPN diagnosis were also excluded. Patients and corresponding controls were followed from date of MPN diagnosis until a diagnosis of a second malignancy, death, or end of follow-up on December 31st 2010.
Statistical analysis
Outcome, diagnosis of a second malignancy, was obtained by cross linking to the Swedish Cancer Register. Separate analyses were performed for 15 common non-hematologic malignancies and for three categories of hematologic malignancies: AML, lymphoma, and multiple myeloma. Patients were followed until the first event within each category of malignancy. Recurrent events in the same category of malignancy were not included in the analysis, and events of other categories of malignancy was ignored in each analysis. Hazard ratios (HRs) were calculated with 95 % confidence intervals (CI) using proportional hazards Cox models adjusting for age (18-49, 50-59, 60-69, 70-79, and 80 years and older), calendar period (1973-82, 1983-92, 1993-2001, and 2002-2009) and sex. The final models included interactions between MPN status and MPN subtype, calendar period, sex, and age, respectively. Graphs of the hazard ratio over follow up time after MPN diagnosis were obtained using flexible parametric survival models, adjusting for the same covariates as in the Cox model. A sensitivity (or landmark) analysis was performed, where all patients with a diagnosis of a second malignancy within the first year of the MPN diagnosis were excluded, with the purpose of reducing the possible effects of detection/surveillance bias shortly after the MPN diagnosis. To estimate the proportion of MPN patients, and the matched controls, that experience non-hematological malignancies cumulative incidence was also calculated for all non-hematologic malignancies using a flexible parametric model whilst accounting for death as a competing event. Additionally, to increase comparability to other studies in the field, standardized incidence ratios (SIR) were calculated, using data from the Swedish Cancer Register, with 95 % confidence intervals (CI).
All analyses were calculated using Stata version 14.
The Regional Ethical Review Board in Stockholm approved the study; informed consent was waived because there was no contact with the study subjects.
Results
A total of 9 379 MPN patients and 35 682 matched controls were identified between 1973 and 2009. Our MPN cohort consisted of 4 214 PV patients, 2 660 ET patients, 1 392 PMF patients, and 1 113 patients with MPN-U. The median age at diagnosis was 67.5 years and there were 4 502 (48 %) men and 4 877 (52%) women (Table 1). The vast majority of the patients, 92%, were identified from the Swedish Cancer Register and 8% were identified from the Inpatient Register.
Table 1.
Distribution of patients with myeloproliferative neoplasms and their matched population controls. PV=polycythemia vera, ET=essential thrombocythemia, PMF=primary myelofibrosis, MPN-U=myeloproliferative neoplasm, unclassifiable, n=number
| Patients (n) | Controls (n) | |
|---|---|---|
| Men | 4 502 (48%) | 16 992 (48%) |
| Women | 4 877 (52%) | 18 690 (52%) |
| Age 18-49 | 1 004 | 4 016 |
| Age 50-59 | 1 341 | 5 363 |
| Age 60-69 | 2 235 | 8 938 |
| Age 70-79 | 3 087 | 12 032 |
| Age 80+ | 1 712 | 5 333 |
| Median age | 67.5 years | 66.6 years |
| Period 1973-1982 | 1 545 | 5 995 |
| Period 1983-1992 | 2 213 | 8 468 |
| Period 1993-2001 | 2 692 | 10 160 |
| Period 2002-2009 | 2 929 | 11 059 |
| PV | 4 214 (45%) | - |
| ET | 2 660 (28%) | - |
| PMF | 1 392 (15%) | - |
| MPN-U | 1 113 (12%) | - |
| Total | 9 379 | 35 682 |
In the MPN cohort, there were 1 192 patients with a second non-hematologic malignancy included in the analyses and 4 758 in the control cohort. In the analysis of all non-hematologic malignancies, the median time of follow up was 7.7 years, and median time of follow-up to outcome was 6.1 years. The HR of all non-hematologic malignancies was 1.6 (95% CI 1.5-1.7) compared to controls, and was significantly elevated across all age groups, all calendar periods, and all MPN subtypes. The HR of all non-hematologic malignancies tended to be higher in patients aged 80 years and older at diagnosis, HR 2.2 (1.8-2.6) compared to controls. The HRs were 1.7 (1.3-2.1), 1.5 (1.3-1.8), 1.4 (1.3-1.6), and 1.5 (1.4-1.7) in patients aged 18-49, 50-59, 60-69, and 70-79 years at the time of MPN diagnosis, respectively in relation to controls, (p-value for interaction <0.0001). The HR of all non-hematologic malignancy was similar in men and women; 1.6 (1.5-1.7) and 1.5 (1.4-1.7) respectively, compared to controls, similar for the different MPN subtypes; PV 1.5 (1.4-1.7), ET 1.6 (1.4-1.8), PMF 1.5 (1.2-1.9), and MPN-U 1.9 (1.5-2.5), and similar for the different calendar periods: 1.6 (1.4-1.8) in patients diagnosed between 1973-1982, 1.5 (1.4-1.7) 1983-1992, 1.6 (1.4-1.8) 1993-2001, and 1.7 (1.4-2.0) in 2001-2009.
Among non-hematologic malignancies, the highest increased risk was observed for non-melanoma skin cancer (HR 2.8; 2.4-3.3) in MPN-patients compared to controls (Table 2). The risk increase of non-melanoma skin cancer was significantly elevated in all MPN subtypes, age categories, and calendar periods. There was a trend towards a more pronounced risk-increase in men with a HR of 3.4 (2.8-4.2) and 2.3 (1.9-2.8) in women, compared to corresponding controls. The HRs for developing kidney cancer, brain cancer, endocrine cancers, malignant melanoma, pancreas cancer, lung cancer, and head and neck cancer were also significantly elevated (Table 2).
Table 2.
Hazard ratio of cancers in all patients with myeloproliferative neoplasms compared to matched controls. MPN=myeloproliferative neoplasm, HR=hazard ratio, CI=confidence interval
| Type of cancer |
HR | 95% CI | Number of events in MPN patients |
|---|---|---|---|
| All non-hematologic | 1.6 | 1.5 - 1.7 | 1185 |
| Non-melanoma skin cancer | 2.8 | 2.4 - 3.3 | 252 |
| Brain | 2.8 | 1.9 - 4.2 | 37 |
| Kidney | 2.8 | 2.0 - 4.0 | 49 |
| Endocrine organs | 2.5 | 1.6 - 3.8 | 33 |
| Malignant melanoma | 1.9 | 1.4 - 2.7 | 53 |
| Pancreas | 1.8 | 1.2 - 2.6 | 32 |
| Lung | 1.7 | 1.4 - 2.2 | 92 |
| Head and neck | 1.7 | 1.2 - 2.7 | 32 |
| Esophagus and stomach | 1.6 | 1.2 - 2.2 | 53 |
| Breast | 1.4 | 1.1 - 1.7 | 106 |
| Prostate | 1.2 | 1.1 - 1.4 | 190 |
| Liver and gallbladder | 1.3 | 0.9 - 1.9 | 31 |
| Colon, rectum, and anus | 1.1 | 0.9 - 1.3 | 129 |
| Female genital organs | 1.2 | 0.9 - 1.5 | 73 |
| Urinary tract excluding kidney | 1 | 0.7 - 1.3 | 46 |
For hematologic malignancies, there was an expected high HR for developing AML, HR 46.0 (35.6-64.9) (Table 3). An increased risk was also observed for lymphomas, HR 2.6 (2.0-3.3). There was a tendency towards a larger risk increase of lymphoma in patients with PMF, followed by patients with MPN-U and ET (Table 3). In total, there were 90 patients with lymphoma in the MPN cohort; 67 non-Hodgkin lymphomas of which 11 were diffuse large B-cell lymphomas and 14 chronic lymphocytic leukemia (CLL), 8 patients with Hodgkin lymphoma and 15 with unspecified lymphoma. Moreover, the elevated risk of developing multiple myeloma was of borderline significance, HR 1.7 (1.0-3.0).
Table 3.
Hazard ratio of hematologic malignancies in patients with myeloproliferative neoplasms (MPN) compared to matched controls, stratified by MPN subtype. MPN=myeloproliferative neoplasm, PV=polycythemia vera, ET=essential thrombocythemia, PMF=primary myelofibrosis, MPN-U=myeloproliferative neoplasm, unclassifiable, AML=acute myeloid leukemia, HR=hazard Ratio, CI=confidence interval, n=number
| MPN subtype |
AML HR (95% CI) n = 278 |
Lymphoma HR (95% CI) n = 90 |
Multiple myeloma HR (95% CI) n = 16 |
|---|---|---|---|
| All MPN | 46.0 (32.6 - 64.9) | 2.6 (2.0 – 3.3) | 1.7 (1.0 - 3.0) |
| PV | 38.1 (23.5 - 62.0) | 1.9 (1.3 - 2.8) | 1.6 (0.7 - 3.5) |
| ET | 26.1 (13.4 - 50.9) | 2.3 (1.3 - 3.9) | 1.4 (0.5 - 3.6) |
| PMF | 99.2 (41.0 - 240.1) | 6.0 (3.4 - 10.8) | 9.0 (1.8 - 44.0) |
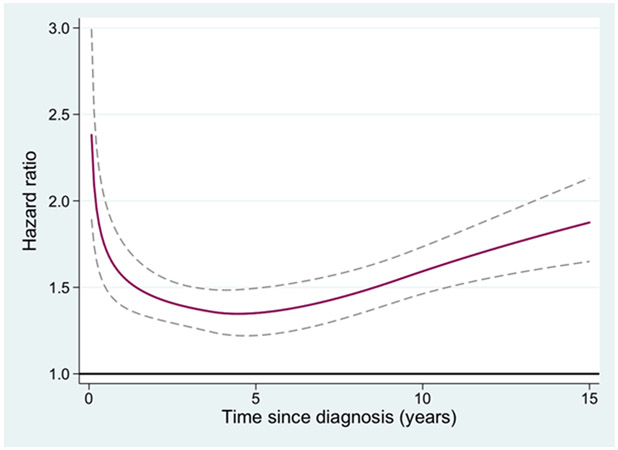
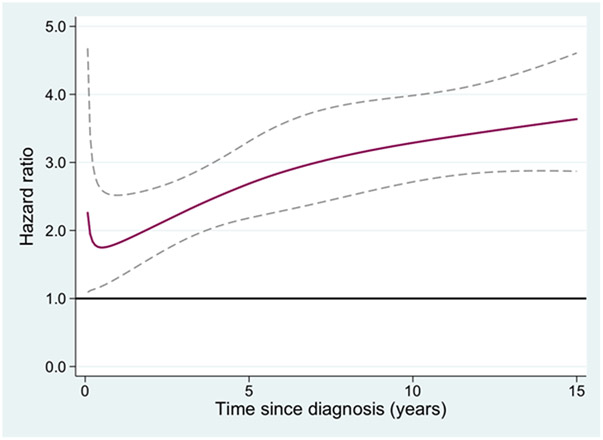
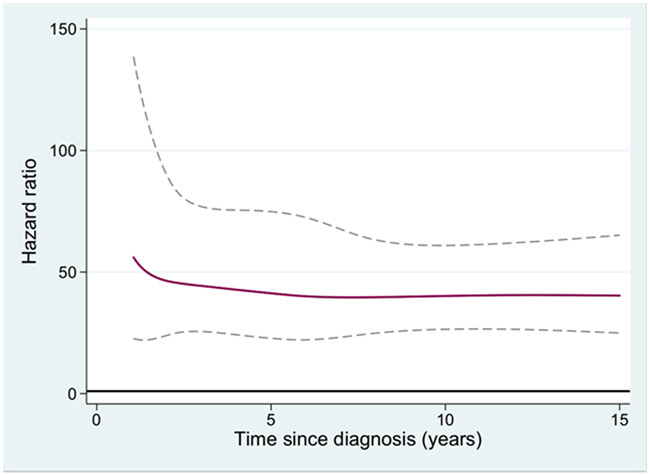
The HR of second malignancy for MPN patients compared to population controls, in relation to follow up time after MPN diagnosis was also analyzed. For all non-hematologic malignancies (Figure 1), non-melanoma skin cancer (Figure 2), and melanoma the HR was increasing with time from diagnosis. To elucidate if non-melanoma skin cancer and melanomas were the major contributors to the increasing HR with time from MPN diagnosis for all non-hematologic malignancies, a separate analysis of all non-hematologic malignancies excluding all skin cancers was performed. The HR of that new outcome was 1.4 (1.3-1.5), with a remaining tendency of an increasing HR over time from MPN diagnosis (not shown). The hazard ratio of AML in relation to time from the MPN diagnosis is presented in Figure 3, and the rate of transformation was not to be affected by duration of MPN.
Figure 1.
Hazard ratio of all second non-hematologic malignancies for patients with myeloproliferative neoplasm (MPN) compared to matched population controls, in relation to time from MPN diagnosis
Figure 2.
Hazard ratio of second non-melanoma skin cancer for patients with myeloproliferative neoplasms (MPN) compared to matched population controls, in relation to time from MPN diagnosis
Figure 3.
Hazard ratio of second acute myeloid leukemia for patients with myeloproliferative neoplasm (MPN) compared to matched population controls, in relation to time from MPN diagnosis
In the sensitivity analysis, where all patients with malignancies within the first year of the MPN diagnosis were excluded, the overall HR of non-hematologic malignancies remained unchanged; HR 1.5 (1.4-1.6). For the majority of malignancies, the risk increase remained significant in the sensitivity analysis, with the exception of cancer of the prostate, which were of borderline significance in the primary analysis. The elevated risk of developing endocrine and kidney cancer was lower in the sensitivity analysis than in the primary analysis, with HRs of 2.3 (1.4-3.7) and 2.4 (1.7-3.6), respectively.
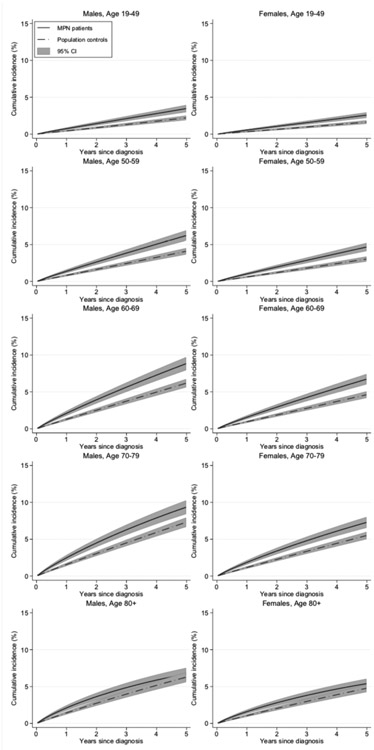
The cumulative incidence for all non-hematologic malignancies in all MPN patients during the last calendar period shows that the proportion diagnosed with second malignancies is significantly larger in MPN patients than controls in all age groups but he oldest, and similar among men and women, see figure 4. The SIRs are generally slightly smaller than the HRs, see table 4. SIR for all non-hematologic malignancy was 1.4 (1.3-1.5) in PV, 1.4 (1.2-1.5 in ET and 1.5 (1.2-1.8) in PMF.
Figure 4.
Cumulative incidences with 95 % confidence intervals, of all non-hematologic malignancies in patients with myeloproliferative neoplasm (MPN) subdivided by sex and age group, compared to matched controls, for the last calendar period, 2002-2009.
Table 4.
Standardised incidence ratio (SIR) in all MPN patients compared to the general population. MPN=myeloproliferative neoplasm, CI=confidence interval
| Type of cancer |
SIR | 95 %CI |
|---|---|---|
| All non hematologic | 1.4 | 1.3 - 1.5 |
| Non-melanoma skin cancer | 3.3 | 2.9 - 3.8 |
| Brain | 2.1 | 1.5 - 2.8 |
| Kidney | 2 | 1.5 - 2.7 |
| Endocrine organs | 2 | 1.4 - 2.9 |
| Malignant melanoma | 1.9 | 1.4 - 2.7 |
| Pancreas | 1.3 | 0.9 - 1.9 |
| Lung | 1.3 | 1.1 - 1.6 |
| Head and neck | 1.4 | 1.0 - 2.0 |
| Esophagus and stomach | 1.4 | 1.0 - 1.8 |
| Breast | 1 | 0.8 - 1.2 |
| Prostate | 1.1 | 0.96 - 1.3 |
| Liver and gallbladder | 1.2 | 0.9 - 1.8 |
| Colon, rectum, and anus | 1 | 0.8 - 1.2 |
| Female genital organs | 1.4 | 1.1 - 1.7 |
| Urinary tract excluding kidney | 0.9 | 0.7 - 1.2 |
| Acute myeloid leukemia | 33.9 | 30.1 - 38.1 |
| Lymphoma | 2.1 | 1.7 - 2.6 |
| Multiple myeloma | 1.2 | 0.7 - 1.9 |
Discussion
In this large population-based study of 9 379 patients with MPN, we found a significantly increased risk of second non-hematologic and hematologic malignancies. The relative risk of all non-hematologic malignancies was increased by 60 % compared to matched controls. The HR of all non-hematologic malignancy increased with time from diagnosis, thus the longer the patient had lived with the MPN, the higher was the relative risk of a second malignancy compared to population controls. From our results, it is obvious that a long follow-up time is necessary in the study of second malignancy in MPN. Our findings highlight the need for awareness among clinicians managing MPN patients to ensure early detection of second malignancies in these patients.
The risk of non-melanoma skin cancer constituted the largest risk increase, and was more accentuated in our results than in previous works.(10) In our study, the relative risk of kidney cancer was higher shortly after MPN diagnosis, a potential effect of the diagnostic work-up often including imaging to assess spleen size, but an elevated risk of kidney cancer persisted during follow-up time compared to the general population, as has been indicated previously.(10, 12, 19) The risk increase for brain cancer that we observed in our study, is a novel finding, but should be interpreted with caution due to limited number of events. In all, the results of our study provide new information on the risk of second malignancies as well as validating and expanding on previous findings.
The risk increase for AML is well known and a 10-year incidence of 2.3-10 % in PV, 2-5 % in ET and 8-20 % in PMF are previously recognized.(20) The HR of AML tended to be lower in the earlier calendar periods and may be caused by a higher degree of underreporting of AML and underestimation of risks during those years. The HR of AML was constant in relation to time from MPN diagnosis, suggesting that the risk of transformation does not increase with duration of MPN disease, in contrast to for example skin cancers. The risk of developing lymphoma in MPN patients was 2.6-fold increased in our study, which is similar to the increased risk described previously.(6-8) The risk increase that we found was higher than that reported by Masarova et al(8), which might be attributable to the fact that our study is population-based and loss to follow up is minimized. An association with CLL has been previously described in the literature, and it has been shown that MPN patients have an increased probability of having circulating CLL-like B-cell clones compared to age-matched controls.(21, 22) Second hematologic malignancies in the myeloid as well as the lymphoid cell lineage is a main concern in patients with MPN.
The difference in HRs and SIRs was expected and may be explained by the fact that MPN patients and the matched controls have been excluded on basis of prior malignancies, but the same exclusion criteria is not possible to apply in the general population used for comparison in the SIR calculations. This introduces a possible bias and risk of underestimating the risk of second malignancies in MPN patients, and is an important reason for us to present HRs as our main results. Another advantage of the modelling approach used in our paper is that we can estimate how the HRs change over time since MPN diagnosis.
There may be several possible mechanisms for the increased cancer risk in MPN patients. Most MPN patients receive one or more types of cytoreductive treatment during their disease course. The first national Swedish guidelines were published in 1998 and suggested HU or radioactive phosphorus as first line cytoreductive treatment for patients with PV and ET, and HU or interferon-α for patients with PMF. Current Nordic guidelines recommends HU as the first line of cytoreductive treatment in the majority of patients, the exception is in patients under 60 years of age where interferon-α is preferred.(23) An increased risk of non-melanoma skin cancer during HU treatment has been reported in smaller studies and numerous case reports.(9, 24, 25) It is also implied that HU may act as a photosensitizer and thus in combination with UV exposure increase the risk of skin cancer.(26) In addition, both the disease and its treatment may be associated with a certain degree of immunosuppression. Furthermore, over-activation of the JAK/STAT pathway as well as several other MPN-associated mutations for example in TET2, ASXL1, TP53, NRAS, KRAS and LNK (27, 28) occur in both hematological and non-hematologic tumors,(29-32), and although they are acquired mutations,(33-35) this may suggest a common genetic pathophysiology between MPN and solid malignancies. Furthermore, MPN patients have an increased risk of having non-hematologic malignancies prior to their MPN diagnosis.(12, 36) First-degree relatives of MPN patients have a significantly increased risk of MPN and CLL as well as melanoma and brain cancer,(37) suggesting a germline susceptibility to MPN and other malignancies. Most likely, the explanation of the increased risk of second malignancies in patients with MPN is multifactorial where a combination of cytoreductive treatment, genetic predisposition and acquired mutations, as well as immune-related effects all may contribute to an increased cancer risk.
The strengths of the study are that it is population-based and contains data from high quality health registers, ensuring generalizability of our findings. It is a large study including 9 379 patients and 35 682 matched controls over a time period of 37 years with minimal loss-to-follow-up. Limitations of this study include lack of individual information on treatment and mutational states in the registers. The number of reported ET patients has increased over time, implying an underreporting of ET in the earlier calendar periods, but may also be due to changes in disease classification(1, 38) Since MPN patients are continuously monitored by hematologists/oncologists, there is also a possibility of surveillance bias. The minimal changes in the result between the primary analysis and the sensitivity analysis suggest that detection bias around the time of diagnosis was not a major concern.
Further research is needed to understand the mechanisms of the increased cancer risk, and to what extent cytoreductive medications add to the risk. Follow up evaluation of the increased cancer risk is also recommended in the future when MPN treatment choices likely will change in favor of immunomodulatory treatments e.g. JAK-inhibitors. In summary, our study provides robust population-based support of an increased risk of both non-hematologic and hematologic malignancies in patients with MPN.
Acknowledgment
We thank the “Swedish Initiative for research on Microdata in the Social and Medical sciences” (SIMSAM) and Rozita Broumandi for valuable assistance in collecting the information from the Swedish registers.
Research funding: The regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, Blodcancerfonden, The Cancer Research Foundations of Radiumhemmet, The Adolf H. Lundin Charitable Foundation, The Memorial Sloan Kettering Core Grant (P30 CA008748), Swedish Cancer Society
Footnotes
Previously presented: Presented in part as an oral contribution at European Hematology Association 21st Congress June 10th, 2016 Copenhagen, Denmark.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Swerdlow SH, International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: International Agency for Research on Cancer; 2008. 439 p. p. [Google Scholar]
- 2.Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(6):761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hultcrantz M, Kristinsson SY, Andersson TM, Landgren O, Eloranta S, Derolf AR, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(24):2995–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124(16):2507–13; quiz 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbui T, Thiele J, Passamonti F, Rumi E, Boveri E, Ruggeri M, et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: an international study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(23):3179–84. [DOI] [PubMed] [Google Scholar]
- 6.Vannucchi AM, Masala G, Antonioli E, Chiara Susini M, Guglielmelli P, Pieri L, et al. Increased risk of lymphoid neoplasms in patients with Philadelphia chromosome-negative myeloproliferative neoplasms. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(7):2068–73. [DOI] [PubMed] [Google Scholar]
- 7.Rumi E, Passamonti F, Elena C, Pietra D, Arcaini L, Astori C, et al. Increased risk of lymphoid neoplasm in patients with myeloproliferative neoplasm: a study of 1,915 patients. Haematologica. 2011;96(3):454–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masarova L, Newberry KJ, Pierce SA, Estrov Z, Cortes JE, Kantarjian HM, et al. Association of lymphoid malignancies and Philadelphia-chromosome negative myeloproliferative neoplasms: Clinical characteristics, therapy and outcome. Leukemia research. 2015;39(8):822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kissova J, Ovesna P, Penka M, Bulikova A, Kiss I. Second malignancies in philadelphia-negative myeloproliferative neoplasms-single-center experience. Anticancer research. 2014;34(5):2489–96. [PubMed] [Google Scholar]
- 10.Frederiksen H, Farkas DK, Christiansen CF, Hasselbalch HC, Sorensen HT. Chronic myeloproliferative neoplasms and subsequent cancer risk: a Danish population-based cohort study. Blood. 2011;118(25):6515–20. [DOI] [PubMed] [Google Scholar]
- 11.Khanal N, Giri S, Upadhyay S, Shostrom VK, Pathak R, Bhatt VR. Risk of second primary malignancies and survival of adult patients with polycythemia vera: A United States population-based retrospective study. Leukemia & lymphoma. 2016;57(1):129–33. [DOI] [PubMed] [Google Scholar]
- 12.Fallah M, Kharazmi E, Sundquist J, Hemminki K. Higher risk of primary cancers after polycythaemia vera and vice versa. British journal of haematology. 2011;153(2):283–5. [DOI] [PubMed] [Google Scholar]
- 13.Masarova L, Cherry M, Newberry KJ, Estrov Z, Cortes JE, Kantarjian HM, et al. Secondary solid tumors and lymphoma in patients with essential thrombocythemia and polycythemia vera - single center experience. Leukemia & lymphoma. 2016;57(1):237–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Susini MC, Masala G, Antonioli E, Pieri L, Guglielmelli P, Palli D, et al. Risk of second cancers in chronic myeloproliferative neoplasms. Blood. 2012;119(16):3861–2; author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 15.Andreasson B, Lofvenberg E, Westin J. Management of patients with polycythaemia vera: results of a survey among Swedish haematologists. European journal of haematology. 2005;74(6):489–95. [DOI] [PubMed] [Google Scholar]
- 16.Berlin NI. Diagnosis and classification of the polycythemias. Seminars in hematology. 1975;12(4):339–51. [PubMed] [Google Scholar]
- 17.Barlow L, Westergren K, Holmberg L, Talback M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta oncologica. 2009;48(1):27–33. [DOI] [PubMed] [Google Scholar]
- 18.Turesson I, Linet MS, Bjorkholm M, Kristinsson SY, Goldin LR, Caporaso NE, et al. Ascertainment and diagnostic accuracy for hematopoietic lymphoproliferative malignancies in Sweden 1964–2003. International journal of cancer Journal international du cancer. 2007;121(10):2260–6. [DOI] [PubMed] [Google Scholar]
- 19.Najean Y Association of renal carcinoma and polycythemia vera: 5 cases in which nephrectomy preceded and did not influence the clinical course of the polycythemia. Nouvelle revue francaise d'hematologie. 1991;33(1):9–10. [PubMed] [Google Scholar]
- 20.Cerquozzi S, Tefferi A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood cancer journal. 2015;5:e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miltiades P, Lamprianidou E, Kerzeli IK, Nakou E, Papamichos SI, Spanoudakis E, et al. Three-fold higher frequency of circulating chronic lymphocytic leukemia-like B-cell clones in patients with Ph-Myeloproliferative neoplasms. Leukemia research. 2015. [DOI] [PubMed] [Google Scholar]
- 22.Laurenti L, Tarnani M, Nichele I, Ciolli S, Cortelezzi A, Forconi F, et al. The coexistence of chronic lymphocytic leukemia and myeloproliperative neoplasms: a retrospective multicentric GIMEMA experience. American journal of hematology. 2011;86(12):1007–12. [DOI] [PubMed] [Google Scholar]
- 23.Nordic care program for patients with Essential Thrombocythemia, Polycythemia Vera and Primary Myelofibrosis http://nmpn.org/index.php/guidelines/17-nmpn-care-program-2017/file: Nordic MPN Study Group; 2017. [Available from: http://nmpn.org/index.php/guidelines/17-nmpn-care-program-2017/file. [Google Scholar]
- 24.Saraceno R, Teoli M, Chimenti S. Hydroxyurea associated with concomitant occurrence of diffuse longitudinal melanonychia and multiple squamous cell carcinomas in an elderly subject. Clinical therapeutics. 2008;30(7):1324–9. [DOI] [PubMed] [Google Scholar]
- 25.Wiechert A, Reinhard G, Tuting T, Uerlich M, Bieber T, Wenzel J. [Multiple skin cancers in a patient treated with hydroxyurea]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete; 2009;60(8):651–2, 4. [DOI] [PubMed] [Google Scholar]
- 26.Turner ML. Sun, drugs, and skin cancer: a continuing saga. Arch Dermatol. 2010;146(3):329–31. [DOI] [PubMed] [Google Scholar]
- 27.Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123(14):2220–8. [DOI] [PubMed] [Google Scholar]
- 28.Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129(6):667–79. [DOI] [PubMed] [Google Scholar]
- 29.Bian EB, Zong G, Xie YS, Meng XM, Huang C, Li J, et al. TET family proteins: new players in gliomas. Journal of neuro-oncology. 2014;116(3):429–35. [DOI] [PubMed] [Google Scholar]
- 30.Song F, Amos CI, Lee JE, Lian CG, Fang S, Liu H, et al. Identification of a melanoma susceptibility locus and somatic mutation in TET2. Carcinogenesis. 2014;35(9):2097–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, et al. The role of JAK-STAT signaling within the CNS. Jak-Stat. 2013;2(1):e22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Functional Katoh M. and cancer genomics of ASXL family members. British journal of cancer. 2013;109(2):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Filippi P, Zecca M, Lisini D, Rosti V, Cagioni C, Carlo-Stella C, et al. Germ-line mutation of the NRAS gene may be responsible for the development of juvenile myelomonocytic leukaemia. British journal of haematology. 2009;147(5):706–9. [DOI] [PubMed] [Google Scholar]
- 34.Ruijs MW, Verhoef S, Rookus MA, Pruntel R, van der Hout AH, Hogervorst FB, et al. TP53 germline mutation testing in 180 families suspected of Li-Fraumeni syndrome: mutation detection rate and relative frequency of cancers in different familial phenotypes. J Med Genet. 2010;47(6):421–8. [DOI] [PubMed] [Google Scholar]
- 35.Schaub FX, Looser R, Li S, Hao-Shen H, Lehmann T, Tichelli A, et al. Clonal analysis of TET2 and JAK2 mutations suggests that TET2 can be a late event in the progression of myeloproliferative neoplasms. Blood. 2010;115(10):2003–7. [DOI] [PubMed] [Google Scholar]
- 36.Pettersson H, Knutsen H, Holmberg E, Andreasson B. Increased incidence of another cancer in myeloproliferative neoplasms patients at the time of diagnosis. European journal of haematology. 2015;94(2):152–6. [DOI] [PubMed] [Google Scholar]
- 37.Landgren O, Goldin LR, Kristinsson SY, Helgadottir EA, Samuelsson J, Bjorkholm M. Increased risks of polycythemia vera, essential thrombocythemia, and myelofibrosis among 24,577 first-degree relatives of 11,039 patients with myeloproliferative neoplasms in Sweden. Blood. 2008;112(6):2199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaffe ES, World Health Organization. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon Oxford: IARC Press ; Oxford University Press; (distributor); 2001. 351 p. p. [Google Scholar]