Abstract
We have previously reported that high aldehyde dehydrogenase (ALDH) enzyme activity in breast cancer cells results in breast cancer stem cell (BCSC) properties by upregualting Notch-1 and epithelial mesenchymal markers. This results in chemoresistance in breast cancer. Here, we examined the functional and clinical significance of ALDH expression by measuring the ALDH levels in breast cancer tissues by immunohistochemistry. There was a significantly higher ALDH expression in higher grade breast cancer tumor tissues (Grade- II and III) versus normal breast tissues. Injection of BCSC (ALDH+ and CD44+/CD22−) cells resulted in aggressive tumor growth in athymic mice versus ALDH− cells. The ALDH+ and CD44+/CD22− tumors grow rapidly and are larger than ALDH− tumors which were slow growing and smaller. Molecularly, ALDH+ tumors expressed higher expression of Notch-1 and EMT markers than ALDH− tumors. Oral administration of the naturally occurring Psoralidin (Pso, 25 mg/kg of body weight) significantly inhibited the growth in ALDH+ and ALDH− tumors as well. Psoralidin inhibited Notch-1 mediated EMT activation in ALDH+ and ALDH− tumors-this confirms our in vitro findings. Our results suggest that Notch-1 could be an attractive target and inhibition of Notch-1 by Psoralidin may prevent pathogenesis of breast cancer as well as metastasis.
INTRODUCTION
Breast cancer (BCa) is one of the most common cancers in women. Each year it affects more than 1 million women worldwide (232,670 cases in U.S.) and is responsible for 400,000 patient deaths (40,000 in U.S.A) [1]. Although, BCa mortality has decreased, it remains the leading cause of cancer death with limited long-term survival (21%) [2,3]. In general, breast tumors are comprised of heterogeneous populations of cells. Of these, 1–2% are breast cancer stem cells (BCSCs) and are believed to initiate and promote tumorigenesis [4]. There is increasing evidence that these small fractions of BCSCs are responsible for metastasis, tumor recurrence, and resistance to chemotherapeutic agents [5–10]. Previously, we and others reported that chemotherapeutic agents such as docetaxel, doxorubicin, or 5-flurouracil (5-FU) fail to inhibit the growth of BCSCs or to preferentially target and kill differentiated breast cancer cells [11,12]. Hence, this is a key challenge for clinicians to palliate an incurable disease and decrease cancer mortality.
BCSCs are characterized by important cell-surface markers such as CD44+/CD24−/low, Lin−, and functional markers like high aldehyde dehydrogenase activity (ALDH+) or the presence of an ABC transporter-dependent Hoechst side population. These have all been used to characterize BCSCs [13]. Recent studies have suggested that an ideal strategy for treatment is targeting key pathways that determine BCSCs phenotype and self-renewal [14–21]. However, targeting self-renewal pathways to eradicate BCSCs can be toxic as these pathways are also involved in normal stem cell homeostasis. Hence, it is important to target cancer stem cells specifically or at least their pathways involved in self-renewal, maintenance, and differentiation [22–24]. We and others have reported that activation of Notch-1 signaling plays a diverse role in regulating self-renewal, proliferation, and apoptosis of normal and cancer stem cells. It is well known that aberrant activation of Notch-1 is implicated in many cancer types including BCa [25–27]. It also enhances the metastatic phenotype of BCa [28,29].
An epithelial mesenchymal transition (EMT) program is initiated by multiple extracellular events. It orchestrates many transcriptional and translational processes and signal transduction pathways that moves the cells to a mesenchymal state-this is the first step in metastasis [12]. Of these, Notch-1 signaling is one of the key players that regulate EMT in breast cancer and BCSCs [30]. Well-established facts have shown that mutation or non-functional E-cadherin and higher expression of β-catenin expressions are causative factors for EMT and BCa [31,32]. In addition, many transcription factors (Snail, Slug, and ZEB1) have been shown to directly repress E-cadherin function and others (Twist and ZEB1) could be indirectly involved [33]. Importantly, Notch-1 signaling regulates these EMT markers and transcription factors.
The existence of BCSCs has profound implications on cancer chemoprevention and therapy [34,35]. Hence, the aim of this study is to identify novel preventive and/or therapeutic agents that specifically target BCSCs and BCa cells without causing toxicity to normal cells. Earlier, we have reported that Psoralidin (Pso) is a major bioactive compound (>99% purity) derived from kernel seeds of an herbal plant (Psoralea corylifolia; Leguminosae family) [36]. It is extensively used in traditional medicine to treat gynecological bleeding and vitiligo [37,38]. This small molecule effectively targets Notch-1 signaling and inhibits EMT and suppresses cell proliferation in the BCSCs and BCa.
Here, we explored the in vivo tumorigenicity of ALDH+, ALDH− and CD44+/CD22− cells as well as the in vivo efficacy of a potent natural compound Pso inhibiting tumor growth in xenograft models. Our results demonstrated that ALDH+ tumors exhibited a higher expression of Notch-1, β-catenin and slug than ALDH− tumors and oral administration of Pso inhibited the tumor growth of ALDH+, CD44+/CD22− and ALDH− xenografts and molecular studies from tumor tissues suggested that Pso effectively inhibited Notch-1 activation and downregulated EMT markers. These results highlight the important role of Notch-1 and suggest that it can be a novel therapeutic target for BCa.
Materials and Methods
Cell lines and reagents:
Human BC epithelial cell line MDA-MB-231 was purchased from the American Type Culture Collection (Manassas, VA, USA). MDA-MB-231 cells were maintained in DMEM containing L-glutamine and sodium pyruvate, supplemented with 10% fetal bovine serum and 1% antibiotic and antimycotic solution in a humidified atmosphere of 5% CO2 at 370C in an incubator. ALDH+ and CD44+/CD22− cells were procured from CELPROGEN and were expanded and maintained in stem cell expansion and undifferentiation media. Psoralidin was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). BD Matrigel (High concentration) was purchased from BD Biosciences.
Aldefluor assay for separation of the ALDH+ population by flow cytometry
BCSCs were identified and isolated from MDA-MB-231 BCCs, on the basis of high ALDH enzyme activity using ALDEFLUOR kit for ALDH-based cell detection (StemCell Technologies, Durham, NC, USA) as we described earlier [30]. Briefly, 1×106 cells were suspended in 1 ml ALDEFLUOR buffer and incubated for 45 min at 370C and cells were centrifuged, and resuspended in 0.5 ml ALDEFLOUR assay buffer. ALDH activity was measured on the basis of the amount of fluorescent reaction product produced. Cells with high ALDH activity were labeled as ALDH+, and cells with no ALDH activity were labeled as ALDH−.
Immunohistochemistry (IHC) analysis of human BCa tissue microarray (TMA)
For immunohistochemical analysis human BCa TMA (Cat no. BRC1509) was purchased from Pantomics (Richmond, CA, USA). For each grade the TNM classification is provided in the product data sheet. Breast cancer patients (normal (representing hyperplasia or fibroadenoma) = 5; grade-I = 8, grade-II = 42, and grade-III =14) were studied. BCa tissue array slide was stained with primary antibody for ALDH1, followed by secondary antibody incubation, and was analyzed under a light microscope. The tissue microarray (TMA) slide was viewed and scored by two board certified pathologists. Each tissue core was evaluated for ALDH1 staining as follows: Location of staining as either cytoplasmic or nuclear, the pattern of staining as either granular or diffuse, and staining intensity as weak, moderate or strong. Each tissue core was scored on a scale of 0 to 3+, with ALDH1 expression in normal tissue defining the baseline score of 1+. A score of 0 was no staining, a score 1+ was either granular staining or weak staining, a score of 2+ was moderate diffuse staining, and a score of 3+ was strong diffuse staining. A composite score for each tissue core was calculated by multiplying the two values together to normalize the heterogeneity of staining and multifocal nature of the tumor by two different pathologists.
Xenograft Studies
All animals were housed under pathogen-free conditions, and experiments were performed in accordance with Institutional Animal Care & Use Committee (IACUC). Balb/c athymic nude mice (nu/nu) were purchased from The Jackson Laboratory and used at 6 week of age. For subcutaneous tumor xenograft studies, approximately 1 ×105 ALDH+, CD44+/CD22− and 5 × 105 ALDH− cells were subcutaneously implanted into the right/left flanks of female nude mice. Each control and treated group consists of minimum 6 to 11 mice. The mice were monitored twice weekly, and tumor volumes were measured once a week
Immunohistochemical Analysis of Xenografts
For histological examination, tumor samples derived from ALDH− and ALDH+ xenografts were fixed and stained with hematoxylin and eosin (H&E) and were examined under the light microscope. Similarly, tumor sections were stained with primary antibodies for Notch-1 (cat no. N4788), β-catenin (cat no. 9562L), N-cadherin (cat no. SC393933), pAKT (cat no. 4060), Snail (cat no. SC271977) and Slug (cat no. Ab27568).
Protein Extraction and Western Blotting
Total protein extracts from ALDH− and ALDH+ tumors were prepared with Tissue Extraction Reagent (Life Technologies) according to the manufacturer’s protocol. Western blotting was performed using specific antibodies against Notch-1, Presenillin1 (cat. No:A00881,Gene script), HES1 (SC-13844), NFkB (p65)(SC-8008), Actin (SC-1616) (Santa Cruz Biotechnologies, Dallas, TX), Bcl 2 (cat.no-2876), AKT(cat.no-4691), β-catenin (cat.no-9582), E-cadherin (cat no. 24E10), MMP-9 (cat.no-2270), Snail (cat.no-3879), Slug (cat.no-9585) (Cell Signaling, Danvers, MA). Positive bands were detected with the enhanced chemiluminescence method.
Real time PCR
Total RNA was isolated from control and treated tumor tissue using Qiagen RNeasy Mini kit (cat. no - 74104). Reverse transcription of 1ug RNA was performed by Applied Biosysytems Taqman reverse transcription kit (cat. no- N8080234). Real time analysis was done by Applied Biosystems® step one plus Real time PCR. Specific gene primers were purchased from Invitrogen.
Docking studies
The 3D crystal structure of the nicaristin (PDB ID: 4R12) was downloaded from protein data bank (www.rcsb.org) as a pdb file. The downloaded pdb file was prepared for docking by converting it to pdbqt file using mgl Tools [39]. The preprocessed structure was reviewed. The receptor was defined by placing the grid box on entire protein. An in house program was used to calculate grid box parameters. The grid box parameters were Center_x =16.435, Center_y=3.903, Center_z=20.314, size_x=74.012, size_y=73.708, size_z=65.92. A configuration file was prepared using the above mentioned parameters. Molecular docking was performed using the Autodock VinaXB program [40].
Statistics
Statistical analysis was done by Prism 6 software (GraphPad Software Inc, La Jolla, CA, USA). The data is presented as Mean and standard deviation. Significant differences between groups were evaluated by unpaired t-test. P value less than 0.05 was considered significant.
RESULTS
High expression of ALDH correlated with BCa tumor grade:
Previous studies have shown a higher expression of BCSC markers during breast cancer progression. To confirm whether ALDH expression correlates with different tumor grades, we performed IHC to assess ALDH expression in a breast cancer TMA. Of all of the BCa samples, 60% had low expression of ALDH in normal tissues, and 62.5% had moderate expression in grade-I (score-1+), There were 29% with higher expression in Grade-II (score-2+), and 19% had strong expression in grade-III (score-3+) of BCa specimens (Figure 1).
Figure 1:
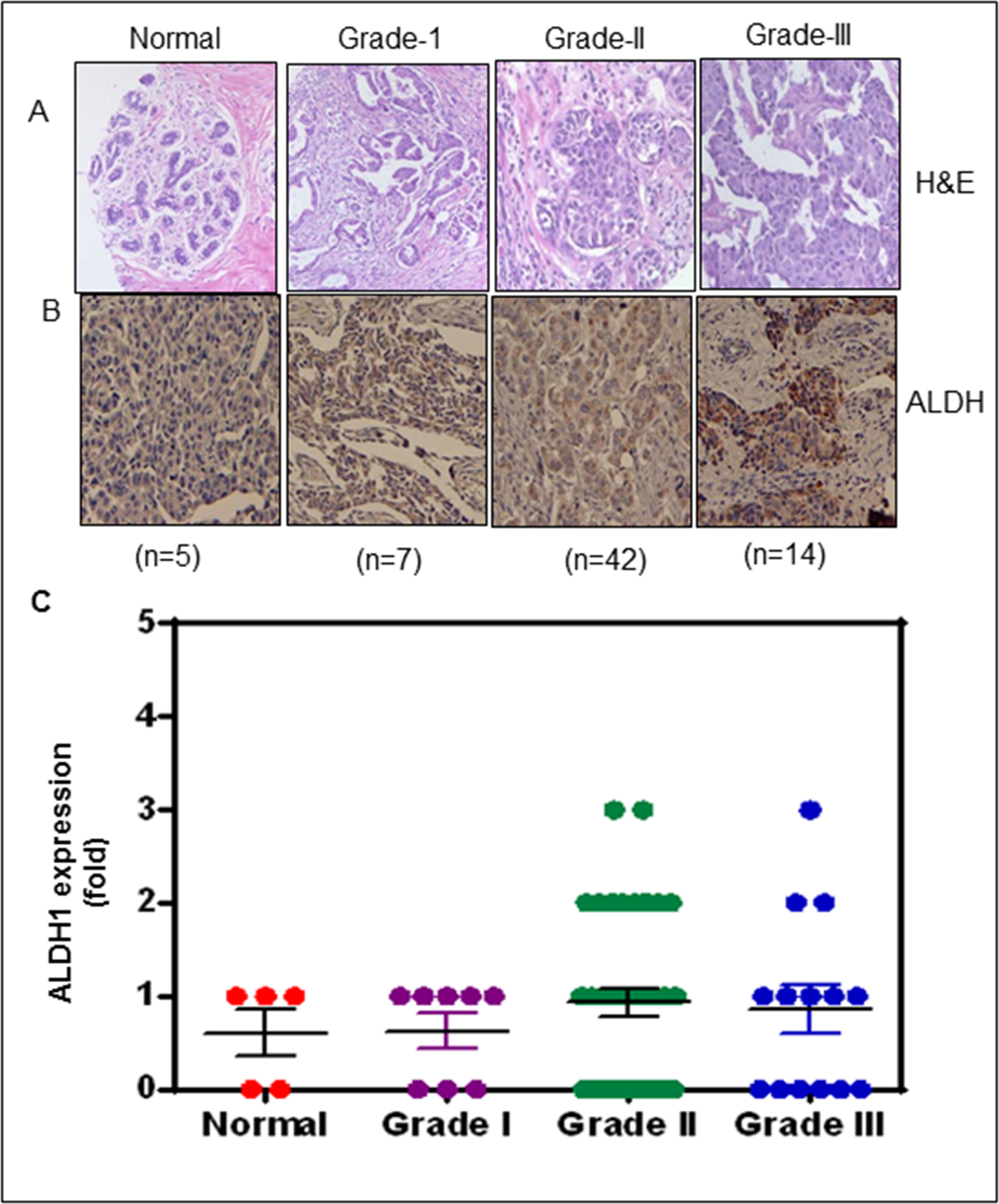
Human BCa tissue microarray (TMA) illustrating the expression of ALDH1 in BCa patients. (A) H&E staining for human BCa (TMA). (B) Immunoreactivity for ALDH1 in representative sections of human BCa. (B) Scatter plot demonstrating ALDH1 expression score based on labelling intensity and given as mean with SEM.
Oral administration of Pso inhibited CD44+/CD22−, ALDH− and ALDH+ tumor xenografts
Our earlier work suggested that Pso inhibits the growth of ALDH+ and ALDH− BCa in cell culture models. Here, we validated the anti-cancer stem cell activity of Pso on xenograft models.
Once the tumor reached 50–100 mm3, the animals were divided into two groups. Control groups received vehicle control (sesame oil) by oral gavage. The other group received Pso (25 mg/kg) 5 days a week for 4 weeks. After Pso treatment CD44+/CD22−, ALDH+ and ALDH− tumor growth was inhibited as compared with vehicle control treated animals (Figure 2a, 2b, 2c). We noted that ALDH+ and CD44+/CD22− tumors showed more rapid and aggressive growth than ALDH− tumors. In fact, not only did this delay tumor growth (3–4 weeks), but smaller tumor volumes were also observed in ALDH− tumors. These in vivo results suggest that BCSC cells are highly tumorigenic and more aggressive than BC cells those devoid stem cells. Pso inhibits tumor growth in the case of both ALDH− and ALDH+ xenografts.
Figure 2:
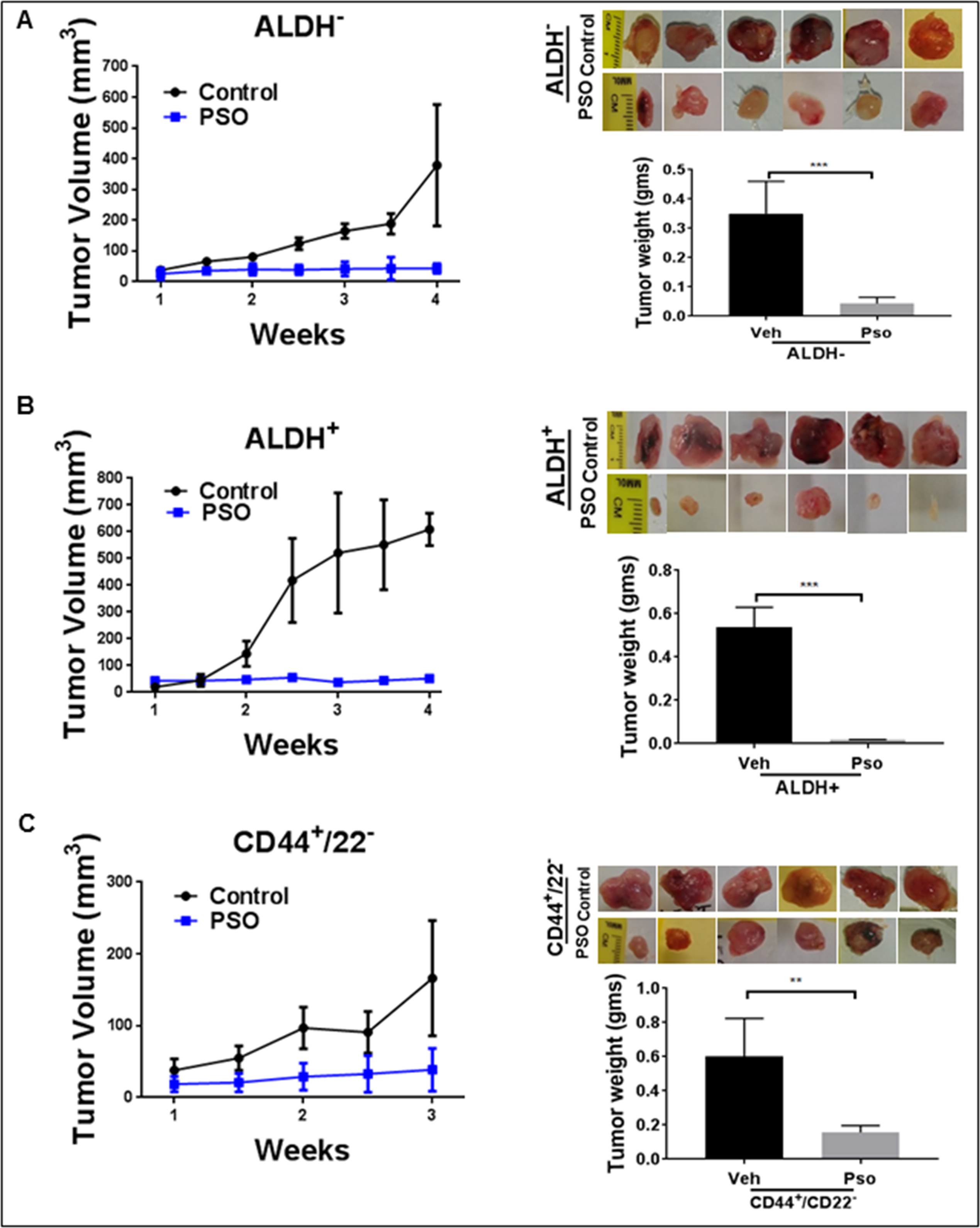
Pso inhibits tumor growth in both ALDH− and ALDH+ cell derived xenografts in nude mice. ALDH+, CD44+ / CD22− and ALDH− cells in a 50-μL final volume (1:1) of phosphate-buffered saline (PBS) and Matrigel were injected subcutaneously into separate flanks of the mouse. Tumor volumes were monitored once a week for 4 weeks. (A) This graph represents the tumor volumes (mm3) and weight for the ALDH− tumors treated with Pso. (B) The graph represents the tumor volumes and weights of ALDH+ tumors treated with Pso. (C) The graph represents the tumor volumes and weights of CD44+/CD22− tumors treated with Pso for 3 weeks. Tumors were extracted and weighed after the mice were sacrificed.
Psoralidin acts as a gamma secretase inhibitor
We evaluated the binding ability of Psoralidin with gamma secretase using molecular docking studies. Binding pockets of all four components of gamma secretase were explored. It appears that Psoralidin binds to nicarstin part of the protein. We evaluated different binding modes and their affinities. The best docked pose yielded a score of −8.5 kcal/mol. This suggests that Psoralidin binds to nicarstin in the micro molar concentration range. It appears that the hydroxyl group of Psoralidin makes polar contacts with the side chain and main chain of ASN 132 and aromatic rings of Psoralidin makes pi-pi stacking interaction with TYR 154. Also the solvent water molecules seem to stabilize the binding of Psoralidin in the binding pocket as shown in figure 3C.
Figure 3:
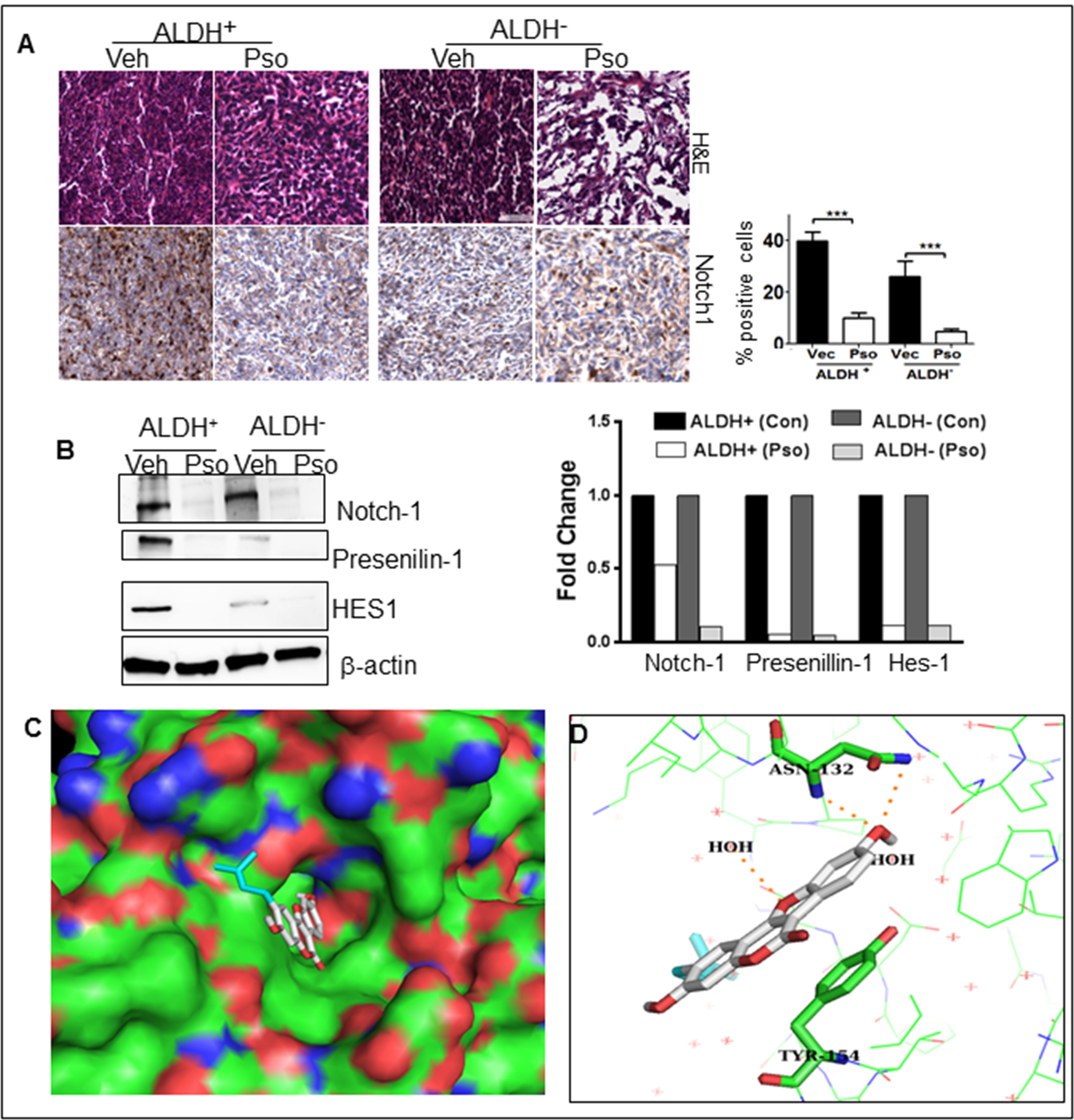
Pso inhibits Notch-1 signaling in ALDH− and ALDH+ cells. (A) Tumor tissues were processed for H&E and immunohistochemical staining for Notch-1. Total of 300–400 cells were counted and out of that Notch-1 positive cells were counted and are represented in the graph as percent. (B) ALDH+(Control), ALDH+(Pso), ALDH− (Control) and ALDH− (Pso) tumor were utilized to prepare whole cell lysates. Western blot analysis was done for Notch-1, Presenilin1 and HES1. β-actin was used as a control for equal loading. The graph represents the densitometric analysis (Fold) for each protein normalized to β-actin. (C) Binding pose of Psoralidin in the binding pocket of nicarstin domain of gamma secretase (PDB ID: 4R12). Psoralidin is represented as white stick model and the binding pocket is represented in surface model. (D) Psoralidin is shown as a white stick model; the protein (nicarstin) is shown as a lines. The interacting amino acids ASN 132 and TYR154 are represented in green stick model. The figure was prepared with PYMOL [58].
Pso inhibited Notch-1 signaling in ALDH+ and ALDH− xenografts
Inhibition of Notch-1 signaling in both ALDH+ and ALDH− cells by Pso were established before. To ascertain antitumor activity of Pso by inhibiting Notch-1 expression, tumor tissues from ALDH+ and ALDH− tumors were analyzed for any immunohistochemical changes in the expression of Notch-1. A higher expression of Notch-1 and Presenilin-1 in ALDH+ tumors versus the ALDH− tumors was observed. There was significant downregulation of Notch-1 expression in both Pso treated ALDH+ and ALDH− tumors tissues (Figure 3a). To confirm further, cell lysates were prepared from control and treated tumors and subjected to Western blot analysis. As expected, ALDH+ tumors expressed higher endogenous levels of Notch-1, Presenilin-1, HES-1 versus ALDH− cells. The Pso significantly inhibited the expressions of Notch-1 and downstream effectors such as Presenilin-1 and HES-1 (Figure 3b).
Activation or nuclear localization of AKT is an essential and key component for the proliferation of BSCSs. There is also molecular crosstalk between Notch-1 signaling and AKT signaling [41]. Hence, we confirmed pAKT expression in ALDH+ and ALDH− tumor tissue. Versus ALDH− tumor tissues, the ALDH+ cells showed intense nuclear localization of pAKT expression in control cells; however, Pso-treatment down-regulated pAKT expression in both ALDH+ and ALDH− cells by IHC staining (Figure 4a). Furthermore, Western blot analysis confirmed downregulation of pAKT expression in tumor tissues treated with Pso (Figure 4b). To ascertain whether the downstream signaling products of AKT such as NF-κB (p65) and Bcl-2 were also affected in Pso-treatment, we checked both p65 and Bcl-2 expression in control as well as Pso-treated tumors (Figure 4b). A higher expression of p65 and Bcl-2 were seen in ALDH+ tumors and Pso inhibited these pro-survival molecules in both cell types (Figure 4a & b).
Figure 4:
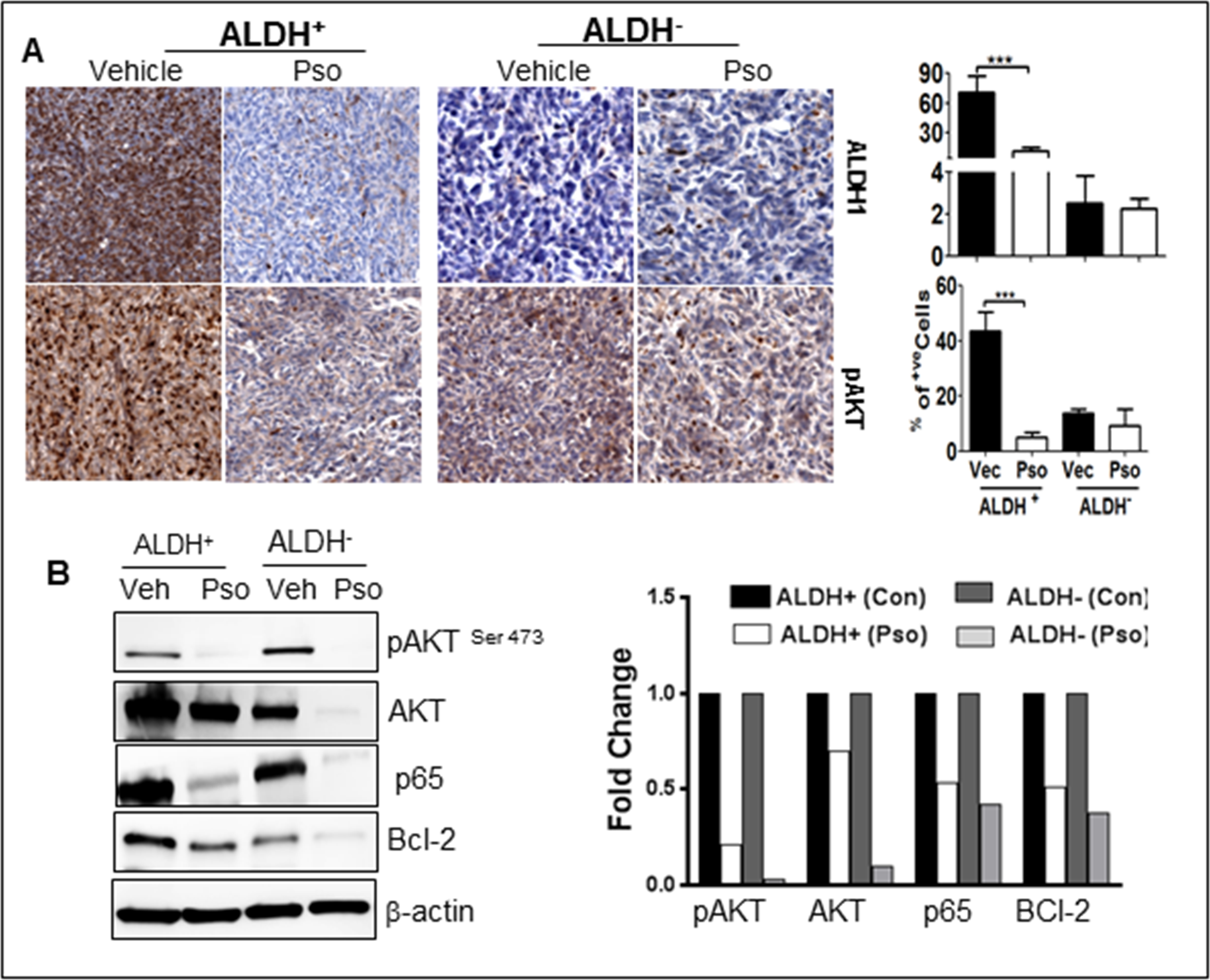
Pso inhibits pro-survival signaling in ALDH+ and ALDH− cells. (A) Tumor tissues were processed for immunohistochemical staining for pAKT (pro-survival).Total of 300–400 cells were counted and out of that pAKT positive cells were counted and are represented in the graph as percent. (B) ALDH+(Control), ALDH+(Pso), ALDH− (Control) and ALDH− (Pso) tumor were utilized to prepare whole cell lysates. Western blot analysis was done for AKT, phosphorylated-AKT (pAKT), p65 and Bcl-2. was used as a control for equal loading. The graph represents the densitometric analysis (Fold) for each protein normalized to β-actin.
Psoralidin restricted EMT in ALDH+, CD44+/CD22− and ALDH− xenografts
Next, we determined the expression of EMT markers because Notch-1 signaling potentially influences the EMT in BCSCs. We also reported that inhibition of Notch-1 hinders the expression of EMT genes in ALDH− and ALDH+ cells. An immunohistochemical examination revealed a clear increase in the expression of β-catenin, Snail and Slug in ALDH+ tumors (Figure 5). Further down regulation of these EMT markers (β-catenin, Snail, Slug and MMP-9) was seen in Pso-treated ALDH− and ALDH+ tumors by IHC as well as western blot analysis (Figure 6a). In addition, gene expression analysis also confirmed EMT downregulation after Pso treatment in ALDH+ and CD44+/CD22− xenografts (Figure 6b). Finally, we performed Retic staining, a marker for angiogenesis in xenograft tumor tissues. ALDH+ tumors exhibited dense micro vessels as compared to ALDH− tumors and Pso significantly inhibited the micro vessel formation in both tumor types (Figure 6c) suggesting Pso could be a potent compound to inhibit angiogenesis in breast cancer models. These findings further confirm the role of Notch-1-mediated EMT in BCSCs. All of these results indicate that Pso is a potent natural compound that inhibits the growth of both BCSC and BC cell-derived tumor xenografts.
Figure 5:
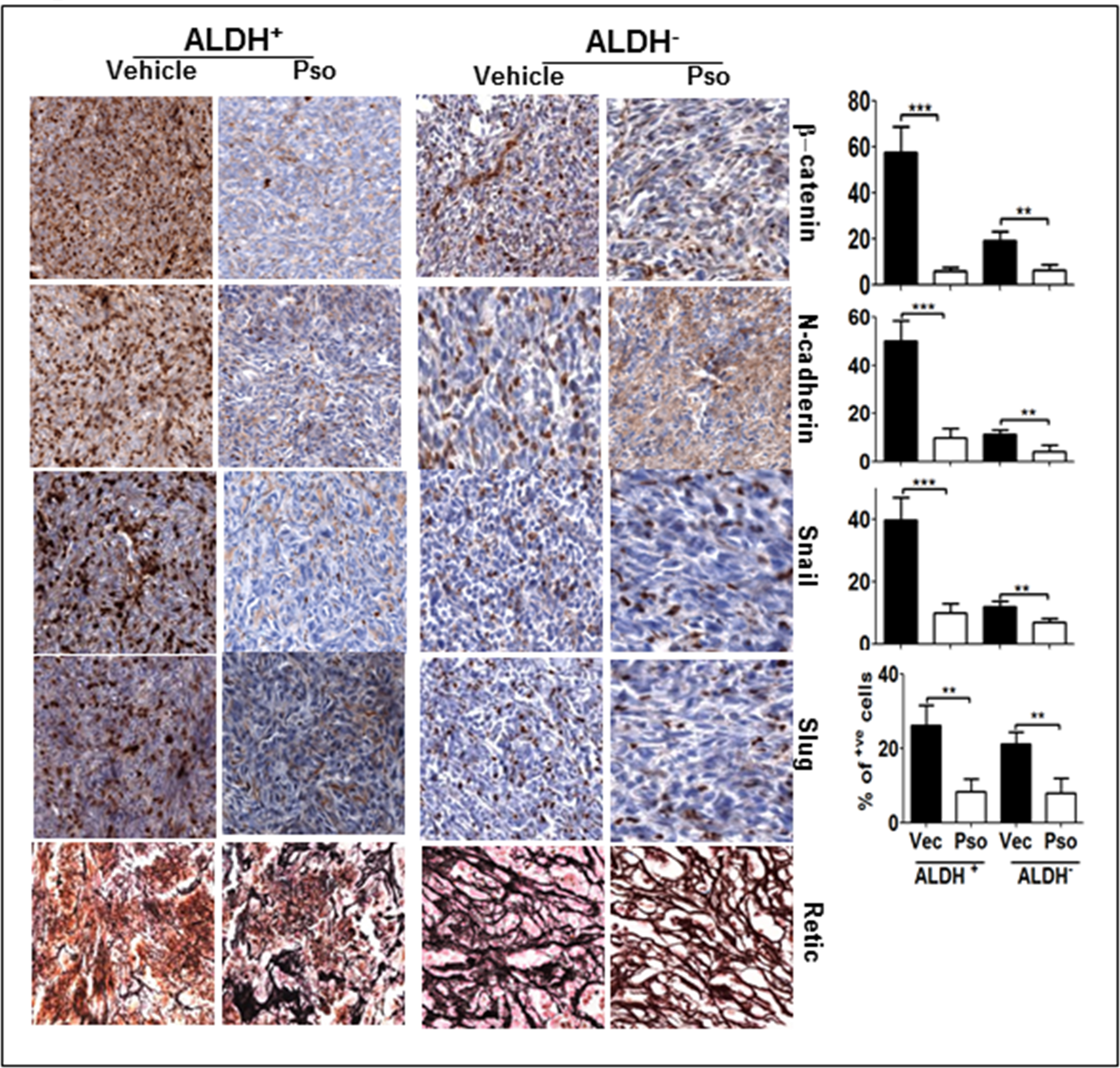
Pso inhibits EMT signaling in ALDH+ and ALDH−. (A) Tumor tissues were processed for immunohistochemical staining for β-catenin, N-cadherin, snail and slug. Total of 300–400 cells were counted and out of that β-catenin, N-cadherin, Snail and Slug positive cells were counted and are represented in the graph as percent.
Figure 6:
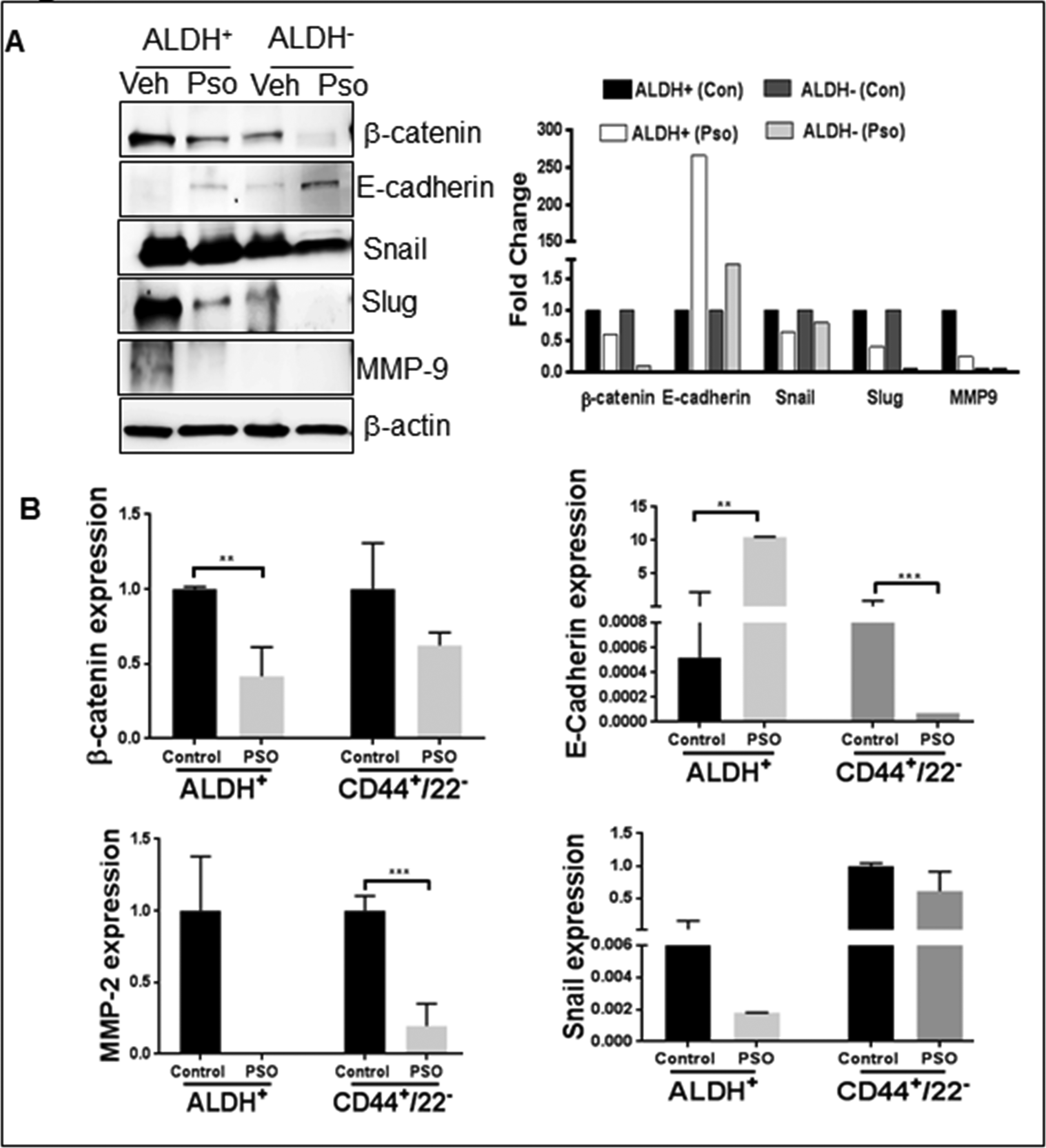
(A) ALDH+ (Control), ALDH+(Pso), ALDH− (Control) and ALDH− (Pso) tumor were utilized to prepare whole cell lysates. Western blot analysis was done for β-catenin, E-cadherin, Snail, Slug and MMP9. β-actin was used as a control for equal loading. The graph represents the densitometric analysis (Fold) for each protein normalized to Actin. (B) Real time PCR was performed to check gene level expression of EMT markers in ALDH+ and CD44+/CD22− tumors after Pso treatment. Gene expression difference was represented as fold change.
Discussion
Our results highlight three important findings: (i) ALDH expression could be an ideal stem cell marker for BCSCs, (ii) Notch-1 is an attractive target to eradicate BCSCs and BCa cells, and (iii) Pso is a potent molecule that inhibits both ALDH+, CD44+/CD22− and ALDH− tumors by targeting Notch-1 signaling.
Many cancer stem cell markers have been identified including CD133, CD44+/CD22−, and ALDH1+ in which CD133 is widely used as a cell surface marker in many cancer types [42] including breast cancer [43]. However, many groups commonly use ALDH1 in BCa, and this study confirms that high ALDH1 expression correlates with disease progression [13,44–47]. Our study confirms the previous observations that ALDH expression correlates with tumor grade, metastasis and poor outcome (32,17). Although, our sample size is small, these findings correlated with a previous publication stating that ALDH1 expression correlates with drug resistance as well as aggressive and metastatic phenotypes in breast cancer [13,48]. The evidence shows that BCSC (ALDH+) are multipotent and govern many signaling pathways responsible for aggressive growth, chemo-resistance, metastasis (EMT) and recurrence of the disease. Hence, researchers are developing small molecules that target particular signaling pathways to completely eliminate BCa. In our in vivo experiments, we observed mice injected with less than 5000 ALDH+ cells developed tumors within two months and rapid tumor growth were observed after 10 weeks, however, we injected 100 times higher ALDH− cells to form tumors within the time frame.
In one such attempt in our previous study, we studied Notch-1 signaling in the context of BCSCs. Notch signaling plays diverse roles and regulates various cellular processes i.e., self-renewal, maintenance, differentiation, proliferation, and apoptosis of stem cells. Aberrant Notch signaling is implicated in breast carcinogenesis because it is involved in regulating the various stages of mammary gland development-and more importantly-EMT [12,49,50]. Consistent with our earlier findings and others, this study confirms that activation of Notch-1 plays a critical role in the aggressiveness and invasiveness of breast cancer [51,52].
This study is a continuation of our previous findings stating that Pso inhibited Notch-1 activation in both ALDH+ and ALDH− BCa cells. We found that oral administration of Pso effectively suppressed both tumor types in nude mice models. Most of the natural products were tested against many cancer types by oral administration. This is a more suitable or preferred route of administration. Many chemopreventive agents effectively suppressed tumor growth in Xenograft models. Very recently, Li et.,al [53] demonstrated that intravenous injection of triptolide-a Chinese herbal medicine-effectively suppressed the BCSC induced tumors more effectively. Similarly, other well-known chemopreventive agents such as Withaferin-A [54] and benzyl isothiocyanate [55] BITC inhibited the BSCS tumor growth in mouse mammary tumor virus-neu (MMTV-neu) transgenic mice. From our studies, Pso is a non-toxic agent that effectively suppresses both ALDH+ and ALDH− tumors without causing significant organ toxicity.
Molecular analysis suggested that Pso inhibited Notch-1 expression in both tumor types, and it is well established that higher expression of Notch-1 is correlated with poor overall survival (32% survival rate) independent of estrogen receptor (ER) status in BCa [56]. Molecular docking studies suggest pso binds to gamma secretase and inhibit the Notch1 signaling in both BCSC and BC cells in vitro and in vivo. It has been demonstrated that the metastatic role of Notch-1 which activates EMT by up-regulating mesenchymal markers including β-catenin, snail, slug, vimentin, etc. as well as by down-regulating epithelial markers i.e. E -cadherin in BCa [50,57]. Our results are consistent with these findings.
Our study revealed that high ALDH enzyme activity is correlated with BCa pathogenesis, metastasis and poor prognosis. This ALDH high population of cells depicts high expression of Notch-1 signaling. This in turn activates many genes and transcription factors that are involved in breast carcinogenesis and metastasis. Pso is a natural molecule with low toxicity and is effective against breast cancer cell populations. Pso clearly inhibited tumor growth in ALDH+, CD44+/CD22− and ALDH− tumors and is a potent molecule to target BCa in clinical settings.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 2.Stockler M, Wilcken NR, Ghersi D, Simes RJ. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer. Cancer Treat Rev 2000;26(3):151–168. [DOI] [PubMed] [Google Scholar]
- 3.Gangopadhyay S, Nandy A, Hor P, Mukhopadhyay A. Breast cancer stem cells: a novel therapeutic target. Clin Breast Cancer 2013;13(1):7–15. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100(7):3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia 2007;9(11):882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol 2008;26(17):2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velasco-Velazquez MA, Popov VM, Lisanti MP, Pestell RG. The role of breast cancer stem cells in metastasis and therapeutic implications. Am J Pathol 2011;179(1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsang JY, Huang YH, Luo MH et al. Cancer stem cell markers are associated with adverse biomarker profiles and molecular subtypes of breast cancer. Breast Cancer Res Treat 2012;136(2):407–417. [DOI] [PubMed] [Google Scholar]
- 9.Velasco-Velazquez MA, Homsi N, De La Fuente M, Pestell RG. Breast cancer stem cells. Int J Biochem Cell Biol 2012;44(4):573–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santilli G, Binda M, Zaffaroni N, Daidone MG. Breast cancer-initiating cells: insights into novel treatment strategies. Cancers (Basel) 2011;3(1):1405–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shafee N, Smith CR, Wei S et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res 2008;68(9):3243–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smalley M, Piggott L, Clarkson R. Breast cancer stem cells: obstacles to therapy. Cancer Lett 2013;338(1):57–62. [DOI] [PubMed] [Google Scholar]
- 13.Ginestier C, Hur MH, Charafe-Jauffret E et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1(5):555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proia DA, Kuperwasser C. Reconstruction of human mammary tissues in a mouse model. Nat Protoc 2006;1(1):206–214. [DOI] [PubMed] [Google Scholar]
- 15.Hideshima T, Catley L, Yasui H et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood 2006;107(10):4053–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leleu X, Jia X, Runnels J et al. The Akt pathway regulates survival and homing in Waldenstrom macroglobulinemia. Blood 2007;110(13):4417–4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem 1998;273(32):19929–19932. [DOI] [PubMed] [Google Scholar]
- 18.Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 1996;10(12):1443–1454. [DOI] [PubMed] [Google Scholar]
- 19.Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim Biophys Acta 2011;1815(2):197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izrailit J, Reedijk M. Developmental pathways in breast cancer and breast tumor-initiating cells: therapeutic implications. Cancer Lett 2012;317(2):115–126. [DOI] [PubMed] [Google Scholar]
- 21.Angeloni V, Tiberio P, Appierto V, Daidone MG. Implications of stemness-related signaling pathways in breast cancer response to therapy. Semin Cancer Biol 2015;31:43–51. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Dontu G, Mantle ID et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 2006;66(12):6063–6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res 2004;6(6):R605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smalley MJ, Dale TC. Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev 1999;18(2):215–230. [DOI] [PubMed] [Google Scholar]
- 25.van Es JH, van Gijn ME, Riccio O et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005;435(7044):959–963. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Li Y, Banerjee S et al. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J Cell Biochem 2010;109(4):726–736. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto Y, Maitra A, Ghosh B et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell 2003;3(6):565–576. [DOI] [PubMed] [Google Scholar]
- 28.Cao YW, Wan GX, Sun JP et al. Implications of the Notch1-Snail/Slug-epithelial to mesenchymal transition axis for lymph node metastasis in infiltrating ductal carcinoma. Kaohsiung J Med Sci 2015;31(2):70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao YW, Li WQ, Wan GX et al. Correlation and prognostic value of SIRT1 and Notch1 signaling in breast cancer. J Exp Clin Cancer Res 2014;33:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suman S, Das TP, Damodaran C. Silencing NOTCH signaling causes growth arrest in both breast cancer stem cells and breast cancer cells. Br J Cancer 2013;109(10):2587–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincan E, Barker N. The upstream components of the Wnt signalling pathway in the dynamic EMT and MET associated with colorectal cancer progression. Clin Exp Metastasis 2008;25(6):657–663. [DOI] [PubMed] [Google Scholar]
- 32.Lombaerts M, van Wezel T, Philippo K et al. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer 2006;94(5):661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009;9(4):265–273. [DOI] [PubMed] [Google Scholar]
- 34.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001;414(6859):105–111. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Wicha MS, Schwartz SJ, Sun D. Implications of cancer stem cell theory for cancer chemoprevention by natural dietary compounds. J Nutr Biochem 2011;22(9):799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das TP, Suman S, Damodaran C. Induction of reactive oxygen species generation inhibits epithelial-mesenchymal transition and promotes growth arrest in prostate cancer cells. Mol Carcinog 2014;53(7):537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chopra B, Dhingra AK, Dhar KL. Psoralea corylifolia L. (Buguchi) - folklore to modern evidence: review. Fitoterapia 2013;90:44–56. [DOI] [PubMed] [Google Scholar]
- 38.Xiao G, Li G, Chen L et al. Isolation of antioxidants from Psoralea corylifolia fruits using high-speed counter-current chromatography guided by thin layer chromatography-antioxidant autographic assay. J Chromatogr A 2010;1217(34):5470–5476. [DOI] [PubMed] [Google Scholar]
- 39.Morris GM, Huey R, Lindstrom W et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem 2009;30(16):2785–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koebel MR, Schmadeke G, Posner RG, Sirimulla S. AutoDock VinaXB: implementation of XBSF, new empirical halogen bond scoring function, into AutoDock Vina. J Cheminform 2016;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jain MV, Jangamreddy JR, Grabarek J et al. Nuclear localized Akt enhances breast cancer stem-like cells through counter-regulation of p21(Waf1/Cip1) and p27(kip1). Cell Cycle 2015;14(13):2109–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grosse-Gehling P, Fargeas CA, Dittfeld C et al. CD133 as a biomarker for putative cancer stem cells in solid tumours: limitations, problems and challenges. J Pathol 2013;229(3):355–378. [DOI] [PubMed] [Google Scholar]
- 43.Bock C, Kuhn C, Ditsch N et al. Strong correlation between N-cadherin and CD133 in breast cancer: role of both markers in metastatic events. J Cancer Res Clin Oncol 2014;140(11):1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunju LP, Cookingham C, Toy KA, Chen W, Sabel MS, Kleer CG. EZH2 and ALDH-1 mark breast epithelium at risk for breast cancer development. Mod Pathol 2011;24(6):786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morimoto K, Kim SJ, Tanei T et al. Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor, positive human epidermal growth factor receptor type 2, and high Ki67 expression. Cancer Sci 2009;100(6):1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charafe-Jauffret E, Ginestier C, Iovino F et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res 2010;16(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Lv DL, Duan JJ et al. ALDH1A1 expression correlates with clinicopathologic features and poor prognosis of breast cancer patients: a systematic review and meta-analysis. BMC Cancer 2014;14:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan H, Wu N, Huang Y et al. Aldehyde dehydrogenase 1 expression correlates with the invasion of breast cancer. Diagn Pathol 2015;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrison H, Farnie G, Brennan KR, Clarke RB. Breast cancer stem cells: something out of notching? Cancer Res 2010;70(22):8973–8976. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Zhao X, Shao S et al. Notch1 induces epithelial-mesenchymal transition and the cancer stem cell phenotype in breast cancer cells and STAT3 plays a key role. Int J Oncol 2015;46(3):1141–1148. [DOI] [PubMed] [Google Scholar]
- 51.Grudzien P, Lo S, Albain KS et al. Inhibition of Notch signaling reduces the stem-like population of breast cancer cells and prevents mammosphere formation. Anticancer Res 2010;30(10):3853–3867. [PubMed] [Google Scholar]
- 52.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010;29(34):4741–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Liu R, Yang Y et al. Triptolide-induced in vitro and in vivo cytotoxicity in human breast cancer stem cells and primary breast cancer cells. Oncol Rep 2014;31(5):2181–2186. [DOI] [PubMed] [Google Scholar]
- 54.Kim SH, Singh SV. Mammary cancer chemoprevention by withaferin A is accompanied by in vivo suppression of self-renewal of cancer stem cells. Cancer Prev Res (Phila) 2014;7(7):738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim SH, Sehrawat A, Singh SV. Dietary chemopreventative benzyl isothiocyanate inhibits breast cancer stem cells in vitro and in vivo. Cancer Prev Res (Phila) 2013;6(8):782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reedijk M, Odorcic S, Chang L et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res 2005;65(18):8530–8537. [DOI] [PubMed] [Google Scholar]
- 57.Shao S, Zhao X, Zhang X et al. Notch1 signaling regulates the epithelial-mesenchymal transition and invasion of breast cancer in a Slug-dependent manner. Mol Cancer 2015;14(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schrödinger. The PyMOL Molecular Graphics System, version; LLC: New York,. 2012; 1.5.0.4. [Google Scholar]


