Abstract
Simple Summary
Many countries have already restricted the use of antibiotics in preventing diarrhea and improving the growth of weaning piglets. Therefore, exploring alternatives to antibiotics is an urgent unmet need. Bacillus licheniformis-fermented feed additive (BLF)-containing probiotics and antimicrobial substances can diminish the diarrhea incidence of weaning piglets. However, limited information is available in terms of the parallel supplementation of antibiotics and BLF in the diet of weaning piglets. For practical application, this information is important to assess whether BLF can be used as an antibiotic substitute. In this study, the effects of BLF or in combination with bacitracin (antibiotics) on weaning piglets were evaluated. The results showed that the half replacement of bacitracin with BLF leads to positive effects on the alleviation of diarrhea incidence and modification of cecal microbiota in weaning piglets.
Abstract
This study investigated the potential of a Bacillus licheniformis-fermented feed additive (BLF) as an antibiotic substitute in weaning piglets. Ninety-six crossbred piglets were randomly allotted into four treatments with three replicate pens per treatment and eight pigs per pen. Piglets were fed diets as follows: a basal diet as control, a basal diet supplemented with bacitracin (30 mg/kg of bacitracin methylene disalicylate), a basal diet supplemented with BLF (1 g/kg of the Bacillus licheniformis-fermented feed additive), and a basal diet supplemented with bacitracin and BLF (15 mg/kg of bacitracin methylene disalicylate and 0.5 g/kg of the Bacillus licheniformis-fermented feed additive). The results showed that replacing all or half the bacitracin with BLF both reduced the incidence of diarrhea in weaning piglets from day 1 to 14. Principal coordinates analysis and a species abundance heat map showed that distinct clusters were formed between groups. Replacing all the bacitracin with BLF reduced bacterial evenness in the cecal digesta of weaning piglets, while the inhibitory effect on bacterial evenness was reversed in the group treated with bacitracin in combination with BLF. These results indicated that the half replacement of bacitracin with BLF was able to decrease the incidence of diarrhea and modify cecal microbiota composition in weaning piglets, suggesting that a Bacillus licheniformis-fermented feed additive has good potential as a suitable alternative to antibiotics use in the swine industry.
Keywords: antibiotic, Bacillus licheniformis, diarrhea, microbiota, piglet
1. Introduction
Intestinal diseases and diarrhea in weaning piglets caused by environmental stress and conditioned pathogens are major economic problems in the swine industry worldwide [1]. Intestinal microbiota disturbances after weaning are associated with a high incidence of diarrhea and lead to poor growth rate in piglets [2]. The use of antibiotics as growth promoters has been effective at reducing post-weaning diarrhea and promoting immunomodulatory and anti-inflammatory responses, thereby improving the growth of weaning piglets [3,4]. However, multi-drug resistant pathogens and antibiotic residues in animal products are becoming a global health concern due to the overuse of antibiotics in animal production. Many countries have banned antibiotic growth promoters for animal growth promotion. Hence, finding acceptable alternatives to antibiotics in the prevention of diarrhea in weaning piglets is important.
Several alternatives to antibiotics have been developed in recent years, among which probiotics have been prominent [5,6,7]. It has been reported that in weaning piglets, probiotic supplementation in diets can have a similar effect to antibiotics [8]. The prevention of post-weaning diarrhea by the application of probiotics or probiotic-derived functional metabolites has also been investigated in weaning piglets [9,10,11]. Among the probiotic strains that have been studied, Bacillus licheniformis has been reported to have a strong ability to produce digestive enzymes and antimicrobial peptides that enhance nutrient utilization and inhibit pathogen growth [12,13,14]. Furthermore, the combined use of B. licheniformis and Bacillus subtilis have been found to decrease the morbidity and mortality rates, ameliorate enteritis, and improve the growth performance of weaning piglets [15,16]. B. licheniformis supplementation has also been reported to improve the incidence of diarrhea in weaning piglets [17]. Our recent study also demonstrated that a B. licheniformis-fermented feed additive (BLF) has a beneficial effect on the alleviation of the diarrhea incidence of weaning piglets [11]. More importantly, bacterial communities in the feces were clearly distinct in the weaning piglets treated with antibiotics and those treated with BLF [11].
The combination of antibiotics and probiotics, as well as comparisons of these feed additives, have been evaluated in broilers and piglets [18,19,20]. The half replacement of antibiotics with Bacillus amyloliquefaciens has been found to improve the growth performance, antioxidant activity, and digestive enzyme activity in weaning piglets [18,19]. However, to the best of our knowledge, limited information is available in terms of the parallel supplementation of bacitracin and BLF in the diet of weaning piglets. It is important to comparatively evaluate the beneficial effects of bacitracin and BLF for practical application. Therefore, this study aimed to investigate the effects of BLF on the growth performance, diarrhea incidence, and cecal microbiota of weaning piglets as a substitute for antibiotics.
2. Materials and Methods
2.1. BLF and Antibiotics
B. licheniformis (American Type Culture Collection 12713) was obtained from the Food Industry Research and Development Institute (Hsinchu, Taiwan). Protocols for BLF preparation in this study were conducted as described previously [21]. Briefly, sterile fermentation substrates were inoculated with the inoculum of B. licheniformis and incubated at 30 °C for 6 days. Fermented feeds were dried at 50 °C and then homogenized through mechanical agitation. The B. licheniformis spore and B. licheniformis-derived antimicrobial peptide (surfactin) quantities in BLF were 5 × 1011 CFU/g and 10 mg/g, respectively. Bacitracin methylene disalicylate was obtained from Nice Garden Industrial (Taipei, Taiwan).
2.2. Animals and Diets
All the procedures used in this experiment were approved by the Institutional Animal Care and Use Committee of National Ilan University (IACUC, protocol number 109-4). Ninety-six crossbred castrated male and female piglets ((Landrace × Yorkshire) × Duroc) at 28 days old with an average initial body weight of 9.31 ± 0.638 kg were randomly divided into four groups in a completely randomized design. Each group had three replicates with eight piglets per replicate. The dietary treatments comprised a basal diet as control (C), a basal diet supplemented with 30 mg/kg of bacitracin (bacitracin methylene disalicylate) (A), a basal diet supplemented with 1.0 g/kg of BLF (5 × 108 CFU/kg of feed) (BLF), and a basal diet supplemented with 15 mg/kg of bacitracin (bacitracin methylene disalicylate) and 0.5 g/kg of BLF (2.5 × 108 CFU/kg of feed) (AF). Diets were formulated to meet or exceed the nutrient requirement recommendation by National Research Council (Nutrient Requirements for Swine, 2012, Table 1). In BLF-treated groups, the soybean meal in the basal diet was equally replaced with BLF. Food and water were offered ad libitum throughout the experimental period. The experimental period was 28 days. All piglets were adapted to the pens and the basal diets for 7 days before starting the experiment. The piglets were housed in pens with slatted metal floors (2.5 m × 4.0 m). The room temperature was 30 °C at the arrival of the piglets and was then gradually decreased and kept at 24 °C until the end of the experiment. A 10 light: 14 dark photoperiod was applied throughout the experiment. The growth performance parameters (average body weight, average daily weight gain, average daily feed intake, and feed conversion ratio) were measured every week.
Table 1.
Composition of basal diets.
| Item | d 1 to 28 1 |
|---|---|
| Ingredient, g kg−1 | |
| Corn, yellow | 570.0 |
| Soybean meal, 44% crude protein (CP) | 260.0 |
| Dried whey | 85.0 |
| Soybean oil | 35.0 |
| Fish meal, 60% CP | 30.0 |
| CaHPO4 | 12.0 |
| CaCO3, 38% Ca | 5.0 |
| Salt | 4.0 |
| L-Lysine, 98% | 3.5 |
| Vitamin, premix 2 | 1.0 |
| Mineral, premix 3 | 1.0 |
| Choline, 50% | 0.8 |
| Fermented feed additive | 0 |
| Chemical composition, g kg−1 | |
| Crude protein | 191.2 |
| Phenylalanine and Tyrosine | 15.5 |
| Lysine | 14.5 |
| Methionine and Cystine | 6.5 |
| Analyzed calcium | 8.0 |
| Analyzed total phosphorus | 7.0 |
| Metabolizable energy (ME), kcal/kg | 3567.63 |
1 Fed in meal form to all piglets; 2 supplied per kg diet: vitamin A, 6000 international unit (IU); vitamin D, 900 IU; vitamin E, 30 IU; vitamin K3, 3 mg; vitamin B2, 6 mg; pantothenic acid, 18 mg; niacin, 60 mg; and vitamin B12, 30 μg; 3 supplied per kg diet: Fe, 140 mg; Zn, 100 mg; Cu, 20 mg; Mn, 4 mg; I, 0.2 mg; and Se, 0.1 mg.
2.3. Analysis of Diarrhea Incidence
The consistency of the fresh excreta was evaluated daily according to 4 levels: 0, solid; 1, semi-solid; 2, semi-liquid; and 3, liquid. Levels 0 and 1 were considered normal, while levels 2 and 3 were considered diarrhea. The incidence of diarrhea in each pen was recorded daily and calculated as described previously [10]
2.4. Small Intestinal Morphology
Two piglets per replicate pen were randomly chosen (based on the average weight of each replicate) at the end of the experiment (day 28) and euthanized by electrical stunning followed by exsanguination. Three replicates (6 piglets per treatment, n = 3) were used for the examination of intestinal histology. Two-centimeter-long segments of the duodenum, jejunum, and ileum were taken from the middle of each part and immediately fixed in 10% neutral buffered formalin (Origin Pure Biosci and Tech, Taipei City, Taiwan). All specimens were sectioned at a thickness of 5 μm (3 cross-sections from each sample) on a rotary microtome (Thermo Scientific, Waltham, MA, USA), followed by staining with hematoxylin and eosin. An image analyzer system composed of an Olympus CKX41 microscope (Olympus Corporation, Tokyo, Japan) and the Visiopharm image software (Hørsholm, Denmark) was used for estimating intestinal morphology. The villus length, crypt depth, and villus-to-crypt ratio of each segment were measured randomly on 30 villi in one piglet.
2.5. 16S Ribosomal RNA Sequencing and Analysis
Two piglets per replicate (6 piglets per treatment, n = 3) were randomly chosen (based on the average weight of each replicate) at the end of the experiment (day 28) and euthanized by electrical stunning followed by exsanguination. The cecal digesta from 2 piglets were freshly collected and then pooled for cecal microbiota analysis. The total genomic DNA from cecal digesta was extracted using a QIAamp DNA Microbiome kit (QIAGEN, Germantown, MD, USA). Purified genomic DNA was quantified on a Quibt 2.0 Fluorometer (Thermo Scientific, Waltham, MA, USA). The PCR amplification of the variable regions 3 and 4 of the 16S rRNA gene was conducted using region-specific primers (341F-805R). The PCR products were purified by agarose gel electrophoresis, and the QIAquick Gel Extraction kit (QIAGEN, Germantown, MD, USA) was used for the recovery of DNA from the gels. All sequencing libraries were generated using TruSeq Nano DNA Library Prep kits (Illumina, San Diego, CA, USA), and the libraries were quantified on the Qubit 2.0 Fluorometer (Thermo Scientific, Waltham, MA, USA) and Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The libraries were subjected to paired-end sequencing (2 × 300 bp) by the Illumina MiSeq platform. Amplicon sequences were clustered into operational taxonomic units (OTUs) using a similarity threshold of 97% in the UCHIME software (version 4.2) and the mothur software (version 1.39.5). The obtained sequences were aligned to the Genomes Online Database (gold.jgi.doe.gov) to determine the OTUs’ phylogeny. Low abundance OTUs (<0.005% of total reads) were removed. A Venn diagram was constructed to present the number of unique and shared OTUs (version 1.6.17). Taxonomic assignments and alpha diversity analysis were made using a naïve Bayesian classification method (rdp.cme.msu.edu) and the QIIME 2 software (version 2017.4, GitHub, San Francisco, CA, USA), respectively. The principal component analysis (PCA), principal coordinate analysis (PCoA), and beta diversity were calculated using the weighted and unweighted UniFrac metric (a distance metric used for comparing bacterial communities) and performed in the QIIME 2 software. The R corrplot package (v.0.84, GitHub, San Francisco, CA, USA) was used to visualize the correlation matrices.
2.6. Statistical Analysis
Data were analyzed by a one-way ANOVA through the general linear model procedure of statistical analysis system (SAS) (version 9.4, 2012; SAS Institute, Cary, NC, USA). Replicates were used as the experimental unit. Means were compared using Tukey’s honestly significant difference test. A p value between 0.05 and 0.1 was considered a trend, and a p value of less than 0.05 was statistically significant. The PCA, PCoA, and beta diversity were performed in the QIIME software using UniFrac distances coupled with standard multivariate statistics. p values were adjusted for multiple comparisons using the Benjamini–Hochberg false discovery rate method. The correlations between cecal microbiota, diarrhea incidence, and growth performance were analyzed using Pearson’s correlation coefficient.
3. Results
3.1. Effect of BLF as an Antibiotics Substitute on Growth Performance, Diarrhea Incidence, and Small Intestine Morphology
All piglets were healthy over the experimental period. Though the changes in body weight between groups were not statistically significant, a trend of increased body weight was observed with the supplementation of BLF in combination with bacitracin (27.24 kg) compared with the control group (26.40 kg) (p = 0.066). Though it did not reach statistical significance, the half replacement of bacitracin with BLF caused a trend in improving the average daily weight gain in weaning piglets at 1–28 days (0.63 kg/d/head) compared with the control group (0.601 kg/d/head) (p = 0.061). There were no significant differences between groups in average daily feed intake and feed conversion ratio over the experimental period. The incidence of diarrhea in the group treated with bacitracin was significantly decreased compared with the control group at 1–14 days (p < 0.05) (Table 2). Similar to bacitracin, replacing all the bacitracin or the half replacement of bacitracin with BLF also reduced the diarrhea incidence in weaning piglets compared with the control group at 1–14 days (p < 0.05) (Table 2). No significant difference was observed in the incidence of diarrhea between the group treated with bacitracin alone, BLF alone, and BLF in combination with bacitracin (Table 2). There were no significant differences between groups in diarrhea incidence at 15–28 days (Table 2). Though the changes in diarrhea incidence between groups were not statistically significant at 1–28 days, a trend of decreased diarrhea incidence in weaning piglets was observed with the supplementation of bacitracin compared with the control group (p = 0.058) (Table 2).
Table 2.
Effect of bacitracin and Bacillus licheniformis-fermented feed additive (BLF) on the diarrhea incidence of weaning piglets.
| Item | C 1 | A 2 | BLF 3 | AF 4 | SEM 5 | p-Value |
|---|---|---|---|---|---|---|
| Diarrhea, % | ||||||
| 1–14 d | 16.06 6,a | 6.84 b | 10.11 b | 9.22 b | 1.264 | 0.009 |
| 15–28 d | 11.26 | 8.11 | 8.41 | 6.59 | 0.855 | 0.373 |
| 1–28 d | 13.66 | 7.48 | 9.27 | 7.91 | 0.968 | 0.058 |
1 C = Basal diet; 2 A = basal diet supplemented with 30 mg/kg bacitracin; 3 BLF = basal diet supplemented with 1 g/kg BLF; 4 AF = basal diet supplemented with 15 mg/kg bacitracin and 0.5 g/kg BLF; 5 SEM = standard error of mean; 6 data are mean values of 3 replicate per treatment (8 piglets per replicate); a,b means that have no superscript in common are significantly different from each other (p < 0.05).
The villus length was longer in the small intestine of the duodenum in the antibiotics alone group compared with the control group (p < 0.05) (Table 3). Though it did not reach statistical significance, replacing all the bacitracin with BLF caused a trend in decreasing crypt depth in the small intestine of the jejunum (p = 0.062) (Table 3). Similar to bacitracin, replacing all bacitracin with BLF increased the ratio of villus length to crypt depth in the small intestine of the ileum compared with the control group (p < 0.05) (Table 3).
Table 3.
Effects of bacitracin and BLF on small intestine morphology.
| Item | C 1 | A 2 | BLF 3 | AF 4 | SEM 5 | p-Value | |
|---|---|---|---|---|---|---|---|
| Villus length (μm) | Duodenum | 519.35 6,b | 668.85 a | 597.35 ab | 611.00 ab | 26.546 | 0.008 |
| Jejunum | 401.70 | 480.35 | 425.75 | 466.70 | 13.067 | 0.251 | |
| Ileum | 260.49 | 536.25 | 635.05 | 490.75 | 52.362 | 0.199 | |
| Crypt depth (μm) | Duodenum | 156.00 | 134.55 | 139.75 | 107.25 | 9.851 | 0.818 |
| Jejunum | 170.95 | 107.25 | 97.5 | 102.05 | 11.641 | 0.062 | |
| Ileum | 114.40 | 117.00 | 146.25 | 160.55 | 10.841 | 0.743 | |
| Villus length: Crypt depth | Duodenum | 3.44 | 5.32 | 4.28 | 5.81 | 0.374 | 0.528 |
| Jejunum | 2.38 | 4.89 | 4.71 | 4.59 | 0.424 | 0.138 | |
| Ileum | 2.34 b | 4.69 a | 4.39 a | 3.08 ab | 0.324 | 0.035 | |
1 C = Basal diet; 2 A = basal diet supplemented with 30 mg/kg bacitracin; 3 BLF = basal diet supplemented with 1 g/kg BLF; 4 AF = basal diet supplemented with 15 mg/kg bacitracin and 0.5 g/kg BLF; 5 SEM = standard error of mean; 6 data are mean values of 3 replicate per treatment (2 piglets per replicate); a,b means that have no superscript in common are significantly different from each other (p < 0.05).
3.2. Effect of BLF as an Antibiotics Substitute on the Cecal Bacterial Microbiota
After the quality-based trimming of raw data, the averages of high-quality reads from the cecal digesta of weaning piglets fed only a basal diet, bacitracin, BLF, or BLF in combination with bacitracin were 84,566, 97,807, 87,001, and 89,312, respectively. The average OTUs in the four aforementioned groups were 20,139, 23,336, 21,042, and 21,133, respectively (Table 4). Alpha diversity results showed that no significant difference was observed in the cecal species richness (Chao1 and Fisher alpha) between groups (Table 4). In contrast, one of the cecal species evenness estimators (Shannon) was decreased in the group treated with BLF alone compared with the control group (p < 0.05) (Table 4). The Venn diagram indicated that 201 OTUs (core overlap) were shared by four of the plotted groups (Figure 1). The numbers of unique OTUs found in the four aforementioned groups were 563, 156, 1113, and 193, respectively. On one hand, 176 OTUs were found in both the control group and the group treated with bacitracin alone, and 36 OTUs were found in both the control group and the group treated with BLF alone. In contrast, 193 OTUs were found in both the control group and the group treated with BLF in combination with bacitracin. The PCA indicated statistically significant discrimination among the groups (Figure 2a). The PCoA of quantitative traits and qualitative traits revealed that the microbiota of cecal samples among the groups were clearly separated (Figure 2b,c). The beta diversity analysis based on quantitative traits and qualitative traits also revealed that the cecal microbiota among the groups was clearly differentiated (Figure 2d,e). As found through quantitative analysis, replacing all bacitracin with BLF exhibited a unique cecal bacterial structure. However, the cecal bacterial structure between groups was clearly separated based on qualitative analysis.
Table 4.
The microbial alpha diversity in the cecal digesta of weaning piglets. OTU: operational taxonomic unit.
| Item | Effective Reads | Number of OTUs | Chao1 | Fisher Alpha | Shannon | Simpson Reciprocal |
|---|---|---|---|---|---|---|
| C 1 | 84,566.00 7 | 20,138.67 | 1352.67 | 250.88 | 7.62 a | 48.81 ab |
| A 2 | 97,807.33 | 23,335.67 | 1105.00 | 189.72 | 7.41 a | 39.44 bc |
| BLF 3 | 87,001.33 | 21,042.00 | 951.67 | 160.89 | 6.98 b | 36.59 c |
| AF 4 | 89,312.00 | 21,133.00 | 1156.67 | 204.37 | 7.64 a | 50.96 a |
| SEM 5 | 2244.360 | 514.455 | 53.385 | 12.787 | 0.083 | 2.189 |
| Adjusted p-Value 6 | 0.167 | 0.167 | 0.172 | 0.202 | 0.006 | 0.054 |
1 C = Basal diet; 2 A = basal diet supplemented with 30 mg/kg bacitracin; 3 BLF = basal diet supplemented with 1 g/kg BLF; 4 AF = basal diet supplemented with 15 mg/kg bacitracin and 0.5 g/kg BLF; 5 SEM = standard error of mean; 6 adjusted p-value: false discovery rate correction using the Benjamini–Hochberg procedure; 7 data are mean values of 3 replicate per treatment (2 piglets per replicate); a–c means that have no superscript in common are significantly different from each other (p < 0.05).
Figure 1.
Venn diagram showing the number of OTUs shared and unique between groups. The overlapping regions between the ellipses illustrate the OTUs that were shared between the C, A, BLF, and AF groups (n = 3). The value of each region represents the number of OTUs corresponding to the region.
Figure 2.
Advanced analysis of the bacterial communities of cecal digesta. (A) Principal component analysis plots of the cecal digesta of the C, A, BLF, and AF groups (n = 3). Principal coordinate analysis plots of quantitative traits (weighted UniFrac distances) (B) and qualitative traits (unweighted UniFrac distances) (C) of the cecal bacterial communities from the C, A, BLF, and AF groups (n = 3). (D) The beta diversity index of the cecal digesta from the C, A, BLF, and AF groups based on weighted UniFrac distances (n = 3). (E) The beta diversity index of the cecal digesta from the C, A, BLF, and AF groups based on unweighted UniFrac distances (n = 3).
3.3. Effects of BLF as an Antibiotics Substitute on the Cecal Bacterial Taxonomic Composition
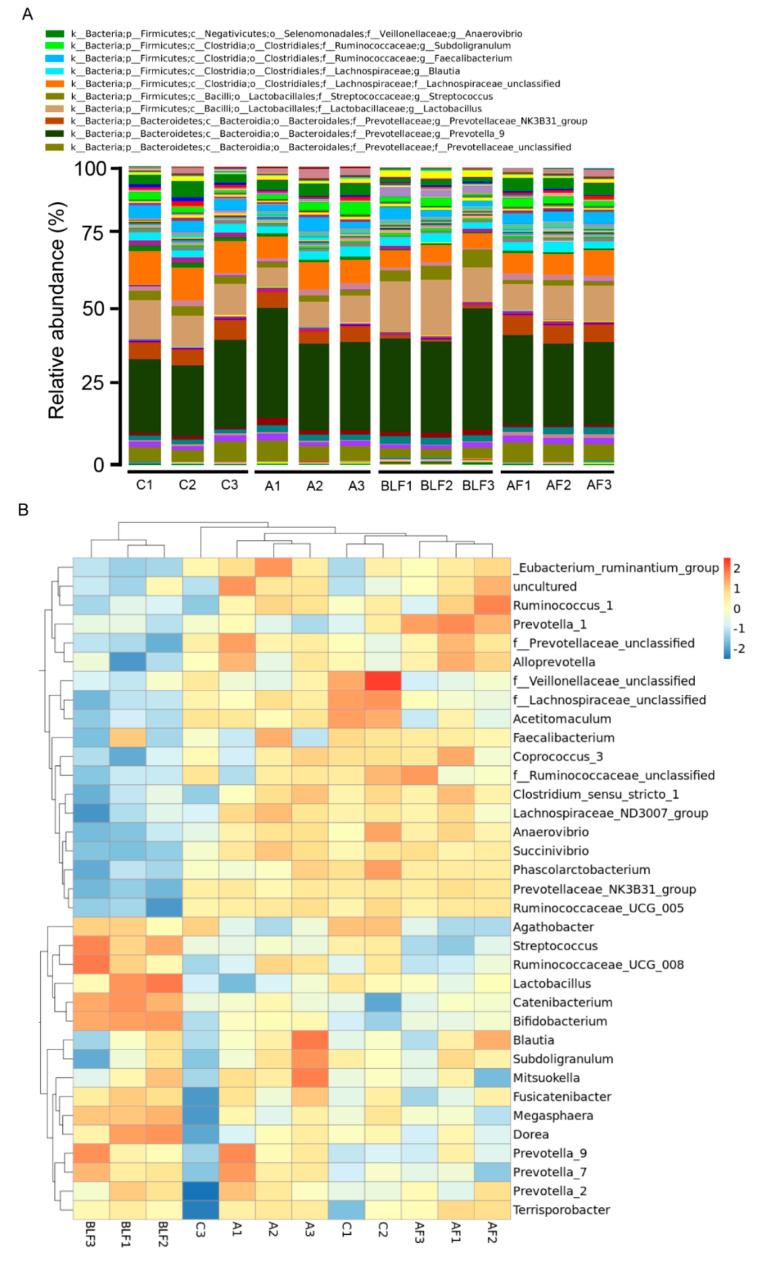
The results of bacterial taxonomy in the cecal digesta of weaning piglets are shown in Table 5. At the phylum level, no significant differences were found in the abundance of the Firmicutes and Bacteroidetes phyla between groups. Replacing all the bacitracin with BLF decreased the abundance of the Actinobacteria phylum compared with groups treated with antibiotics alone (p < 0.05). The Proteobacteria phylum abundances were increased in the group treated with BLF alone compared with other groups (p < 0.001). Replacing all the bacitracin with BLF increased the abundances of the Bacilli, Erysipelotrichia, and Coriobacteriia classes compared with other groups (p < 0.05). The Gammaproteobacteria class abundances were lower in the groups treated with BLF alone compared with the group treated with bacitracin alone (p < 0.05). The Aeromonadales order abundances were lower in the groups treated with BLF alone compared with the group treated with bacitracin alone (p < 0.05). Replacing all the bacitracin with BLF increased the abundance of the Lactobacillales, Erysipelotrichales, and Coriobacteriales orders compared with other groups (p < 0.05). The Lachnospiraceae family abundances were lower in all treatment groups compared with the control group (p < 0.05). The abundances of the Streptococcaceae and Erysipelotrichaceae families were increased in the groups treated with BLF alone compared with other groups (p < 0.05). Replacing all the bacitracin with BLF reduced the abundances of the Succinivibrionaceae and Muribaculaceae families compared with the group treated with BLF in combination with bacitracin (p < 0.05). The Peptostreptococcaceae family abundances were higher in all treatment groups compared with the control group (p < 0.05). Replacing all the bacitracin or half the bacitracin with BLF decreased the abundance of the Lachnospiraceae_unclassified genus compared with the control group (p < 0.05). The half replacement of bacitracin with BLF increased the abundance of the Prevotellaceae NK3B31 group genus compared with the group treated with bacitracin alone, while replacing all the bacitracin with BLF decreased the abundance of the Prevotellaceae NK3B31 group genus (p < 0.001). Replacing all the bacitracin with BLF increased the abundance of the Streptococcus genus compared with other groups (p < 0.05). Compared with the control group, the Agathobacter genus abundances were reduced in the group treated with bacitracin and the group treated with BLF in combination with bacitracin (p < 0.05). Replacing all the bacitracin with BLF decreased the abundance of the Acetitomaculum genus compared with the control group and the group treated with bacitracin alone (p < 0.05). An overview of the taxonomy at the genus level and a heat map of the 35 most abundant genera in the cecal digesta are shown in Figure 3. The results of the heat map showed that similar bacterial community clusters, such as the Anaerovibrio, Succinivibrio, Phascolarctobacterium, Prevotellaceae NK3B31 group, and Ruminococcaceae_UCG_005 genera were observed between the groups treated with basal diet, bacitracin alone, and BLF in combination with bacitracin. In contrast, replacing all bacitracin with BLF resulted in unique bacterial community clusters compared with other groups, such as the Streptococcus, Ruminococcaceae_UCG_008, Lactobacillus, Catenibacterium, and Bifidobacterium genera. In addition, some bacterial community clusters were decreased in the groups treated with BLF alone, such as the Anaerovibrio, Succinivibrio, Phascolarctobacterium, Prevotellaceae NK3B31 group, and Ruminococcaceae_UCG_005 genera. Some overlaps in bacterial community clusters were observed between the groups treated with bacitracin alone and the groups treated with BLF alone, such as the Fusicatenibacter, Prevotella_9, Prevotella_7, Prevotella_2, and Terrisporobacter genera.
Table 5.
Comparison of the abundances of bacterial taxa in the cecal digesta of weaning piglets.
| Item | Relative Abundance (%) 1 | Adjusted | ||||
|---|---|---|---|---|---|---|
| C 2 | A 3 | BLF 4 | AF 5 | SEM 6 | p-Value 7 | |
| Phylum | ||||||
| Firmicutes | 54.30 8 | 45.79 | 51.99 | 48.05 | 1.655 | 0.379 |
| Bacteroidetes | 43.46 | 50.24 | 45.50 | 49.12 | 1.630 | 0.468 |
| Actinobacteria | 1.43 ab | 2.84 a | 0.36 b | 1.88 ab | 0.309 | 0.018 |
| Proteobacteria | 0.47 c | 0.82 b | 2.05 a | 0.67 bc | 0.188 | <0.001 |
| Class | ||||||
| Bacteroidia | 43.46 | 50.23 | 45.50 | 49.12 | 1.630 | 0.468 |
| Clostridia | 31.90 | 27.92 | 22.21 | 27.86 | 1.279 | 0.059 |
| Bacilli | 14.20 b | 9.91 b | 20.67 a | 12.18 b | 1.315 | 0.012 |
| Negativicutes | 7.64 | 7.19 | 5.45 | 7.12 | 0.465 | 0.468 |
| Gammaproteobacteria | 1.36 ab | 2.75 a | 0.34 b | 1.81 ab | 0.302 | 0.018 |
| Erysipelotrichia | 0.54 b | 0.77 b | 3.61 a | 0.86 b | 0.387 | <0.001 |
| Coriobacteriia | 0.21 b | 0.17 b | 0.87 a | 0.25 b | 0.089 | <0.001 |
| Order | ||||||
| Bacteroidales | 43.35 | 50.09 | 45.44 | 49.04 | 1.625 | 0.469 |
| Clostridiales | 31.90 | 27.92 | 22.21 | 27.86 | 1.279 | 0.059 |
| Lactobacillales | 14.20 b | 9.90 b | 20.67 a | 12.18 b | 1.316 | 0.012 |
| Selenomonadales | 7.64 | 7.19 | 5.45 | 7.12 | 0.465 | 0.469 |
| Aeromonadales | 1.08 ab | 2.23 a | 0.09 b | 1.63 a | 0.276 | 0.025 |
| Erysipelotrichales | 0.54 b | 0.77 b | 3.61 a | 0.86 b | 0.387 | <0.001 |
| Coriobacteriales | 0.21 b | 0.17 b | 0.87 a | 0.25 b | 0.088 | <0.001 |
| Family | ||||||
| Prevotellaceae | 42.59 | 49.70 | 45.20 | 48.49 | 1.645 | 0.490 |
| Lachnospiraceae | 21.10 a | 16.30 b | 13.66 b | 15.43 b | 0.876 | 0.003 |
| Lactobacillaceae | 11.38 | 7.96 | 15.79 | 10.65 | 1.012 | 0.074 |
| Ruminococcaceae | 8.48 | 8.41 | 6.44 | 8.88 | 0.475 | 0.406 |
| Veillonellaceae | 6.66 | 6.52 | 5.22 | 6.33 | 0.380 | 0.618 |
| Streptococcaceae | 2.82 b | 1.94 b | 4.88 a | 1.53 b | 0.414 | 0.003 |
| Clostridiaceae_1 | 1.47 | 1.78 | 0.86 | 1.90 | 0.156 | 0.109 |
| Acidaminococcaceae | 0.97 | 0.67 | 0.22 | 0.79 | 0.103 | 0.093 |
| Succinivibrionaceae | 1.08 ab | 2.23 a | 0.09 b | 1.63 a | 0.276 | 0.028 |
| Erysipelotrichaceae | 0.54 b | 0.77 b | 3.61 a | 0.86 b | 0.387 | <0.001 |
| Muribaculaceae | 0.39 a | 0.26 bc | 0.19 c | 0.31 ab | 0.024 | <0.001 |
| Peptostreptococcaceae | 0.52 b | 1.21 a | 1.13 a | 1.39 a | 0.114 | 0.019 |
| Genus | ||||||
| Prevotella 9 | 25.97 | 32.05 | 34.31 | 28.47 | 1.380 | 0.179 |
| Lactobacillus | 11.38 | 7.96 | 15.79 | 10.65 | 1.012 | 0.081 |
| Lachnospiraceae_unclassified | 11.06 a | 8.09 b | 5.45 c | 7.51 bc | 0.639 | 0.004 |
| Prevotellaceae NK3B31 group | 5.71 ab | 4.76 b | 1.00 c | 6.16 a | 0.624 | <0.001 |
| Prevotellaceae_unclassified | 5.18 | 5.74 | 3.24 | 6.12 | 0.417 | 0.081 |
| Faecalibacterium | 3.80 | 2.88 | 2.76 | 3.65 | 0.286 | 0.644 |
| Streptococcus | 2.82 b | 1.94 b | 4.88 a | 1.53 b | 0.414 | 0.004 |
| Anaerovibrio | 3.64 ab | 3.65 ab | 1.46 b | 3.87 a | 0.360 | 0.080 |
| Blautia | 2.55 | 2.95 | 2.68 | 2.94 | 0.133 | 0.720 |
| Subdoligranulum | 1.71 | 2.78 | 1.68 | 2.28 | 0.275 | 0.644 |
| Agathobacter | 1.80 a | 0.01 b | 0.83 ab | 0.01 b | 0.238 | 0.006 |
| Alloprevotella | 1.81 | 1.82 | 1.36 | 2.19 | 0.114 | 0.108 |
| Prevotella 2 | 1.56 | 2.09 | 2.25 | 2.08 | 0.114 | 0.179 |
| Acetitomaculum | 1.48 a | 0.89 b | 0.40 c | 0.63 bc | 0.127 | <0.001 |
| Clostridium_sensu_stricto_1 | 1.46 | 1.78 | 0.86 | 1.90 | 0.156 | 0.123 |
1 Bacterial taxa present at relative abundances of greater than 0.5% and the proportion of unidentified bacteria is 0.022%; 2 C = basal diet; 3 A = basal diet supplemented with 30 mg/kg bacitracin; 4 BLF = basal diet supplemented with 1 g/kg BLF; 5 AF = basal diet supplemented with 15 mg/kg bacitracin and 0.5 g/kg BLF; 6 SEM = standard error of mean; 7 adjusted p value: false discovery rate correction using the Benjamini–Hochberg procedure; 8 data are mean values of 3 replicate per treatment (2 piglets per replicate); a–c means that have no superscript in common are significantly different from each other (p < 0.05).
Figure 3.
Taxonomic composition analysis of cecal digesta. (A) The microbiota compositions at the genus level for 16S rRNA sequences in cecal digesta. Composition of the major taxonomic groups at the genus level in samples collected from the C, A, BLF, and AF groups (n = 3). (B) Species abundance heat map showing the abundance distribution of the dominant 35 genera (Y-axis) among bacterial communities between groups (X-axis) (n = 3). Values are normalized by Z-score.
3.4. Association between the Average Abundance of the Genera, Diarrhea Incidence, and Growth Performance
The Prevotella 9, Prevotellaceae_unclassified, Blautia, and Subdoligranulum genera abundances were negatively associated with the incidence of diarrhea, whereas the Lactobacillus, Lachnospiraceae_unclassified, Faecalibacterium, Streptococcus, and Anaerovibrio genera abundances were positively associated with the incidence of diarrhea (Figure 4a). The Prevotella 9 and Streptococcus genera abundances were negatively associated with body weight (BW) and the average daily weight gain (ADG). In contrast, the Faecalibacterium, Anaerovibrio, Blautia, and Subdoligranulum genera abundances were positively correlated with body weight and average daily weight gain. In addition, the Faecalibacterium, Blautia, and Subdoligranulum genera abundances were negatively correlated with the feed conversion ratio (FCR), whereas the Prevotella 9 genus abundances were positively correlated with the FCR (Figure 4b).
Figure 4.
The correlation analysis between cecal microbiota, diarrhea incidence, and growth performance in weaning piglets. (a) Relationship between diarrhea incidence and abundant genera. (b) Relationship between growth performance and abundant genera. The size and shade of each circle represents the magnitude of correlation. The blue circle and red circle represent positive and negative correlations, respectively.
4. Discussion
In this study, we demonstrated for the first time that the full or half replacement of bacitracin with BLF reduced the incidence of diarrhea in weaning piglets. The principal coordinates analysis of qualitative traits and a heat map of species abundance revealed distinct clusters between the groups treated with bacitracin alone, BLF alone, and bacitracin in combination with BLF. The Prevotellaceae NK3B31 group, Prevotellaceae_unclassified, and Anaerovibrio genera abundances were higher in the cecal digesta of the group treated with bacitracin in combination with BLF. The Prevotella 9, Prevotellaceae_unclassified, Blautia, and Subdoligranulum genera abundances in the cecal digesta were negatively correlated with the diarrhea incidence of weaning piglets.
Previous studies have generally focused on the effects of multi-strains of Bacillus species-based direct-fed microbes on the growth performance and diarrhea incidence in weaning piglets [15,17,22]. The combined use of B. licheniformis and B. subtilis in weaning piglets was previously found to ameliorate daily weight gain and the feed conversion ratio, as well as to reduce the morbidity and mortality rates associated with diarrhea [15]. The dietary supplementation of Bacillus species-based probiotics (B. lichenformis, B. subtilis, and Bacillus coagulans) improved the average daily weight gain and feed efficiency in growing–finishing pigs [22]. Furthermore, the dietary supplementation of a combined mixture of B. lichenformis and Clostridium butyricum decreased the diarrhea incidence in weaning piglets [17]. Supplementing a BLF in the diet of weaning piglets alleviated the diarrhea incidence [11]. In the present study, we demonstrated that, similar to the effects of antibiotics, replacing all the bacitracin with BLF still reduced diarrhea incidence in weaning piglets. The half replacement of bacitracin with BLF not only alleviated the diarrhea incidence but also had a tendency to increase the body weight and average daily weight gain of weaning piglets. The live or spore form of B. licheniformis is the most widely used formula of probiotics for the prevention of post-weaning diarrhea in the diet of weaning piglets [15,17,22,23]. B. licheniformis-derived metabolites, such as antimicrobial peptides, are generally not included in the diet of weaning piglets. Here, the fermented feed additive contained the live B. licheniformis and B. licheniformis-derived antimicrobial peptide surfactin. It has been reported that surfactin isolated from BLF can inhibit the growth of diarrhea-associated pathogens, such as Clostridium perfringens and Brachyspira hyodysenteriae [14,24]. Surfactin also exhibited an anti-inflammatory effect without cytotoxicity in vitro [25,26]. In addition to B. licheniformis-derived surfactin, B. licheniformis reduced the pathogens in the intestine by competitive exclusion and increased nutrient utilization [12,27]. The antibiotic used in the present study, bacitracin, can be produced by B. licheniformis and has potent antibacterial activity, primarily against Gram-positive organisms [28,29]. Therefore, compared with previous studies, the mechanism or efficiency of BLF on the improvement of daily weight gain and the alleviation of diarrhea incidence in weaning piglets may be different, as the half replacement of bacitracin with BLF resulted in a superior average daily weight gain in weaning piglets compared with the groups treated with bacitracin or BLF alone. B. licheniformis and surfactin in the fermented products is likely to still be active in the gastrointestinal tract when simultaneously supplemented with bacitracin. Taken together, these findings demonstrate that the dietary supplementation of BLF has beneficial effects on the prevention of post-weaning diarrhea in piglets. The effects of replacement of antibiotics with different concentrations of BLF on the growth performance and diarrhea incidence in weaning piglets remain to be investigated in the future. In addition, the effects of a half dose of antibiotic treatment alone, a half dose of BLF treatment alone, and a half dose of antibiotics in combination with a half dose of BLF treatment on weaning piglets still need to be confirmed.
The intestinal microbiota are also critical in providing resistance against pathogen colonization [30]. Pathogen invasion and environmental stress are associated with post-weaning diarrhea by altering the intestinal microbiota composition [31]. Antibiotics are known to reduce the incidence of post-weaning diarrhea by destroying pathogenic bacteria and changing the gut microbial composition in piglets [1]. Imbalanced fecal microbiota have been observed in response to antibiotic prophylaxis in poultry and pigs [11,32]. In addition to antibiotics, gut microbial composition and diversity can be shaped by probiotics [32]. Many countries have prohibited the use of antibiotic growth promoters as feed additives, but livestock has become more prone to infection by pathogens, thereby negatively affecting production. Therefore, it is imperative to explore effective alternatives to antibiotic growth promoters in infectious disease prevention in animal production. The effect of replacing or reducing antibiotics with probiotics on the performance and gut microbiota of pigs has been increasingly investigated [18,19,23,33]. It has been reported that the simultaneous supplementation of B. licheniformis and antibiotics cannot improve the growth performance of weaning piglets [23]. Body weight and gut microbiota have not been found to be affected by the supplementation of Bacillus species-based probiotics in combination with antibiotic administration in piglets during lactation and post-weaning [33]. Replacing all the antibiotics or the half replacement of antibiotics with B. amyloliquefaciens has been found to increase growth performance, antioxidant capacity, and digestive enzyme activity compared with the groups treated with antibiotics alone [18,19]. However, the half and total replacement of antibiotics with B. amyloliquefaciens was found to not alter the α-diversity of the microbial population in the feces of weaning piglets [19]. However, a recent study demonstrated that bacterial species richness and evenness in the feces decreased in weaning piglets fed BLF or antibiotics [11]. In the present study, we demonstrated that no difference was found in the bacterial species richness in the cecal digesta between groups. These findings imply that the BLF or bacitracin differently regulate the microbiota between cecal digesta and fecal content in weaning piglets. Replacing all the bacitracin with BLF reduced the bacterial species evenness compared with the control group, while the effects were reversed in the group treated with BLF in combination with bacitracin. Mirroring the results of a previous study [11], a principal coordinate analysis with quantitative traits showed that bacterial communities were clearly distinct in the cecal digesta for groups treated with bacitracin alone, BLF alone, or both the bacitracin and the BLF. These findings demonstrated that the full of half replacement of bacitracin with BLF can have a different impact on the cecal microbiota of weaning piglets.
The gut commensal Prevotella species mainly contribute to polysaccharide breakdown and short-chain fatty acid production in the gut of weaning piglets [34,35]. The Prevotella 9 genus is one of the dominant species in the gut and is associated with diarrhea incidence in weaning piglets. It has been demonstrated that the supplementation of fiber in the diet of piglets decreased the diarrhea incidence and increased the Prevotella 9 genus abundances [35]. The Prevotella 9 genus abundances in the fecal microbiota of diarrheic piglets were lower than those in non-diarrheic piglets [36]. The Prevotella 9 genus abundances in the feces are negatively associated with the diarrhea incidence in weaning piglets, and supplementation with BLF has been shown to be able to increase the abundance of the Prevotella 9 genus in the feces [11]. In the current study, the Prevotella 9 genus abundances in the cecal digesta were also negatively associated with the diarrhea incidence of weaning piglets. Since the highest abundance of the Prevotella 9 genus in the cecal digesta was not observed in the piglets with the superior growth performance (BLF in combination with the bacitracin-treated group), the Prevotella 9 genus abundances in the cecal digesta were not positively correlated with the growth performance of weaning piglets, indicating that the Prevotella 9 genus cannot be used as an indicator of growth performance in weaning piglets. Alternatively, the Blautia and Subdoligranulum genera in the cecal digesta may be potential indicators of growth performance and diarrhea incidence in weaning piglets. Previous studies have reported that the Blautia genus, a short-chain fatty acid-producing bacteria, is positively correlated with growth performance and negatively correlated with diarrhea incidence in weaning piglets [11,37]. The Subdoligranulum genus is known to be involved in the conversion of lactate to butyrate and has been positively associated with feed efficiency in pigs [38,39]. Taken together, the abundance of the Prevotella 9 genus in the gastrointestinal tract plays a critical role in the prevention of diarrhea incidence in weaning piglets. However, how these bacteria interact with each other in response to bacitracin or BLF or both to alleviate diarrhea in weaning piglets remains to be elucidated. Since the pH, short-chain fatty acid concentration, and pro-oxidative and anti-oxidative balance of the cecal digesta are associated with gut microbiota, whether the changes of microbiota in response to antibiotics or BLF or both in the cecal digesta of weaning piglets also regulate these parameters remain to be investigated.
5. Conclusions
These findings confirmed that the half replacement of bacitracin with BLF has beneficial effects on the reduction of diarrhea incidence in weaning piglets. Similar to bacitracin, the full or half replacement of bacitracin with BLF can reduce diarrhea incidence. Furthermore, BLF and bacitracin differentially modulate the cecal microbiota of weaning piglets. Therefore, based on our research, a Bacillus licheniformis-fermented feed additive could be a feasible alternative to antibiotics in the piglet diet for the reduction of antibiotic use.
Acknowledgments
The authors would like to thank Genomics (New Taipei City, Taiwan) for the 16S rRNA sequencing support.
Author Contributions
Conceptualization, Y.-H.Y.; methodology, K.-H.L. and Y.-H.Y.; validation, K.-H.L. and Y.-H.Y.; formal analysis, K.-H.L. and Y.-H.Y.; investigation, K.-H.L. and Y.-H.Y.; resources, Y.-H.Y.; data curation, Y.-H.Y.; writing—original draft preparation, Y.-H.Y.; writing—review and editing, Y.-H.Y.; supervision, Y.-H.Y.; project administration, Y.-H.Y.; funding acquisition, Y.-H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Council of Agriculture, grant number 109AS-12.1.2-ST-a4.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Vondruskova H., Slamova R., Trckova M., Zraly Z., Pavlik I. Alternatives to antibiotic growth promoters in prevention of diarrhoea in weaned piglets: A review. Vet. Med. Czech. 2010;55:199–224. doi: 10.17221/2998-VETMED. [DOI] [Google Scholar]
- 2.Dou S., Gadonna-Widehem P., Rome V., Hamoudi D., Rhazi L., Lakhal L., Larcher T., Bahi-Jaber N., Pinon-Quintana A., Guyonvarch A., et al. Characterisation of early-life fecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea. PLoS ONE. 2017;12:e0169851. doi: 10.1371/journal.pone.0169851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhouma M., Fairbrother J.M., Beaudry F., Letellier A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017;59:31. doi: 10.1186/s13028-017-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niewold T.A. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult. Sci. 2007;86:605–609. doi: 10.1093/ps/86.4.605. [DOI] [PubMed] [Google Scholar]
- 5.Khan R.U., Naz S. The applications of probiotics in poultry production. Worlds Poult. Sci. J. 2013;69:621–632. doi: 10.1017/S0043933913000627. [DOI] [Google Scholar]
- 6.Ahmed S.T., Hoon J., Mun H.S., Yang C.J. Evaluation of Lactobacillus and Bacillus-based probiotics as alternatives to antibiotics in enteric microbial challenged weaned piglets. Afr. J. Microbiol. Res. 2014;8:96–104. [Google Scholar]
- 7.Abudabos A.M., Al-Batshan H.A., Murshed M.A. Effects of prebiotics and probiotics on the performance and bacterial colonization of broiler chickens. S. Afr. J. Anim. Sci. 2015;45:419–428. doi: 10.4314/sajas.v45i4.8. [DOI] [Google Scholar]
- 8.Kritas S.K., Morrison R.B. Evaluation of probiotics as a substitute for antibiotics in a large pig nursery. Vet. Rec. 2005;156:447. doi: 10.1136/vr.156.14.447. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y., Dun Y., Li S., Zhao S., Peng N., Liang Y. Effects of Bacillus subtilis KN-42 on growth performance, diarrhea and faecal bacterial flora of weaned piglets. Asian-Australas J. Anim. Sci. 2014;27:1131–1140. doi: 10.5713/ajas.2013.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Y.H., Su L.W., Horng Y.B., Yu Y.H. Effects of soybean meal fermented by Lactobacillus species and Clostridium butyricum on growth performance, diarrhea incidence and fecal bacteria in weaning piglets. Ann. Anim. Sci. 2019;19:1051–1062. doi: 10.2478/aoas-2019-0042. [DOI] [Google Scholar]
- 11.Hung D.Y., Cheng Y.H., Chen W.J., Hua K.F., Pietruszka A., Dybus A., Lin C.S., Yu Y.H. Bacillus licheniformis-fermented products reduce diarrhea incidence and alter the fecal microbiota community in weaning piglets. Animals. 2019;9:1145. doi: 10.3390/ani9121145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozs M., Manczinger L., Vágvölgyi C., Kevei F. Secretion of a trypsin-like thiol protease by a new keratinolytic strain of Bacillus licheniformis. FEMS Microbiol. Lett. 2001;205:221–224. doi: 10.1111/j.1574-6968.2001.tb10951.x. [DOI] [PubMed] [Google Scholar]
- 13.Thaniyavarn J., Roongsawang N., Kameyama T., Haruki M., Imanaka T., Morikawa M., Kanaya S. Production and characterization of biosurfactants from Bacillus licheniformis F2.2. Biosci. Biotechnol. Biochem. 2003;67:1239–1244. doi: 10.1271/bbb.67.1239. [DOI] [PubMed] [Google Scholar]
- 14.Horng Y.B., Yu Y.H., Dybus A., Hsiao F.S., Cheng Y.H. Antibacterial activity of Bacillus species-derived surfactin on Brachyspira hyodysenteriae and Clostridium perfringens. AMB Express. 2019;9:188. doi: 10.1186/s13568-019-0914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexopoulos C., Georgoulakis I.E., Tzivara A., Kyriakis C.S., Govaris A., Kyriakis S.C. Field evaluation of the effect of a probiotic-containing Bacillus licheniformis and Bacillus subtilis spores on the health status, performance, and carcass quality of grower and finisher pigs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004;51:306–312. doi: 10.1111/j.1439-0442.2004.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W., Zhu Y.H., Zhou D., Wu Q., Song D., Dicksved J., Wang J.F. Oral administration of a select mixture of Bacillus probiotics affects the gut microbiota and goblet cell function following Escherichia coli challenge in newly weaned pigs of genotype MUC4 that are supposed to be enterotoxigenic E. coli F4ab/ac receptor negative. Appl. Environ. Microbiol. 2017;83:e02747-16. doi: 10.1128/AEM.02747-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zong X., Wang T.H., Lu Z.Q., Song D.G., Zhao J., Wang Y.Z. Effects of Clostridium butyricum or in combination with Bacillus licheniformis on the growth performance, blood indexes, and intestinal barrier function of weanling piglets. Livest. Sci. 2019;220:137–142. doi: 10.1016/j.livsci.2018.12.024. [DOI] [Google Scholar]
- 18.Wang Y., Wu Y., Wang B., Cao X., Fu A., Li Y., Li W. Effects of probiotic Bacillus as a substitute for antibiotics on antioxidant capacity and intestinal autophagy of piglets. AMB Express. 2017;7:52. doi: 10.1186/s13568-017-0353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu S., Cao X., Wu Y., Mei X., Xu H., Wang Y., Zhang X., Gong L., Li W. Effects of probiotic Bacillus as an alternative of antibiotics on digestive enzymes activity and intestinal integrity of piglets. Front. Microbiol. 2018;9:2427. doi: 10.3389/fmicb.2018.02427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trela J., Kierończyk B., Hautekiet V., Józefiak D. Combination of Bacillus licheniformis and salinomycin: Effect on the growth performance and GIT microbial populations of broiler chickens. Animals. 2020;10:889. doi: 10.3390/ani10050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng Y.H., Horng Y.B., Dybus A., Yu Y.H. Bacillus licheniformis-fermented products improve growth performance and intestinal gut morphology in broilers under Clostridium perfringens challenge. J. Poult. Sci. 2020 doi: 10.2141/jpsa.0200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balasubramanian B., Li T., Kim I.H. Effects of supplementing growing-finishing pig diets with Bacillus spp. probiotic on growth performance and meat-carcass grade quality traits. Revista Brasileira de Zootecnia. 2016;45:93–100. doi: 10.1590/S1806-92902016000300002. [DOI] [Google Scholar]
- 23.Collinder E., Cardona M.E., Berge G.N., Norin E., Stern S., Midtvedt T. Influence of zinc bacitracin and Bacillus licheniformis on microbial intestinal functions in weaned piglets. Vet. Res. Commun. 2003;27:513–526. doi: 10.1023/A:1026043623194. [DOI] [PubMed] [Google Scholar]
- 24.Lin E.R., Cheng Y.H., Hsiao F.S.H., Proskura W.S., Dybus A., Yu Y.H. Optimization of solid-state fermentation conditions of Bacillus licheniformis and its effects on Clostridium perfringens-induced necrotic enteritis in broilers. Revista Brasileira de Zootecnia. 2019;48:e20170298. doi: 10.1590/rbz4820170298. [DOI] [Google Scholar]
- 25.Park S.Y., Kim Y. Surfactin inhibits immunostimulatory function of macrophages through blocking NK-kappaB, MAPK and Akt pathway. Int. Immunopharmacol. 2009;9:886–893. doi: 10.1016/j.intimp.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Liu C., Dong B., Ma X., Hou L., Cao X., Wang C. Anti-inflammatory activity and mechanism of surfactin in lipopolysaccharide-activated macrophages. Inflammation. 2015;38:756–764. doi: 10.1007/s10753-014-9986-y. [DOI] [PubMed] [Google Scholar]
- 27.Xu S., Lin Y., Zeng D., Zhou M., Zeng Y., Wang H., Zhou Y., Zhu H., Pan K., Jing B., et al. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci. Rep. 2018;8:1744. doi: 10.1038/s41598-018-20059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernlohr R.W., Novelli G.D. Some characteristics of bacitracin production by Bacillus licheniformis. Arch. Biochem. Biophys. 1960;87:232–238. doi: 10.1016/0003-9861(60)90166-1. [DOI] [Google Scholar]
- 29.Butaye P., Devriese L.A., Haesebrouck F. Antimicrobial growth promoters used in animal feed: Effects of less well known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 2003;16:175–188. doi: 10.1128/CMR.16.2.175-188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickard J.M., Zeng M.Y., Caruso R., Núñez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017;279:70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gresse R., Chaucheyras-Durand F., Fleury M.A., Van de Wiele T., Forano E., Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y.C., Yu Y.H. Bacillus licheniformis–fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2019;99:1432–1443. doi: 10.1016/j.psj.2019.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poulsen A.R., Jonge N., Nielsen J.L., Højberg O., Lauridsen C., Cutting S.M., Canibe N. Impact of Bacillus spp. spores and gentamicin on the gastrointestinal microbiota of suckling and newly weaned piglets. PLoS ONE. 2018;13:e0207382. doi: 10.1371/journal.pone.0207382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flint H.J., Bayer E.A. Plant cell wall breakdown by anaerobic microorganisms from the mammalian digestive tract. Ann. N. Y. Acad. Sci. 2008;1125:280–288. doi: 10.1196/annals.1419.022. [DOI] [PubMed] [Google Scholar]
- 35.Adams S., Kong X., Jiang H., Qin G., Sossah F.L., Che D. Prebiotic effects of alfalfa (Medicago sativa) fiber on cecal bacterial composition, short-chain fatty acids, and diarrhea incidence in weaning piglets. RSC Adv. 2019;9:13586–13599. doi: 10.1039/C9RA01251F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J., Du L., Li X., Zhong H., Ding Y., Liu Z., Ge L. Identification of the core bacteria in rectums of diarrheic and non-diarrheic piglets. Sci. Rep. 2019;9:18675. doi: 10.1038/s41598-019-55328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J.J., Gao M.X., Song X.J., Zhao L., Li Y.W., Hao Z.H. Changes in bacterial diversity and composition in the faeces and colon of weaned piglets after feeding fermented soybean meal. J. Med. Microbiol. 2018;67:1181–1190. doi: 10.1099/jmm.0.000766. [DOI] [PubMed] [Google Scholar]
- 38.Brown C.T., Davis-Richardson A.G., Giongo A., Gano K.A., Crabb D.B., Mukherjee N., Casella G., Drew J.C., Ilonen J., Knip M., et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE. 2011;6:e25792. doi: 10.1371/journal.pone.0025792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reyer H., Oster M., McCormack U.M., Muráni E., Gardiner G.E., Ponsuksili S., Lawlor P.G., Wimmers K. Host-microbiota interactions in ileum and caecum of pigs divergent in feed efficiency contribute to nutrient utilization. Microorganisms. 2020;8:563. doi: 10.3390/microorganisms8040563. [DOI] [PMC free article] [PubMed] [Google Scholar]