Abstract
Background
A previous RTS,S/AS01B vaccine challenge trial demonstrated that a 3-dose (0-1-7–month) regimen with a fractional third dose can produce high vaccine efficacy (VE) in adults challenged 3 weeks after vaccination. This study explored the VE of different delayed fractional dose regimens of adult and pediatric RTS,S/AS01 formulations.
Methods
A total of 130 participants were randomized into 5 groups. Four groups received 3 doses of RTS,S/AS01B or RTS,S/AS01E on a 0-1-7–month schedule, with the final 1 or 2 doses being fractional (one-fifth dose volume). One group received 1 full (month 0) and 1 fractional (month 7) dose of RTS,S/AS01E. Immunized and unvaccinated control participants underwent Plasmodium falciparum–infected mosquito challenge (controlled human malaria infection) 3 months after immunization, a timing chosen to potentially discriminate VEs between groups.
Results
The VE of 3-dose formulations ranged from 55% (95% confidence interval, 27%–72%) to 76% (48%–89%). Groups administered equivalent formulations of RTS,S/AS01E and RTS,S/AS01B demonstrated comparable VE. The 2-dose group demonstrated lower VE (29% [95% confidence interval, 6%–46%]). All regimens were well tolerated and immunogenic, with trends toward higher anti-circumsporozoite antibody titers in participants protected against infection.
Conclusions
RTS,S/AS01E can provide VE comparable to an equivalent RTS,S/AS01B regimen in adults, suggesting a universal formulation may be considered. Results also suggest that the 2-dose regimen is inferior to the 3-dose regimens evaluated.
Clinical Trial Registration
Keywords: RTS, S/AS01, delayed fractional dose, 3-month challenge, Plasmodium falciparum, malaria, immunogenicity, safety, efficacy, controlled human malaria infection
We explored the ability of different delayed fractional dose-regimens of RTS,S/AS01-formulations to increase vaccine efficacy using CHMI. The pediatric RTS,S/AS01E-formulation was comparably efficacious with an equivalent RTS,S/AS01B regimen in adults. The two-dose regimen evaluated was inferior to the three-dose regimens.
After a period of unprecedented success in reducing in morbidity and mortality rates from Plasmodium falciparum malaria, from 2000 to 2015, progress has stalled. In recent years, malaria incidence has increased in high-disease-burden countries, as have parasite resistance to antimalarial drugs and mosquito resistance to insecticides [1]. In 2018 alone, an estimated 228 million malaria cases and 405 000 deaths worldwide due to malaria occurred [1]. An effective malaria vaccine could potentially help offset these roadblocks and provide an important tool for malaria control, especially in young children. In addition, if the vaccine can be given to prevent infection in all age groups, it can reduce community transmission and help achieve malaria elimination [1, 2].
To date, RTS,S/AS01 is the only malaria vaccine to receive a positive regulatory review and opinion from the European Medicines Agency, based on the results of a large phase III evaluation that demonstrated moderate vaccine efficacy (VE) against malaria [3–5]. The vaccine is currently being evaluated for its potential use as a seasonal malaria vaccine in young children [6, 7], and in a large-scale pilot implementation program under the Expanded Programme of Immunisation. The latter is conducted in young children from 3 countries in sub-Saharan Africa to further assess safety, logistical feasibility, and impact against severe disease and death in a real-life setting [8].
Nevertheless, the continued development of a malaria vaccine will be a key component of future integrated malaria control and elimination programs [2]. Developmental efforts are continuing to improve the efficacy and durability of RTS,S/AS01, using fractional doses of vaccine and modifications in both schedule and number of doses, in an attempt to optimize impact and minimize costs of manufacture and delivery. It is critical to evaluate whether the pediatric RTS,S/AS01E vaccine formulation, which contains half of the active ingredients compared with the adult RTS,S/AS01B formulation, elicits comparable efficacy against infection when used in persons of all ages, including adults who contribute to onward transmission, because this would allow for wider use.
Controlled human malaria infection (CHMI) vaccine studies, including those of RTS,S/AS01 in malaria-naive adults, have greatly accelerated the evaluation of malaria vaccines and allowed further evaluation of successful candidates in the field [9–11]. CHMI has historically been tested within 3–4 weeks after the final immunization [12, 13]. We reasoned that performing a malaria challenge several months after the last RTS,S/AS01 immunization would provide data regarding the durability of the protective response as well as providing a useful barometer to measure subtle differences between vaccine groups that differ in the number of vaccine doses and size of the dose used at each immunization.
Recently, use of a fractional third dose of RTS,S/AS01B (one-fifth dose volume), and changes in the schedule of administration from 0, 1, 2 and months to 0, 1, and 7 months resulted in very high levels of protection in a cohort of adults when CHMI was performed 3 weeks after final vaccination [14]. There is also evidence from nonmalaria vaccine studies that reducing vaccine antigen concentrations does not result in inferior immunogenicity [15, 16]. We therefore speculated that applying a delayed fractional dose regimen to the pediatric formulation (RTS,S/AS01E) might provide improved protection In this article, we present results of a study where we explored VE of different delayed fractional dose regimens of adult (RTS,S/AS01B) and pediatric (RTS,S/AS01E) formulations of RTS,S/AS01.
METHODS
Study Design
This trial was a phase IIa, open-label, randomized, controlled, single-center study performed at the Walter Reed Army Institute of Research in Silver Spring, Maryland. The protocol was approved by the Walter Reed Army Institute of Research Institutional Review Board. The study was conducted according to the protocol and in compliance with International Conference on Harmonization/Good Clinical Practice guidelines. Written informed consent was obtained from each participant before study procedures were initiated (ClinicalTrials.gov identifier: NCT03162614).
Study Participants
Men and nonpregnant, nonlactating women, military and civilian, aged 18–55 years (inclusive), were recruited from the Baltimore–Washington, DC, region by noncoercive means to participate in this trial. Participants were eligible for inclusion if they were able to consent and comply with study procedures, were in good general health and without any serious acute or chronic illness as determined by history, physical examination, and laboratory screening tests, were without allergies or prior reactions to any component of the study vaccines, and did not have a history of malaria, malaria vaccination, or recent malaria exposure and/or use of drugs with antimalarial properties. Refer to the Supplementary Materials for additional details.
Study Vaccines and Vaccination
RTS,S/AS01 is manufactured by GSK Biologicals (GSK). RTS,S/AS01B, the adult formulation, contains 50 µg of RTS,S [17], along with AS01B, an adjuvant system containing 50 µg of Monophosphoryl Lipid A, 50 µg of QS-21 (Quillaja saponaria Molina, fraction 21; licensed by GSK from Antigenics, a wholly owned subsidiary of Agenus), and liposome [18, 19] in a 0.5-mL dose. RTS,S/AS01E, the pediatric formulation, contains 25 µg of RTS,S and an adjuvant system AS01E, (25 µg of MPL, QS-21, and liposomes) in a 0.5-mL dose. Fractionated dosages were 0.1 mL and thus contained one-fifth of the antigen and respective adjuvants present in the full dose. Vaccines were administered intramuscularly in the deltoid muscle of the arm. All 3-dose groups (AduFx, 2PedFx, Adu2Fx, and PedFx) were immunized on a 0-1-7–month schedule, and the Adu1Fx group at 0 and 7 months (Table 1).
Table 1.
Vaccine Dose Details for All Study Treatment Groups
| Study Group | Vaccination Months | RTS,S Antigen Administered, μg | Adjuvant Administered, μg | Volume Administered per Vaccination, mL |
||
| Per Vaccination | Total | Per Vaccination | Total | |||
| AduFx | 0-1-7 | 50-50-10 | 110 | 50-50-10 | 110 | 0.5-0.5-0.1 |
| 2PedFx | 0-1-7 | 50-50-10 | 110 | 50-50-10a | 110 | 1.0-1.0-0.2 |
| PedFx | 0-1-7 | 25-25-5 | 55 | 25-25-5 | 55 | 0.5-0.5-0.1 |
| Adu2Fx | 0-1-7 | 50-10-10 | 70 | 50-10-10 | 70 | 0.5-0.5-0.1 |
| Adu1Fx | 0-…-7 | 50-…-10 | 60 | 50-…-10 | 60 | 0.5-…-0.1 |
aAdministered in 1.0ml (double) doses.
Study Treatments
Study participants were assigned to 1 of 5 treatment groups (Table 1). Participants were allocated to study groups using a randomization system on the internet (SBIR).
Efficacy Assessment
All vaccinated study participants, along with an infectivity control group that did not receive any study vaccine, were subjected to a CHMI challenge 3 months after the last vaccination. We opted for delayed CHMI, instead of challenging participants 3–4 weeks after the final vaccine dose. We reasoned that the delay could provide a measure of vaccine protective durability in the context of logistical feasibility and could potentially better differentiate efficacy and immunogenicity between the different treatment groups.
Details of the CHMI methods have been published elsewhere [12, 20]. Briefly, all participants were challenged through the bite of 5 Anopheles stephensi mosquitoes infected with P. falciparum (3D7, a clone of the NF54 strain) and were subsequently assessed for the presence of parasitemia by means of daily thick blood smear reading (as described elsewhere [21]) from 5 to 19 days after CHMI, and then every other day up to 28 days after CHMI. Participants with confirmed malaria infection were treated with standard courses of chloroquine phosphate or atovaquone-proguanil.
Safety Assessments
Solicited local (injection site) and general (systemic) adverse events (AEs) were monitored for 7 days after each vaccination. AEs were graded 1–3, with grade 3 indicating severities preventing normal everyday activities, redness or swelling >100 mm or fever >39.0°C (>102.1°F). Unsolicited AEs were recorded for 30 days after each vaccination and after CHMI. Serious AEs were captured for the duration of the study. All solicited local AEs were considered causally related to vaccination. Causality of other AEs and serious AEs was assessed by the investigator. Hematological and biochemical tests for safety assessment were performed at various time points during the trial.
Immunogenicity Assessments
Antibody levels against the R32LR circumsporozoite protein (CSP) repeat region were measured using standard enzyme-linked immunosorbent assays (ELISAs) [22]. Antibody levels against full-length recombinant CSP and C-terminal CSP pf16 peptide were assessed by ELISA [22]. Further details are presented in the supplementary materials.
To demonstrate the avidity of the antibodies in the assay, we used 1 mol/L ammonium thiocyanate (anti-CSP repeat region and 4 mol/L urea (full-length and C-terminal CSP) as a chaotropic reagent in ELISA-based avidity assays, as described elsewhere [22]. The avidity index was calculated as the ratio of antibody concentrations with or without chaotropic reagent. Levels of antibody against the hepatitis B surface antigen component of the vaccine (anti-HBs) were measured by means of chemiluminometric immunoassay (Centaur; Siemens Healthcare).
Statistical Analyses
All analyses were conducted using SAS software in SAS Drug Development, in accordance with the predefined analysis plan. The sample size was determined based on logistical feasibility and the ability to conduct the CHMI and follow-up of the study participants safely. The primary end point was the occurrence of confirmed P. falciparum parasitemia (defined by a positive blood smear) after sporozoite challenge in each study group versus infectivity controls. The study power assumptions and the objectives are presented in the Supplementary Material.
All safety analyses were descriptive and performed on the intent-to-treat set that included all participants who received ≥1 dose of a study vaccine and the infectivity controls. Analyses for efficacy and immunogenicity were performed on the per-protocol set, which included all participants in the intent-to-treat set who received all vaccinations in accordance with procedures, requirements and limitations specified in the study protocol, who underwent the P. falciparum challenge, and for whom relevant data were available. All P values were computed for informative purposes and were not adjusted for multiplicity. Further details on statistical analyses are available in the Supplementary Materials.
Data Sharing
The results of this study are available on the GSK Clinical Study Register (www.gsk-clinicalstudyregister.com). For interventional studies that evaluate our medicines, anonymized patient-level data will be made available to independent researchers, subject to review by an independent panel, at www.clinicalstudydatarequest.com within 6 months of publication.
RESULTS
Demographic Characteristics
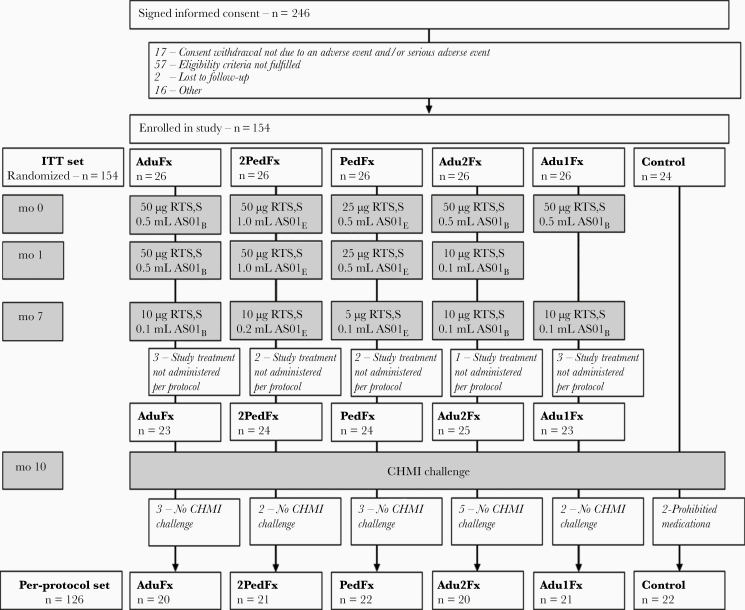
A total of 154 participants were enrolled in this trial (26 in each vaccine group and 24 infectivity controls), and 126 participants underwent the CHMI challenge (Figure 1). Participants were predominantly white and male, and their mean age was 31.1 years. Details are provided in Supplementary Table 1.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram. Boxes in gray indicate treatment procedures. The intent-to-treat (ITT) set included all participants who received ≥1 dose of a study vaccine and the infectivity controls and was used for analyses of safety. The per-protocol set included all participants in the ITT set who received all vaccinations in accordance with procedures, requirements and limitations specified in the study protocol, who underwent the Plasmodium falciparum challenge, and for whom relevant data were available. AduFx, Adu2Fx, Adu1Fx, PedFx, and 2PedFx indicate study treatment groups. Treatment differences are presented in the individual boxed for months 0, 1, and 7. The protocol for controlled human malaria infection (CHMI) is presented in Methods. Reasons for withdrawal categorized as “Other” include travel, missed vaccination, exclusion criteria met, inability to participate in challenge, pregnancy, and family emergency. Two participants in the infectivity control group were administered presumptive antimalaria medication prohibited in the study protocol (prohibited medication).
Determining VE
After CHMI at 3 months after the last vaccination, 22 of 24 infectivity controls (91.7%), and 46 of 104 vaccinated participants(44.2%) presented with confirmed P. falciparum infection. Of note, 2 participants in the infectivity control group were not included in the per-protocol analysis for VE, because they withdrew their consent on days 17 and 20 after challenge and were presumptively treated with antimalarial medication. Both participants remained negative for smear-confirmed parasitemia until consent withdrawal. The VE for the intent-to-treat set that includes these participants is presented in Supplementary Figure 1.
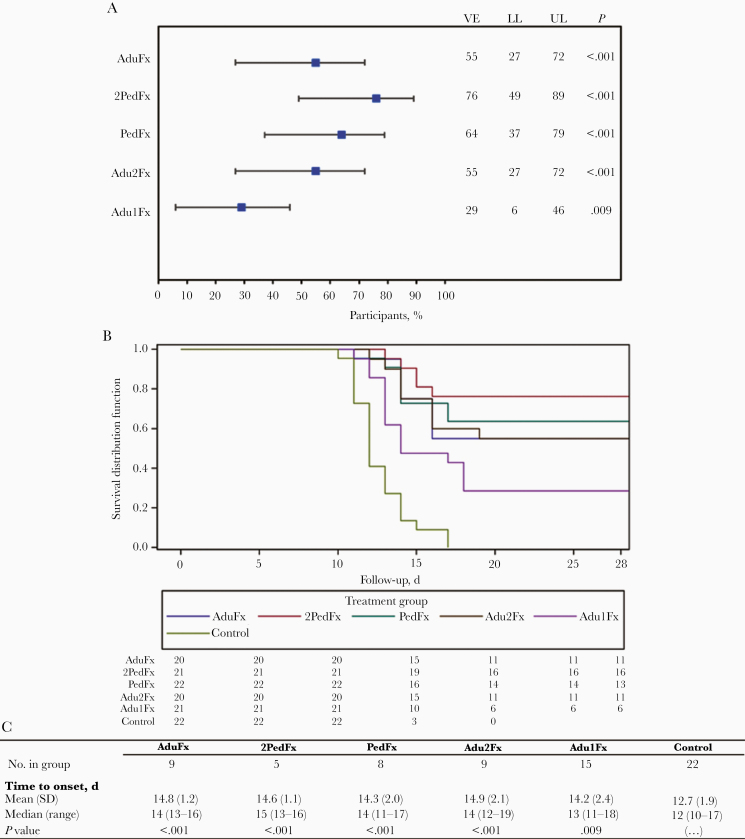
Confirmed parasitemia was significantly reduced in participants receiving a 3-dose regimen compared to infectivity controls (P < .001 for all groups). The VE of all 3-dose regimens with the adult or pediatric RTS,S formulations ranged from 55% (95% confidence interval, 27%–72%) to 76% (49%–89%). VE was lower in the 2-dose Adu1Fx group (29% [95% confidence interval, 6%–46%]; P = .009) than in controls (Figure 2A).
Figure 2.
Vaccine efficacy (VE) and occurrence of parasitemia. A, VE in the prevention of confirmed Plasmodium falciparum parasitemia for all 5 study groups. Error bars indicate 95% confidence intervals (CIs). B, Kaplan-Meier survival curve for the time to onset of confirmed P. falciparum parasitemia. C, Mean times to onset of confirmed P. falciparum parasitemia in all study groups. P values represent comparison with the infectivity control group. Abbreviations: LL, lower limit of the 95% CI; UL, upper limit of the 95% CI.
Participants in the 2PedFx group received the same concentrations of the RTS,S antigen and the AS01 adjuvant system as participants in the AduFx group, and the VEs were comparable (Figure 2A). The AduFx group were administered the same regimen as participants in the previous study assessing VE in response to administration of a delayed fractional dose [22]).
There were no apparent differences in VE whether 2 fractional doses of RTS,S/AS01B or various dosages of the pediatric formulations were used. Time to onset of parasitemia was delayed in all study groups compared with infectivity controls (Figure 2B and 2C and Supplementary Figure 1) with a difference in survival time between the 2-dose and 3-dose groups.
Immunogenicity
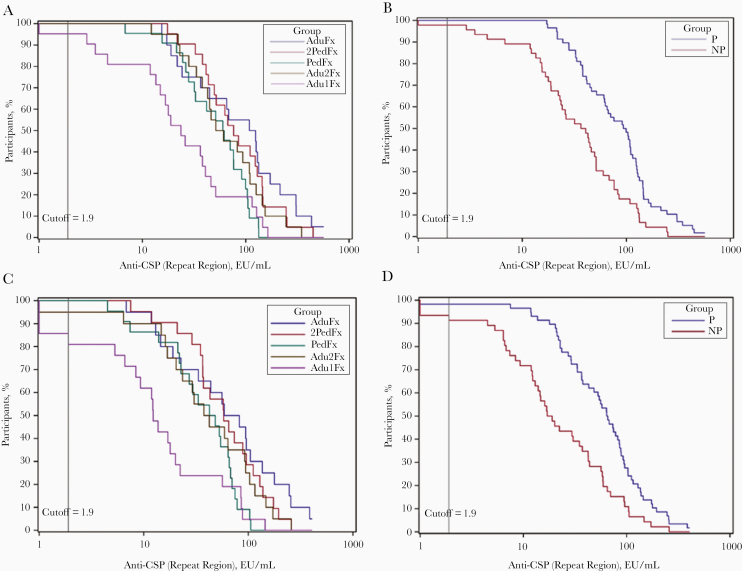
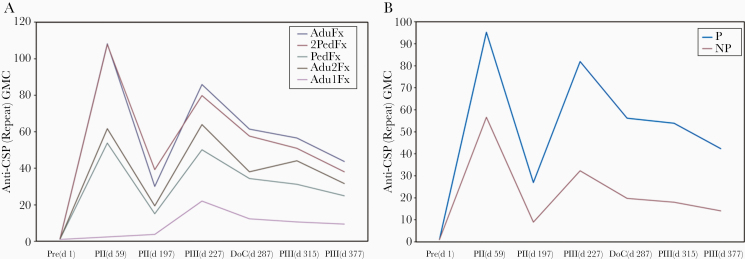
All immunizations with 3 doses induced anti-CSP (repeat region) antibody responses by the second vaccine dose (Table 2 and Figure 3). Anti-CSP (repeat region) antibody concentrations decreased over time but increased after the fractional dose at month 7 to levels comparable to those seen after the second dose (Table 2). These kinetics were comparable for all groups (Figure 4 and Supplementary Figure 2). On the day of challenge, no differences in anti-CSP (repeat region) antibodies were apparent between groups that received 3 vaccine doses. Anti-CSP geometric mean concentrations were lower in participants in the 2-dose group at all time points tested. This difference was statistically significant compared with the AduFx group (P < .001; data not shown). All participants in the 3-dose groups were seropositive for anti-CSP (repeat region) antibodies 1 month after the final vaccination, whereas in the 2-dose group only 95% of participants were seropositive at that time point.
Table 2.
Anti–Plasmodium falciparum Circumsporozoite Protein (Repeat Region) and Anti–Hepatitis B Surface Antigen Antibody Response
| Geometric Mean Concentration (95% Confidence Interval)) | |||||
|---|---|---|---|---|---|
| Antibody Response | AduFx | 2PedFx | PedFx | Adu2Fx | Adu1Fx |
| Anti-CSP (repeat region) | n = 20 |
n = 21 |
n = 22 |
n = 20 |
n = 21 |
| Pre | 1.0 (1.0–1.0) |
1.0 (1.0–1.0) |
1.0 (1.0–1.0) |
1.0 (1.0–1.0) |
1.0 (1.0–1.0) |
| mo 2 | 108.2 (70.1–167.0) |
107.8 (73.0–159.1) |
53.8 (39.0–74.1) |
61.7 (47.6–79.9) |
… |
| mo 7 | 30.0 (17.9–50.5) |
39.3 (23.8–64.8) |
15.1 (9.3–24.5) |
19.4 (13.2–28.5) |
3.8 (2.0–7.2) |
| mo 8 | 85.9 (50.8–145.5) |
79.8 (54.7–116.6) |
50.2 (35.5–70.9) |
64.0 (42.8–95.8) |
22.1 (12.0–40.8) |
| mo 10 (DoC) | 61.4 (34.7–108.8) | 57.6 (38.2–87.1) |
34.4 (23.1–51.1) |
38.1 (20.8–69.9) |
12.3 (6.2–24.4) |
| mo 11 | 56.6 (32.5–98.4) |
51.0 (33.3–78.0) |
31.3 (21.2–46.3) |
44.1 (27.8–70.1) |
10.6 (5.2–21.5) |
| mo 13 | 43.7 (24.9–76.8) | 38.1 (24.3–59.8) | 25.0 (16.5–37.9) | 31.7 (19.6–51.3) | 9.5 (4.8–19.0) |
| Anti-HBS | n = 20 | n = 21 | n = 21 | n = 20 | n = 21 |
| Pre | 44.1 (15.6–124.3) |
68.8 (20.4–231.7) | 45.7 (15.2–137.5) | 21.8 (9.0–52.6) | 16.0 (6.5–39.6) |
| mo 2 | 42 616.7 (12 325.1–147 356.7) |
28 894.8 (8705.8–95 903.3) | 26 149.3 (8588.7– 79 615.2) | 14 710.6 (3837.0–56 398.8) | … |
| mo 7 | 19 369.3 (8325.5–45 062.6) |
15 358.8 (5971.2–39 505.1) |
11 269.1 (4892.1–25 958.8) | 6846.7 (2273.5–20 618.8) | 2031.7 (715.9–5766.4) |
| mo 8 | 45 959.9 (27 093.8–77 962.8) |
30 994.5 (15 352.8–62 572.4) |
28 560.6 (15 532.4–52 516.4) | 26 717.0 (12 916.3–55 263.3) | 35 620.9 (22 337.4–56 803.7) |
| mo 10 (DoC) | 36 266.3 (21 828.7–60 252.9) |
20 712.2 (10 050.4–42 684.5) |
19 126.1 (9889.0–36 991.5) | 13 911.3 (4939.4–39 179.9) | 16 376.8 (9798.1–27 372.8) |
| mo 11 | 33 027.7 (19 159.7–56 933.4) |
18 527.8 (8862.8–38 732.5) |
17 465.5 (8798.2–34 671.2) | 16 685.0 (8192.0–33 982.9) | 12 609.9 (7499.3–21 203.5) |
| mo 13 | 27 823.0 (15 910.1–48 655.8) | 15 083.7 (6953.8–32 718.4) | 13 716.0 (6456.7–29 136.9) | 11 257.6 (5164.7–24 538.5) | 10 975.2 (5889.9–20 451.3) |
Abbreviations: Anti-HBs, antibody to hepatitis B surface antigen; CSP, circumsporozoite protein; DoC, day of controlled human malaria infection challenge; Pre, prevaccination sampling time point.
Figure 3.
Reverse cumulative distribution curves for anti–circumsporozoite protein (CSP) (repeat region) antibodies, by treatment and protection status against the controlled human malaria infection (CHMI) challenge. A, Reverse cumulative curves for anti-CSP (repeat region) antibodies for all 5 treatment groups 1 month after administration of the delayed fractional dose. B, Reverse cumulative curves for anti-CSP (repeat region) antibodies for all participants stratified by protection status 1 month after administration of the delayed fractional dose. C, Reverse cumulative curves for anti-CSP (repeat region) antibodies for all 5 treatment groups on the day of CHMI challenge. D, Reverse cumulative curves for anti-CSP (repeat region) antibodies for all participants stratified by protection status on the day of CHMI challenge. Abbreviations: EU, enzyme-linked immunosorbent assay units; NP, stratified group of participants not protected against malaria in the CHMI challenge; P, stratified group of participants protected against malaria in the CHMI challenge.
Figure 4.
Anti–Plasmodium falciparum circumsporozoite protein (CSP) antibody response, by treatment and protection status against the controlled human malaria infection challenge. A, Anti-CSP (repeat region) antibody geometric mean concentrations (GMCs) for all 5 treatment groups. B, Anti-CSP (repeat region) antibody GMCs for all participants stratified by protection status. Abbreviations: Abbreviations: NP, stratified group of participants not protected against malaria in the CHMI challenge; P, stratified group of participants protected against malaria in the CHMI challenge; Pre(d1), prevaccination (day 1) sampling time point; PII(d59), day 59 (month 2) sampling time point; PII(d197), day 197 (month 7) sampling time point; PIII(d227), day 227 (month 8) sampling time point 1 month after administration of the delayed fractional dose; DoC (d287), day 287 (month 10) sampling time point (day of CHMI challenge); PIII(d315), day 315 (month 11) sampling time point; PIII(d377), day 377 (month 13) sampling time point.
The kinetics of the anti-CSP (full-length) and anti-CSP (C-terminus) responses followed a comparable pattern for all groups and decreased over time after the month 7 immunization (Supplementary Table 2). Irrespective of treatment group, all protected individuals had significantly higher anti-CSP (repeat region) concentrations than the unprotected participants from 1 month after the final vaccination until the study’s end (P < .001).
Anti-CSP (repeat region) avidity indices at 1 month after the final vaccination and on the day of the CHMI were within the same range for all study groups, and no differences were apparent between protected and unprotected participants (Supplementary Figure 3). Findings were comparable for anti-CSP (full-length and C-terminus) antibody avidity (Supplementary Figure 4).
Anti-HBs antibody geometric mean concentrations markedly increased after vaccination in all study groups (Table 2), and all participants were seroprotected 1 month after the last vaccine dose. Anti-HBs antibody concentrations did not differ between participants who were protected and those who were unprotected after CHMI (Supplementary Figure 5).
Safety
All vaccine regimens were well tolerated. The percentages of solicited and unsolicited AEs reported after each vaccine dose were comparable between groups (Table 3). Injection site pain was the most frequently reported solicited local AE, and fatigue and headache were the most frequently reported solicited general AEs. Detailed on local and general solicited AEs are presented in Supplementary Tables 3 and 4. Chills and upper respiratory tract infections were the most frequently reported unsolicited AEs after vaccination (Supplementary Table 5). No AEs were reported after the challenge, and no serious AEs were reported during the study period. No clinically significant abnormalities were seen in hematological or biochemical parameters.
Table 3.
Solicited and Unsolicited Adverse Events Reported Within 7 Days After Vaccine Dose
| Adverse Events | Adverse Event, % (95% Confidence Interval) | ||||
|---|---|---|---|---|---|
| AduFx (75 Doses) | 2PedFx (75 Doses) |
PedFx (76 Doses) | Adu2Fx (77 Doses) |
Adu1Fx (49 Doses) | |
| Any event | |||||
| Any symptom | 85 (75–92) |
88 (78–94) |
82 (71–90) |
82 (71–90) |
82 (68–91) |
| Any local symptom | 77 (66–86) |
76 (65–85) |
78 (67–86) |
70 (59–80) |
76 (61–87) |
| Any general symptom | 60 (48–71) | 72 (60–82) | 59 (47–70) | 53 (42–65) | 57 (42–71) |
| Grade 3 event | |||||
| Any symptom | 4 (1–11) |
5 (1–13) | 11 (5–20) | 3 (0–9) | 10 (3–22) |
| Any local symptom | 0 (0–5) |
1 (0–7) | 4 (1–11) | 0\ (0–5) | 2 (0–11) |
| Any general symptom | 4 (1–11) | 4 (1–11) | 8 (3–16) | 3 (0–9) | 8 (2–20) |
| Event causally related to vaccination | |||||
| Any symptom | 85 (75–92) |
87 (77–93) | 82 (71–90) |
79 (68–88) | 82 (68–91) |
| Any local symptom | 77 (66–86) |
76 (65–85) | 78 (67–86) |
70 (59–80) | 76 (61–87) |
| Any general symptom | 59 (47–70) | 65 (53–76) | 55 (43–67) | 47 (35–58) | 55 (40–69) |
Discussion
This clinical trial was conducted to further explore the findings of a previous clinical trial that showed a substantial increase in VE when the last dose of a 3-dose regimen was fractionated to one-fifth the standard dose and delayed to month 7 instead of month 2 [14]. In the current study, we investigated 5 delayed fractional dose vaccine regimens using different vaccine doses and schedules to evaluate their impact on vaccine immunogenicity and VE after a CHMI challenge conducted 3 months after the administration of the final vaccine dose. Our results show that all vaccine regimens were efficacious and immunogenic, but, compared to 3-dose regimens (2 full doses followed by a delayed fractional dose), VE and anti-CSP (repeat region) antibody concentrations were reduced in participants who were only administered 2 vaccine doses (1 full dose and a delayed fractional dose). Interestingly, groups that were administered 3 vaccine doses showed comparable efficacy, irrespective of whether they received the adult (RTS,S/AS01B) or pediatric (RTS,S/AS01E) vaccine dosage.
Antibody responses to the NANP-repeat region of CSP were elicited by all vaccine schedules. While the antibody concentrations at day of challenge was lower in the PedFx and 2PedFx groups that received 3 doses of RTS,S/AS01E than in the AduFx group, only responses of the 2-dose group was significantly lower than in the AduFx group. A trend for lower antibody responses was found in unprotected participants and particularly in those of the 2-dose group. Following the kinetics of the antibody response, concentrations declined gradually and were increased again after the third dose. However, the third fractional dose could not increase the antibody response above the response induced by the second full dose.
The impact of dose reductions on vaccine immunogenicity has previously been investigated, and results from nonmalaria vaccine studies have indicated that antigen dosage may be substantially reduced without negative effects on immunogenicity [15, 16]. In the current study, dose reductions, providing either 1 full adult dose of RTS,S/AS01B and 2 fractional doses (Adu2Fx) or 2 full pediatric doses of RTS,S/AS01E and a fractional pediatric dose (PedFx) did not seem to affect VE and the delay to parasitemia, but these findings warrant confirmation in field trials.
Moreover, this similarity in VE between the 3-dose regimens is remarkable in light of the delay in the execution of the CHMI challenge. We reasoned that by extending the delay between the third (fractional) vaccine dose and the CHMI challenge from 3 weeks after the final vaccination to 3 months, we would better be able to assess trends in efficacy associated with vaccine regimen difference. As expected owing to decay in VE over time, when CHMI occurred 3 months after completion of the vaccination regimen, VE in our 3-dose delayed fractional dose groups was lower than that seen in a previous study where challenged occurred 3 weeks after completion of the vaccination course [14]. Interestingly, both VE and the delay to parasitemia were comparable between groups for all 3-dose treatment regimens, suggesting that reductions in RTS,S/AS01 antigen and adjuvant content may be viable without compromising protection against malaria. We are currently evaluating whether an additional fractional dose administered 1 year after the last vaccination can restore VE in a CHMI model.
VE findings in the different treatment groups are reflective of the induced anti-CSP antibody responses. In line with a very large body of work, RTS,S/AS01 induced a robust immune response in all treatment groups. Compared with 3-dose regimens, the 2-dose RTS,S/AS01B regimen induced a substantially lower anti-CSP (repeat region) response that may be associated with the reduction in VE seen in that group. Further supporting the association between anti-CSP (repeat region) antibody geometric mean concentrations and VE, antibody concentrations were lower in individuals in whom parasitemia developed after CHMI challenge. These data support and add to prior studies of RTS,S showing that efficacy can be associated with anti-CSP (repeat region) antibody concentration [12, 14, 23, 24]. A true correlate or immunological surrogate is lacking and may implicate additional immunological effector mechanisms that are being investigated in other studies.
Consistent with findings of prior studies, the highest anti-CSP (repeat region) concentrations in the AduFx and 2PedFx groups, who received the highest cumulative dosages of antigen and adjuvant, were observed after the second immunization and were not boosted after the third immunization beyond that observed after 2 doses. Within the constraints set by the relatively small sample sizes of treatment groups, it is notable that the relative increase in anti-CSP (repeat region) antibodies after the last fractional dose was greatest in the Adu2Fx group, which received 2 fractional doses of RTS,S/AS01B at both the second and third immunizations, and the PedFx group, which received the lowest cumulative amounts of vaccine over the entire 3-dose regimen.
We previously hypothesized that the increased VE might be explained by an increase in anti-CSP (repeat region) avidity, because higher avidity was observed with the delayed fractional dose regimen compared with the standard-dose 0-1-2–month regimen [14]. In our trial, we were unable to substantiate this hypothesis, because anti-CSP (repeat region, full-length, and C-terminus) antibody avidity indices were all within the same range for all treatment groups, and antibody avidity indices did not substantially differ between protected and unprotected participants. Although we did not have a comparator group receiving 3 full doses on a 0-1-2–month schedule, and we therefore cannot directly verify or refute the previous hypothesis, antibody avidity indices in our study were comparable to those seen in the month 0-1-7–month delayed fractional dose group [14].
Of note, as with antibody concentrations, antibody characteristics have not been consistently associated with protection. Avidity of NANP-specific antibodies, evaluated in field trials with (full dose) RTS,S/AS01E in 0-1-2– and 0-1-7–month vaccine regimens, was found not to be associated with protection from clinical malaria in children [25, 26]. In contrast, it was shown that protection against clinical malaria disease in RTS,S/AS01E-vaccinated infants and children enrolled in the RTS,S/AS01 phase 3 efficacy trial could be explained by anti-CSP immunoglobulin G concentrations and avidity and that these are affected by age, site, and prevaccination levels [27, 28]. Because the antibody concentrations and antibody avidities induced by the fractional dose schedules did not differ significantly between protected and unprotected participants, investigations on antibody functionality are needed to discover and validate potential correlates of protection.
The association between RTS,S/AS01-induced immune responses and VE is complicated and needs to be better understood to further improve RTS,S/AS01-induced protection against malaria. Work is underway to evaluate the function of anti-CSP antibodies, a deeper appreciation of the fine specificity of NANP repeat region and C-terminal antibodies, as well as investigations on the early cellular immune mechanisms leading to antibody production after a delayed fractional dose of RTS,S/AS01B.
In conclusion, in the largest CHMI challenge trial to date, we show that the pediatric dosage of RTS,S/AS01E, when compared with the adult RTS,S/AS01B dosage, provides comparable efficacy when administered to adults in a 3-dose delayed fractional dose regimen. This suggests a universal formulation may be considered. The results also suggest that a 2-dose regimen, as used in this study, is inferior to the 3-dose regimens evaluated.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The study staff and trial partners thank the study participants for their participation and support of malaria clinical research. They are also grateful for the help and dedication of the following groups and individuals, from the Walter Reed Army Institute of Research (WRAIR): the staffs of the Human Subjects Protection Branch, Clinical Trials Center, Insectary, Malaria Vaccine Branch, Core Immunology Laboratories, the medical monitor Captain Ramiro Gutierrez, the subject ombudsmen, Ruthie Ratcliffe, and Michael Hrycyk, Joanne Gbenjo, Julie Ake, Patrick Twomey, Anne Preston, and the many individuals from both WRAIR and the Naval Medical Research Center who provided malaria microscopy support; from GSK: Marc Cullinane, Muriel Debois, Elena Smith, Laurence Vanbeselaere, Nathalie Baudson, Fabienne Danhier (Modis on behalf of GSK), and Jarno Jansen (Modis on behalf of GSK), for editorial support and assistance; and from PATH: Scott Gregory and Emily Locke for clinical sample management, Sahlah Dubel and Kyung Park for project management, and David C. Kaslow, Ulrike Wille-Reece, and Ashley Birkett for input into study design.
Additional members of the RTS,S Malaria Vaccine Working Group. WRAIR: Susan B. Cicatelli, Elizabeth H. Duncan, Kristin T. Mills, Christine E. Lee, Judith E. Epstein, Jessica J. Cowden, Michele D. Spring, Melinda J. Hamer, Nathanial K. Copeland, Viseth Ngauy, Donna M. Tosh, Justin M. Curley, Jason W. Bennett, Mark Riddle, Paige E. Waterman, Michael A. Koren, Jack N. Hutter, Elke Bergmann-Leitner, Jennifer Kooken, Evelina Angov, and Kyle Peterson; and GSK: Aurélia Leprince and Linda Murray.
Author contributions. All authors participated either in the design and implementation or the analysis and interpretation of the study, as well as the development of the manuscript. All authors had full access to the data and granted their final approval of the manuscript before submission for publication.
Disclaimer. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense, nor do they reflect the views and decisions of the World Health Organization. GlaxoSmithKline Biologicals was involved in all stages of the study conduct and analysis.
Financial support. This work was supported by PATH–Malaria Vaccine Initiative, the US Department of Defense, were provided by GlaxoSmithKline Biologicals (provision of experimental products, sponsorship of study and publication, and all costs associated with the development and publishing of the current article)
Potential conflicts of interest. M. T., E. J., D. M., M. L., W. R. B., and O. O. A. are employees of and M. T., E. J., D. M., W. R. B., and O. O. A. have restricted shares in the GSK group of companies. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 67th Annual Meeting of the American Society of Tropical Medicine and Hygiene, New Orleans, Louisiana, October 28–November 2, 2018; presentation LB-5597.
Contributor Information
RTS,S Malaria Vaccine Working Group:
Susan B Cicatelli, Elizabeth H Duncan, Kristin T Mills, Christine E Lee, Judith E Epstein, Jessica J Cowden, Michele D Spring, Melinda J Hamer, Nathanial K Copeland, Viseth Ngauy, Donna M Tosh, Justin M Curley, Jason W Bennett, Mark Riddle, Paige E Waterman, Michael A Koren, Jack N Hutter, Elke Bergmann-Leitner, Jennifer Kooken, Evelina Angov, Kyle Peterson, Aurélia Leprince, and Linda Murray
References
- 1. World Health Organization–Malaria Vaccine Funders Group. 2013 Update to the Malaria Vaccine Technology Roadmap. 2013. Available at: https://www.who.int/immunization/sage/meetings/2013/april/6_Draft_roadmap_update_v_5_March.pdf?ua=1. Accessed 28 January 2020. [Google Scholar]
- 2. Rabinovich RN, Drakeley C, Djimde AA, et al. malERA: an updated research agenda for malaria elimination and eradication. PLoS Med 2017; 14:e1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. RTS,S Clinical Trials Partnership. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med 2014; 11:e1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 386:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. RTS,S Clinical Trials Partnership. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011; 365:1863–75. [DOI] [PubMed] [Google Scholar]
- 6. ClinicalTrials.gov. Seasonal malaria vaccination (RTS,S/AS01) and seasonal malaria chemoprevention (SP/AQ) (RTSS-SMC) https://clinicaltrials.gov/ct2/show/NCT03143218.
- 7. Greenwood B, Dicko A, Sagara I, et al. Seasonal vaccination against malaria: a potential use for an imperfect malaria vaccine. Malar J 2017; 16:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. Malaria Vaccine Implementation Programme—Programme Advisory Group. https://www.who.int/immunization/research/committees/malaria_vaccine_implementation_group/en/. Accessed 28 January 2020.
- 9. Draper SJ, Angov E, Horii T, et al. Recent advances in recombinant protein-based malaria vaccines. Vaccine 2015; 33:7433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffman SL, Vekemans J, Richie TL, Duffy PE. The march toward malaria vaccines. Am J Prev Med 2015; 49:S319–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ockenhouse CF, Magill A, Smith D, Milhous W. History of U.S. military contributions to the study of malaria. Mil Med 2005; 170:12–6. [DOI] [PubMed] [Google Scholar]
- 12. Stoute JA, Slaoui M, Heppner DG, et al. RTS,S Malaria Vaccine Evaluation Group. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med 1997; 336:86–91. [DOI] [PubMed] [Google Scholar]
- 13. Kester KE, Cummings JF, Ofori-Anyinam O, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis 2009; 200:337–46. [DOI] [PubMed] [Google Scholar]
- 14. Regules JA, Cicatelli SB, Bennett JW, et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis 2016; 214:762–71. [DOI] [PubMed] [Google Scholar]
- 15. Frey SE, Couch RB, Tacket CO, et al. Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med 2002; 346:1265–74. [DOI] [PubMed] [Google Scholar]
- 16. Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med 2004; 351:2295–301. [DOI] [PubMed] [Google Scholar]
- 17. Garçon N, Heppner DG, Cohen J. Development of RTS,S/AS02: a purified subunit-based malaria vaccine candidate formulated with a novel adjuvant. Expert Rev Vaccines 2003; 2:231–8. [DOI] [PubMed] [Google Scholar]
- 18. Alderson MR, McGowan P, Baldridge JR, Probst P. TLR4 agonists as immunomodulatory agents. J Endotoxin Res 2006; 12:313–9. [DOI] [PubMed] [Google Scholar]
- 19. Kensil CR, Kammer R. QS-21: a water-soluble triterpene glycoside adjuvant. Expert Opin Investig Drugs 1998; 7:1475–82. [DOI] [PubMed] [Google Scholar]
- 20. Gordon DM, McGovern TW, Krzych U, et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J Infect Dis 1995; 171:1576–85. [DOI] [PubMed] [Google Scholar]
- 21. Tamminga C, Sedegah M, Regis D, et al. Adenovirus-5-vectored P. falciparum vaccine expressing CSP and AMA1. Part B: safety, immunogenicity and protective efficacy of the CSP component. PLoS One 2011; 6:e25868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clement F, Dewar V, Van Braeckel E, et al. Validation of an enzyme-linked immunosorbent assay for the quantification of human IgG directed against the repeat region of the circumsporozoite protein of the parasite Plasmodium falciparum. Malar J 2012; 11:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kester KE, Cummings JF, Ockenhouse CF, et al. Phase 2a trial of 0, 1, and 3 month and 0, 7, and 28 day immunization schedules of malaria vaccine RTS,S/AS02 in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine 2008; 26:2191–202. [DOI] [PubMed] [Google Scholar]
- 24. Ockenhouse CF, Regules J, Tosh D, et al. Ad35.CS.01-RTS,S/AS01 heterologous prime boost vaccine efficacy against sporozoite challenge in healthy malaria-naïve adults. PLoS One 2015; 10:e0131571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ajua A, Lell B, Agnandji ST, et al. The effect of immunization schedule with the malaria vaccine candidate RTS,S/AS01E on protective efficacy and anti-circumsporozoite protein antibody avidity in African infants. Malar J 2015; 14:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olotu A, Clement F, Jongert E, et al. Avidity of anti-circumsporozoite antibodies following vaccination with RTS,S/AS01E in young children. PLoS One 2014; 9:e115126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dobaño C, Sanz H, Sorgho H, et al. Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy. Nat Commun 2019; 10:2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. White MT, Verity R, Griffin JT, et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis 2015; 15:1450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.