Abstract
Research shows fluctuations in drinking across the menstrual cycle among women with alcohol use disorder (AUD), but little work has investigated moderators of these fluctuations. This study examined drinking and craving intensity across the menstrual cycle, and the moderating effect of baseline depression and emotional distress during the mid-late luteal phase and/or menses, among women receiving AUD treatment. Fifty-nine regularly cycling women reported menstrual history and baseline depression. Over three months of treatment, they kept daily logs of drinks, alcohol cravings, and menstruation (yes/no). Emotional distress during the mid-late luteal phase and/or menses of their most recent menstrual cycle was also assessed during treatment. Menstrual cycle phase was estimated for each within-treatment day. Mixed model analyses tested main and interactive effects of menstrual cycle phase, baseline depression, and emotional distress during the mid-late luteal phase and/or menses on daily drinks and craving intensity. Women drank most during the mid-late luteal phase and menses compared to other phases. Among women with lower baseline depression, those with lower distress during the mid-late luteal phase and/or menses reported more intense cravings during the mid-late luteal phase (ΔM=.77, p=.000) and menses (ΔM=.51, p=.012); those with higher distress reported more intense cravings during menses, compared to all other phases (p<.01). Among women with higher baseline depression, craving intensity remained consistently high. Results document more drinking during the mid-late luteal phase and menses and suggest that cycle-related distress and depression moderate the alcohol-menstrual association among women in AUD treatment.
Keywords: menstrual cycle phase, alcohol consumption, alcohol cravings, depression, women
Negative mood states constitute one of the most common drinking triggers for women with alcohol use disorder (AUD; Karpyak et al., 2016; Peltier et al., 2019). Affective models of alcohol use suggest that alcohol cravings mediate the association between negative affect and greater alcohol consumption (Bujarski & Ray, 2014; Witkiewitz, Bowen, & Donovan, 2011). Thus, alcohol cravings that occur in response to distressing situations may be associated with heightened risk of drinking and/or relapse for women with alcohol problems (Boykoff et al., 2010; Moore et al., 2014). One source of negative affect that might intensify alcohol cravings and thus influence drinking behavior among women is phase of the menstrual cycle.
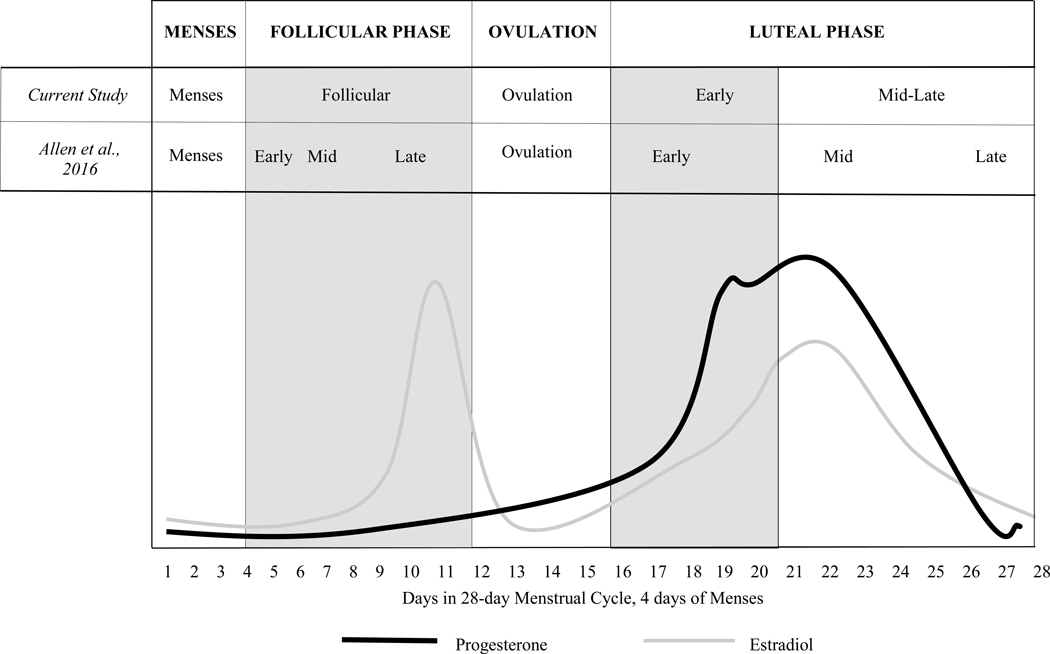
Hormonal fluctuations that accompany the menstrual cycle have been linked to changes in both psychological and physiological processes, including an increase in negative mood states such as irritability, fatigue, depression, and anxiety, during the mid-late luteal phase (often termed “premenstrual” in previous research) and/or during menses (Farage, Osborn, & MacLean, 2008; Moos et al., 1969), and positive mood during ovulation (Aganoff & Boyle, 1994). Higher serum levels of the ovarian hormone progesterone are associated with lower stress reactivity (Fox, Sofuoglu, Morgan, Tuit, & Sinha, 2013), and increases in estradiol have been associated with negative mood and implicated in depression (Gordon, Eisenlohr-Moul, Rubinow, Schrubbe, & Girdler, 2016; Lasiuk & Hegadoren, 2007). Progesterone levels are lowest during menses and the follicular phase of the menstrual cycle, begin to rise after ovulation, peak during the mid-luteal phase, then drop during the late luteal phase, after which a new cycle begins. Estradiol levels peak just prior to ovulation, drop precipitously afterward, and then, in a pattern similar to that of progesterone, increase during the early luteal phase and drop during mid-late luteal phase (see Figure 1). If women with AUD do indeed experience mood-related alcohol cravings and/or drink as a result of negative emotional states, then they may especially do so at times during the menstrual cycle when they experience heightened negative affect. For some women, such changes in negative affect may be influenced by hormonal fluctuations.
Figure 1.
Fluctuation of Progesterone and Estradiol Levels during the Phases of a 28-Day Menstrual Cycle, as Defined in the Current Study and by Allen et al. (2016)
Alcohol consumption and menstrual cycle phase in women without AUD
The literature on the alcohol-menstrual association is modest and inconclusive across samples of women without AUD (Carroll, Lustyk, & Larimer, 2015), perhaps in part because of the variability in defining drinking parameters and clinical characteristics of the samples. Drinking parameters for these samples range from “social” drinking, to moderate drinking, to unspecified levels of drinking, all defined inconsistently across studies (Harvey & Beckman, 1985; Holdstock & de Wit, 2003; Joyce et al., 2018; Marks, Hair, Klock, Ginsburg, & Pomerleau, 1994; Pomerleau, Cole, Lumley, Marks, & Pomerleau, 1994; Sutker, Libet, Allain, & Randall, 1983; Tobin, Schmidt, & Rubinow, 1994). Some studies on women without AUD have documented greater drinking frequency or intensity during the late luteal phase (often referred to as “premenstrual;” e.g., Harvey & Beckman, 1985; Joyce et al., 2018) or menses (e.g., Marks et al., 1994). However, other research on women without AUD has failed to find phase-related differences in alcohol consumption (e.g., Holdstock & de Wit, 2003; Pomerleau et al., 1994; Sutker et al., 1983; Tobin et al., 1994). In addition, although drinking frequency and intensity have been examined, other drinking characteristics such as alcohol craving intensity have not.
A few studies among women without AUD have examined changes in drinking to relieve negative affect across the menstrual cycle. Sutker et al. (1983) documented more frequent drinking to relieve tension or dysphoria during menses. Joyce et al. (2018) found that drinking to cope with negative affect increased during the late luteal phase and menses and also predicted a higher number of drinks during menses. The late luteal phase and menses have been associated with the greatest psychological distress (Farage et al., 2008; Moos et al., 1969) and are also characterized by dropping or low levels of progesterone and estradiol. Associations between declining progesterone and greater alcohol use have been documented in recent research using biological assays to confirm ovarian hormone levels among college women without AUD. For instance, Martel, Eisenlohr-Moul, and Roberts (2017) reported associations between higher risk of drinking and binge drinking and perimenstrual decreases in progesterone, as well as associations between greater alcohol use and preovulatory increases in estradiol. Another study found that a decrease in progesterone increased the likelihood of alcohol consumption on negative mood days (Holzhauer, Wemm, Wulfert, & Cao, 2020).
Alcohol consumption and menstrual cycle phase in women with AUD
Only a few studies have examined the alcohol-menstrual association among women with AUD. Investigating this association in clinical populations is important because triggers related to the menstrual cycle could exacerbate problem drinking and/or heighten relapse risk. The ability to predict fluctuations in alcohol cravings could inform targets for clinical intervention for AUD by identifying phases in a woman’s menstrual cycle that may present high drinking risk. As early as 1963, Podolsky observed that “premenstrual tension” was associated with increased drinking in women with AUD (Podolsky, 1963). Other early studies also documented greater alcohol consumption during the late luteal phase of the menstrual cycle (Allen, 1996; Belfer, Shader, Carroll, & Harmatz, 1971). Epstein et al. (2006) measured menstrual cycle phase and drinking behavior prospectively in a sample of alcohol dependent women. Forty-eight (69%) of the 70 women who provided data on drinking triggers prior to AUD treatment identified the late luteal phase (for participants, labeled the “premenstrual” phase) as a drinking trigger. A subsample of 12 regularly cycling women reported fewer, less frequent cravings but significantly greater drinking frequency during the late luteal phase compared to other non-menses days of the menstrual cycle. Thus, some women with AUD report phase-related differences in alcohol consumption and/or cravings. However, moderators of the alcohol-menstrual association have not been examined in this population.
A separate but related literature on smoking and menstrual cycle phase could inform the alcohol-menstrual association. Growing evidence indicates that higher levels of progesterone and lower levels of estradiol may be associated with decreased smoking behavior (Wetherill, Franklin, & Allen, 2017). However, this literature remains mixed. Like the alcohol-menstrual association, the smoking-menstrual association is likely influenced by individual difference factors. The late luteal phase is marked by negative affect and emotion dysregulation (Farris, Abrantes, & Zvolensky, 2019), and negative affective symptoms have been shown to be associated with greater smoking severity during this phase (Pang, Andrabi, & Leventhal, 2017). Clinical conditions may also play a moderating role; for instance, female smokers with depression exhibit less variation in physiological reactivity to nicotine across the menstrual cycle compared to their non-depressed counterparts (Allen et al., 2013).
Disparate findings within the alcohol-menstrual literature
The alcohol-menstrual literature is marked by a number of limitations. Research on women without AUD is compromised by methodological variability, such as the use of unclear or inconsistent drinking parameters in characterizing samples. There is also a dearth of research on the alcohol-menstrual association among women with AUD. Most studies rely on retrospective self-report of the menstrual cycle and/or inconsistent delineations of menstrual cycle phases. In addition, previous studies have focused upon drinking frequency and/or intensity but not alcohol cravings. Given the role of negative affect in driving alcohol cravings among women with alcohol problems (Boykoff et al., 2010; Moore et al., 2014), as well as variation in negative affect across the menstrual cycle (Farage et al., 2008; Moos et al., 1969), research should also examine how alcohol cravings might vary by menstrual cycle phase.
Another explanation for the seemingly disparate findings within the alcohol-menstrual literature is heterogeneity in the way that menstrual cycle phase affects drinking behavior. Individual difference factors such as state and trait negative affect might influence the alcohol-menstrual association among women with AUD, as appears to be the case among female smokers (Allen et al., 2013). Given the high prevalence of depressive disorders among women with AUD (Goldstein, Dawson, Chou, & Grant, 2012), negative affect and co-occurring depression may moderate the alcohol-menstrual association. In addition, with respect to ovarian hormones, estradiol has been implicated in the development of depression (Walf & Frye, 2006; Balzer, Duke, Hawke, & Steinbeck, 2015), and progesterone and its metabolites have been shown to function differently among women with depression and other internalizing disorders, compared to women without such conditions (Klatzkin, Morrow, Light, Pedersen, & Girdler, 2006; Rasmusson et al., 2006; Schüle, Nothdurfter, & Rupprecht, 2014). Both estradiol and progesterone affect neurological and physiological processes that contribute to mood and internalizing symptoms, such as serotonin activity and hypothalamic-pituitary-adrenal (HPA) axis functioning (Gordon et al., 2016; Lasiuk & Hegadoren, 2007). Women with depression may have distinct patterns of hormonal fluctuation (e.g., blunted fluctuations, or different “peaks” of these hormones across the cycle) and may thus experience the influence of hormones on mood and stress differently from women without depression. Such differences may, in turn, carry implications for alcohol use and alcohol cravings, especially among women with AUD. However, no study has explicitly examined the moderating effect of depressed mood on fluctuations in alcohol consumption and cravings across the menstrual cycle in women with AUD.
The present study
Research has documented the frequent occurrence of alcohol cravings in response to negative affect among women with alcohol problems (Boykoff et al., 2010; Moore et al., 2014), the dysphoria known to accompany certain phases of the menstrual cycle (Farage et al., 2008; Moos et al., 1969), the purported mood regulatory effects of ovarian hormones (Fox et al., 2013; Rasmusson et al., 2006), and associations between ovarian hormone levels and alcohol use, at least in women without AUD (Holzhauer et al., 2020; Martel et al., 2017). Based on prior work, it is reasonable to expect that women with AUD may experience differential drinking risk during times of the menstrual cycle that are characterized by negative affect. Past research implicates the late luteal phase (often termed the “premenstrual” phase) and menses in women with and without AUD, though somewhat inconsistently and with an emphasis on drinking frequency and/or intensity but not alcohol craving intensity. In addition, little research has investigated potential moderators among women with AUD. Mood-related moderators represent particularly promising candidates for study because of known associations between negative mood and certain menstrual cycle phases, as well as the mood-regulating role of the ovarian hormones that underlie the menstrual cycle.
The purpose of the current study is to extend past work on fluctuations in alcohol use across the menstrual cycle by (a) using a sample of women with AUD; (b) using daily monitoring of drinking behavior and menstrual cycles rather than retrospective recall; and (c) examining both drinking intensity and alcohol craving intensity. Extrapolating from previous reports (Allen, 1996; Belfer et al., 1971; Carroll et al., 2015; Epstein et al., 2006), we hypothesized that women with AUD would report greater alcohol consumption and alcohol craving intensity during the mid-late luteal phase and/or menses. We also examined the effect of two mood-related moderators, comorbid depression at baseline, and self-reported emotional distress during the mid-late luteal phase and/or menses, on associations between menstrual cycle phase and alcohol use and cravings.
Methods
Participants
Participants were drawn from a larger study of 168 women enrolled in an outpatient treatment research program for women with AUD (Epstein et al., 2018; McCrady, Epstein, Hallgren, Cook, & Jensen, 2016). Women eligible for the main trial were age 18 or older, lacked gross cognitive deficits or recent psychotic symptoms, did not have current physiological drug dependence, and were married or in a current heterosexual, committed romantic relationship (the latter criterion of which was related to a couples-based treatment arm in the parent study; see below). All women met criteria for current DSM-IV-TR (American Psychiatric Association, 2000) alcohol dependence and had consumed at least one drink in the 30 days prior to the initial telephone screening. The current study subsample included women who self-reported premenopausal status, endorsed at least one full menstrual cycle with menses during 3 months of treatment, and reported not using hormone-based birth control. The parent study was approved by the Rutgers University Institutional Review Board, and all participants provided informed consent at the in-person intake.
Procedure
For the parent study, participants were recruited from the community via flyers, newspaper or on-line advertisements, and outreach to community medical and mental health treatment providers. Potential participants completed a brief telephone screen to determine preliminary eligibility which was then confirmed in an in-person intake with a study clinician. At a subsequent baseline research interview, participants completed self-report questionnaires and semi-structured interviews, and were remunerated ~25. Women were then randomly assigned to one of two study treatments within each of an individual or couple cognitive-behavior therapy for AUD arm (for additional detail, see Epstein et al., 2018; McCrady, Epstein, Cook, Jensen, & Ladd, 2011; McCrady et al., 2016). Participants subsequently began a 12-session, 3-month course of weekly, manual-guided outpatient treatment conducted by masters or doctoral level study clinicians (see McCrady et al., 2016). Of the 70 women who were menstruating and not using hormone-based birth control, 59 attended at least one session of therapy and provided sufficient within-treatment menstrual and drinking data (i.e., covering at least one menstrual cycle) for inclusion in the present analysis.
Measures
Demographic Form and Menstrual History Questionnaires were created by the research team to assess basic identifying information including age, marital status, ethnic background, occupational status, education, and income. Baseline menstrual and hormonal birth control status was also assessed. Menstrual status was assessed with the question(s), “Are you post-menstrual? If yes, age of onset _____.” For women who answered “no” to this question, we examined daily log data (described below) to identify those women who reported at least one regular menstrual cycle in the three months of treatment. Participants selected their current method(s) of birth control from options including (among others) hormonal birth control; women who selected any of the hormonal birth control methods were not included in the present analysis.
Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV; First, Spitzer, Gibbon, & Williams, 2002). The SCID-IV is a semi-structured interview that assesses current and lifetime Axis I disorders using DSM-IV (APA, 2000) criteria. Good inter-rater reliabilities have been reported for the SCID-IV, with a mean reported kappa of 0.87 in a previous study (Epstein et al., 2006). In our sample, the SCID-IV was used to diagnose current DSM-IV Axis I conditions, including alcohol dependence.
The Timeline Follow Back (TLFB; Sobell & Sobell, 1992) is a reliable calendar-style record of alcohol consumption that uses drinking patterns and cues to prompt recall. Timeline data were used to determine drinking frequency (percent drinking days) and drinking intensity (standard drinks per drinking day) during the 3 months prior to the last drink before the baseline interview.
Daily Drinking Log (DDL) cards are a self-report, paper and pencil daily record, used in this study to record alcohol use, alcohol craving intensity, and menses during treatment. Participants were instructed to carry one card per day with them and complete a card in real time every day during the 3-month treatment window. No daily prompting was done, but, each week, patients brought all (7) between-session daily cards (as well as their patient workbook) to the study treatment session. During the first part of every weekly agenda review, the importance of the DDLs was emphasized, and completed cards were discussed collaboratively by therapist and patient to examine triggers, cravings, and strategies applied. If cards were not completed fully, the therapist used motivational enhancement techniques to encourage completion and brainstormed strategies to resolve obstacles to adequate completion for the upcoming week. The importance of the DDLs was then reiterated at the end of every treatment session for all participants. Data from the cards included daily alcohol use (converted to number of standard drinks); the frequency and intensity of alcohol cravings (the latter using a Likert scale of 1–7, with higher scores indicating greater intensity); triggers associated with each use and craving; and menstrual status for each day. For the latter, participants circled “yes,” “no” or “n/a” to the question, “Do you have your menstrual period today?” DDL data were used (a) to create our main dependent variables, namely, daily number of standard drinks (a measure of drinking intensity) and daily intensity of alcohol cravings; and (b) to delineate menstrual cycle phase for each day of data based on the daily self-reported menses days (described further below). DDL data are highly correlated with retrospective TLFB data (McCrady, Epstein, & Hirsch, 1999). In addition, the use of real time diary methodology follows previous guidelines for menstrual research with self-report data (Sommer, 1986).
Beck Depression Inventory-II (BDI-II; Beck, Steer, & Carbin, 1988) is a 21-item measure that assesses the severity of depression symptoms. Each item is rated on a scale from 0 (absence of symptom) to 3 (worst severity of symptom), and total scores range from 0–63. The BDI-II was administered at baseline; Cronbach’s alpha in the current sample was 0.91.
Menstrual Distress Questionnaire Form-C (MDQ-C; Moos, 1968) assesses eight mood symptoms (loneliness, anxiety, mood swings, crying, irritability, tension, feeling sad or blue, and restlessness) experienced during the mid-late luteal phase and menses of the most recent menstrual cycle on the following scale: 0 (“no experience of symptom”), 1 (“present, mild”), 2 (“present, moderate”), 3 (“present, strong”), 4 (“present, severe”). Study therapists administered the MDQ-C after women submitted the first DDL card that indicated current menses. Women were instructed to complete the MDQ-C retrospectively for the time period from the end of the last menstrual cycle to the first day of current menses. Scores from the mid-late luteal phase and menses were summed (with possible combined scores ranging from 0 to 64) for both statistical and conceptual reasons. First, distress during the mid-late luteal phase was highly correlated with distress during menses (r=.79). In addition, both phases involve dropping, then low, progesterone and estradiol levels, and past research has shown both phases to be associated with negative mood and increased alcohol use. Thus, to reduce collinearity, we created a single moderator variable indicating self-reported emotional distress during the mid-late luteal phase and/or menses.
Delineation of Menstrual Cycle Phase for Each Participant
Based on the timing of each participant’s menses as self-reported in real time on daily logs, every within-treatment day was categorized as falling within one of five menstrual cycle phases. Phases are consistent with recent recommendations for definitions (Allen et al., 2016; see discussion section for further explanation). (1) We first coded menses (all bleeding days), then anchored all remaining days to menses and coded all days. We designated all non-menses days as follows: (2) follicular phase (all days between end of menses and beginning of ovulation); (3) ovulation (−16 through −12 days before onset of bleeding); (4) early luteal phase (−11 through −8 days before onset of bleeding); (5) mid-late luteal phase (with the late luteal phase often termed “premenstrual” in previous research; −7 through −1 day before onset of bleeding). Because most menstrual cycles are not 28 days in length, and because menses was our anchor for cycle phase delineation, we calculated backwards (as outlined above) from the first day of each reported menses to estimate phases. All women reported at least one menses during the study; thus, we were able to code each within-treatment day as falling within one of these estimated phases.
Data Analytic Plan
Descriptive statistics were computed to characterize the sample on demographics, alcohol use, menstrual cycle symptoms, and depressed mood. Fixed effects linear Mixed Model Analysis was conducted in SPSS 22. Daily number of drinks and mean daily intensity of alcohol cravings from the three months of treatment were entered as the dependent variable in two separate models. Menstrual cycle phase (categorical variable), baseline depression (continuous measure of BDI-II score), and self-reported emotional distress during the mid-late luteal phase and/or menses (continuous measure) served as independent and interactive predictors (i.e., with all main effects, two-way interactions, and a three-way interaction term entered). Results reported in Table 1 use menses as the reference group; simple effects of significant main and interaction effects were then examined using different reference groups. Baseline depression and self-reported emotional distress during the mid-late luteal phase and/or menses were both entered into analyses as continuous predictors. However, for visual graphing purposes (see Figure 2), we categorize women with lower/higher baseline depression or lower/higher self-reported emotional distress during the mid-late luteal phase and/or menses (i.e., one standard deviation below/above the mean).
Table 1.
Mixed Models Analysis Results - Estimates of Fixed Effects
| Daily Number of Drinks | Daily Craving Intensity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed effects | Est | t | p | 95% CI, Lower Limit | 95% CI, Upper Limit | Est | t | p | 95% CI, Lower Limit | 95% CI, Upper Limit |
| (Intercept) | 0.308 | 7.420 | 0.000 | 0.226 | 0.390 | 2.111 | 21.352 | 0.000 | 1.917 | 2.305 |
| Follicular Phase (FP) | −0.134 | −2.634 | 0.009 | −0.234 | −0.034 | −0.270 | −2.220 | 0.027 | −0.509 | −0.031 |
| Ovulation (O) | 0.029 | 0.291 | 0.771 | −0.168 | 0.226 | −0.378 | −1.816 | 0.070 | −0.786 | 0.030 |
| Early Luteal Phase (eLP) | −0.143 | −1.720 | 0.087 | −0.307 | 0.021 | −0.295 | −1.533 | 0.125 | −0.672 | 0.082 |
| Mid-Late Luteal Phase (mlLP) | −0.034 | −0.576 | 0.565 | −0.152 | 0.083 | −0.092 | −0.660 | 0.509 | −0.365 | 0.181 |
| Menses (M) | REF | --- | --- | --- | --- | REF | --- | --- | --- | --- |
| Baseline Depression (BDI-II) | −0.007 | −1.698 | 0.09 | −0.016 | 0.001 | −0.046 | −5.214 | 0.000 | −0.064 | −0.029 |
| mlLP/M Emotional Distress (MED) | 0.008 | 2.838 | 0.005 | 0.002 | 0.013 | 0.017 | 2.441 | 0.015 | 0.003 | 0.030 |
| FP*BDI-II | 0.006 | 1.18 | 0.239 | −0.004 | 0.016 | 0.021 | 1.910 | 0.056 | −0.001 | 0.042 |
| O*BDI-II | −0.008 | −0.696 | 0.487 | −0.029 | 0.014 | 0.030 | 1.336 | 0.182 | −0.014 | 0.074 |
| eLP*BDI-II | 0.005 | 0.674 | 0.501 | −0.01 | 0.021 | 0.022 | 1.244 | 0.214 | −0.012 | 0.056 |
| mlLP*BDI-II | 0.002 | 0.303 | 0.762 | −0.01 | 0.013 | 0.021 | 1.715 | 0.087 | −0.003 | 0.046 |
| M*BDI-II | REF | --- | --- | --- | --- | REF | --- | --- | --- | --- |
| FP*MED | −0.002 | −0.645 | 0.520 | −0.009 | 0.005 | 0.003 | 0.383 | 0.702 | −0.013 | 0.020 |
| O*MED | −0.007 | −0.88 | 0.38 | −0.022 | 0.008 | 0.006 | 0.404 | 0.686 | −0.022 | 0.034 |
| eLP*MED | −0.009 | −1.657 | 0.099 | −0.021 | 0.002 | −0.008 | −0.623 | 0.533 | −0.035 | 0.018 |
| mlLP*MED | −0.001 | −0.186 | 0.853 | −0.009 | 0.007 | −0.015 | −1.554 | 0.120 | −0.034 | 0.004 |
| M*MED | REF | --- | --- | --- | --- | REF | --- | --- | --- | --- |
| BDI-II*MED | 0.000 | −0.996 | 0.321 | −0.001 | 0.000 | 0.000 | −0.276 | 0.782 | −0.001 | 0.001 |
| FP*BDI-II*MED | 0.000 | 1.097 | 0.274 | 0.000 | 0.001 | 0.000 | 0.226 | 0.821 | −0.001 | 0.002 |
| O*BDI-II*MED | 0.000 | 0.368 | 0.713 | −0.001 | 0.002 | 0.003 | 1.956 | 0.051 | 0.000 | 0.006 |
| eLP*BDI-II*MED | 0.000 | 0.671 | 0.503 | −0.001 | 0.001 | 0.003 | 2.411 | 0.016 | 0.001 | 0.005 |
| mlLP*BDI-II*MED | 0.001 | 1.36 | 0.175 | 0.000 | 0.001 | 0.002 | 2.499 | 0.013 | 0.000 | 0.004 |
| M*BDI-II*MED | REF | --- | --- | --- | --- | REF | --- | --- | --- | --- |
Note: Bolded values represent simple effects interpreted due to significant main or interactive fixed effects in the mixed models analysis.
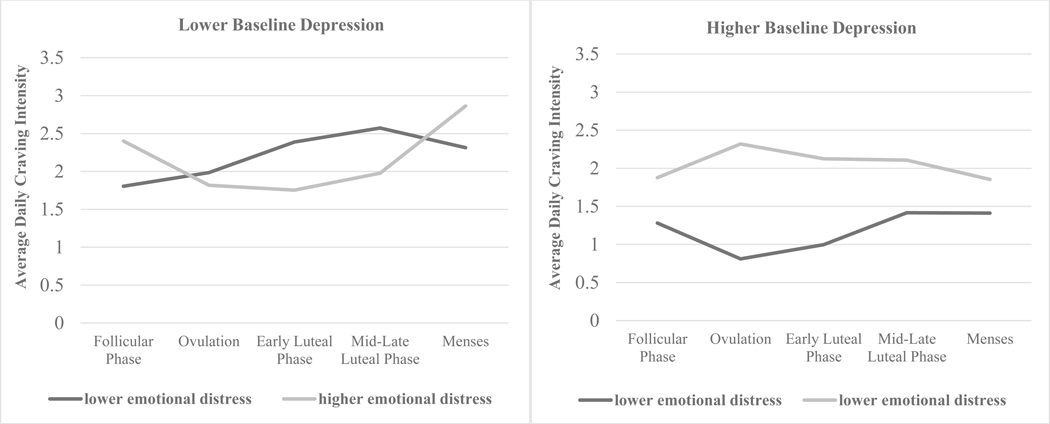
Figure 2.
Interaction of Menstrual Cycle Phase and Lower/Higher Self-reported Emotional Distress in the Mid-Late Luteal Phase and/or Menses among Women with Lower or Higher Baseline Depression
Note: To graph this three-way interaction effect, estimates are based on one standard deviation (BDI-II SD=10.40) below the mean for “lower baseline depression” and one standard deviation above the mean for “higher baseline depression”. Scores for baseline depression and self-reported emotional distress in the mid-late luteal phase and/or menses were entered as continuous variables in the mixed model analyses. Significant differences across menstrual cycle phases were found only among women with lower baseline depression. See results for details.
Results
The mean sample age (n=59) was 40.4 (SD=6.3) years, 86.4% were married, 93.2% were Caucasian, and 50.8% were employed full-time, with an average of 15.4 (SD=2.0) years of education and an average household income of ~100,977 (SD=~45.74). During the 90 days prior to the last drink before baseline, participants reported a mean of 67.9 (SD=25.2) percent drinking days and a mean of 6.1 (SD=3.2) drinks per drinking day. At baseline, the sample reported a moderate level of depression (BDI-II score, M=20.17, SD=10.40, range 0–43). For report of daily drinking and cravings, women completed DDLs on an average of 98.6% (SD=5.5%) of the total possible within-treatment days. The mean, median, and modal length of menses as reported on the DDLs was five days. The sample self-reported a moderate level of emotional distress during the mid-late luteal phase and/or menses (M=23.26, SD=15.02, range 0–54) of their most recent menstrual cycle.
Daily number of drinks.
The first model tested the main and interactive effects of menstrual cycle phase, baseline depression, and self-reported emotional distress during the mid-late luteal phase and/or menses on the number of drinks consumed daily during the within-treatment time frame (with zeroes entered for days on which no drinking occurred). Results are presented in Table 1. No significant interaction terms emerged, but there was a main effect of both menstrual cycle phase (F=2.60, p=.037) and baseline depression (F=5.15, p=.024) on the number of drinks consumed per day. Women consumed fewer daily drinks during the follicular phase compared to menses (p=.009); the difference between daily drinks consumed during the mid-late luteal and follicular phases was not significant (p=.058). In addition, women with higher levels of baseline depression consumed less alcohol overall than those with lower levels of baseline depression (p=.001). Main, fixed effects analyses showed that women reporting higher emotional distress during the mid-late luteal phase and/or menses did not differ from those reporting lower distress with respect to daily drinks during any menstrual cycle phase (p=.062).
Intensity of daily alcohol cravings.
The second model tested the main and interactive effects of menstrual cycle phase, baseline depression, and self-reported emotional distress during the mid-late luteal phase and/or menses on the intensity of alcohol cravings for every day during treatment (with zeroes entered for days on which no cravings were reported). Results are shown in Table 1. Analyses showed a significant main effect of baseline depression (F=22.24, p=.000) and self-reported emotional distress during the mid-late luteal phase and/or menses (F=11.46, p=.001), but not overall menstrual cycle phase (F=1.85, p=.117). However, results also revealed a significant three-way interaction of menstrual cycle phase, baseline depression, and self-reported emotional distress during the mid-late luteal phase and/or menses. The three-way interaction is shown in Figure 2. As stated above, although baseline depression and self-reported emotional distress during the mid-late luteal phase and/or menses were both entered into analyses as continuous predictors, to facilitate visual graphing, we refer to women with lower/higher baseline depression or lower/higher self-reported emotional distress during the mid-late luteal phase and/or menses (i.e., one standard deviation below/above the mean). Women with lower levels of baseline depression exhibited significant fluctuations in alcohol craving intensity across the menstrual cycle, whereas women with higher levels of baseline depression did not show such fluctuations regardless of their self-reported emotional distress during the mid-late luteal phase and/or menses. Among women with lower levels of baseline depression, those with relatively low self-reported emotional distress during the mid-late luteal phase and/or menses reported significantly more intense alcohol cravings during the mid-late luteal phase (ΔM = .77, p=.000) and menses (ΔM =.51, p=.012), particularly compared to the follicular phase. Women with lower levels of baseline depression but higher self-reported emotional distress during the mid-late luteal phase and/or menses reported significantly higher alcohol craving intensity during menses compared with ovulation (ΔM = 1.05, p=.007), the early luteal phase (ΔM = 1.11, p=.004), and the mid-late luteal phase (ΔM = 0.89, p=.002). Alcohol craving intensity was higher during menses than during the follicular phase, but this difference was not statistically significant (ΔM = .47, p=.073). Finally, women with higher levels of baseline depression did not exhibit fluctuations in alcohol craving intensity across menstrual cycle phases regardless of their self-reported emotional distress during the mid-late luteal phase and/or menses (all p-values for change in mean alcohol craving intensity were >.05).
Discussion
The purpose of the present study was to examine fluctuations in alcohol consumption and alcohol cravings across the menstrual cycle among women in treatment for AUD. Consistent with some prior work, our results highlighted associations between menstrual cycle phase and drinking characteristics (drinking intensity and alcohol craving intensity). In addition, the association between menstrual cycle phase and alcohol craving intensity was moderated by the presence of comorbid baseline depression and self-reported emotional distress during the mid-late luteal phase (which is often referred to as the “premenstrual” phase in previous research) and/or during menses.
This sample of women with AUD consumed significantly more daily drinks during menses (a time of low progesterone/estradiol), and at a trend level (p=.058) during the mid-late luteal phase (a time of quickly decreasing progesterone/estradiol), compared to the follicular phase (a time of low progesterone/rising estradiol), thus partially corroborating previous reports of greater drinking frequency or intensity during the late luteal phase and menses (Harvey & Beckman, 1986; Joyce et al., 2018; Marks et al., 1994). Because the mid-late luteal phase and menses are associated with negative mood states (Farage et al., 2008; Moos et al., 1969) and decreasing or low progesterone/estradiol, these results suggest that these women may be turning to alcohol to cope with this distress. This interpretation is consistent with prior work implicating craving as a mediator of the association between negative affect and greater alcohol use (Bujarski & Ray, 2014; Witkiewitz et al., 2011), as well as the heightened risk associated with craving that accompanies distressing situations (Boykoff et al., 2010; Moore et al., 2014).
With regard to daily intensity of alcohol cravings, women with lower levels of baseline depression reported menstrual cycle-related fluctuations in alcohol craving intensity, with the mid-late luteal phase or menses (i.e., times of decreasing or low progesterone and estradiol) representing peak intensity. Those with lower self-reported emotional distress during the mid-late luteal phase and/or menses reported more intense alcohol cravings during those phases compared to the follicular phase, whereas those with higher self-reported emotional distress during these phases reported more intense alcohol cravings during menses, compared to the other phases. Interestingly, women with lower emotional distress during the mid-late luteal phase and/or menses reported peak alcohol craving intensity in the mid-late luteal phase, which then decreased slightly during menses, whereas women with higher emotional distress during the mid-late luteal phase and/or menses reported peak alcohol craving intensity during menses. From the mid to late luteal phase, estradiol and progesterone levels are dropping at a quick rate. These rapidly shifting hormone levels may influence emotional distress in nuanced ways during these times of the menstrual cycle, thereby driving alcohol cravings for some women but not others. If women are indeed differentially vulnerable to hormonal fluctuations, as has been suggested by some researchers (e.g., Soares & Zitek, 2008), this may potentiate negative emotion and alcohol cravings among women with AUD at different points in the menstrual cycle. Future research should continue to examine menstrual cycle-related changes in mood and menstrual cycle-related distress to elucidate potential associations between menstrual cycle phase and alcohol cravings among women with AUD.
In our sample, women with higher levels of baseline depression exhibited no fluctuations in alcohol craving intensity across the menstrual cycle, regardless of their self-reported menstrual cycle-related emotional distress. Instead, they reported a consistently high level of alcohol craving intensity across the cycle. These women may experience a high level of overall emotional distress throughout the cycle (due to their depression), such that they are relatively unresponsive to the purported phase- and hormone-related changes in emotional reactivity. This interpretation is consistent with reports from the smoking literature indicating less phase-related variation in physiological reactivity to nicotine among depressed, compared to non-depressed, women (Allen et al., 2013). These combined findings are also consistent with reports that depressed women exhibit low levels of the progesterone metabolite allopregnanolone, which is thought to be linked to the hormone’s mood regulatory effects (Klatzkin et al., 2006; Rasmusson et al., 2006; Schüle et al., 2014; Sripada et al., 2013). Whereas women without depression experience the emotionally protective effects of peak progesterone during ovulation (and, conversely, the negative emotional consequences of progesterone withdrawal, notably during the mid-late luteal phase and menses), those with depression, who may not experience hormone-related mood changes, experience a heightened level of negative affect throughout the cycle. For women with AUD, this constant state of negative affect may manifest as sustained alcohol craving intensity. Again, further research is necessary to examine this possibility.
Because hormone assays were not collected in this study, we temper attribution of our findings to fluctuations in hormones. However, understanding the role of ovarian hormones in mood regulation is an important avenue for future research among women receiving AUD treatment. Progesterone and its metabolites are associated with stress and emotion regulation, and lower levels of these metabolites have been found among women with depression (Klatzkin et al., 2006; Schüle et al., 2014). The current findings suggest that the mid-late luteal phase (when progesterone levels are dropping) and menses (when progesterone levels are lowest) are times of increased drinking, and, among women in AUD treatment with lower baseline depression, increased craving. This suggests that progesterone may mediate the association between menstrual cycle phase and drinking and/or alcohol cravings. In our study, women with higher baseline depression also reported higher alcohol craving intensity across the cycle, which may be mediated by chronically low levels of progesterone or a failure of progesterone metabolization. Our findings also suggest the potential role of estradiol in explaining women’s alcohol cravings and use across the menstrual cycle. Like progesterone, estradiol levels quickly decline and reach their lowest during the mid-late luteal phase and menses. Research has implicated estradiol in risk-taking, negative affect, and depression among women (Balzer et al., 2015; Gordon et al., 2016). The nature of the specific associations among estradiol, progesterone, and mood is complex and requires further research. The association between estradiol and psychological outcomes may not be linear (Balzer et al., 2015); for example, some research suggests that it is the ratio of progesterone to estradiol that may predict these outcomes (Farage et al., 2008).
Our study findings should be interpreted in the context of our study limitations. Although the sample is comparable to or somewhat larger than other sample sizes in the relevant literature, it is nonetheless relatively small. However, the intensive daily measurements over three months allowed us to examine within-person fluctuations, thus mitigating sample size limitations to some degree. It is possible that the menstrual cycles of some study women were reciprocally affected by their heavy alcohol consumption (Augustyńska et al., 2007), thus influencing when menstrual symptoms were experienced. In addition, because the women were engaged in treatment, they may have had enhanced awareness of drinking cues, including menstrual cycle triggers (Epstein et al., 2006). For instance, some women who might otherwise have experienced phase-related alcohol cravings may have felt fewer such cravings due to their success addressing high-risk situations.
Another limitation is that premenopausal status was assessed through self-report; it is possible that some participants may have experienced menstrual irregularities associated with perimenopause which, in turn, may have influenced study findings. Menstrual cycle phase was also assessed through self-report without biological samples to verify hormonal status, which limited our ability to delineate sub-phases of the menstrual cycle, where more nuanced fluctuations in ovarian hormones that could drive associated changes in behavior are thought to occur. However, our data were collected using a real time daily diary methodology that followed previous guidelines for menstrual research (Sommer, 1986), and menstrual cycle phase delineations overlapped almost perfectly with recent recommendations by Allen et al. (2016; see Figure 1). Where our delineations primarily diverge from theirs is that we do not distinguish between the mid- and late-luteal sub-phases. For example, for a 28-day cycle with 4 menses days, the correspondence of our phases with those of Allen et al. (2016) is as follows: menses (days 14, consistent with Allen et al.); follicular phase (days 5–11, comprising Allen et al.’s early, mid, and late follicular sub-phases across days 4–12); ovulation (days 12–16, comprising Allen et al.’s ovulation on days 13–15); early luteal phase (days 17–20, comprising Allen et al.’s early luteal sub-phase on days 16–20); and mid-late luteal phase (days 21–28, comprising Allen et al.’s mid- and late-luteal sub-phases on days 21–23 and days 24–28, respectively). Although we were unable to separate the mid- and late-luteal sub-phases, the majority of our findings were focused on the combined mid-late luteal phase and menses, which were clearly delineated because women were reporting, in real time, whether or not they were having menses.
These limitations notwithstanding, the results of this study contribute to the literature on the alcohol-menstrual association. This study is one of only a few that address this association in women with AUD and replicates and extends Epstein et al. (2006), a small pilot study and the only prior study to use a daily diary method in this population. In addition, although prior work has measured drinking frequency and intensity, other drinking variables such as the intensity of alcohol cravings have not appeared extensively in the literature. Our findings suggest that the mid-late luteal phase and/or menses represent high-risk times during the menstrual cycle for the experience of alcohol cravings as well as for drinking frequency and intensity.
Our findings also suggest that depressed mood and the self-reported experience of emotional distress during the mid-late luteal phase and/or menses (when progesterone and estradiol levels are decreasing or at their lowest) could serve as important moderators of the association between menstrual cycle phase and drinking behavior. To our knowledge, ours is the first study to examine these particular moderators of the alcohol-menstrual association among women with AUD. Future work should continue to investigate the alcohol-menstrual association in a variety of women with AUD with and without depression, including women with known premenstrual dysphoric disorder, women with a range of drinking levels, and women not actively pursuing treatment. Continuing this line of research is important because it could help identify particular times during the menstrual cycle that a woman may face heightened drinking triggers and, in turn, increased risk for problem drinking and/or relapse. For instance, in the smoking literature, research indicates that the timing of quit date selection could influence the likelihood of success (Allen, Allen, Lunos, & Hatsukami, 2009; Allen, Bade, Center, Finstad, & Hatsukami, 2008). If similar findings were documented among women with AUD regarding drinking cessation, they could be used to optimize treatment outcomes.
Acknowledgments
The parent study was funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant R01 AA07070 to Drs. McCrady and Epstein. The authors declare no financial conflicts of interest.
References
- Aganoff JA, & Boyle GJ (1994). Aerobic exercise, mood states and menstrual cycle symptoms. Journal of Psychosomatic Research, 38, 183–192. doi: 10.1016/0022-3999(94)90114-7 [DOI] [PubMed] [Google Scholar]
- Allen D. (1996). Are alcoholic women more likely to drink premenstrually? Alcohol & Alcoholism, 31, 145–147. doi: 10.1093/oxfordjournals.alcalc.a008125 [DOI] [PubMed] [Google Scholar]
- Allen AM, McRae-Clark AL, Carlson S, Saladin ME, Gray KM, Wetherington CL, … & Allen SS (2016). Determining menstrual phase in human biobehavioral research: A review with recommendations. Experimental and Clinical Psychopharmacology, 24, 1–11. doi: 10.1037/pha0000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Allen AM, Kotlyar M, Lunos S, Absi M, & Hatsukami D. (2013). Menstrual phase and depressive symptoms differences in physiological response to nicotine following acute smoking abstinence. Nicotine & Tobacco Research, 15, 1091–1098. doi: 10.1093/ntr/nts236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Allen AM, Lunos S, & Hatsukami DK (2009). Patterns of self-selected smoking cessation attempts and relapse by menstrual phase. Addictive Behaviors, 34, 928–931. doi: 10.1016/j.addbeh.2009.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Bade T, Center B, Finstad D, & Hatsukami D. (2008). Menstrual phase effects on smoking relapse. Addiction, 103, 809–821. doi: 10.1111/j.1360-0443.2008.02145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders, Text revision (4th ed. - TR). Washington, DC: Author. [Google Scholar]
- Augustyńska B, Ziółkowski M, Odrowąż-Sypniewska G, Kiełpinski A, Gruszka M, & Kosmowski W. (2007). Menstrual cycle in women addicted to alcohol during the first week following drinking cessation: Changes of sex hormones levels in relation to selected clinical features. Alcohol & Alcoholism, 42, 80–83. doi: 10.1093/alcalc/agI094 [DOI] [PubMed] [Google Scholar]
- Balzer BW, Duke SA, Hawke CI, & Steinbeck KS (2015). The effects of estradiol on mood and behavior in human female adolescents: A systematic review. European Journal of Pediatrics, 174(3), 289–298. doi: 10.1007/s00431-014-2475-3 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Carbin MG (1988). Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review, 8(1), 77–100. doi: 10.1016/0272-7357(88)90050-5 [DOI] [Google Scholar]
- Belfer ML, Shader RI, Carroll M, & Harmatz JS (1971). Alcoholism in women. Archives of General Psychiatry, 25, 540. doi: 10.1001/archpsyc.1971.01750180060010 [DOI] [PubMed] [Google Scholar]
- Boykoff N, Schneekloth TD, Hall-Flavin D, Loukianova L, Karpyak VM, Stevens SR, et al. (2010). Gender differences in the relationship between depressive symptoms and craving in alcoholism. The American Journal on Addictions, 19, 352–356. doi: 10.1111/j.1521-0381.2010.00057.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, & Ray LA (2014). Negative affect is associated with alcohol, but not cigarette use in heavy drinking smokers. Addictive Behaviors, 39, 1723–1729. doi: 10.1016/j.addbeh.2015.07.019 [DOI] [PubMed] [Google Scholar]
- Carroll HA, Lustyk MKB, & Larimer ME (2015). The relationship between alcohol consumption and menstrual cycle: A review of the literature. Archives of Women’s Mental Health, 18, 773–781. doi: 10.1007/s00747-015-0568-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein EE, Rhines KC, Cook S, Zdep-Mattocks B, Jensen NK, McCrady BS (2006). Changes in alcohol craving and consumption by phase of menstrual cycle in alcohol dependent women. Journal of Substance Use, 11, 323–332. doi: 10.1080/14659890500419717 [DOI] [Google Scholar]
- Epstein EE, McCrady BS, Hallgren KA, Cook S, Jensen NK, & Hildebrandt T. (2018). A randomized trial of female-specific cognitive behavior therapy for alcohol dependent women. Psychology of Addictive Behaviors, 32(1), 1–15. doi: 10.1037/adb00000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage MA, Osborn TW, & MacLean AB (2008). Cognitive, sensory, and emotional changes associated with the menstrual cycle: A review. Archives of Gynecology and Obstetrics. doi: 10.1007/s00404-008-0708-2 [DOI] [PubMed] [Google Scholar]
- Farris SG, Abrantes AM, & Zvolensky MJ (2019). Emotional distress and tobacco demand during the menstrual cycle in female smokers. Cognitive Behavior Therapy, 48, 177–183. doi: 10.1080/1650603.2018.1494208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JW (2002). Structured clinical interview for DSM-IV-TR Axis I disorders, research version, patient edition (SCID-I/P). New York: Biometric Research, New York State Psychiatric Institute. [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, Tuit KL, & Sinha R. (2013). The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: Impact of gender and cue type. Psychoneuroendocrinology, 38, 1532–1544. doi: 10.1016/j.psyneuen.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RB, Dawson DA, Chou SP, & Grant BF (2012). Sex differences in prevalence and comorbidity of alcohol and drug use disorders: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Studies on Alcohol and Drugs, 73(6), 938–950. Doi: 10.15288/jsad.2012.73.938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Eisenlohr-Moul TA, Rubinow DR, Schrubbe L, & Girdler SS (2016). Naturally occurring changes in estradiol concentrations in the menopause transition predict morning cortisol and negative mood in perimenopausal depression. Clinical Psychological Science, 4(5), 919–935. doi: 10.1177/2167702616647924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SM, & Beckman LJ (1985). Cyclic fluctuation in alcohol consumption among female social drinkers. Alcoholism: Clinical and Experimental Research, 9, 465–471. doi: 10.1111/j.1530-0277.1985.tb05584x [DOI] [PubMed] [Google Scholar]
- Holdstock L, & de Wit H. (2000). Effects of ethanol at four phases of the menstrual cycle. Psychopharmacology, 150, 374–382. doi: 10.1007/s002130000461 [DOI] [PubMed] [Google Scholar]
- Holzhauer CG, Wemm SF, Wulfert E, & Cao Z. (2020). Fluctuations in progesterone moderate the relationship between daily mood and alcohol use in young adult women. Addictive Behaviors, 101, 106–146. doi: 10.1016/j.addbeh.2019.106146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce KM, Hudson A, O’Connor R, Thompson K, Hodgin M, Perrot T, & Stewart SH (2018). Changes in coping and social motives for drinking and alcohol consumption across the menstrual cycle. Depression and Anxiety, 35, 313–320. doi: 10.1002/da.22699 [DOI] [PubMed] [Google Scholar]
- Karpyak VM, Biernacka JM, Geske JR, Abulseoud OA, Brunner MD, Chauhan M, … & Onsrud DA (2016). Gender-specific effects of comorbid depression and anxiety on the propensity to drink in negative emotional states. Addiction, 111(8), 1366–1375. doi: 10.1111/add.13386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow AL, Light KC, Pedersen CA, & Girdler SS (2006). Histories of depression, allopregnanolone responses to stress, and premenstrual symptoms in women. Biological Psychology, 71, 2–11. doi: 10.1016/j.biopsycho.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Lasiuk GC, & Hegadoren KM (2007). The effects of estradiol on central serotonergic systems and its relationship to mood in women. Biological Research for Nursing, 9(2), 147–160. doi: 10.1177/1099800407305600 [DOI] [PubMed] [Google Scholar]
- Marks JL, Hair CS, Klock SC, Ginsburg BE, & Pomerleau CS (1994). Effects of menstrual phase on intake of nicotine, caffeine, and alcohol and nonprescribed drugs in women with late luteal phase dysphoric disorder. Journal of Substance Abuse, 6, 235–243. doi: 10.1016/s0899-3289(94)90265-8 [DOI] [PubMed] [Google Scholar]
- Martel MM, Eisenlohr-Moul T, & Roberts B. (2017). Interactive effects of ovarian steroid hormones on alcohol use and binge drinking across the menstrual cycle. Journal of Abnormal Psychology, 126, 1104–1113. doi: 10.1037/abn0000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrady BS, Epstein EE, Cook S, Jensen N, & Ladd B. (2011). What do women want? Alcohol treatment choices, treatment entry and retention. Psychology of Addictive Behaviors, 25,521–529. doi: 10.1037/a0024037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrady BS, Epstein EE, Hallgren KA, Cook S, & Jensen NK (2016). Women with alcohol dependence: A randomized trial of couple versus individual plus couple therapy. Psychology of Addictive Behaviors, 30, 287–299. doi: 10.1037/adb0000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrady BS, Epstein EE, & Hirsch LS (1999). Maintaining change after conjoint behavioral alcohol treatment for men: Outcomes at 6 months. Addiction, 94, 1381–1396. doi: 10.1046/j.1360-0443.1999.949138110.x [DOI] [PubMed] [Google Scholar]
- Moore TM, Seavey A, Ritter K, McNulty JK, Gordon KC, & Stuart GL (2014). Ecological momentary assessment of the effects of craving and affect on risk for relapse during substance abuse treatment. Psychology of Addictive Behaviors, 28, 619–624. doi: 10.1037/a0034127 [DOI] [PubMed] [Google Scholar]
- Moos RH (1968). The development of a menstrual distress questionnaire. sychosomatic Medicine, 30, 853–867. doi: 10.1097/00006842-196811000-00006 [DOI] [PubMed] [Google Scholar]
- Moos RH, Kopell BS, Melges FT, Yalom ID, Lunde DT, Clayton RB, & Hamburg DA (1969). Fluctuations in symptoms and moods during the menstrual cycle. Journal of Psychosomatic Research, 13, 37–44. doi: 10.1016/0022-3999(69)09917-8 [DOI] [PubMed] [Google Scholar]
- Pang RD, Andrabi N, & Leventhal AM (2017). Premenstrual symptoms and factors implicated in smoking cessation among woman smokers. Experimental and Clinical Psychopharmacology, 25, 235–241. doi: 10.1037/pha0000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, & McKee SA (2019). Sex differences in stress-related alcohol use. Neurobiology of Stress, 100149. doi: 10.1016/j.ynstr.2019.100149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky E. (1963). The woman alcoholic and premenstrual tension. Journal of the American Medical Women’s Association, 18, 816. [PubMed] [Google Scholar]
- Pomerleau CS, Cole PA, Lumley MA, Marks JL, & Pomerleau OF (1994). Effects of menstrual phase on nicotine, alcohol, and caffeine intake in smokers. Journal of Substance Abuse, 6, 227–234. doi: 10.1016/s0899-3289(94)90253-4 [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, … & Guidotti A. (2006). Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biological Psychiatry, 60, 704–713. doi: 10.1016/j.biopsych.2006.03.026 [DOI] [PubMed] [Google Scholar]
- Schüle C, Nothdurfter C, & Rupprecht R. (2014). The role of allopregnanolone in depression and anxiety. Progress in Neurobiology, 113, 79–87. doi: 10.1016/j.pneurobio.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Soares CN, & Zitek B. (2008). Reproductive hormone sensitivity and risk for depression across the female life cycle: A continuum of vulnerability? Journal of Psychiatry and Neuroscience, 33(4), 331–343. [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back: A technique for assessing self-reported ethanol consumption In Allen J. & Litten RZ (Eds.), Measuring alcohol consumption: Psychosocial and biological methods (pp. 41–72). Totowa, NJ: Humana Press. [Google Scholar]
- Sommer B. (1986). Task force report on guidelines for menstrual cycle research. Society for Menstrual Cycle Research Newsletter, 2, 1–2. [Google Scholar]
- Sripada RK, Marx CE, King AP, Ramptono JC, Ho SS, & Liberzon I. (2013). Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biological Psychiatry, 73, 1045–1053. doi: 10.1016/j.biopsych.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutker PB, Libet JM, Allain AN, & Randall CL (1983). Alcohol use, negative mood states, and menstrual cycle phases. Alcoholism: Clinical and Experimental Research, 7, 327–331. doi: 10.1111/j.1530-0277.1983.tb05472.x [DOI] [PubMed] [Google Scholar]
- Tobin MB, Schmidt PJ, & Rubinow DR (1994). Reported alcohol use in women with premenstrual syndrome. The American Journal of Psychiatry, 151, 1503–1504. doi: 10.1176/ajp.151.10.1503 [DOI] [PubMed] [Google Scholar]
- Walf AA, & Frye CA (2006). A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology, 31(6), 1097–1111. doi: 10.1038/sj.npp.1301067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Franklin TR, & Allen SS (2016). Ovarian hormones, menstrual cycle phase, and smoking: A review with recommendations for future studies. Current Addiction Reports, 3, 1–8. doi: 10.1007/s40429-015-0093-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S, & Donovan DM (2011). Moderating effects of a craving intervention on the relation between negative mood and heavy drinking following treatment for alcohol dependence. Journal of Consulting and Clinical Psychology, 79, 54–63. doi: 10.1037/a0022283 [DOI] [PMC free article] [PubMed] [Google Scholar]