Abstract
Simple Summary
In this study, we analyzed the microRNA (miRNA) sequencing libraries of the hen ovary at four different developmental stages (15, 20, 30, and 68 W) and found 209 differently expressed (DE) miRNAs in the six comparisons of the four stages, which might be involved in ovarian growth and functions. In particular, five key miRNAs (gga-miR-2954, gga-miR-6634-5p, gga-miR-449b-5p, gga-miR-449c-3p, and gga-miR449c-5p) targeting 23 predicted genes were obtained in the interaction network and functional enrichment analysis through a combination of DE miRNAs and differentially expressed genes (DEGs), which might be involved in cell differentiation and proliferation, steroid hormone biosynthesis, and angiogenesis in ovarian development. Our findings can contribute to a better understanding of the role of functional miRNAs in ovarian development and the ovulatory cycle during the four developmental stages of hens.
Abstract
It is well-known that multiple functional miRNAs are found in mammals’ ovaries, which are linked not only to ovarian development, but also to maturation and apoptosis. However, there is still a lack of knowledge regarding the role of miRNAs in the hen ovary. In the present study, we analyzed the miRNA sequencing libraries of ovaries at the four different developmental stages of hens (15, 20, 30, and 68 W) and a total of 677 known miRNAs and 61 novel miRNAs were identified. In total, 209 of them were differently expressed miRNAs (DE miRNAs) obtained from comparisons of the four stages, including 84 upregulated and 125 downregulated DE miRNAs. Furthermore, the five key DE miRNAs gga-miR-2954, gga-miR-6634-5p, gga-miR-449b-5p, gga-miR-449c-3p, and gga-miR449c-5p were screened using an analysis of the miRNA-mRNA interaction network and functional enrichment annotated in seven significantly enriched pathways, such as endocytosis, lysine degradation, the biosynthesis of amino acids, and the MAPK signaling pathway, which may primarily participate in cell differentiation and proliferation, steroid hormone biosynthesis, and angiogenesis by targeting the related genes. For instance, gga-miR-449 family members were predicted to target 15 genes, including TGFB1, TPM1, TPM3, and CAMKB2, which were reported to regulate follicular growth, selection, and the ovulatory cycle. Taken together, our results illustrate the ovarian miRNA profiles of the four classic developmental stages of hens and highlight the significant role of miRNAs in ovarian development and functions. However, in-depth research needs to be carried out to validate the potential functional miRNAs found in this study.
Keywords: DE miRNAs, ovarian function, target genes, different stages, hens
1. Introduction
The ovarian status with age-alteration is directly associated with the laying mechanism of hens, which regularly triggers ovulation and maintains the laying process of chickens. Each ovarian follicle is comprised of a follicular theca layer, a granulosa layer, and stroma surrounding an oocyte, and the structure and numbers of follicular cells differ significantly in ovarian developmental stages [1]. It has been reported that there are numerous regulatory factors involved in the regulation of follicular development and ovarian functions in chickens, such as IGFs, TGF-β, SF-1, and FOXL2, which can regulate the expression of related genes in follicular selection, growth and maturation, cell differentiation, and steroid hormone biosynthesis through various signaling pathways [2,3,4,5].
MicroRNAs (miRNAs) are well-known to be short noncoding RNAs, around 21–25 nt in length, that are found widely in eukaryotic cells and negatively regulate the transcription of gene expression by binding the 3’UTR region of the target gene [6]. In the few last years, a large number of functional miRNAs in mammalian ovaries have been shown to play important roles in the regulation of follicular development and atresia, cell proliferation and apoptosis, steroid hormone biosynthesis, and ovarian diseases [7,8,9,10,11]. For example, miR126-3p can promote porcine follicular cell proliferation by regulating PIK3R2 expression, and several miRNAs, including miR-26b, miR-34a, miR-125a, and miR-92a, can induce follicular granular cell apoptosis by targeting their individual genes [12,13]. Moreover, miR-375, miR-873, and miR-202 can influence the biosynthesis of steroid hormones in the ovary by inhibiting the expression of key genes [14,15]. In avian ovaries, miR-26a-5p was found to promote follicular theca cell proliferation, which was differently expressed in the mature and immature ovaries of chickens [16]. Additionally, miR-1b-3p was reported to possibly regulate the follicle development of hens [17]. However, research into functional miRNAs in the ovarian development and functions of chickens is still lagging behind and the process whereby the whole miRNA profiles in poultry ovaries are integrated during the reproductive process remains unclear. Therefore, the objective of our study was to explore the novel miRNAs that regulate follicular growth and the ovulatory cycle during the ages of 15, 20, 30, and 68 W, which represent the four critical stages of ovarian development: Initial development, first laying, and the high and low laying periods, respectively.
2. Materials and Methods
2.1. Ethics Statement
All experimental animals were treated while following the regulations for the administration of affairs concerning experimental animals. The animal care methods were approved by the Henan Agricultural University Institutional Animal Care and Use Committee (Permit Number: 19-0068).
2.2. Sample Collection, RNA Isolation, and Quality Analysis
A total of 36 Hy-line brown laying hens were collected at 15, 20, 30, and 68 weeks of age and were anesthetized by an intravenous wing injection of pentobarbital sodium (3%, 30 mg/kg body weight). Under deep anesthesia, each individual was euthanized by jugular vein bleeding. Then, we separated the whole ovarian tissues from all of the individuals, and froze and stored them at −80 ℃ in an ultra-freezer for the subsequent procedures. The total RNA of ovarian tissues was extracted using the Trizol RNA extraction reagent (Invitrogen, Carlsbad, CA, USA), and a total of 12 RNA samples was obtained, with each sample mixing three tissues at each age, in order to eliminate the differences between individuals. The purity and concentration of the RNA were determined using a NanoPhotometer® spectrophotometer (IMPLEN, Westlake Village, CA, USA) and a Qubit® RNA Assay Kit (Life Technologies, Carlsbad, CA, USA). The integrity of the RNA was evaluated using a Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, Palo Alto, CA, USA).
2.3. Library Preparation and Sequencing
Three micrograms of total RNA per sample were prepared as the input for the small RNA library. The preparation and sequencing of the library were carried out using NEBNext® Mutiple Small RNA library Prep Set (NEB, Lpswich, MA, USA) and the Illumina Hiseq 2500/2000 platform (Illumina, San Diego, CA, USA) to generate 50 bp single-end reads. The quality of the library was measured using an Agilent Bioanalyzer 2100 system (Agilent Technologies, Palo Alto, CA, USA) with DNA High Sensitivity Chips. A total of 12 small RNA libraries were obtained in this study.
2.4. Distribution and Identification of miRNA
Low quality reads, 3′ adapter/insert tag deletion, 5′ adapter contaminants, and Ploy A/T/G/C in the raw data were removed to obtain clean reads using Illumina CASAVA (version 1.8, http://www.illumina.com/) in the preprocessing. A length of 18–30 nt from the clean reads was filtered for the downstream analysis. Known miRNA alignments were identified using miRBase 20.0 and described those that started with “gga-miR-”. Novel miRNA was predicted using miREvo and mirdeep2 [18,19], describing that which started with “novel-”.
2.5. Differential Expression of miRNA and mRNA and Clustering Analysis
The miRNA and mRNA expression levels were presented as the transcript per million (TPM) and fragments per kilo-base of exon per million fragments mapped (FPKM), respectively [20,21]. The differential expression of miRNA and mRNA between every two ages was performed using the DESeq R package (version 3.4.2, http://www.R-project.org/). An adjusted p value < 0.05 and |log2 (fold change)| > 1 were set as the thresholds to identify significantly differently expressed miRNAs (DE miRNAs) and differently expressed mRNA (DE mRNA). Furthermore, a Venn diagram was produced using the limma package in R (version 3.32.5, http://www.bioconductor.org/packages/release/bioc/html/limma.html). Hierarchical clustering analyses were used to demonstrate the expression profiles of miRNAs during different ovarian development stages of hens.
2.6. Target Gene Prediction, Enrichment, and Interaction Network Analysis
The target genes of miRNAs were predicted by psRobot_tar in miRanda for animals and integrated with the differentially expressed gene (DEG) profiles to reinforce the accuracy of prediction [22]. In this study, the raw profile of mRNA was submitted in the NCBI database and contributed to analyzing the prediction of the target gene (more details in the Data Availability section). The negative correlation of miRNA and mRNA was selected by calculating the Pearson value. Cytoscape (version 3.7.2, http://www.cytoscape.org/) was used for the miRNA-mRNA interaction network analysis. All of the potential target genes of DE miRNAs among the combinations of the four developmental stages were used for gene ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses, which were implemented by the GOseq R package (version 2.12, http://www.geneontology.org/) and KOBAS software (version 2.0, http://www.genome.jp/kegg/). GO terms with a p-value < 0.01 and KEGG pathways with p < 0.05 were considered to be significantly enriched.
2.7. Validation of miRNA Expression
To validate the RNA-seq of the miRNA data, we analyzed five highly expressed miRNAs using reverse transcription real-time PCR (RT-qPCR). Bulge-loop miRNA RT-qPCR primer sets (RiboBio, Guangzhou, China) were used to amplify miRNAs and the process of RT-qPCR was performed, following the manufacturer’s recommendation. The results were analyzed using the 2−∆∆Ct method. The primer sequences of the selected miRNAs and internal reference-chicken U6 RNA were designed via RiboBio (RiboBio, Guangzhou, China).
3. Results
3.1. Sequencing Analysis
In the sequencing libraries, we obtained 12,739,770 raw reads, 12,604,754 clean reads, and 11,760,062 mapped reads, with a 99.76% Q20 content on average, which indicated the high quality of the Illumina sequencing. In addition, the miRNA sequences of the ovaries in the four developmental stages were generally 21–23 nt in length, occupying 83.99% (Table 1). A total of 738 miRNAs were generated in our transcriptome data, including 677 known miRNAs and 61 novel miRNAs, which were used for the following analyses.
Table 1.
Overview of sequencing data for small RNA.
| Samples | Raw Reads | Clean Reads | Clean Ratio (%) | Mapped Reads | Mapped Ratio (%) | Q20 (%) | Frequency Percent of 21–23 nt (%) |
|---|---|---|---|---|---|---|---|
| O15_1 | 12,668,758 | 12,485,845 | 98.56 | 9,568,340 | 94.1 | 99.81 | 79.25 |
| O15_2 | 17,026,099 | 16,850,839 | 98.97 | 11,052,700 | 96.31 | 99.76 | 80.54 |
| O15_3 | 11,521,207 | 11,369,282 | 98.68 | 11,614,036 | 95.84 | 99.69 | 79.78 |
| O20_1 | 13,211,633 | 13,123,238 | 99.33 | 10,360,873 | 94.65 | 99.69 | 77.09 |
| O20_2 | 13,225,084 | 13,073,265 | 98.85 | 10,436,434 | 95.82 | 99.73 | 86.56 |
| O20_3 | 11,191,768 | 11,061,677 | 98.84 | 10,414,140 | 96.03 | 99.7 | 85.07 |
| O30_1 | 12,553,362 | 12,421,066 | 98.95 | 10,578,899 | 95.37 | 99.74 | 83.34 |
| O30_2 | 11,766,007 | 11,652,830 | 99.04 | 11,540,385 | 95.37 | 99.81 | 87.53 |
| O30_3 | 11,300,865 | 11,136,431 | 98.54 | 12,259,060 | 96.56 | 99.7 | 87.78 |
| O68_1 | 16,894,428 | 16,730,280 | 99.03 | 12,135,277 | 96.15 | 99.67 | 85.81 |
| O68_2 | 11,172,817 | 11,062,162 | 99.01 | 15,619,129 | 95.14 | 99.82 | 87.39 |
| O68_3 | 10,345,214 | 10,290,138 | 99.47 | 15,541,471 | 94.27 | 99.85 | 87.72 |
| Average | 12,739,770 | 12,604,754 | 98.94 | 11,760,062 | 95.47 | 99.76 | 83.99 |
Note: Each sample name consists of letters and numerals. The capital letters with numerals 15, 20, 30, and 68 correspond to the tissues of the ovary and the four different weeks of developmental stages, while the numerals 1, 2, and 3 correspond to the three samples in each stage. This naming convention is the same as that used in the following tables and figures.
3.2. DE miRNA Analysis
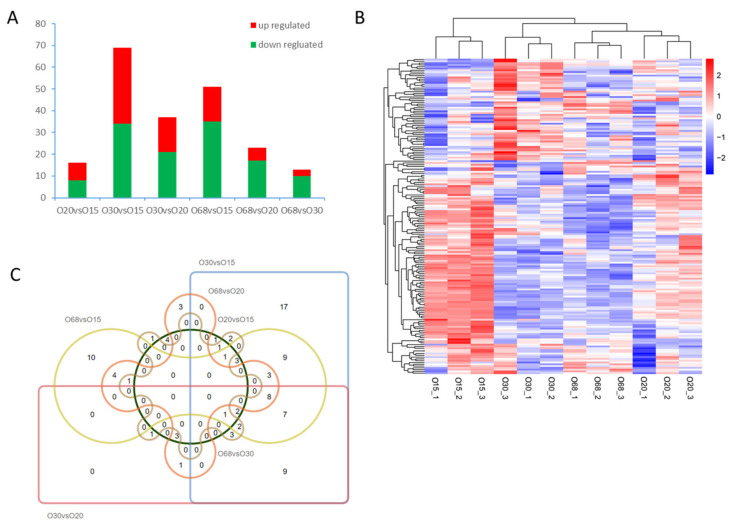
To reveal the distribution of DE miRNAs, a bar graph, heatmap, and Venn diagram were developed to demonstrate the up- and downregulated miRNAs, the expression profiles, and the co-expressed/specific miRNAs among the combinations of different developmental stages. In total, 209 DE miRNAs were found in our data, with 84 upregulated and 125 downregulated DE miRNAs. In particular, the maximum number of DE miRNAs (69) was obtained in the comparison of O30 and O15, which indicated variation in the preliminary and vigorous growth stages of the hen ovary compared to the others (Figure 1A). Furthermore, there were similar expression models of miRNAs between O30 and O68, most of which were different from those in O15 and O30 (Figure 1B). In the Venn diagrams, no co-expressed miRNAs were found when considering all six combinations (Figure 1C). However, were eight co-expressed DE miRNAs (e.g., gga-miR-135a-5p, gga-miR-202-5p, gaa-miR-31-5p, gga-miR-34a-5p, gga-miR-449a, gga-miR-449b-5p, gga-miR-449d-5p, and gga-miR-499-5p) were obtained in the comparisons of O30 vs. O15, O68 vs. O15, O30 vs. O20, and O68 vs. O20, which might be involved in the physiological functions of the ovary before and after the egg-laying of hens.
Figure 1.
The distribution of differentially expressed microRNAs (DE miRNAs) in different developmental stages. (A) The numbers of upregulated and downregulated DE miRNAs among the six comparison groups. (B) Hierarchical clustering heatmap of the DE miRNA expression profiles in the four developmental stages. (C) Venn diagrams of the DE miRNAs among the six comparison groups.
3.3. Interaction Network Analysis of miRNA-mRNA
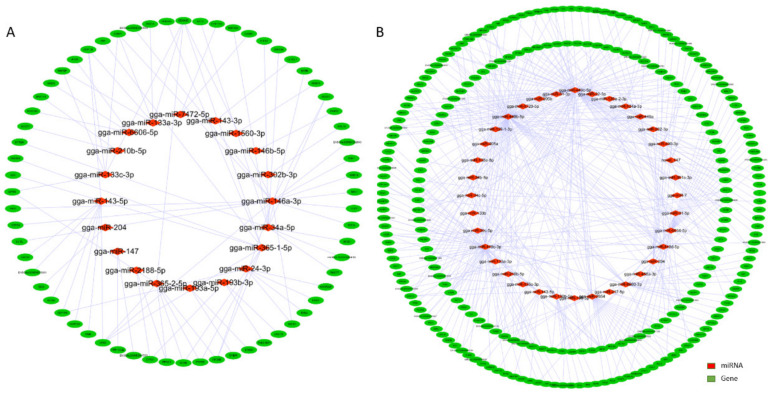
To investigate the potential interaction of miRNA-mRNA, a miRNA-mRNA network was constructed based on a comparison of the predicted target genes of all DE miRNAs and DEG profiles among the six combinations, as well as the negative correlation of DE miRNA-mRNA with the different expression models at the four ages of the hens. Within the interaction network, 51 miRNAs and 290 predicted genes formed 479 negative miRNA-mRNA regulatory networks, among which there were 86 upregulated miRNAs with downregulated genes and 393 downregulated miRNAs with upregulated genes (Figure 2A,B). The top five miRNAs associated with the largest number of target genes were gga-miR-449b-5p, gga-miR449c-5p, gga-miR-449c-3p, gga-miR-6634-5p, and gga-miR-2954, respectively. Notably, all of them were downregulated DE miRNAs during the different developmental stages. The concrete profile of the interaction network, the expression levels, and Pearson’s correlation analyses of DE miRNAs and DE mRNAs are listed in Supplementary Table S1.
Figure 2.
The interaction network of DE miRNA-differentially expressed gene (DEG). (A) The interaction network of the upregulated miRNA and downregulated target genes. (B) The interaction network of downregulated miRNAs and upregulated target genes. The red rhombus and green oval represent the miRNAs and predicted target genes, respectively.
3.4. Functional Enrichment Analysis
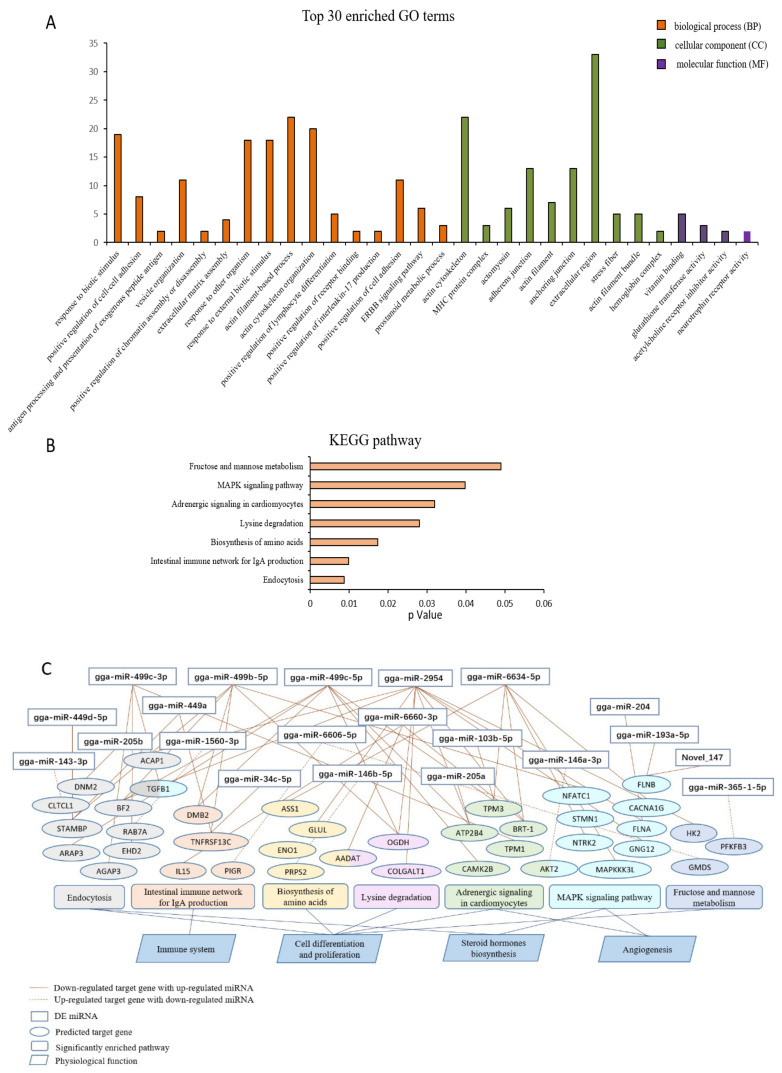
Using the predicted target genes of the DE miRNAs obtained from the interaction network analysis, we performed a GO and KEGG enrichment analysis to reveal the potential role of DE miRNAs in the ovarian function. The top 30 enriched GO terms are shown in Figure 3A, indicating that the most enriched BP, CC, and MF terms responded to the biotic stimulus, actin cytoskeleton, and vitamin binding, respectively. Moreover, the BP terms mainly focused on the responses to external stimuli, cell differentiation and proliferation, and the immune system, which are strongly associated with tissue development. Furthermore, it was revealed that 101 target genes were annotated in 92 pathways, among which seven significantly enriched pathways were obtained, including endocytosis, the intestinal immune network for IgA production, the biosynthesis of amino acids, lysine degradation, adrenergic signaling in cardiomyocytes, the MAPK signaling pathway, and fructose and mannose metabolism (p < 0.05) (Figure 3B). To further explore the key miRNAs and predicted target genes, we constructed a potential correlation network of the miRNAs-gene-pathway-function and demonstrated that gga-miR-499c-3p, gga-miR-499b-5p, gga-miR-499c-5p, gga-miR-2954, and gaa-miR-6634-5p were widely involved in the regulation of multiple target genes in various significantly enriched pathways (Figure 3C). In particular, the predicted target genes of gga-miR-449c-5p and gga-miR-2954–TGFB1 were involved in both the endocytosis and MAPK signaling pathways, and AADAT was involved in both the biosynthesis of amino acids and lysine degradation, respectively. Interestingly, the five key miRNAs were the same as those obtained in the interaction network of miRNA-mRNA, which suggests that these miRNAs have play major roles in ovarian development and the ovulatory cycle, such as cell differentiation and proliferation, steroid hormone biosynthesis, and angiogenesis. The concrete profiles of GO and KEGG enrichment analysis are listed in Supplementary Tables S2 and S3, respectively.
Figure 3.
Functional enrichment analysis for DE miRNAs-DEGs. (A) The top 30 gene ontology (GO) terms from the GO enrichment analysis of the predicted target genes. (B) The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway significant enrichment analysis of predicted target genes. (C) The potential correlation network of miRNA-gene-pathway-function.
3.5. Integrated Analysis of Key DE miRNAs
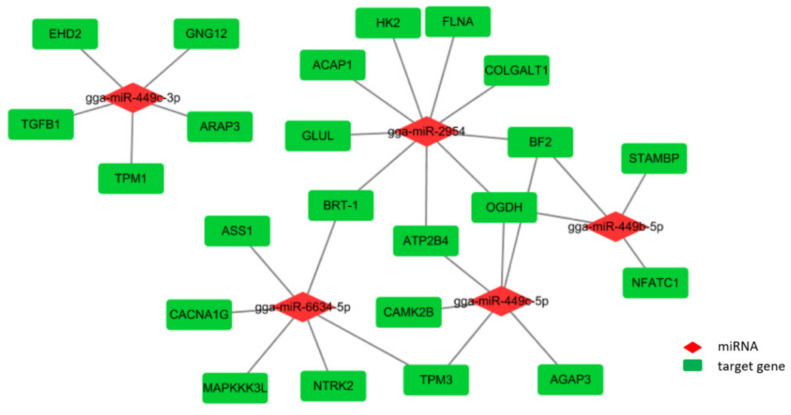
According to the above analysis of the interaction network and enrichment annotation, we integrated the key possible miRNA-mRNA networks that may modulate the ovarian function during the different developmental stages. It was shown that the five key miRNAs associated with a total of 23 target genes formed 30 combined pairs in this network, among which gga-miR-449b-5p was co-expressed with DE miRNAs in the comparisons of O30 vs. O15, O30 vs. O20, O68 vs. O15, and O68 vs. O20, as previously mentioned, which might regulate the expression of NFATC1, STAMBP, BF2, and OGDH. Meanwhile, OGDH and BF2 were both targets of gga-miR-449b-5p, gga-miT-449c-5p, and gga-miR-2954. TPM3 was the target of gga-miR-449c-5p and gga-miR-6634-5p, and BRT-1 was the target of gga-miR-2954 and gga-6634-5p. All of the results indicate that these key miRNAs might be involved in the physiological process of ovarian development and function through the related signaling pathways (Figure 4).
Figure 4.
Integrated key miRNA-mRNA network. The red rhombus and green rectangle represent the key miRNAs and predicted target genes, respectively.
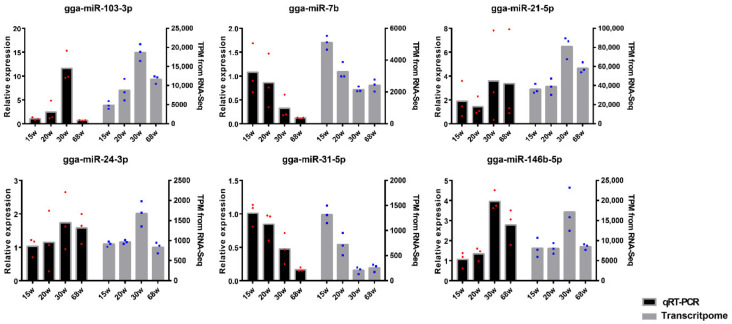
3.6. Validation of miRNA Profiles by qRT-PCR
Using these data, we validated six highly expressed miRNAs (e.g., gga-miR-103-3p, gga-miR-7b, gga-miR-21-5p, gga-miR-24-3p, gga-miR-31-5p, and gga-miR-146b-5p) using qRT-PCR (Figure 5). All of the expression results are consistent with those in RNA-seq data and provided a great reliability for conducting the subsequent research.
Figure 5.
The validation of highly expressed miRNAs between qRT-PCR and the transcriptome. The left y-axis of the above graph shows the value of relative expression and the right y-axis shows the value of transcript per million (TPM). The red and blue dots correspond to the three biological replicates.
4. Discussion
The ovarian morphology and functions were extremely variable in the different developmental stages of the hens. The ovary developed very slowly, with several primary and secondary follicles appearing before sexual maturation, while the rapid growth of the ovary mainly occurred after laying the first egg [23]. The ovarian shape during the peak laying period was much larger than that in the other periods and consisted of numerous follicles with various sizes to maintain the process of frequent egg laying [24]. Then, the capacity of egg production declined with age due to a high rate of follicle atresia in the ovary [25]. Based on this result, we sought to assess whether functional miRNAs play a crucial role in ovarian development and functions along with the changing age of hens. Therefore, in this study, we determined the miRNA profiles in the four classic stages of ovarian development (15, 20, 30, and 68 W) using RNA-seq, which also represented the first study analyzing all four different stages of hens’ ovarian development. A total of 677 known miRNAs and 61 novel miRNAs were identified, and 209 DE miRNAs were obtained among the comparisons of the four stages of development. Throughout all the DE miRNAs, there were 84 upregulated and 125 downregulated DE miRNAs in our data, of which eight co-existing DE miRNAs (gga-miR-135a-5p, gga-miR-202-5p, gaa-miR-31-5p, gga-miR-34a-5p, and gga-miR-449 family members) were observed in the comparisons of O30 vs. O15, O30 vs. O15, O30 vs. O20, O68 vs. O15, and O68 vs. O15 using Venn diagrams, likely related to the ovarian maturation and ovulation mechanism. Furthermore, miR-202-5p, miR-34a, miR-31-5p, and miR-135a have been reported to be involved in ovarian granulosa cellular proliferation, apoptosis, and steroidogenesis in mammals [7,26,27,28]. However, the role of mi-449 family members in the ovary remains unclear, except for miR-499a and miR-449b, which have been proven to lead to cell cycle arrest in ovarian cancer cells [29].
In the GO and KEGG enrichment analyses, we found that the BP categories of the top 30 GO terms and the significantly enriched pathways were both involved in the regulation of tissue development and the immune system, which responded to the ovarian alteration status with age. To more deeply excavate the key miRNAs and target genes, we developed an interaction network of miRNA-mRNA and enrichment analyses of the target genes, indicating that gga-miR-2954, gga-miR-6634-5p, gga-miR-449b-5p, gga-miR-449c-3p, and gga-miR-449c-5p did not only widely target multiple genes, but were also significantly enriched in seven pathways, including the endocytosis, biosynthesis of amino acids, and MAPK signaling pathways (p < 0.05). In addition, all of these genes were downregulated by differently expressed miRNAs.
Then, we filtered 23 target genes in the binding of the five key miRNAs that may be associated with ovarian development and function and obtained a total of 30 combined pairs. Some studies have reported gga-miR-2954 to be an essential miRNA related to the sex differences of poultry [30,31]. However, our results predict that it is an important DE miRNA among the developmental stages of hens, which was also determined in the granulosa and thecal layer of geese ovarian follicles, indicating that gga-miR-295 may be related to follicle selection [32]. Unfortunately, there was no evidence demonstrating the role of gga-miR-6634-5p in the ovarian development and function of hens. Furthermore, it was interesting that the gga-miR-449 family members co-existed in the comparison of before (15 and 20 W) and after (30 and 68 W) the continuous laying periods and were involved in all seven significantly enriched pathways by targeting 15 genes, among which TGFB1 was linked to endocytosis and the MAPK signaling pathway that play a major role in steroid hormones synthesis and follicular development [33,34]; TPM1 was involved in the regulation of angiogenesis by reducing the VEGF expression [35]; and TPM3, ATP2B4, CAMK2B, and NFATC1 might also be associated with angiogenesis. Clearly, both steroidogenesis and angiogenesis in follicles significantly differed and played a crucial role in follicular selection, maturation, and the ovulatory cycle during the ovarian developmental stages of hens, in which the miR-449 family may contribute to regulation by targeting the related genes [36,37]. The miR-449 family has been suggested to be an indicator of a weak expression in cancer cells, inhibiting cell proliferation and migration to suppress tumor development in various tissues of mammals, such as the liver, stomach, and ovary [38,39,40]. However, the function of miR-449 in the normal ovaries of poultry is still unknown. Therefore, further research must be performed to explore the roles of key miRNAs in ovarian functions.
5. Conclusions
In summary, our study highlighted the role of miRNAs in ovarian development and functions, first outlining the transcriptome analysis of miRNAs during the four classic ovarian developmental stages of hens. Meanwhile, it was indicated that five key DE miRNAs, including gga-miR-449b-5p, gga-miR-499c-3p, gga-miR-449c-5p, gga-miR-2954, and gga-miR-6634-5p, might play a major role in the process of follicular development, steroidogenesis, and angiogenesis. Therefore, our findings will contribute to a better understanding of the role of miRNAs in ovarian development and physiological functions during the four stages of the hen ovary.
Acknowledgments
We would like to thank the poultry germplasm resources farm for feeding and providing the trial animals, and all the students of the Henan Innovative Engineering Research Center of Poultry Germplasm Resource for their contribution to sample collection and data analysis.
Abbreviations
IGF: insulin-like growth factor; TGF-β: transforming growth factor-beta; SF-1: steroidogenic factor 1; FOXL2: Forkhead Box L2; UTR: untranslated region; PIK3R2: phosphoinsitide-3-kinase regulatory subunit 2; TPM: transcript per million; FPKM: fragments per kilo-base of exon per million fragments mapped; DE miRNAs: differentially expressed miRNAs; DEG: differentially expressed genes; GO: Gene Ontology; KEGG: Kyoto Encyclopedia of Genes and Genomes; RT-qPCR: reverse transcription real-time PCR; BP: biological process; CC: cellular component; MF: molecular function; MAPK: mitogen-activated protein kinase; TGFB1: transforming growth factor beta 1; AADTA: aminoadipate aminotransferase; NFATC1: nuclear factor of activated T cells 1; STAMBP: STAM binding protein; BF2: MHC class I; OGDH: oxoglutarate dehydrogenase; BRT-1: beta-tropomyosin; TPM3: tropomyosin 3; TPM1: tropomyosin 1; VEGF: vascular endothelial growth factor; ATP2B4: ATPase plasma membrane Ca2+ transporting 4; CAMK2B: calcium/calmodulin-dependent protein kinase II beta.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/10/9/1680/s1: Supplementary Table S1. A list of the interaction network, the expression levels, and Pearson’s correlation coefficient of DE miRNAs and DE mRNAs; Supplementary Table S2. A list of the top 30 significantly enriched GO terms of all the target genes of DE miRNAs in the ovary during the four development stages of hens (p < 0.01); Supplementary Table S3. A list of the significant KEGG classification of target genes of DE miRNAs in the ovary during the four development stages of hens (p < 0.05).
Author Contributions
Experimental design, Y.-D.T. and X.-T.K.; writing and conducted most of the data analysis, J.L.; validation and preparation of experimental materials, C.L. and Q.L.; part of the data analysis, W.-T.L., H.L., and G.-X.L.; Y.-D.T., X.-J.L., and X.-T.K. acquired the funding for the study. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Science Foundation of China (Project No. 31872987), China Agriculture Research System (NO. CARS-40-K04), Scientific Studio of Zhongyuan Scholars (30601985), and NSFC-Henan Province Joint Fund (U1704233).
Conflicts of Interest
All the authors declare that there are no conflicts of interest.
References
- 1.Zakaria A.H., Miyaki T., Imai K. The Effect of Aging on the Ovarian Follicular Growth in Laying Hens. Poult. Sci. 1983;62:670–674. doi: 10.3382/ps.0620670. [DOI] [PubMed] [Google Scholar]
- 2.Barua A., Yoshimura Y., Tamura T. Effects of ageing and oestrogen on the localization of immunoglobulin-containing cells in the chicken ovary. J. Reprod. Fertil. 1998;114:11–16. doi: 10.1530/jrf.0.1140011. [DOI] [PubMed] [Google Scholar]
- 3.Wang J., Gong Y. Transcription of CYP19A1 is directly regulated by SF-1 in the theca cells of ovary follicles in chicken. Gen. Comp. Endocrinol. 2017;247:1–7. doi: 10.1016/j.ygcen.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Onagbesan O.M., Vleugels B., Buys N., Bruggeman V., Safi M., Decuypere E. Insulin-like growth factors in the regulation of avian ovarian functions. Domest. Anim. Endocrinol. 1999;17:299–313. doi: 10.1016/S0739-7240(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 5.Onagbesan O., Bruggeman V., Decuypere E. Intra-ovarian growth factors regulating ovarian function in avian species: A review. Anim. Reprod. Sci. 2009;111:121–140. doi: 10.1016/j.anireprosci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Bushati N., Cohen S.M. microRNA Functions. Annu. Rev. Cell Dev. Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 7.Maalouf S.W., Liu W., Pate J.L. MicroRNA in ovarian function. Cell Tissue Res. 2015;363:7–18. doi: 10.1007/s00441-015-2307-4. [DOI] [PubMed] [Google Scholar]
- 8.Sen A., Prizant H., Light A., Biswas A., Hayes E., Lee H.-J., Barad D., Gleicher N., Hammes S.R. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc. Natl. Acad. Sci. USA. 2014;111:3008–3013. doi: 10.1073/pnas.1318978111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebremedhn S., Salilew-Wondim D., Ahmad I., Sahadevan S., Hossain M., Hoelker M., Rings F., Neuhoff C., Tholen E., Looft C., et al. MicroRNA Expression Profile in Bovine Granulosa Cells of Preovulatory Dominant and Subordinate Follicles during the Late Follicular Phase of the Estrous Cycle. PLoS ONE. 2015;10:e0125912. doi: 10.1371/journal.pone.0125912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yerushalmi G.M., Salmon-Divon M., Ophir L., Yung Y., Baum M., Coticchio G., Fadini R., Mignini-Renzini M., Canto M.D., Machtinger R., et al. Characterization of the miRNA regulators of the human ovulatory cascade. Sci. Rep. 2018;8:15605. doi: 10.1038/s41598-018-33807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J., Xu Y.-X., Liu H., Pan Z. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod. Biol. Endocrinol. (RBE) 2019;17:9. doi: 10.1186/s12958-018-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y., Chen Q., Qin R., Zhang K., Li H. MicroRNA-449a reduces cell survival and enhances cisplatin-induced cytotoxicity via downregulation of NOTCH1 in ovarian cancer cells. Tumor Biol. 2014;35:12369–12378. doi: 10.1007/s13277-014-2551-3. [DOI] [PubMed] [Google Scholar]
- 13.Worku T., Rehman Z.U., Talpur H.S., Bhattarai D., Ullah F., Malobi N., Kebede T., Yang L. MicroRNAs: New Insight in Modulating Follicular Atresia: A Review. Int. J. Mol. Sci. 2017;18:333. doi: 10.3390/ijms18020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donadeu F.X., Sontakke S.D., Ioannidis J. MicroRNA indicators of follicular steroidogenesis. Reprod. Fertil. Dev. 2017;29:906. doi: 10.1071/RD15282. [DOI] [PubMed] [Google Scholar]
- 15.Yu C., Li M., Wang Y., Liu Y., Yan C., Pan J., Liu J., Cui S. miR-375 mediates CRH signaling pathway in inhibiting E2 synthesis in porcine ovary. Reproduction. 2017;153:63–73. doi: 10.1530/REP-16-0323. [DOI] [PubMed] [Google Scholar]
- 16.Kang L., Yang C., Wu H., Chen Q., Huang L., Li X., Tang H., Jiang Y. miR-26a-5p Regulates TNRC6A Expression and Facilitates Theca Cell Proliferation in Chicken Ovarian Follicles. DNA Cell Biol. 2017;36:922–929. doi: 10.1089/dna.2017.3863. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Hou L., Sun Y., Xing J., Jiang Y., Kang L. Single nucleotide polymorphism rs737028527 (G>A) affect miR-1b-3p biogenesis and effects on chicken egg-laying traits. Anim. Reprod. Sci. 2020;218:106476. doi: 10.1016/j.anireprosci.2020.106476. [DOI] [PubMed] [Google Scholar]
- 18.Friedländer M.R., Mackowiak S.D., Li N., Chen W., Rajewsky N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012;40:37–52. doi: 10.1093/nar/gkr688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen M., Shen Y., Shi S., Tang T. miREvo: An integrative microRNA evolutionary analysis platform for next-generation sequencing experiments. BMC Bioinform. 2012;13:140. doi: 10.1186/1471-2105-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou L., Chen J., Li Z., Li X., Hu X., Huang Y., Zhao X., Liang C., Wang Y., Sun L., et al. Integrated Profiling of MicroRNAs and mRNAs: MicroRNAs Located on Xq27.3 Associate with Clear Cell Renal Cell Carcinoma. PLoS ONE. 2010;5:e15224. doi: 10.1371/journal.pone.0015224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pertea M., Kim D., Pertea G.M., Leek J.T., Salzberg S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enright A.J., John B., Gaul U., Tuschl T., Sander C., Marks D.S. MicroRNA targets in Drosophila. Genome Biol. 2003;5:R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson F.E., Renema R.A., Oosterhoff H.H., Zuidhof M., Wilson J.L. Carcass Traits, Ovarian Morphology and Egg Laying Characteristics in Early Versus Late Maturing Strains of Commercial Egg-Type Hens. Poult. Sci. 2001;80:37–46. doi: 10.1093/ps/80.1.37. [DOI] [PubMed] [Google Scholar]
- 24.Johnson A.L., Woods D.C. Dynamics of avian ovarian follicle development: Cellular mechanisms of granulosa cell differentiation. Gen. Comp. Endocrinol. 2009;163:12–17. doi: 10.1016/j.ygcen.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Waddington D., Perry M.M., Gilbert A.B., Hardie M.A. Follicular growth and atresia in the ovaries of hens (Gallus domesticus) with diminished egg production rates. J. Reprod. Fertil. 1985;74:399–405. doi: 10.1530/jrf.0.0740399. [DOI] [PubMed] [Google Scholar]
- 26.Yu H.-Y., Pan S.-S. MiR-202-5p suppressed cell proliferation, migration and invasion in ovarian cancer via regulating HOXB2. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2256–2263. doi: 10.26355/eurrev_202003_20491. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z., Chen C.-Z., Xu M.-Q., Zhang L.-Q., Liu J.-B., Gao Y., Jiang H., Yuan B., Zhang J.-B. MiR-31 and miR-143 affect steroid hormone synthesis and inhibit cell apoptosis in bovine granulosa cells through FSHR. Theriogenology. 2019;123:45–53. doi: 10.1016/j.theriogenology.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Wei Y., Lu S., Hu Y., Guo L., Wu X., Liu X., Sun Y. MicroRNA-135a Regulates VEGFC Expression and Promotes Luteinized Granulosa Cell Apoptosis in Polycystic Ovary Syndrome. Reprod. Sci. 2020;27:1436–1442. doi: 10.1007/s43032-020-00155-0. [DOI] [PubMed] [Google Scholar]
- 29.Yuan J.-M., Shi X.-J., Sun P., Liu J.-X., Wang W., Li M., Ling F.-Y. Downregulation of cell cycle-related proteins in ovarian cancer line and cell cycle arrest induced by microRNA. Int. J. Clin. Exp. Med. 2015;8:18476–18481. [PMC free article] [PubMed] [Google Scholar]
- 30.Luo G., Hafner M., Shi Z., Brown M., Feng G.-H., Tuschl T., Wang X.-J., Li X. Genome-wide annotation and analysis of zebra finch microRNA repertoire reveal sex-biased expression. BMC Genom. 2012;13:727. doi: 10.1186/1471-2164-13-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warnefors M., Mössinger K., Halbert J., Studer T., VandeBerg J.L., Lindgren I., Fallahshahroudi A., Jensen P., Kaessmann H. Sex-biased microRNA expression in mammals and birds reveals underlying regulatory mechanisms and a role in dosage compensation. Genome Res. 2017;27:1961–1973. doi: 10.1101/gr.225391.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q., Hu S., Wang Y., Deng Y., Yang S., Hu J., Li L., Wang J. mRNA and miRNA Transcriptome Profiling of Granulosa and Theca Layers from Geese Ovarian Follicles Reveals the Crucial Pathways and Interaction Networks for Regulation of Follicle Selection. Front. Genet. 2019;10 doi: 10.3389/fgene.2019.00988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu G., Kang L., Wei Q., Cui X., Wang S., Chen Y., Jiang Y. Expression and Regulation of MMP1, MMP3, and MMP9 in the Chicken Ovary in Response to Gonadotropins, Sex Hormones, and TGFB11. Biol. Reprod. 2014;90:57. doi: 10.1095/biolreprod.113.114249. [DOI] [PubMed] [Google Scholar]
- 34.Rosairo D., Kuyznierewicz I., Findlay J., Drummond A. Transforming growth factor-beta: Its role in ovarian follicle development. Reproduction. 2008;136:799–809. doi: 10.1530/REP-08-0310. [DOI] [PubMed] [Google Scholar]
- 35.Wang J., Tang C., Yang C., Zheng Q., Hou Y. Tropomyosin-1 Functions as a Tumor Suppressor with Respect to Cell Proliferation, Angiogenesis and Metastasis in Renal Cell Carcinoma. J. Cancer. 2019;10:2220–2228. doi: 10.7150/jca.28261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebedeva I.Y., A Lebedev V., Grossmann R., Parvizi N. Age-dependent role of steroids in the regulation of growth of the hen follicular wall. Reprod. Biol. Endocrinol. (RBE) 2010;8:15. doi: 10.1186/1477-7827-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D., Lee J., Johnson A.L. Vascular endothelial growth factor and angiopoietins during hen ovarian follicle development. Gen. Comp. Endocrinol. 2016;232:25–31. doi: 10.1016/j.ygcen.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Lizé M., Klimke A., Dobbelstein M. MicroRNA-449 in cell fate determination. Cell Cycle. 2011;10:2874–2882. doi: 10.4161/cc.10.17.17181. [DOI] [PubMed] [Google Scholar]
- 39.Kheir T.B., Futoma-Kazmierczak E., Skanderup A.J., Krogh A., Bardram L., Hother C., Grønbæk K., Federspiel B., Lund A.H., Friis-Hansen L. miR-449 inhibits cell proliferation and is down-regulated in gastric cancer. Mol. Cancer. 2011;10:29. doi: 10.1186/1476-4598-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandbothe M., Buurman R., Reich N., Greiwe L., Vajen B., Gürlevik E., Schäffer V., Eilers M., Kühnel F., Vaquero A., et al. The microRNA-449 family inhibits TGF-β-mediated liver cancer cell migration by targeting SOX4. J. Hepatol. 2017;66:1012–1021. doi: 10.1016/j.jhep.2017.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.