Abstract
Introduction
Virtual Communities of Practice (VCoP) or knowledge-sharing virtual communities offer ubiquitous access to information and exchange possibilities for people in similar situations, which might be especially valuable for the self-management of patients with chronic diseases. In view of the scarce evidence on the clinical and economic impact of these interventions on chronic conditions, we aim to evaluate the effectiveness and cost-effectiveness of a VCoP in the improvement of the activation and other patient empowerment measures in patients with ischaemic heart disease (IHD).
Methods and analysis
A pragmatic randomised controlled trial will be performed in Catalonia, Madrid and Canary Islands, Spain. Two hundred and fifty patients with a recent diagnosis of IHD attending the participating centres will be selected and randomised to the intervention or control group. The intervention group will be offered participation for 12 months in a VCoP based on a gamified web 2.0 platform where there is interaction with other patients and a multidisciplinary professional team. Intervention and control groups will receive usual care. The primary outcome will be measured with the Patient Activation Measure questionnaire at baseline, 6, 12 and 18 months. Secondary outcomes will include: clinical variables; knowledge (Questionnaire of Cardiovascular Risk Factors), attitudes (Self-efficacy Managing Chronic Disease Scale), adherence to the Mediterranean diet (Mediterranean Diet Questionnaire), level of physical activity (International Physical Activity Questionnaire), depression (Patient Health Questionnaire), anxiety (Hospital Anxiety Scale-A), medication adherence (Adherence to Refill Medication Scale), quality of life (EQ-5D-5L) and health resources use. Data will be collected from self-reported questionnaires and electronic medical records.
Ethics and dissemination
The trial was approved by Clinical Research Ethics Committee of Gregorio Marañón University Hospital in Madrid, Nuestra Señora de Candelaria University Hospital in Santa Cruz de Tenerife and IDIAP Jordi Gol in Barcelona. The results will be disseminated through workshops, policy briefs, peer-reviewed publications, local/international conferences.
Trial registration number
ClinicalTrials.gov Registry (NCT03959631). Pre-results.
Keywords: coronary heart disease, general medicine (see internal medicine), geriatric medicine, adult cardiology
Strengths and limitations of this study.
We will experimentally test an innovative learning intervention based on a Virtual Community of Practice (VCoP) for patient empowerment, for which the literature lacks experimental evaluations.
VCoP can enhance communication between community members in different geographic locations and even from different time zones.
Participation rate can be low as similar experiences have shown; we will include the active role of a community manager, weekly emails as reminders and a gamified competitive score system to boost participation.
Since all randomised patients will be required a minimum level of digital literacy so, the results could not be generalised to all patients.
Patients belonging to the control group and intervention group could receive a different type of self-management support depending on the centres where the care is provided.
Introduction
In Western countries, ischaemic heart disease (IHD) is a major public concern, and although mortality from IHD has been significantly reduced since 2000, it remains as a leading cause of death (50.6 deaths/100 000 inhabitants in Spain and 106.6 deaths/100 000 inhabitants in the USA in 2016).1 In Spain, 32 325 people died from IHD in 2017, according to the National Institute of Statistics.2 Patients with IHD may have a stable disease or an acute coronary syndrome, which could present with or without ST segment elevation. In addition, some patients may have left ventricular dysfunction and heart failure.3–5
For the treatment of IHD, in addition to the pharmacological treatment and, if necessary, interventional procedures, it is essential to manage cardiovascular risk factors such as smoking cessation, blood pressure, lipids and diabetes control, adherence to a Mediterranean diet, active lifestyle and prevent obesity. Moreover, for the secondary prevention of IHD, cardiac rehabilitation programmes are beneficial for patients, improving exercise capacity, quality of life and psychological well-being.6–8 The active role of the patient is crucial, along with the support of healthcare providers to achieve a successful secondary prevention of IHD.
The empowerment and self-management of patients with chronic conditions are becoming one of the main objectives in healthcare, especially in primary care (PC). The European EMPATHiE project9 defines the empowered patient as one who ‘has control over the management of the conditions of their daily life, actively tries to improve his/her quality of life and has the necessary knowledge, skills, attitudes and self-perception to adjust his/her behaviour and work in partnership with others when necessary, to achieve optimal well-being’.
One of the domains included in patient empowerment is the level of patient activation. Patient activation incorporates a combination of knowledge about the illness, ability and self-confidence in the management of the medical conditions.10 It is associated with healthy behaviours, good chronic disease metrics and reduced morbidity and unplanned hospitalisations.11–15
Interventions aimed at empowerment are intended to provide patients (and their informal caregivers, when appropriate) with the ability to participate in decisions related to their illness to the extent they wish, develop self-confidence, self-esteem and skills to face the physical, emotional and social impact of the disease in their daily lives.16 17
Virtual Communities of Practice (VCoP) offer ubiquitous access to information and exchange possibilities for people in similar situations, which is especially valuable in patients with chronic diseases. A CoP is a group of individuals who participate in a common activity and experience and create a shared identity and deepen their knowledge and experience in the area through a continuous interaction that strengthens their relationships.18 In this context, a group of patients with the same illness such as IHD, could benefit from an intervention of these characteristics where they can share resources and information in addition to having the possibility of receiving peer and professional support.
There is little research on the effect of VCoP in terms of their clinical and economic impact and on the empowerment of patients with chronic diseases, especially with IHD.19 20 We propose to address this gap and, thus, present the protocol of a randomised controlled trial, which mainly aims to evaluate the effectiveness and cost-effectiveness of a VCoP to improve the activation and other measures related with patient empowerment in patients with IHD.
Methods and analysis
This protocol has been prepared in accordance with the Standard Protocol Items: Recommendations for Interventional Trials checklist (online supplemental additional file 1).21
bmjopen-2020-037374supp001.pdf (76.1KB, pdf)
Study design
We plan a pragmatic randomised controlled multicentre trial (e-mpodera2), with two parallel arms and 18-month follow-up.
Study setting
The setting of the intervention will be a virtual setting. Usual care will be provided at primary care practices (PCPs) and outpatient specialised clinics in Catalonia, Madrid and Canary Islands in Spain.
Eligibility criteria
Patients with a recent diagnosis of IHD will be screened for the following eligibility criteria:
Inclusion criteria
Age ≥18 years; active diagnosis in the electronic medical record (EMR) of IHD (International Classification of Primary Care Second Edition - ICPC-2 codes K74-76; or International Classification of Diseases 9th Edition - ICD-9 codes 410, 411, 411.8, 413, 414 and 414.9) in the year prior to inclusion in the study; internet at home or smartphone; be able to follow the requirements of the study (eg, digital literacy); have signed the informed consent (online supplemental additional file 2).
bmjopen-2020-037374supp002.pdf (104.8KB, pdf)
Exclusion criteria
Institutionalised, terminal illness, physical or mental disability that limits the ability to answer the questionnaires or when telephone/email contact is not available in the PCPs/hospitals’ databases.
Interventions
VCoP group
‘e-mpodera2’ is a gamified VCoP on a web 2.0 platform based on the exchange of experiences and knowledge through participatory learning.22 It will provide educational, playful elements and tools that will facilitate the learning and transfer of knowledge and attitudes among patients with IHD and with healthcare professionals. The structure and components will be designed according to the needs and specifications of patients with IHD recruited in an earlier stage using a cocreation methodology with face-to-face sessions and virtual activities (forums and interactions) that incorporated a personalised itinerary—Patient Journey Map—(published elsewhere) and with the use of various types of content including readings, resources, videos, games and virtual sessions.22
Patients will have access to multidisciplinary professional support as needed and according to what was identified in the content-design stage (published elsewhere) that will potentially include general practitioners, cardiologists, psychologists, self-care and self-management specialists, nutritionist and others as necessary. Various thematic areas related to the empowerment of patients and self-care of IHD will be progressively covered: health competence, self-efficacy and activation improvement, behavioural changes, lifestyle/signs/symptoms monitoring, technical skills, chronic disease acceptance and shared decision-making. Special emphasis will be given to the changes recommended by European Guidelines23 for self-management of IHD including monitoring changes in symptoms, stress management, mental health and adherence to medication, diet, exercise plans, sodium cholesterol, and alcohol restriction and tobacco abstinence. The active role of a community manager, weekly emails as reminders and a gamified competitive score system will boost participation.
Usual care group
Patients allocated to both the intervention and the control group will continue with their usual self-care and professional care according to the local guidelines.3–5
Outcomes measures
Primary outcome
The primary outcome will be the patient activation level using the Patient Activation Measure (PAM) questionnaire that assesses activation in patients with chronic diseases.12 The questionnaire consists of 13 items that assess knowledge, skills and confidence of people for self-care, measured by a Likert 1–4 scale with a total score between 0 and 100 (100 identifies the patients with the highest level of activation). The Spanish translated version has been validated in patients with chronic diseases and has demonstrated a similar behaviour to the original instrument with good validity and reliability properties.24 It has been used in previous studies by this research team.25
Secondary outcomes
For the effectiveness of the VCoP, we will record the following secondary measures:
Clinical variables such as body mass index, lipid profile (High-density lipoprotein cholesterol - HDL-C, Low-density lipoprotein cholesterol - LDL-C), smoking status, number and frequency of angina episodes will be collected through researcher developed online questionnaire that will be fulfilled by healthcare professionals combined with information from the EMR.
Knowledge about the disease will be assessed through a self-administered online questionnaire based on the Questionnaire of Cardiovascular Risk Factors,26–28 previously translated from the English version and adapted to the Spanish population.
Patients’ attitudes to self-care will be evaluated using the self-administered Self-efficacy Managing Chronic Disease Scale (SMCDS),29 translated into Spanish30 and used in patients with heart failure.31
Adherence to the Mediterranean diet will be assessed with the Mediterranean diet questionnaire,32 validated in the Spanish population in the PREDIMED (Prevención con Dieta Mediterránea) study.33–35
Physical activity will be measured using the International Physical Activity Questionnaire (IPAQ), translated and adapted to the Spanish language.36 Patients will be classified into three categories (low, medium and high) according to the index of physical activity (product of the intensity—in Metabolic Equivalents, METs—by the frequency) and the duration of the activity.
Depressive disorders will be detected by the Patient Health Questionnaire-9 (PHQ-9),37 validated in Spanish with similar behaviour to the original and good acceptance.38
Anxiety will be assessed using the Hospital Anxiety and Depression Scale (HADS scale),39 a 14-item questionnaire validated in PC in Spain,40 41 with special interest and usefulness in the context of PC. It is a measure composed of two subscales (HADS-A: anxiety and HADS-D: depression), of 7 items each that are scored from 0 to 3. The authors recommend a threshold of eight points to detect possible cases of anxiety. One of the main virtues of this tool is the suppression of somatic symptoms. However, in patients with IHD, it underestimates people with depression,42 while the subscale HADS-A has good specificity and predictive value for measuring anxiety in this PC.43
Adherence to medication will be assessed with the Adherence Refill and Medication Scale (ARMS),44 validated in Spain and used to measure adherence to medication in patients with chronic diseases. It consists of 12 questions and there is no cut-off point, the lower the score, the better the adherence. To quantify adherence, a value of 1–4 (never, sometimes, almost always or always) is assigned to each of the responses according to a Likert-type scale.
Quality of life related to health (HRQoL) will be described and assessed with the EQ-5D-5L index,45 46 a generic and standardised instrument developed by the EuroQoL Group, and prepared in several languages, including Spanish, and used in PC.47 It relates the HRQoL with the amount of life and offers a score for the gains in health, the Quality Adjusted Life Year (QALY). The descriptive EQ-5D-5L system comprises five dimensions (mobility, personal care, daily activities, pain/discomfort and anxiety/depression).
Explanatory and adjustment variables
Sociodemographic: age, sex, nationality, Autonomous Community of residence (Catalonia, Madrid or Canary Islands), marital status (married/partner, single, separated/divorced, widowed), living alone (yes/no), educational level (incomplete primary education, complete primary education, secondary education, university or equivalent studies), income level and employment status.48
Morbidity-related: type of IHD (stable angina, unstable angina, myocardial infarction), duration of IHD (months), current diagnosis of heart failure in EMR (K86), left ventricular ejection fraction (30%, 30%–35%, 35%–45%, >45%), New York Heart Association (NYHA) functional classification (I–IV), number and description of chronic concomitant diseases,49 pharmacological treatment (acetylsalicylic acid or clopidogrel/ticagrelor/prasugrel, beta-blockers, statins, ACE inhibitors, angiotensin II receptor blockers, other treatments), cardiac catheterisation (yes/no) and participation in a cardiac rehabilitation programme before and during the study period (yes/no).
Use of healthcare resources: primary care (PC) visits, visits to the emergency department, visits to specialists, number of hospitalisations, lengths of stay, prescribed medications, use of diagnostic tests.
Loss of productivity: self-administered questionnaire about work absences related to the illness.
Use of the VCoP: number of logins into the platform and time spent using the platform.
This information will be collected online from a patient self-reported questionnaire that the research team will elaborate combined with information from the EMR. VCoP use data will be collected through the platform database.
Adverse events
All significant adverse events as well as unintended consequences for each group will be collected and described by the site researcher, nominated for each PCP and hospital, and reported to the core team. A special form to report trial-related adverse events has been developed and distributed.
Participant timeline
Primary and secondary outcome measures will be collected before the start of the VCoP intervention and at 6, 12 and 18 months. See table 1.
Table 1.
Schedule of enrolment, interventions, and assessments (SPIRIT checklist)
| Timepoint | Study period | ||||
| Preallocation | Postallocation | Close-out | |||
| Enrolment | Baseline | 6 months | 12 months | 18 months | |
| Eligibility screen | X | ||||
| Informed consent | X | ||||
| Interventions | |||||
| VCoP | |||||
| Usual care | |||||
| Assessments | |||||
| PAM | X | X | X | X | |
| Sociodemographic and clinical variables | X | X* | X* | X* | |
| Knowledge | X | X | X | X | |
| SMCDS | X | X | X | X | |
| Mediterranean Diet Questionnaire | X | X | X | X | |
| IPAQ | X | X | X | X | |
| PHQ-9 | X | X | X | X | |
| HADS-A | X | X | X | X | |
| ARMS-e | X | X | X | X | |
| EQ-5D-5L | X | X | X | X | |
| Use of resources | X | X | X | ||
| Use of VCoP | |||||
| Adverse events | |||||
*Follow-up of just clinical variables.
ARMS-e, Adherence Refill and Medication Scale; HADS, Hospital Anxiety and Depression Scale; IPAQ, International Physical Activity Questionnaire; PAM, Patient Activation Measure; PHQ-9, Patient Health Questionnaire; SMCDS, Self-efficacy Managing Chronic Disease Scale; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials; VCoP, Virtual Community of Practice.
Sample size
Assuming an alpha error of 0.05 and power of 80%, the necessary number of patients to detect, by means of independent two-sample t-test, an average minimal important difference of 4 points (SD 10) in the PAM questionnaire12 24 between the intervention and usual care group, is 200 patients (100 per arm). Assuming a 20% loss to follow-up, the required sample increases to 250 (125 per arm).
Recruitment
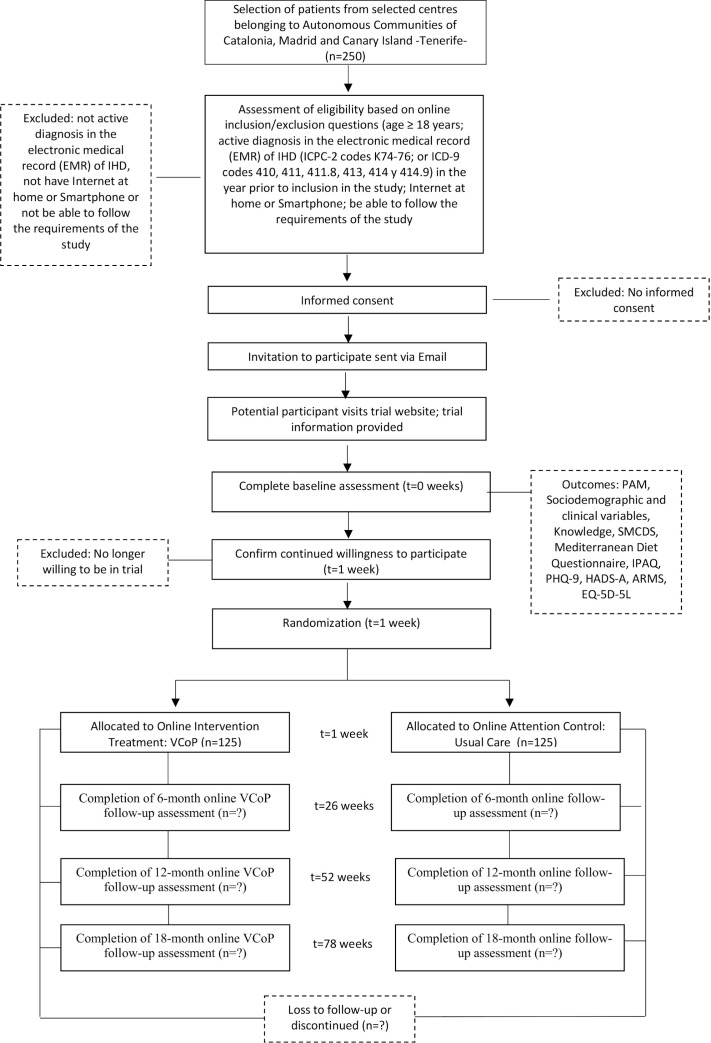
Patient recruitment will be organised on each Autonomous Community (Catalonia, Madrid or Canary Islands). The recruitment will be supported by informative meetings with directors and healthcare professionals (general practitioners, nurses, cardiologists) from the participating centres. In these meetings, a 10-minute presentation describing the study aim, planned time frame and tasks to be carried out by healthcare professionals, expected resources utilisation and funding procedures will be detailed. Patients that fulfil inclusion criteria will be actively encouraged by their healthcare professionals to participate by providing information about the trial and collecting their informed consent and contact details (eg, phone number/email). The research team will invite potential participants via phone and mail to access the ‘e-mpodera2’ platform where they will be provided with a unique registration code (figure 1). Patients will be consecutively included in the study; recruitment will be continuous until the sample size is reached.
Figure 1.
Flow of participants. ARMS, Adherence Refill and Medication Scale; CdPV, Comunidad de Práctica Virtual; HADS-A, Hospital Anxiety and Depression Scale; IPAQ, International Physical Activity Questionnaire; PAM, Patient Activation Measure; PHQ-9, Patient Health Questionnaire; SMCDS, Self-efficacy Managing Chronic Disease Scale.
Allocation and blinding
Two hundred and fifty patients will be randomly assigned to the intervention (VCoP) or control group. The randomisation, stratified by centre, will be central and automatically performed by the online ‘e-mpodera2’ platform and the assigned group will be communicated to the patient once he or she has entered the platform and completed baseline assessment (figure 1). Lack of knowledge of the randomisation sequence by the professionals who participate in the recruitment of patients will, therefore, be ensured. The intervention group will be taken directly to the registration page of ‘e-mpodera2’ VCoP, where they will receive a personalised message to welcome them into the platform. To warrant patient participation and cooperation, this type of intervention cannot be blinded to patients. Data analysis will be blinded to the assignment of the intervention.
Data management
In order to maintain participant confidentiality, all information will be stored with anonymised ID code numbers. The ID code numbers will be unrelated to participants’ identifiers, except in a central file with the participants’ contact details. All data will be stored on an electronic database management system located on a secure server with password-controlled access provided for research data collection. Databases will be designed to avoid downloading inappropriate values for every variable. Trial monitoring will be the responsibility of the core research team in charge of all quality control activities, assessing adherence to the trial protocol: timely work plan execution and comprehensiveness of data acquisition and data quality.
The Research Ethics Committees, the representatives of the Health Authority in matters of inspection and the personnel authorised by the Promoter, may only access to check personal data, clinical study procedures and compliance with the rules of good clinical practice (always maintaining the confidentiality of information).
Statistical analysis
Sociodemographic and clinical baseline variables for both groups will be analysed by descriptive methods (mean (SD), median (range), n (%)). The VCoP effect on the primary and secondary outcomes will be examined by means of multilevel linear regression, with the intervention, measurement time (0, 6, 12 and 18 months) and their interaction as fixed effects (along with other potential covariates), random intercepts for patients and general practitioner (GP), and unstructured covariance to account for within-subject correlations. We will also analyse the three-way interaction intervention×time×centre, since usual care could vary between centres, leading to differential intervention effects. We expect to recruit a sufficient number of GPs to allow their inclusion in the model as a random intercept, but we will perform a sensitivity analysis as well as excluding this component. Between-group differences at each time-point will be compared by means of Wald’s χ2 test.
We will perform the analyses on an intention-to-treat basis (a sensitivity analysis on the per-protocol population will be also performed). Multiple imputation will be used for missing data, if applicable (Markov Chain Monte Carlo multivariate imputation algorithm, with 10 imputations per variable). Analyses will be carried out with the statistical software R V.4.0.2 (http://www.R-project.org/).
Cost-effectiveness analysis of the VCoP
We will carry out an economic evaluation, from baseline to 18-month follow-up, in which the costs and the results of the VCoP will be compared with the usual care following the recommendations of the guidelines for the management of patients with IHD,3–5 during the period of the clinical trial. The accepted analytical methods by the scientific community will be followed.50 The analysis will take both the perspective of the National Health System and of the social perspective. Therefore, direct healthcare costs and indirect costs will be included. The direct costs per patient will be calculated based on the use of healthcare resources, and the indirect costs will be estimated, focusing on productivity losses due to IHD, applying the human capital approach. In addition to including the short-term costs (development and implementation of the VCoP), the costs observed during the follow-up will be included. We do not plan to consider opportunity costs in our cost-effectiveness analysis from the social perspective, as we understand that patients will use their free time on the VCoP and therefore they will not spend work or productive time not generating a cost for the system. The use of resources will be obtained from a patient self-reported questionnaire described in the outcome section. In addition, information about work absences related to the illness will be requested. The classic costs estimation approach will be followed, multiplying the use of resources by their unit cost. The unit costs will be obtained from the eHealth cost database (Oblikue Consulting) and from public sources such as rates and retail prize. The main outcome measure will be the incremental cost per gained QALY. The utilities for the estimation of the QALYs will be obtained through the EQ-5D-5L questionnaire45 that will be completed by the patient at the beginning of the study and at each follow-up visit. Results of the cost-effectiveness analysis will be summarised as the incremental cost-effectiveness ratio (ICER). ICER is the ratio of the differences in costs to the differences in observed effects. Non-parametric methods based on bootstrap simulations will be used to calculate CIs in the ICER. The same non-parametric methods will be used to calculate the acceptability curve that represents the probability that each choice will be cost-effective for different cost-effectiveness thresholds. The willingness-to-pay threshold is defined at Euro 25 000/QALY on the basis of the values most recently reported in the Spanish literature.51 Finally, deterministic sensitivity analyses (one, two or several ways) will be carried out in order to assess the impact of the parameters on the cost-effectiveness results of the VCoP.
Patient and public involvement
This protocol was developed without patient or public involvement. A group of patients with IHD will actively participate in a content-design previous stage using a cocreation methodology with face-to-face sessions and virtual activities.
Ethics and dissemination
Informed consent will be obtained from each participant before randomisation. The project received ethics approval from the local Committees at each participating Autonomous Community: Clinical Research Ethics Committee of Gregorio Marañón University Hospital in Madrid, Nuestra Señora de Candelaria University Hospital in Santa Cruz de Tenerife and from the coordinating centre IDIAP Jordi Gol in Barcelona (19/053-P). Patients will be personally informed by their physicians or nurses about the study and the possibility to participate during a programmed consultation. They will receive written information of the proposed research project, including information regarding the aims of the project, the duration of the participants’ involvement, the expected benefits to the participant and the procedures involved in the participation. Recruiters will emphasise that enrolment in the study is voluntary and that participants can withdraw at any moment of the project and that any decision they take in this respect will have no bearing on the medical care received. Once patients have signed the written informed consent, a researcher from the ‘e-mpodera2’ team will contact them via phone and/or mail to provide further information along with the necessary data (username and password) to login into the online platform. Additionally, recruiters will highlight that information generated by the study will be published, but no identification details will be divulged. Patients and healthcare providers will be informed of whom to contact in case of any query and research staff will be available to answer questions.
We will prepare presentations to disseminate the study findings to healthcare stakeholders and patients, and at relevant national and international conferences. We aim to publish the results of the trial in peer-reviewed journals.
Trial status
The recruitment of patients in each region will start in September 2020. The estimated end date of the recruitment for this study is December 2020.
Supplementary Material
Footnotes
Twitter: @Inst_Donabeidan
Contributors: AIG-G wrote the initial draft of the protocol. CO is the guarantor of the trial. AIG-G, CO, LP-P, DK, VP-H and MB conceived the project. SG-E, AR-R and CV-N provided the methodological guidance. AT-C, VR-G, AT-C, JM-R, JCO-R, SD-S, LM-C, JG-G, NV-C, AR-A, JCdC, JMB-F, M-ET-B, MMB, YdR-G and ABR-P are co-supervisors of this project, providing advice at all stages of the development of the protocol, and contributed to the revision of the manuscript. All authors read and approved the final manuscript.
Funding: This study has been funded by Instituto de Salud Carlos III through the project ‘PI18/01404, PI18/01397, PI18/01333’, Co-funded by European Regional Development Fund, (ERDF) ‘A way of shaping Europe’.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wilkins E, Wilson L, Wickramasinghe K, et al. European cardiovascular disease statistics. 192 2017 edition Brussels: Eur Hear Network, 2017. [Google Scholar]
- 2.Instituto Nacional de Estadística Ine base. Available: http://www.ine.es/inebmenu/indice.htm#12 [Accessed 27 Feb 2018].
- 3.Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2018;39:119–77. 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 4.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–77. 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 5.Roffi M, Patrono C, Collet J-P, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2016;37:267–315. 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 6.García-Porrero E, Andrés-Esteban E, de Pablo-Zarzosa C, et al. Cardiología preventiva Y rehabilitación. Revista Española de Cardiología 2010;63:40–8. 10.1016/S0300-8932(10)70139-1 [DOI] [PubMed] [Google Scholar]
- 7.Piepoli MF, Corrà U, Dendale P, et al. Challenges in secondary prevention after acute myocardial infarction: a call for action. Eur J Prev Cardiol 2016;23:1994–2006. 10.1177/2047487316663873 [DOI] [PubMed] [Google Scholar]
- 8.Anderson L, Thompson DR, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016:CD001800. 10.1002/14651858.CD001800.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EMPATHiE Consortium EMPATHiE, empowering patients in the management of chronic diseases. final summary report 2014.
- 10.Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff 2013;32:207–14. 10.1377/hlthaff.2012.1061 [DOI] [PubMed] [Google Scholar]
- 11.Greene J, Hibbard JH. Why does patient activation matter? an examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med 2012;27:520–6. 10.1007/s11606-011-1931-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skolasky RL, Green AF, Scharfstein D, et al. Psychometric properties of the patient activation measure among multimorbid older adults. Health Serv Res 2011;46:457–78. 10.1111/j.1475-6773.2010.01210.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salyers MP, Matthias MS, Spann CL, et al. The role of patient activation in psychiatric visits. Psychiatr Serv 2009;60:1535–9. 10.1176/ps.2009.60.11.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmittdiel J, Mosen DM, Glasgow RE, et al. Patient assessment of chronic illness care (PACIC) and improved patient-centered outcomes for chronic conditions. J Gen Intern Med 2008;23:77–80. 10.1007/s11606-007-0452-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosen DM, Schmittdiel J, Hibbard J, et al. Is patient activation associated with outcomes of care for adults with chronic conditions? J Ambul Care Manage 2007;30:21–9. 10.1097/00004479-200701000-00005 [DOI] [PubMed] [Google Scholar]
- 16.Náfrádi L, Nakamoto K, Schulz PJ. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS One 2017;12:e0186458. 10.1371/journal.pone.0186458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Risling T, Martinez J, Young J, et al. Evaluating patient Empowerment in association with eHealth technology: Scoping review. J Med Internet Res 2017;19:e329. 10.2196/jmir.7809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li LC, Grimshaw JM, Nielsen C, et al. Evolution of Wenger's concept of community of practice. Implement Sci 2009;4:11. 10.1186/1748-5908-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliva A. Psychosocial intervention 2013;22:15–23. [Google Scholar]
- 20.Pinilla J, Barber P BG. El coste de la enfermedad potencialmente prevenible en España. Madrid; 2017. [Google Scholar]
- 21.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586–7. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bermejo-Caja CJ, Koatz D, Orrego C, et al. Acceptability and feasibility of a virtual community of practice to primary care professionals regarding patient empowerment: a qualitative pilot study. BMC Health Serv Res 2019;19:403 10.1186/s12913-019-4185-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Clinical Guideline Centre MI - Secondary Prevention: Secondary Prevention in Primary and Secondary Care for Patients Following a Myocardial Infarction: Partial Update of NICE CG48. London: Royal College of Physicians; 2013. [PubMed] [Google Scholar]
- 24.Moreno-Chico C, González-de Paz L, Monforte-Royo C, et al. Adaptation to European Spanish and psychometric properties of the patient activation measure 13 in patients with chronic diseases. Fam Pract 2017;34:627–34. 10.1093/fampra/cmx022 [DOI] [PubMed] [Google Scholar]
- 25.González-González AI, Orrego C, Perestelo-Perez L, et al. Effectiveness of a virtual intervention for primary healthcare professionals aimed at improving attitudes towards the empowerment of patients with chronic diseases: study protocol for a cluster randomized controlled trial (e-MPODERA project). Trials 2017;18:505. 10.1186/s13063-017-2232-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alm-Roijer C, Stagmo M, Udén G, et al. Better knowledge improves adherence to lifestyle changes and medication in patients with coronary heart disease. Eur J Cardiovasc Nurs 2004;3:321–30. 10.1016/j.ejcnurse.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 27.Alm-Roijer C, Fridlund B, Stagmo M, et al. Knowing your risk factors for coronary heart disease improves adherence to advice on lifestyle changes and medication. J Cardiovasc Nurs 2006;21:E24–31. 10.1097/00005082-200609000-00015 [DOI] [PubMed] [Google Scholar]
- 28.Saffi MAL, Jacques de Macedo Junior LJ, Trojahn MM, et al. Validity and reliability of a questionnaire on knowledge of cardiovascular risk factors for use in Brazil. Rev Esc Enferm USP 2013;47:1083–9. 10.1590/S0080-623420130000500011 [DOI] [PubMed] [Google Scholar]
- 29.Lorig KR, Ritter PL. Hispanic chronic disease self-management. Nurs Res 2003;52:361–9. 10.1097/00006199-200311000-00003 [DOI] [PubMed] [Google Scholar]
- 30.Ritter PL, Lorig K. The English and Spanish self-efficacy to manage chronic disease scale measures were validated using multiple studies. J Clin Epidemiol 2014;67:1265–73. 10.1016/j.jclinepi.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 31.Salvadó-Hernández C, Cosculluela-Torres P, Blanes-Monllor C, et al. Insuficiencia cardiaca en atención primaria: actitudes, conocimientos Y autocuidado. Atención Primaria 2018;50:213–21. 10.1016/j.aprim.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trichopoulou A, Costacou T, Bamia C, et al. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med Overseas Ed 2003;348:2599–608. 10.1056/NEJMoa025039 [DOI] [PubMed] [Google Scholar]
- 33.Martínez-González MA, García-Arellano A, Toledo E, et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One 2012;7:e43134. 10.1371/journal.pone.0043134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sánchez-Tainta A, Zazpe I, Bes-Rastrollo M, et al. Nutritional adequacy according to carbohydrates and fat quality. Eur J Nutr 2016;55:93–106. 10.1007/s00394-014-0828-3 [DOI] [PubMed] [Google Scholar]
- 35.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. 10.1056/NEJMoa1200303 [DOI] [PubMed] [Google Scholar]
- 36.Mantilla Toloza SC, Gómez-Conesa A. El Cuestionario Internacional de Actividad Física. un instrumento adecuado en El seguimiento de la actividad física poblacional. Revista Iberoamericana de Fisioterapia y Kinesiología 2007;10:48–52. 10.1016/S1138-6045(07)73665-1 [DOI] [Google Scholar]
- 37.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. primary care evaluation of mental disorders. patient health questionnaire. JAMA 1999;282:1737. 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 38.Diez-Quevedo C, Rangil T, Sanchez-Planell L, et al. Validation and utility of the patient health questionnaire in diagnosing mental disorders in 1003 General Hospital Spanish inpatients. Psychosom Med 2001;63:679–86. 10.1097/00006842-200107000-00021 [DOI] [PubMed] [Google Scholar]
- 39.Quintana JM, Padierna A, Esteban C, et al. Evaluation of the psychometric characteristics of the Spanish version of the hospital anxiety and depression scale. Acta Psychiatr Scand 2003;107:216–21. 10.1034/j.1600-0447.2003.00062.x [DOI] [PubMed] [Google Scholar]
- 40.Herrero MJ, Blanch J, Peri JM, et al. A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen Hosp Psychiatry 2003;25:277–83. 10.1016/S0163-8343(03)00043-4 [DOI] [PubMed] [Google Scholar]
- 41.Terol-Cantero MC, Cabrera-Perona V, Martín-Aragón M. Revisión de estudios de la Escala de Ansiedad Y Depresión Hospitalaria (HAD) en muestras españolas. An Psicol 2015;31:494. [Google Scholar]
- 42.Moryś JM, Bellwon J, Adamczyk K, et al. Depression and anxiety in patients with coronary artery disease, measured by means of self-report measures and clinician-rated instrument. Kardiol Pol 2016;74:53–60. 10.5603/KP.a2015.0116 [DOI] [PubMed] [Google Scholar]
- 43.Bunevicius A, Staniute M, Brozaitiene J, et al. Screening for anxiety disorders in patients with coronary artery disease. Health Qual Life Outcomes 2013;11:37. 10.1186/1477-7525-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.González-Bueno J, Calvo-Cidoncha E, Sevilla-Sánchez D, et al. [Spanish translation and cross-cultural adaptation of the ARMS-scale for measuring medication adherence in polypathological patients]. Aten Primaria 2017;49:459–64. 10.1016/j.aprim.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramos-Goñi JM, Craig BM, Oppe M, et al. Handling data quality issues to estimate the Spanish EQ-5D-5L value set using a hybrid interval regression approach. Value Health 2018;21:596–604. 10.1016/j.jval.2017.10.023 [DOI] [PubMed] [Google Scholar]
- 47.Herdman M, Badia X, Berra S. El EuroQol-5D: Una alternativa sencilla para La medición de la calidad de vida relacionada Con La salud en atención primaria. Atención Primaria 2001;28:425–9. 10.1016/S0212-6567(01)70406-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes. Circulation 2018;137:2166–78. 10.1161/CIRCULATIONAHA.117.029652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Halloran J, Miller GC, Britt H. Defining chronic conditions for primary care with ICPC-2. Fam Pract 2004;21:381–6. 10.1093/fampra/cmh407 [DOI] [PubMed] [Google Scholar]
- 50.Glick H, Doshi J, Sonnad S, et al. Economic evaluation in clinical trials. 1st ed Oxford: Oxford University Press, 2007. [Google Scholar]
- 51.Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ 2018;27:746–61. 10.1002/hec.3633 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-037374supp001.pdf (76.1KB, pdf)
bmjopen-2020-037374supp002.pdf (104.8KB, pdf)