Abstract
Introduction
Metformin and diets aimed at promoting healthy body weight are the first line in treating type 2 diabetes mellitus (T2DM). Clinical practice, backed by clinical trials, suggests that many individuals do not reach glycaemic targets using this approach alone. The primary aim of the Personalised Medicine in Pre-diabetes—Towards Preventing Diabetes in Individuals at Risk (PREDICT) Study is to test the efficacy of personalised diet as adjuvant to metformin in improving glycaemic control in individuals with dysglycaemia.
Methods and analysis
PREDICT is a two-arm, parallel group, single-masked randomised controlled trial in adults with pre-diabetes or early-stage T2DM (with glycated haemoglobin (HbA1c) up to 8.0% (64 mmol/mol)), not treated with glucose-lowering medication. PREDICT is conducted at the Clinical Research Facility at the Garvan Institute of Medical Research (Sydney). Enrolment of participants commenced in December 2018 and expected to complete in December 2021. Participants are commenced on metformin (Extended Release, titrated to a target dose of 1500 mg/day) and randomised with equal allocation to either (1) the Personalised Nutrition Project algorithm-based diet or (2) low-fat high-dietary fibre diet, designed to provide caloric restriction (75%) in individuals with body mass index >25 kg/m2. Treatment duration is 6 months and participants visit the Clinical Research Facility five times over approximately 7 months. The primary outcome measure is HbA1c. The secondary outcomes are (1) time of interstitial glucose <7.8 mmol/L and (2) glycaemic variability (continuous glucose monitoring), (3) body weight, (4) fat mass and (5) abdominal visceral fat volume (dual-energy X-ray absorptiometry), serum (6) low-density lipoprotein cholesterol (7) high-density lipoprotein cholesterol and (8) triglycerides concentrations, (9) blood pressure, and (10) liver fat (Fibroscan).
Ethics and dissemination
The study has been approved by the St Vincent’s Hospital Human Research Ethics Committee (File 17/080, Sydney, Australia) and the Weizmann Institutional Review Board (File 528-3, Rehovot, Israel). The findings will be published in peer-reviewed open access medical journals.
Trial registration number
Keywords: diabetes & endocrinology, nutrition & dietetics
Strengths and limitations of this study.
The randomised controlled design testing a novel diet against standard of care may lead to a new tool to manage dysglycaemia in individuals requiring metformin.
Conducted in an adult Australian population, the study findings may not be applicable to other populations.
The algorithm used to devise the personalised diet relies on accurate recording of the dietary intake by the participants.
The dietary intervention requires use of a smartphone application which may limit its applicability to some populations.
Introduction
Type 2 diabetes mellitus (T2DM) and its preceding medical condition, pre-diabetes, are significant risk factors for cardiovascular disease, and most affected individuals demonstrate additional metabolic risk factors, such as hypertension, dyslipidaemia, excess weight and fatty liver.1 T2DM affects approximately 422 million adults globally,2 with an additional 352 million individuals at increased risk, having pre-diabetes (prevalence estimates of impaired glucose tolerance (IGT)).3 4 Individuals diagnosed with pre-diabetes or T2DM are encouraged to adopt a healthy lifestyle and, if overweight, to lose weight.5 The majority of individuals with T2DM are treated with metformin, which is the eighth most prescribed medication in the USA.6–8
Metformin, an oral biguanide, is the first-line treatment for individuals with newly diagnosed T2DM and, in some cases, for the prevention of diabetes in individuals with pre-diabetes.7 Metformin is an ideal medication to initiate for management of T2DM or for prevention of diabetes, because it does not cause hypoglycaemia and has a favourable, although modest, effect on body weight.9 Metformin monotherapy is insufficient to achieve glycaemic control in a large proportion of treated individuals.10–12 Findings from the Diabetes Prevention Program in individuals with pre-diabetes suggested that the glycaemic efficacy of metformin depends on the magnitude of weight loss,13 explaining 64% of the diabetes risk reduction, with additional 17% explained by decreases in fasting insulin and pro-insulin at 3 years of follow-up.13 14
The current treatment guidelines in T2DM recommend prescribing metformin in combination with a healthy lifestyle, enabling weight loss.15 The most recent nutritional guidelines for individuals with T2DM or pre-diabetes are no longer supporting a universal ideal dietary macronutrient distribution; instead, the guidelines suggest individualised eating plans.5
In the pioneering Personalised Nutrition Project (PNP),16 Zeevi et al developed an algorithm that predicts an individual’s postprandial glycaemic response (PPGR) to meals. The algorithm incorporates the individual’s personal data (eg, age, gender, body mass index (BMI)), blood tests (eg, glycated haemoglobin (HbA1c)), dietary features, continuous glucose monitor (CGM)-derived data and gut microbiome features, and trained on data previously collected in 800 individuals. Personally tailored dietary plans based on the algorithm were trialled in a small group of individuals with pre-diabetes and shown to improve glycaemic variability and PPGR over 7 days.
The primary objective of the Personalised Medicine in Pre-diabetes—Towards Preventing Diabetes in Individuals at Risk (PREDICT) Study is to compare glycaemic control, measured by HbA1c, following 6 months of metformin, prescribed with either (1) the PNP algorithm-based diet or (2) low-fat high-dietary fibre (LFHF) diet, based on the Australian Healthy Eating Guide17 and the American Association of Clinical Endocrinologists guide for medical care of patients with obesity,18 in individuals with pre-diabetes or early-stage T2DM naive to glucose-lowering pharmacotherapy.
The secondary objectives of the PREDICT Study are to compare the effect of the PNP diet versus LFHF diet when prescribed with metformin on: (1) time of interstitial glucose <7.8 mmol/L, (2) glycaemic variability, (3) weight, (4) body fat mass, (5) abdominal visceral fat volume, (6) serum low-density lipoprotein (LDL) cholesterol concentration (7) serum high-density lipoprotein cholesterol concentration, (8) serum triglycerides concentration, (9) blood pressure and (10) liver fat. The exploratory objectives of the study are to test the effect of the treatment on the gut microbiome.
Methods
Study design, setting and population
The study is a two-arm, parallel group, single-masked randomised controlled trial. Adults with pre-diabetes or early-stage T2DM who are not treated with glucose-lowering medications are randomised, with equal allocation, to either the PNP or LFHF diet arms, in both arms participants are commenced on metformin Extended Release (XR) 1500 mg/day treatment for 6 months. All the study visits are performed at the Clinical Research Facility at the Garvan Institute of Medical Research (Sydney). Metagenomics and data processing for the personalised dietary interventions are performed at the Weizmann Institute of Science (Rehovot).
Patient and public involvement
Patients or the public were not involved in the design or other aspects of the research.
Eligibility
Adults (20–70 years old) with pre-diabetes or recently (in the last 6 months) diagnosed T2DM with (HbA1c ≤8.0% (64 mmol/mol)), not pregnant or planning to become pregnant during, and for at least 3 months after the study, are recruited (box 1). A wide age range was selected to encompass different populations of individuals managing their pre-diabetes for short or long durations and to increase the likelihood to recruit the sample size in a timely manner. The HbA1c cap at 8.0% (64 mmol/mol) was selected to ensure that individuals with T2DM are relatively well controlled. Blood tests indicative of normo-glycaemia, HbA1c above 8.0% (64 mmol/mol), liver enzymes (alanine aminotransferase and/or aspartate aminotransferase) over three times the normal range limit and estimated glomerular filtration rate (eGFR)19 lower than 45 mL/min/1.73 m2 are grounds for exclusion. Individuals treated with glucose-lowering medication other than metformin in the last 24 months, or metformin in the last 3 months, will be excluded. Individuals with conditions or treatments that affect glycaemia (eg, oral steroids), impact weight (eg, bariatric surgery, weight loss medications) or the gut microbiome (eg, inflammatory bowel disease, coeliac, frequent antibiotic treatment) will be excluded. Participants who have had a cardiovascular event in the previous 6 months or received an investigational new drug within the last 6 months will be excluded. Participants with conditions that may interfere with the ability to understand the requirements of the study and those who refuse treatment with metformin or refuse to use the smartphone application will be excluded (box 1).
Box 1. Inclusion and exclusion criteria.
Inclusion criteria
Men and women 20–70 years of age.
Pre-diabetes (IFG* or IGT* and/or glycated haemoglobin (HbA1c) 5.7%–6.4% (39–46 mmol/mol)).
Individuals diagnosed with type 2 diabetes mellitus in the last 6 months with HbA1c ≤8.0% (64 mmol/mol).
Willingness to provide written informed consent, participate and comply with the study.
Exclusion criteria
Women planning pregnancy during the study or 3 months after study completion.
Individuals with type 1 diabetes, neoplastic disease (in the last 3 years), cardiovascular event (−6 months), chronic gastrointestinal disorders.
Liver enzymes alanine aminotransferase and/or aspartate aminotransferase >3 times normal range limit.
eGFR**<45 mL/min/1.73 m2.
Normo-glycaemia.
HbA1c >8.0% (64 mmol/mol).
Current or recent (within 24 months) treatment with a glucose-lowering medication other than metformin, current or recent (within 3 months) treatment with metformin, current treatment with an oral steroid, immunosuppressive medications, antibiotics (within 3 months).
Alcohol or substance abuse.
Participants who had received an investigational new drug within the last 6 months.
Participants involved in another clinical study.
Participants who have had bariatric surgery.
Participants who actively lose weight.
Participants with conditions that may interfere with the ability to understand the requirements of the study, refuse treatment with metformin or refuse to use the smartphone application.
*Impaired fasting glucose (IFG): fasting plasma glucose 5.6–6.9 mmol/L and/or impaired glucose tolerance (IGT): 2-hour plasma glucose during 75 g oral glucose tolerance test 7.8–11.0 mmol/L.
**Estimated glomerular filtration rate (eGFR) calculated as reported.19
Recruitment
The study is advertised in general practitioners (GPs), endocrinologists and dieticians’ practices in the Sydney metropolitan area, and through targeted social media campaigns. A collaboration with Blacktown Mt Druitt Hospital (Western Sydney) has been established in 2019 for the purpose of recruitment. During a hospital screening programme ran between 2016 and 2018, 17.3% and 30.2% of individuals visiting the emergency department (ED) at Blacktown Mt Druitt Hospital have had HbA1c values indicative of diabetes and pre-diabetes, respectively.20 Since September 2019, individuals visiting the ED who have had a blood test indicative of pre-diabetes receive a letter prompting them to contact the PREDICT team and encouraging they share the result with a GP.
To date (February 2020), 38 participants were enrolled. Of the 38 participants enrolled, 20 completed, 13 are ongoing, and 5 withdrew before the end of the treatment (13% dropout rate). Recruitment of participants is expected to complete in December 2021.
Participants contacting the team receive the participant information sheet (online supplemental material) via email or post. Experienced clinical research nurse/associate provides details about the study over the phone. Willing participants are referred to a commercial pathology to perform an oral glucose tolerance test (OGTT, 75 g) and HbA1c test. They are asked to sign a consent form after reading the participant information sheet explaining the possible risks of undergoing the OGTT and HbA1c tests prior to performing the blood tests. If the blood tests indicate either T2DM (with HbA1c ≤8.0% (64 mmol/mol)), or impaired fasting glucose or impaired glucose tolerance or HbA1c ≥5.7 (39 mmol/mol), they are invited to a screening and enrolment visit at the Garvan Clinical Research Facility.
bmjopen-2020-037859supp001.pdf (227.2KB, pdf)
A stool collection kit for metagenomics OMNIgene GUT (OMR-200; DNA Genotek) is mailed to participants prior to the screening/enrolment visit. Participants collect the sample according to the manufacturer’s instructions the day before the visit and keep the sample at room temperature. At the Clinical Research Facility, the sample is vortexed, centrifuged for a few seconds and material aliquoted into cryo vials and kept in −70°C freezer. One vial is transferred to facilities at the Weizmann Institute of Science and stored at −20°C until DNA extraction.
The pretreatment data are collected across the screening/enrolment and the baseline visits.
Screening/enrolment procedures and measurements
During the screening/enrolment visit, participants sign the study informed consent form (online supplemental material) and undergo medical examination by a physician. Participants have their weight, height, waist and hip circumference and blood pressure measured. Basal metabolic rate (BMR) is estimated using bioelectrical impedance analysis (BIA, used for calculating the energy requirement, see the Energy target section). Blood samples are collected to evaluate liver (liver enzymes) and kidney (creatinine and eGFR) function and full blood count. Glucose monitor (FreeStyle Libre Pro, Abbott, Germany) is attached for a period of 14 days.
A link to download the PNP smartphone application is sent to the participants prior to the screening/enrolment visit along with a short video demonstrating the app use. They are asked to log-in to the app with a personal (re-identifiable) code provided by email, and to familiarise with the app in preparation for a training session with the dietician. During the screening/enrolment visit, the dietitian practices with the participants browsing the food database, selecting food and beverage items and indicating the amount consumed. Participants are taught to add frequently consumed foods to a favourites list, which makes future search of food items easier. When the CGM is on, participants are asked to carry on with their usual routine and to record all meals, snacks and drinks using the app. The period between the screening/enrolment and the baseline visits (4–6 weeks) serves as the ‘run-in’ period.
Randomisation
Randomisation is performed between the screening/enrolment and baseline visits. Individuals are randomised with 1:1 allocation into the two arms in rounds of four to six individuals each, with randomisation performed within each round using the minimisation programme for allocation of subjects to parallel groups, modified from Saghaei et al.21 They are stratified by gender, age (20–49 or 50–70 years), BMI (<25.0 or >25.1 kg/m2) and HbA1c (<5.7 (39 mmol/mol) or >5.8% (40 mmol/mol)). To avoid bias, the randomisation is performed by a study investigator located at the Weizmann Institute who does not interact with the study participants. The study nurses and physicians who have direct contact with the participants are blinded to the randomisation order, however due to the nature of the intervention, the study dietician is not blinded to the treatment allocation.
Baseline visit: measurements
The baseline visit is performed approximately 4–6 weeks after the screening/enrolment visit (table 1). Participants attend the Clinical Research Facility following an overnight fast. Blood is drawn for serum lipids measurement and anthropometric measures and blood pressure are taken. Arterial stiffness (pulse wave analysis, AtCor Medical, Australia) is measured twice and average recorded, as described.22 This is followed by measurement of resting energy expenditure, carbohydrate and fat oxidation over 30 min by indirect calorimetry (Quark, Cosmed, Italy).23 Body composition is assessed using dual-energy X-ray absorptiometry (DXA, Lunar Prodigy, GE Healthcare). Specifically, total body fat mass and fat-free mass (enCORE software), the android and gynoid region, and visceral fat (CoreScan software, GE Healthcare) are recorded.24 Liver steatosis (controlled attenuation parameter, CAP) and liver fibrosis (liver stiffness measurements, LSMs) are assessed using FibroScan (Touch 502 by Echosens) by a trained technician. CAP and LSMs have been reported to correlate closely with steatosis and fibrosis assessed using the gold standard liver biopsy.25 A physical activity monitor (ActivePal, Pal Technologies) is applied on the thigh for a period of 14 days. At the end of the baseline visit, the participants practice using the app with the dietician.
Table 1.
Study timeline of activities and measurements taken at each of the study events and visits
| Location and time | Measurements | |
| Pre-screening | Phone −35±10 days |
|
| Local pathology |
|
|
| Screening/enrolment (non-fasting) |
Clinical Research Facility −30±15 days |
|
| Home |
|
|
| Baseline (fasting) |
Clinical Research Facility 0, treatment clock starts |
|
| 3 months (fasting) |
Clinical Research Facility +90±10 days |
|
| Home |
|
|
| 5.5 months (non-fasting) |
Clinical Research Facility +166±10 days |
|
| Local pathology |
|
|
| 6 months (fasting) |
Clinical Research Facility +180±10 days |
|
| Home |
|
|
| 12 months | Local pathology |
|
| Home |
|
*Questionnaire includes hunger/fullness, dietary habits and dislikes, physical activity, medications, dietary supplements, personal and family history of disease, food-frequency questionnaire.
†Gut microbiome features from stool samples collected at 3 and 6 months will be compared with baseline (exploratory outcome).
‡The Laboratory for the Study of Human Ingestive Behavior, Pennsylvania State University are the copyright holders of the Diet Satisfaction Questionnaire.30
§Questionnaire includes diet (are you following the diet you were allocated to?), metformin (are you continuing the metformin treatment and dosage) and current body weight.
BIA, bioelectrical impedance analysis; BMI, body mass index; DXA, dual-energy X-ray absorptiometry; HbA1c, glycated haemoglobin; OGTT, oral glucose tolerance test; REE, resting energy expenditure; RMR, resting metabolic rate; RQ, respiratory quotient.
Prediction of PPGR using the algorithm
The prediction of PPGR in PREDICT follows the modelling framework described in Zeevi et al16 and is performed between the screening/enrolment and baseline visits. Time-stamped food records from the app, CGM and other data collected during the enrolment visit at the Garvan are shared with a mathematician at the Weizmann Institute of Science where data processing occurs, on an Institutional secured server. The data, together with the stool metagenome sequencing data are integrated with the Weizmann Institute’s database to develop personalised algorithms for predicting each individual’s PPGR. A database of recipes of meals (n=233) and smaller meals (‘snacks’, n=249) varying in macronutrient composition to generate feedback on the PPGR to pre-consumed meals has been created. Using the participants' features, personalised PPGRs are calculated for every meal and snack in the database based on nutrient composition, and energy-adjusted quintile cut-offs of PPGR are used to create personalised meal ratings ranging from 1 to 5 (corresponding with ‘excellent’, ‘good’, ‘medium’, ‘bad’ and ‘very bad’). The predictive model, originally trained on data collected in an Israeli adult cohort,16 has been shown to be predictive of PPGR in a US cohort of healthy adults (n=327) consuming a Western-style diet.26
Interventions
Both arms use the PNP mobile app to select meals/foods. In 2018, the app, developed at the Weizmann Institute of Science, was adapted to Australian consumers including the Australian food database (AUSNUT 2011–2013)27 of approximately 5700 food items.
Personalised diet (PNP diet arm)
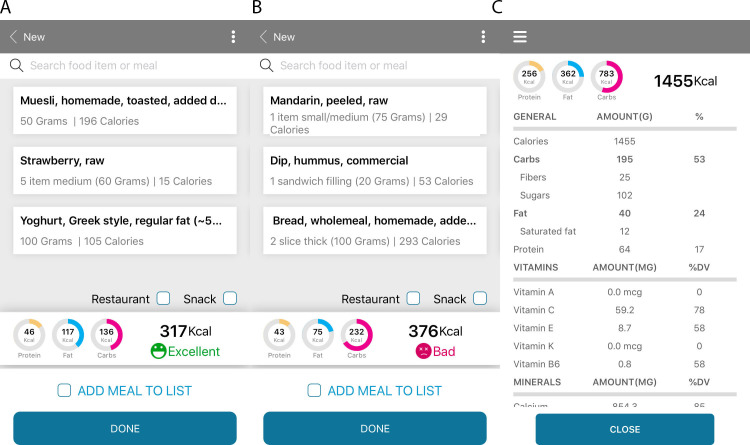
Participants in the PNP diet arm receive personalised feedback on each of their food item/meal choice and are asked to consult with the app in real-time to select the recommended meal for them. The feedback is color-coded with a traffic light system; green (‘good’ and ‘excellent’), yellow (‘medium’) and red (‘bad’ or ‘very bad’) PPGR (figure 1A, B). Participants are advised to aim for as many ‘good’ and ‘excellent’ scores, occasional ‘medium’, and to avoid ‘bad’ and ‘very bad’ scores. When receiving bad scores, they are advised to trial substituting, adding or removing ingredients from the meal to improve the score. In individuals with hypercholesterolemia, a special set of recipes containing reduced saturated fat are uploaded into the smartphone app. Special recipes are available for individuals practising vegetarianism or avoiding dairy, eggs and fish/seafood.
Figure 1.
Screenshots of the smartphone application used daily by participants in the Personalised Medicine in Pre-diabetes—Towards Preventing Diabetes in Individuals at Risk Study. Participants randomised to the personalised diet arm receive scores for each meal. Panels (A) and (B) depict two meal options selected by an individual in the study where two isocaloric breakfasts are predicted to result in modest (A) or exaggerated (B) postprandial glycaemic responses. The daily energy intake and macronutrient breakdown are provided to each of the study participants (C).
LFHF arm
The diet comparator chosen in the present study (the standard of care, LFHF) follows the Australian Healthy Eating Guide17 principles. The LFHF diet is designed to provide approximately 30% of the total daily energy intake from fat, of which up to 10% of the fat is saturated fat, 50%–55% of energy from low glycaemic load carbohydrates, 20%–25% from protein and 30 g/day of dietary fibre. The diet is rich in legumes, poor in white grains and added sugar. Overall, the comparator diet chosen is considerably different from the diet of the average adult Australian.28 A database of recipes (n=110 meals and n=80 snacks) following the LFHF nutrient content has been created based on the AUSNUT 2013 recipes.27 Food items such as sugary drinks, processed meat, candies, sugar and cream were excluded from the LFHF recipes. Similar to the PNP arm, recommended meals scaled to the individual’s energy target are uploaded into the participants’ app, taking into account the individual’s dietary restrictions and likes. Similar to the PNP arm, participants of the LFHF arm are encouraged to choose from the recipes uploaded for them or browse the food database (5700 food items) to design their own meals, as long as they follow the general dietary guidelines. Participants of the LFHF arm are instructed to consult with the total daily energy and macronutrient breakdown charts to ensure they follow the recommended diet (figure 1C).
Energy target and using the app to select meals in real-time (both arms)
The energy requirement calculation is based on RMR estimated by BIA (Tanita, TBF-300 by Wedderburn) and on the Mifflin equation.29 The two values are multiplied by a physical activity factor of 1.4 (lightly active) then averaged, and the value compared with the average daily energy intake of at least 7 days, extracted from the time-stamped meals recorded using the app. In participants with BMI >25 kg/m2, energy target of 75% is prescribed. Participants of both arms are encouraged to consume three bigger (breakfast, lunch, dinner) and three smaller (snack) meals spread throughout the day. An email, along with a short video summarising the principles of the diet and app use is sent to the participants (different sets of email and video to each arm) in the first week of the treatment period.
Metformin (relevant to both study arms)
Metformin (XR) is dispensed by the St Vincent’s Hospital (SVH) Pharmacy (Sydney) at baseline (for a period of 3 months) and at the 3 months visit (to last until the end of the study). The target dose (1500 mg/day) is titrated over 3 weeks to minimise gastrointestinal intolerance. A target dose of 1000 mg/day is set for participants with mild to moderately decreased eGFR (45–59 mL/min/1.73 m2), or participants who cannot tolerate the higher dose. A standardised dose of 1500 mg/day, rather than 2000 mg/day, was selected to suit both participants with pre-diabetes and T2DM, while minimising intolerance. Participants are instructed to take the medication with the evening meal and record it daily using a medication recording screen in the app or in paper logbooks.
Monitoring and adherence evaluation (relevant to both study arms)
The dietician reviews scores calculated programmatically based on the frequency of using the app and on meeting the daily energy target, along with the time-stamped meals consumed by the participants daily. In the PNP arm, the score incorporates the proportion of meals achieving the desired (‘excellent’ and ‘good’) scores, while in the LFHF arm, the proportion of days in which dietary fat ≤35%, saturated fat ≤10%, carbohydrates ≥45% and dietary fibre ≥15 g. The dietician contacts individuals who need encouragement to achieve better scores. Participants of the two treatment groups receive the same attention according to their adherence. Time devoted to each individual by the dietician is recorded for later analysis purposes. Satisfaction with the diet is assessed using the Diet Satisfaction Questionnaire30 at 6 months (table 1).
Adherence to the metformin is based on pill counting at the 6 months’ visit and on logs of daily dose using the app or logbooks.
Physical activity and other confounders
Participants are asked to maintain the same level of physical activity throughout the study. Physical activity is monitored at two time points during the study using ActivePal (table 1). The device records time (start and duration) and type (quiet, standing and steps) of activity and 14 days’ worth of data, stored in the device, downloaded on return. Information about background medications and nutritional supplements is collected before the start of the treatment using questionnaires (table 1). Participants are asked to report any change in medications at each of the study visits and use the medication screen in the smartphone app.
Primary and secondary outcomes: measurements
Participants attend the Clinical Research Facility five times during the study, over approximately 7 months. Primary and secondary endpoint measures are collected before the start of the intervention (across two visits: screening/enrolment and baseline) and at 3 and 6 months of treatment. Table 1 outlines the measurements obtained at each of the study visits/events.
Study outcomes
The primary outcome measure is change in HbA1c from baseline to 6 months of treatment. Furthermore, a comprehensive glycaemia assessment is enabled through continuous glucose monitoring. Interstitial glucose concentrations are recorded every 15 min using CGM for 14 days before the start of the intervention (in the run-in period) and after 3 and 6 months of intervention. The sensor stores the data for the duration of the recording, while the participants are blinded to the glucose readings. The data are downloaded on return of the sensor. Time of the day with glucose readings below 7.8 mmol/L before versus after the treatment will be compared. Glycaemic variability,31 including (1) mean amplitude of glucose excursion (a measure of the variation of glucose concentrations from the mean), (2) the SD and the (3) mean postprandial area under the curve will also be assessed. Furthermore, fasting plasma glucose, 1-hour and 2-hour plasma glucose post 75 g glucose assessment are repeated after 6 months and will be compared with the baseline values. HbA1c test is repeated 6 months after treatment cessation (at 12 months, table 1), along with a short questionnaire, including weight, diet and medication status.
Weight, waist and hip circumferences are recorded at 3 months and 6 months of treatment and compared with baseline. Fat, fat-free mass and android/gynoid fat distribution and visceral fat measurements by DXA are repeated at 6 months of treatment.24 Similarly, resting energy expenditure and fat/carbohydrate oxidation is measured after 6 months of treatment.
Hepatic steatosis is common in pre-diabetes and T2DM1 32 and prevention of liver steatohepatitis is key target in individuals with pre-diabetes or T2DM. Metformin primarily targets the liver, inhibiting lipogenesis and increasing fatty acid oxidation; therefore, a beneficial effect on liver lipid and fibrosis with metformin has been assumed.33 However, comprehensive meta-analyses of randomised clinical trials concluded that reduction in both steatosis and fibrosis with metformin were underwhelming.34 35 Liver fibroscan measure is repeated after 6 months of treatment.
Blood pressure and pulse wave analysis measurements are repeated at 3 and 6 months of treatment and serum lipids measured after 6 months of treatment.
Safety/adverse events monitoring
Gastrointestinal side effects are the most common adverse effects of metformin and may occur in 20%–30% of individuals.36 37 Specifically, abdominal discomfort, nausea, diarrhoea and anorexia are common.36 While the gastrointestinal adverse effects are transient, in approximately 5% of individuals the symptoms may persist and result in cessation of metformin.37 Vitamin B12 concentrations may be lower with metformin, if metformin is administered for a long duration.38 39 The mechanism(s) responsible for the lower plasma B12 concentrations are unclear. A very rare, but potentially fatal complication of metformin use is lactic acidosis, mainly in patients with renal impairment.36 40 In PREDICT, individuals with severe renal impairment are excluded. Metformin is titrated over 3 weeks to negate the potential gastrointestinal side effects. Adverse events are recorded and monitored over the phone and during the study visits.
Statistical analysis
Sample size calculation
Based on the primary outcome measure HbA1c, to detect a clinically meaningful difference of 0.4% in the change of HbA1c from baseline between the study arms at 6 months, assuming SD of 1% for both groups,41 with 80% power at two-sided significance level of 0.05, a sample size of 106 for each arm is required. Hence, with an estimated dropout rate of 20%, we aim to enrol 132 individuals to each arm, totalling 264 individuals in the study.
Analysis plan
The intention-to-treat approach will be used for efficacy analysis. A likelihood-based mixed model repeated measures approach will be used for the primary efficacy analysis. The primary outcome measure HbA1c at baseline, 3 and 6 months will be the dependent variable and intervention by time interaction will be the fixed effects, and participants will be treated as random effect. The primary time specific comparison will be the difference in least square mean between intervention (PNP) and control (LFHF) diet at 6 months’ treatment. The differences between the groups after 3 months of treatment will also be examined. Missing data will be handled directly through maximum-likelihood estimation via mixed modelling. To control for potential confounding effects, demographic and clinical covariates (eg, age, gender, baseline BMI and background medications) will be adjusted as necessary in the model. To account for reduced metformin dose due to intolerance or eGFR 45–59 mL/min/1.73 m2, metformin dose status (1500 mg/day (normal) dose/reduced dose) will be also adjusted in the model. Piecewise linear mixed model will be used to compare trend change between arms in different periods (0–3 months and 3–6 months). Different statistical analysis strategies including t-test, Mann-Whitney U test, Χ2 test, linear/generalised linear regression and mixed model will be used based on the type and distribution of the outcome measures. Mediation analysis will be carried out to explore if the weight loss mediates the intervention effect on glycaemia and estimate indirect and direct effects and the proportion mediated (how much of the total intervention effect works through weight loss). We expect some degree of weight loss in all participants, as has been reported for metformin.10 42 43 The effect of the diet intervention mediated by metformin adherence on the study outcomes will also be tested. Subgroup analyses can be further performed to explore the intervention effect in specific subcohorts, for instance, the group of participants who have diabetes at baseline, the group of participants who achieve adherence standard and maintain the desired metformin dose; the group of participants with BMI >25 kg/m2 at baseline and others. The potential impact of the COVID-19 pandemic on the study outcomes may be explored, including comparisons of adherence and outcomes across groups of participants enrolled and followed-up pre-pandemic, during and post-pandemic period. Sensitivity analysis related to the impact of COVID-19 may be conducted.
Laboratory testing
HbA1c is analysed using high performance liquid chromatography (Bio-Rad D-100, Bio-Rad Laboratories), plasma glucose using the Cobas 8000 (Roche), and liver and renal function tests using the Atellica platform (Siemens). Serum lipid profile is analysed by a spectrophotometric assay (Advia 2400 Chemistry System (Siemens Medical Solutions Diagnostics)), with LDL calculated using the Friedewald equation. Metagenomic DNA from the stool samples is purified using DNeasy PowerMag Soil DNA extraction kit (Qiagen) optimised for Tecan automated platform. Next-Generation Sequencing libraries are prepared using Nextera DNA library prep (Illumina) and sequenced on a NovaSeq sequencing platform (Illumina). Sequencing is performed with 100 bp single end reads with the depth of 10 million reads per sample. Host DNA is detected by mapping reads to the human genome with inclusive parameters, and those reads removed. Bacterial relative abundance estimation is performed by mapping bacterial reads to species-level genome bins representative genomes.44 Mapping is performed using Bowtie45 and abundance estimated by calculating the mean coverage of unique genomic regions across the 50% most densely covered areas, as previously described.46
Confidentiality and data storage
Each participant is associated with an individual program-generated code used to identify their study documents, data and specimens collected during the study. The re-identifiable code is documented in the participant’s record and on all study documents. Study data are collected and managed using REDCap electronic data capture tools47 48 hosted at the Garvan Institute of Medical Research. Some coded data are shared with essential personnel at the Weizmann Institute of Science on institutional Dropbox. Data collected in the form of paper hard copies are kept in locked cabinets and electronic files on a password-protected folder with access granted to the Garvan study team. Re-identifiable blood, stool, plasma and serum samples will be kept at the Garvan Institute’s freezer facility. All the study questionnaires are disseminated using REDCap.
Dissemination of results
The results of the study will be disseminated to healthcare professionals via open access publications in medical journals, without any restrictions. On completion of data analysis, the participants will be invited to an information session at the Garvan Institute of Medical Research with the study investigator(s) where the findings of the study will be shared and discussed. Individual letters are disseminated to the study participants after the 6 months’ treatment visit (approximately 7–8 months from study enrolment) summarising individual results (eg, baseline and post-treatment weight, body fat, liver fat, HbA1c, fasting, 1-hour and 2-hour plasma glucose concentrations). The participants are encouraged to share their individual results with the GP.
Ethics and dissemination
The study has been approved by the SVH Human Research Ethics Committee (HREC) (File 17/080, Sydney, Australia) and the Weizmann Institutional Review Board (IRB) (File 528-3, Rehovot, Israel). Protocol modifications are communicated to the SVH HREC, the Weizmann IRB, the trial registry (ClinicalTrials.gov), the study investigators and the study participants (if relevant). De-identified participants’ data that underlie the findings reported in the research article will be available immediately following publication, ending 5 years following the article publication, to researchers who provide a methodologically sound proposal with the aim to achieve the aims reported in the approved project proposal.
Supplementary Material
Acknowledgments
We would like to thank Dr David Jung from UNSW (Sydney, Australia) for assistance in the set-up of the REDCap data collection tool and Ms Rebecca Hickey for performing the Fibroscan measurements.
Footnotes
Twitter: @SamochaBonetD
TDH and AG contributed equally.
Contributors: DS-B, JRG, ES and EE contributed to the conception and design of the study. TDH, AG and DS-B drafted the manuscript. AG and ZL contributed to the study design and the statistical plan. ZL contributed to the sample size estimation. AG, DK, RR, KT, EC, MD, JRS and T-MH contributed to the collection of the data. All authors revised and approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Funding: This work is supported by the Garvan Research Foundation and the St Vincent’s Clinic Foundation, Sydney, Australia. The study sponsor is the Garvan Institute of Medical Research, 384 Victoria Street, Darlinghurst, NSW 2020, Australia, +61 2 92958100.
Disclaimer: The study sponsor and funding bodies have no role in the study design, collection, management, analysis and interpretation of data, writing of the report, and the decision to submit the report for publication.
Competing interests: EE and ES are paid consultants of the company DayTwo. MD has received travel support and speaker fees from Gilead, Abbvie and Merck. All other authors declare they have no conflict of interest.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Stefan N, Fritsche A, Schick F, et al. . Phenotypes of prediabetes and stratification of cardiometabolic risk. Lancet Diabetes Endocrinol 2016;4:789–98. 10.1016/S2213-8587(16)00082-6 [DOI] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30. 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hostalek U. Global epidemiology of prediabetes - present and future perspectives. Clin Diabetes Endocrinol 2019;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IDF Diabetes Atlas 8th edition, 2017. International Diabetes Federation. Available: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/134-idf-diabetes-atlas-8th-edition.html
- 5.Evert AB, Dennison M, Gardner CD, et al. . Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–54. 10.2337/dci19-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahlqvist E, Storm P, Käräjämäki A, et al. . Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361–9. 10.1016/S2213-8587(18)30051-2 [DOI] [PubMed] [Google Scholar]
- 7.Bailey CJ. Metformin: historical overview. Diabetologia 2017;60:1566–76. 10.1007/s00125-017-4318-z [DOI] [PubMed] [Google Scholar]
- 8.Davies MJ, D'Alessio DA, Fradkin J, et al. . Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care 2018;41:2669–701. 10.2337/dci18-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florez JC. The pharmacogenetics of metformin. Diabetologia 2017;60:1648–55. 10.1007/s00125-017-4335-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samocha-Bonet D, Debs S, Greenfield JR. Prevention and treatment of type 2 diabetes: a Pathophysiological-Based approach. Trends Endocrinol Metab 2018;29:370–9. 10.1016/j.tem.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 11.Kahn SE, Haffner SM, Heise MA, et al. . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–43. 10.1056/NEJMoa066224 [DOI] [PubMed] [Google Scholar]
- 12.TODAY Study Group, Zeitler P, Hirst K, et al. . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–56. 10.1056/NEJMoa1109333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lachin JM, Christophi CA, Edelstein SL, et al. . Factors associated with diabetes onset during metformin versus placebo therapy in the diabetes prevention program. Diabetes 2007;56:1153–9. 10.2337/db06-0918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aroda VR, Knowler WC, Crandall JP, et al. . Metformin for diabetes prevention: insights gained from the diabetes prevention Program/Diabetes prevention program outcomes study. Diabetologia 2017;60:1601–11. 10.1007/s00125-017-4361-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42:S13–28. 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- 16.Zeevi D, Korem T, Zmora N, et al. . Personalized nutrition by prediction of glycemic responses. Cell 2015;163:1079–94. 10.1016/j.cell.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 17.Australian Government National Health and Medical Research Council Australian guide to healthy eating, 2015. Available: https://www.eatforhealth.gov.au/guidelines/australian-guide-healthy-eating2019
- 18.Garvey WT, Mechanick JI, Brett EM, et al. . American association of clinical endocrinologists and American College of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract 2016;22 Suppl 3:1–203. 10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 19.Lamb EJ, Tomson CRV, Roderick PJ, et al. . Estimating kidney function in adults using formulae. Ann Clin Biochem 2005;42:321–45. 10.1258/0004563054889936 [DOI] [PubMed] [Google Scholar]
- 20.Meyerowitz-Katz G, Seelan S, Gaur P, et al. . Detecting the hidden burden of pre-diabetes and diabetes in Western Sydney. Diabetes Res Clin Pract 2019;151:247–51. 10.1016/j.diabres.2019.04.019 [DOI] [PubMed] [Google Scholar]
- 21.Saghaei M, Saghaei S. Implementation of an open-source customizable minimization program for allocation of patients to parallel groups in clinical trials. J Biomed Sci Eng 2011;04:734–9. 10.4236/jbise.2011.411090 [DOI] [Google Scholar]
- 22.Kozan P, Blythe JC, Greenfield JR, et al. . The effect of buffering high acid load meal with sodium bicarbonate on postprandial glucose metabolism in Humans-A randomized placebo-controlled study. Nutrients 2017;9:861. 10.3390/nu9080861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen DL, Liess C, Poljak A, et al. . Phenotypic characterization of insulin-resistant and insulin-sensitive obesity. J Clin Endocrinol Metab 2015;100:4082–91. 10.1210/jc.2015-2712 [DOI] [PubMed] [Google Scholar]
- 24.Tang A, Coster ACF, Tonks KT, et al. . Longitudinal changes in insulin resistance in normal weight, overweight and obese individuals. J Clin Med 2019;8. 10.3390/jcm8050623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eddowes PJ, Sasso M, Allison M, et al. . Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1717–30. 10.1053/j.gastro.2019.01.042 [DOI] [PubMed] [Google Scholar]
- 26.Mendes-Soares H, Raveh-Sadka T, Azulay S, et al. . Assessment of a personalized approach to predicting postprandial glycemic responses to food among individuals without diabetes. JAMA Netw Open 2019;2:e188102. 10.1001/jamanetworkopen.2018.8102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Australian food, supplement and nutrient database (AUSNUT) 2011-2013: food standards Australia New Zealand; 2016.
- 28.Grech A, Rangan A, Allman-Farinelli M. Macronutrient Composition of the Australian Population’s Diet; Trends from Three National Nutrition Surveys 1983, 1995 and 2012. Nutrients 2018;10:1045 10.3390/nu10081045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mifflin MD, St Jeor ST, Hill LA, et al. . A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7. 10.1093/ajcn/51.2.241 [DOI] [PubMed] [Google Scholar]
- 30.James BL, Loken E, Roe LS, et al. . Validation of the diet satisfaction questionnaire: a new measure of satisfaction with diets for weight management. Obes Sci Pract 2018;4:506–14. 10.1002/osp4.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Ther 2009;11 Suppl 1:S-55–S-67. 10.1089/dia.2008.0132 [DOI] [PubMed] [Google Scholar]
- 32.Marjot T, Moolla A, Cobbold JF, et al. . Non-Alcoholic fatty liver disease in adults: current concepts in etiology, outcomes and management. Endocrine Reviews 2020;41:66–117. 10.1210/endrev/bnz009 [DOI] [PubMed] [Google Scholar]
- 33.Green CJ, Marjot T, Tomlinson JW, et al. . Of mice and men: is there a future for metformin in the treatment of hepatic steatosis? Diabetes Obes Metab 2019;21:749–60. 10.1111/dom.13592 [DOI] [PubMed] [Google Scholar]
- 34.Musso G, Cassader M, Rosina F, et al. . Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia 2012;55:885–904. 10.1007/s00125-011-2446-4 [DOI] [PubMed] [Google Scholar]
- 35.Sawangjit R, Chongmelaxme B, Phisalprapa P, et al. . Comparative efficacy of interventions on nonalcoholic fatty liver disease (NAFLD): a PRISMA-compliant systematic review and network meta-analysis. Medicine 2016;95:e4529. 10.1097/MD.0000000000004529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howlett HC, Bailey CJ. A risk-benefit assessment of metformin in type 2 diabetes mellitus. Drug Saf 1999;20:489–503. 10.2165/00002018-199920060-00003 [DOI] [PubMed] [Google Scholar]
- 37.McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia 2016;59:426–35. 10.1007/s00125-015-3844-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Jager J, Kooy A, Lehert P, et al. . Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ 2010;340:c2181. 10.1136/bmj.c2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinstatler L, YP Q, Williamson RS, et al. . Association of biochemical B12 deficiency with metformin therapy and vitamin B12 supplements. Diabetes Care 2012;35:327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeFronzo R, Fleming GA, Chen K, et al. . Metformin-Associated lactic acidosis: current perspectives on causes and risk. Metabolism 2016;65:20–9. 10.1016/j.metabol.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 41.Jönsson T, Granfeldt Y, Ahrén B, et al. . Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol 2009;8:35. 10.1186/1475-2840-8-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knowler WC, Barrett-Connor E, Fowler SE, et al. . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, et al. . 10-Year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet 2009;374:1677–86. 10.1016/S0140-6736(09)61457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasolli E, Asnicar F, Manara S, et al. . Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell 2019;176:649–62. 10.1016/j.cell.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods 2012;9:357–9. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korem T, Zeevi D, Suez J, et al. . Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science 2015;349:1101–6. 10.1126/science.aac4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris PA, Taylor R, Minor BL, et al. . The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris PA, Taylor R, Thielke R, et al. . Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-037859supp001.pdf (227.2KB, pdf)