Abstract
Objective
Dietary patterns and practices can predispose or protect against metabolic syndrome (MetS) in humans. Despite the growing problem of MetS in adults, the underpinning dietary behaviour is poorly understood. We determined the dietary patterns and practices relevant to MetS in adults with central obesity attending a mission hospital in Kenya.
Study design
Descriptive, cross-sectional.
Setting
Outpatient clinic of a mission-based hospital in Nairobi.
Participants
Adults (N=404) aged 18–64 years diagnosed with central obesity as per the International Diabetes Federation definition for MetS.
Primary outcomes
Anthropometric measurements, clinical-biochemical markers and dietary components, quantity and frequency of food intake, as well as time-lapse between consumption of dinner and sleeping.
Results
A high (87.2%) prevalence of MetS was observed for respondents who reported consumption of large amount of carbohydrates (p<0.001), proteins (p<0.001), processed/fast foods (p<0.001) and sugar (p=0.009). Frequent consumption of legumes (p<0.001), nuts (p<0.001), fruits (p<0.001) and vegetables (p=0.021) was linked to reduced MetS. Additionally, longer interval between eating dinner and going to bed was associated with reduced MetS.
Conclusion
Regular consumption of fruits, vegetables, legumes and nuts, as well as observing sometime after eating dinner before sleeping, was the dietary pattern significantly associated with a lower risk of MetS. Whereas, consumption of a large quantity of carbohydrates, proteins, processed/fast foods and sugar is likely to predispose to MetS. The findings underscore the need to focus on specific dietary intake patterns including frequency, quantity, quality and variety for MetS prevention and management. The MetS-related interventions could be implemented during individual consultation, group and community health messaging sessions.
Keywords: nutrition & dietetics, hypertension, public health
Strengths and limitations of this study.
This was the first study conducted among the informal settlements (slums) in Kenya that determined the association between dietary intake patterns and metabolic syndrome.
The relatively large sample size increases the possibility of replication and generalisation of the findings.
The use of widely recognised and validated dietary questionnaires is another strength of the study.
The study was limited by its cross-sectional design.
The self-reported dietary patterns and practices may suffer from information bias.
Introduction
A dietary pattern refers to the quantity, variety or combination of different foods in a diet and the frequency with which they are habitually consumed. The importance of nutritious dietary patterns and practices on metabolic syndrome (MetS) cannot be overstated. Indeed, components of MetS namely: central obesity, raised blood glucose, elevated blood pressure (BP) and dyslipidaemia1 are closely linked to dietary behaviour. Dietary patterns and practices have been implicated in the risk for MetS.2 3 Several important factors including genetic, unhealthy eating habits and urbanisation have been cited as risk factors for MetS,4 however, unhealthy dietary pattern plays a major role in the incidence of MetS. Unhealthy diet characterised by consumption of processed/fast foods has been reported to be associated with MetS and cardiovascular diseases (CVDs).3 5 Whereas, a healthy dietary pattern characterised by regular consumption of fruits and vegetables,6 legumes7 8 and nuts9 is strongly associated with a lower risk of MetS. Saturated fatty acids mainly from processed/fast foods as well as refined carbohydrates are the major dietary factors fully responsible for the occurrence of MetS.10 Lifestyle modification involving adjusting the type, quality and quantity of diet is attributed to reduction of the risk for MetS.5 11 Of significance is the fact that MetS is linked to high propensity of escalation into non-communicable diseases (NCDs) notably hypertension and type 2 diabetes and the resultant morbidity and mortality.12 13 Indeed, individuals with MetS are more than seven times more likely to develop diabetes and twofold likely to develop and die from CVDs.11 13
A growing epidemic of MetS has been observed globally with sub-Saharan and developing countries bearing the biggest burden. For example, according to the International Diabetes Federation (IDF), the global prevalence of MetS is approximately 25%.14 MetS just like the NCDs is disproportionally heavy in sub-Saharan Africa with a resultant burden on the health system and economy. For example, the prevalence of MetS has been found to be 35.1% in Nigeria,15 35.9% in Ghana,16 39.0% in Cameroon17 and 42.1% in Egypt.18 In Kenya, the prevalence of MetS has been reported at 25.6%.19
The growing burden of MetS in Africa is associated with nutritional transition14 20 characterised by intake of high energy-dense foods,21 high quantity,3 lack of variety and quality of foods.22 Kenya is experiencing a rapid epidemiological and nutritional transition accompanied by increased consumption of unhealthy dietary pattern characterised by high intakes of refined carbohydrates, processed/fast foods, sugar-sweetened beverages, and low fruits and vegetables.2 Evidence by Kimani et al20 showed that patients with hypertension who daily consumed vegetables and fruits had lower rates of obesity, hypertension and cholesterol levels, some of the components of MetS. Elsewhere, reports from one of Nairobi’s slums showed a high prevalence of overweight and abdominal obesity related to consumption of low vegetables and fruits.22 The reports and other documented evidence underscore the importance of diet in relation to MetS and its related components providing a window of prevention through awareness creation using health facilities or community as avenues for the interventions.
Unhealthy dietary pattern characterised by consumption of high-calorie diet such as processed/fast foods that are high in fats and sugars promotes obesity compared with low-energy foods, for example fruits and vegetables.23 The high-calorie diets are causally linked to insulin resistance, type 2 diabetes, dyslipidaemia and high BP—the main components of MetS.24 Moreover, diet has been associated with risk for high BP and poor hypertension control.20 Although some studies in Kenya have demonstrated the association between nutrition and MetS, they are limited in number and methodological rigour. For well-thought evidence guided dietary/nutritional-oriented public health interventions on MetS studies are required. We sought to determine dietary patterns/practices (frequency intake of fruits, vegetables, legumes, nuts, processed and/or fast foods, proportion of protein and carbohydrate, amount of salt and sugar, as well as time interval between taking dinner and sleeping) relevant to MetS among Kenyan adults with central obesity attending a mission hospital in Nairobi.
Methods and materials
The study methods and materials have been well elaborated in our published work.25 This is part of the larger community-based lifestyle intervention study for managing MetS among adults in an ongoing project.
Study setting
The study was executed at St Mary’s Mission Hospital—a faith-based health facility located in Langata constituency Nairobi County. The hospital provides affordable services to a large low-income-earning population from the neighbouring Kibera, Mukuru-Kwa-Njenga and Kuwinda slums. Specifically, Kibera is the largest and poorest slum in Africa, with individual resident’s average monthly income of US$39 per household.26 The hospital has an inpatient bed capacity of 350 offering medical, surgical, maternity, paediatric, postnatal, newborn unit, operating theatre, inpatient gynaecology and physiotherapy services. Additionally, the facility has a 24-hour outpatient department that offers general outpatient care, maternal and child health, diabetic and hypertension, nutrition, dental, eye, pharmacy, laboratory and imaging services, as well as HIV/AIDS prevention treatment and care services. The hypertension–diabetic clinic operates on daily basis from Monday to Friday serving about 600 patients per month. The clinic is run by a team of professionals comprising of physicians, nurses, nutritionists, laboratory technicians, pharmacists and social workers.
Study design, sampling methods and respondents
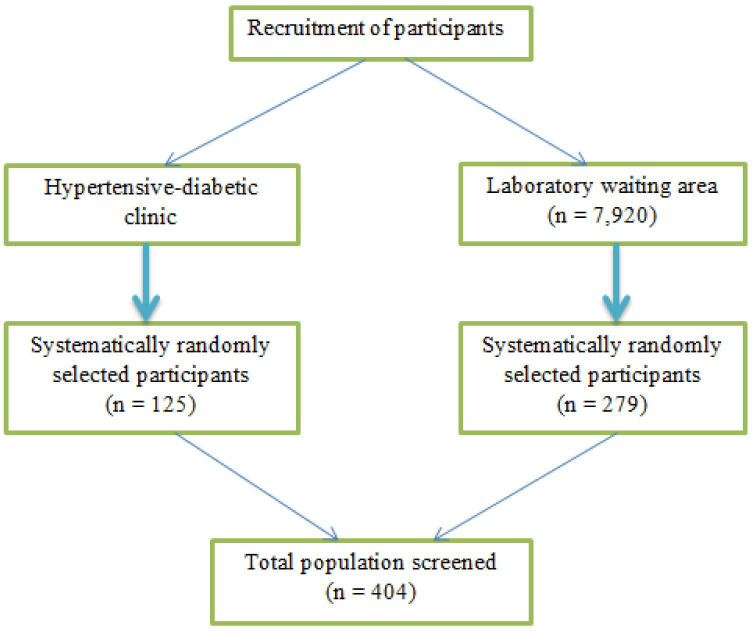
A cross-sectional study involving adults (N=404) aged 18–64 years. For inclusion into the study, we considered central obesity (waist circumference (WC) ≥94 cm for men and ≥80 cm for women) as the primary criteria for screening for the other MetS components as per the IDF guidelines.1 We excluded pregnant and lactating women, individuals with contraindication for exercise due to serious diseases such as CVDs, cancer, mental illness and physical disability. However, some known patients with hypertension or diabetes were included based on the components of MetS. A systematic random sampling method was used to recruit the study respondents. During the study period, the clinic served for about 600 adults with hypertension and/or diabetes per month, which translated to 1800 patients in 3 months equivalent to the duration for completion of the data collection. Thus, the sampling interval was determined by dividing the target population (1800) in a 3-month period by the number of patients with hypertension–diabetes calculated to be screened for MetS (n=125) to get the sample interval of 14. Accordingly, every 14th client with hypertension/diabetes was included in the study after consenting until the desired sample size was achieved. An additional 279 participants who included those attending outpatients and their accompanying visitors were screened using systematic random sampling as they waited at the main laboratory waiting area of the hospital. During the same period, the hospital’s main laboratory served for about 2640 adult clients (aged 18–64) per month, equivalent to 7920 in a 3-month period, the duration required to complete the data collection. Then, the total population for 3 months (7920) was divided by the initially adjusted sample size (375) to get a sample interval of 21. Then every 21st participant was included in the screening until the desired sample size was achieved (figure 1).
Figure 1.
Flow chart showing recruitment of the participants.
Data collection tools and procedures
Data were collected using a researcher-assisted structured questionnaire adopted from the WHO STEPwise approach to NCD risk factor surveillance.27 The questionnaire had four categories including sociodemographics, dietary intake patterns and practices, anthropometrics and biochemical markers. The data were collected by two trained research assistants with a bachelor’s degree in nursing training background. The blood glucose and lipid levels were collected and analysed by two experienced laboratory technicians.
Assessment of dietary patterns/practices
A pre-intervention and post-intervention dietary intake patterns including quantity, variety and the frequency with which the respondents habitually consumed were assessed using 32 food frequency questionnaires adopted from the WHO STEPwise approach to NCD risk factor surveillance,27 with some modifications to fit the Kenyan dietary context. The respondents were asked how frequently they consumed a particular food product, and the quantity usually they eat per food item by making comparisons with the specific reference portions. Frequency intake of fruits, vegetables, legumes, nuts, processed/fast foods, quantity or proportion of protein and carbohydrate, amount of salt and sugar, as well as time interval between taking dinner and sleeping, was assessed by asking the respondents how often a week they consumed these foods. The recommended frequency of legume and nut intake is four to five times per week and therefore we grouped the responses as (often ≥four times/week, sometimes (two to three times/week and rarely (≤one time/week). Whereas the recommended frequency of fruit and vegetable intake is four to five times a day, and thus we grouped as daily and not daily. Consumption of processed/fast foods was categorised as (always ≥5 days/week, sometimes 2–4 days/week, rarely <2 days/week). Common household measuring equipment including measuring cups, spoons and plates were shown to assist the participants in the estimation of the amounts to avoid measurement bias. As part of the dietary pattern assessment, the dietary approach to stop hypertension (DASH) eating plan was used to estimate the quantity or proportion of protein (eg, meat, eggs, whole milk), carbohydrate (eg, ugali, bread, chapatti, rice, maize, potatoes, pasta) and vegetables/fruits as well as frequency of legume and nut consumption. According to the DASH diet, vegetables and/or fruits constitute one-half of a plate at each meal. One-quarter of the plate is filled with carbohydrates and the remaining one-quarter is filled with plant proteins like legumes, soy products, nuts and seed proteins to control high BP. Animal proteins in the diet should mainly compose of lean meats, low-fat dairy, eggs and fish. We measured the proportion of a meal by drawing a plate into four parts. Then we asked the respondents to estimate the proportion of vegetables and/or fruits, carbohydrate and proteins they usually fill their plate. The processed and/or fast foods such as chips, sandwiches, hamburgers, fried chicken, French fries, sausages, samosas, pizza, hot dogs, ice cream among others were captured using specific questions focused on such theme integrated in the questionnaire. Time interval between taking dinner and sleeping was assessed by asking respondents at what time do they usually eat their dinner. The responses were: before 20:00, between 20:00 and 21:00, between 21:00 and 22:00 and after 22:00. Then, they were asked the time they usually go to bed to sleep. The responses were: before 21:00, between 21:00 and 22:00, between 22:00 and 23:00, between 23:00 and midnight and after midnight.
Anthropometric variables
The anthropometric parameters included weight, height, WC and hip circumference (HC). These parameters were measured using standard measurement tools. The body weight was measured to the nearest 0.1 kg using a Sohenle mechanical weighing scale with the respondent in light clothing. The height (m) was measured using a portable stadiometer to the nearest 0.5 cm, with subjects standing upright on a flat surface without shoes, the back of the heels and the occiput on the equipment. The height and weight was used to calculate the body mass index that is the ratio of weight (kg) over height in m2. The WC was taken at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest to the nearest 0.1 cm using a procedure by WHO. The HC was taken at the greatest posterior protuberance of the buttocks to the nearest 0.1 cm using a flexible tape.28
Clinical variables (BP and heart rate)
BP and heart rates were measured using OMRON automatic BP monitor (model: M3; HEM-141-E, serial no: 20170916247VG, Japan) after a rest period of 5–10 min in a sitting position. The BP was measured two times with a 5 min time interval with the mean of the two measurements being recorded. Elevated BP as a component of MetS was defined as ≥130/85 mm Hg.1 A systolic BP of 120–139 mm Hg and/or diastolic BP of 80–89 mm Hg were considered as prehypertension. Hypertension was defined as systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg.29 The heart rate was measured for 1 min as well by the BP monitor.
Biochemical variables
The blood samples for fasting blood glucose (FBG), lipids (triglycerides (TGs) and high-density lipoprotein cholesterol (HDL-C)) levels were drawn and analysed following an overnight fasting of 8–12 hours. FBG sample was obtained from the respondents’ finger using HemoCue B-Glucose photometer (photometer, 1995). A sample of 3 mL of blood was obtained from the brachial vein following standard infection prevention procedures to determine TGs and HDL-C values. Each blood sample was labelled with the participants’ number to avoid errors of recording. Raised FBG level was defined as FBG level ≥5.6 mmol/L.1 Pre-diabetes and diabetes were defined as FBG of 5.6–6.9 mmol/L and ≥7 mmol/L, respectively.30 Raised TG was defined as TGs level ≥1.7 mmol/L irrespective of gender. Whereas, low HDL-C level was defined as HDL-C <1.03 mmol/L in men and <1.29 mmol/L in women.1
Validity and reliability of the study tools
The WHO STEPwise approach to NCD risk factor surveillance questionnaire27 was used to collect the data. Additionally, the tools were reviewed for content validity by experts in the field of CVD and nutrition to ascertain relevance and completeness. The recommendations and suggestions were incorporated in the final questionnaire. To measure reliability of the questionnaire, a test re-test method was employed, whereby a repeat pre-test was carried out after 3 weeks, and Cohen’s kappa statistic was used to measure the level of agreement of the two results. The result of the repeated questions had a kappa value of 0.91 therefore, the questionnaire was considered reliable.
Patient and public involvement
There were no patients and members of the public involved in developing the research design and questions. This research was solely done by researchers without involving patients or public in any part of the research work. However, this research involved human participants. The results will be disseminated to the public during health message sharing sessions in the study area as well as seminars/conferences.
Definition of MetS
MetS was defined using the IDF criteria1 to include central obesity (WC of ≥94 cm for men and ≥80 cm for women) that was compulsory. The criteria also included other components namely: (1) raised TGs level ≥1.7 mmol/L (≥150 mg/dL) or history of specific treatment for the lipid abnormality; (2) reduced HDL-C <1.03 mmol/L (<40 mg/dL) in men and <1.29 mmol/L (<50 mg/dL) in women or history of specific treatment for the lipid abnormality; (3) elevated BP: systolic BP ≥130 mm Hg or diastolic BP ≥85 mm Hg or on treatment for previously diagnosed hypertension; (4) raised FBG level of ≥100 mg/dL (≥5.6 mmol/L) or previously diagnosed type 2 diabetes mellitus. The respondents had to display at least two of the four metabolic abnormalities in addition to the central obesity.
Data analyses
Statistical analyses were performed using the SPSS V.22. Descriptive data were analysed using proportions and summarised in frequency tables. The Χ2 test of independence and binary logistic regression were used to determine associations between categorical variables such as frequency of specific food consumption, prevalence of MetS and its related components. A multiple logistic regression model with backward conditional was carried out to determine the dietary-related variables independently contributed to the occurrence of MetS. Backward conditional method was specified with removal at p<0.05 to determine the independent predictors of MetS as it removes the confounding variables until no further variables can be removed without a statistically insignificant loss of fit (last or reduced model). The fitness model was also performed to describe the variance and classification of MetS. A p value of less than 0.05 was considered to be significant.
Results
Demographic characteristics of the respondents
Respondents totalling 404 were recruited with mean age of 42.5±11.9 (mean±SD) years. Most (59.2%, n=239) of them were between 31 and 50 years. A high proportion of the respondents were married (76.0%, n=307), women (54.5%, n=220), Protestants (59.7%, n=241) and self-employed (52.2%, n=211). Respondents with secondary level education were 48.8% (n=197). Economically family monthly income was reported to be between US$100 and US$300 (45.5%; n=184) (table 1).
Table 1.
Demographic characteristics of the respondents
| Characteristics | Number | % |
| Age (years) | ||
| ≤30 | 68 | 16.8 |
| 31–40 | 124 | 30.7 |
| 41–50 | 115 | 28.5 |
| >50 | 97 | 24 |
| Gender | ||
| Male | 184 | 45.5 |
| Female | 220 | 54.5 |
| Marital status | ||
| Married | 307 | 76 |
| Single | 69 | 17.1 |
| Divorced | 6 | 1.5 |
| Separated | 11 | 2.7 |
| Widowed | 10 | 2.5 |
| Cohabiting | 1 | 0.2 |
| Religion | ||
| Protestant | 241 | 59.7 |
| Catholic | 131 | 32.4 |
| Muslim | 32 | 7.9 |
| Level of education | ||
| No formal education | 7 | 1.7 |
| Primary | 75 | 18.6 |
| Secondary | 197 | 48.8 |
| College/university | 125 | 30.9 |
| Occupation | ||
| Government employee | 15 | 3.7 |
| Non-government employee | 111 | 27.5 |
| Self-employed | 211 | 52.2 |
| Unemployed | 67 | 16.6 |
| Family monthly income (US$) | ||
| Less than 100 | 67 | 16.6 |
| 101–300 | 184 | 45.5 |
| 301–500 | 66 | 16.3 |
| Over 500 | 49 | 12.1 |
| No response | 38 | 9.4 |
| Total | 404 | 100 |
Relationship between the DASH eating plan and MetS-related components
The DASH diet was used to determine quantity or proportion of meals usually consumed by the respondents. According to the DASH diet, at each meal, vegetables and/or fruits constitute one-half of a plate, carbohydrates one-quarter of the plate and the remaining one-quarter of the plate to be filled with plant proteins like legumes, soy products, nuts and seed proteins. Animal proteins in the diet should be limited to lean meats, low-fat dairy, eggs and fish. Most (77%; n=311) of the respondents reported consuming less than the recommended portion of a plate as vegetables and/or fruits. Further analysis revealed that those who consumed less than the recommended portion of a plate as vegetables and/or fruits were more likely to have MetS (χ²=32.004, p<0.001), elevated BP (χ²=116.082, p<0.001) and raised FBG level (χ²=5.590, p=0.018) compared with those who frequently took the recommended portion as vegetables/fruits. Most, 70.8% (n=286) of the respondents, frequently consumed a large proportion (over one-quarter of a plate) of a carbohydrate diet as part of the main meals. Respondents who frequently consumed a large proportion of carbohydrate foods were more likely to have MetS (χ²=33.866, p<0.001) and elevated BP (χ²=79.690, p<0.001) compared with those who ate the recommended amounts (≤25% portion of a plate). With regards to protein consumption, slightly above a quarter (29%; n=117) of the respondents frequently consumed a large proportion (over one-quarter of a plate) of protein diet as part of the main meals. Respondents who frequently consumed large proportion of foods rich in proteins were more likely to have MetS (χ²=13.122, p<0.001), elevated BP (χ²=33.342, p<0.001) and raised FBG level (χ²=6.393, p=0.011) compared with those who ate the recommended amounts (less than or 25% portion of a plate) (table 2).
Table 2.
Relationship between the DASH eating plan and metabolic syndrome (MetS)-related components
| MetS and its components | Proportion of a plate filled with vegetables and/or fruits | Total | χ2 | Df | P value | |
| Less than half of a plate | Half and above of a plate | |||||
| MetS | N (%) | N (%) | N (%) | 32.004 | 1 | <0.001 |
| Yes | 287 (81.5) | 65 (18.5) | 352 (100) | |||
| No | 24 (46.2) | 28 (53.8) | 52 (100) | |||
| Total | 311 (77.0) | 93 (23.0) | 404 (100) | |||
| Blood pressure (BP) | 116.082 | 1 | <0.001 | |||
| Elevated BP | 248 (93.2) | 18 (6.8) | 266 (100) | |||
| Normal BP | 63 (45.7) | 75 (54.3) | 138 (100) | |||
| High-density lipoprotein cholesterol (HDL-C) | 1.084 | 1 | 0.298 | |||
| Reduced HDL-C | 231 (75.7) | 74 (24.3) | 305 (100) | |||
| Normal HDL-C | 80 (80.8) | 19 (19.2) | 99 (100) | |||
| Triglycerides (TGs) | 1.729 | 1 | 0.189 | |||
| Raised TGs | 207 (79) | 55 (21) | 262 (100) | |||
| Normal TGs | 104 (73.2) | 38 (26.8) | 142 (100) | |||
| Fasting blood glucose (FBG) | 5.59 | 1 | 0.018 | |||
| Raised FBG level | 76 (86.4) | 12 (13.6) | 88 (100) | |||
| Normal FBG level | 235 (74.4) | 81 (25.6) | 316 (100) | |||
| Proportion of a plate filled with carbohydrates | ||||||
| ≤one-quarter of a plate | >one-quarter of a plate | |||||
| MetS | N (%) | N (%) | N (%) | 33.866 | 1 | <0.001 |
| Yes | 85 (24.1) | 267 (75.9) | 352 (100) | |||
| No | 33 (63.5) | 19 (36.5) | 52 (100) | |||
| Total | 118 (29.2) | 286 (70.8) | 404 (100) | |||
| BP | 79.69 | 1 | <0.001 | |||
| Elevated BP | 39 (14.7) | 227 (85.3) | 266 (100) | |||
| Normal BP | 79 (57.2) | 59 (42.8) | 138 (100) | |||
| HDL-C | 0.054 | 1 | 0.816 | |||
| Reduced HDL-C | 90 (29.5) | 215 (70.5) | 305 (100) | |||
| Normal HDL-C | 28 (28.3) | 71 (71.7) | 99 (100) | |||
| TGs | 2.974 | 1 | 0.085 | |||
| Raised TGs | 69 (26.3) | 193 (73.7) | 262 (100) | |||
| Normal TGs | 49 (34.5) | 93 (65.5) | 142 (100) | |||
| FBG | 1.554 | 1 | 0.213 | |||
| Raised FBG level | 21 (23.9) | 67 (76.1) | 88 (100) | |||
| Normal FBG level | 97 (30.7) | 219 (69.3) | 316 (100) | |||
| Total | 118 (29.2) | 286 (70.8) | 404 (100) | |||
| Proportion of a plate filled with protein | ||||||
| ≤one-quarter of a plate | >one-quarter of a plate | |||||
| MetS | N (%) | N (%) | N (%) | 13.122 | 1 | <0.001 |
| Yes | 239 (67.9) | 113 (32.1) | 352 (100) | |||
| No | 48 (92.3) | 4 (7.7) | 52 (100) | |||
| Total | 287 (71.0) | 117 (29.0) | 404 (100) | |||
| BP | 33.342 | 1 | <0.001 | |||
| Elevated BP | 164 (61.7) | 102 (38.3) | 266 (100) | |||
| Normal BP | 123 (89.1) | 15 (10.9) | 138 (100) | |||
| HDL-C | 0.464 | 1 | 0.496 | |||
| Reduced HDL-C | 214 (70.2) | 91 (29.8) | 305 (100) | |||
| Normal HDL-C | 73 (73.7) | 26 (26.3) | 99 (100) | |||
| TGs | 0.238 | 1 | 0.626 | |||
| Raised TGs | 184 (70.2) | 78 (29.8) | 262 (100) | |||
| Normal TGs | 103 (72.5) | 39 (27.5) | 142 (100) | |||
| FBG | 6.393 | 1 | 0.011 | |||
| Raised FBG level | 53 (60.2) | 35 (39.8) | 88 (100) | |||
| Normal FBG level | 234 (74.1) | 82 (25.9) | 316 (100) | |||
| Total | 287 (71) | 117 (29) | 404 (100) | |||
DASH, dietary approach to stop hypertension.
Frequency of fruit and vegetable intake in relation to MetS and related components
Of the respondents, some, 17.8% (n=72) and 41.1% (n=166), daily consumed fruits and vegetables, respectively. Further analysis showed a significant association between daily consumption of fruits and vegetables and MetS with some of its components. Respondents who did not consume fruits daily were more likely to have MetS (χ²=14. 276, p<0.001), elevated BP (χ²=13.505, p<0.001) and raised FBG level (χ²=9. 301, p=0.002) compared with those who frequently consumed fruits. Similarly, respondents who did not daily consume vegetables were more likely to have MetS (χ²=5.313, p=0.021) and elevated BP (χ²=24.677, p<0.001) compared with those who ate daily (table 3).
Table 3.
Frequency of fruit and vegetable intake in relation to metabolic syndrome (MetS) and related components
| MetS and its components | Frequency of fruit intake | Total | χ2 | Df | P value | |
| Daily | Not daily | |||||
| MetS | N (%) | N (%) | N (%) | 14.276 | 1 | <0.001 |
| Yes | 53 (15.1) | 299 (84.9) | 352 (100) | |||
| No | 19 (36.5) | 33 (63.5) | 52 (100) | |||
| Total | 72 (17.8) | 332 (82.2) | 404 (100) | |||
| Blood pressure (BP) | 13.505 | 1 | <0.001 | |||
| Elevated BP | 34 (12.8) | 232 (87.2) | 266 (100) | |||
| Normal BP | 38 (27.5) | 100 (72.5) | 138 (100) | |||
| High-density lipoprotein (HDL) | 1.029 | 1 | 0.31 | |||
| Reduced HDL | 51 (16.7) | 254 (83.3) | 305 (100) | |||
| Normal HDL | 21 (21.2) | 78 (78.8) | 99 (100) | |||
| Triglycerides (TGs) | 2.403 | 1 | 0.121 | |||
| Raised TGs | 41 (15.6) | 221 (84.4) | 262 (100) | |||
| Normal TGs | 31 (21.8) | 111 (78.2) | 142 (100) | |||
| Fasting blood glucose (FBG) | 9.301 | 1 | 0.002 | |||
| Raised FBG level | 6 (6.8) | 82 (93.2) | 88 (100) | |||
| Normal FBG level | 66 (20.9) | 250 (79.1) | 316 (100) | |||
| Total | 72 (17.8) | 332 (82.2) | 404 (100) | |||
| Frequency of vegetable intake | ||||||
| Daily | Not daily | |||||
| MetS | N (%) | N (%) | N (%) | 5.313 | 1 | 0.021 |
| Yes | 137 (38.9) | 215 (61.1) | 352 (87.1) | |||
| No | 29 (55.8) | 23 (44.2) | 52 (12.9) | |||
| Total | 166 (41.1) | 238 (58.9) | 404 (100) | |||
| BP | 24.677 | 1 | <0.001 | |||
| Elevated BP | 86 (32.3) | 180 (67.7) | 266 (65.8) | |||
| Normal BP | 80 (58) | 58 (42) | 138 (34.2) | |||
| HDL | 0.748 | 1 | 0.387 | |||
| Reduced HDL | 129 (42.3) | 176 (57.7) | 305 (75.5) | |||
| Normal HDL | 37 (37.4) | 62 (62.6) | 99 (24.5) | |||
| TGs | 0.019 | 1 | 0.89 | |||
| Raised TGs | 107 (40.8) | 155 (59.2) | 262 (64.9) | |||
| Normal TGs | 59 (41.5) | 83 (58.5) | 142 (35.1) | |||
| FBG | 0.204 | 1 | 0.652 | |||
| Raised FBG level | 38 (43.2) | 50 (56.8) | 88 (100. 0) | |||
| Normal FBG level | 128 (40.5) | 188 (59.5) | 316 (100.0) | |||
| Total | 166 (41.1) | 238 (58.9) | 404 (100.0) | |||
Association between legume and nut consumption and MetS-related components
According to the DASH eating plan, the recommended frequency of legume and nut consumption is four to five times per week. Less than a quarter (20.5%; n=83) of the respondents included legumes/pulses in their meals as recommended (four to five times in a week). Similarly, a small proportion (15.1%; n=61) of the respondents consumed nuts 4–5 days in a week as part of their meals. Further analysis with binary logistic regression showed a significant association between frequency of legume and nut consumption and MetS. Respondents who consumed legumes less than the recommended frequency were more likely to have MetS (OR=125.8; p<0.001), elevated BP (OR=2.3; p=0.001), low HDL-C (crude OR/COR=3.0; p<0.001), raised TGs (OR=4.1; p<0.001) and raised FBG level (adjusted OR=2.2; p=0.037) compared with those who ate them as recommended. Respondents who sometimes (two to three times/week) consumed nuts were more likely to have MetS (OR=105.6; p<0.001), elevated BP (COR=5.4; p<0.001), reduced HDL-C (OR=3.0; p<0.001), raised TGs (OR=6.2; p<0.001) and raised FBG level (OR=3.2; p=0.011) compared with those who included nuts as recommended (four to five times/week) (table 4).
Table 4.
Association between legume and nut consumption and metabolic syndrome-related components
| Frequency of legume intake | Metabolic syndrome | Total | COR (95% CI) | P value | |
| Yes | No | ||||
| Always | 40 (48.2) | 43 (51.8) | 83 (100) | 1 | |
| Sometimes | 78 (91.8) | 7 (8.2) | 85 (100) | 125.8 (29.3–539.9) | <0.001 |
| Rarely | 234 (99.2) | 2 (0.8) | 236 (100) | 10.5 (2.1–51.6) | 0.004 |
| Total | 352 (87.1) | 52 (12.9) | 404 (100) | ||
| Frequency of legume intake | Blood pressure (BP) | ||||
| Elevated BP | Normal BP | ||||
| Often | 43 (51.8) | 40 (48.2) | 83 (100) | 1 | |
| Sometimes | 54 (63.5) | 31 (36.5) | 85 (100) | 2.3 (1.4–3.9) | 0.001 |
| Rarely | 169 (71.6) | 67 (28.4) | 236 (100) | 1.4 (0.9–2.4) | 0.167 |
| Total | 266 (65.8) | 138 (34.2) | 404 (100) | ||
| Frequency of legume intake | High-density lipoprotein (HDL) | ||||
| Low HDL | Normal HDL | ||||
| Often | 48 (57.8) | 35 (42.2) | 83 (100) | 1 | |
| Sometimes | 67 (78.8) | 18 (21.2) | 85 (100) | 3.0 (1.8–5.2) | <0.001 |
| Rarely | 190 (80.5) | 46 (19.5) | 236 (100) | 1.1 (0.6–2.0) | 0.739 |
| Total | 305 (75.5) | 99 (24.5) | 404 (100) | ||
| Frequency of legume intake | Triglycerides (TGs) | ||||
| Raised TGs | Normal TGs | ||||
| Often | 33 (39.8) | 50 (60.2) | 83 (100) | 1 | |
| Sometimes | 57 (67.1) | 28 (32.9) | 85 (100) | 4.1 (2.4–6.9) | <0.000 |
| Rarely | 172 (72.9) | 64 (27.1) | 236 (100) | 1.3 (0.8–2.3) | 0.309 |
| Total | 262 (64.9) | 142 (35.1) | 404 (100) | ||
| Frequency of legume intake | Fasting blood glucose (FBG) | ||||
| Raised FBG level | Normal FBG level | ||||
| Often | 10 (12) | 73 (88) | 83 (100) | 1 | |
| Sometimes | 24 (28.2) | 61 (71.8) | 85 (100) | 2.2 (1.0–4.5) | 0.037 |
| Rarely | 54 (22.9) | 182 (77.1) | 236 (100) | 0.8 (0.4–1.3) | 0.325 |
| Total | 88 (21.8) | 316 (78.2) | 404 (100) | ||
| Frequency of nut intake | Metabolic syndrome | Total | COR (95% CI) | P value | |
| Yes | No | ||||
| Often | 19 (31.1) | 42 (68.9) | 61 (100) | 1 | |
| Sometimes | 94 (94.9) | 5 (5.1) | 99 (100) | 105.6 (37.4–298.4) | <0.001 |
| Rarely | 239 (98) | 5 (2) | 244 (100) | 2.5 (0.7–9.0) | 0.147 |
| Total | 352 (87.1) | 52 (12.9) | 404 (100) | ||
| Frequency of nut intake | BP | ||||
| Elevated BP | Normal BP | ||||
| Often | 22 (36.1) | 39 (63.9) | 61 (100) | 1 | |
| Sometimes | 60 (60.6) | 39 (39.4) | 99 (100) | 5.4 (3.0–9.9) | <0.001 |
| Rarely | 184 (75.4) | 60 (24.6) | 244 (100) | 2.0 (1.2–3.3) | 0.007 |
| Total | 266 (65.8) | 138 (34.2) | 404 (100) | ||
| Frequency of nut intake | HDL | ||||
| Reduced HDL | Normal HDL | ||||
| Often | 35 (57.4) | 26 (42.6) | 61 (100) | 1 | |
| Sometimes | 74 (74.7) | 25 (25.3) | 99 (100) | 3.0 (1.7–5.5) | <0.001 |
| Rarely | 196 (80.3) | 48 (19.7) | 244 (100) | 1.4 (0.8–2.4) | 0.254 |
| Total | 305 (75.5) | 99 (24.5) | 404 (100) | ||
| Frequency of nut intake | TGs | ||||
| Raised TGs | Normal TGs | ||||
| Often | 17 (27.9) | 44 (72.1) | 61 (100) | 1 | |
| Sometimes | 73 (73.7) | 26 (26.3) | 99 (100) | 6.2 (3.3–11.5) | <0.001 |
| Rarely | 172 (70.5) | 72 (29.5) | 244 (100) | 0.9 (0.5–1.4) | 0.547 |
| Total | 262 (64.9) | 142 (35.1) | 404 (100) | ||
| Frequency of nut intake | FBG | ||||
| Raised FBG level | Normal FBG level | ||||
| Often | 6 (9.8) | 55 (90.2) | 61 (100) | 1 | |
| Sometimes | 19 (19.2) | 80 (80.8) | 99 (100) | 3.2 (1.3–7.8) | 0.011 |
| Rarely | 63 (25.8) | 181 (74.2) | 244 (100) | 1.5 (0.8–2.6) | 0.194 |
| Total | 88 (21.8) | 316 (78.2) | 404 (100) | ||
COR, crude OR.
Consumption of processed and/or fast foods relative to MetS and related components
Slightly over one-third (36.4%; n=147) of the respondents always consumed processed and/or fast foods, of which, 96.6% presenting with MetS. Further analysis showed a significant association between frequency of eating processed/fast foods and MetS. Respondents who always consumed processed/fast foods were more likely to have MetS (χ²=66.34; p<0.001) compared with those who rarely ate those types of food. Moreover, respondents who sometimes ate processed/fast foods were more likely to have elevated BP (OR=6.3; p<0.001), low HDL-C (OR=2.5; p=0.002), raised TGs (OR=2.6; p<0.001) and raised FBG level (OR=5.8; p<0.001) compared with those who rarely ate these foods (table 5).
Table 5.
Relationship between consumption of processed/fast foods and metabolic syndrome-related components
| Frequency of eating processed/ fast foods | Metabolic syndrome | Total | χ2 | Df | P value | |
| Yes | No | |||||
| Rarely | 90 (70.3) | 38 (29.7) | 128 (100) | 66.34 | 2 | <0.001 |
| Sometimes | 120 (93.0) | 9 (7.0) | 129 (100) | |||
| Always | 142 (96.6) | 5 (3.4) | 147 (100) | |||
| Total | 352 (87.1) | 52 (12.9) | 404 (100) | |||
| Blood pressure (BP) | COR (95% CI) | P value | ||||
| Elevated BP | Normal BP | |||||
| Rarely | 65 (49.2) | 67 (50.8) | 132 (100) | 1 | ||
| Sometimes | 79 (60.8) | 51 (39.2) | 130 (100) | 6.288 (3.51–11.27) | <0.001 | |
| Always | 122 (85.9) | 20 (14.1) | 142 (100) | 3.938 (2.18–7.10) | <0.001 | |
| Total | 266 (65.8) | 138 (34.2) | 404 (100) | |||
| High-density lipoprotein (HDL) | ||||||
| Low HDL | Normal HDL | |||||
| Rarely | 88 (66.7) | 44 (33.3) | 132 (100) | 1 | ||
| Sometimes | 99 (76.2) | 31 (23.8) | 130 (100) | 2.458 (1.39–4.34) | 0.002 | |
| Always | 118 (83.1) | 24 (16.9) | 142 (100) | 1.540 (0.85–2.79) | 0.156 | |
| Total | 305 (75.5) | 99 (24.5) | 404 (100) | |||
| Triglycerides (TGs) | ||||||
| Raised TGs | Normal TGs | |||||
| Rarely | 67 (50.8) | 65 (49.2) | 132 (100) | 1 | ||
| Sometimes | 92 (70.8) | 38 (29.2) | 130 (100) | 2.562 (1.55–4.23) | <0.001 | |
| Always | 103 (72.5) | 39 (27.5) | 142 (100) | 1.091 (0.64–1.85) | 0.747 | |
| Total | 262 (64.9) | 142 (35.1) | 404 (100) | |||
| Fasting blood glucose (FBG) | ||||||
| Raised FBG | Normal FBG level | |||||
| Rarely | 13 (9.8) | 119 (90.2) | 132 (100) | 1 | ||
| Sometimes | 20 (15.4) | 110 (84.6) | 130 (100) | 5.787 (2.98–11.25) | <0.001 | |
| Always | 55 (38.7) | 87 (61.3) | 142 (100) | 3.477 (1.94–6.24) | <0.001 | |
| Total | 88 (21.8) | 316 (78.2) | 404 (100) | |||
COR, crude OR.
Consumption of sugar and salt in relation to MetS-related components
Most (62.6%; n=253) of the respondents consumed more than the recommended (more than five teaspoons) amount of sugar in a day. With regards to salt intake, the majority, 57.9% (n=234) of the respondents added salt to their meal right before they ate or as they were eating it. Further analysis revealed respondents who consumed more than the recommended amount of sugar were more likely (χ²=6.917, p=0.009) to have MetS. However, raised FBG level was significantly (χ²=25.099, p<0.001) related to consumption of less than five teaspoons of sugar per day. Respondents who added salt to foods right before they ate were more likely (χ²=21.718, p<0.001) to have elevated BP compared with those who did not add salt after the food has been cooked. However, respondents who did not add salt after the food has been cooked were more likely to have both raised TGs (χ²=9.697, p=0.002) and FBG (χ²=10.028, p=0.002) levels compared with those who added salt while they were eating their meals (table 6).
Table 6.
Consumption of sugar and salt in relation to metabolic syndrome (MetS)-related components
| MetS and its components | Sugar consumption status | Total | χ2 | Df | P value | |
| ≤5 tsp per day | >5 tsp per day | 6.917 | 1 | 0.009 | ||
| MetS | N (%) | N (%) | N (%) | |||
| Yes | 123 (34.9) | 229 (65.1) | 352 (100) | |||
| No | 28 (53.8) | 24 (46.2) | 52 (100) | |||
| Total | 151 (37.4) | 253 (62.6) | 404(100) | |||
| Blood pressure (BP) | 0.016 | 1 | 0.9 | |||
| Elevated BP | 100 (37.6) | 166 (62.4) | 266 (100) | |||
| Normal BP | 51 (37) | 87 (63) | 138 (100) | |||
| High-density lipoprotein (HDL) | 1.428 | 1 | 0.232 | |||
| Reduced HDL | 109 (35.7) | 196 (64.3) | 305 (100) | |||
| Normal HDL | 42 (42.4) | 57 (57.6) | 99 (100) | |||
| Triglycerides (TGs) | 0.438 | 1 | 0.508 | |||
| Raised TGs | 101 (38.5) | 161 (61.5) | 262 (100) | |||
| Normal TGs | 50 (35.2) | 92 (64.8) | 142 (100) | |||
| Fasting blood glucose (FBG) | 25.099 | 1 | <0.001 | |||
| Raised FBG level | 53 (60.2) | 35 (39.8) | 88 (100) | |||
| Normal FBG level | 98 (31) | 218 (69) | 316 (100) | |||
| Total | 151 (37.4) | 253 (62.6) | 404 (100) | |||
| Adds salt to meals at the table | Total | |||||
| Yes | No | |||||
| MetS | N (%) | N (%) | N (%) | 0.113 | 1 | 0.736 |
| Yes | 205 (58.2) | 147 (41.8) | 352 (100) | |||
| No | 29 (55.8) | 23 (44.2) | 52 (100) | |||
| Total | 234 (57.9) | 170 (42.1) | 404 (100) | |||
| BP | 21.718 | 1 | <0.001 | |||
| Elevated BP | 176 (66.2) | 90 (33.8) | 266 (100) | |||
| Normal BP | 58 (42) | 80 (58) | 138 (100) | |||
| HDL | 0.006 | 1 | 0.936 | |||
| Reduced HDL | 177 (58) | 128 (42) | 305 (100) | |||
| Normal HDL | 57 (57.6) | 42 (42.4) | 99 (100) | |||
| TGs | 9.697 | 1 | 0.002 | |||
| Raised TGs | 137 (52.3) | 125 (47.7) | 262 (100) | |||
| Normal TGs | 97 (68.3) | 45 (31.7) | 142 (100) | |||
| FBG | 10.028 | 1 | 0.002 | |||
| Raised FBG level | 38 (43.2) | 50 (56.8) | 88 (100) | |||
| Normal FBG level | 196 (62) | 120 (38) | 316 (100) | |||
| Total | 234 (57.9) | 170 (42.1) | 404 (100) | |||
Time interval after consumption of dinner and sleeping in relation to MetS-related components
Less than a quarter (19.6%; n=79) of the respondents observed more than a 2-hour interval between taking dinner and sleeping. Analysis with binary logistic regression revealed a significant association between the time interval of taking dinner and sleeping with MetS. Respondents who observed more than a 2-hour interval between eating dinner and sleeping were 20% (p<0.001), 40% (p=0.001), 50% (p=0.027) and 50% (p=0.011) less likely to have MetS, elevated BP, reduced HDL-C and raised TGs, respectively, compared with those who observed less than a 1-hour time interval between eating dinner and sleeping (table 7).
Table 7.
Relationship between time interval of taking dinner and sleeping relative to metabolic syndrome-related components
| Time interval between taking dinner and sleep | Metabolic syndrome | Total | COR (95% CI) | P value | |
| Yes | No | ||||
| Less than 1 hour | 97 (90.7) | 10 (9.3) | 107 (100) | 1 | |
| 1–2 hours | 201 (92.2) | 17 (7.8) | 218 (100) | 0.2 (0.1–0.5) | <0.001 |
| More than 2 hours | 54 (68.4) | 25 (31.6) | 79 (100) | 0.2 (0.1–0.4) | <0.001 |
| Total | 352 (87.1) | 52 (12.9) | 404 (100) | ||
| Blood pressure (BP) | |||||
| Elevated BP | Normal BP | ||||
| Less than 1 hour | 69 (64.5) | 38 (35.5) | 107 (100) | 1 | |
| 1–2 hours | 157 (72) | 61 (28) | 218 (100) | 0.6 (0.3–1.0) | 0.059 |
| More than 2 hours | 40 (50.6) | 39 (49.4) | 79 (100) | 0.4 (0.2–0.7) | 0.001 |
| Total | 266 (65.8) | 138 (34.2) | 404 (100) | ||
| High-density lipoprotein (HDL) | |||||
| Reduced HDL | Normal HDL | ||||
| Less than 1 hour | 92 (86) | 15 (14) | 107 (100) | 1 | |
| 1–2 hours | 164 (75.2) | 54 (24.8) | 218 (100) | 0.3 (0.1–0.5) | <0.001 |
| More than 2 hours | 49 (62) | 30 (38) | 79 (100) | 0.5 (0.3–0.9) | 0.027 |
| Total | 305 (75.5) | 99 (24.5) | 404 (100) | ||
| Triglycerides (TGs) | |||||
| Raised TGs | Normal TGs | ||||
| Less than 1 hour | 62 (57.9) | 45 (42.1) | 107 (100) | 1 | |
| 1–2 hours | 156 (71.6) | 62 (28.4) | 218 (100) | 0.9 (0.5–1.6) | 0.76 |
| More than 2 hours | 44 (55.7) | 35 (44.3) | 79 (100) | 0.5 (0.3–0.9) | 0.011 |
| Total | 262 (64.9) | 142 (35.1) | 404 (100) | ||
| Fasting blood glucose (FBG) | |||||
| Raised FBG level | Normal FBG level | ||||
| Less than 1 hour | 21 (19.6) | 86 (80.4) | 107 (100) | 1 | |
| 1–2 hours | 50 (22.9) | 168 (77.1) | 218 (100) | 1.1 (0.5–2.3) | 0.752 |
| More than 2 hours | 17 (21.5) | 62 (78.5) | 79 (100) | 0.9 (0.5–1.7) | 0.796 |
| Total | 88 (21.8) | 316 (78.2) | 404 (100) | ||
COR, crude OR.
Multivariable analysis for dietary risk factors of MetS
Binary logistic regression analysis was performed to model MetS (presence or absence) as a dependent variable and the independent variables that revealed significant association at p<0.05 during the bivariate analysis. Accordingly, the logistic model included the following factors: proportion of a plate filled with vegetables and/or fruits; frequency of processed/fast food, fruit, vegetable, legume and nut intake; sugar consumption status; and time interval between taking dinner and sleep. Backward conditional method was specified with removal at p<0.05 to determine the independent predictors of MetS as it removes the confounding variables until no further variables can be removed without a statistically insignificant loss of fit (last or reduced model). After considering all, frequency of processed/fast food, legume and nut intake was independently associated with MetS. The fitness model according to Hosmer and Lemeshow Test was 0.248, which indicates the model fits. Respondents who often consumed processed/fast foods were three times (95% CI: 1.48–7.29, p=0.003) more likely to develop MetS compared with those who rarely consumed legumes. Respondents who rarely and sometimes consumed legumes were six (95% CI: 3.27–12.52, p<0.001) and four (95% CI: 1.24–10.83, p=0.022) times, respectively, more likely to develop MetS compared with those who always consumed legumes. With regards to nut intake, respondents who rarely consumed nuts were seven (95% CI: 3.68–13.62, p<0.001) and four (95% CI: 1.36–9.54, p=0.011) times, respectively, more likely to develop MetS compared with those who always consumed nuts (table 8).
Table 8.
Multivariable analysis for dietary risk factors of metabolic syndrome
| Variable | AOR | 95% CI | P value | |
| Lower | Upper | |||
| Full/first model | ||||
| Proportion of plate filled with vegetables and/or fruits | ||||
| Less than half of a plate | 2.15 | 0.63 | 7.29 | 0.221 |
| Half and above of a plate | Ref | |||
| Frequency of processed/fast food intake | ||||
| Always | 3.192 | 1.246 | 6.903 | 0.004 |
| Sometimes | 2.132 | 0.775 | 5.648 | 0.109 |
| Rarely | Ref | |||
| Frequency of fruit intake | ||||
| Not daily | 2.015 | 0.526 | 7.716 | 0.306 |
| Daily | Ref | |||
| Frequency of vegetable intake | ||||
| Not daily | 1.048 | 0.293 | 3.744 | 0.942 |
| Daily | Ref | |||
| Frequency of legume intake | ||||
| Rarely | 6.19 | 2.624 | 11.725 | <0.001 |
| Sometimes | 3.702 | 1.096 | 10.248 | 0.039 |
| Often | Ref | |||
| Frequency of nut intake | ||||
| Rarely | 6.667 | 3.78 | 12.811 | <0.001 |
| Sometimes | 4.718 | 1.934 | 9.18 | 0.012 |
| Always | Ref | |||
| Sugar consumption status | ||||
| >5 tsp per day | 1.505 | 0.426 | 5.315 | 0.525 |
| ≤5 tsp per day | Ref | |||
| Time interval between taking dinner and sleep | ||||
| Less than 1 hour | 4.21 | 0.819 | 21.644 | 0.085 |
| 1–2 hours | 3.824 | 0.908 | 16.11 | 0.061 |
| More than 2 hours | Ref | |||
| Reduced/last model | ||||
| Frequency of processed/fast food intake | ||||
| Always | 3.286 | 1.482 | 7.289 | 0.003 |
| Sometimes | 3.358 | 0.864 | 13.053 | 0.08 |
| Rarely | Ref | |||
| Frequency of legume intake | ||||
| Rarely | 6.395 | 3.267 | 12.519 | <0.001 |
| Sometimes | 4.28 | 1.235 | 10.832 | 0.022 |
| Often | Ref | |||
| Frequency of nut intake | ||||
| Rarely | 7.081 | 3.68 | 13.622 | <0.001 |
| Sometimes | 4.03 | 1.359 | 9.538 | 0.011 |
| Often | Ref | |||
AOR, adjusted OR.
Discussion
Our findings underscore the relationship between dietary patterns/practices and MetS in adults with central obesity. In sum, we report that MetS is linked with frequent consumption of: large proportion of carbohydrates, proteins, processed/fast foods, large amounts of sugars, less than 50% portion of a plate filled as vegetables and/or fruits, and adding salt to food. However, adequate and/or frequent consumption of: fruits, vegetables, legumes and nuts were linked with protection against MetS. Additionally, those who observed more than a 2-hour interval between eating dinner and sleeping were less likely to have MetS. Although these findings show the importance of diet practices/patterns in the development and sustainability of MetS—a well-documented narrative, such link has also been locally reported with intake of refined carbohydrate, processed/fast foods, sugar-sweetened beverages, low fruits and vegetables20 22 as the main associated risks.
Consumption of large amounts of carbohydrates and proteins, as well as small proportion of vegetables and/or fruits, was linked to higher prevalence of MetS, elevated BP and raised FBG level. The aforementioned does not meet the DASH diet criteria. The DASH eating plan has been shown to be beneficial on MetS.9 31 However, excess consumption of carbohydrates3 and specifically animal-based protein foods32 increases the risks for MetS. Importantly, consumption of unhealthy diet characterised by a high-calorie content is known risk factor for obesity, a principal element for various metabolic-clinical abnormalities such as dyslipidaemia, high BP, insulin resistance features of MetS.21 The cardiometabolic protective effects of fruits and vegetables can be attributed to their richness in vitamins, minerals, phytochemicals, fibres, potassium, magnesium and antioxidants. Fruits and vegetables are rich in phytochemicals and flavonoids which have been reported to support cardioprotective properties.33 Both fruits and vegetables are also rich in soluble dietary fibres that may decrease the intestinal absorption for cholesterol and bile salts, thus controlling their levels.34 Consumption of high-fibre diets such as fruits and vegetables slows absorption of foods in the gut, resulting in a regulated release of insulin from the pancreas, thus maintaining normal glucose level. Furthermore, vegetables and fruits are rich in potassium, an important cofactor for BP regulation. Mechanistically, when serum potassium level is low, sodium and water retention increases resulting in high BP.35 36
We show that the prevalence of MetS and related components was inversely associated with legume consumption. The findings are consistent with reported beneficial effect of legumes on MetS,7 8 TGs and BP.37 The cardiometabolic protective effect of dietary legumes is attributed to the high content of viscous soluble fibres which contributes to slow absorption of carbohydrates, cholesterol and bile salts in the intestine resulting in improved blood sugar control38 and blood lipid levels.34 Similarly, regular consumption of nuts is associated with lower risk of MetS and related components. The beneficial effect of regular consumption of nuts on MetS,9 central obesity, type 2 diabetes, BP and TGs39 is well established. Nuts may exert protective effect on MetS through several mechanisms. First, they are rich in both macronutrients and micronutrients including unsaturated fatty acids, fibre, non-sodium minerals, tocopherols and bioactive phytochemicals such as polyphenols and phytosterols.40 These biomolecules have cardioprotective effect via improving inflammation, oxidative stress and endothelial function. The mechanisms can improve insulin secretion and sensitivity and thus reduce the risk of type 2 diabetes, dyslipidaemia, central obesity and hypertension.41 Furthermore, dietary fibres from nuts have cholesterol38 and blood glucose34 reducing effects. Nuts have magnesium that can reduce peripheral inflammation improving insulin resistance as well as stimulating production of vasodilators specifically nitric oxide and prostacyclins,42 hence controlling both blood glucose and BP.
As regards to frequent consumption of processed/fast foods, they were directly linked to MetS (elevated BP, low HDL-C, raised TGs and FBG levels). These findings are consistent with reports showing frequently consumption of processed/fast foods increases chances of having MetS.3 5 Processed/fast foods are high in refined carbohydrates, cholesterol, salt, processed sugars—MetS-friendly food but poor in whole grains, fruits and vegetables.43 Additionally, excessive sugar consumption (more than five teaspoons in a day) is a risk factor for MetS. Intake of below 25 g (five teaspoons) per person is recommended by the WHO to prevent NCDs notably hypertension and diabetes.44 Added sugars and/or sugar-sweetened beverages are linked with central obesity,44 45 dyslipidaemia,46 insulin resistance,47 type 2 diabetes,47 48 high BP49 and MetS.45 50 Surprisingly, we found consumption of less sugar per day to be associated with raised FBG levels. This finding could have been attributed to the known patients with diabetes recruited into the study who were more likely to have been taking less or no sugar as per the recommendation compared with non-diabetics.
Adding salt to foods after the food has been cooked was shown to be associated with elevated BP. The relationship between salt consumption and high BP is well documented.32 51 Dietary guideline by WHO recommends daily salt intake of less than 5 g (one teaspoon) per person to help prevent high BP, reduce risk of heart disease and stroke in adults.52 Of particular interest in our study finding is that low-salt consumption was significantly associated with both raised TGs and FBG levels. Indeed, reducing or restriction salt intake has a beneficial effect on lowering BP. However, an unwanted side effect that we also show is consuming less salt has increased risk of elevated levels of blood cholesterol.53 The mechanisms associated with low salt intake and hyperlipidaemia can be explained by the fact that limited sodium intake reduces body water content and in an attempt to revert the low plasma volume, epinephrine, renin and angiotensin increase. These hormones inhibit insulin action, causing insulin resistance54 and consequently high insulin level in the blood inhibits lipid metabolism and increases blood cholesterol.55 With regards to the raised FBG level in relation to low salt intake, there is so far no explanation to this association. However, it could be due to mechanisms associated with the aforementioned hormones.55
A finding of interest is the short time interval between taking dinner and going to bed and likelihood of MetS. This is related to the fact that quantity of food consumption is directly associated with shunting of blood into the mesenteric system resulting into early sleepiness. This finding is consistent with reports that eating too close to bedtime is a risk factor for obesity,56–58 dyslipidaemia,58 MetS and hyperglycaemia,59 60 diabetes and cardiovascular morbidity.61 Indeed, eating an early dinner allows the body time to burn off those unwanted calories before going to sleep62 and thus reduces the risks of CVDs. Whether, the likelihood of sleepiness relative to the quantity and quality of food was not a subject of our investigation. However, reports show carbohydrate-rich food is associated with sleepiness due to their possibility of increasing plasma concentration of tryptophan—a precursor for serotonin and sleep-inducing agent.63
Strengths and limitations of this study
This was the first study conducted among the informal settlements (slums) in Kenya that determined the association between dietary intake patterns and MetS. The relatively large sample size increases the possibility of replication and generalisation of the findings. The use of widely recognised and validated dietary questionnaires is another strength of the study. The findings reinforce the importance of dietary consideration in public health interventions addressing MetS and related cardiovascular problems. The study was limited by its cross-sectional design. The self-reported dietary patterns and practices may suffer from information bias. The current study was conducted in Nairobi, the capital city of Kenya, where consumption of processed and/or fast foods as well as sweetened beverages is common, thus, generalisability to the rural areas in the country may not be possible.
In conclusion, regular consumption of fruits, vegetables, legumes and nuts, as well as observing sometime after eating dinner before sleeping, was the dietary pattern significantly associated with reduced risk of MetS. However, consumption of a large quantity of carbohydrates, proteins, processed/fast foods and sugar is likely to predispose to MetS. The findings underscore the need to focus on specific dietary intake patterns including frequency, quantity, quality and variety for MetS prevention and management. The MetS-related interventions could be implemented during individual consultation, group and community health messaging sessions.
Supplementary Material
Acknowledgments
The authors would like to thank all the laboratory staff of St Mary’s Mission Hospital who participated in the biochemical analysis of this data.
Footnotes
Contributors: OTO, SK and MW conceptualised and designed the study. OTO acquired the data, carried out the analyses, interpreted the data and drafted the article. SK and WM critically reviewed the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Obtained.
Ethics approval: Ethical approval to conduct this study was obtained from Kenyatta National Hospital-University of Nairobi Ethical Review Committee (KNH-UoN ERC) (approval number P430.07/2017). The institutional permission was granted by the administration of the St Mary’s Mission Hospital. Consent was obtained from the study participants prior to data collection after an explanation on study aim and objectives. The participants were assured of confidentiality, privacy, anonymity and non-coercive nature of the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The dataset analysed during the current study is available from the corresponding author on a reasonable request.
References
- 1.Alberti KGMM, Eckel RH, Grundy SM, et al. . Harmonizing the metabolic syndrome: a joint interim statement of the International diabetes Federation Task force on epidemiology and prevention; National heart, lung, and blood Institute; American heart association; world heart Federation; international atherosclerosis Society; and international association for the study of obesity. Circulation 2009;120:1640–5. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 2.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep 2018;20:12. 10.1007/s11906-018-0812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suliga E, Kozieł D, Cieśla E, et al. . Dietary patterns in relation to metabolic syndrome among adults in Poland: a cross-sectional study. Nutrients 2017;9:1366. 10.3390/nu9121366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lind PM, Risérus U, Salihovic S, et al. . An environmental wide association study (EWAS) approach to the metabolic syndrome. Environ Int 2013;55:1–8. 10.1016/j.envint.2013.01.017 [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Monforte M, Sánchez E, Barrio F, et al. . Metabolic syndrome and dietary patterns: a systematic review and meta-analysis of observational studies. Eur J Nutr 2017;56:925–47. 10.1007/s00394-016-1305-y [DOI] [PubMed] [Google Scholar]
- 6.Li X-T, Liao W, Yu H-J, et al. . Combined effects of fruit and vegetables intake and physical activity on the risk of metabolic syndrome among Chinese adults. PLoS One 2017;12:e0188533. 10.1371/journal.pone.0188533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosseinpour-Niazi S, Mirmiran P, Mirzaei S, et al. . Cereal, fruit and vegetable fibre intake and the risk of the metabolic syndrome: a prospective study in the Tehran lipid and glucose study. J Hum Nutr Diet 2015;28:236–45. 10.1111/jhn.12242 [DOI] [PubMed] [Google Scholar]
- 8.Sala-Vila A, Estruch R, Ros E. New insights into the role of nutrition in CVD prevention. Curr Cardiol Rep 2015;17:26. 10.1007/s11886-015-0583-y [DOI] [PubMed] [Google Scholar]
- 9.Babio N, Toledo E, Estruch R, et al. . Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ 2014;186:E649–57. 10.1503/cmaj.140764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czekajło A, Różańska D, Zatońska K, ´zanska DR, et al. . Association between dietary patterns and metabolic syndrome in the selected population of Polish adults—results of the pure Poland study. Eur J Public Health 2019;29:335–40. 10.1093/eurpub/cky207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Suzuki T, Hirata K, Elkind MSV, et al. . Metabolic syndrome, endothelial dysfunction, and risk of cardiovascular events: the Northern Manhattan study (NOMAS). Am Heart J 2008;156:405–10. 10.1016/j.ahj.2008.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford ES, Li C, Sattar N, et al. . Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care 2008;31:1898–904. 10.2337/dc08-0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev 2015;16:1–12. 10.1111/obr.12229 [DOI] [PubMed] [Google Scholar]
- 15.Sabir AA, Jimoh A, Iwuala SO, et al. . Metabolic syndrome in urban city of north-western Nigeria: prevalence and determinants. Pan Afr Med J 2016;23:19. 10.11604/pamj.2016.23.19.5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gyakobo M, Amoah AG, Martey-Marbell D-A, et al. . Prevalence of the metabolic syndrome in a rural population in Ghana. BMC Endocr Disord 2012;12:25. 10.1186/1472-6823-12-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dandji MBS, Zambou FN, Dangang DSB, et al. . Prevalence of metabolic syndrome in adult men of the Dschang health district in Western-Cameroon. World J Nutrit Health 2018;6:1–10. 10.12691/jnh-6-1-1 [DOI] [Google Scholar]
- 18.H Maklady FA, Kamal HM, El-Eraky AZ, et al. . Prevalence of metabolic syndrome among adults in Suez canal area. Egyptian Heart J 2014;66:15 10.1016/j.ehj.2013.12.043 [DOI] [Google Scholar]
- 19.Omuse G, Maina D, Hoffman M, et al. . Metabolic syndrome and its predictors in an urban population in Kenya: a cross sectional study. BMC Endocr Disord 2017;17:37. 10.1186/s12902-017-0188-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuel K, Mirie W, Chege M, et al. . Association of lifestyle modification and pharmacological adherence on blood pressure control among patients with hypertension at Kenyatta national Hospital, Kenya: a cross-sectional study. BMJ Open 2029;2:e023995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012;70:3–21. 10.1111/j.1753-4887.2011.00456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hulzebosch A, van de Vijver S, Oti SO, et al. . Profile of people with hypertension in Nairobi's slums: a descriptive study. Global Health 2015;11:26. 10.1186/s12992-015-0112-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babio N, Bulló M, Basora J, et al. . Adherence to the Mediterranean diet and risk of metabolic syndrome and its components. Nutr Metab Cardiovasc Dis 2009;19:563–70. 10.1016/j.numecd.2008.10.007 [DOI] [PubMed] [Google Scholar]
- 24.DiNicolantonio JJ, Lucan SC, O’Keefe JH. The evidence for saturated fat and for sugar related to coronary heart disease. Prog Cardiovasc Dis 2016;58:464–72. 10.1016/j.pcad.2015.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okube OT, Kimani ST, Mirie W. Gender differences in the pattern of socio-demographics relevant to metabolic syndrome among Kenyan adults with central obesity at a mission hospital in Nairobi, Kenya. High Blood Press Cardiovasc Prev 2020;27:61–82. 10.1007/s40292-020-00360-7 [DOI] [PubMed] [Google Scholar]
- 26.Desgroppes A, Taupin S. Kibera: the biggest slum in Africa. Les Cahiers de l’Afriquede l’Est 2011;44:23–34. [Google Scholar]
- 27.World Health Organization The WHO step-wise approach to non-communicable disease risk factor surveillance, 2017. [Google Scholar]
- 28.World Health Organization Western Pacific region, International association for the study of obesity, International obesity Task force: the Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia, 2000. [Google Scholar]
- 29.Chobanian AV, Bakris GL, Black HR, et al. . The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560–72. 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization International diabetes Federation: definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation, 2006. [Google Scholar]
- 31.Saneei P, Fallahi E, Barak F, et al. . Adherence to the DASH diet and prevalence of the metabolic syndrome among Iranian women. Eur J Nutr 2015;54:421–8. 10.1007/s00394-014-0723-y [DOI] [PubMed] [Google Scholar]
- 32.Cheng M, Wang H, Wang Z, et al. . Relationship between dietary factors and the number of altered metabolic syndrome components in Chinese adults: a cross-sectional study using data from the China health and nutrition survey. BMJ Open 2017;7:e014911. 10.1136/bmjopen-2016-014911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steemburgo T, Dall'Alba V, Almeida JC, et al. . Intake of soluble fibers has a protective role for the presence of metabolic syndrome in patients with type 2 diabetes. Eur J Clin Nutr 2009;63:127–33. 10.1038/sj.ejcn.1602902 [DOI] [PubMed] [Google Scholar]
- 34.Visioli F. Nutritional support in the pharmacological treatment of metabolic syndrome. Eur J Pharmacol 2011;668:S43–9. 10.1016/j.ejphar.2011.05.083 [DOI] [PubMed] [Google Scholar]
- 35.Rheinschild E. Fruit and veggies rich in potassium may be key to lowering blood pressure, 2017. Available: https://news.usc.edu/119637/how-tolower-your-blood-pressure-eat-more-fruit-and-veggies
- 36.Savica V, Bellinghieri G, Kopple JD. The effect of nutrition on blood pressure. Annu Rev Nutr 2010;30:365–401. 10.1146/annurev-nutr-010510-103954 [DOI] [PubMed] [Google Scholar]
- 37.Bazzano LA, Thompson AM, Tees MT, et al. . Non-soy legume consumption lowers cholesterol levels: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis 2011;21:94–103. 10.1016/j.numecd.2009.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouchenak M, Lamri-Senhadji M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: a review. J Med Food 2013;16:185–98. 10.1089/jmf.2011.0238 [DOI] [PubMed] [Google Scholar]
- 39.Blanco Mejia S, Kendall CWC, Viguiliouk E, et al. . Effect of tree nuts on metabolic syndrome criteria: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2014;4:e004660. 10.1136/bmjopen-2013-004660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ros E, Hu FB. Consumption of plant seeds and cardiovascular health: epidemiological and clinical trial evidence. Circulation 2013;128:553–65. 10.1161/CIRCULATIONAHA.112.001119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ros E. Health benefits of nut consumption. Nutrients 2010;2:652–82. 10.3390/nu2070652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbagallo M, Dominguez LJ, Galioto A, et al. . Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Aspects Med 2003;24:39–52. 10.1016/S0098-2997(02)00090-0 [DOI] [PubMed] [Google Scholar]
- 43.Paniagua JA, Pérez-Martinez P, Gjelstad IMF, et al. . A low-fat high-carbohydrate diet supplemented with long-chain n-3 PUFA reduces the risk of the metabolic syndrome. Atherosclerosis 2011;218:443–50. 10.1016/j.atherosclerosis.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization Guideline: sugars intake for adult and children. Geneva: World Health Organization, 2015. [PubMed] [Google Scholar]
- 45.Bray GA, Popkin BM. Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes?: health be damned! pour on the sugar. Diabetes Care 2014;37:950–6. 10.2337/dc13-2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welsh JA, Sharma A, Cunningham SA, et al. . Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation 2011;123:249–57. 10.1161/CIRCULATIONAHA.110.972166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanhope KL. Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit Rev Clin Lab Sci 2016;53:52–67. 10.3109/10408363.2015.1084990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DiNicolantonio JJ, O'Keefe JH, Lucan SC. Added fructose: a principal driver of type 2 diabetes mellitus and its consequences. Mayo Clin Proc 2015;90:372–81. 10.1016/j.mayocp.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 49.DiNicolantonio JJ, Lucan SC. The wrong white crystals: not salt but sugar as aetiological in hypertension and cardiometabolic disease. Open Heart 2014;1:e000167. 10.1136/openhrt-2014-000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denova-Gutiérrez E, Talavera JO, Huitrón-Bravo G, et al. . Sweetened beverage consumption and increased risk of metabolic syndrome in Mexican adults. Public Health Nutr 2010;13:835–42. 10.1017/S1368980009991145 [DOI] [PubMed] [Google Scholar]
- 51.Oh YS, Appel LJ, Galis ZS, et al. . National heart, lung, and blood Institute Working Group report on salt in human health and sickness: building on the current scientific evidence. Hypertension 2016;68:281–8. 10.1161/HYPERTENSIONAHA.116.07415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization Guideline: sodium intake for adults and children. Geneva: World Health Organization, 2012. [PubMed] [Google Scholar]
- 53.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev 2017;4:CD004022. 10.1002/14651858.CD004022.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev 2011;11:CD004022. 10.1002/14651858.CD004022.pub3 [DOI] [PubMed] [Google Scholar]
- 55.Soleimani M. Insulin resistance and hypertension: new insights. Kidney Int 2015;87:497–9. 10.1038/ki.2014.392 [DOI] [PubMed] [Google Scholar]
- 56.Bechtold DA, Loudon ASI. Hypothalamic clocks and rhythms in feeding behaviour. Trends Neurosci 2013;36:74–82. 10.1016/j.tins.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 57.Drapeau V, Gallant AR. Homeostatic and circadian control of food intake: clinical strategies to prevent overconsumption. Curr Obes Rep 2013;2:93–103. 10.1007/s13679-012-0038-3 [DOI] [Google Scholar]
- 58.Yoshida J, Eguchi E, Nagaoka K, et al. . Association of night eating habits with metabolic syndrome and its components: a longitudinal study. BMC Public Health 2018;18:1366. 10.1186/s12889-018-6262-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soga Y, Shirai C, Ijichi A. [Association between daily lifestyle and the risk of metabolic syndrome among young adults in Japan. An analysis of Kobe city young adult health examination data]. Nihon Koshu Eisei Zasshi 2013;60:98–106. [PubMed] [Google Scholar]
- 60.Nakajima K, Suwa K. Association of hyperglycemia in a general Japanese population with late-night-dinner eating alone, but not breakfast skipping alone. J Diabetes Metab Disord 2015;14:16. 10.1186/s40200-015-0147-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akerstedt T, Wright KP. Sleep loss and fatigue in shift work and shift work disorder. Sleep Med Clin 2009;4:257–71. 10.1016/j.jsmc.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baron KG, Reid KJ, Kern AS, et al. . Role of sleep timing in caloric intake and BMI. Obesity 2011;19:1374–81. 10.1038/oby.2011.100 [DOI] [PubMed] [Google Scholar]
- 63.Afaghi A, O’Connor H, Chow CM. High-glycemic-index carbohydrate meals shorten sleep onset. Am J Clin Nutr 2007;85:426–30. 10.1093/ajcn/85.2.426 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.