Abstract
Objectives
To make practical and evidence-based recommendations on improving understanding of bleeding and thrombosis with pediatric extracorporeal life support and to make recommendations for research directions.
Data Sources
Evaluation of literature and consensus conferences of pediatric critical care and extracorporeal life support experts.
Study Selection
A team of 10 experts with pediatric cardiac and extracorporeal membrane oxygenation experience and expertise met through the Pediatric Cardiac Intensive Care Society to review current knowledge and make recommendations for future research to establish “best practice” for anticoagulation management related to extracorporeal life support.
Data Extraction/Synthesis
The first of a two-part white article focuses on clinical understanding and limitations of medications in use for anticoagulation, including novel medications. For each medication, limitations of current knowledge are addressed and research recommendations are suggested to allow for more definitive clinical guidelines in the future.
Conclusions
No consensus on best practice for anticoagulation exists. Structured scientific evaluation to answer questions regarding anticoagulant medication and bleeding and thrombotic events should occur in multicenter studies using standardized approaches and well-defined endpoints. Outcomes related to need for component change, blood product administration, healthcare outcome, and economic assessment should be incorporated into studies. All centers should report data on patients receiving extracorporeal life support to a registry. The Extracorporeal Life Support Organization registry, designed primarily for quality improvement purposes, remains the primary and most successful data repository to date. (Pediatr Crit Care Med 2019; 20:1 027–1033)
Keywords: anticoagulation, extracorporeal, membrane oxygenation, hemorrhage, heparin, pediatrics, thrombosis
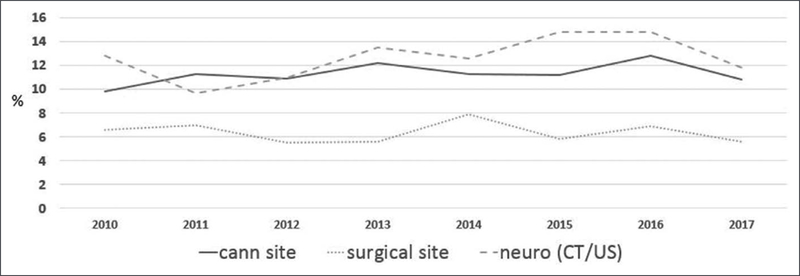
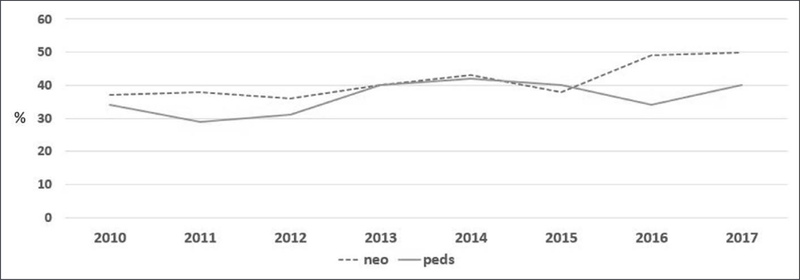
Use of extracorporeal membrane oxygenation (ECMO) for pediatric patients continues to expand (1). Critical illness and exposure of blood to the ECMO circuit create a prothrombotic state and a risk of bleeding. The primary mediator of clot formation when the blood contacts nonbiologic ECMO surface is thrombin. Activation of thrombin is associated with two common complications during ECMO: bleeding and thrombosis. Generation of thrombin leads to consumption of platelets and coagulation factors. In areas of native tissue injury, this consumption impairs the ability of normal coagulation and thus bleeding can result. Concurrently, thrombin production also stimulates fibrin production, platelet activation, and other factors in the coagulation pathway to shift the balance toward a prothrombotic state. The proteins that help to balance this procoagulant effect are antithrombin, protein C, and protein S. Despite advances in understanding of the coagulation pathways in the body, improvements in production of ECMO that is less stimulating to the blood, and laboratory testing to define the levels of factors important in the coagulation pathway, thrombotic and bleeding complications remain a major cause of morbidity and mortality during ECMO support (2). Outcomes such as neonatal hemorrhagic events and circuit component clotting rates have not changed significantly over the last 6 years (Figs. 1 and 2). Specific guidelines and recommendations for management of anticoagulation, bleeding, and monitoring during ECMO are lacking, and anticoagulation algorithms remain institution specific (2).
Figure 1.
Neonatal hemorrhagic complications. Data abstracted from the International Extracorporeal Life Support Organization Registry, with permission, January 2019. Cann = cannulation, CT = computerized tomography, neuro = neurologic, US = ultrasound.
Figure 2.
Mechanical circuit component clots. Data abstracted from the International Extracorporeal Life Support Organization Registry, with permission, January 2019. Neo = neonatal, Peds = pediatrics
The aim of the first of this two-part white article is to describe current and novel anticoagulation medications. Anticoagulation monitoring is addressed in the second portion of this work. An expert panel offers a description of the pertinent clinical effects of anticoagulants and their limitations. Definitive clinical guidelines on anticoagulation are still not possible so the panel offers specific recommendations, with level of agreement, on future research that will help identify optimal anticoagulation strategies.
METHODS
The Pediatric Cardiac Intensive Care Society (PCICS) research subcommittee on scientific statements and white articles comprised of 10 international members. Each member was chosen by the research committee Chairs on the basis of their expertise and reputation in the field of clinical research. All subcommittee members meet on a monthly basis lay means of conference calls and also meet face-to-face at the annual PCICS scientific meeting.
A literature search process was developed with the goal of making the methodology transparent and reproducible similar to that used by the American College of Cardiology (ACC)/ American Heart Association (AHA) scientific statements (3). However, given the lack of available evidence, only level (quality of evidence) was used for included articles. As the purpose of the project was to offer a roadmap for future research, a systemic review was not performed. The quality of the studies was assessed by careful screening of the methods and results. We used the ACC and AHA Guideline Recommendation Classification System, updated in 2015, to compare the strength and quality of evidence between studies (4). During a consensus meeting, all experts without disclosed confiict of interest rated the recommendations.
RESULTS
Current Medications in Use for Anticoagulation
Unfractionated Heparin
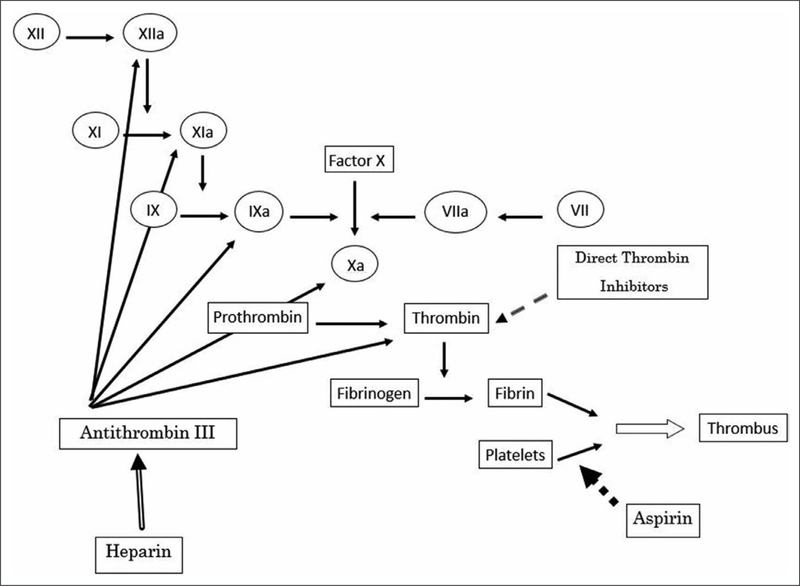
The majority of pediatric ECMO centers continue to use unfractionated heparin (UFH) for anticoagulation during ECMO. UFH consists of heparin molecules arrayed in different sizes of saccharide chains (5). UFH exerts the majority of its anticoagulant effect by binding and activating anti thrombin (Fig. 3). The binding of heparinoid particles alters the structure of the antithrombin molecule making the antithrombin/heparin complex more than 1,000-fold more potent than antithrombin alone in inhibiting factors IIa (thrombin), Xa, IXa, XIa, and XIIa, with predominant effects on thrombin and factor Xa (6). The larger heparin molecules can accelerate the inhibition of proteases, like thrombin, by up to 17,000-fold (7). Only 1/3 of heparin molecules have the specific pentasaccharide sequence needed to bind antithrombin. Further, heparin also consists of larger and smaller chains. Only the longer chains are able to catalyze the antithrombin/ heparin complex and inhibit both thrombin and factor Xa. Shorter chains only inhibit factor Xa. UFH preparations are heterogeneous in terms of size of the heparin molecules as well as those with the binding sequence for antithrombin. Heparin is excreted via the kidneys, with larger molecules cleared faster than smaller ones (8).
Figure 3.
Coagulation cascade and targets of anticoagulants.
Heparin also has anti-inflammatory effects, exerts effects on other coagulation factors, and inhibits osteoclast formation as well. Through an indirect mechanism, it has effects on the tissue factor pathway inhibitor. In addition to antithrombin, heparin also binds to plasma proteins, endothelial cells, and macrophages (9). Heparin is cleared by rapid and saturable binding to endothelial cells and macrophages which is dose dependent, and via renal clearance, which is a slower process (8). All of these factors explain the variable anticoagulation effects noted with heparin administration. We recommend that all clinicians who treat patients with UFH understand the mechanisms of action, the expected difference in response in neonates due to developmental differences in antithrombin levels, the limitations of its efficacy and the rationale for its variable anticoagulant effects. Improved understanding may help the pediatric ECMO community develop more valid research efforts to determine if heparin is the “best” anticoagulant and how to optimize administration and monitoring to reduce bleeding and thrombosis.
Antithrombin
Antithrombin plays an important role as an anticoagulant, inactivating factor II (thrombin), factor Xa, and other serine proteases in the clotting cascade (10). Antithrombin binds to clotting factors via its reactive center, forming an irreversible complex and inhibiting the clotting factors activity. The antithrombin molecule also contains a heparinbinding site. Binding of endogenous or exogenous heparins amplifies the ability of antithrombin to inactivate clotting factors. Antithrombin is found circulating in plasma, as well as bound to the endothelial surface of blood vessels.
Antithrombin activity is commonly measured by a functional assay that measures the ability of antithrombin to inhibit factor Xa or factor II. Results are reported as a percent (international units/deciliter), with the normal range often described as between 80% and 120% in adults. Normal antithrombin activity level varies with maturational status, with neonates having antithrombin activity normally around 65% (range, 39–87%) (11). Antithrombin activity increases to adult levels within 6 months (11). Antithrombin activity can be affected by liver injury (as it is produced and degraded in the liver), cardiopulmonary bypass (CPB), sepsis/disseminated intravascular coagulation, critical illness, and other conditions.
Maintaining antithrombin activity is vital to balance the coagulant and anticoagulant pathways in the body. In a normal situation, thrombin released from the site of endothelial injury into the bloodstream is neutralized by antithrombin and thus thrombus formation is limited. When antithrombin activity is low, this free thrombin in the plasma will not be bound to antithrombin and thus excessive clot formation may occur. Severe antithrombin deficiency has been reported to be associated with risk of thrombosis (12). Patients with hereditary antithrombin deficiency may have antithrombin ranges of 35–60%, and these patients are reported to have higher occurrences of deep venous thrombosis (13).
The administration of antithrombin during ECMO has risen exponentially, without rigorous examination of its effect, optimal dosing, and differences with maturational state. The majority of work regarding antithrombin and extracorporeal circuits comes from the CPB literature. High levels of antithrombin during CPB were associated with a decrease in hemostatic activation (14).
Studies of antithrombin during ECMO have noted variable results and suffer from a lack of standardized processes for ECMO practice, equipment, and endpoints. New data on genetic variation which has shown that there is latent antithrombin may also be important in the coagulation process. This may also have implications for anticoagulation management and evaluation in the future. Of particular concern in the current era of increasing antithrombin use, Wong et al ( 15) found that patients who received antithrombin administration on ECMO had a higher rate of hemorrhagic and thrombotic events (Level C-limited data [LD]). We suggest that an evaluation of expected antithrombin levels in critical illness, in patients on ECMO and those who are not, be undertaken prior to a trial to establish efficacy and safety of antithrombin replacement. A detailed description of a research approach to antithrombin replacement can be found under the proposed studies section at the end of this report.
Novel Medications for Anticoagulation in Pediatrics
Direct Thrombin Inhibitors
Heparin therapy has many disadvantages as noted above, and as monitoring and adjustment algorithms have not markedly decreased bleeding and thrombotic complications with ECMO, clinicians have searched for new methods of anticoagulation. Direct thrombin inhibitors (DTIs) are competitive inhibitors of thrombin that do not require a cofactor (Fig. 3). They are potentially good candidates for use on ECMO secondary to their short half-lives, independence of antithrombin, more predictable effect than UFH, effects on both bound and free thrombin, and antiplatelet properties (16, 17) (Level C-LD). Three parenteral DTIs have been Food and Drug Administration approved in adults: arg- atroban, bivalirudin, and lepirudin. Hirudin is available under the recombinant names of lepirudin and desirudin. Hirudin is cleared by the kidneys and accumulates in renal insufficiency. In addition, antibodies can develop to lepirudin and result in anaphylaxis upon repetitive exposure to the drug. Bivalirudin is also an analog of hirudin and its duration of action is short (about 25min) before it becomes cleaved from thrombin. It undergoes proteolytic degradation and only 20% is excreted through the kidney, thereby leading to less accumulation in hepatic and renal dysfunction (18). Argatroban has a half-life of 45 minutes. As argatroban is metabolized in the liver, it must be used with caution in patients with hepatic failure. It can be useful especially in patients with renal failure because it does not require renal elimination. There are theoretical advantages of bivalirudin over argatroban and lepirudin on ECMO, namely its largely proteolytic degradation and shorter half-life. Use of DTIs is currently increasing in the ECMO population (19) with a paucity of formal studies of risks and benefits.
A main concern with the parenteral DTIs is that unlike UFH, they do not have direct antidotes. Recombinant factor VII has been reported to reverse the anticoagulant effects of DTIs in ex vivo samples although clinical data are sparse (20) (Level C-LD). Another disadvantage is that there is no readily available laboratory assay to accurately measure the therapeutic effects. DTIs prolong prothrombin time, activated partial thromboplastin time (aPTT), international normalized ratio, and activated clotting time (ACT) in a linear fashion at low, but not high doses. None of these assays are validated for DTI monitoring, and all are affected by multiple hemostatic factors that may cause spurious results (21). The current “Chest” guidelines recommend aPTT for chronic monitoring and ACT for monitoring of procedural anticoagulation (22), although these recommendations are not specific to ECMO (Level B-nonrandomized [NR]). Dedicated DTI assays such as plasma-diluted thrombin time or ecarin bleeding time are currently available in only a few centers, and their efficacy in ECMO is not established (21).
Although DTIs have been successfully used where UFH is contraindicated, there is a paucity of data on safely and efficacy for children on ECMO (Table 1). Although some reports highlight the safety and efficacy of bivalirudin in ECMO patients, other case reports note that circuit thrombosis occurs quickly in circumstances where ECMO flow is emergently stopped. Patient thrombosis in the form of pulmonary embolus or other thrombosis has also been noted (18, 24, 27). Overall, however, the pharmacologic profile of the DTIs, especially bivalirudin, makes them good candidates for further controlled clinical trials in pediatrics. Ongoing single-center studies of DTI pharmacokinetics during ECMO and studies comparing a DTI to UFH will inform such future trials. We recommend a comparison study of heparin versus a DTI be conducted as a multicenter randomized controlled trial with a protocol that is as uniform as possible for circuitry, anticoagulation algorithm and blood product replacement triggers. In particular, patient thrombotic events and need for ECMO component change due to clot formation should be recorded in each study.
TABLE 1.
Selected Direct Thrombin Inhibitor Studies
| Reference | Year | Patient Population (n) | Study Design | Findings |
|---|---|---|---|---|
| Moffett and Teruya (19) | 2014 | Pediatrics (Pediatric Health Information System registry) | Administrative database review to determine frequency of use for each DTI | Argatroban 70%, bivalirudin 23%, lepirudin 7% |
| Nagle et al (18) | 2013 | Pediatrics (12) | Case series-ECMO using DTI for HIT, heparin resistance, or thrombosis | -41.7% survival -High interpatient dose variability: 0.045–0.48 mg/kg/hr, and increased dose requirement over time -Eight required circuit change. |
| Ranucci et al (23) | 2011 | Adults/pediatrics (21) | Retrospective comparison of eight patients requiring postcardiotomy ECMO treated with heparin and 13 treated with bivalirudin | -Bivalirudin group had longer aPTT, activated clotting time, and lower blood loss -Heparin group received more platelet, plasma, and antithrombin -Five children in each group with one surviving in each |
| Pieri et al (24) | 2013 | Adults (20) | Retrospective cohort of 10 patients receiving bivalirudin on ECMO vs 10 heparin controls | -aPTT variability (>20% compared with previous sample) higher in heparin group -No difference in bleeding, thromboembolic events, or mortality |
| Menk et al (25) | 2017 | Adults (39) | Case-control study (39 patients with acute respiratory distress syndrome on ECMO receiving argatroban vs 39 matched patients receiving heparin) | -No significant differences in major and minor bleeding complications, thromboembolic events, rates of transfusion, and device-associated complications -Over time on ECMO, there were significantly fewer aPTT values below the targeted aPTT goal in the argatroban group vs the heparin group (p < 0.05) |
| Beiderlinden et al (26) | 2007 | Adults (9) | Case series of patients receiving argatroban for suspected HIT | -At argatroban infusion rates of 0.2 g/kg/min, aPTT, and thrombin times reached targeted levels -No oxygenator or extracorporeal system clotting was observed over an average duration of argatroban infusion of 4 d |
aPTT = activated partial thromboplastin time, DTI = direct thrombin inhibitors, ECMO = extracorporeal membrane oxygenation, HIT = heparin-induced thrombocytopenia.
Antiplatelet Therapy
Aspirin (acetyl salicylic acid) irreversibly inhibits platelet cyclooxygenase-1 and inhibits the production of thromboxane-A2 (28) (Fig. 3). Platelet activation leading to aggregation and increased clot burden occurs with exposure to the ECMO circuit. It is reasonable that antiplatelet therapy, in addition to whatever anticoagulant medication is being administered, might reduce clotting within the ECMO circuit and in the patient. Antiplatelet therapy is standard in patients supported with ventricular assist devices and other extracorporeal devices. Randomized trials in patients with carotid artery disease and following device placement exist and offer good roadmaps to design an appropriate trial in ECMO (29). To date, only case series on the use of antiplatelet agents in ECMO are available (30,31).
Bein et al (31) report on the use of low-dose aspirin as an adjunct to heparin to provide anticoagulation in a series of patients (n = 15) supported with pumpless (extracorporeal lung assist. Compared with a historical matched cohort (n = 15) treated with heparin alone, the addition of aspirin improved oxygen transfer across the membrane and was associated with fewer oxygenator changes. One patient in the aspirin group compared with five patients in the heparin-only group died.
New genetic data used to assess efficacy of these medications are arising and may provide guidance on their use (32). Aspirin resistance assays may also be important in assessing the effect of aspirin in individual patients. Currently, there is no information on the safety and efficacy of the use of aspirin in pediatric patients supported with ECMO. Thus, recommendations on the use of aspirin as an adjunct to anticoagulation in ECMO cannot be made, but could be an important area of future investigation. We recommend that aspirin, after adequate postcannulation hemostasis is achieved, and heparin should be compared with heparin alone with regards to bleeding and thrombotic complications and that thromboelastography with platelet mapping, or other measures of platelet function, should be included in the trial design.
DISCUSSION
Proposed Future Research Direction
ECMO has experienced an exponential rise in clinical application, especially in patients with cardiac failure. Anticoagulation is necessary in the extracorporeal circuit, and bleeding and thrombosis remain the most frequent and devastating complications related to ECMO.
Despite single-center reports and data mining of large ECMO databases, “best practice” to reduce bleeding and thrombosis within the ECMO circuit and the patient remains elusive. Poorly standardized definitions of bleeding or thrombosis and how these events are categorized limit our ability to define best practice. Even more difficult issues include variable pumps, circuits, and other ECMO components across centers. Singlecenter reports have noted decreased bleeding and/or thrombotic events, specifically after introduction of anticoagulation protocols. A recent report demonstrated a decrease in bleeding events after heparin protocol implementation which demonstrates that these protocols should be routinely used within pediatric ECMO programs. Besides the clinical benefit, these protocols will aid in research efforts if they can be standardized across centers (33). This will be necessary to achieve widespread improvement as single-center experiences are challenging to generalize. Once better guidance can be produced on protocols that improve anticoagulation broadly, work can begin to explore ways to adjust these protocols for individual variation.
How then should we establish best practice to eliminate the devastating effects of bleeding and thrombosis in ECMO patients? It is evident that our current means of clinical investigation is lacking.
We propose a “roadmap” for investigation that includes clear definitions for bleeding and thrombosis, mandatory data fields that provide specific information on bleeding and thrombosis events with timing, methods to validate data and prevent interrater ‘variability issues, and full reporting of patients to data registries such as Extracorporeal Life Support Organization or specific databases for a research question. In addition, standardization of ECMO equipment and anticoagulation practices and testing regimens between sites who are researching anticoagulation is important. Finally, we will need to consider developmental differences in hemostasis when designing trials and anticoagulation strategies.
As important is standardizing patient care practices between research sites. Although this may limit the number of centers who can work together on a defined project, it is not an insurmountable task. The importance of this aspect of designing studies on anticoagulation is illustrated by noting the variability in practice that has been described. The large National Institutes of Health-sponsored evaluation of bleeding and thrombosis in children receiving ECMO is an example of how center ‘variability can affect bleeding and thrombosis (2). All centers were large, experienced, ECMO sites, but variability in anticoagulation practice, testing, circuitry, and transfusion triggers existed and the occurrence of bleeding and thrombosis was ‘variable between sites. Although not statistically significant, mortality among sites also varied (2). Other studies have also noted the variability in anticoagulation practice and lack of protocol adherence even in sites where such protocols were in place (34).
This must be addressed as we move forward. In this article, we have discussed several anticoagulants with their advantages and disadvantages. Many of these remain incompletely understood. To move the field of pediatric ECMO forward and establish best practices in anticoagulation, limiting variability in study design must be a guiding principle.
CONCLUSIONS
Proposed Studies
Randomized trials of bivalirudin and/or argatroban versus heparin using standardized circuitry and standardized drug titration protocols should be conducted after their safety has been established in pediatrics. Measured endpoints should include the need for circuit component change, bleeding and thrombotic events, amount of blood products administered, number of required dosing changes to maintain anticoagulation in desired range and percent of time values were in the desired range, and survival to ECMO decannulation and to hospital discharge.
- A trial of antithrombin replacement should be conducted. Suggestions include the following:
- Establishing baseline levels of antithrombin in children with critical illness, in patients on ECMO and those who are not, at ‘varying ages (<30 d, 1–6 mo, and older).
-
A standard dosing infusion protocol designed to maintain levels in a predetermined range should be developed to establish a dosing regimen prior to the study.
-
i.The above may also be compared with a bolus infusion regimen. As the two currently available forms of antithrombin replacement have varying half-lives, these aspects must be considered in the study design.
-
i.
-
An antithrombin trial should compare bleeding, thrombosis, heparin dosing, and blood product use to assess the safety and efficacy of antithrombin replacement.
-
i.Cost analysis should also be considered due to the expense of antithrombin replacement.
-
i.
ACKNOWLEDGMENTS
We acknowledge Sandra Staveski, PhD, RN, APRN, CPNP-AC, and Jean Connor, PhD, RN, CPNP, EAAN, for bringing the team of experts together and providing guidance and structure to the consensus meetings. We thank Justin Horowitz for help creating the figures.
Footnotes
See also p. 1089
The work for this project occurred during monthly phone meetings and at each of the institutions listed above for the authors.
Dr. Reddy’s institution received funding from the National Institutes of Health and the American Heart Association. Dr. Thiagarajan’s institution received funding from Bristol Myers Squibb and Pfizer. Dr. Dalton received funding from Innovative ECMO Concepts (consultant), and she disclosed off-label product use of extracorporeal membrane oxygenation. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Thiagarajan RR, Barbara RP, Rycus PT, et al. ; ELSO member centers: Extracorporeal life support organization registry international report 2016. ASAIO j 2017; 63:60–67 [DOI] [PubMed] [Google Scholar]
- 2.Dalton HJ, Garcia-Fi l ion P, Holubkov R, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr Crit Care Med 2015; 16:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JL: Evolution of the ACC/AHA clinical practice guidelines i n perspective: Guiding the guidelines. J Am Coll Cardiol 2015; 65:2735–2738 [DOI] [PubMed] [Google Scholar]
- 4.Halperi n JL, Levine GN, Al-Khatib SM, et al. : Further evolution of the ACC/AHA clinical practice guideline recommendation classification system: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 67:1572–1574 [DOI] [PubMed] [Google Scholar]
- 5.Ryerson LM, Lequier LL: Anticoagulation management and monitoring during pediatric extracorporeal life support: A review of current issues. Front Pediatr 2016; 4:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu YI, Sheffield WP, Blajchman MA: Defining the heparin-binding domain of antithrombin. Blood Coagul Fibrinolysis 1994; 5:83–95 [DOI] [PubMed] [Google Scholar]
- 7.Willemze A, Ciaccia A, Church S. Heparin, not dermatan sulfate, promotes the “substrate-like” behavior of L444R recombinant heparin cofactor 2 in thrombin formation In: Chemistry and Biology of serpins. Laragh J, Brenner B (Eds). New York, NY, Springer Science+Business Media, 1997, pp 278–279 [Google Scholar]
- 8.Hirsh J, Raschke R: Heparin and low-molecular-weight heparin: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004; 126;188S–203S [DOI] [PubMed] [Google Scholar]
- 9.Ryerson LM, Bruce AK, Lequier L, et al. : Administration of antithrombin concentrate in infants and children on extracorporeal life support improves anti coagulation efficacy. ASAIO J 2014; 60:559–563 [DOI] [PubMed] [Google Scholar]
- 10.Perry DJ: Antithrombin and its inherited deficiencies. Blood Rev 1994; 8:37–55 [DOI] [PubMed] [Google Scholar]
- 11.Andrew M, Paes B, Milner R, et al. : Development of the human coagulation system i n the full-term i nfant. Blood 1987; 70:165–172 [PubMed] [Google Scholar]
- 12.Previtali E, Bucciarelli P, Passamonti SM, et al. : Risk factors for venous and arterial thrombosis. Blood Transfus 2011; 9:120–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demers C, Ginsberg JS, Hirsh J, et al. : Thrombosis in antithrombin-III- deficient persons. Report of a large kindred and literature review. Ann Intern Med 1992; 116:754–761 [DOI] [PubMed] [Google Scholar]
- 14.Avidan MS, Levy JH, van Aken H, et al. : Recombinant human antithrombin III restores heparin responsiveness and decreases activation of coagulation in heparin-resistant patients during cardiopulmonary bypass. J Thorac Cardiovasc Surg 2005; 130:107–113 [DOI] [PubMed] [Google Scholar]
- 15.Wong TE, Nguyen T, Shah SS, et al. : Antithrombin concentrate use in pediatric extracorporeal membrane oxygenation: A multicenter cohort study. Pediatr Crit Care Med 2016; 17:1170–1178 [DOI] [PubMed] [Google Scholar]
- 16.Di Nisio M, Middeldorp S, Buller HR: Direct thrombin inhibitors. N Engl J Med 2005; 353:1028–1040 [DOI] [PubMed] [Google Scholar]
- 17.Annich G, Adachi I: Anticoagulation for pediatric mechanical circulatory support. Pediatr Crit Care Med 2013; 14:S37–S42 [DOI] [PubMed] [Google Scholar]
- 18.Nagle EL, Dager WE, Duby JJ, et al. : Bivalirudin in pediatric patients maintained on extracorporeal life support. Pediatr Crit Care Med 2013; 14:e182–e188 [DOI] [PubMed] [Google Scholar]
- 19.Moffett BS, Teruya J: Trends in parenteral direct thrombin inhibitor use in pediatric patients: Analysis of a large administrative database. Arch Pathol Lab Med 2014; 138:1229–1232 [DOI] [PubMed] [Google Scholar]
- 20.Young G, Yonekawa KE, Nakagawa PA, et al. : Recombinant activated factor VII effectively reverses the anticoagulant effects of heparin, enoxapa- rin, fondaparinux, argatroban, and bivalirudin ex vivo as measured using thromboelastography. Blood Coagul Fibrinolysis 2007; 18:547–553 [DOI] [PubMed] [Google Scholar]
- 21.Latham GJ, Jefferis Kirk C, Falconer A, et al. : Challenging argatroban management of a child on extracorporeal support and subsequent heart transplant. Semin Cardiothorac VascAnesth 2016; 20:168–174 [DOI] [PubMed] [Google Scholar]
- 22.Linkins LA, Dans AL, Moores LK, et al. : Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence- Based Clinical Practice Guidelines. Chest 2012; 141: e495S–e530S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranucci M, Ballotta A, Kandil H, et al. : Bivalirudin-based versus conventional heparin anticoagulation for postcardiotomy extracorporeal membrane oxygenation. Crit Care 2011; 15:R275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pieri M, Agracheva N, Bonaveglio E, et al. : Bivalirudin versus heparin as an anticoagulant during extracorporeal membrane oxygenation: A case-control study. J Cardiothorac Vase Anesth 2013; 27:30–34 [DOI] [PubMed] [Google Scholar]
- 25.Menk M, Briem P, Weiss B, et al. : Efficacy and safety of argatroban in patients with acute respiratory distress syndrome and extracorporeal lung support. Ann Intensive Care 2017; 7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beiderlinden M, Treschan T, Gorlinger K, et al. : Argatroban in extracorporeal membrane oxygenation. Artif Organs 2007; 31:461–465 [DOI] [PubMed] [Google Scholar]
- 27.Koster A, Weng Y, Bottcher W, et al. : Successful use of bivalirudin as anticoagulant for ECMO in a patient with acute HIT. Ann Thorac Surg 2007; 83:1865–1867 [DOI] [PubMed] [Google Scholar]
- 28.Schror K: Aspirin and platelets: The antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin Thromb Hemost 1997; 23:349–356 [DOI] [PubMed] [Google Scholar]
- 29.Barrera JG, Rojas KE, Balestrini C, et al. : Early results after syn chronous carotid stent placement and coronary artery bypass graft in patients with asymptomatic carotid stenosis. J Vase Surg 2013; 57:58S–63S [DOI] [PubMed] [Google Scholar]
- 30.Staudacher DL, Biever PM, Benk C, et al. : Dual Antiplatelet Therapy (DAPT) versus no antiplatelet therapy and incidence of major bleeding in patients on venoarterial extracorporeal membrane oxygenation. PLoS One 2016; 11 :e0159973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bein T, Zimmermann M, Philipp A, et al. : Addition of acetylsalicylic acid to heparin for anticoagulation management during pumpless extracorporeal lung assist. ASAIO J 2011; 57:164–168 [DOI] [PubMed] [Google Scholar]
- 32.BergmeijerTO RenyJL, Pakyz RE, et al. ; ICPC Investigators: Genomewide and candidate gene approaches of clopidogrel efficacy using pharmacodynamic and clinical end points-Rationale and design of the International Clopidogrel Pharmacogenomics Consortium (ICPC). Am Heart J 2018; 198:152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nair AG, Oladunjoye OO, Trenor CC III, et al. : An anticoagulation protocol for use after congenital cardiac surgery. J Thorac Cardiovasc Surg 2018; 156:343–352.e4 [DOI] [PubMed] [Google Scholar]
- 34.Bembea MM, Annich G, Rycus P, et al. : Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med 2013; 14:e77–e84 [DOI] [PMC free article] [PubMed] [Google Scholar]