Abstract
Purpose
Adenosine triphosphate (ATP) is involved in the diameter regulation of retinal vessels. The compound has been shown to induce both constriction and dilatation, but the detailed mechanisms underlying these effects and the site of action of the compound are not known in detail. Therefore, the purpose of the present study was to investigate whether the vasoactive effects of ATP on retinal vessels depend on intra- and extravascular application, and to study whether the effects differ at different vascular branching levels.
Methods
Diameter changes in arterioles, pre-capillary arterioles, and capillaries were studied in perfused porcine hemiretinas (n = 48) ex vivo after intra- and extravascular application of the nondegradable ATP analogue ATP-γ-S or ATP in the presence or not of antagonists to the CD73/ecto-5′-nucleotidase (AOPCP), the P2-purinergic receptor (PPADS), the A3-adenosine receptor (MRS1523), and the synthesis of cyclooxygenase products (ibuprofen).
Results
Intravascular ATP-induced constriction and extravascular ATP-induced dilatation of retinal arterioles, pre-capillary arterioles and capillaries, and dilatation was inhibited by ibuprofen. Both constriction and dilatation of arterioles were inhibited by antagonizing ATP degradation. Furthermore, constriction at all three branching levels was antagonized by blocking the A3 purinoceptor, whereas constriction in arterioles and pre-capillary arterioles was antagonized by blocking the P2 purinoceptor.
Conclusions
ATP affects the diameter of retinal arterioles, pre-capillary arterioles, and capillaries through different pathways, and the effects depend on whether the compound is administered intravascularly or extravascularly. This may form the basis for selective interventions on retinal vascular disease with differential involvement of vessels at different branching levels.
Keywords: adenosine triphosphate, diameter regulation, perfused retina, ex vivo model
The purine nucleotide adenosine triphosphate (ATP) can modulate retinal blood flow in health and disease.1 It has been proposed that the activity of ATP and its degradation products are involved in the pathophysiology of diabetic retinopathy,2 which is supported by the finding of increased ATP concentrations in eyes from patients with proliferative diabetic retinopathy.3 Animal studies have shown that extravascular application of ATP induces contraction of rat vascular pericytes4 and arterioles5 that are mediated by P2X receptors. In bovine tissue, ATP can induce contraction of arterioles6 and retinal vascular pericytes.7 In porcine retinal arterioles, extravascular application of ATP agonists induces vasodilatation8,9 and the ATP degradation product adenosine has dual effects on the tone of retinal arterioles with contraction and relaxation mediated by different receptors.10 Recently, attention has been directed at different effects of intra- and extravascular application of vasoactive compounds11 and the role of pre-capillary arterioles and capillaries for regulating retinal blood flow.12–14 However, the effects of different routes of application of ATP and the role of the compound for the diameter regulation of smaller retinal vessels have not been studied in detail.
Therefore, the diameter of retinal arterioles, pre-capillary arterioles, and capillaries were studied in perfused porcine hemiretinas after intra- and extravascular application of ATP, alone and in the presence of antagonists known to antagonize ATP-induced constriction and dilatation.
Materials and Methods
Solutions
Physiological saline solution (PSS 1.6) contained in mmol/l: NaCl 119, KCl 4.7, MgSO4 1.17, NaHCO3 25.0, KH2PO4 1.18, CaCl2 1.6, EDTA 0.026, Glucose 5.5, and HEPES 10.0 (pH = 7.4). PSS 0.0 was PSS 1.6 without CaCl2. KPSS was PSS 1.6 where NaCl had been replaced with equimolar amounts of KCl resulting in a K+ concentration of 124 mmol/l. PSS-HEPES was PSS 1.6 without KH2PO4, EDTA, and HEPES reduced to a concentration of 5.0 (pH = 7.53).
Chemicals
Dissolved in PSS 1.6 (from Sigma-Aldrich, Glostrup, Denmark): fluorescein isothiocyanate-dextran (FITC) to a concentration of 2.5 * 10−3 mol/l. Adenosine 5′-triphosphate disodium salt hydrate (ATP) and adenosine 5′’-[γ-thio]triphosphate tetralithium salt (ATP-γ-S) both to a concentration of 10−4 mol/l. From Cayman Chemicals, Europe, Tallinn, Estonia: the thromboxane receptor agonist 9,11-dideoxy-9α,11α-methanoepoxyprosta-5z,13E-dien-1-oic acid (U46619) to a concentration of 10−5 mol/l.
Dissolved in Distilled Water9,10
(from Tocris Bioscience, Avonmouth, Bristol, UK): The ecto-5′-nucleotidase/CD73 inhibitor α,β-Methyleneadenosine 5′-diphosphate sodium salt (AOPCP) to a concentration of 10−4 mol/l. From Sigma-Aldrich, Glostrup, Denmark: the nonselective P2 purinoreceptor antagonist Pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt hydrate (PPADS) to a concentration of 10−5 mol/l. The selective A3 adenosine receptor antagonist 3-Propyl-6-ethyl-5-[(ethylthio)carbonyl]-2 phenyl-4-propyl-3-pyridine carboxylate (MRS 1523) to a concentration of 10−5 mol/l. The cyclooxygenase (COX) inhibitor ibuprofen to a concentration of 10−5 mol/l. Papaverine in a concentration of 10−2 mol/l to obtain maximal vasodilatation.
Tissue
Porcine eyes from Danish Landrace pigs (age approximately 6 months and weight 84–85 kg) were collected daily from a local abattoir (Danish Crown, Horsens, Denmark). The use of this tissue for research does not require approval from animal ethics authorities in Denmark. The pigs were anaesthetized with CO2 and exsanguinated, followed by enucleation of one eye from each animal. The eyes were immersed in PSS-HEPES at 5°C for the transport to the laboratory, and the time from enucleation to the initiation of the experiment never exceeded 90 minutes.
Dissection
The eye was placed in an eye holder (MTS, the Wetlab Company, Innsbruck, Austria) and fixed with needles through Tenon's capsule and remaining periocular soft tissue. The anterior part of the eye was removed by a frontal section between the ora serrata and the equator using a razor blade (Industry blade model 420-4; Jydsk Barberblade Fabrik, Aalborg, Denmark). In the remaining posterior part of the eye, the vitreous body was removed using anatomic forceps (stainless steel round 115 mm type 1605.0115; Hounisen, Skanderborg, Denmark) and replaced with PSS 0.0. The preparation was placed under a stereo microscope (Stemi 100; Carl Zeiss Microscopy), and it was ensured that the first branching of the superior arteriole was located more than 5 mm from the optic disc. Subsequently, the retina was divided into an upper and a lower half with a horizontal cut through the optic disc using scissors (Troutman type 23121; Bausch & Lomb, Heidelberg, Germany). The upper hemiretina contained a complete vascular segment supplied by one main arteriole and drained to one main venule. This preparation was separated from the underlying choroid and sclera by injection of PSS 0.0 using a blunt syringe (Beaver-Visitec International, Abingdon, Oxfordshire, UK) and was transferred to the tissue chamber using a spoon (Partou-Spatula Spoon 185 mm; Hounisen, Skanderborg, Denmark).
Mounting
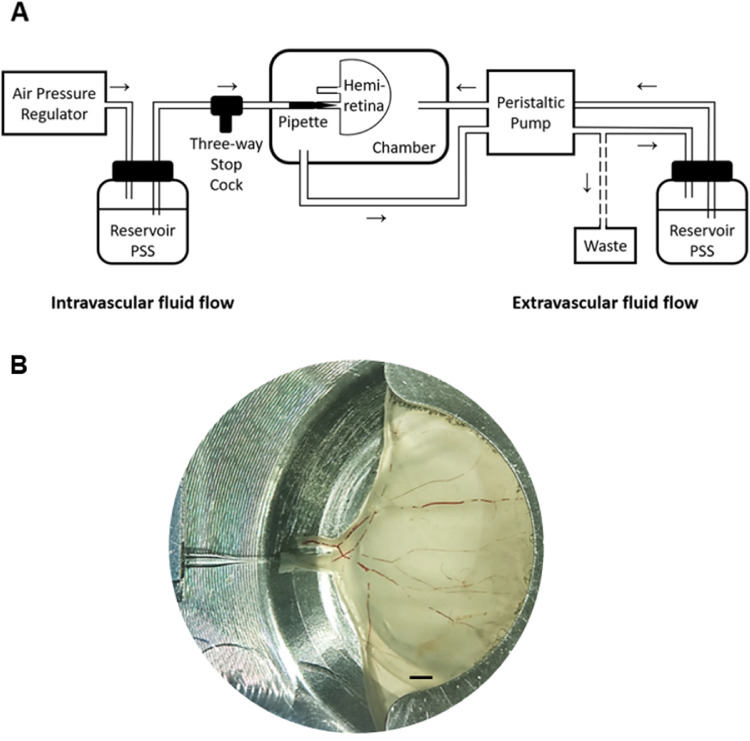
The experimental setup has been described in detail previously11 and is shown in Figure 1. In short, a tissue chamber has been designed to hold a porcine hemiretina while controlling pH, temperature, and oxygen saturation. The chamber contains two perfusion systems, one for perfusing the retinal vascular system (intravascular fluid flow) and another system for continuously renewing the chamber fluid surrounding the hemiretina (extravascular fluid flow). Intravascular perfusion was performed through the primary arteriole, which was mounted on a pipette using a pair of forceps (Dumont #5 Inox tip; Dumont, Switzerland) and secured by surgical ligatures (Ethilon 10-0; Johnson & Johnson, Birkeroed, Denmark). The pipette had been prepared from a glass tube with an inner diameter of 600 µm (Drummond, Birmingham, AL, USA) drawn in a pipette puller (Model P-97 Flaming/Brown Micropipette puller; Sutter Instruments, Novitas, CA, USA) to a tip diameter of approximately 25 µm. The pipette was in turn connected to a three-way stop cock for application of intravascular compounds. Both systems were supplied by a reservoir containing PSS and perfusion was driven by an air pressure regulator that created a stable pressure of 80 mm Hg (the intravascular flow) and a peristaltic pump (Minipuls 3; Glison, Dunstable, UK) with a speed of 18.5 mL/min (the extravascular flow). The intravascular flow was drained from the larger retinal venule into the tissue chamber and in turn drained to a waste container.
Figure 1.
The experimental setup. (A) The perfusion system. Arrows indicate the direction of flow in, respectively, the intravascular (left) and the extravascular (right) perfusion systems. (B) An upper porcine hemiretina mounted in the tissue chamber. Stagnant erythrocytes are seen in the larger vessels. Bar = 2 mm.
After mounting of the preparation, the tissue chamber was placed in a fluorescence microscope (Nikon Eclipse Ti; Nikon, Japan). The intravascular system was flushed with PSS 0.0 to remove erythrocytes and avoid clotting. Subsequently, perfusion of both the intravascular and the extravascular systems with PSS 1.6 was commenced, the preparation was heated to 37°C and adjusted to pH 7.4 (Mettler Toledo SevenGo DuoPro pH-meter; SG68, Glostrup, Denmark), and bubbling with 5% CO2 in atmospheric air was commenced. The preparation was allowed to equilibrate for 50 minutes.
Diameter Measurements
After equilibration, the intravascular system was perfused with PSS FITC in order to increase the contrast of the blood vessels. Vessels at three branching levels were identified as follows: (1) larger arteriole: the arteriole after the second dichotomous branching from the primary arteriole that was nearest to the central retina (baseline diameter: mean = 85.6 µm, range = 60.8–108.9 µm, n = 48), (2) pre-capillary arteriole: the branch from the large arteriole directly supplying the capillary network, which had the longest course in focus (baseline diameter: mean = 18.2 µm, range = 11.2–29.7 µm, n = 48), (3) capillary: the branch from the pre-capillary arteriole with the longest course in focus and with a diameter less than 10 µm (baseline diameter: mean = 5.5 µm, range = 2.8–9.3 µm, n = 48). The tissue chamber was positioned on a moveable table in the microscope that allowed the saving of the coordinates of the positions at the three different vessel branches. Each vessel position was recorded for approximately 30 seconds using a camera (DS-Qi1Mc-U3 8 bit; Nikon, Japan) through microscope objectives resulting in a magnification of ×4 (arterioles), ×10 (pre-capillary arterioles), and ×20 (capillaries). A diameter recording of all three branching levels in a preparation was performed within 5 minutes.
Protocols
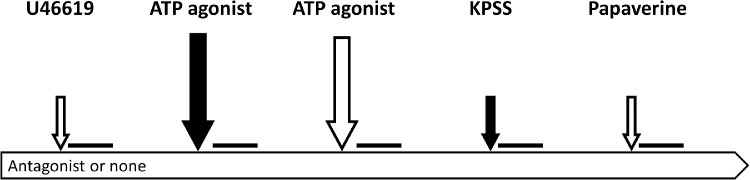
The sequence of events during an experiment is shown in Figure 2. In order to enhance the contrast of the vessel borders, PSS FITC was added intravascularly before each diameter recording, either together with vasoactive compounds or alone when vasoactive compounds were added extravascularly. Each step was followed by a diameter recording.
Figure 2.
The experimental protocol. White arrows indicate extravascular administration and solid arrows intravascular administration of vasoactive compounds that were followed by diameter recordings represented by the horizontal lines. Large arrows: Addition of the ATP agonist. Small arrows: Addition of compounds for establishing the reference diameters using U46619 (preconstriction), KPSS (viability and maximal constriction) and papaverine (maximal dilatation).
Six experimental series each consisting of eight individual experiments were performed. Two different protocols were used, one (protocol A) for the first two series and another (protocol B) for the remaining four series:
Protocol A
-
1.
Extravascular application of U46619 (10−5 mol/l) to obtain preconstriction,11 followed by equilibration for 10 minutes.
-
2.
Intravascular ATP agonist (10−4 mol/l), followed by equilibration for 2 minutes.
-
3.
Extravascular ATP agonist (10−4 mol/l), followed by equilibration for 2 minutes.
-
4.
Intravascular application of KPSS to obtain maximal vasoconstriction and test viability, followed by equilibration for 5 minutes. The vessel was considered to be nonviable if KPSS-induced constriction was < 10%.15
-
5.
Extravascular papaverin (10−4 mol/l) to determine the maximal vasodilatation, followed by equilibration for 10 minutes.
The protocol was repeated with ATP replaced by the nondegradable analogue ATP-γ-S.
Control Experiments
In two experiments, protocol A with ATP as agonist was repeated with steps 2 and 3 performed in the reverse order. This showed no influence of the order of intra- and extravascular application of ATP on the diameter response.
Protocol B
The steps in protocol A with ATP as agonist were repeated, except that either PPADS (10−5 mol/l), MRS 1523 (10−5 mol/l), ibuprofen (10−5 mol/l), or AOPCP (10−4 mol/l) was added to the fluids to be present throughout the experiment.
Data Analysis
The diameter recordings were replayed using the imaging software NIS-Elements AR (4.00 build 798; Nikon) and the image showing the sharpest delineation of each blood vessel was selected. The images were color inverted using IrfanView (version 4.37) and were uploaded to Matlab (version R2017b; Mathworks, Natick, MA, USA). The Automated Retinal Image Analyzer (ARIA) plug-in16,17 for Matlab was used to identify the vessel border and to calculate the vessel diameters at an interval of one pixel along the vessel, representing increments of 1.5 µm on arterioles, 0.6 µm on pre-capillary arterioles, and 0.3 µm on capillaries. Diameter values were collected from a distance of 25 pixels from the branching points defining the start and the end of the vessel, except for the starting point of larger vessels that was 25 pixels from the image frame (Fig. 3), and for pre-capillary arterioles and capillaries the end point could also be where the vessel came out of focus. The average number of diameter measurements collected along the vessel segments were for arterioles: mean (range): 843 (218–1280); pre-capillary arterioles: 527 (166–1244); and for capillaries: 339 (90–899). The diameter values were saved in Excel (Microsoft Excel 2016; Microsoft, Redmond, WA, USA) for further analysis.
Figure 3.
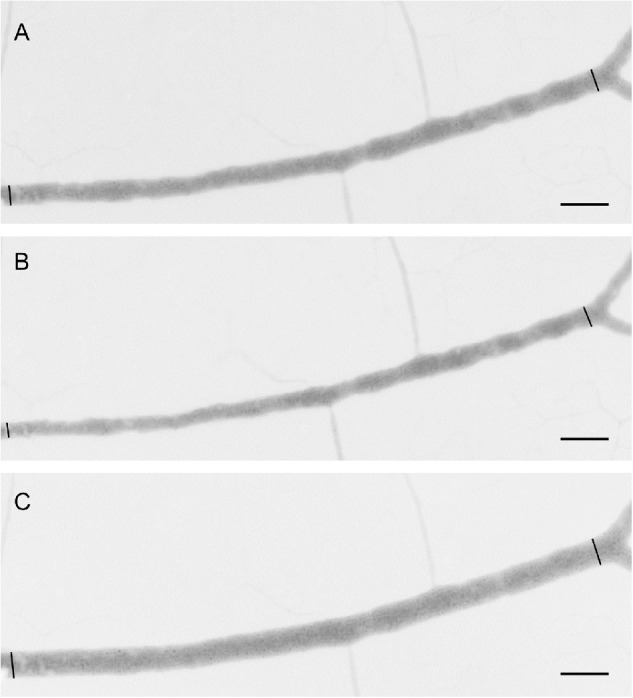
Representative example of a larger arteriole after application of (A) U46619 to induce preconstriction, (B) intravascular ATP, and (C) extravascular ATP. Diameter measurements (n = 1188) were obtained from a position 25 pixels from the image frame to the left and until a position 25 pixels from the following bifurcation. Bar = 100 µm.
For each experimental condition, the diameter of positions along the vascular segment were calculated as the average of five successive individual diameter measurements. At each position, the diameter was normalized using the diameter at maximum constriction induced by KPSS as 0% and the diameter at maximal dilatation with papaverine as 100% from the same position, and the normalized responses at all positions along the segment were averaged to represent the overall diameter response of that segment. The diameter responses of ATP were presented as the change in the diameter from the U46619-induced preconstriction. The effects of ATP-γ-S and ATP together with antagonists were shown with the response of ATP alone as reference.
Statistical Analysis
The analyses were performed using Excel and GraphPad Prism 8 (GraphPad Software Inc., La Jolla, CA, USA).
The Friedman test followed by the uncorrected Dunn's test was used to test if intra- and extravascular ATP resulted in a significant change of the diameter from the preconstriction level.
The Mann–Whitney test was used to test if the diameter response induced by ATP in the presence of each of the antagonists differed from the response of ATP alone as reference.
Results
Figure 3 shows an example of the diameter response of a larger retinal arteriole after preconstriction, and intravascular and extravascular application of ATP.
Figure 4 shows that intravascular application of ATP induced constriction whereas extravascular application of the compound induced dilatation of vessels at all three branching levels (P < 0.05 for all comparisons).
Figure 4.
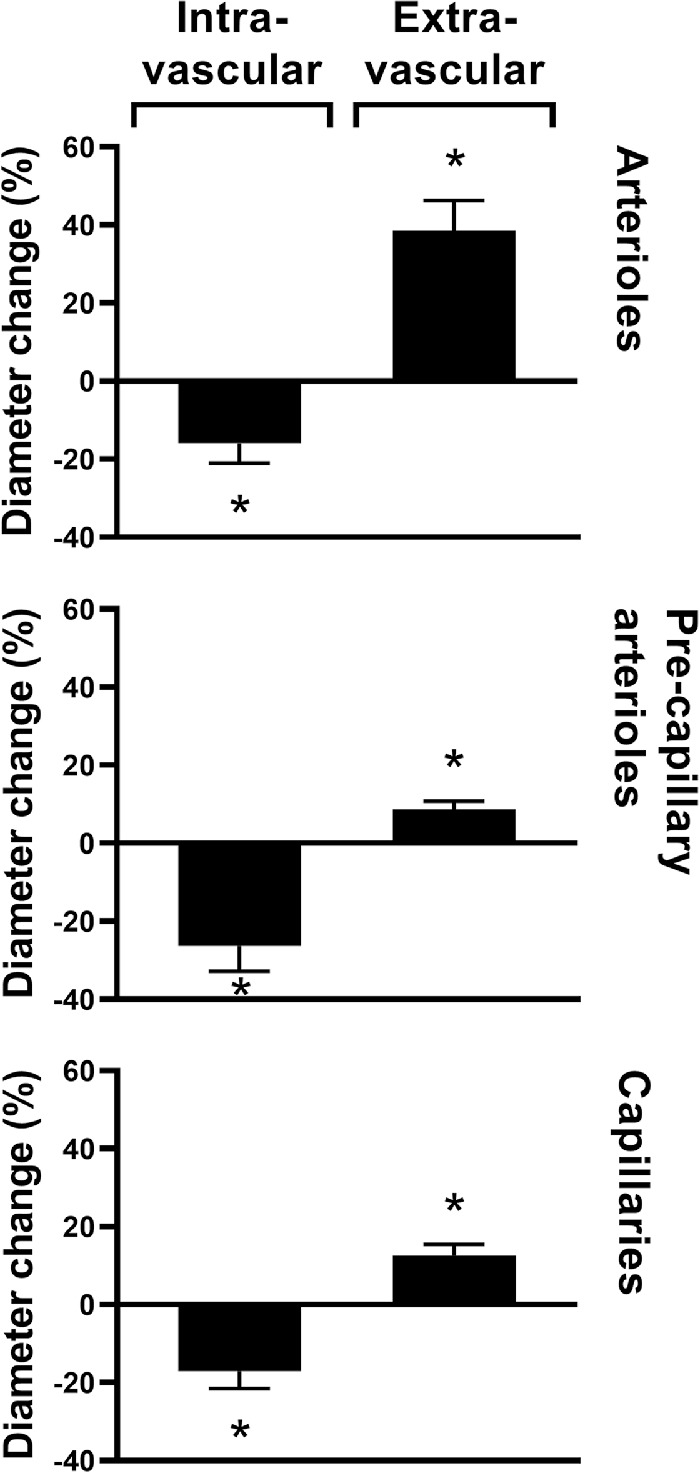
Changes in the diameters of the three studied vessel branches in percent (mean ± SEM) from preconstriction after intra- and extravascular application of ATP. Asterisks indicate P < 0.05 (n = 8 for all series).
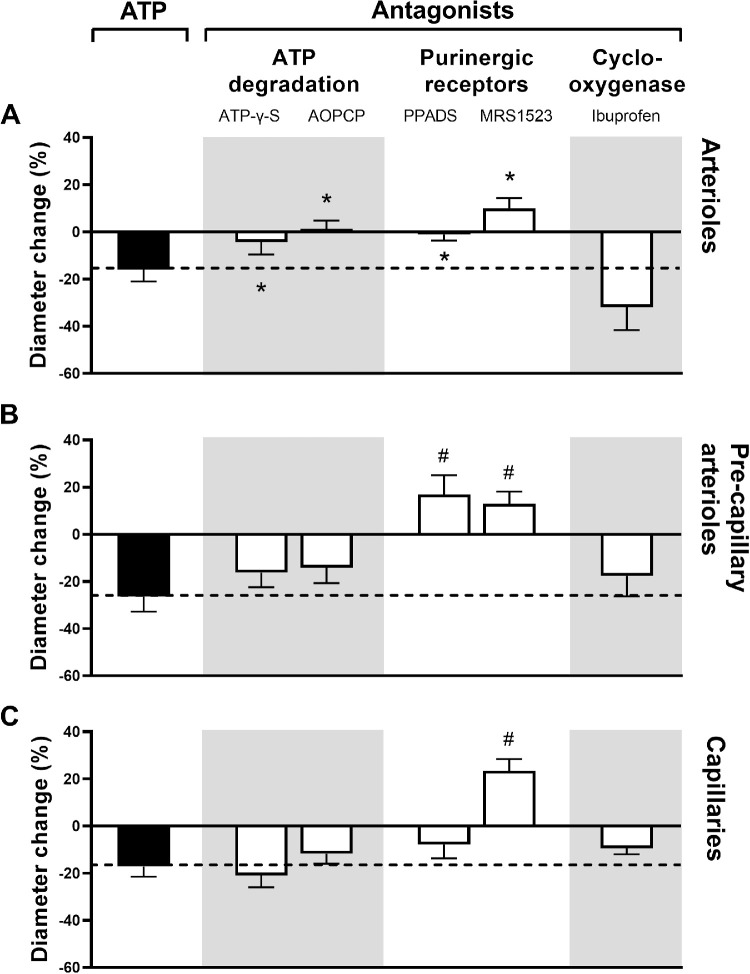
Figure 5 shows that constriction induced by intravascular ATP in arterioles was blocked by all antagonists (P < 0.04 for all comparisons) except for the COX inhibitor ibuprofen (P = 0.44), in the pre-capillary arterioles, the constriction was blocked by the A3 adenosine receptor antagonist MRS 1523 (P < 0.01) and the nonselective P2 purinoreceptor antagonist PPADS (P < 0.01), but was not significantly affected by the other antagonists (P > 0.19 for all comparisons), whereas the constriction of capillaries was blocked by the A3 adenosine receptor antagonist MRS 1523 (P < 0.01), but was not significantly affected by the other antagonists (P > 0.16 for all comparisons).
Figure 5.
The diameter change in percent (mean ± SEM) after intravascular application of ATP (solid columns), the level of which is continued with a dashed line in order to facilitate the interpretation of the effects of antagonists (white columns). Asterisks indicate P < 0.05 and pound signs P < 0.01 (n = 8 for all series).
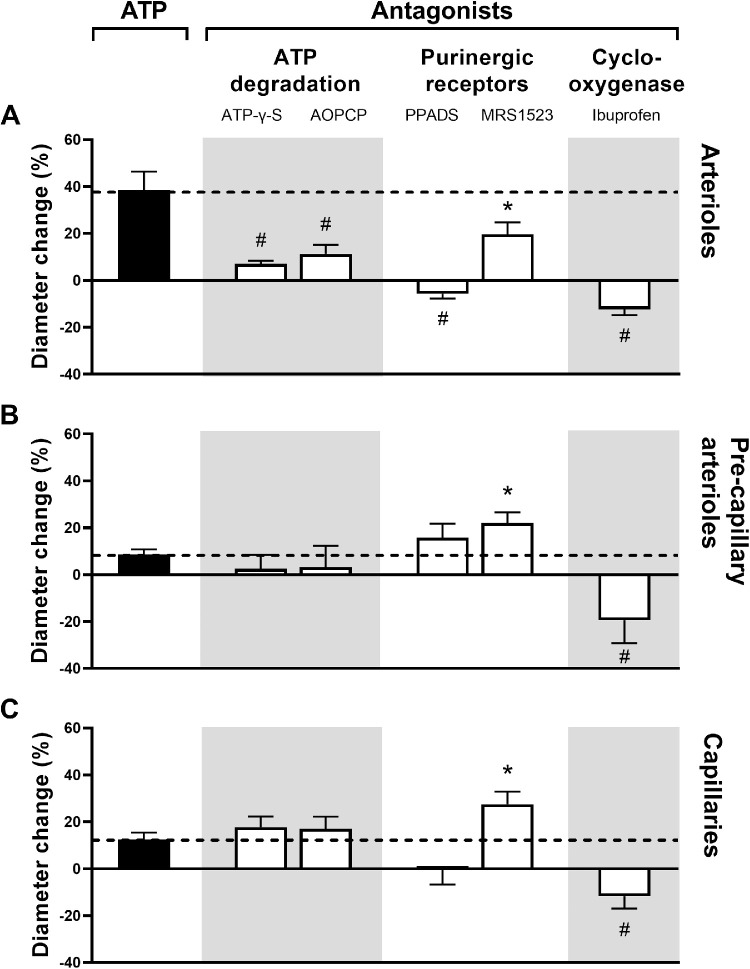
Figure 6 shows that dilatation induced by extravascular ATP in arterioles was significantly reduced by all antagonists (P < 0.05 for MRS 1523 and P < 0.01 for all other comparisons), in the pre-capillary arterioles, the dilatation was significantly antagonized by ibuprofen (P = 0.01) and significantly enhanced by MRS 1523 (P = 0.03), but was not significantly affected by the other antagonists (P > 0.23 for all comparisons), and in the capillaries, dilatation was blocked by ibuprofen (P < 0.01) and significantly enhanced by MRS 1523 (P < 0.05), but was not significantly affected by the other antagonists (P > 0.23).
Figure 6.
The diameter change in percent (mean ± SEM) after extravascular application of ATP (solid columns), the level of which is continued with a dashed line in order to facilitate the interpretation of the effects of antagonists (white columns). Asterisks indicate P < 0.05 and pound signs P < 0.01 (n = 8 for all series).
Discussion
The present study extends current knowledge of the vasoactive effects of ATP on retinal vessels by showing differential effects of the compound after intra- and extravascular application and different effects on retinal vessels at different branching level. The experimental conditions represented a simplification from the normal physiological situation because the retinal vessels were perfused with buffer instead of whole blood, the preparation was not in contact with the retinal pigment epithelium supplied by a choroidal circulation, and by the absence of normal neuronal activity induced by light stimulation. However, the experimental setup allowed the study of diameter changes in serially connected vessels from a hemiretinal segment during pharmacological interventions. This may have reflected elements of diameter regulation in retinal vessels that are active in vivo and constitutes a step on the path toward studying diameter regulation in the retinal vascular system in the living organism.
The fact that constriction of larger arterioles induced by intravascular ATP was reduced when ATP was substituted with the nondegradable ATP analogue and during blocking of ATP-degradation indicates that the constriction had been induced by a degradation product of ATP. Conversely, ATP induced constriction of pre-capillary arterioles and capillaries were unaffected by substitution of ATP with the nondegradable ATP analogue and by blocking of ATP-degradation. This suggests that the diameter response in these branches had been due to a direct effect of ATP, although it cannot be excluded that ATP degradation products had also contributed to the response. The constriction can be expected not to have involved COX products because the inhibition of prostaglandin synthesis with ibuprofen had no effect on the response. The inhibition of ATP-induced constriction at all three vascular branching levels in the presence of an A3 receptor antagonist is in accordance with previous findings that purinergic stimulation can constrict porcine retinal arterioles mediated by A3 receptors10 and shows that this response potential is more widely distributed in the vessels determining retinal vascular resistance. Conversely, the blocking of P2 purinoceptors could only antagonize ATP-induced constriction of arterioles and pre-capillary arterioles. This supports findings that circulating ATP can induce constriction of pre-capillary arterioles mediated by P2 receptors in the cerebral circulation.18 The absence of this antagonizing effect in capillaries as well as the fact that constriction of arterioles but not pre-capillary arterioles and capillaries depended on ATP degradation products supports the notion that ATP-induced constriction is mediated by different pathways at different vascular branching levels. This may be related to variations in the distribution of purinergic receptors throughout the vascular bed, as observed outside the eye.19 Purinergic receptors have been identified on endothelial cells in the rat kidney,20 and in the mouse aorta the contracting effect of ATP depends on this cell type.21 However, the compounds may also act directly on the vascular smooth muscle cells after passage of the purinergic agonist through the endothelial cell layer.22 Therefore, it is still unknown how the effect of intravascular ATP is transferred to vascular smooth cells to result in vasoconstriction. This should be the subject of further investigation.
The fact that the diameter responses of intra- and extravascular application of ATP were opposite is suggestive. The dilatation after extravascular application was blocked by ibuprofen, which indicates that a COX product originating from the abluminal side of the vessel had been involved in the response. This supports observations from myograph studies of porcine retinal arterioles where ATP-induced dilatation was shown to depend on the prostaglandin (PGE) synthesized in the perivascular retina.23 Similarly to the constriction of arterioles induced by intravascular ATP, the dilatation of these vessels induced by extracellular ATP depended on ATP degradation and stimulation of A3 and P2 receptors. The blocking of both constriction and dilatation by the same antagonist may be due to lack of specificity to receptor subtypes with opposite effects because the P2 receptor antagonist blocked both P2X and P2Y receptors.24
The finding supports previous observations that extravascular ATP can induce dilatation of retinal vessels that is mediated by P2 purinergic receptors.25 The source of extravascular ATP in vivo may be retinal Müller cells26 that may stimulate the release of PGEs with vasodilating effects.27,28 This is consistent with findings that ATP-induced dilatation of porcine retinal arterioles is preceded by activity in perivascular cells that connect the retinal arterioles with the perivascular retina.8 The vasodilating effect of extravascular ATP may explain that retinal arterioles are dilated in patients with diabetes29 in whom ATP has been found to be increased in the vitreous body3 and a particular role of A3 and P2 receptors is supported by the lack of effect on the diameter of retinal vessels of blocking other purinergic receptors in vivo.30
The findings of the present study may explain the observed dual effects of the ATP degradation product adenosine on porcine arterial segments studied in a myograph setup.10 Here, the dilatation induced when the purine was applied to the extravascular part of the excised vascular segment may have been supplemented with a constrictive effect when the compound could enter the vascular lumen from the open ends of the studied vascular segments. Differences in the balance between these constricting and dilating effects on retinal vessels may explain species variations so that extravascular application of ATP can induce constriction in the rat4,5 and cow,6,7 and dilatation in pigs.31
Dual effects of vasoactive compounds have previously been shown in the eye32 as well as in extraocular tissues,33,34 and may allow modulation of blood flow through the distribution of receptors mediating, respectively, constriction and dilatation. The present findings suggest that this principle participates in the regulation of retinal vascular diameters mediated by ATP. The finding might potentially be exploited for future therapies aimed at treating retinal diseases, such as diabetic retinopathy, where vascular dysfunction and disturbances in purinergic metabolism are involved in the disease pathogenesis.
Acknowledgments
Supported by Aarhus University, Fight for Sight Denmark, The Synoptik Foundation, The VELUX Foundation, Maskinfabrikant Jochum Jensen og hustru Marie Jensen's Foundation, and Isenkræmmer Christian Reventlow Poulsen's Foundation.
Disclosure: C. Ernst, None; P. Skov Jensen, None; C. Aalkjaer, None; T. Bek, None
References
- 1. Pournaras CJ, Rungger-Brändle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Pro Eye Res. 2008; 27: 284–330. [DOI] [PubMed] [Google Scholar]
- 2. Liou GI, Ahmad S, Naime M, Fatteh N, Ibrahim AS. Role of adenosine in diabetic retinopathy. J Ocul Biol Dis Inform. 2011; 4: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loukovaara S, Sahanne S, Jalkanen S, Yegutkin GG. Increased intravitreal adenosine 5′-triphosphate, adenosine 5′-diphosphate and adenosine 5′-monophosphate levels in patients with proliferative diabetic retinopathy. Acta Ophthalmol. 2015; 93: 67–73. [DOI] [PubMed] [Google Scholar]
- 4. Kawamura H, Sugiyama T, Wu DM, et al.. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2003; 551: 787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kur J, Newman EA. Purinergic control of vascular tone in the retina. J Physiol. 2014; 592: 491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ziganshina A, Zihanshin BA, Ziganshin AU. Dual effects of ATP on isolated arterioles of the bovine eye. Pharmacol Res. 2012; 66: 170–176. [DOI] [PubMed] [Google Scholar]
- 7. Das A, Frank RN, Weber ML, Kennedy A, Reidy CA, Mancini MA. ATP causes retinal pericytes to contract in vitro. Exp Eye Res. 1988; 46(3): 349–362. [DOI] [PubMed] [Google Scholar]
- 8. Misfeldt MW, Pedersen SM, Bek T. Perivascular cells with pericyte characteristics are involved in ATP- and PGE(2)-induced relaxation of porcine retinal arterioles in vitro. Invest Ophthalmol Vis Sci. 2013; 54: 3258–3264. [DOI] [PubMed] [Google Scholar]
- 9. Riis-Vestergaard MJ, Bek T. Purinergic mechanisms and prostaglandin E receptors involved in ATP-induced relaxation of porcine retinal arterioles in vitro. Ophthalmic Res. 2015; 54(3): 135–142. [DOI] [PubMed] [Google Scholar]
- 10. Riis-Vestergaard MJ, Misfeldt MW, Bek T. Dual effects of adenosine on the tone of porcine retinal arterioles in vitro. Invest Ophthalmol Vis Sci. 2014; 55(3): 1630–1636. [DOI] [PubMed] [Google Scholar]
- 11. Skov Jensen P, Pedersen SMM, Aalkjaer C, Bek T. The vasodilating effects of insulin and lactate are increased in precapillary arterioles in the porcine retina ex vivo. Acta Ophthalmol. 2016; 94(5): 454–462. [DOI] [PubMed] [Google Scholar]
- 12. Puro DG. Retinovascular physiology and pathophysiology: new experimental approach/new insights. Prog Retin Eye Res. 2012; 31(3): 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skov Jensen P, Aalkjaer C, Bek T. Differential effects of nitric oxide and cyclo-oxygenase inhibition on the diameter of porcine retinal vessels with different caliber during hypoxia ex vivo. Exp Eye Res. 2017; 160: 38–44. [DOI] [PubMed] [Google Scholar]
- 14. Skov Jensen P, Aalkjaer C, Bek T. The vasodilating effect of glucose differs among vessels at different branching level in the porcine retina ex vivo. Exp Eye Res. 2019; 179: 150–156. [DOI] [PubMed] [Google Scholar]
- 15. Torring MS, Aalkjaer C and Bek T. Constriction of porcine retinal arterioles induced by endothelin-1 and the tromboxane analogue U46619 in vitro decreases with increasing vascular branching level. Acta Ophthalmol. 2014; 92: 232–237. [DOI] [PubMed] [Google Scholar]
- 16. Bankhead P. ARIA - Retinal Vessel Detection Using MATLAB. Available at: http://petebankhead.github.io/ARIA/. Last Accessed August 30, 2016.
- 17. Bankhead P, Scholfield CN, McGeown JG, Curtis TM. Fast retinal vessel detection and measurement using wavelets and edge location refinement. PLoS One. 2012; 7: e32435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewis CJ, Ennion SJ, Evans RJ. P2 purinoceptor-mediated control of rat cerebral (pial) microvasculature; contribution of P2X and P2Y receptors. J Physiol. 2000; 527(2): 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gitterman DP, Evans RJ. Properties of P2X and P2Y receptors are dependent on artery diameter in the rat mesenteric bed. Br J Pharmacol. 2000; 131(8): 1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tölle M, Jankowski V, Schuchardt M, et al.. Adenosine 5'-tetraphosphate is a highly potent purinergic endothelium-derived vasoconstrictor. Circ Res. 2008; 103(10): 1100–1108. [DOI] [PubMed] [Google Scholar]
- 21. Ansari HR, Nadeem A, Tilley SL, Mustafa SJ. Involvement of COX-1 in A3 adenosine receptor-mediated contraction through endothelium in mice aorta. Am J Physiol Heart Circ Physiol. 2007; 293(6): H3448–H3455. [DOI] [PubMed] [Google Scholar]
- 22. Burnstock G & Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2013; 66: 102–192. [DOI] [PubMed] [Google Scholar]
- 23. Holmgaard K & Bek T. ATP-induced relaxation of porcine retinal arterioles in vitro depends on prostaglandin E synthesized in the perivascular retinal tissue. Invest Ophthalmol Vis Sci. 2010; 51: 5168–5175. [DOI] [PubMed] [Google Scholar]
- 24. Jarvis MF. The RBI handbook of receptor classification and signal transduction. Drug Dev. Res. 1996; 37(4): 259–260. Web. [Google Scholar]
- 25. Newman EA. Glia Retinal, Squire LR. Encyclopedia of Neuroscience. Amsterdam: Elsevier Ltd; 2009; 8: 225–232. [Google Scholar]
- 26. Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Müller cells. J Neurosci. 2001; 21: 2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crecelius AR, Kirby BS, Richards JC, et al.. Mechanisms of ATP-mediated vasodilation in humans: modest role for nitric oxide and vasodilating prostaglandins. Am J Physiol Heart Circ Phyciol. 2011; 301: H1302–H1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newman EA. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Phil Trans R Soc B. 2015; 370: 20140195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tilma KK, Bek T.. Topical treatment for 1 week with latanoprost but not diclofenac reduces the diameter of dilated retinal arterioles in patients with type 1 diabetes mellitus and mild retinopathy. Acta Ophthalmol. 2012; 90(8): 750–755. [DOI] [PubMed] [Google Scholar]
- 30. Dons-Jensen Petersen L, Bøtker H-E, Bek T. The diameter of retinal arterioles is unaffected by intravascular administration of the adenosine A2A receptor agonist regadenoson in normal persons. Biomed Hub. 2019; 4: 500563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Misfeldt MW, Aalkjaer C, Simonsen U, Bek T. Novel cellular bouton structure activated by ATP in the vascular wall of porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2010; 51(12): 6681–6687. [DOI] [PubMed] [Google Scholar]
- 32. Kringelholt S, Simonsen U, Bek T. Dual effect of prostaglandins on isolated intraocular porcine ciliary arteries. Acta Ophthalmol. 2013; 91: 498–504. [DOI] [PubMed] [Google Scholar]
- 33. Astin M. Effects of prostaglandin E2, F2alpha, and latanoprost acid on isolated ocular blood vessels in vitro. J Ocul Pharmacol Ther. 1998; 14: 119–128. [DOI] [PubMed] [Google Scholar]
- 34. Vysniauskiene I, Allemann R, Flammer J, Haefliger IO. Vasoactive responses of U46619, PGF2alpha, latanoprost, and travoprost in isolated porcine ciliary arteries. Invest Ophthalmol Vis Sci. 2006; 47: 295–298. [DOI] [PubMed] [Google Scholar]