Abstract
Purpose
The sunset glow fundus (SGF) appearance in Vogt-Koyanagi-Harada (VKH) disease was evaluated by means of adaptive binarization of patients’ fundus photographs.
Methods
Twenty-nine Japanese patients with acute VKH were enrolled in this study. We evaluated one eye of each patient, and thereby divided the patients into two groups; SGF+ and SGF− at 6 months after treatment. We compared patient age, gender, and spherical equivalent refractive error (SERE) and choroidal thickness measured using optical coherence tomography. We also compared the choroidal vascular appearance index (CVAI), derived by adaptive binarization image processing of fundus photographs, between the two groups. Measurements of choroidal thickness and CVAI were taken at the onset of disease, and 1, 3, and 6 months after treatment. The sunset glow index (SGI), as previously reported, was calculated using color fundus photographs, and compared to the CVAI.
Results
Eight patients (27.6%) were categorized into the SGF+ group. At all time points, the mean CVAI in the SGF+ group was significantly greater than that in the SGF− group. No significant difference was observed in choroidal thicknesses at any time point. The SGI was significantly greater in the SGF+ group at 6 months.
Conclusions
CVAI could be a new predictive biomarker for the development of SGF in patients with VKH disease.
Translational Relevance
Detecting SGF is important for management of patients with VKH, and CVAI may indicate the possibility of developing into SGF, although the color fundus photographs do not yet show SGF at that time.
Keywords: Vogt-Koyanagi-Harada disease, sunset glow fundus, adaptive binarization, uveitis
Introduction
Vogt-Koyanagi-Harada (VKH) is a systemic autoimmune disorder that affects organs, including the eyes, that have melanocytes in their tissues.1 In some patients with VKH, progression into chronic recurrences of granulomatous uveitis occurs because depigmentation of the choroid results from choroidal melanocyte damage.2,3 In those cases, the fundus shows orange discoloration called “sunset glow fundus (SGF)”.1,4 There are few objective measures of SGF. The first one is the sunset glow index (SGI), which was reported by Suzuki, and is an objective evaluation of the color balance in color fundus photographs.5 The second measure is polarization-sensitive optical coherence tomography (PS-OCT), which was reported by Miura et al., and can provide an in vivo objective evaluation of choroidal melanin loss underlying the SGF in chronic VKH disease.6 Unfortunately, PS-OCT is not a widely used form of optical coherence tomography (OCT) and thus it is not an option at many facilities.
Although ocular inflammation, progressing subclinically in chronic VKH disease with SGF, is sight-threatening, detection of such progression is still challenging. For example, Keino et al. reported that 67.5% of patients showed SGF, although chronic clinical ocular inflammation was apparent in only 17.5% of the patients.7 Murata et al. have reported that signs of subclinical ocular inflammation were detected in most patients with VKH with SGF by indocyanine green angiography.8 As an alternative, early post-treatment choroidal thickness was reported to be an index to alert physicians to future progression to SGF.9
Recently, we defined the choroidal vascular appearance index (CVAI) as the visibility of choroidal vessels in color fundus photographs, based on adaptive binarization imaging processing.10 Our previous study indicated that the CVAI was correlated with choroidal thickness. In this study, we investigated whether the CVAI adds specific information to the SGF, like the SGI does, and whether the progression of SGF can be predicted by evaluating the CVAI.
The aim of this study was to investigate how CVAI changes with choroidal thickness, and how SGF progresses because of decreasing melanin in patients with VKH disease, to determine whether CVAI could be a predictive biomarker for the development of SGF.
Methods
Study Design and Eligibility
Between January 2016 and May 2019, a total of 29 Japanese patients with acute VKH were enrolled in this retrospective, observational study. We excluded recurrent cases, patients suspected of having a recurrent case with SGF at the initial visit, and cases with strong anterior chamber inflammation. The current study was performed in accordance with the Declaration of Helsinki and with approval from the ethics committees of Hyogo College of Medicine (No. 2426).
Study Protocol
The data we extracted from medical records included patient age, gender, and spherical equivalent refractive error (SERE). Measurements of choroidal thickness, SGI, and the CVAI were taken at the onset of disease, and 1, 3, and 6 months after treatment. The diagnosis of VKH was made in accordance with the revised criteria proposed by the International Nomenclature Committee.11 We used the data for the right eye of each patient. No patients underwent intraocular surgery. All patients were treated with systemic corticosteroids from the disease onset, starting with steroid pulse therapy.
Measurement of CVAI
As introduced above, the CVAI is the choroidal vasculature appearance index of the binarization image from color fundus pictures.1 Briefly, CVAI = CVR/IR × 1000, where CVR is the number of pixels of the choroidal blood vessel region and IR is the number of pixels in the imaging region. CVAI was calculated using original software and we confirmed the repeatability of the software results. A total of 116 color fundus photographs and 116 OCT images were evaluated.
We used color fundus photographs (TRC-50DX, Topcon, Tokyo, Japan) with similar brightness, contrast, and color balance characteristics. Choroidal thickness under the fovea was measured with the enhanced depth-imaging technique on an OCT device (Spectralis, Heidelberg, Germany).12,13 Choroidal thickness that could not be measured because the inner scleral border was not visible on OCT (over 800 µm) was set to 800 µm. As a result, the choroidal thickness was set to 800 µm in 19 images (14.7%).
Measurement of the Sunset Glow Index
To compare the color balance of the fundus photographs, we calculated the SGI, as in the previous studies.5,6,14 The formula is as follows: SGI = Lred / (Lred + Lgreen + Lblue), where Lred, Lgreen, and Lblue represent the mean luminance of the red, green, and blue (R, G, and B) channels, respectively.
Study End Points
The primary end point was the difference in the CVAI between the fundus pictures from patients with and without SGF. All eyes were classified into one of two groups, SGF+ and SGF−, based on the fundus photographs at 6 months post-treatment. Photographs of each eye were individually evaluated by two blinded observers (Y.K. and H.F.) regarding the presence of SGF. When the two observers disagreed, a third (H.I.) judged the photograph and the three observers together reached a consensus. Comparisons of CVAI with choroidal thickness and SGI, between the two groups at each time point, were secondary end points. We also assessed the baseline characteristics of all patients.
Statistical Analyses
For continuous variables, the mean, median, and range were calculated. For discrete variables, the number of values in each category and the percentages in each category were calculated. Group differences were assessed using the Student's t-test or Wilcoxon signed-rank test for continuous variables and Fisher's extract test or the Pearson χ2 test for categorical variables.
We assessed the accuracy of each screening test using receiver operating characteristic (ROC) curve analysis. ROC analysis provides several advantages over sensitivity and specificity determination for a single cutoff. The formula for calculation of the “true positive rate” was true positive/(true positive + false negative); the formula for calculation of the “false positive rate” was false positive/(false positive + true negative). Calculating the area under the ROC curve (AUC) provides a summary of discriminative ability for a screening test and allows quick comparison of discriminative ability among different screening tests. The AUC has a value from 0.5 to 1.0, where 1.0 represents perfect ability to discriminate between, for example, children without vision disorders and children with vision disorders, and 0.5 represents discrimination resulting from pure chance.15 An AUC greater than 0.9 is considered excellent; greater than 0.8 through 0.9 is very good; 0.7 to 0.8 is good; 0.6 to 0.7 is average, and less than 0.6 is considered poor.16 Analyses were performed with JMP Pro (version 14.0.0; SAS Institute Inc., Cary, NC). For all analyses, P values were reported, as well as two-sided 95% confidence intervals for point estimates. Statistical significance was determined when P values were < 0.05.
Results
Baseline Characteristics
Altogether, we used 116 fundus photographs and OCT images from 29 patients with VKH (mean age of 50.7 ± 18.8 years, range = 13–81 years). All patients had characteristic symptoms of VKH in both eyes. Of the 29 patients, 8 (27.6%) showed SGF. Baseline characteristics, age, SERE, choroidal thickness, CVAI, and SGI in all eyes for the SGF− group and SGF+ group are shown in the Table. Briefly, no significant differences in any parameters were observed between the SGF− and SGF+ groups at baseline.
Table.
Baseline Characteristics of Patients With or Without Sunset Glow Fundus (SGF)
| Total | SGF− Group (n = 21) | SGF+ Group (n = 8) | SGF− Versus SGF+ P Value | |
|---|---|---|---|---|
| Age, y | 50.7 ± 18.8 | 54.3 ± 17.6 | 41.3 ± 19.7 | 0.09 |
| SERE, D | −1.13 ± 3.24 | −0.69 ± 2.55 | −2.28 ± 4.61 | 0.24 |
| Choroidal thickness, µm | 693 ± 130 | 698 ± 132 | 679 ± 133 | 0.74 |
| CVAI, % | 0.041 ± 0.022 | 0.040 ± 0.024 | 0.046 ± 0.019 | 0.549 |
| SGI | 0.59 ± 0.048 | 0.59 ± 0.050 | 0.59 ± 0.040 | 0.89 |
CVAI, choroidal vascular appearance index; SERE, spherical equivalent refractive error; SGI, sunset glow index.
Primary End Point: CVAI in Patients With and Without SGF
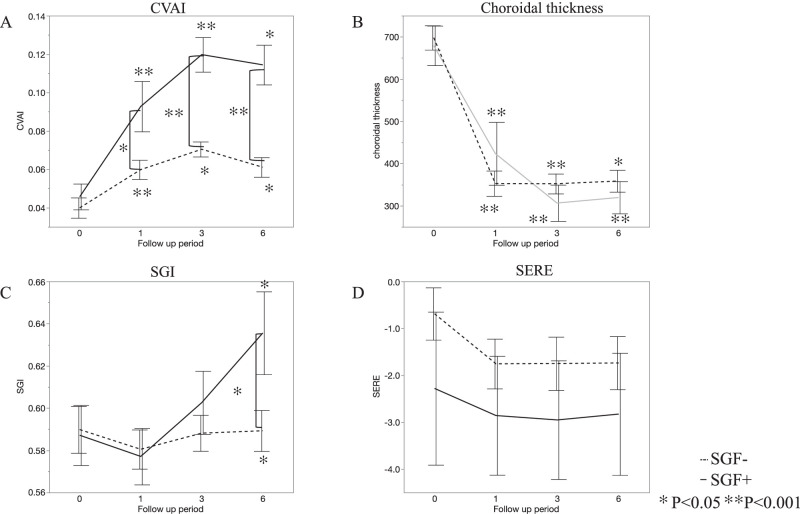
The mean CVAI was significantly greater at 1, 3, and 6 months than at baseline in both of the SGF− and SGF+ groups (P < 0.0001, P = 0.0028, and P = 0.0047 in the SGF− group, and P < 0.0001, P < 0.0001, and P = 0.0026 in the SGF+ group, respectively). The mean CVAI values in the SGF+ group at 1, 3, and 6 months were significantly greater than those in the SGF− group (P = 0.072, P < 0.0001, and P < 0.0001, respectively, Fig. 1A).
Figure 1.
Changes in the choroidal vascular index (CVAI), choroidal thickness, sunset glow index (SGI) and spherical equivalent refractive error (SERE) in the SGF− and SGF+ groups. (A) The mean CVAI was significantly elevated at all post-treatment examinations in both groups, compared with baseline. The mean CVAI in the SGF+ group at 1, 3, and 6 months was significantly greater than that in the SGF− group (P = 0.072, P < 0.0001, and P < 0.0001, respectively). (B) The mean choroidal thickness decreased significantly from baseline across time in both groups; however, no significant differences were found between the SGF− and SGF+ groups. (C) The mean SGI did not change significantly from baseline at 1, 3 or 6 months in the SGF− group. However, it was significantly greater at 6 months than at baseline in the SGF+ group and was significantly greater than that in the SGF− group at that time. (D) The mean SERE did not change significantly from baseline at any point in either the SGF− or SGF+ groups. Additionally, no significant difference was found between the groups at any point.
Typical color fundus photographs, the corresponding adaptive binarization images, basic picture for CVAI, histograms of pixel values for each channel of the RGB image, raw data for SGI, and OCT images are shown in Figure 2. The extent of the whitish area in the adaptive binarization images from the SGF+ group tended to exceed that in adaptive binarization images from the SGF− group at 1, 3, and 6 months. SGI showed no change during the follow-up period in the SGF– group, but significantly increased (P = 0.038) at 6 months in the SGF + group. In the histograms, each channel of the RGB image (excluding the frame part) is shown. OCT images in both groups showed rapid resolution of the retinal detachments after the therapy.
Figure 2.
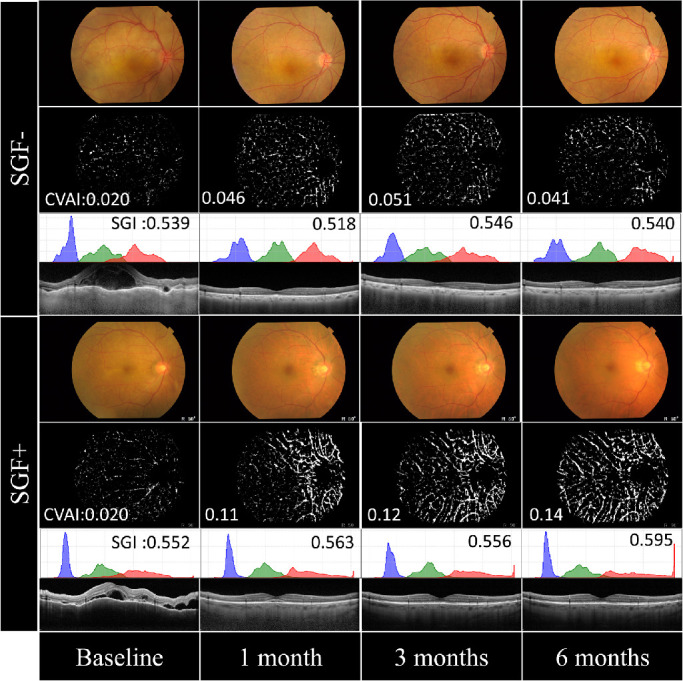
Typical color fundus photographs and corresponding adaptive binarization images, histograms of pixel values and optical coherence tomography (OCT) images. Color fundus photographs and corresponding adaptive binarization images, histograms of pixel values and OCT images from an SGF− patient (upper) and an SGF+ patient (lower). There was no change in appearance across time in the adaptive binarization images from the SGF− patient. However, the whitish area in the adaptive binarization images from the SGF+ patient tended to increase during the follow-up period. The histograms show each channel of the RGB image (excluding the frame part), and the x- and y-axes are drawn on the same scale used in the other panels. In the OCT images, subretinal detachment greatly improved after therapy.
Secondary End Points
Choroidal Thickness, SGI, and SERE With and Without SGF
The mean choroidal thickness was significantly thinner at 1 (353 µm), 3 (352 µm), and 6 months (359 µm) post-treatment than at baseline (697 µm; P < 0.0001 for each time) in the SGF− group, and at 1 (424 µm), 3 (306 µm), and 6 months (320 µm) post-treatment than at baseline (679 µm) in the SGF+ group (P < 0.0001, P < 0.0001, and P = 0.0018, respectively). No significant difference was found between the SGF− and SGF+ groups at any point (baseline, 1, 3, and 6 months; P = 0.74, P = 0.30, P = 0.33, and P = 0.42, respectively, Fig. 1B).
The mean SGI did not differ significantly between its baseline (0.590) and the values at 1 month (0.580), 3 months (0.588), and 6 months (0.589) in the SGF− group (P = 0.50, P = 0.90, and P = 0.97, respectively). However, the SGI was significantly greater at 6 months (0.636) than at baseline (0.587) in the SGF+ group (P = 0.038). No significant difference at baseline, or 1 and 3 months was found between the SGF− and SGF+ groups (P = 0.85, P = 0.84, and P = 0.39, respectively), but that at 6 months (0.64) in the SGF+ group was significantly greater than that (0.59) in the SGF− group (P = 0.027; Fig. 1C).
The mean SERE did not change significantly from baseline at any time point in either the SGF− or SGF+ group, and no significant difference was observed between the two groups at any time (Fig. 1D).
ROC Curves and AUCs Between CVAI and SGI
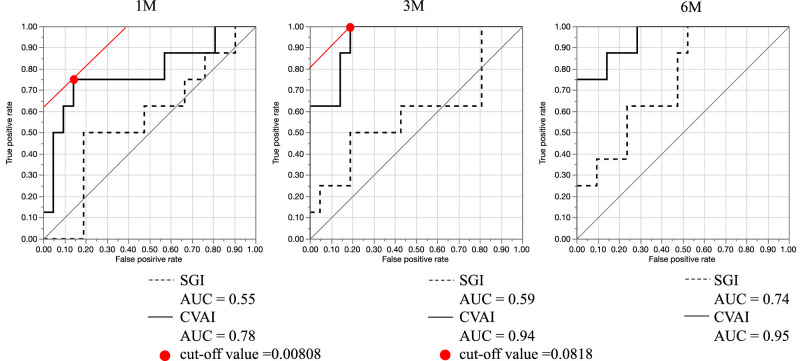
The AUCs for CVAI ROC curves were 0.78 at 1 month, 0.94 at 3 months, and 0.95 at 6 months. For the SGI, the corresponding AUCs were 0.55 at 1 month, 0.59 at 3 months, and 0.74 at 6 months, indicating that the CVAI was more sensitive at all time points.
Furthermore, on the basis of the Youden index, the cutoff value for CVAI that later developed into SGF was 0.0808 at 1 month and 0.0818 at 3 months (Fig. 3).
Figure 3.
Receiver operating characteristic (ROC) curves and areas under the curve (AUCs) obtained from CVAI and sunset glow index (SGI) in patients with or without sunset glow fundus (SGF). ROC curves and AUC values based on the choroidal vasculature appearance index (CVAI) and sunset glow index (SGI) in patients with or without SGF at 1 month (left), 3 months (middle), and 6 months (right) after treatment. The red dots show the cutoff values.
Discussion
This study demonstrated that the CVAI was greater in the SGF+ group than in the SGF− group at 1, 3, and 6 months after treatment, despite no significant difference in choroidal thickness being found between the SGF− and SGF+ groups at those time points, and no significant difference in SGI at 1 and 3 months. Therefore, the CVAI could be the most sensitive marker to detect SGF in the early phase of VKH disease.
The appearance of SGF is associated with thinning of the choroid during the convalescent phase of VKH disease.17 In the convalescent phase, depigmentation of the choroids occurs 2 to 6 months after disease onset.18 The convalescent stage with SGF was reportedly characterized by mild-to-moderate inflammatory cell infiltration.4 Fong et al. suggested that stromal scarring causes shrinkage and dropout of small vessels in the choroid in the convalescent stage.19 Nakai et al. reported that SGF occurs because, in addition to the loss of melanocytes, tissue damage after inflammation can result in tissue necrosis, fibrosis, and, finally, choroidal thinning.3,17 However, in the present study, no significant difference in choroidal thickness was found between the SGF− and SGF+ groups. Hirooka et al. reported that the mean central choroidal thickness at 1 week after the start of treatment was significantly greater in eyes with SGF than in eyes without SGF, and that the cutoff choroidal thickness value at 1 week (to signal future progression to SGF) was 410 µm.20 In the present study, although no significant difference was found, the mean choroid thickness in the SGF+ group was greater at 1 month (424 vs. 353 µm) than in the SGF− group, and smaller at 3 months (306 vs. 352 µm) and 6 months (319 vs. 359 µm). These results suggest that spontaneous inflammation and thick choroid in the early phase after the start of treatment may be a risk factor for progression to SGF. Therefore, earlier detection of the SGF or earlier prediction of progression to SGF could lead to a reduction in chronic inflammation, by promoting early treatments.
Regarding the diagnosis of SGF, according to a previous study, the mean SGI was 0.649 in SGF+ and 0.578 in SGF−.6 Here, the mean SGI was 0.636 in the SGF+ group and 0.589 in the SGF−. Furthermore, the CVAI differed earlier between the SGF− and SGF+ groups than did the SGI. ROC curve analyses and AUCs indicated that the CVAI was a good biomarker at 1 month and an excellent biomarker at 3 and 6 months, whereas SGI was poor at 1 and 3 months, and only a good biomarker at 6 months. Therefore, as a predictive biomarker, CVAI is more sensitive than SGI in the early stage of VKH disease. In the evaluation of CVAI, each color fundus photograph was separated into 8-bit RBG (red, green, blue) components, and the R-component image was used in the detection of choroidal vessels. The R component was also used for SGI calculation, however, not to evaluate the choroidal vessels but rather the redness of the color fundus photographs, as determined by both the choroidal and retinal vessels. Therefore, we propose that during the development of the SGF, choroidal vascular-related changes occur before the fundus becomes reddish. The orange-red discoloration of SGF is due to depigmentation of the inflamed choroid,18 and the choroidal stroma contains a large number of melanocytes with melanin.21 Miura et al. provided objective evaluation of melanin loss in chronic VKH disease by using PS-OCT, and eyes with SGF showed a lack of choroidal melanin and the preservation of melanin in the retinal pigment epithelium.6 Thus, the decrease in melanin content may increase the transparency of choroidal vessels. We suggest that in the process of decreasing choroidal melanin, the medium and large choroidal vessels are the first to become more visible, and second that redness develops because of further thinning of the choroidal stroma and severe decrease in melanin, causing the CVAI to differ between the SGF− and SGF+ groups before SGI differences occur. Furthermore, of 8 patients with SGF, only 1 developed the SGF at 3 months after treatment, but the other 7 patients progressed to the SGF by 6 months. As described above, CVAI in SGF+ patients already tended to be greater than in SGF− patients by 1 or 3 months. Therefore, CVAI may indicate the possibility of developing into SGF, although the color fundus pictures do not yet show any SGF at that time. Moreover, CVAI changed more over time than did SGI. CVAI was approximately 2.6 times greater at 3 months (0.12 ± 0.026) and 2.5 times greater at 6 months (0.11 ± 0.029) than it was at baseline (0.046 ± 0.019), whereas SGI was only approximately 10% greater at 6 months (0.64 ± 0.055) than at baseline (0.59 ± 0.040).
The results showed that the mean SERE was smaller in the SGF+ group than in the SGF−, although no significant difference was found. It has been reported that the choroid thins with aging and with myopic degeneration,22,23 because choroidal thickness was significantly correlated with the degree of tessellation,14 and thinning of the choroid is associated with progression to SGF.17 It was reported that eyes with significant myopic progression developed severe SGF within 4.3 ± 3.1 years, and the mean refractive error was −3.17 diopter (D) in progression of myopia in > −1.0 D and −2.89 D in no progression myopia group and no significant difference was found.24 Additionally, no significant difference was observed in our short follow-up study, thus we think that a smaller SERE may be a risk factor leading to SGF, and that myopia progresses over a long interval.
This study had several limitations. First, it was a retrospective study and conducted at a single center, with a relatively small number of patients and the number of SGF+ patients was particularly small (all of whom were Japanese). Second, the broad range of ages (13–81 years old) of the included patients was a limitation because choroidal thickness is strongly influenced by age. Third, in this study, adaptive binarization was used for image processing. Unlike the normal binarization, adaptive binarization determines whether a certain pixel is assigned a 0 or 1 based on peripheral pixel values. Normally, choroidal blood vessels have a higher pixel value than the retina, thus it was possible to extract the choroidal vessels only by adaptive binarization. However, when the pixel values became higher overall, due to progression of the SGF, choroidal vessels may not be detected accurately by adaptive binarization. Therefore, a long-term observational study is needed to evaluate the progression of the SGF.
In conclusion, CVAI could be a novel predictive biomarker to indicate the development of SGF in patients with VKH disease.
Acknowledgments
The authors thank Claire Barnes, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Disclosure: Y. Komuku, None; H. Ishikawa, None; A. Ide, None; T. Matsuoka, None; H. Fukuyama, None; T. Okadome, None; F. Gomi, None
References
- 1. O'Keefe GAD, Rao NA. Vogt-Koyanagi-Harada disease. Surv Ophthalmol. 2017; 62: 1–25. [DOI] [PubMed] [Google Scholar]
- 2. Rao NA. Pathology of Vogt-Koyanagi-Harada disease. Int Ophthalmol. 2007; 27: 81–85. [DOI] [PubMed] [Google Scholar]
- 3. Inomata H, Sakamoto T.. Immunohistochemical studies of Vogt-Koyanagi-Harada disease with sunset sky fundus. Curr Eye Res. 1990; 9(Suppl): 35–40. [DOI] [PubMed] [Google Scholar]
- 4. Read RW, Rechodouni A, Butani N, et al.. Complications and prognostic factors in Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2001; 131: 599–606. [DOI] [PubMed] [Google Scholar]
- 5. Suzuki S. Quantitative evaluation of “sunset glow” fundus in Vogt-Koyanagi-Harada Disease. Jpn J Ophthalmol. 1999; 43: 327–333. [DOI] [PubMed] [Google Scholar]
- 6. Miura M, Makita S, Yasuno Y, et al.. Polarization-sensitive optical coherence tomographic documentation of choroidal melanin loss in chronic Vogt-Koyanagi-Harada disease. Investig Ophthalmol Vis Sci. 2017; 58: 4467–4476. [DOI] [PubMed] [Google Scholar]
- 7. Keino H, Goto H, Usui M. Sunset glow fundus in Vogt-Koyanagi-Harada disease with or without chronic ocular inflammation. Graefe's Arch Clin Exp Ophthalmol. 2002; 240: 878–882. [DOI] [PubMed] [Google Scholar]
- 8. Murata T, Sako N, Takayama K, et al.. Identification of underlying inflammation in Vogt-Koyanagi-Harada disease with sunset glow fundus by multiple analyses. J Ophthalmol. 2019; 2019: 3853794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirooka K, Saito W, Namba K, et al.. Early post-treatment choroidal thickness to alert sunset glow fundus in patients with Vogt-Koyanagi-Harada disease treated with systemic corticosteroids. Wallace GR, ed. PLoS One. 2017; 12: e0172612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Komuku Y, Ide A, Fukuyama H, et al.. Choroidal thickness estimation from colour fundus photographs by adaptive binarisation and deep learning, according to central serous chorioretinopathy status. Sci Rep. 2020; 10: 5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Read RW, Holland GN, Rao NA, et al.. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: Report of an international committee on nomenclature. Am J Ophthalmol. 2001; 131: 647–652. [DOI] [PubMed] [Google Scholar]
- 12. Spaide RF, Koizumi H, Pozonni MC, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146: 496–500. [DOI] [PubMed] [Google Scholar]
- 13. Margolis R, Spaide RF.. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147: 811–815. [DOI] [PubMed] [Google Scholar]
- 14. Yoshihara N, Yamashita T, Ohno-Matsui K, Sakamoto T. Objective analyses of tessellated fundi and significant correlation between degree of tessellation and choroidal thickness in healthy eyes. PLoS One. 2014; 9(7): e103586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanley JA, McNeil BJ.. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982; 143: 29–36. [DOI] [PubMed] [Google Scholar]
- 16. Choi BCK. Slopes of a receiver operating characteristic curve and likelihood ratios for a diagnostic test. Am J Epidemiol. 1998; 148: 1127–1132. [DOI] [PubMed] [Google Scholar]
- 17. Nakai K, Gomi F, Ikuno Y, et al.. Choroidal observations in Vogt-Koyanagi-Harada disease using high-penetration optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2012; 250: 1089–1095. [DOI] [PubMed] [Google Scholar]
- 18. Goto H, Rao NA. Sympathetic ophthalmia and Vogt-Koyanagi-Harada syndrome. Int Ophthalmol Clin. 1990; 30: 279–285. [DOI] [PubMed] [Google Scholar]
- 19. Fong AH, Li KK, Wong D. Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt-Koyanagi-Harada disease. Retina. 2011; 31(3): 502–509. [DOI] [PubMed] [Google Scholar]
- 20. Hirooka K, Saito W, Namba K, et al.. Early post-treatment choroidal thickness to alert sunset glow fundus in patients with Vogt-Koyanagi-Harada disease treated with systemic corticosteroids. PLoS One. 2017; 12(2): e0172612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weiter JJ, Delori FC, Wing GL, Fitch KA. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Investig Ophthalmol Vis Sci. 1986; 27: 145–152. [PubMed] [Google Scholar]
- 22. Ding X, Li J, Zeng J, et al.. Choroidal thickness in healthy Chinese subjects. Investig Opthalmology Vis Sci. 2011; 52: 9555. [DOI] [PubMed] [Google Scholar]
- 23. Harb E, Hyman L, Gwiazda J, et al.. Choroidal thickness profiles in myopic eyes of young adults in the correction of myopia evaluation trial cohort. Am J Ophthalmol. 2015; 160: 62–71.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takahashi H, Takase H, Terada Y, Mochizuki M, Ohno-Matsui K. Acquired myopia in Vogt–Koyanagi–Harada disease. Int Ophthalmol. 2019; 39: 521–531. [DOI] [PubMed] [Google Scholar]