Abstract
Purpose
To evaluate the potential of lactoferrin (Lf) as a diagnostic biomarker for ocular diseases using a meta-analytic approach.
Methods
All original studies reporting an estimate of the average Lf concentration in healthy subjects and those affected by ocular diseases were searched up to March 2020. The DerSimonian and Laird method was used to calculate the random effects pooled mean difference and the corresponding 95% confidence interval (CI) in Lf concentration between healthy subjects and those affected by dry eye (DE), Sjögren syndrome (SS), and diabetic retinopathy, separately. The presence of between-study heterogeneity was evaluated using the Cochran's Q test and the I2 index. Stratified analyses were performed to assess potential sources of heterogeneity and influence and cumulative analyses to evaluate the robustness of the results obtained. Publication bias was also evaluated using funnel plot and the Egger's test.
Results
The pooled mean differences in Lf concentrations between healthy subjects and those with DE, Sjögren syndrome, and diabetic retinopathy were respectively 0.62 (95% CI, 0.35–0.89) for DE, 3.78 (95% CI, −6.64 to 14.17), and 0.19 (95% CI, −4.00 to 4.39). Regarding DE, the stratified analysis showed that geographical area (P value Q test < 0.0001) and sample size (P < 0.0005) were sources of heterogeneity. Moreover, no study substantially influenced the results obtained and the pooled mean difference became statistically significant after a sample size of 220. Publication bias may affect the results of DE.
Conclusions
The results of the current meta-analysis suggest that Lf level in tears is a good candidate as dry eye syndrome diagnostic biomarker.
Keywords: lactoferrin, biomarker, tears, meta-analysis, ocular disease
Lactoferrin (Lf), also known as lactotransferrin, is a nonheme iron-binding protein belonging to the transferrin family. Lf has several biological functions, including antimicrobial and immunomodulatory activities.1 Thanks to its multifunctional protective character, this glycoprotein is ubiquitous and it is present in different mucosal secretions such as tears, saliva, milk, and nasal secretions, among others.2 During the last decades, different techniques have been used to measure Lf concentration in tears, such as gel electrophoresis,3 high-performance liquid chromatography,4 mass spectrometry,3,5 ELISA,6 or diagnostic test kits.7,8
Several studies report Lf concentration in tears of healthy subjects6,9 and patients with ocular diseases, such as dry eye (DE) syndrome10–12 and keratoconus (KC).13 In particular, patients with ocular diseases seem to have lower levels of Lf compared with healthy subjects. Thus, it has been suggested14,12 that altered Lf concentrations might represent a potential diagnostic biomarker for diagnosis of ocular diseases in a simple and noninvasive way, thanks to the accessibility of tears and the convenience of tear sampling.
As defined by the FDA-NIH BEST Resource,15 a diagnostic biomarker “is used to detect or confirm the presence of a disease or condition of interest or to identify individuals with a subtype of the disease”.16 Therefore, the first step needed to evaluate the potential of Lf as a diagnostic biomarker for ocular diseases is to determine whether the difference in Lf concentration between subjects with ocular disease and healthy controls reported in several studies is significant and reliable, in order to be further validated as a candidate biomarker for ocular diseases. Since only a few studies have been published on this issue and some of them are limited by low sample size, we performed a meta-analysis to exploit the whole evidence available in the literature and provide a precise estimate of the mean difference (MD) of Lf concentration between subjects with ocular disease and healthy controls to evaluate the potential of Lf as a diagnostic biomarker for ocular diseases.
Methods
The current meta-analysis was conducted and reported following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.
Search Strategy
A literature search was carried out using PubMed, Web of science and Scopus from inception of each database up to March 2020 to identify all articles reporting the average levels of Lf in the tear film of patients with ocular diseases and of healthy controls. The following search strategy was considered: (“Lactoferrin” OR “Lactotransferrin” OR “Proteomic”, OR “Tears”) AND (“Ocular disease” OR “Dry-Eye” OR “Sjögren disease” OR “Diabetic retinopathy” OR ”Keratoconus”). Terms were included in the search strategy both as MeSH and text terms. Moreover, the references of the retrieved publications where checked to identify potential additional relevant publications missed by the PubMed search.
Inclusion Criteria
Studies were included in the meta-analysis if they (i) were original studies conducted on humans, (ii) were written in the English language, (iii) included both subjects affected by ocular disease and healthy controls, and (iv) reported the average concentration of Lf in the tear film of both healthy and ill subjects and the corresponding measure of variability, such as standard error, standard deviation, variance, confidence intervals (CIs), or data allowing its calculation. When the same data were used for more than one publication, only the most complete publication was selected.
Data Collection
The following information was extracted from the retrieved studies: first author, publication year, country, disease affecting the subjects (e.g., DE, keratoconus), group-specific sample size, proportion of females in the sample, mean age of the subjects, diagnostic criteria of the diseases, method used for tear sampling, method used to assess Lf concentration (ELISA, mass spectrometry, etc.), estimate of the average Lf concentration, and the corresponding variability measure in ill and healthy patients. For all studies, Lf concentration was transformed to milligrams per milliliter. When studies reported the relative Lf concentration, a transformation from relative to absolute concentration was performed considering the total protein content reported in the same study or, when this value was absent, referring to the work by Versura et al.,12 where the average protein content was estimated to be 9.89 ± 2.28 and 6.44 ± 2.1 mg/mL for controls and patients with DE, respectively.
Statistical Analysis
For each study, the MD of Lf concentration between healthy and ill subjects was calculated as and the corresponding standard error as , where and are the mean values, s2healthy and s2illthe standard deviations, and nhealthy and nill the sample sizes of healthy and ill subjects, respectively. For each ocular disease with at least three estimates of MD, the fixed model and the random effect model of DerSimonian and Laird were applied to estimate the pooled mean difference (pMD) and the corresponding 95% CI. In presence of high between-studies heterogeneity, evaluated by means of the Cochran's Q test and the I2 index (values of the index of >50% imply high heterogeneity) we reported the random effect model estimates. In the presence of high heterogeneity and less than five studies, we applied a more robust method for the calculation of the CI the Hartung Knapp Sidik Jonkman.17
A stratified analysis was carried out to evaluate several potential sources of heterogeneity when at least two study-specific MD estimates were available for each stratum. We considered the following potential sources of heterogeneity: tear sampling methods, Lf concentration estimation methods, diagnostic criteria (symptoms and clinical examination vs. symptoms and clinical examination and/or diagnostic test), average age, female proportion, geographical area, and the sample size. Age, female proportion, and samples size were categorized according to their median values. The presence of heterogeneity among strata was tested using the Cochran's Q test.
To verify the robustness of findings, an influence analysis was carried out testing the change of pMD obtained excluding one study at a time. Moreover, a cumulative analysis was carried out to assess the effect of sample size on the pMD. Finally, publication bias was visually assessed through the funnel plot and tested using the Egger's test.
Results
The flow diagram for article inclusion in this study is shown in Figure 1. The PubMed search retrieved 190 studies. An additional 93 studies were identified using Scopus and Web of Science (73 and 20, respectively), leading to the identification of 283 studies overall. We excluded 72 studies (approximately 25%) because they were unrelated to the study issue according to title and abstract and approximately the same percentage of papers (approximately 22%; n = 61) was excluded because they were not original articles. Another 12 and 21 papers (about 4% and 7%) were excluded because they were either animal studies or not written in English, respectively. A further 96 studies (approximately 34%) were excluded because they did not report average Lf concentrations in both categories of healthy and ill subjects and/or the corresponding variability measure or enough data for their calculation, and two studies (approximately 1%) were excluded because reporting duplicated data. Finally, 19 studies7,11,12,14,18–32 (approximately 7% of the articles found in the first instance) were included in the meta-analysis.
Figure 1.
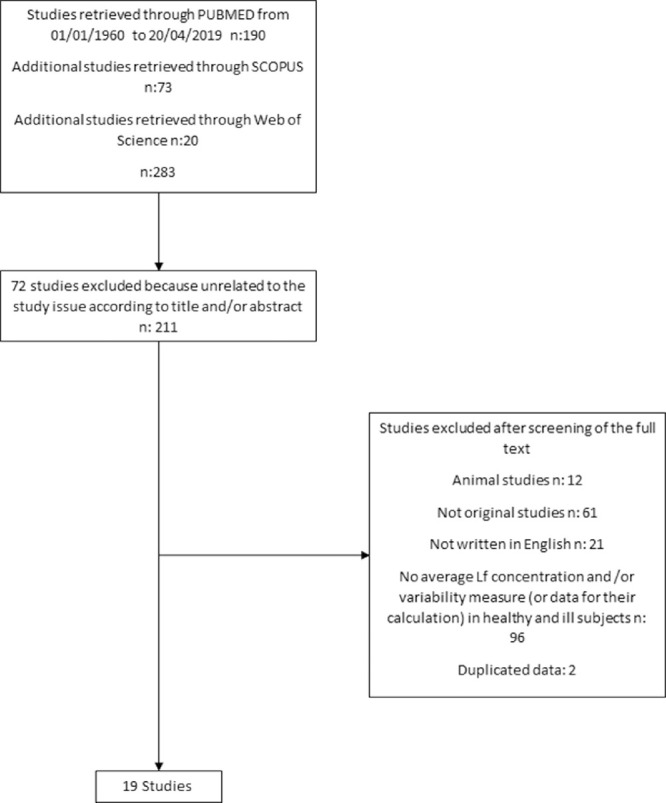
Selection flow chart for inclusion of the studies in the meta-analysis.
The main characteristics of the selected studies are reported in Table 1. Among those, wight (approximately 42%) were performed in Europe, six (approximately 32%) in Asia, and five (approximately 26%) in the United States. The Lf concentration in tears has been studied mainly for two different conditions, both of which, to some extent, concern the so-called DE syndrome. Indeed, 14 studies (approximately 74%) were focused on patients with keratoconjunctivitis sicca (KS), which is often referred to as the condition of having DE, and 4 (approximately 21%) studies concerned patients with Sjögren syndrome (SS), an autoimmune disease in which DE is one of the primary symptoms. Two studies provide data concerning diabetic retinopathy (DR), one study was dedicated to keratoconus and another one to corneal melting and chronic conjunctivitis. The sample size ranged from 19 subjects25 to 205 subjects12; the proportion of females from 44%30 to 100%11; and the mean age from 28.93 years.32 to 63.17 years.28 Nine studies11,12,20,22–24,31–33 based the diagnosis of the ocular disease of interest only on signs, symptoms, personal or familial history and clinical evaluation, eight studies7,18,21,25–28 considered, in addition to these features, a tear test (Schirmer test, Rose Bengal test, etc.), and two studies14,19 considered only a tear test. The most used method to collect tears in these studies was by a capillary tube (12 of 19), followed by Schirmer strips or polyester wicks (n = 7). Lf concentration was quantified by using gel electrophoresis in seven studies, ELISA in five studies, and other methods in three studies. Finally, 14 studies reported Lf absolute concentration, whereas 5 studies reported the Lf relative concentration. Two studies18,31 reported the average Lf concentration for different grades of DE. For these studies, only the estimate associated with grade I disease, that is, the lowest disease severity, was selected to provide more conservative pooled estimate of the MD, because the average Lf concentration for DE grade I is expected to display less remarkable differences from that of control subjects than higher grades. One study27 reported the average Lf concentration for both proliferative and nonproliferative DR; from this study, only the estimates related to nonproliferative DR was selected. Finally, the study of Markusse et al.23 reported the average Lf concentration for a group of subjects with suspected but not confirmed SS; this group of patients was not included in the analysis since different from either SS or control patients.
Table 1.
Main Characteristics of the Studies Included in the Meta-Analysis
| First Author, Year (ref) | Country | Sample Size | Proportion of Females | AgeMean (Standard Deviation) | Diagnostic Criteria | Tear Sampling Method | Method for Lf Quantification | Lf Mean Concentration (Standard Deviation) |
|---|---|---|---|---|---|---|---|---|
| Stuchell et al., 198119 | US | 32 KS 66 C | NA | NA | Signs, symptoms, clinical evaluation, and/or tear test (tear osmolarity) | Schirmer strip | Gel electrophoresis | KS: 1.54 (0.82) mg/mL* C: 1.37 (1.02) mg/mL |
| Farris et al., 198620 | US | 24 KS 26 C | Overall: 76% | Overall†: 59.52 (13.40) KS: 59 (13.00) C: 60 (14.00) | Signs, symptoms, clinical evaluation | Schirmer strip | Gel electrophoresis | KS: 1.71 (1.19) mg/mL* C: 2.16 (1.50) mg/mL |
| Boukes et al., 198721 | NL | 2 Corneal melting 58 CONJ 30 Idiopathic DE 7 SS 28 C | NA | NA | Signs, symptoms, clinical evaluation, and/or tear test (Schirmer test) | Schirmer strip | HPLC | Corneal melting: 2.11 (1.84) mg/mL‡ CONJ: 4.02 (2.79) mg/mL DE: 0.74 (0.704) mg/mL SS: 0.64 (0.47) mg/mL C: 3.96 (2.98) mg/mL |
| Lucca et al., 199022 | US | 20 KS 20 C | KS: 85% C: 85% | KS:62 (NA) C:62 (NA) | Signs, symptoms, clinical evaluation | Capillary tube | Lactoplate | KS: 1.34 (0.78) mg/mL* C: 1.18 (0.56) mg/mL |
| Yolton et al., 19917 | US | 37 DE 12 C | Overall:57% | DE: 44.05 (19.52) C: 33.17 (13.81) | Signs, symptoms, clinical evaluation, and/or tear test (tear break-up time, Rose Bengal test) | Schirmer strip | Lactoplate | DE: 1.86 (0.67) mg/mL C: 1.95 (0.56) mg/mL |
| Markusse et al., 199323 | NL | 44 Primary SS 21 Non-SS 24 C | SS: 91% Non-SS: 90% C: 100% | SS: 55 (NA) Non-SS: 47 (NA) C: 55 (NA) | Signs, symptoms, clinical evaluation | Schirmer strip | Radioimmunoassay | SS: 3.82 (5.28) mg/mL§ Non-SS: 2.86 (5.19) mg/mL C: 0.86 (1.48) mg/mL |
| Stolwijk et al., 199424 | NL | 29 DR 26 C | NA | DR: 46.3 (10.4) C: 39.7 (11.5) | Signs, symptoms, clinical evaluation | Capillary tube | HPLC | DR: 1.29 (0.47) mg/mL C: 1.16 (0.38) mg/mL |
| Solomon et al., 200125 | US | 9 SS 10 C | SS: 89% C: 60% | SS: 68 (9.1) C: 32 (6.6) | Signs, symptoms, clinical evaluation, and/or tear test (Schirmer test, fluorescein staining, elevated serum autoantibody titers) | Polyester wick | ELISA | SS: 0.8 (0.40) mg/mL C: 13.5 (0.70) mg/mL |
| Ohashi et al., 200326 | JP | 71 DE 23 SS 16 C | DE: 77% SS: 96% C: 75% | DE: 48 (16) SS: 58 (10) C: 30 (4) | Signs, symptoms, clinical evaluation, and/or tear test (tear break-up time, Rose Bengal test) | Capillary tube | ELISA | DE: 0.69 (0.55) mg/mL SS: 0.13 (0.22) mg/mL C: 2.05 (1.12) mg/mL |
| Wang et al., 200514 | JP | 145 DE 54 C | NA | NA | Signs, symptoms, clinical evaluation, and/or tear test (Schirmer test, tear break-up time) | Schirmer strip | Lactoplate | DE: 0.8 (0.7) mg/mL C: 1.77 (0.82) mg/mL |
| Yu et al., 200827 | CN | 20 NP-DR 20 P-DR 20 C | NA | NA | Signs, symptoms, clinical evaluation, and/or tear test (Schirmer test) | Capillary tube | Lowry protein assay | NP-DR: 1.14 (0.48) mg/mL P-DR: 1.10 (0.24) mg/mL C: 1.67 (0.43) mg/mL |
| Versura et al., 201028 | IT | 60 Evaporative DE 30 C | DE: 70% C: 73% | DE: 64.2 (22.3) C: 61.1 (17.8) | Signs, symptoms, clinical evaluation, and/or tear test (tear break-up time, Schirmer test) | Capillary tube | Gel electrophoresis | DE: 1.30 (0.17) mg/mL‡ C: 2.43 (0.34) mg/mL |
| Balasubramanian et al., 201229 | UK | 26 KC 28 C | KC: 50% C: 54% | KC: 35.77 (7.96) C: 35.71 (8.13) | Signs, symptoms, clinical evaluation | Capillary tube | ELISA | KC: 0.67 (0.28) mg/mL C: 1.13 (0.29) mg/mL |
| Yanwei et al., 201230 | CN | 40 DE 35 C | DE: 63% C: 54% | DE: 39 (13) C: 36 (12) | Signs, symptoms, clinical evaluation, and/or tear test (tear break-up time, Schirmer test, fluorescent staining) | Capillary tube | Radial immunodiffusion kit | DE: 1.10 (0.79) mg/mL C: 1.95 (0.25) mg/mL |
| Versura et al., 201312 | IT | 160 DE 45 C | DE: 85% C: 62% | DE: 51.09 (16.35) C: 44.70 (9.50) | Signs, symptoms, clinical evaluation | Micropipette | Gel electrophoresis | DE: 1.4 (0.76) mg/mL C: 2.11 (0.74) mg/mL |
| Lee et al., 201418 | KR | 24 DE (6 grade I, 6 grade II, 6 grade III, 6 grade IV) 6 C | NA | DE: 39.2 (15) C: 34.1 (13.9) | Signs, symptoms, clinical evaluation, and/or tear test (tear break-up time, Schirmer test) | Capillary tube | Gel electrophoresis | DE grade I: 0.64 (0.04) mg/mL‡ DE grade II: 0.62 (0.05) mg/mL DE grade III: 0.60 (0.04) mg/mL DE grade IV: 0.26 (0.05) mg/mL C: 0.64 (0.04) mg/mL |
| Careba et al., 201511 | RO | 33 DE 33 C | 100% | NA | Signs, symptoms, clinical evaluation | Capillary tube | Gel electrophoresis | DE:1.22 (0.44) mg/mL‡ C: 2.37 (0.74) mg/mL |
| Kim et al., 201631 | KR | 52 DE (10 grade I, 10 grade II, 22 grade III, 10 grade IV) 29 C | DE: 60% C: 41% | DE: 52.37 (10.18) C: 50.69 (10.37) | Signs, symptoms, clinical evaluation | Capillary tube | ELISA | DE grade I: 0.27 (0.11) mg/mL DE grade II: 0.27 (0.19) mg/mL DE grade III: 0.10 (0.09) mg/mL DE grade IV: 0.06 (0.04) mg/mL C: 0.41 (0.19) mg/ml |
| Constantin et al., 201932 | RO | 12 DE 18 C | NA | DE: 38.58 (10.62) C: 22.50 (11.35) | Signs, symptoms, clinical evaluation | Capillary tube | ELISA | DE: 1.43 (0.18) mg/mL C: 1.79 (0.09) mg/mL |
C, healthy controls; CN, China; CONJ, chronic conjunctivitis; HPLC, high-performance liquid chromatography; IT, Italy; JP, Japan; KC, keratoconus; KR, South Korea; KS, keratoconjunctivitis sicca; NA, not available; NL, the Netherlands; Non-SS, suspected SS whose diagnosis was not confirmed; NP-DR, nonproliferative DR; P-DR, Proliferative DR; RO, Romania; SS, Sjögren's syndrome.
Calculated as average of the strata.
Converted from mg/dL.
Converted from mg/L.
Converted from relative percentage in mg/mL.
DE Syndrome
Figure 2 reports the study-specific MD of Lf concentrations between healthy and DE subjects, the pMD estimate, the corresponding 95% CI, and the I2 index used to quantify heterogeneity.
Figure 2.
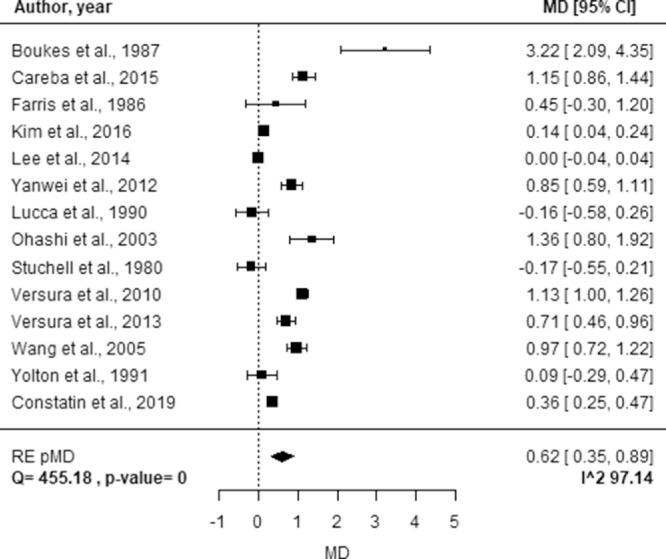
Forest plot of study-specific MDs of Lf concentration between healthy controls and subjects affected by DE. Squares represent the study-specific, relative MD. The size of the squares reflects the study-specific statistical weight, that is, the inverse of the variance. Horizontal lines represent 95% CIs. The diamond represents the pMD estimate with corresponding 95% CIs. Q and P value are the statistic tests used to assess heterogeneity and the corresponding P value; I^2 is the value of the I2 index.
For this disease, the pMD obtained from the random effect model was 0.62 (95% CI, 0.35–0.89), suggesting that subjects affected by DE have a significant lower Lf concentration compared with healthy subjects. However, the pooled estimate was characterized by high heterogeneity (I2 = 97.14%). The analysis of potential sources of heterogeneity is reported in Table 2. A significant difference in the pMD of Lf concentration was found only for the geographical area (P < 0.0001) and sample size (P = 0.0005). In particular, greater pMD were observed in studies conducted in Europe and Asia (pMDs of 1.07 and 0.59 respectively) compared with those conducted in the United States (pMD of −0.03) and larger sample size (pMD of 0.86 vs. 0.23).
Table 2.
The pMDs and Corresponding 95% CIs of Lf Concentration Between Groups of Healthy Controls and DE Subjects by Strata of Potential Sources of Heterogeneity
| Strata | N | MD | 95% CI | I2 | P Value |
|---|---|---|---|---|---|
| Tear sampling methods | |||||
| Capillary tubes | 9 | 0.60 | (0.28 to 0.91) | 97.92% | 0.6780 |
| Paper strips | 5 | 0.76 | (0.04 to 1.49) | 92.35% | |
| Methods to quantify Lf concentration | |||||
| Gel electrophoresis | 6 | 0.55 | (−0.03 to 1.13) | 98.49% | 0.7971 |
| ELISA* | 3 | 0.45 | (−0.88 to 1.77) | 91.55% | |
| Other | 5 | 0.80 | (0.21 to 1.39) | 92.12% | |
| Diagnostic criteria | 0.2215 | ||||
| Signs, symptoms, clinical evaluation | 6 | 0.45 | (0.16 to 0.73) | 91.56% | |
| Signs, symptoms, clinical evaluation, and/or tear test | 8 | 0.82 | (0.30 to 1.34) | 98.17% | |
| Age, years | |||||
| ≤48 | 5 | 0.47 | (0.14 to 0.80) | 95.59% | 0.9915 |
| >48 | 5 | 0.47 | (−0.09 to 1.02) | 97.43% | |
| Females, % | |||||
| ≤76 | 5 | 0.54 | (−0.01 to 1.09) | 97.42% | 0.5931 |
| >76* | 4 | 0.76 | (−0.30 to 1.81) | 89.88% | |
| Country | |||||
| Asia | 5 | 0.59 | (0.26 to 0.93) | 96.59% | < 0.0001 |
| Europe | 5 | 1.07 | (0.60 to 1.55) | 96.25% | |
| US | 4 | −0.03 | (−0.25 to 0.18) | 0.00% | |
| Sample size | |||||
| ≤62 | 7 | 0.23 | (0.01 to 0.44) | 91.47% | 0.0005 |
| >62 | 7 | 0.85 | (0.57 to 1.14) | 87.90% |
CIs calculated using the Hartung Knapp Sidik Jonkman method.
The influence analysis, reported in Figure 3, showed that neither the pMD nor heterogeneity were substantially affected by a single study. The pMD varied from 0.53 (95% CI, 0.26–0.79), excluding the study by Boukes et al.,21 to 0.68 (95% CI, 0.40–0.96), excluding the study by Stuchell et al.,19 whereas the I2 index varied from 95.08% excluding the study of Versura et al.28 to 97.36% excluding the study by Farris et al.20 or Yolton et al.7
Figure 3.
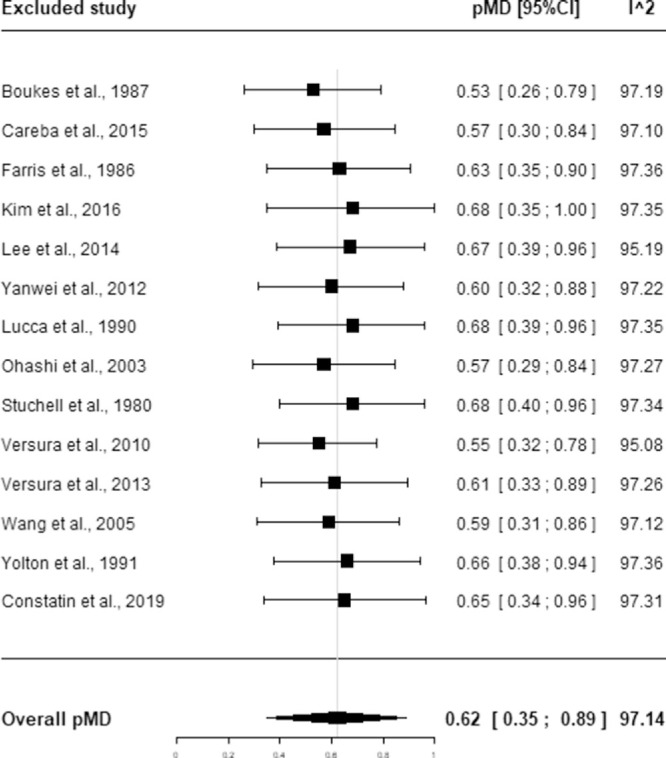
Influence analysis for DE. Squares represent the pMD obtained excluding the indicated study. Horizontal lines represent 95% CIs. The diamond represents the overall pMD estimate with corresponding 95% CIs obtained in the main analysis. I^2 is the value of the I2 index.
Figure 4 shows the results of the cumulative analysis aimed to assess the impact of sample size on the pMD estimate. A statistically significant pMD was observable only when the sample size was greater than 220 subject and the pooled estimate tended to stabilize when the sample size was greater than 600 subjects.
Figure 4.
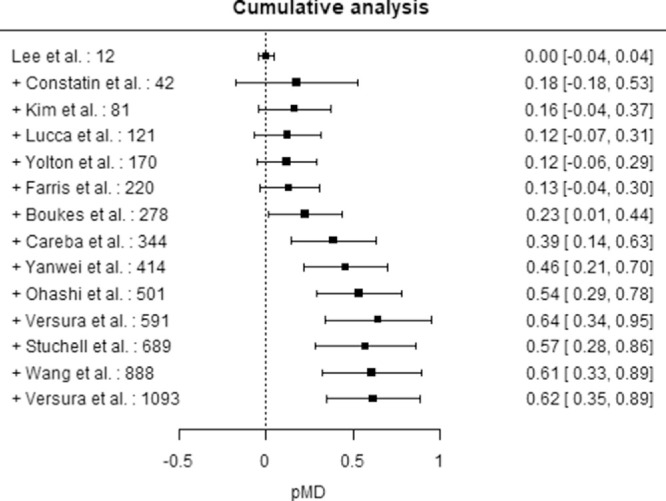
Cumulative analysis by sample size – DE. Squares represent the pMD obtained adding a study at a time ordered by increasing sample size indicated study. Horizontal lines represent 95% CIs.
Other Diseases
The study-specific MD, the pMD, the corresponding 95% CI, and the I2 index are reported in Figure 5 for SS and Figure 6 for DR. No statistically significant difference was observed in average Lf concentration for SS (pMD, 3.78; 95% CI, −6.64 to 14.17) and DR (pMD, 0.19; 95% CI, −4.00 to 4.39) relative to healthy controls. High heterogeneity was found for both SS (I2 = 99.68%) and DR (I2 = 92.21%).
Figure 5.
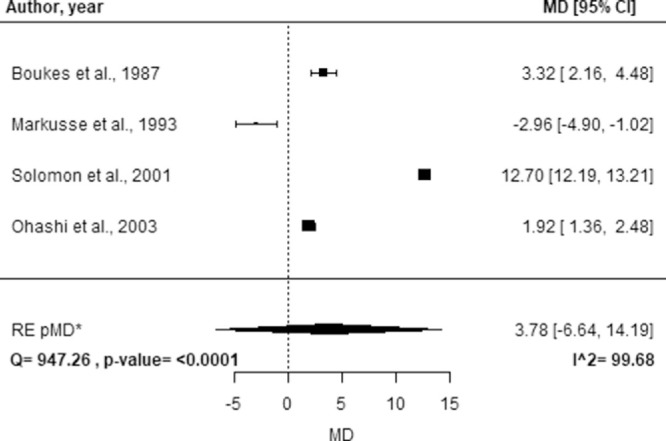
Forest plot of study-specific MDs of Lf concentration between groups of healthy controls and subjects affected by SS. Squares represent the study-specific, relative MD. The size of the squares reflects the study-specific statistical weight, i.e. the inverse of the variance. Horizontal lines represent 95% CIs. The diamond represents the pMD estimate with corresponding 95% CIs. Q and p-value are the statistic tests used to assess heterogeneity and the corresponding p-value; I^2 is the value of the I2 index. *CIs calculated using the Hartung Knapp Sidik Jonkman Hartung Knapp Sidik Jonkman method.
Figure 6.
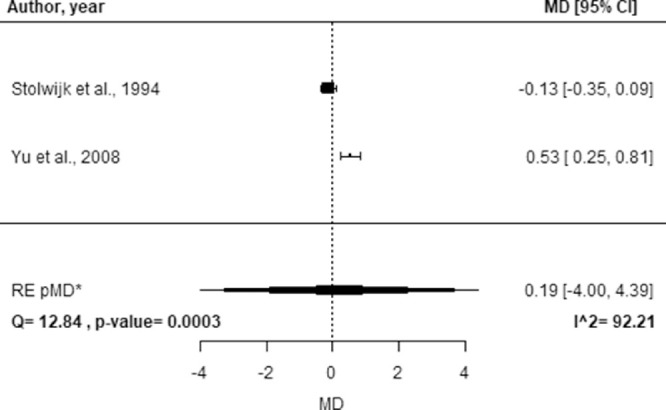
Forest plot of study-specific MDs of Lf concentration between healthy controls and subjects affected by DR. Squares represent the study-specific, relative MD. The size of the squares reflects the study-specific statistical weight, that is, the inverse of the variance. Horizontal lines represent 95% CIs. The diamond represents the pMD estimate with corresponding 95% CIs. Q and P value are the statistic tests used to assess heterogeneity and the corresponding p-value; I^2 is the value of the I2 index. *CIs calculated using the Hartung Knapp Sidik Jonkman method.
Publication bias may have affected the results obtained for DE because asymmetry was observed in the funnel plot and confirmed by the Egger's test (P = 0.0076; Fig. 7). Publication bias was not assessed for SS and DR owing to the low number of studies evaluating those diseases.
Figure 7.
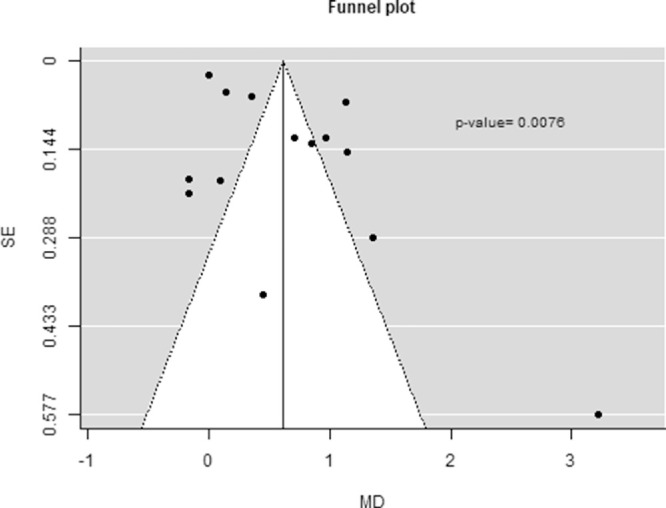
Funnel plot to assess publication bias and P value of the Egger's test
Discussion
The results of this meta-analysis show a significant decrease in Lf concentrations in the tears of subjects affected by DE. This finding is particularly relevant because DE is an ocular condition occurring quite frequently and often in association with systemic diseases.
Considering the high heterogeneity of the data, a subgroup analysis was performed, which suggested that the MD was strongly influenced mainly by country and sample size, the impact of the latter factor was confirmed by the cumulative analysis. The results of this analysis suggest that a too low sample may lead to a lack statistically significant difference in the average Lf concentrations between healthy subjects and those affected by DE.
This report is the first to highlight a difference in Lf concentration in human tears according to the geographic origin of the subject. This difference in Lf expression has been reported previously just for human breast milk. Lien et al.34 measured Lf concentration in milk from women living in five different continents and found a modest but significant variability. A recent study analyzed additional factors besides ethnicity, by measuring Lf levels in milk from Chinese women: body mass index and diet did not influence Lf concentration, although ethnicity and age did.35
Although previous analyses on tears36 showed that Lf is significantly upregulated in samples from females, and similar conclusions were obtained by analyzing other human specimens, such as saliva,37 no statistically significant difference in MD was observed in this study between genders. This result is in line with others that reported no difference at all.38,39 Differently from geographical area (i.e., ethnicity), sample size is a parameter that is not related to the subjects, rather to the study design; indeed, a small sample size and/or a small prefiltering can lead to an overfitting of the model proposed.40
Nonetheless, it should be pointed out that the definition and diagnostic criteria of DE have changed over time. Until the early 2000s, DE was mainly considered as a disorder of the tear film owing to tear deficiency or excessive evaporation causing some ocular symptoms.41 In 2007, the report of the International Dry Eye WorkShop of the Tear Film and Ocular Surface Society defined DE as a multifactorial disease of the tears and ocular surface accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.42 In 2017, Craig et al.43 proposed a few revisions and defined DE as a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles. In the 2017 diagnosis report, Dry Eye WorkShop II listed questionnaires, tear film tests, and epithelial abnormalities as DE diagnostic tests.44 The proposed classification scheme included patients with DE symptoms without obvious DE signs. Other authors proposed slightly different DE definitions and specific lists of symptoms, signs, and test procedures.45–48 Overall, the change of DE definition over time and in different Tear Film and Ocular Surface Society and the Dry Eye WorkShop reports has no evident effects on the studies collected here, where the definition of DE was based mainly on signs and symptoms, frequently associated to tear test, with no specific differences over time, nor in the pMD of Lf concentration. Noteworthy, a difference in Lf concentrations was observed for DE, but not for SS and DR, relative to healthy controls. In the last years, a huge effort has been made to clarify the causes and mechanisms behind different conditions such as DE and SS,41,49–52 which were originally considered similar or even the same. However, this result may be due to the low number of studies available in the scientific literature reporting Lf concentrations in subjects affected by SS or DR, leading to low power in detecting a statistically significant pMD and to a lack of precision in the estimates.
This study has several implications. First, it allows one to estimate the MD of Lf concentration between healthy and DE subjects considering a large sample size (1093 subjects), leading to more precise estimates compared with single studies. The larger sample size implies a greater power in detecting a statistically significant MD in Lf concentration between groups. Second, stratification and sensitivity analyses were carried out, allowing the evaluation of potential sources of heterogeneity and bias in the conduction of the study. However, some limitations need to be discussed. The low number of studies reporting Lf concentrations in individuals affected by SS and DR do not allow to provide a reliable estimate of the MD for these diseases. Moreover, the results obtained need to be taken with caution owing to the high between-study heterogeneity found. Finally, it could be argued that publication bias may have affected the results regarding DE. However, since the most precise estimates were associated to low MD, the pooled estimate obtained could be an underestimation of the real MD between subjects affected by DE and healthy subjects, therefore the potential of Lf as diagnostic biomarker for DE should not be questioned.
Conclusions
This is the first meta-analysis aimed at highlighting the potential role of tear Lf as a diagnostic biomarker for ocular diseases suggesting Lf as a good candidate as DE biomarker.
A key role of Lf in DE should not be surprising. In fact, thanks to its ability to chelate iron, Lf can counteract oxidative stress in the tear film,53–55 which is thought to be associated with the inflammation occurring in DE disease.56 A study supporting this hypothesis reported that a systemic administration of this protein in mice led to a decrease in oxidative damage and to an enhancement of tear function.57 Such treatment would be useful for prevention or treatment of early DE. For example, Pastori et al.58 proposed a Lf-loading of contact lenses, whose wear is a known risk factor for DE development.59
To compare data across studies and to validate Lf as a diagnostic biomarker, there is still a need for further development of standardized protocols of tear collection, processing and storage.60 Moreover, future studies will need to select a large and homogeneous population with well-defined diseases etiology.61
For these reasons, tear Lf monitoring may be a useful strategy to early diagnosis of DE, discriminating it from similar pathologies. Nevertheless, further investigations based on individual data are needed to test the hypothesis generated by the current work and to evaluate whether Lf may be useful to discriminate also subjects with SS and DR.
In particular, it would be advisable to set an appropriate sample size and to use the most suitable techniques to both quantify Lf concentration and identify subjects affected by ocular diseases.
Acknowledgments
Disclosure: E. Ponzini, None; L. Scotti, None; R. Grandori, None; S. Tavazzi, None; A. Zambon, None
References
- 1. Sánchez L, Calvo M, Brock JH. Biological role of lactoferrin. Arch Dis Child. 1992; 67(5): 657–661, doi: 10.1136/adc.67.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. González-Chávez SA, Arévalo-Gallegos S, Rascón-Cruz Q. Lactoferrin: structure, function and applications. Int J Antimicrob Agents. 2009; 33(4): 301.e1–301e8, doi: 10.1016/j.ijantimicag.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 3. Kijlstra A, Kuizenga A, van der Velde M, van Haeringen NJ. Gel electrophoresis of human tears reveals various forms of tear lactoferrin. Curr Eye Res. 1989; 8(6): 581–588, doi: 10.3109/02713688908995757. [DOI] [PubMed] [Google Scholar]
- 4. Sitaramamma T, Shivaji S, Rao GN. HPLC analysis of closed, open, and reflex eye tear proteins. Indian J Ophthalmol. 1998; 46(4): 239–245. [PubMed] [Google Scholar]
- 5. Masoudi S, Zhong L, Raftery MJ, Stapleton FJ, Willcox MD. Method development for quantification of five tear proteins using selected reaction monitoring (SRM) mass spectrometry. Invest Ophthalmol Vis Sci. 2014; 55(2): 767–775, doi: 10.1167/iovs.13-12777. [DOI] [PubMed] [Google Scholar]
- 6. Kijlstra A, Jeurissen SH, Koning KM. Lactoferrin levels in normal human tears. Br J Ophthalmol. 1983; 67(3): 199–202, doi: 10.1136/bjo.67.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yolton DP, Mende S, Harper A, Softing A. Association of dry eye signs and symptoms with tear lactoferrin concentration. J Am Optom Assoc. 1991; 62(3): 217–223. [PubMed] [Google Scholar]
- 8. Yamada K, Takaki S, Komuro N, Suzuki K, Citterio D. An antibody-free microfluidic paper-based analytical device for the determination of tear fluid lactoferrin by fluorescence sensitization of Tb3+. Analyst. 2014; 139(7): 1637–1643, doi: 10.1039/c3an01926h. [DOI] [PubMed] [Google Scholar]
- 9. Esmaeelpour M, Watts PO, Boulton ME, Cai J, Murphy PJ. Tear film volume and protein analysis in full-term newborn infants. Cornea. 2011; 30(4): 400–404, doi: 10.1097/ICO.0b013e3181f22cd9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abe T, Nakajima A, Matsunaga M, Sakuragi S, Komatsu M. Decreased tear lactoferrin concentration in patients with chronic hepatitis C. Br J Ophthalmol. 1999; 83(6): 684–687, doi: 10.1136/bjo.83.6.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Careba I, Chiva A, Totir M, Ungureanu E, Gradinaru S. Tear lipocalin, lysozyme and lactoferrin concentrations in postmenopausal women. J Med Life. 2015; 8(Spec Issue): 94–98. [PMC free article] [PubMed] [Google Scholar]
- 12. Versura P, Bavelloni A, Grillini M, Fresina M, Campos EC. Diagnostic performance of a tear protein panel in early dry eye. Mol Vis. 2013; 19: 1247–1257. [PMC free article] [PubMed] [Google Scholar]
- 13. Lema I, Brea D, Rodríguez-González R, Díez-Feijoo E, Sobrino T. Proteomic analysis of the tear film in patients with keratoconus. Mol Vis. 2010; 16: 2055–2061. [PMC free article] [PubMed] [Google Scholar]
- 14. Wang H-F, Fukuda M, Shimomura Y. Diagnosis of dry eye. Semin Ophthalmol. 2005; 20(2): 53–62, doi: 10.1080/08820530590931115. [DOI] [PubMed] [Google Scholar]
- 15. FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource. Food and Drug Administration (US); 2016. Accessed July 28, 2020, http://www.ncbi.nlm.nih.gov/books/NBK326791/. [PubMed] [Google Scholar]
- 16. Villani E, Vujosevic S.. Foreword: biomarkers and surrogate endpoints in ophthalmic clinical research. Invest Ophthalmol Vis Sci. 2017; 58(6): BIOi–BIOii, doi: 10.1167/iovs.17-22128. [DOI] [PubMed] [Google Scholar]
- 17. Sidik K, Jonkman JN.. A simple confidence interval for meta-analysis. Stat Med. 2002; 21(21): 3153–3159, doi: 10.1002/sim.1262. [DOI] [PubMed] [Google Scholar]
- 18. Lee SH, Park M-Y, Kim KW, Wee SW, Kim JC. Zinc finger protein in severe dry eye syndrome. Curr Eye Res. 2014; 39(5): 431–438, doi: 10.3109/02713683.2013.851705. [DOI] [PubMed] [Google Scholar]
- 19. Stuchell RN, Farris RL, Mandel ID. Basal and reflex human tear analysis: II. Chemical analysis: lactoferrin and lysozyme. Ophthalmology. 1981; 88(8): 858–862, doi: 10.1016/S0161-6420(81)34938-0. [DOI] [PubMed] [Google Scholar]
- 20. Farris RL, Stuchell RN, Mandel ID. Tear osmolarity variation in the dry eye. Trans Am Ophthalmol Soc. 1986; 84: 250–268. [PMC free article] [PubMed] [Google Scholar]
- 21. Boukes RJ, Boonstra A, Breebaart AC, et al.. Analysis of human tear protein profiles using high performance liquid chromatography (HPLC). Doc Ophthalmol. 1987; 67(1-2): 105–113, doi: 10.1007/bf00142704. [DOI] [PubMed] [Google Scholar]
- 22. Lucca JA, Nunez JN, Farris RL. A comparison of diagnostic tests for keratoconjunctivitis sicca: lactoplate, Schirmer, and tear osmolarity. CLAO J. 1990; 16(2): 109–112. [PubMed] [Google Scholar]
- 23. Markusse HM, van Haeringen NJ, Swaak AJ, Hogeweg M, de Jong PT. Tear fluid analysis in primary Sjögren's syndrome. Clin Exp Rheumatol. 1993; 11(2): 175–178. [PubMed] [Google Scholar]
- 24. Stolwijk TR, Kuizenga A, van Haeringen NJ, Kijlstra A, Oosterhuis JA, van Best JA. Analysis of tear fluid proteins in insulin-dependent diabetes mellitus. Acta Ophthalmol (Copenh). 1994; 72(3): 357–362, doi: 10.1111/j.1755-3768.1994.tb02773.x. [DOI] [PubMed] [Google Scholar]
- 25. Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001; 42(10): 2283–2292. [PubMed] [Google Scholar]
- 26. Ohashi Y, Ishida R, Kojima T, et al.. Abnormal protein profiles in tears with dry eye syndrome. Am J Ophthalmol. 2003; 136(2): 291–299, doi: 10.1016/s0002-9394(03)00203-4. [DOI] [PubMed] [Google Scholar]
- 27. Yu L, Chen X, Qin G, Xie H, Lv P. Tear film function in type 2 diabetic patients with retinopathy. Ophthalmologica. 2008; 222(4): 284–291, doi: 10.1159/000140256. [DOI] [PubMed] [Google Scholar]
- 28. Versura P, Nanni P, Bavelloni A, et al.. Tear proteomics in evaporative dry eye disease. Eye (Lond). 2010; 24(8): 1396–1402, doi: 10.1038/eye.2010.7. [DOI] [PubMed] [Google Scholar]
- 29. Balasubramanian SA, Pye DC, Willcox MDP. Levels of lactoferrin, secretory IgA and serum albumin in the tear film of people with keratoconus. Exp Eye Res. 2012; 96(1): 132–137, doi: 10.1016/j.exer.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 30. Yanwei L, Wei Z, Yu Z. The relationship between dry eye and lactoferrin levels in tears. Asian Biomedicine. 2012; 6(1): 81–85, doi: 10.5372/1905-7415.0601.130. [DOI] [Google Scholar]
- 31. Kim WS, Wee SW, Lee SH, Kim JC. Angiogenin for the diagnosis and grading of dry eye syndrome. Korean J Ophthalmol. 2016; 30(3): 163–171, doi: 10.3341/kjo.2016.30.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Constantin MM, Corbu CG, Tanase C, et al.. Spin probe method of electron paramagnetic resonance spectroscopy – a qualitative test for measuring the evolution of dry eye syndrome under treatment. Anal Methods. 2019; 11(7): 965–972, doi: 10.1039/C8AY02783H. [DOI] [Google Scholar]
- 33. Balasubramanian SA, Pye DC, Willcox MDP. Levels of lactoferrin, secretory IgA and serum albumin in the tear film of people with keratoconus. Exp Eye Res. 2012; 96(1): 132–137, doi: 10.1016/j.exer.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 34. Lien E, Jackson J, Kuhlman C, Pramuk K, Lönnerdal B, Janszen D. Variations in concentrations of lactoferrin in human milk: a nine country survey. Adv Exp Med Biol. 2004; 554: 423–426, doi: 10.1007/978-1-4757-4242-8_55. [DOI] [PubMed] [Google Scholar]
- 35. Cai X, Duan Y, Li Y, et al.. Lactoferrin level in breast milk: a study of 248 samples from eight regions in China. Food Funct. 2018; 9(8): 4216–4222, doi: 10.1039/C7FO01559C. [DOI] [PubMed] [Google Scholar]
- 36. Ananthi S, Santhosh RS, Nila MV, Prajna NV, Lalitha P, Dharmalingam K. Comparative proteomics of human male and female tears by two-dimensional electrophoresis. Exp Eye Res. 2011; 92(6): 454–463, doi: 10.1016/j.exer.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 37. Bezwoda WR, Baynes RD, Khan Q, Mansoor N. Enzyme linked immunosorbent assay for lactoferrin. Plasma and tissue measurements. Clin Chim Acta. 1985; 151(1): 61–69, doi: 10.1016/0009-8981(85)90235-9. [DOI] [PubMed] [Google Scholar]
- 38. Daniel E, Duriasamy M, Ebenezer GJ, null Shobhana, Job CK. Elevated free tear lactoferrin levels in leprosy are associated with type 2 reactions. Indian J Ophthalmol. 2004; 52(1): 51–56. [PubMed] [Google Scholar]
- 39. Sonobe H, Ogawa Y, Yamada K, et al.. A novel and innovative paper-based analytical device for assessing tear lactoferrin of dry eye patients. Ocular Surface. 2019; 17(1): 160–166, doi: 10.1016/j.jtos.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 40. Hernández B, Parnell A, Pennington SR. Why have so few proteomic biomarkers “survived” validation? (Sample size and independent validation considerations). Proteomics. 2014; 14(13-14): 1587–1592, doi: 10.1002/pmic.201300377. [DOI] [PubMed] [Google Scholar]
- 41. Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995; 21(4): 221–232. [PubMed] [Google Scholar]
- 42. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5(2): 75–92, doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 43. Craig JP, Nichols KK, Akpek EK, et al.. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017; 15(3): 276–283, doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 44. Wolffsohn JS, Arita R, Chalmers R, et al.. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017; 15(3): 539–574, doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 45. Behrens A, Doyle JJ, Stern L, et al.. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006; 25(8): 900–907, doi: 10.1097/01.ico.0000214802.40313.fa. [DOI] [PubMed] [Google Scholar]
- 46. Baudouin C, Aragona P, Van Setten G, et al.. Diagnosing the severity of dry eye: a clear and practical algorithm. Br J Ophthalmol. 2014; 98(9): 1168–1176, doi: 10.1136/bjophthalmol-2013-304619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hyon JY, Kim H-M, Lee D, et al.. Korean guidelines for the diagnosis and management of dry eye: development and validation of clinical efficacy. Korean J Ophthalmol. 2014; 28(3): 197–206, doi: 10.3341/kjo.2014.28.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shimazaki J. Definition and diagnostic criteria of dry eye disease: historical overview and future directions. Invest Ophthalmol Vis Sci. 2018; 59(14): DES7–DES12, doi: 10.1167/iovs.17-23475. [DOI] [PubMed] [Google Scholar]
- 49. Murube J. Antecedents of Sjogren syndrome. Ocul Surf. 2010; 8(2): 49–59, doi: 10.1016/s1542-0124(12)70068-x. [DOI] [PubMed] [Google Scholar]
- 50. Pflugfelder SC. Tear dysfunction and the cornea: LXVIII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2011; 152(6): 900–909.e1, doi: 10.1016/j.ajo.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramos-Remus C, Suarez-Almazor M, Russell AS. Low tear production in patients with diabetes mellitus is not due to Sjögren's syndrome. Clin Exp Rheumatol. 1994; 12(4): 375–380. [PubMed] [Google Scholar]
- 52. Vitali C, Bombardieri S, Jonsson R, et al.. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002; 61(6): 554–558, doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clamp JR, Creeth JM.. Some non-mucin components of mucus and their possible biological roles. Ciba Found Symp. 1984; 109: 121–136, doi: 10.1002/9780470720905.ch9. [DOI] [PubMed] [Google Scholar]
- 54. Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995; 41(12 Pt 2): 1819–1828. [PubMed] [Google Scholar]
- 55. Lim SY, Raftery MJ, Goyette J, Hsu K, Geczy CL. Oxidative modifications of S100 proteins: functional regulation by redox. J Leukoc Biol. 2009; 86(3): 577–587, doi: 10.1189/jlb.1008608. [DOI] [PubMed] [Google Scholar]
- 56. Seen S, Tong L.. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018; 96(4): e412–e420, doi: 10.1111/aos.13526. [DOI] [PubMed] [Google Scholar]
- 57. Kawashima M, Kawakita T, Inaba T, et al.. Dietary lactoferrin alleviates age-related lacrimal gland dysfunction in mice. PLoS ONE. 2012; 7(3): e33148, doi: 10.1371/journal.pone.0033148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pastori V, Tavazzi S, Lecchi M. Lactoferrin-loaded contact lenses: eye protection against oxidative stress. Cornea. 2015; 34(6): 693–697, doi: 10.1097/ICO.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 59. Kojima T, Ibrahim OMA, Wakamatsu T, et al.. The impact of contact lens wear and visual display terminal work on ocular surface and tear functions in office workers. Am J Ophthalmol. 2011; 152(6): 933–940.e2, doi: 10.1016/j.ajo.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 60. Tamhane M, Cabrera-Ghayouri S, Abelian G, Viswanath V. Review of biomarkers in ocular matrices: challenges and opportunities. Pharm Res. 2019; 36(3): 40, doi: 10.1007/s11095-019-2569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kempsell KE, Ball G, Szakmany T. Issues in biomarker identification, validation and development for disease diagnostics in public health. Exp Rev Molec Diagnost. 2016; 16(4): 383–386, doi: 10.1586/14737159.2016.1133300. [DOI] [PubMed] [Google Scholar]


