Abstract
Current treatment options for older and relapsed or refractory (R/R) acute myeloid leukemia (AML) patients are limited and represent an unmet need. Based on preclinical studies showing strong anti-leukemic effects in vivo, this phase I dose-escalation study assessed the safety and preliminary clinical activity of the oral exportin-1 inhibitor, selinexor, in combination with the hypomethylating agent, decitabine 20 mg/m2, in adults with R/R AML and in older (age ≥60) untreated AML patients. There were no protocol-defined dose limiting toxicities. The recommended phase 2 dose of selinexor was 60mg (~35 mg/m2) given twice-weekly. Notable grade ≥3 toxicities included asymptomatic hyponatremia (68%), febrile neutropenia (44%), sepsis (44%), hypophosphatemia (36%), and pneumonia (28%). In 25 patients, the overall response rate was 40%. Modification of selinexor to a flat dose of 60mg, twice-weekly for two weeks after decitabine, improved tolerability of the regimen and demonstrated preliminary clinical activity in poor-risk patients with AML.
Keywords: Acute myeloid leukemia, decitabine, selinexor, clinical trials
INTRODUCTION
Acute myeloid leukemia (AML) is an aggressive and biologically heterogeneous disease characterized by clonal accumulation and expansion of leukemic blasts in the peripheral blood and bone marrow [1]. Long-term survival for patients with relapsed/refractory (R/R) disease and older (>60 years) AML patients remains poor, thereby underscoring the need for more innovative therapies that are both effective and well-tolerated.
Selective Inhibitor of Nuclear Export (SINE) compounds are a novel group of orally bioavailable, small-molecule inhibitors of the nuclear transport protein, exportin-1 (XPO1). XPO1 is the primary nuclear exporter of over 200 cargo proteins through the nuclear pore complex, including nearly all known tumor suppressor proteins (TSPs) and growth regulators [2]. XPO1 is overexpressed in multiple cancer types, [3–6] including AML, [7,8] and causes aberrant cytoplasmic localization of TSPs leading to their functional inactivation. Thus, XPO1-mediated nuclear export is one of the mechanisms that many cells, including leukemic blasts, use to inactivate TSPs. SINE compounds bind to and prevent XPO1-mediated transport of TSPs, leading to their accumulation in the nucleus and causing leukemia cell death.
Pre-clinical studies have demonstrated that selinexor (SINE KPT-330), displays potent cytotoxic activity in AML cell lines and in mouse models, [7–9] supporting XPO1 inhibition as a promising therapeutic target in the treatment of AML. Furthermore, a recently published phase 1 study reported that selinexor was safe and acceptably tolerated with supportive care as monotherapy in patients with R/R AML and produced an objective response rate of 14% [10].
Our group previously reported that sequential treatment of AML blasts with the hypomethylating agent, decitabine, followed by selinexor enhances the antileukemic effects of selinexor in vitro and in a MV4–11 xenograft model [11]. We reasoned that these effects were mediated by the re-expression of a subset of TSPs that are epigenetically silenced via DNA methylation, and trafficked to the cytoplasm by XPO1 [11]. As such, we hypothesized that combining selinexor, with decitabine as a backbone, in a phase I open-label non-randomized dose escalation study would be a tolerable combination that would enhance antileukemic effects in AML and improve disease responses in both newly diagnosed and R/R AML patients.
METHODS
Eligibility
Patients >18 years old with histologically confirmed diagnoses of R/R AML (excluding acute promyelocytic leukemia), or previously untreated older adults (>60 years) unfit for chemotherapy, Eastern Cooperative Oncology Group (ECOG) status <2 (Table SI) and with no standard treatment options were eligible for enrollment. Patients with secondary AML or therapy related AML were also eligible. Patients who received decitabine or azacitidine as prior treatment for myelodysplastic syndrome or AML remained eligible; however, treatment with these agents was not permitted within 6 months of study entry. Patients who had relapsed after allogeneic stem cell transplant were also eligible. Exclusion criteria included active CNS malignancy at the time of enrollment, serious systemic infection (e.g., HIV on anti-retroviral therapy, hepatitis A/B/C), uncontrolled active infections, or treatment with any other investigational anticancer drug within 14 days prior to enrollment. Descriptions of the inclusion/exclusion criteria for this study are provided in the Supporting Information.
Study Design
The protocol was approved by the Institutional Review Board and is in accordance with the Declaration of Helsinki, the International Conference on Harmonisation-Good Clinical Practice (ICHGCP) and local laws. The primary objectives were to determine safety, tolerability and the recommended phase 2 dose (RP2D). The secondary objectives were to assess the overall response rate (ORR), rate and duration of CR/CRi, and pharmacodynamics of selinexor in combination with decitabine.
Adults with R/R AML and patients over age 60 with untreated AML received standard 10-day decitabine induction at 20mg/m2 intravenously on days 1-10 for up to four 28-day cycles in combination with oral selinexor, initially, on days 11, 13, 18, 20, 25 and 27. The dosing schedule was later amended to days 11, 13, 18 and 20 after two patients enrolled on dose level 1 (selinexor 23 mg/m2) withdrew consent due to grade 1 or grade 2 gastrointestinal toxicities, specifically nausea and anorexia. Dose escalation proceeded in standard 3+3 cohorts across four dose levels of selinexor: 23 mg/m2, 30 mg/m2, 40 mg/m2 and 55 mg/m2. Bone marrow (BM) assessments were done for all patients after cycle 1 and following completion of subsequent cycles of treatment only if there were no circulating blasts in the peripheral blood. Patients with <5% bone marrow blasts proceeded with five days of maintenance decitabine at 20 mg/m2 on days 1-5 and selinexor, at the same dose used during induction, on days 6, 8, 13 and 15 until disease progression or until development of unacceptable toxicities warranting treatment discontinuation.
Safety
Safety and tolerability were evaluated by assessments of drug-related dose limiting toxicity (DLT), adverse event (AE) reports, physical examinations, and laboratory safety evaluations. National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v4.03 was used for grading of all AEs, with the exception of hyponatremia, and investigators assessed causality as either unrelated, unlikely related or possibly, probably or definitely related.
Baseline cytogenetic/molecular and correlative studies
Cytogenetic analyses were performed using unstimulated short-term (24-, 48-, and 72-hour) cultures with or without a direct method and G-banding. The criteria used to describe a cytogenetic clone and description of karyotype followed the recommendations of the International System for Human Cytogenetic Nomenclature [12]. The diagnosis of normal karyotype was based on ≥20 metaphases analyzed in BM specimens subjected to short-term (24- or 48-hour) unstimulated cultures. DNA samples were analyzed for presence of recurrent gene mutations in AML using targeted amplicon sequencing on the MiSeq (Illumina) platforms. Variants were called using an adaptation of GATK best practices workflow, and variants were filtered and aggregated with Mucor [13]. We used a cut off of ≥5% of variant allele frequency (VAF) to call a mutation. NPM1 and CEBPA mutation results were confirmed in a clinical PCR/Sanger assay. FLT3-internal tandem duplications (FLT3-ITD) were evaluated using capillary electrophoresis fragment analysis, as described previously [14]. Analysis of Genome-wide DNA methylation was performed using DNA isolated from 16 AML patient samples before decitabine and selinexor treatment. DNA was converted using sodium bisulfite and subjected to the Infinium Methylation Assay (Illumina) and analyzed using Infinium MethylationEPIC BeadChip arrays (Illumina), reporting the DNA methylation levels of approximately 850,000 CpG sites genome-wide.
Response Criteria
Objective disease response was assessed according to the revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in AML [15]. Stable disease was defined as not meeting criteria for other response categories for at least one cycle.
Statistical Analysis
Baseline characteristics of 25 patients enrolled in this trial were described using median and range for continuous variables and frequency and percentage for categorical variables. Adverse events were summarized among all patients across all cycles, and also stratified by dose levels and grade levels. Response was summarized using frequency and percentage. Overall response was defined as the number of patients who achieved any level of clinical response (e.g. CR, CRi, MLFS), and overall response rate was estimated with an exact 95% confidence interval. Progression-free survival (PFS) was defined as the time from date of first treatment until progression date or date of death from any cause, censoring patients who were alive and progression free at the date of last follow up. Patients who started a new therapy prior to progression were censored at the time of transferring to new therapy (n=6, 4 patients went to transplant, 1 participated in another clinical trial and 1 transferred to decitabine). Overall survival (OS) was define as the time from starting treatment till death date from any cause, censoring patients at the date last known alive. Kaplan-Meier survival curves for OS and PFS were generated among all patients. The survival functions by response status were compared using the Mantel-Byar method [16].
RESULTS
Patient Characteristics
Between April 2014 and October 2016, 25 patients with R/R AML (n=20), or newly diagnosed older adults over age 60 (n=5) were enrolled in this single-institution phase 1 trial (Fig 1). The median follow-up was 21.8 months (range, 9.2-30.8 months). The median age of the patient population was 60 years (range, 23-83 years). Six out of 20 (30%) patients with R/R AML had received more than three prior lines of treatment. No patients in our study received prior hypomethylating agent therapy for the treatment of antecedent myelodysplastic syndrome or AML. Patient demographics and characteristics are shown in Table I.
Figure 1.
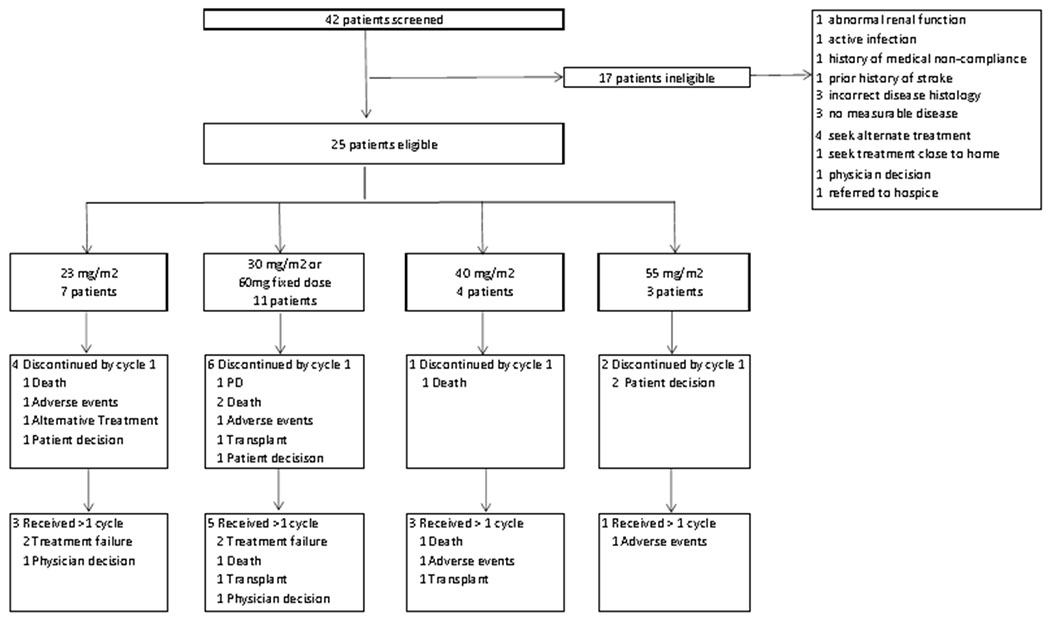
CONSORT diagram of phase I selinexor and decitabine trial
Table 1.
Pre-treatment patient characteristics (n=25)
| Characteristics | n (%) | |
|---|---|---|
| Demographics | ||
| Age | ||
| Median (Range) | 60 (23–83) | |
| Gender | ||
| Female | 13 (52.0) | |
| Male | 12 (48.0) | |
| Race | ||
| Black | 4 (16.0) | |
| White | 21 (84.0) | |
| Disease Characteristics | ||
| Refractory | 8 (32.0) | |
| Relapsed | 12 (48.0) | |
| Untreated | 5 (20.0) | |
| Number of previous treatments | ||
| 0 | 5 (20.0) | |
| 1 | 2 (8.0) | |
| 2 | 4 (16.0) | |
| 3 | 8 (32.0) | |
| 4 | 6 (24.0) | |
| History of CNS disease | ||
| Yes | 1 (4.0) | |
| No | 24 (96.0) | |
| History of extramedullary disease | ||
| Yes | 1 (4.0) | |
| No | 24 (96.0) | |
| ELN 2017 genetic group | ||
| Favorable | 4 (16.7) | |
| Intermediate | 13 (54.2) | |
| Adverse | 7 (29.2) | |
| Unknown | 1 | |
| FLT3 ITD mutation | ||
| Yes | 5 (20.8) | |
| No | 19 (79.2) | |
| Unknown | 1 | |
| CEBPA | ||
| Yes | 1 (4.2) | |
| No | 21 (95.8) | |
| Unknown | 3 | |
| NPM1 mutation | ||
| Yes | 7 (29.2) | |
| No | 16 (70.8) | |
| Unknown | 2 | |
| ECOG at baseline | ||
| 0 | 9 (36.0) | |
| 1 | 16 (64.0) | |
| Prior MDS | ||
| Yes | 1 (4.0) | |
| No | 24 (96.0) | |
| Prior MPN | ||
| Yes | 0 (0) | |
| No | 25 (100) | |
| Baseline Hematological Measures | ||
| Bone Marrow Blast % | ||
| Median (Range) | 51 (2-92) | |
| Peripheral Blood Blast % | ||
| Median (Range) | 6 (0-92.4) | |
| White Blood Count, K/uL | ||
| Median (Range) | 2.7 (0.5-61.9) | |
| Absolute Neutrophil Count, K/uL | ||
| Median (Range) | 0.5 (0-80) | |
| Hemoglobin, g/dL | ||
| Median (Range) | 8.4 (6.1-13.6) | |
| Platelet, K/uL | ||
| Median (Range) | 44 (7.3-196) |
Abbreviations: ELN: European Leukemia Net; ECOG PS: Eastern Cooperative Oncology Group Performance Score
Safety
Four dose levels of selinexor ranging from 23-55 mg/m2 were evaluated in combination with a fixed dose of 20 mg/m2 of decitabine. The dosing and treatment schedules can be found in Supporting Information Table SII. There were no protocol defined DLTs in this study, including at the maximum administered dose of 55 mg/m2, where three patients were fully evaluated for DLT. However, two patients treated on the maximum dose level declined continuation of study therapy after cycle 1 due to chronic low-level GI toxicities. Based on this, as well as emerging experience from a separate trial with single-agent selinexor which reported higher rates of sepsis in older patients, dosing of selinexor was changed to a flat dose of 60 mg (approximately 35 mg/m2) given twice-weekly. Since at least 6 patients had already received a corresponding flat dose of 60 mg or higher of selinexor in combination with decitabine on the current trial without a DLT, 60 mg of selinexor was the RP2D and 7 additional patients were treated at this dose level as part of an expansion cohort. The median number of treatment cycles given was 1 (range 1-4) and the median number of treatment cycles given to responders was 2 (range 1-4). There were a total of 13 patients, across all dose levels, who discontinued treatment after one cycle of therapy. Reasons for treatment discontinuation after one cycle included: patient decision to come off therapy or pursue alternate therapy (n=5), death (n=4), adverse events (n=2), transplant (n=1), and progressive disease (n=1) (Fig 1).
Treatment related AEs observed in at least 10% of patients treated across all cycles are shown in Table II. The most common grade ≥3 treatment-related non-hematologic toxicities were asymptomatic hyponatremia (68%), febrile neutropenia (44%), sepsis (44%), hyperglycemia (40%), hypertension (40%), hypophosphatemia (36%), and pneumonia (28%). Treatment-related toxicities attributed specifically to selinexor are shown in Supporting Information Table SIV. The most common non-hematologic toxicities, occurring in over 20% of patients were asymptomatic hyponatremia (92%), fatigue (52%), nausea (48%), diarrhea (36%), anorexia (32%) and weight loss (20%). With the addition of protocol mandated supportive medications, including D2 antagonists, 5-HT3 antagonists, dexamethasone, megestrol and/or olanzapine, these symptoms improved and resulted in fewer elective study withdrawals (Fig 1). For the purposes of DLT assessment, asymptomatic hyponatremia was not graded according to CTCAE v4.03 but rather by using a clinically meaningful plasma sodium cut-off value of <125mmol/L. Sodium <125 mmol/L was graded as a DLT. Specifics of hyponatremia management are described in Supporting Information Table SIII.
Table 2.
All Cause Adverse Events in Over 10% Patients
| 23 mg/m2 | 30 mg/m2 or 60mg fixed dose | 40 mg/m2 | 55 mg/m2 | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1&2 | Grade 3+ | Total (n=7) | Grade 1&2 | Grade 3+ | Total (n=11) | Grade 1&2 | Grade 3+ | Total (n=4) | Grade 1&2 | Grade 3+ | Total (n=3) | Grade 1&2 | Grade 3+ | Total (n=25) | |
| Hematologic toxicities | |||||||||||||||
| Anemia | 0(0) | 7(100) | 7(100) | 1(9) | 9(81) | 10(90) | (0) | 4(100) | 4(100) | (0) | 3(100) | 3(100) | 1(4) | 23(92) | 24(96) |
| aPTT prolonged | 4(57) | (0) | 4(57) | 6(54) | 1(9) | 7(63) | 3(75) | 1(25) | 4(100) | 1(33) | (0) | 1(33) | 14(56) | 2(8) | 16(64) |
| INR increased | 4(57) | 1(14) | 5(71) | 9(81) | (0) | 9(81) | 4(100) | (0) | 4(100) | 3(100) | (0) | 3(100) | 20(80) | 1(4) | 21(84) |
| Leukopenia | (0) | 7(100) | 7(100) | (0) | 10(90) | 10(90) | (0) | 4(100) | 4(100) | (0) | 3(100) | 3(100) | 0(0) | 24(96) | 24(96) |
| Lymphopenia | 1(14) | 6(85) | 7(100) | 3(27) | 7(63) | 10(90) | (0) | 4(100) | 4(100) | (0) | 2(66) | 2(66) | 4(16) | 19(76) | 23(92) |
| Neutropenia | (0) | 6(85) | 6(85) | (0) | 11(100) | 11(100) | (0) | 3(75) | 3(75) | (0) | 2(66) | 2(66) | 0(0) | 22(88) | 22(88) |
| Thrombocytopenia | (0) | 7(100) | 7(100) | (0) | 11(100) | 11(100) | (0) | 4(100) | 4(100) | (0) | 3(100) | 3(100) | 0(0) | 25(100) | 25(100) |
| Gastrointestinal disorders | |||||||||||||||
| Alkaline phosphatase increased | (0) | (0) | (0) | 2(18) | (0) | 2(18) | 2(50) | (0) | 2(50) | (0) | (0) | (0) | 4(16) | 0(0) | 4(16) |
| ALT increased | 2(28) | 1(14) | 3(42) | 3(27) | (0) | 3(27) | 2(50) | 1(25) | 3(75) | 1(33) | (0) | 1(33) | 8(32) | 2(8) | 10(40) |
| Anorexia | 2(28) | (0) | 2(28) | 5(45) | (0) | 5(45) | 2(50) | (0) | 2(50) | 2(66) | (0) | 2(66) | 11(44) | 0(0) | 11(44) |
| AST increased | 2(28) | (0) | 2(28) | 5(45) | (0) | 5(45) | 1(25) | 1(25) | 2(50) | 1(33) | (0) | 1(33) | 9(36) | 1(4) | 10(40) |
| Constipation | 3(42) | 1(14) | 4(57) | 7(63) | (0) | 7(63) | 1(25) | (0) | 1(25) | 2(66) | (0) | 2(66) | 13(52) | 1(4) | 14(56) |
| Diarrhea | 3(42) | 1(14) | 4(57) | 4(36) | (0) | 4(36) | 2(50) | (0) | 2(50) | (0) | 1(33) | 1(33) | 9(36) | 2(8) | 11(44) |
| Hyperbilirubinemia | 1(14) | (0) | 1(14) | 1(9) | (0) | 1(9) | 3(75) | (0) | 3(75) | 2(66) | (0) | 2(66) | 7(28) | 0(0) | 7(28) |
| Mucositis | 2(28) | (0) | 2(28) | 2(18) | (0) | 2(18) | 2(50) | (0) | 2(50) | 1(33) | 1(33) | 2(66) | 7(28) | 1(4) | 8(32) |
| Nausea | 4(57) | (0) | 4(57) | 7(63) | (0) | 7(63) | 2(50) | (0) | 2(50) | 3(100) | (0) | 3(100) | 16(64) | 0(0) | 16(64) |
| Vomiting | 1(14) | (0) | 1(14) | 3(27) | (0) | 3(27) | (0) | (0) | (0) | 1(33) | (0) | 1(33) | 5(20) | 0(0) | 5(20) |
| Electrolyte and nutrition disorders | |||||||||||||||
| Hyperglycemia | 5(71) | 2(28) | 7(100) | 6(54) | 5(45) | 11(100) | 2(50) | 2(50) | 4(100) | 2(66) | 1(33) | 3(100) | 15(60) | 10(40) | 25(100) |
| Hyperkalemia | 1(14) | (0) | 1(14) | 2(18) | (0) | 2(18) | (0) | (0) | (0) | 1(33) | (0) | 1(33) | 4(16) | 0(0) | 4(16) |
| Hypoalbuminemia | 6(85) | (0) | 6(85) | 7(63) | (0) | 7(63) | 4(100) | (0) | 4(100) | 1(33) | 1(33) | 2(66) | 18(72) | 1(4) | 19(76) |
| Hypocalcemia | 2(28) | (0) | 2(28) | 2(18) | (0) | 2(18) | 4(100) | (0) | 4(100) | (0) | (0) | (0) | 8(32) | 0(0) | 8(32) |
| Hypokalemia | 5(71) | (0) | 5(71) | 4(36) | 2(18) | 6(54) | 3(75) | 1(25) | 4(100) | 3(100) | (0) | 3(100) | 15(60) | 3(12) | 18(72) |
| Hypomagnesemia | 1(14) | (0) | 1(14) | 3(27) | (0) | 3(27) | (0) | (0) | (0) | 2(66) | (0) | 2(66) | 6(24) | 0(0) | 6(24) |
| Hyponatremia | 4(57) | 3(42) | 7(100) | 3(27) | 7(63) | 10(90) | (0) | 4(100) | 4(100) | 0(0) | 3(100) | 3(100) | 7(28) | 17(68) | 24(96) |
| Hypophosphatemia | 2(28) | 1(14) | 3(42) | 2(18) | 4(36) | 6(54) | 1(25) | 2(50) | 3(75) | 0(0) | 2(66) | 2(66) | 5(20) | 9(36) | 14(56) |
| Infections and infestations | |||||||||||||||
| Catheter related infection | (0) | 1(14) | 1(14) | 1(9) | 1(9) | 2(18) | (0) | 2(50) | 2(50) | (0) | (0) | (0) | 1(4) | 4(16) | 5(20) |
| Febrile neutropenia | 0(0) | 3(42) | 3(42) | (0) | 5(45) | 5(45) | (0) | 1(25) | 1(25) | (0) | 2(66) | 2(66) | 0(0) | 11(44) | 11(44) |
| Fever | (0) | (0) | (0) | 3(27) | (0) | 3(27) | 1(25) | (0) | 1(25) | (0) | (0) | (0) | 4(16) | 0(0) | 4(16) |
| Pneumonia | (0) | 1(14) | 1(14) | (0) | 3(27) | 3(27) | 1(25) | 2(50) | 3(75) | (0) | 1(33) | 1(33) | 1(4) | 7(28) | 8(32) |
| Sepsis | (0) | 3(42) | 3(42) | (0) | 6(54) | 6(54) | (0) | 2(50) | 2(50) | 0(0) | 0(0) | 0(0) | 0(0) | 11(44) | 11(44) |
| Musculoskeletal and connective tissue disorders | |||||||||||||||
| Bone pain | 2(28) | (0) | 2(28) | 3(27) | (0) | 3(27) | (0) | (0) | (0) | (0) | (0) | (0) | 5(20) | 0(0) | 5(20) |
| Muscle weakness | (0) | (0) | (0) | 2(18) | (0) | 2(18) | 2(50) | (0) | 2(50) | (0) | (0) | (0) | 4(16) | 0(0) | 4(16) |
| Pain in extremity | 0(0) | 0(0) | 0(0) | 1(10) | 0(0) | 1(10) | 2(50) | 0(0) | 2(50) | 2(50) | 0(0) | 2(50) | 5(20) | 0(0) | 5(20) |
| Nervous system disorders | |||||||||||||||
| Headache | (0) | (0) | (0) | 3(27) | 1(9) | 4(36) | (0) | (0) | (0) | (0) | (0) | (0) | 3(12) | 1(4) | 4(16) |
| Peripheral motor neuropathy | (0) | (0) | (0) | 2(18) | (0) | 2(18) | (0) | (0) | (0) | 1(33) | (0) | 1(33) | 3(12) | 0(0) | 3(12) |
| Psychiatric disorders | |||||||||||||||
| Depression | 1(14) | (0) | 1(14) | 2(18) | (0) | 2(18) | (0) | (0) | (0) | (0) | (0) | (0) | 3(12) | 0(0) | 3(12) |
| Skin and subcutaneous tissue disorders | |||||||||||||||
| Rash | (0) | (0) | (0) | 2(18) | (0) | 2(18) | 1(25) | (0) | 1(25) | 1(33) | (0) | 1(33) | 4(16) | 0(0) | 4(16) |
| Skin infections | (0) | (0) | (0) | 2(18) | (0) | 2(18) | 1(25) | 1(25) | 2(50) | (0) | (0) | (0) | 3(12) | 1(4) | 4(16) |
| Vascular disorders | |||||||||||||||
| Hypertension | 2(28) | 2(28) | 4(57) | 4(36) | 4(36) | 8(72) | 1(25) | 3(75) | 4(100) | 1(33) | 1(33) | 2(66) | 8(32) | 10(40) | 18(72) |
| Hypotension | 4(57) | 1(14) | 5(71) | 1(9) | 3(27) | 4(36) | 1(25) | (0) | 1(25) | (0) | (0) | (0) | 6(24) | 4(16) | 10(40) |
| Renal and urinary disorders | |||||||||||||||
| Acute kidney injury | 4(57) | 1(14) | 5(71) | 3(27) | 2(18) | 5(45) | 2(50) | 1(25) | 3(75) | 1(33) | (0) | 1(33) | 10(40) | 4(16) | 14(56) |
| Respiratory, thoracic and mediastinal disorders | |||||||||||||||
| Cough | 1(14) | 0(0) | 1(14) | 3(30) | 0(0) | 3(30) | 3(75) | 0(0) | 3(75) | 1(25) | 0(0) | 1(25) | 8(32) | 0(0) | 8(32) |
| Dyspnea | (0) | (0) | (0) | 5(45) | 1(9) | 6(54) | 2(50) | (0) | 2(50) | 3(100) | (0) | 3(100) | 10(40) | 1(4) | 11(44) |
| Epistaxis | (0) | (0) | (0) | 3(27) | 1(9) | 4(36) | 1(25) | (0) | 1(25) | 1(33) | (0) | 1(33) | 5(20) | 1(4) | 6(24) |
| Pleural effusion | (0) | (0) | (0) | 2(18) | 1(9) | 3(27) | (0) | (0) | (0) | (0) | (0) | (0) | 2(8) | 1(4) | 3(12) |
| Respiratory failure | (0) | (0) | (0) | (0) | 3(27) | 3(27) | (0) | (0) | (0) | (0) | 1(33) | 1(33) | 0(0) | 4(16) | 4(16) |
| Sore throat | (0) | (0) | (0) | 3(27) | (0) | 3(27) | 1(25) | (0) | 1(25) | (0) | (0) | (0) | 4(16) | 0(0) | 4(16) |
| Cardiac disorders | |||||||||||||||
| Ejection fraction decreased | (0) | 2(28) | 2(28) | (0) | (0) | (0) | (0) | (0) | (0) | 1(33) | (0) | 1(33) | 1(4) | 2(8) | 3(12) |
| QTc prolongation | 2(28) | 2(28) | 4(57) | 3(27) | 1(9) | 4(36) | (0) | (0) | (0) | 1(33) | (0) | 1(33) | 6(24) | 3(12) | 9(36) |
| Sinus tachycardia | (0) | (0) | (0) | 2(18) | 1(9) | 3(27) | 3(75) | (0) | 3(75) | 1(33) | (0) | 1(33) | 6(24) | 1(4) | 7(28) |
| General disorders and administration site conditions | |||||||||||||||
| Fatigue | 3(42) | 1(14) | 4(57) | 8(72) | 2(18) | 10(90) | 3(75) | (0) | 3(75) | 2(66) | (0) | 2(66) | 16(64) | 3(12) | 19(76) |
| Limb edema | 3(42) | (0) | 3(42) | 2(18) | (0) | 2(18) | 3(75) | (0) | 3(75) | 1(33) | (0) | 1(33) | 9(36) | 0(0) | 9(36) |
| Non-cardiac chest pain | 1(14) | (0) | 1(14) | 1(9) | (0) | 1(9) | 1(25) | (0) | 1(25) | (0) | (0) | (0) | 3(12) | 0(0) | 3(12) |
| Weight loss | 2(28) | (0) | 2(28) | 1(9) | (0) | 1(9) | (0) | (0) | (0) | 2(66) | (0) | 2(66) | 5(20) | 0(0) | 5(20) |
There were a total of 59 serious adverse events (SAEs) across 21 of the 25 patients who started treatment on this study. Seven of the SAEs were deemed as probably or possibly related to decitabine and selinexor, but none were DLTs. Four of the SAEs were fatal and included sepsis (n=2), acute kidney injury, and respiratory failure. The majority of the remainder of the SAEs resulted in hospitalizations as a result of sepsis, infections and febrile neutropenia.
Four patients died during the first cycle or prior to the first bone marrow biopsy for disease response assessment. Three of these deaths were attributed to AML and one was attributed to typhlitis that was considered as possibly related to decitabine and selinexor.
Efficacy
Among all 25 patients, 10 responded to therapy (40%, 95% CI: 21% to 61%) (Table III). Of the 12 relapsed patients, 3 patients (25%) achieved CR, CRi, or MLFS. Of the 8 primary refractory patients, 3 patients (38%) achieved CR, CRi or morphologic leukemia free state (MLFS). Overall, in 20 patients with R/R AML, 6 responded to therapy; 3 patients achieved CR, 1 had CRi and 2 achieved MLFS. Of the 6 responders with R/R disease, 2 presented with normal karyotype, 2 with complex karyotype, including one patient with therapy-related AML, 1 with inversion 16 and 1 with del(9q). Four of the responders with R/R disease were able to proceed with allogeneic stem cell transplantation and had no evidence of disease at the time of transplant. Four patients with R/R disease died prior to bone marrow examination for disease response assessment and were considered non-responders.
Table 3.
Disease responses, n (%)
| Best response | All (n = 25) | Elderly untreated (n=5) | Relapsed/Refractory (n=20) | 23 mg/m2 (n=7) | 30 mg/m2 or 60mg fixed dose (n=11) | 40 mg/m2 (n=4) | 55 mg/m2 (n=3) |
|---|---|---|---|---|---|---|---|
| ORR (CR/CRi/MLFS) | 10 (40) | 4 (80) | 6 (30) | 2 (29) | 4 (36) | 3 (75) | 1 (33) |
| CR | 5 (20) | 2 (40) | 3 (15) | 2 (29) | 3 (27) | 0 (0) | 0 (0) |
| CRi | 3 (12) | 2 (40) | 1 (5) | 0 (0) | 0 (0) | 2 (50) | 1 (33) |
| MLFS | 2 (8) | 0 (0) | 2 (10) | 0 (0) | 1 (9) | 1 (25) | 0 (0) |
| SD | 10 (40) | 1 (4) | 9 (45) | 4 (57) | 4 (36) | 0 (0) | 2 (67) |
| PD | 1 (4) | 0 (0) | 1 (5) | 0 (0) | 1 (9) | 0 (0) | 0 (0) |
| NE | 4 (16) | 0 (0) | 4 (20) | 1 (14) | 2 (18) | 1 (25) | 0 (0) |
Abbreviations: CR, complete remission; CRi, complete remission with incomplete count recovery; MLFS, morphologic leukemia free state; NE.
In five newly diagnosed older adults over age 60, four responded to therapy; two patients achieved CR and two had CRi. Of these responders, patients presented with normal karyotype (n=2), monosomy 7 (n=1), and del(7q) with +13 (n=1). The latter two patients also achieved cytogenetic remission. Characteristics and outcomes for these patients are shown in Table IV.
Table 4.
Disease characteristics and outcomes for previously untreated AML patients
| Patient No. | Age/Gender | AML Type | Cytogenetics | Molecular Features | No. induction cycles received | No. maintenance cycles received | Disease response | PFS (days) | OS (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 83/F | De novo | Normal | CEBPAdm-/FLT3-/NPM1− | 1 | 0 (received 5-day decitabine maintenance off study) | CR | 264 | 407 |
| 2 | 77/F | De novo | Normal | CEBPAdm-/FLT3-/NPM1+ | 4 | 0 | SD | 233 | 233 |
| 3 | 81/M | tAML | del(7q), +13 | CEBPAdm-/FLT3-/NPM1− | 2 | 1 (disease progression) | CRi | 360 | 392 |
| 4 | 65/F | De novo | Normal | CEBPAdm-/FLT3-ITD+/NPM1− | 2 | 0 (died after cycle 2) | CRi | 76 | 76 |
| 5 | 73/F | De novo | −7 | FLT3-ITD negative (NPM1 and CEBPAdm unknown) | 2 | 1 | CR | 382a | 382a |
censored at last follow-up
Abbreviations: CEBPAdm, CEBPA double mutated; OS, overall survival; PFS, progression free survival; tAML, therapy-related AML
The median overall survival for the entire cohort was 5.9 (95% CI 3.9-10.4) months, and progression free survival (PFS) for the entire cohort was also 5.9 (95% CI 2.4-8.7) months. For responders, PFS was 11.8 months vs 4.4 months for non-responders (P=0.06). OS for responders was 12.9 months compared to 5.9 months for non-responders (P=0.14). Among 8 patients who achieved CR or CRi, 3 proceeded to transplant on day 22, 36 and 43 after responding; 3 relapsed on day 223, 299 and 313 following response; 1 died without relapse on day 1 following response; and 1 was lost to follow-up and died on day 855 following response (Supporting Information Figure S1).
Correlative studies
To assess whether recurrent AML mutations could be associated with treatment response we performed a targeted resequencing on a subset of patients (n=22) for whom DNA was available from BM before treatment. We did not observe any specific association between the presence of any specific mutation and response to therapy Supporting Information Table SV). In particular, we did not note loss of the NPM1 clone, in four NPM1-mutated patients, below a VAF of 5% (Supporting Information Figure S2).
To assess whether a specific pattern of DNA methylation was associated with treatment response, we analyzed the DNA methylation levels of approximately 850,000 CpG sites genome-wide from 16 patients with favorable response (CR or CRi; n=5) and unfavorable response (PD, SD; n=11) using the Infinium MethylationEPIC BeadChip arrays (Illumina). We performed a supervised analysis comparing favorable and unfavorable groups. No differences in CpG methylation levels between groups reached statistical significance (false-discovery rate q<0.2).
DISCUSSION
Current treatment options for older patients with AML who are unfit to receive intensive chemotherapy and those with R/R disease are limited, underscoring a need for evaluation of novel therapies. In this phase 1 clinical trial, patients with R/R AML and older (> 60 years of age) newly diagnosed patients with AML were treated with 10-day decitabine induction cycles followed by escalating doses of selinexor, across four dose levels, ranging from 23 mg/m2-55 mg/m2 in order to determine safety, tolerability and preliminary clinical activity of the combination.
There were no protocol defined DLTs. However, eight patients withdrew consent as a result of grade 1 or grade 2 gastrointestinal toxicities, typically after one cycle of therapy. Eleven patients (44%) developed grade 1 or 2 anorexia, none of which required temporary discontinuation of selinexor. After learning more about the toxicity profile of selinexor and from data from other trials, a 60 mg flat dose for four twice weekly doses was determined to be the RP2D. Using this schedule, we observed improved tolerability as noted by fewer nonhematologic toxicities that resulted in (Table II and Supporting Information Table SIV) less patient withdrawals from the study (Fig 1).
Although the number of older newly diagnosed patients treated with selinexor and decitabine was small, 4 of 5 patients achieved CR/CRi, suggesting there might be a signal of improved disease response compared to newly diagnosed older patients treated only with 10-day decitabine induction (CR/CRi rate of 47%) [17]. This hypothesis would need to be tested in a larger study. Furthermore, in single-agent 10-day decitabine studies, the grade 3 or higher fever and infection rate was reported to be between 55%-58% prior to neutrophil recovery [17,18] compared with a febrile neutropenia rate of 44% in selinexor and decitabine treated patients (Table II).
In contrast to the single-agent experience with selinexor, [10] patients treated with decitabine followed by selinexor experienced higher rates of grade 1 or grade 2 fatigue (76% vs 60%), nausea (64% vs 53%) and diarrhea (44% vs 39%). The combination was also associated with a greater frequency of grade 3 or grade 4 hyponatremia, febrile neutropenia, sepsis, hyperglycemia, hypertension, hypophosphatemia and lung infection compared to single-agent selinexor [10].
Grade ≥ 3 hyperglycemia, was observed in 40% of patients in our study. None of these patients were felt to have hyperglycemia related to selinexor. Two patients developed steroid-induced hyperglycemia due to dexamethasone that was given as a premedication prior to selinexor dosing. Two patients had pre-existing diabetes which worsened either in the setting of sepsis (in the case of one patient) or steroids received as premedication prior to selinexor dosing. The remaining six patients developed transient grade 3 hyperglycemia, typically towards the end of the cycle, lasting 1–3 days, and was always within the context of concurrent sepsis. Only one of these six patients required sliding scale insulin, temporarily, while hospitalized.
Hyponatremia, across all grades, affected 96% of all participants in this study, with 68% of patients experiencing hyponatremia grade 3 or higher. All of the patients who experienced grade ≥ 3 hyponatremia achieved normalization of serum sodium levels upon temporary drug discontinuation and with supportive care (intravenous fluids and salt tablets, if needed) per protocol. Five patients who developed grade 3 hyponatremia on a day they were scheduled to be dosed for selinexor, had the drug held per protocol. The remaining 11 patients developed grade 3 hyponatremia after their final dose of selinexor for the cycle, and their sodium improved to normal prior to the start of the next cycle. In these cases, the drug was not held per protocol criteria, but was not given because the patients had completed all required doses of selinexor for the cycle. Hyponatremia is a common adverse event in a number of selinexor trials and although the precise mechanism is not clear, in the majority of cases, it is thought to be a secondary complication of poor oral intake, dehydration, anorexia, diarrhea and sepsis, all of which were also frequent in patients receiving this therapy. Rare occurrences of hyponatremia have also been attributed to translational hyponatremia as a result of hyperglycemia.
Although modification of selinexor dosing and schedule resulted in improved tolerability, the majority of patients were still only able to receive one cycle of therapy due to grade 1 or 2 nausea or anorexia. Recently, a newer generation of selinexor named eltanexor (KPT-8602) was reported to be as effective as selinexor in preclinical models of chronic lymphocytic leukemia and in AML.[19,20] Eltanexor appears to result in less weight loss and anorexia than selinexor in mice. It is thought that the improved safety profile is due to the fact that eltanexor has less central nervous system penetration than selinexor. Eltanexor is currently being tested in phase 1 clinical trials [21].
The combination of decitabine and selinexor produced an ORR of 40%, which included five patients who achieved CR, three with CRi and two with MLFS. Of the 20 patients with R/R AML, combination therapy produced an ORR of 30%, consisting of three patients with CR, one with CRi and two with MLFS. This finding is encouraging given that in 81 R/R AML patients treated with single agent selinexor, the ORR was 14% [10]. Although 10-day decitabine induction is known to show greater disease activity in the upfront newly diagnosed setting, with CR rates ranging between 30-47%, [17,18,22] patients with R/R AML treated with this regimen have demonstrated a much lower CR rate of 15.7% [18]. As such, the combination of these two agents demonstrates preliminary evidence of improved disease activity, although a larger confirmatory study is needed to support this finding. Importantly, this regimen allowed four out of five R/R responders to proceed with allogeneic stem cell transplant for curative intent. None of these patients had evidence of disease at the time of their transplant.
Five patients treated with this combination were aged 60 years or more and had untreated disease. Of these patients, two achieved CR and two achieved CRi with an ORR of 80%. Some of these responders displayed high-risk cytogenetic features, including one responder with monosomy 7, one with trisomy 13 and another with del(7q); the latter two patients achieved cytogenetic remission as well (Table IV).
The combination of 10-day decitabine followed by twice-weekly doses of selinexor appears to be an active regimen for patients with poor-risk AML, specifically those with R/R AML patients and older adults with untreated disease. Modification of selinexor to a flat dose of 60 mg twice a week for two weeks following decitabine therapy improved tolerability. However, low-grade treatment-related side effects, such as anorexia and fatigue, makes the use of this combination challenging in the elderly population. Future strategies to further improve tolerability in potential confirmatory studies include decreasing the dosing frequency of selinexor to once a week, which is the schedule currently being used in other malignancies, or using next generation SINE compounds, such as eltanexor.
Combination therapies of selinexor with other cytotoxic chemotherapy agents in R/R AML are currently being evaluated [23]. The identification of the subset of patients with AML that are responsive to selinexor as a single agent [10] remains elusive. Further efforts are needed to find predictive biomarkers for response to single and combination therapies.
Supplementary Material
Acknowledgements:
The authors would like to thank the patients who participated in this trial and their families, the coinvestigators, nurses, and study coordinators. This trial was registered at www.clinicaltrials.gov as # NCT02093403. This work supported by R35 CA197734 and the D. Warren Brown Family Foundation (JCB).
Conflict-of-interest disclosure: Research support for B.B. was provided by Karyopharm Therapeutics.
REFERENCES
- 1.Lowenberg B, Downing JA. Acute myeloid leukemia. The New England Journal of Medicine. 1999;341:1051–1062. [DOI] [PubMed] [Google Scholar]
- 2.Fung HY, Chook YM. Atomic basis of CRM1-cargo recognition, release and inhibition. Seminars in cancer biology. 2014. August;27:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdul Razak AR, Mau-Soerensen M, Gabrail NY, et al. First-in-Class, First-in-Human Phase I Study of Selinexor, a Selective Inhibitor of Nuclear Export, in Patients With Advanced Solid Tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016. December;34(34):4142–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gounder MM, Zer A, Tap WD, et al. Phase IB Study of Selinexor, a First-in-Class Inhibitor of Nuclear Export, in Patients With Advanced Refractory Bone or Soft Tissue Sarcoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016. September 10;34(26):3166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuruvilla J, Savona M, Baz R, et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin lymphoma. Blood. 2017. June 15;129(24):3175–3183. [DOI] [PubMed] [Google Scholar]
- 6.Turner JG, Dawson JL, Grant S, et al. Treatment of acquired drug resistance in multiple myeloma by combination therapy with XPO1 and topoisomerase II inhibitors. Journal of hematology & oncology. 2016. August 24;9(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etchin J, Sanda T, Mansour MR, et al. KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. British journal of haematology. 2013. April;161(1):117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranganathan P, Yu X, Na C, et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood. 2012. August 30;120(9):1765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etchin J, Montero J, Berezovskaya A, et al. Activity of a selective inhibitor of nuclear export, selinexor (KPT-330), against AML-initiating cells engrafted into immunosuppressed NSG mice. Leukemia. 2016. January;30(1):190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garzon R, Savona M. A phase I clinical trial of single-agent selinexor in acute myeloid leukemia. 2017. March 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranganathan P, Yu X, Santhanam R, et al. Decitabine priming enhances the antileukemic effects of exportin 1 (XPO1) selective inhibitor selinexor in acute myeloid leukemia. Blood. 2015. April 23;125(17):2689–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simons A, Shaffer LG, Hastings RJ. Cytogenetic Nomenclature: Changes in the ISCN 2013 Compared to the 2009 Edition. Cytogenetic and genome research. 2013;141(1):1–6. [DOI] [PubMed] [Google Scholar]
- 13.Kroll KW, Eisfeld AK, Lozanski G, et al. MuCor: mutation aggregation and correlation. Bioinformatics (Oxford, England). 2016. May 15;32(10):1557–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer research. 2001. October 01;61(19):7233–9. [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003. December 15;21(24):4642–9. [DOI] [PubMed] [Google Scholar]
- 16.DP MNaB. Evaluation of Response-Time Data Involving Transient States: An Illustration Using Heart-Transplant Data. Journal of the American Statistical Association. 1974;69(345):81–86. [Google Scholar]
- 17.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proceedings of the National Academy of Sciences of the United States of America. 2010. April 20;107(16):7473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritchie EK, Feldman EJ, Christos PJ, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leukemia & lymphoma. 2013. September;54(9):2003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etchin J, Berezovskaya A, Conway AS, et al. KPT-8602, a second-generation inhibitor of XPO1-mediated nuclear export, is well tolerated and highly active against AML blasts and leukemia-initiating cells. Leukemia. 2017. January;31(1):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hing ZA, Fung HY, Ranganathan P, et al. Next-generation XPO1 inhibitor shows improved efficacy and in vivo tolerability in hematological malignancies. Leukemia. 2016. December;30(12):2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornell RF, Rossi AC, Baz R, et al. Eltanexor (KPT-8602), a Second-Generation Selective Inhibitor of Nuclear Export (SINE) Compound, in Patients with Refractory Multiple Myeloma. Blood. 2017;130(Suppl 1):3134–3134. [Google Scholar]
- 22.Bhatnagar B, Duong VH, Gourdin TS, et al. Ten-day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leukemia & lymphoma. 2014. July;55(7):1533–7. [DOI] [PubMed] [Google Scholar]
- 23.Ranganathan P, Kashyap T, Yu X, et al. XPO1 Inhibition using Selinexor Synergizes with Chemotherapy in Acute Myeloid Leukemia by Targeting DNA Repair and Restoring Topoisomerase IIalpha to the Nucleus. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016. December 15;22(24):6142–6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


