Abstract
The SARS-CoV-2 pandemic co-occurred with pollen season in Europe 2020 and recent studies suggest a potential link between both. Air samples collected at our measuring station in Leipzig and purified pollen were analyzed for SARS-CoV-2 typical signals or for virus-induced cytopathic effects, to test if the virus could bind to bioaerosols and if so, whether these complexes are infectious. The results show that neither our air samples nor purified pollen were infectious or could act as carrier for virus particles.
Keywords: SARS-CoV-2, Covid-19, Pollen, Particulate matter, Air quality, Cyclone sampler
Graphical abstract

1. Introduction
The analysis of the first pandemic phase of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Europe 2020 demonstrated that infections co-occurred with the pollen season (Brandstetter et al., 2020). Moreover, pollen may interfere with antiviral immunity, e.g. pollen significantly diminish the epithelial response to rhinovirus infection (Gilles et al., 2020). To date it is not known, if pollen allergy has an impact on the prevalence or severity of the SARS-CoV-2 pandemic. In China, allergic diseases, asthma, and COPD were not shown to be risk factors for SARS-CoV-2 infection (Zhang et al., 2020), but in this region the maximum peak was reached during winter time, outside the pollen season and patients were not interviewed for any history of pollinosis.
Importantly, pollen can act as a carrier for various bacteria and moulds (Heydenreich et al., 2012; Obersteiner et al., 2016; Oteros et al., 2019), and SARS-CoV-2 remained viable in aerosols for several hours (van Doremalen et al., 2020). Furthermore, Morawska and Cao (2020) (Morawska and Cao, 2020) speculate that wind-induced dispersion may occur. It was speculated, if bioaerosols may serve as carriers for the virus as regions with high air pollution were more severely affected (Conticini et al., 2020) and just recently it could be demonstrated that SARS-CoV-2 RNA can be present on particulate matter (PM) (Setti et al., 2020). We therefore aimed at investigating whether SARS-CoV-2 can bind to pollen or other kind of particulate matter within bioaerosols sampled at our station in Leipzig and if so, whether these complexes are infectious.
2. Methods
2.1. Sampling of airborne particles and fresh plant pollen
An air quality measurement station at the University of Leipzig Medical center (51.330847 N 12.387845E, height 17.3 m asl) includes: a seven-day recording volumetric pollen trap (Hirst-type trap, Burkhard Manufacturing Co Ltd., Hertfordshier, UK; volumetric flow rate of approx. 10 L/min), a cyclone trap (Burkhard Manufacturing Co Ltd., Hertfordshire, UK; volumetric flow rate of approx. 15 L/min) and a sensor for particulate matter concentrations (FDS15, Dr. Födisch Umweltmesstechnik, Markranstädt, Germany). Airborne particles were collected over 15 days in the first sampling period and over 7 days in all subsequent samples, directly into 1.5 mL micro centrifuge tubes (Camlab Limited, Cambridge, UK). The dry pellet was filled with 200 μL D-PBS-buffer (w/o calcium, w/o magnesium) (Biowest, Nuaillé, France) and a subsample of 40 μL was used for subsequent tests. Individual tests for SARS-CoV-2 were conducted from March 11th–June 6th 2020.
Fresh pollen samples from Betula pendula, Quercus robur and Ostrya carpinifolia were collected (Table A1). Inflorescences were dried in paper bags at 30°C and pollen were sieved through a 70 μm filter (CellTrics, Sysmex, Norderstedt, Germany) and stored dry upon analysis. Highly purified reference material (Alnus glutinosa, Betula pendula and Corylus avellana) were purchased from Allergon AB (Ängelsholm, Sweden).
2.2. Virus isolation
Attempts of SARS-CoV-2 isolation were made using natural environmental air samples (described above, Table 1 ). Following centrifugation at 11,000 rpm (5 min/ room temperature) supernatant was removed and saved for PCR testing (CoV PCR day 0). The pellet was resuspended in DMEM supplemented with nystatin/amphothericin (40 μg/mL/50 μg/mL), seeded onto Vero E6 cells in a 2.5 cm2 petri dish, incubated at 37 °C/5% CO2 and observed for 5 days for the occurrence of virus-induced cytopathic effects (CPE). Negative cultures were split and observed for another 3 days, when 200 μl TCS was obtained and cells were scraped-off (approximately 3 × 106 in 200 μL) and harvested for SARS-CoV-2 PCR. In another set of experiments commercially available highly purified pollen (Alnus glutinosa, Betula pendula and Corylus avellana) were incubated in vitro with SARS-CoV-2 (3 × 104 TCID50 in 0.5 ml DMEM for 1 h), washed 3× (DMEM, centrifuged at 3500 ×g) and then screened for presence of viral RNA and infectious SARS-CoV-2. In yet another separate set of experiments (serving as positive controls), SARS-CoV-2 was spiked to air samples with the indicated genome equivalents (Geq) to exclude the possibility that components of the air samples interfered with virus detection and replication (Table A2, Fig. A1).
Table 1.
SARS CoV-2 detection. Air samples and purified pollen, freshly collected in Leipzig or purchased from Allergon were tested for a SARS-CoV-2 specific signal via RT-PCR on day 0 and day 8 as well as tested for Virus-induced cytopathic effects (CPE) on Vero cells.
| Sample # | Sample description | SARS-CoV-2 PCR day 0a | Virus-induced CPE | SARS-CoV-2 PCR day 8 p.i.b |
|---|---|---|---|---|
| 120 | Week 11.03.-26.03.20 (Air sample) | Negativec | None | Negative |
| 121 | Week 26.03.-02.04.20 (Air sample) | Negative | None | Negative |
| 122 | Week 02.-09.04.20 (Air sample) | Negative | None | Negative |
| 123 | Week 09.-16.04.20 (Air sample) | Negative | None | Negative |
| 124 | Week 16.-23.04.20 (Air sample) | Negative | None | Negative |
| 127 | Week 07.05.-14.05.20 (Air sample) | Negative | None | Negative |
| 128 | Week 14.05.-21.05.20 (Air sample) | Negative | None | Negative |
| 129 | Week 21.05.-28.05.20 (Air sample) | Negative | None | Negative |
| 610 | Betula pendula (purified fresh pollen from Leipzig) | Negative | None | Negative |
| 631 | Ostrya carpinifolia (purified fresh pollen from Leipzig) | Negative | None | Negative |
| 673 | Quercus robur (purified fresh pollen from Leipzig) | Negative | None | Negative |
| A | Alnus glutinosa (Allergon) | Negative | None | Negative |
| B | Betula pendula (Allergon) | Negative | None | Negative |
| C | Corylus avellana (Allergon) | Negative | None | Negative |
| Ad | Alnus glutinosa (Allergon) | Negative | None | Negative |
| Bd | Betula pendula (Allergon) | Negative | None | Negative |
| Cd | Corylus avellana (Allergon) | Negative | None | Negative |
SARS-CoV-2 PCR; measured without prior cell cultivation, day 0.
SARS-CoV-2 PCR; measured after cultivation for 8 days.
Negative: <10 genome equivalents.
Preincubated with SARS-CoV-2 (3 × 104 TCID50) before extensive washing.
2.3. Nucleic acid (NA) extraction
Total NA was extracted from 200 μL of either supernatant of reconstituted airborne particles, cell culture supernatant or resuspended scraped-off-cells, using the DNA and Viral NA Small Volume Kit (Roche, Mannheim, Germany) on the MagNA Pure 96 instrument according to the manufacturer's instructions with the equivalent amount of lysis buffer and eluted in 100 μl. Extracted samples were subsequently tested by SARS-CoV-2-PCR or stored in aliquots at −80 °C until further use.
2.4. SARS-CoV-2 detection
One-Step real time -RT- PCR, targeting either E-gene or N/RdRp gene, was done according to Corman et al. (2020) (Corman et al., 2020) with slight modifications on a LightCycler 2.0 system (Roche, Mannheim, Germany) in 20 μl capillaries with SuperScript™ III Platinum® One-Step Quantitative RT-PCR kit (Thermofisher Scientific. Carlsbad, USA). In short, reaction was set up containing 12.5 μL of 2× reaction buffer, 0.4 μL of MgSO4 (50 mM), 1 μg bovine serum albumin (Roche), primers (E-Sarbeco_F1/R2, Metabion, Munich, Germany) at a final concentration of 0.4 μM each, probe (E-Sarbeco_P1, Tib-Molbiol, Berlin, Germany) at a final concentration of 0.2 μM, and 1 μL SSIII/Platinum Tag. Input-RNA was 5 μl, and 2.6 μl of water was added to reach a final reaction volume of 20 μl. Alternatively RdRp_SARSr-F2/R1 and RdRp_SARSr-P2/P1 were used for amplification and detection of the respective gene. RT was done at 55 °C for 10 min, followed by 3 min denaturation at 94 °C and 45 amplification cycles with denaturation for 15 s at 94 °C, annealing for 30 s at 58 °C and elongation for 30 s at 60 °C. Amplification was monitored after each elongation step at 530 nm.
3. Results
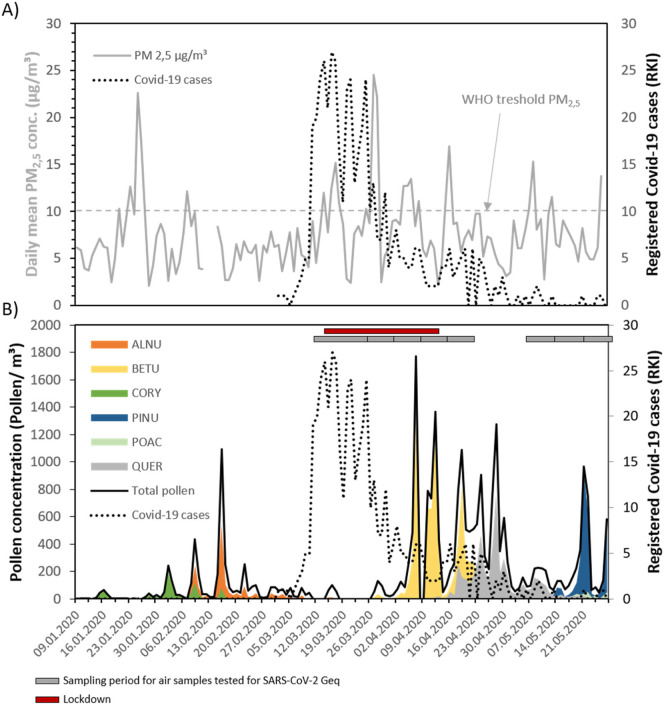
The pollen season in Leipzig, Germany started with a high number of hazel (Corylus sp.) pollen at the beginning of February 2020, followed by alder (Alnus sp.) one week later (Fig. 1 ). Nine days after the last higher Alnus sp. pollen peak (94 pollen/m3), the first Covid-19 cases were documented. Overall, low case numbers were registered in Leipzig in contrast to other German cities, most likely related to an early lockdown (red bar in Fig. 1). The birch (Betula sp.) pollen season started during the decrease of registered Covid-19 cases in April and was followed by pollen emission from oak (Quercus sp.) and pine (Pinus sp.). Particulate matter (PM2,5) concentrations were partially higher than the threshold level of 10 μg/m3 (annual mean) defined by the WHO (dotted grey line in Fig. 1). However, the concentrations were still lower than in regions of Northern Italy (Bianconi et al., 2020; Conticini et al., 2020) or Wuhan (Ma and Kang, 2020) and could be an additional reason for low registered case numbers in Leipzig.
Fig. 1.
COVID 19 cases and pollen and particulate matter concentrations in Leipzig, Germany A) Course of registered Covid-19 cases in Leipzig and daily mean particulate matter (PM2,5)-concentration which were verified with two official stations in Leipzig (“Leipzig-Mitte”, “Leipzig-West”), as well as B) pollen emission for total pollen concentration and most abundant plant species/family (ALNU – Alnus sp., BETU – Betula sp., CORY – Corylus sp., PINU – Pinus sp., POAC – Poaceae, QUER – Quercus sp.), measured at the roof of University of Leipzig Medical Center. Grey bars indicate sampling period for air samples as well as lockdown in Leipzig.
Air samples containing bioaerosols and particulate matter were collected starting with the first wave of infections (grey bars in Fig. 1). In none of these samples SARS-CoV-2 typical signals could be detected at any timepoint either by RT-PCR (Geq) or by analysis of virus-induced cytopathic effects (CPE) on Vero indicator cells (Table 1, Fig. A1).
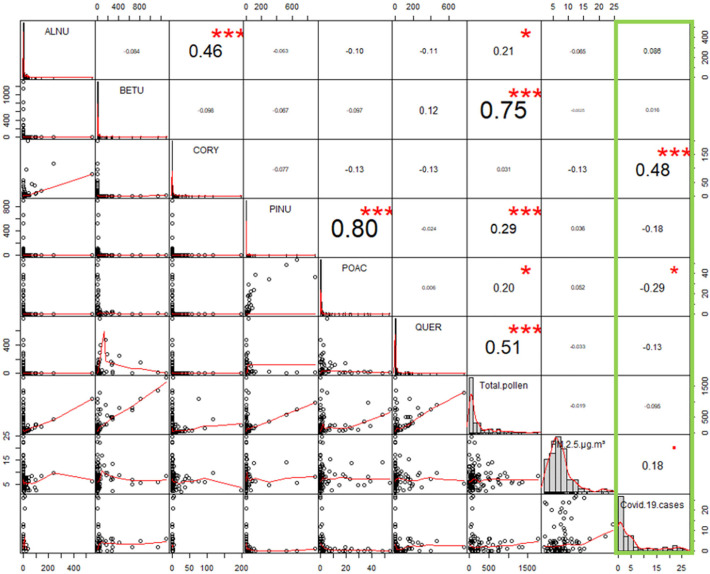
For a detailed analysis of a possible correlation between concentrations of the most abundant pollen, particulate matter and registered Covid-19 cases, a correlation matrix was created with R (package “PerformanceAnalytics”) (Fig. 2 ). The number of registered cases (green box, Fig. 2) was positively correlated with the pollen-concentration of Corylus sp. and negatively correlated with the concentrations of grasses (Poaceae).
Fig. 2.
Correlation matrix for most abundant pollen and COVID19 cases in Leipzig, Germany. Analyses from January 09th - May 27th 2020 for most abundant pollen (ALNU – Alnus sp., BETU – Betula sp., CORY – Corylus sp., PINU – Pinus sp., POAC – Poaceae, QUER – Quercus sp.) and PM2,5 against registered Covid-19 cases (green box). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In addition, pollen of different taxa were directly collected from trees (Betula pendula, Quercus robur and Ostrya carpinifolia), and tested negatively for SARS-CoV-2 signals (Table 1). Next, we wished to determine whether pollen can bind SARS-CoV-2 at all. To address this issue, commercially available, highly purified pollen (Alnus glutinosa, Betula pendula and Corylus avellana) were incubated in vitro with SARS-CoV-2 (3 × 104 TCID50 in 0.5 ml DMEM, i.e. approx. 1 × 107 genome equivalents) for 1 h. Thereafter, pollen was washed, centrifuged as indicated in the supplement before they were screened for presence of viral RNA and infectious SARS-CoV-2. Again, no SARS-CoV-2 typical signal was detected by RT-PCR or CPE at any time point (Table 1). Moreover, we could exclude the possibility that components of the air samples interfered with virus detection since Geq of SARS-CoV-2 spiked to original samples were clearly detected by RT-PCR or CPE (Fig. A1, Table A2).
4. Discussion
In aggregate, our study from Leipzig, Germany could show a positive correlation of Corylus sp. pollen concentration and registered Covid-19 cases, but neither air samples collected during March 11th -June 6th 2020 nor purified Alnus glutinosa, Corylus avellana, Betula pendula, Quercus robur and Ostrya carpinifolia pollen carried or transmitted SARS-CoV-2. We are somewhat consoled by this negative finding since a positive result would have had severe implications for SARS-CoV-2 restrictions, as pollen can be transported over long distances (up to several 100 km) (Hjelmroos, 1991).
It should be noted however, that due to a low number of registered Covid-19 cases in Leipzig, it may be difficult that a measurable virus load can be detected in the air due to low number of emitters, especially since infected persons also had to go into quarantine immediately. Therefore, a negative result for SARS-CoV-2 is not surprising for this case study, but could potentially be different in cities with much higher infection rates. Statistically, it is highly probable to find virus on pollen and particulate matter when the concentration of both is really high also meaning that there is a coalescence effect between droplets and PM. This probably confirms that the spread effect in Leipzig was essentially due to the direct contact human-to-human and not mediated by a vehicle, while in regions with higher PM load, transmission via PM could be an additional pathway (Setti et al., 2020).
We also considered technical limitations to account for our failure to detect SARS-2 CoV-2 signals in air samples and in purified pollen preparations. Air sampling: Several authors have shown, that cyclone samplers are suitable to collect virus particles in the air (D'Arcy et al., 2014; Kim et al., 2018; Verreault et al., 2008; West and Kimber, 2015). D'Arcy et al. (2014) (D'Arcy et al., 2014) successfully detected airborne virus particle in hospital air with a cyclone sampler, which is in accordance with the fact that this technology was originally developed to collect biological warfare agents in the air. The height of our measurement station was chosen to guarantee a representative measurement in contrast to near-ground stations which show higher variability in pollen concentrations (Rojo et al., 2019). Nevertheless, it would be interesting to analyze if SARS-CoV-2 signals in near-ground traps, e.g. on crowded public places can be detected. SARS-CoV-2 detection: Nucleic acids were analyzed by RT-PCR and infectivity was tested by analysis of CPE on Vero indicator cells. We could exclude that suspended air samples or purified pollen interfered with SARS-CoV-2 replication since SARS-CoV-2 spiked to the original samples was readily detected (Fig. A1, Table A2). Our in vitro incubation experiments of highly purified pollen and SARS-CoV-2 were performed in fluid suspension. However, the binding pattern between pollen and virus particles could be potentially different in the air, e.g. due to electrostatic effects. Such effects might be of interest in future studies.
In summary, this is a first study investigating the relation of pollen and SARS-CoV-2 pandemic. The results show that neither air samples nor purified pollen of different taxa were infectious or could act as a carrier for virus particles. This leaves open the possibility that pollen or particulate matter within bioaerosols may affect SARS-CoV-2 susceptibility indirectly for example by perturbing nasal or bronchial epithelial barrier function or anti-viral immunity as shown by others for rhinovirus infection (Gilles et al., 2020).
CRediT authorship contribution statement
The manuscript draft was jointly written by SD, JCS, UGL, RT, TH, GS, MM, MB and JB, all contributing substantially to the final draft, as well as performing experiments (GS, MM, UGL), counting pollen and evaluation of data (MB), collecting samples (TH) or providing conceptual input (JCS, RT, JB, SD).
Declaration of competing interest
SD, TH, GS, MM, MB, JB, UGL and J-CS have nothing to disclose and RT indicated Sanofi Genzyme, Novartis, ALK-Abello, AbbVie, Shire, Fraunhofer Institute IZI and Hautnetz Leipzig e.V. as potential conflicts of interest.
Acknowledgments
Acknowledgement
Studies were co-financed by the German Science Foundation via the iDiv-Flexpool (project number: 34600830-13) and the research project Smart Sensor-based Digital Ecosystem Services (S2DES), funded by the European Social Fund (ESF; Grant Agreement No. 100269858). We especially want to thank the Dr. Födisch Umweltmesstechnik AG for providing the FDS15 sensor. In addition, we want to thank Paul Remmler for the technical support during the installation and maintenance of the measuring station, as well as Marcus Karsten for sampling.
Editor: Jay Gan
Appendix A.
Table A1.
Overview about tested pollen from different plant species, including sample origin, as well as sampling date and location or distributor.
| Species | Sample origin | Sampling date/location or distributor |
|---|---|---|
| Betula pendula | Fresh | 09.04.2020/51.353034 N, 12.432500E |
| Quercus robur | Fresh | 17.04.2020/51.327584 N, 12.395160E |
| Ostrya carpinifolia | Fresh | 09.04.2020/51.333510 N, 12.402796E |
| Betula pendula | Reference material | Allergon, Ängelsholm, Sweden |
| Corylus avellana | Reference material | Allergon, Ängelsholm, Sweden |
| Alnus glutinosa | Reference material | Allergon, Ängelsholm, Sweden |
Table A2.
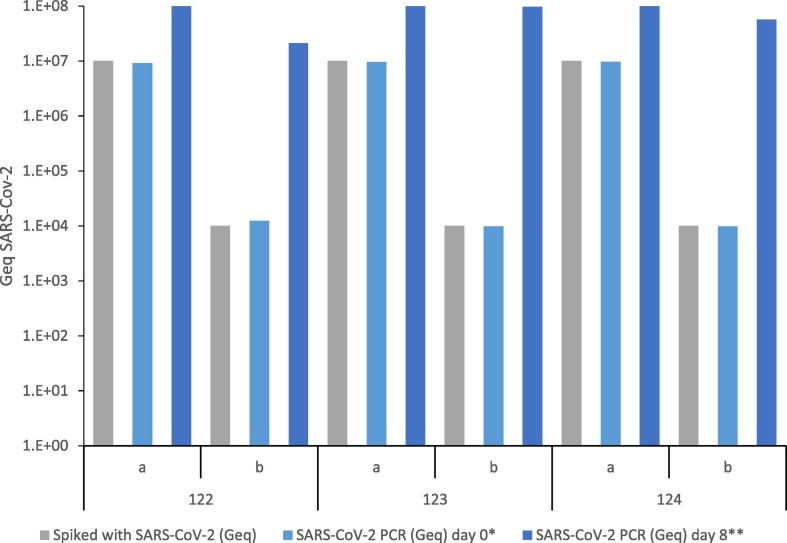
SARS CoV-2 detection – positive controls. Air samples from sampling period 02.-09.04.2020 (sample 122), 09.-16.04.2020 (sample 123) and 16.-23.04-2020 (sample 124) were spiked with a) 1 × 107 and b) 1 × 104 SARS-CoV-2 genome equivalents (Geq) to exclude a potential interference of sample matrix to Geq detection. * SARS-CoV-2 PCR; E, RdRp and N gene, measured without prior cell cultivation, day 0; ** SARS-CoV-2 PCR; E, RdRp and N-Gen, measured after cultivation for 8 days.
| Sample # | Characteristics | Spiked with SARS-CoV-2 (Geq) | SARS-CoV-2 PCR (Geq) day 0a | Virus-induced CPE | SARS-CoV-2 PCR (Geq) day 8b |
|---|---|---|---|---|---|
| 122a | Week 02.-09.04. (Air sample) | 1 × 107 | 0.91 × 107 | +++ | >1 × 108 |
| 122b | Week 02.-09.04. (Air sample) | 1 × 104 | 1.24 × 104 | ++ | 2.1 × 107 |
| 123a | Week 09.-16.04. (Air sample) | 1 × 107 | 0.96 × 107 | +++ | >1 × 108 |
| 123b | Week 09.-16.04. (Air sample) | 1 × 104 | 0.98 × 104 | ++ | 9.7 × 107 |
| 124a | Week 16.-23.04. (Air sample) | 1 × 107 | 0.97 × 107 | +++ | >1 × 108 |
| 124b | Week 16.-23.04. (Air sample) | 1 × 104 | 0.98 × 104 | ++ | 5.7 × 107 |
Note: there was no inhibition of SARS-CoV-2 replication.
SARS-CoV-2 PCR; E, RdRp and N gene, measured without prior cell cultivation, day 0.
SARS-CoV-2 PCR; E, RdRp and N-Gen, measured after cultivation for 8 days.
Fig. A1.
Positive controls. Air samples from sampling period 02.-09.04.2020 (sample 122), 09.-16.04.2020 (sample 123) and 16.-23.04-2020 (sample 124) were spiked with a) 1 × 107 and b) 1 × 104 SARS-CoV-2 genome equivalents (Geq) to exclude a potential interference of sample matrix to Geq detection. * SARS-CoV-2 PCR; E, RdRp and N gene, measured without prior cell cultivation, day 0; ** SARS-CoV-2 PCR; E, RdRp and N-Gen, measured after cultivation for 8 days.
References
- Bianconi V., Bronzo P., Banach M., Sahebkar A., Mannarino M., Pirro M. Particulate matter pollution and the COVID-19 outbreak: results from Italian regions and provinces. Arch. Med. Sci. 2020;16(1) doi: 10.5114/aoms.2020.95336. https://www.termedia.pl/Particulate-matter-pollution-and-the-COVID-19-outbreak-results-from-Italian-regions-and-provinces,19,40670,1,1.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstetter S., Roth S., Harner S., Buntrock-Döpke H., Toncheva A.A., Borchers N., et al. Symptoms and immunoglobulin development in hospital staff exposed to a SARS-CoV-2 outbreak. Pediatr. Allergy Immunol. 2020;31:841–847. doi: 10.1111/pai.13278. https://onlinelibrary.wiley.com/doi/full/10.1111/pai.13278 [DOI] [PubMed] [Google Scholar]
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in northern Italy? Environ. Pollut. 2020;261 doi: 10.1016/j.envpol.2020.114465. http://www.sciencedirect.com/science/article/pii/S0269749120320601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro surveillance bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. https://pubmed.ncbi.nlm.nih.gov/31992387/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcy N., Cloutman-Green E., Klein N., Spratt D.A. Environmental viral contamination in a pediatric hospital outpatient waiting area: implications for infection control. Am. J. Infect. Control. 2014;42(8):856–860. doi: 10.1016/j.ajic.2014.04.014. http://www.sciencedirect.com/science/article/pii/S0196655314006622 [DOI] [PubMed] [Google Scholar]
- Gilles S., Blume C., Wimmer M., Damialis A., Meulenbroek L., Gökkaya M., et al. Pollen exposure weakens innate defense against respiratory viruses. Allergy. 2020;75(3):576–587. doi: 10.1111/all.14047. https://onlinelibrary.wiley.com/doi/full/10.1111/all.14047?casa_token=ov52bDcEZbIAAAAA%3AdxriYJ-F5vPhU1kkyGA_8wBBuZlBEUuf3MwOCu_FCYF_cwu4z40t0gjnfcHHFkI2ck4_DlQ9fyKM9g [DOI] [PubMed] [Google Scholar]
- Heydenreich B., Bellinghausen I., König B., Becker W.-M., Grabbe S., Petersen A., et al. Gram-positive bacteria on grass pollen exhibit adjuvant activity inducing inflammatory T cell responses. Clin Exp Allergy. 2012;42(1):76–84. doi: 10.1111/j.1365-2222.2011.03888.x. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2222.2011.03888.x?casa_token=LbFd6qmnJ0AAAAAA%3A9JhtbjYa-MoNxmrzoiLTSQinLT2FmP0hCh5dRBl-4PSZFEZ0OMJ955xXCTzk0hy6oQa1kIYynKYuWg [DOI] [PubMed] [Google Scholar]
- Hjelmroos M. Evidence of long-distance transport of betula pollen. Grana. 1991;30(1):215–228. [Google Scholar]
- Kim H.-U., Min J., Park G., Shin D., Sung G., Kim T., et al. Electrochemical detection of airborne influenza virus using air sampling system. Aerosol Air Qual. Res. 2018;18:2721–2727. https://scholarworks.bwise.kr/cau/handle/2019.sw.cau/602 UR. [Google Scholar]
- Ma C.-J., Kang G.-U. Air quality variation in Wuhan, Daegu, and Tokyo during the explosive outbreak of COVID-19 and its health effects. Int. J. Environ. Res. Public Health. 2020;17(11):4119. doi: 10.3390/ijerph17114119. https://www.mdpi.com/1660-4601/17/11/4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139 doi: 10.1016/j.envint.2020.105730. http://www.sciencedirect.com/science/article/pii/S016041202031254X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obersteiner A., Gilles S., Frank U., Beck I., Häring F., Ernst D., et al. Pollen-associated microbiome correlates with pollution parameters and the allergenicity of pollen. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0149545. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0149545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteros J., Bartusel E., Alessandrini F., Núñez A., Moreno D.A., Behrendt H., et al. Artemisia pollen is the main vector for airborne endotoxin. J. Allergy Clin. Immunol. 2019;143(1):369–377.e5. doi: 10.1016/j.jaci.2018.05.040. http://www.sciencedirect.com/science/article/pii/S0091674918309990 [DOI] [PubMed] [Google Scholar]
- Rojo J., Oteros J., Pérez-Badia R., Cervigón P., Ferencova Z., Gutiérrez-Bustillo A.M., et al. Near-ground effect of height on pollen exposure. Environ. Res. 2019;174:160–169. doi: 10.1016/j.envres.2019.04.027. http://www.sciencedirect.com/science/article/pii/S0013935119302439 [DOI] [PubMed] [Google Scholar]
- Setti L., Passarini F., de Gennaro G., Barbieri P., Perrone M.G., Borelli M., et al. SARS-Cov-2RNA found on particulate matter of Bergamo in northern Italy: first evidence. Environ. Res. 2020;188:109754. doi: 10.1016/j.envres.2020.109754. http://www.sciencedirect.com/science/article/pii/S0013935120306472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreault D., Moineau S., Duchaine C. Methods for sampling of airborne viruses. Microbiol. Mol. Biol. Rev. 2008;72(3):413–444. doi: 10.1128/MMBR.00002-08. https://mmbr.asm.org/content/72/3/413.short [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J.S., Kimber R.B. Innovations in air sampling to detect plant pathogens. Ann. Appl. Biol. 2015;166(1):4–17. doi: 10.1111/aab.12191. https://onlinelibrary.wiley.com/doi/full/10.1111/aab.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. https://onlinelibrary.wiley.com/doi/full/10.1111/all.14238 [DOI] [PubMed] [Google Scholar]