Abstract
Background & aim
Verify the prevalence of hypovitaminosis D and obesity in elderly patients infected by new coronavirus. The patients developed severe symptoms and were admitted in intensive care unit (ICU) to receive invasive ventilation due to diagnosis of acute respiratory distress syndrome (ARDS).
Methods
A cross-sectional descriptive study composed of elderly (age ≥ 60 years) admitted to the ICU. Were collected demographic (sex, age), anthropometric data, presence of comorbidities (hypertension, diabetes, heart disease, lung, neurological and oncological diseases), severity score in ICU (SAPS III), PaO2/FiO2 ratio, analysis of C-reactive protein (CRP) and serum dosage of 25-hydroxy vitamin D (25 OHD) in the first day of hospitalization to identify elderly with hypovitaminosis D (low values < 30 ng/mL). The diagnosis of obesity in elderly was determined by calculating the body mass index (BMI) ≥ 30 kg/m2.
Results
A total of 176 elderly met the inclusion criteria. 54% were elderly men and mean age of 72.9 ± 9.1 years. The median BMI was 30.5 (28.1–33) kg/m2 with 68.7% having a nutritional diagnosis of obesity and 15.3% had BMI ≥ 35 kg/m2. The most prevalent comorbidities were hypertension (72.2%) and diabetes (40.9%). Prevalence of hypovitaminosis D with values of 25 OHD <30 ng/mL, < 20 ng/mL and <10 ng/mL was 93.8%, 65.9% and 21% respectively. The prevalence of hypovitaminosis D (<30 ng/mL) in obese elderly was 94.2%. There was a negative and significant bivariate correlation between BMI and levels of 25 OHD (r = - 0.15; p = 0.04).
Conclusion
Hypovitaminosis D and obesity in elderly have a high prevalence in critically ill patients in ICU infected by the new coronavirus. Laboratory investigation of vitamin D becomes important, especially in obese elderly patients.
Keywords: SARS-CoV-2, Vitamin D, Obesity, Elderly, ICU, COVID-19
1. Introduction
The pandemic caused by the new coronavirus (SARS-CoV-2) emerged in Wuhan province, China, in November 2019, and has caused infections with varied clinical presentations, ranging from asymptomatic patients, mild flu syndrome to severe respiratory failure. There is still no complete data on how this new human infection behaves, however, based on data from China, the epicenter of the epidemic, it is estimated that 80% of contaminated patients develop infections with mild symptoms and 5% may develop an acute respiratory distress syndrome (ARDS) that requires hospitalization in the intensive care unit (ICU) [1,2], usually after 3–4 days of infection. In Brazil, mortality is estimated at 5.3% [3] and it has already been identified that individuals at higher risk, such as the elderly, obese and chronic diseases (diabetes, hypertension and cancer), confer a high risk for developing severe respiratory infection and consequently evolving to ARDS [4].
ARDS is a clinical condition characterized by an intense systemic inflammatory state and refractory hypoxemia, which can progress to multiorgan failure and death [5]. There is an excessive release of pro-inflammatory cytokines, activation of pro-coagulating factors and increased oxidative stress [6,7]. Consequently, the alveolar edema produced reduces lung compliance, explaining the need to use invasive mechanical ventilation. The systemic inflammatory state has critical metabolic consequences such as hyperglycemia, increased energy expenditure and loss of muscle proteins [8]. Given this demand, many nutritional strategies have been suggested in the course of severely ill patients with ARDS.
Although there is still little evidence regarding serum micronutrient levels in critical care, much less in ARDS, there is a consensus that deficiency may occur, not only due to reduced intake and impaired absorptive processes at this stage, but also due to the high consumption of vitamins and minerals caused by hypermetabolism [9]. However, micronutrients have considerable importance in the course of the illness, especially in ARDS, with emphasis on vitamin D [10]. It is a steroid hormone obtained both endogenously (skin synthesis by sun exposure) and through diet. Although it is critical to maintaining bone homeostasis, it performs numerous functions in the body, mainly in modulating the immune system [10,11].
The prevalence of vitamin D deficiency reaches 50% of patients admitted to the ICU, and this is associated with worse clinical outcomes, such as increased infection/sepsis rate and increased mortality [12]. Therefore, the adequacy of this vitamin to recommended levels can recover the immune system after infectious outbreaks, in addition to supporting better immune protection against viral infections [13,14].
In addition to vitamin D deficiency, obesity is also related to worse outcomes in ICU, explained by changes in the immune response, both acquired and innate and by the perpetuation of a low-grade chronic inflammatory state [15]. In addition, obesity worsens the various comorbidities related to the COVID-19 (Coronavirus Disease - 2019): hypertension, diabetes, lung, and heart diseases [16].
Given the above considerations, this study has as main objective to verify the prevalence of obesity and hypovitaminosis D in elderly patients admitted to the intensive care unit due to SARS-CoV-2.
2. Materials and methods
2.1. Study population
This is a cross-sectional descriptive study and data were collected in the first 24 h of admission to the ICU, at Sancta Maggiore Hospital (Prevent Senior Private Operative, São Paulo, Brazil) in the period from March 15 to April 15, 2020. The healthcare protocols for COVID-19 started in the emergency department and they were continued in the ICU. Patients were consecutively included in the study as they were admitted to the ICU when they met the inclusion criteria.
The inclusion criteria were all consecutive patients admitted in ICU; age equal to or older than 60 years; data collected in the first day of ICU admission; diagnosis of acute respiratory distress syndrome defined as a ratio of arterial oxygen tension over fractional inspired oxygen - PaO2/FiO2 < 300; positive swab from the nasal cavity and oropharynx for detection of viral RNA (ribonucleic acid) for COVID-19 using the reverse-transcription polymerase chain reaction (RT-PCR) technique; computed tomography (CT) scan of the chest showing bilateral interstitial infiltrate pulmonary with a typical “ground-glass” pattern. The exclusion criteria were patients under 60 years old; negative swab for COVID-19 (RT-PCR); patients who had previously used cholecalciferol or calcitriol for any reason in the last month and or went through dialysis.
The study was reviewed and approved by the Research Ethics Committee of the Prevent Senior Institute (CAAE 30608020.9.0000.8114). All procedures were performed following the Declaration of Helsinki.
2.2. Variables and measures
Data of interest were collected for analysis of the population affected by COVID-19: demographic data (sex, age); anthropometric data such as weight (kilograms) and height (meters); severity score in the ICU such as SAPS III (Simplified Acute Physiology Score III) [17]; measurement of the PaO2/FiO2 ratio after orotracheal intubation and mechanical ventilation; the presence of comorbidities (systemic arterial hypertension, diabetes mellitus, chronic kidney disease, chronic obstructive pulmonary disease, asthma, heart diseases, neurological, oncological and immunosuppressed diseases or who use immunosuppressants); CRP (C-reactive protein) measurement in the first day of hospitalization. The data on weight and height were obtained from a survey with the family member or companion who lived with the elderly. From these data, body mass index (BMI) was calculated by dividing weight by height squared (kg/m2). Then, the BMI was stratified according to the cutoff points suggested by the Pan American Health Organization (Organización Panamericana de la Salud, OPS 2002) [18] for the elderly: BMI ≤ 23 kg/m2 (low weight), 23–28 kg/m2 (normal weight), 28 to 30 kg/m2 (overweight) and ≥30 kg/m2 (obesity).
The test requested for the analysis of vitamin D levels was the serum dosage of 25-hydroxy vitamin D (25 OHD), measured by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). The definition of hypovitaminosis D was standardized according to the criteria of the Brazilian Society of Endocrinology and Metabolism (SBEM) defined by a serum level < 20 ng/mL for general population and <30 ng/mL for individuals at risk [19]. As the individuals eligible for this study were all classified as high risk of clinical complications, the cutoff level defined in the study for hypovitaminosis was 25 OHD <30 ng/mL [20]. Severe deficiency was defined by 25 OHD <10 ng/mL [20].
2.3. Statistical analysis
For descriptive analysis, the variables were tested for normality using the Shapiro–Wilk test, normal distribution data expressed as mean and standard deviation (SD) and categorical data expressed as a percentage of frequency. Non-parametric data were described as median and interquartile range (IQR). Spearman correlation test was performed between non-parametric variables. Statistical significance was set at p < 0.05 and 95% confidence interval. Observational data were statistically analyzed using SPSS 24.0 software (version 24.0, SPSS Inc, Chicago, IL).
3. Results
Between March 15 and April 15, 2020, 25 OHD levels were analyzed in 176 elderly patients admitted to the ICU diagnosed with acute respiratory distress syndrome coronavirus 2 (SARS-CoV- 2). The mean age was 72.9 ± 9.1 years and 54% were men, according to the baseline characteristics of elderly who required invasive mechanical ventilation demonstrated in Table 1 .
Table 1.
Baseline characteristics of elderly admitted to the intensive care unit by SARS-CoV-2 who required invasive mechanical ventilation.
| Total of patients (n = 176) | |
|---|---|
| Sex F/M (%) | 81/95 (46/54) |
| Age (years) | 72.9 ± 9.1 |
| Weight (kg) | 82 (73–93) |
| BMI (kg/m2) | 30.5 (28.1–33) |
| SAPS III | 68 (52–84) |
| PaO2/FiO2 ratio | 125 (93.3–159.9) |
| 25 OHD (ng/mL) | 16 (10–21) |
| CRP (mg/L) | 134.5 (77.6–216.3) |
| Comorbidities (%) | |
| Hypertension | 127 (72.2) |
| Diabetes mellitus | 72 (40.9) |
| Heart diseases | 48 (27.3) |
| Lung diseases | 48 (27.3) |
| Neurological diseases | 30 (17) |
| Chronic kidney diseases | 23 (13.1) |
| Oncology | 13 (7.4) |
| Immunosuppressed | 8 (4.5) |
Normal distribution data expressed as mean and standard deviation (SD). Continuous values expressed in median and interquartile range (IQR) and categorical data as a percentage of proportion. F: female; M: male BMI: body mass index; SAPS III: Simplified Acute Physiology Score III; PaO2: arterial oxygen tension; FiO2: fractional inspired oxygen; 25 OHD: 25-hydroxy vitamin D; CRP: C-reactive protein.
The nutritional diagnosis provided by the BMI showed the prevalence rate of obesity (BMI ≥ 30 kg/m2) in 68.7% of the elderly and median BMI of 30.5 (IQR 28.1–33) kg/m2. The findings show that 15.3% of all elderly patients in ICU with SARS-CoV-2 had BMI ≥35 kg/m2.
The serum dosage of 25 OHD performed at the admission to the ICU showed a median value of 16 (IQR 10–21) ng/mL. The prevalence of 25 OHD <30 ng/mL was 93.8%, 25 OHD <20 ng/mL was 65.9% and 25 OHD <10 ng/mL (severe deficiency) was 21%. The prevalence of 25 OHD <30 ng/mL among the obese elderly was 94.2%.
There was a negative correlation between BMI and the serum dosage of 25 OHD with statistical significance (r = - 0.15; p = 0.04). There was no correlation between serum 25 OHD and SAPS III and CRP.
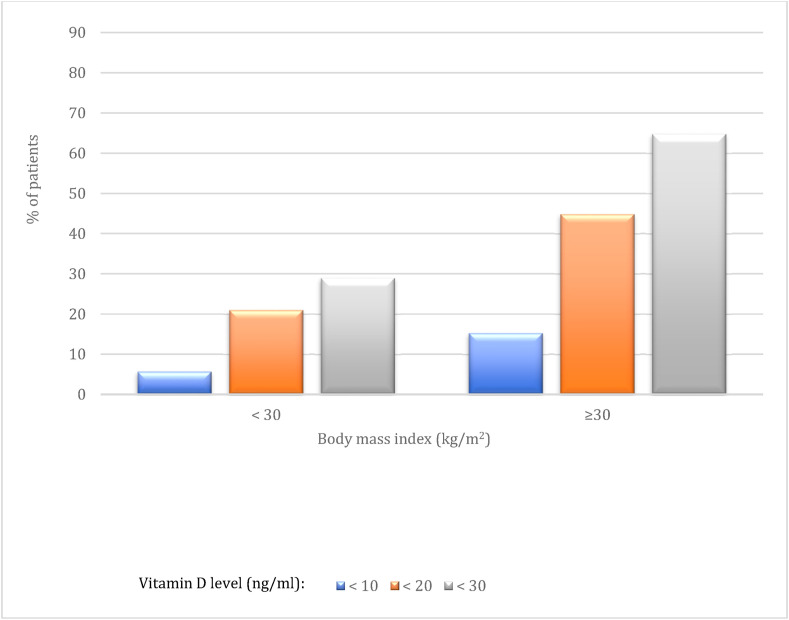
Fig. 1 shows the various prevalence rates of vitamin D levels (25 OHD < 10 ng/mL; < 20 ng/mL; < 30 ng/mL) among the nutritional profiles according to the BMI among severe elderly in the ICU due to SARS-CoV-2.
Fig. 1.
Prevalence of different degrees of hypovitaminosis D (%) according to the results of serum 25-hydroxy vitamin D (25 OHD) measurements distributed between obeses or not obeses stratified by body mass index (BMI).
Regarding the presence of comorbidities, arterial hypertension (72.2%), diabetes mellitus (40.9%), heart disease (27.3%) and lung diseases like asthma or chronic obstructive pulmonary disease (27.3%) showed the highest prevalence among patients admitted to ICU. Chronic kidney disease (13.1%), neurological diseases (17%) and oncological diseases (7.4%) had a lower prevalence.
4. Discussion
In our study, there was a high prevalence rate of low serum levels of 25 OHD (93.8%) and nutritional diagnosis of obesity using BMI (68.7%) among severe elderly patients admitted to ICU by SARS-CoV-2. According to these data, it is suggested that obesity and vitamin D deficiency should be investigated in the evolution of severe cases of COVID-19 requiring ICU admission and mechanical ventilation assisted by ARDS due to the high prevalence of hypovitaminosis D and obesity in elderly patients in critical condition infected by SARS-CoV-2.
Hypovitaminosis D is a common condition among the elderly. Worldwide data show that 5%–25% of the independent elderly population and 60–80% of institutionalized patients are deficient or insufficient in vitamin D [21]. Vitamin D deficiency is also highly prevalent among obese elderly [22].
Many factors contribute to a higher prevalence of hypovitaminosis D among the elderly. There is a decrease in cutaneous vitamin D synthesis after sun exposure due to atrophic changes of the skin itself [23]. Consequently, there is a reduction in the concentration of 7-dehydrocholesterol in the epidermis, resulting in a reduction in the formation of pre-vitamin D3, close to 50% [24,25]. Also, the absence of sun exposure due to mobility problems, fragility, and social isolation are frequent [26].
Multiples reports cite that adequate levels of vitamin D can reduce the risk of viral infections [14,27,28]. The main mechanisms involve the improvement of natural immunity, acquired immunity and physical barriers since vitamin D contributes to the maintenance of the cell tight junctions, gap junctions and adherence junctions [29]. The immunomodulatory functions of vitamin D have received considerable attention because, in addition to its classic role in bone homeostasis involving calcium and phosphorus, vitamin D is a potent immunoregulatory. Vitamin D is thought to modulate immune responses by selective suppression of effector functions, such as the production of inflammatory cytokines and leukocyte infiltration, minimizing inflammation [30,31]. Recent findings also indicate a complex interaction between viral infections and vitamin D, including the induction of antiviral status, functional immunoregulatory characteristics, induction of autophagy and apoptosis [32].
Jakovac H. shows that vitamin D has significant protective effects on SARS-CoV-2 since immune evasion mechanisms initially occur followed by immune hyperreactivity and “cytokine storm”, a common pathogenic mechanism in the development of acute respiratory distress syndrome (ARDS) and the systemic inflammatory response syndrome (SIRS) [33]. Vitamin D can also mitigate the scope of acquired immunity and regenerate the endothelial epithelium, which can be beneficial in reducing the alveolar damage caused by ARDS [34].
In a review article, Wimalawansas S. showed that micronutrient deficiency, especially hypovitaminosis D, increases the risk of developing viral infections, including coronaviruses [35]. In addition, he proposes as an effective strategy, daily supplementation of vitamin D to maintain serum levels of 25 OHD greater than 30 ng/mL.
Another observation from our study was the high prevalence of obesity among critically ill patients with SARS-CoV-2. According to Simonnet A. et al., the prevalence of obesity in patients with SARS-CoV-2 was 47.6% (BMI > 30 kg/m2) and 28.2% (BMI > 35 kg/m2), but not all of these patients required mechanical ventilation [36]. In our study, the prevalence was 68.8% (BMI ≥ 30 kg/m2), but all our patients were elderly people and we included only mechanically ventilated patients with PaO2/FiO2 < 250 ratios.
Obesity-induced inflammation and insulin resistance in adipose tissue can further complicate this scenario [37,38]. The resistance and the lipolytic effects of catecholamines and natriuretic peptide in obese patients mediated by a low amount of beta-2 adrenergic receptors in adipocytes can lead to a reduction in the release of vitamin D stored in adipose tissue [39], explaining the correlation between hypovitaminosis D and obesity.
Low levels of 25 OHD are known to be highly frequent in obese patients. Many explanations are proposed such as altered vitamin D metabolism, behavioral factors such as reduced sun exposure, or reduced intake of foods enriched with vitamin D. In addition, the increase in body fat mass can act as a storage place for vitamin D, since that it is a lipophilic hormone [40]. In a recent meta-analysis involving 35 studies (including 17,245 patients) showed that vitamin D is inversely associated with body fat mass [41].
5. Conclusion
This present study shows an exploratory data with the objective of recording and analyzing the characteristics and the occurrence of hypovitaminosis D and obesity in elderly patients in critical condition by COVID-19. In view of the above considerations, we can conclude that there is a high prevalence of hypovitaminosis D and obesity among elderly patients and these factors should be investigated in the evolution of severe cases of COVID-19 requiring ICU admission and mechanical ventilation assisted by ARDS. In addition, further investigations are needed to stablish an association between obesity and hypovitaminosis D and clinical outcomes in this specific population.
Authorship statement
None of the authors of this manuscript has any financial or personal relationship with other people or organizations that could inappropriately influence this work.
Financial support
Prevent Senior Institute, São Paulo, Brazil.
Declaration of competing interest
None of the co-authors have direct or indirect, financial or other conflicts of interest related to the subject of our report. There is no conflict of interest regarding sponsorship and financing.
Acknowledgements
The authors wish to thank the volunteers for their participation.
References
- 1.Thompson R. Pandemic potential of 2019-nCoV. Lancet Infect Dis. 2020;20(3):280. doi: 10.1016/S1473-3099(20)30068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministério da Saúde Brasil. 2020. Protocolo de Manejo Clínico para o Novo Coronavírus (2019-nCoV)https://www.saude.gov.br/images/pdf/2020/fevereiro/11/protocolo-manejo-coronavirus.pdf [Google Scholar]
- 4.Adhikari S.P., Meng S., Wu Y.J., Mao Y.P., Ye R.X., Wang Q.Z. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dushianthan A., Cusack R., Burgess V.A., Grocott M.P., Calder P. Immunonutrition for adults with ARDS: results from a cochrane systematic review and meta-analysis. Respir Care. 2020;65(1):99–110. doi: 10.4187/respcare.06965. [DOI] [PubMed] [Google Scholar]
- 6.Butt Y., Kurdowska A., Allen T.C. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140(4):345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- 7.Hughes K.T., Beasley M.B. Pulmonary manifestations of acute lung injury: more than just diffuse alveolar damage. Arch Pathol Lab Med. 2017;141(7):916–922. doi: 10.5858/arpa.2016-0342-RA. [DOI] [PubMed] [Google Scholar]
- 8.Preiser J.C., Ichai C., Orban J.C., Groeneveld A.B. Metabolic response to the stress of critical illness. Br J Anaesth. 2014;113(6):945–954. doi: 10.1093/bja/aeu187. [DOI] [PubMed] [Google Scholar]
- 9.Caccialanza R., Laviano A., Lobascio F., Montagna E., Bruno R., Ludovisi S. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020:110835. doi: 10.1016/j.nut.2020.110835. [published online ahead of print, 2020 Apr 3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christopher K.B. Vitamin D and critical illness outcomes. Curr Opin Crit Care. 2016;22(4):332–338. doi: 10.1097/MCC.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 11.Prasad S., Raj D., Warsi S., Chowdhary S. Vitamin D deficiency and critical illness. Indian J Pediatr. 2015;82(11):991–995. doi: 10.1007/s12098-015-1778-3. [DOI] [PubMed] [Google Scholar]
- 12.Braun A., Chang D., Mahadevappa K., Gibbons F.K., Liu Y., Giovannucci E. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39(4):671–677. doi: 10.1097/CCM.0b013e318206ccdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunville C.F., Mourani P.M., Ginde A.A. The role of vitamin D in prevention and treatment of infection. Inflamm Allergy - Drug Targets. 2013;12(4):239–245. doi: 10.2174/18715281113129990046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beard J.A., Bearden A., Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50(3):194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luzi L., Radaelli M.G. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020:1–6. doi: 10.1007/s00592-020-01522-8. [published online ahead of print, 2020 Apr 5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietz W., Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity. 2020 doi: 10.1002/oby.22818. [published online ahead of print, 2020 Apr 1] 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 17.Moreno R.P., Metnitz P.G., Almeida E., Jordan B., Bauer P., Campos R.A. SAPS 3-From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Organización Panamericana de la Salud (OPS) División de Promoción y Protección de la Salud (HPP). Encuesta Multicéntrica: salud bienestar y envejecimiento (SABE) en América Latina y el Caribe. Pan Am Health Organ (PAHO) 2002 http://envejecimiento.csic.es/documentos/documentos/paho-salud-01.pdf [Google Scholar]
- 19.Maeda S.S., Borba V.Z.C., Camargo M.B.R., Silva D.M.W., Borges J.L.C., Bandeira F. Recomendações da Sociedade Brasileira de Endocrinologia e Metabologia (SBEM) para o diagnóstico e tratamento da hipovitaminose D. Arquivos Brasileiros Endocrinol Metabol. 2014;58:411–433. doi: 10.1590/0004-2730000003388. [DOI] [PubMed] [Google Scholar]
- 20.Giustina A., Adler R.A., Binkley N., Bollerslev J., Bouillon R., Dawson-Hughes B. Consensus statement from 2(nd) international conference on controversies in vitamin D. Rev Endocr Metab Disord. 2020;21(1):89–116. doi: 10.1007/s11154-019-09532-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lips P., Duong T., Oleksik A., Black D., Cummings S., Cox D. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86(3):1212–1221. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 22.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosekilde L. Vitamin D and the elderly. Clin Endocrinol. 2005;62(3):265–281. doi: 10.1111/j.1365-2265.2005.02226.x. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher J.C. Vitamin D and aging. Endocrinol Metab Clin N Am. 2013;42(2):319–332. doi: 10.1016/j.ecl.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill T.R., Granic A., Aspray T.J. Vitamin D and ageing. Subcell Biochem. 2018;90:191–220. doi: 10.1007/978-981-13-2835-0_8. [DOI] [PubMed] [Google Scholar]
- 26.Oudshoorn C., van der Cammen T.J., McMurdo M.E., van Leeuwen J.P., Colin E.M. Ageing and vitamin D deficiency: effects on calcium homeostasis and considerations for vitamin D supplementation. Br J Nutr. 2009;101(11):1597–1606. doi: 10.1017/S0007114509338842. [DOI] [PubMed] [Google Scholar]
- 27.Lang P.O., Aspinall R. Vitamin D status and the host resistance to infections: what it is currently (not) understood. Clin Therapeut. 2017;39(5):930–945. doi: 10.1016/j.clinthera.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;(4):12. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwalfenberg G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res. 2011;55(1):96–108. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- 30.Helming L., Böse J., Ehrchen J., Schiebe S., Frahm T., Geffers R. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106(13):4351–4358. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- 31.Jadhav N.J., Gokhale S., Seervi M., Patil P.S., Alagarasu K. Immunomodulatory effect of 1, 25 dihydroxy vitamin D(3) on the expression of RNA sensing pattern recognition receptor genes and cytokine response in dengue virus infected U937-DC-SIGN cells and THP-1 macrophages. Int Immunopharm. 2018;62:237–243. doi: 10.1016/j.intimp.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Teymoori-Rad M., Shokri F., Salimi V., Marashi S.M. The interplay between vitamin D and viral infections. Rev Med Virol. 2019;29(2):e2032. doi: 10.1002/rmv.2032. [DOI] [PubMed] [Google Scholar]
- 33.Jakovac H. COVID-19 and vitamin D-Is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318(5):E589. doi: 10.1152/ajpendo.00138.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kakodkar P., Kaka N., Baig M.N. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19) Cureus. 2020;12(4):e7560. doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wimalawansa S.J. Global Epidemic of Coronavirus—COVID-19: what can we do to minimize risks. Eur J Biomed. 2020;7(3):432–438. [Google Scholar]
- 36.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. [published online ahead of print, 2020 Apr 9] Obesity. 2020 doi: 10.1002/oby.22831. 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Migliaccio S., Di Nisio A., Mele C., Scappaticcio L., Savastano S., Colao A. Obesity and hypovitaminosis D: causality or casualty? Int J Obes Suppl. 2019;9(1):20–31. doi: 10.1038/s41367-019-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelczyńska M., Grzelak T., Walczak M., Czyżewska K. Hypovitaminosis D and adipose tissue - cause and effect relationships in obesity. Ann Agric Environ Med. 2016;23(3):403–409. doi: 10.5604/12321966.1219177. [DOI] [PubMed] [Google Scholar]
- 39.Lamendola C.A., Ariel D., Feldman D., Reaven G.M. Relations between obesity, insulin resistance, and 25-hydroxyvitamin D. Am J Clin Nutr. 2012;95(5):1055–1059. doi: 10.3945/ajcn.111.032060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J., Hupfeld C.J., Taylor S.S., Olefsky J.M., Tsien R.Y. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005;437(7058):569–573. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- 41.Calton E.K., Keane K.N., Newsholme P., Zhao Y., Soares M.J. The impact of cholecalciferol supplementation on the systemic inflammatory profile: a systematic review and meta-analysis of high-quality randomized controlled trials. Eur J Clin Nutr. 2017;71(8):931–943. doi: 10.1038/ejcn.2017.67. [DOI] [PubMed] [Google Scholar]