Abstract
Background
The prediction of COVID-19 disease behavior in the early phase of infection is challenging but urgently needed. MuLBSTA score is a scoring system that predicts the mortality of viral pneumonia induced by a variety of viruses, including coronavirus, but the scoring system has not been verified in novel coronavirus pneumonia. The aim of this study was to validate this scoring system for estimating the risk of disease worsening in patients with COVID-19.
Methods
This study included the patients who were treated between April 1 st and March 13 th , 2020. The patients were classified into mild, moderate, and severe groups according to the extent of respiratory failure. MuLBSTA score was applied to estimate the risk of disease worsening in each severity group and we validated the utility of the scoring system.
Results
A total of 72 patients were analyzed. Among the 46 patients with mild disease, 17 showed disease progression to moderate or severe disease after admission. The model showed a sensitivity of 100% and a specificity of only 34.5% with a cut-off value of 5 points. Among the 55 patients with mild or moderate disease, 6 deteriorated to severe disease, and the model showed a sensitivity of 83.3% and a specificity of 71.4% with a cut-off value of 11 points.
Conclusions
This study showed that MuLBSTA score is a potentially useful tool for predicting COVID-19 disease behavior. This scoring system may be used as one of the criteria to identify high-risk patients worsening to life-threatening status.
Keywords: COVID-19, SARS-CoV-2, Viral pneumonia, MuLBSTA score
1. Introduction
An infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), named as coronavirus infected disease 2019 (COVID-19), recently emerged and spread rapidly worldwide in early 2020. More than 4,000,000 cases have been diagnosed and over 300,000 infected people have died [1]. In previous reports, disease remained mild in approximately 80% of patients and developed to severe in the rest [2]. A major clinical problem regarding this viral infection is that some patients show rapid worsening of disease, including respiratory failure, even though they present with mild disease at first admission [3]. Estimation of the risk of rapid worsening in the early phase of hospital admission is beneficial for the identification of potentially high-risk patients. To date, several risk factors such as age, smoking history, critical disease status, history of diabetes, high hypersensitive troponin I levels, leukocytosis, neutrophilia, D-dimer levels, and hypnotics administration, have been identified [[4], [5], [6], [7], [8]]. Regarding the risk prediction model, a previous report is available that present the utility of the host risk score, which is calculated by the 3 background parameters, including an age of more than 50 years, male, and the presence of hypertension [9]. However, the accuracy of this model is unclear.
MuLBSTA score is an early warning model for predicting mortality in viral pneumonias, including those caused by influenza virus, adenovirus, bocavirus, human rhinovirus, parainfluenza virus, coronavirus, respiratory syncytial virus, enterovirus, and human metapneumovirus [10]. Six indexes; multilobular infiltration, lymphopenia, bacterial coinfection, smoking history, hypertension, and age are included in this score. Although many clinicians have emphasized the clinical importance of these indexes [11,12], few reports have validated this model. Here, we verified the ability of MuLBSTA score to predict disease behavior after admission and its utility for guiding intensive care unit (ICU) admission.
2. Patients and methods
2.1. Study population and data collection
Seventy-two patients who were diagnosed with COVID-19 and treated in our hospital from April 1st to May 13th, 2020 were enrolled. Severity was defined as follows: mild for patients who do not need supplemental oxygen, moderate for patients who need supplemental oxygen of less than 4 L/min, and severe for patients who need supplemental oxygen of more than 5 L/min or intubation. The criteria is based on COVID-19 guideline presented by Japanese ministry of Health, Labour and Welfare, in which “mild” for asymptomatic or only complaining cough, “moderate 1” for complicating pneumonia without needing supplemental oxygen, “moderate 2” for complicating pneumonia with needing supplemental oxygen, and “severe” for needing intubation or ICU admission [13]. Namely, “mild” in our definition correspond to “mild” and “moderate 1” in the guideline, “moderate” to “moderate 2”, and “severe” to “severe”. Multilobular infiltration, lymphopenia, bacterial coinfection, smoking history, hypertension, and age, which are included in MuLBSTA score, were retrospectively reviewed. In addition, sex, body mass index (BMI), several other comorbidities, platelet count, ferritin level, CRP level, and d-dimer level were also reviewed.
2.2. Scoring systems
MuLBSTA score was calculated as follows: MuLBSTA score = (presence of multilobular infiltration, +5) + (absolute lymphocyte count < 800/μL, +4) + (bacterial infection, +4) + (smoking history: current, +3; ex, +2; never, +0) + (history of hypertension, +2) + (age ≥ 60, +2) [10]. For comparison with the other risk prediction model, the host risk score was estimated as follows: host risk score = (age ≥ 50, +1) + (male, +1) + (presence of hypertension, 1) [9].
2.3. Outcomes
These patients were retrospectively followed to assess their disease progression or deterioration. Disease progression, defined as the increase in severity from mild to moderate or severe, is related to the judgement of hospital admission. Disease deterioration, defined as the development from mild or moderate to severe, is related to ICU referral. We evaluated whether MuLBSTA score significantly predicted these two outcomes.
2.4. Statistics
Continuous variables were expressed as means ± standard deviation (SD). The Mann-Whitney U test and Fisher's exact test were applied to compare the risk factors, including MuLBSTA score, between the groups. The cut-off value of MuLBSTA score for predicting disease behavior was determined by receiver operating characteristic (ROC) curve analysis and validated by Fisher's exact test. The logistic regression model was applied for the modification of MuLBSTA score. A p-value of <0.05 was considered as significant. The institutional review board at our hospital approved this study (M2020-025).
3. Results
3.1. Patient characteristics at admission
Seventy-two patients (50 men and 22 women, mean age 57.3 ± 19.4) were studied (Table 1 ). The mean BMI was 24.3 ± 5.5. Eight and 24 patients had a current and past smoking history, respectively. Twenty-seven patients (37.5%) had hypertension, 11 (15.1%) had diabetes mellitus, 7 (9.7%) had asthma, 5 (6.9%) had chronic obstructive pulmonary diseases, 4 (5.6%) had coronary artery diseases, 1 (1.4%) had liver disease, 4 (5.6%) had renal diseases, and 1 (1.4%) had cancer. Fifty-nine patients (81.9%) exhibited multilobular infiltration, and 24 (33.3%) were treated as having bacterial coinfection. Severity at admission was mild in 46 patients, moderate in 9, and severe in 17. The mean MuLBSTA score assessed at admission was 9.4 ± 4.9 points. The mean days from symptom onset to admission was 11.4 ± 5.8 days, and the mean observation period after admission was 11.3 ± 8.4 days. Three patients (4.1%) died. The most frequently selected regimens as a primary treatment were ciclesonide (CIC) in patients with mild disease, favipiravir (FPV) and CIC in patients with moderate disease, and FPV and hydroxychloroquine (HCQ) in patients with severe disease (Table 2 ). When the disease developed, additional regimens including FPV, HCQ, Tocilizumab (TCZ), or corticosteroids were administered.
Table 1.
Patients’ characteristics.
| Total (n = 72) | |
|---|---|
| Male | 50 (69.4%) |
| Age | 57.3 ± 19.4 |
| BMI | 24.3 ± 5.5 |
| Smoking history | |
| (current/ex/never) | 8/24/40 |
| Comorbidity | |
| Hypertension | 27 (37.5%) |
| Diabetes mellitus | 11 (15.1%) |
| Asthma | 7 (9.7%) |
| Chronic obstructive pulmonary disease | 5 (6.9%) |
| Coronary heart disease | 4 (5.6%) |
| Liver disease | 1 (1.4%) |
| Renal disease | 4 (5.6%) |
| Cancer | 1 (1.4%) |
| Number of lobes infiltrated | |
| (5/4/3/2/1/0/NA∗) | 40/9/5/5/6/6/1 |
| Bacterial co-infection | 24 (33.3%) |
| Severity at admission | |
| (mild/moderate/severe) | 46/9/17 |
| MuLBSTA score | 9.4 ± 4.9 |
| Days from onset to admission | 11.4 ± 5.8 |
| Observation period (day) | 11.3 ± 8.4 |
| Death | 3 (4.1%) |
NA; Not Assessed.
Table 2.
Primary treatment regimen.
| Mild disease (n = 46) | |
| No treatment | 3 |
| CIC | 30 |
| FPV + CIC |
13 |
| Moderate disease (n = 9) | |
| No treatment | 1 |
| CIC | 1 |
| FPV | 1 |
| FPV + CIC | 4 |
| FPV + HCQ | 1 |
| FPV + CIC + HCQ |
1 |
| Severe disease (n = 17) | |
| FPV | 4 |
| FPV + CIC | 2 |
| FPV + HCQ | 6 |
| FPV + TCZ | 5 |
CIC, ciclesonide; FPV, favipiravir; HCQ, hydroxychloroquine; TCZ, tocilizumab.
3.2. Clinical course after admission
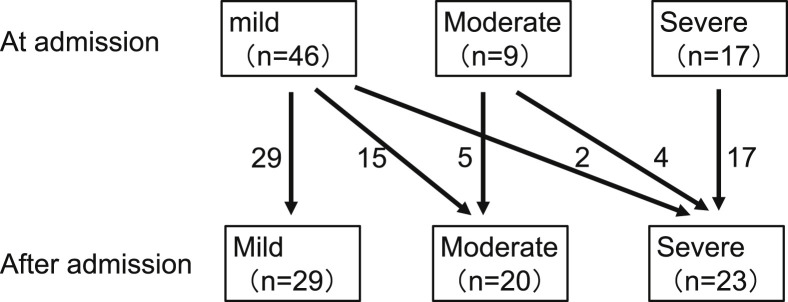
Among the 46 patients with mild disease, 15 developed moderate disease and 2 developed severe disease (Fig. 1 ). These 17 patients were regarded as the progression group, while the remaining 29 were regarded as the stable group. Among the 55 patients with mild or moderate disease, 6 developed severe disease. These 6 patients were regarded as the deterioration group, while the remaining 49 were regarded as the non-deterioration group.
Fig. 1.
Changes in severity after admission. Forty-six patients had mild disease at admission, of which 15 progressed to moderate and 2 deteriorated to severe. These 17 patients were regarded as the progression group, while the rest were regarded as the stable group. Fifty-five patients had mild or moderate disease at admission, of which 6 deteriorated to severe. These 6 cases were regarded as deterioration group, while the rest were regarded as the non-deterioration group.
3.3. Evaluation of disease progression risk in patients with mild disease
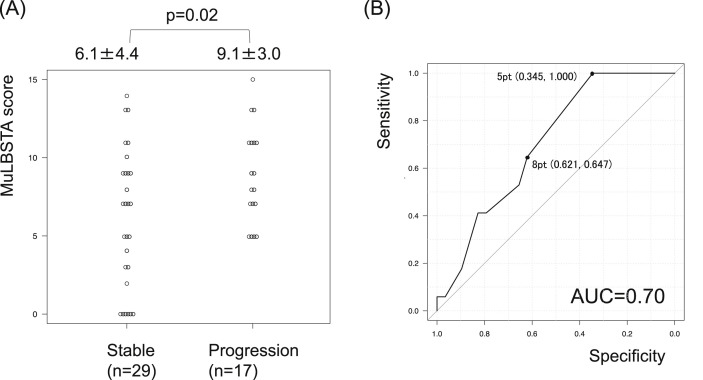
Compared to the stable group, the progression group had a significantly higher MuLBSTA score (6.1 ± 4.4 points vs 9.1 ± 3.0 points, p = 0.02) (Fig. 2 A, Table 3 ). Among the parameters of MuLBSTA score, significantly higher percentages of multilobular infiltration (58.6% vs 94.1%, p = 0.02) and significantly lower lymphocyte counts (1454 ± 642/μL vs 945 ± 283/μL, p < 0.01) were seen in the progression group compared to the stable group. In addition, the BMI and serum CRP level were significantly higher (20.9 ± 3.0 vs 26.0 ± 6.1, p = 0.02, and 2.1 ± 3.0 mg/dl vs 7.9 ± 6.5 mg/dl, p < 0.01, respectively) and the platelet count was significantly lower (29.0 ± 9.8 × 104/μl vs 18.3 ± 7.6 × 104/μl, p < 0.01) in the progression group compared to the stable group. There was also a significant difference in the days from symptom onset to admission between the groups (14.1 ± 7.0 days in the stable group vs 9.1 ± 3.4 days in the progression group, p < 0.01). When 5 points was used as the cut-off value for disease progression in ROC curve analysis, the model showed a sensitivity of 100% but a specificity of only 34.5% (Fig. 2B). In other words, disease progression did not occur if MuLBSTA score was less than 5 points at admission.
Fig. 2.
Predictive model of disease progression in patients with mild disease. The distribution of points reveals a significant increase in MuLBSTA score in the progression group (A). When 5 points was used as the cut-off value for disease progression, the model showed a sensitivity of 100% but a specificity of only 34.5% (B).
Table 3.
Comparison between the stable group and the progression group.
| Mild disease (n = 46) | Stable (n = 29) | Progression (n = 17) | |
|---|---|---|---|
| MuLBSTA score | 6.1 ± 4.4 | 9.1 ± 3.0 | p = 0.02 |
| Multilobe infiltrates | 17 (58.6%) | 16 (94.1%) | p = 0.02 |
| Lymphocyte (/μl) | 1454 ± 642 | 945 ± 283 | p < 0.01 |
| Bacterial coinfection | 2 (7.1%) | 2 (11.1%) | p = 0.62 |
| Smoking history | |||
| (current/ex/never) | 4/8/16 | 1/9/8 | p = 0.38 |
| hypertension | 8 (27.6%) | 7 (41.2%) | p = 0.52 |
| Age≧60 | 11 (37.9%) | 6 (35.3%) | p = 1.00 |
| Male | 14 (48.3%) | 13 (76.5%) | p = 0.07 |
| BMI | 20.9 ± 3.0 | 26.0 ± 6.1 | p = 0.02 |
| Platelet ( × 104/μl) | 29.0 ± 9.8 | 18.3 ± 7.6 | p < 0.01 |
| CRP (mg/dl) | 2.1 ± 3.0 | 7.9 ± 6.5 | p < 0.01 |
| Ferritin (ng/ml) | 462 ± 523 | 1059 ± 833 | p = 0.05 |
| D-dimer (μg/ml) | 1.0 ± 1.1 | 2.9 ± 4.6 | p = 0.13 |
| Days from onset to admission | 14.1 ± 7.0 | 9.1 ± 3.4 | p < 0.01 |
3.4. Evaluation of deterioration risk in patients with mild or moderate disease
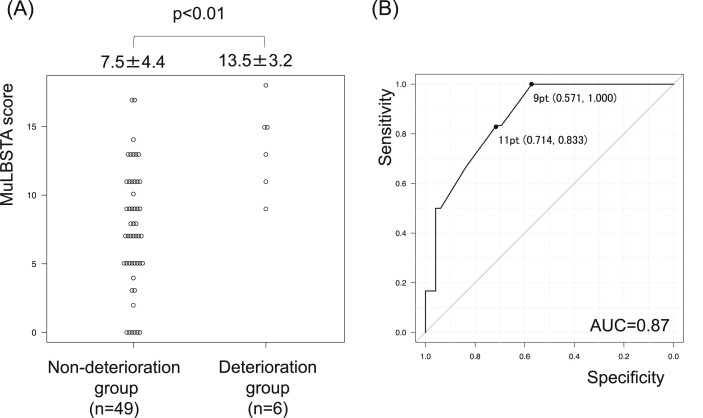
Compared to the non-deterioration group, the deterioration group exhibited a significantly higher MuLBSTA score (7.5 ± 4.4 vs 13.5 ± 3.2, p < 0.01) (Fig. 3 A, Table 4 ). Among the parameters of MuLBSTA score, significantly lower lymphocyte counts (1222 ± 605/μL vs 682 ± 116/μL, p = 0.01) and significantly higher percentages of bacterial coinfection (12.2% vs 50.0%, p = 0.05) were seen in the deterioration group compared to the non-deterioration group. In addition, the platelet count was significantly lower (24.8 ± 10.3 × 104/μl vs 14.3 ± 4.3 × 104/μl, p = 0.01) and serum CRP level was significantly higher (5.1 ± 6.7 mg/dl vs 11.5 ± 8.7 mg/dl, p = 0.03) in the deterioration group compared to the non-deterioration group. In this analysis, a significant difference was not observed in the days from symptom onset to admission between the groups (12.1 ± 6.2 days vs 9.3 ± 4.4 days, p = 0.14). When 11 points was used as the cut-off value for the deterioration in ROC curve analysis, the model showed a sensitivity of 83.3% and a specificity of 71.4% (AUC = 0.87) (Fig. 3B).
Fig. 3.
Predictive model of deterioration in patients with mild or moderate disease. The distribution of points reveals a significant increase in MuLBSTA score in the deterioration group (A). When 11 points was used as the cut-off value for disease deterioration, the model showed a sensitivity of 83.3% and specificity of only 71.4% (B).
Table 4.
Comparison between deterioration group and non-deterioration group.
| Mild and moderate disease (n = 55) | Non-deterioration (n = 49) | Deterioration (n = 6) | |
|---|---|---|---|
| MuLBSTA score | 7.5 ± 4.4 | 13.5 ± 3.2 | p < 0.01 |
| Multilobe infiltrates | 36 (73.5%) | 6 (100.0%) | p = 0.32 |
| Lymphocyte (/μl) | 1222 ± 605 | 682 ± 116 | p = 0.01 |
| Bacterial coinfection | 6 (12.2%) | 3 (50.0%) | p = 0.05 |
| Smoking history | |||
| (current/ex/never) | 5/18/26 | 1/2/3 | p = 0.84 |
| hypertension | 14 (28.6%) | 3 (50.0%) | p = 0.36 |
| Age≧60 | 20 (40.8%) | 3 (50.0%) | p = 0.69 |
| Male | 28 (57.1%) | 6 (100.0%) | p = 0.07 |
| BMI | 23.3 ± 5.5 | 24.4 ± 2.0 | p = 0.19 |
| Platelet ( × 104/μl) | 24.8 ± 10.3 | 14.3 ± 4.3 | p = 0.01 |
| CRP (mg/dl) | 5.1 ± 6.7 | 11.5 ± 8.7 | p = 0.03 |
| Ferritin (ng/ml) | 707 ± 664 | 582 ± 790 | p = 0.38 |
| D-dimer (μg/ml) | 4.8 ± 15.8 | 2.9 ± 4.3 | p = 0.38 |
| Days from onset to admission | 12.1 ± 6.2 | 9.3 ± 4.4 | p = 0.14 |
3.5. Comparison with the host risk score
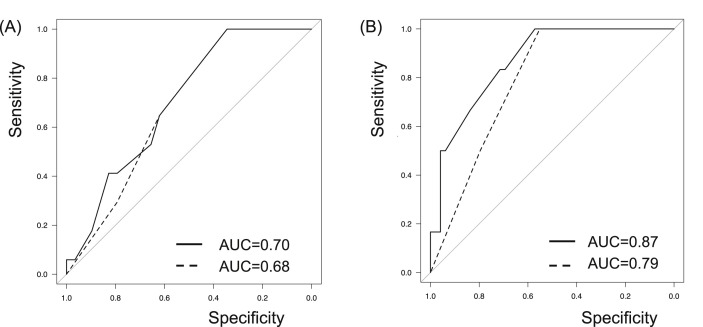
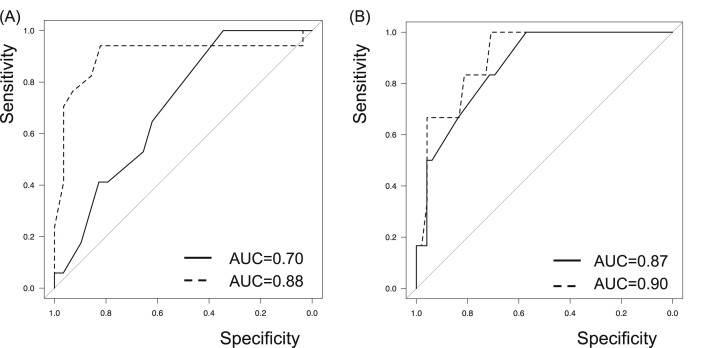
The host risk score was also applied for evaluation of disease progression and deterioration risks. Although the AUC values of MuLBSTA score determined by ROC curve analysis were higher than those of the host risk score, MuLBSTA score could not predict disease progression or deterioration more accurately than the host risk score with significance (Fig. 4 ).
Fig. 4.
Predictive model with the host risk score. MuLBSTA score is demonstrated in solid line and the host risk score is demonstrated in dashed line. The host risk score also correlated with the disease behavior, including disease progression (A) and deterioration (B). The AUC values of MuLBSTA score determined by ROC curve analysis were higher than those of the host risk score, though there were not statistically significant differences for predicting progression risk (AUC values of 0.70 vs 0.68, p = 0.74) and deterioration risk (0.87 vs 0.79, p = 0.33).
3.6. Modification of MuLBSTA score
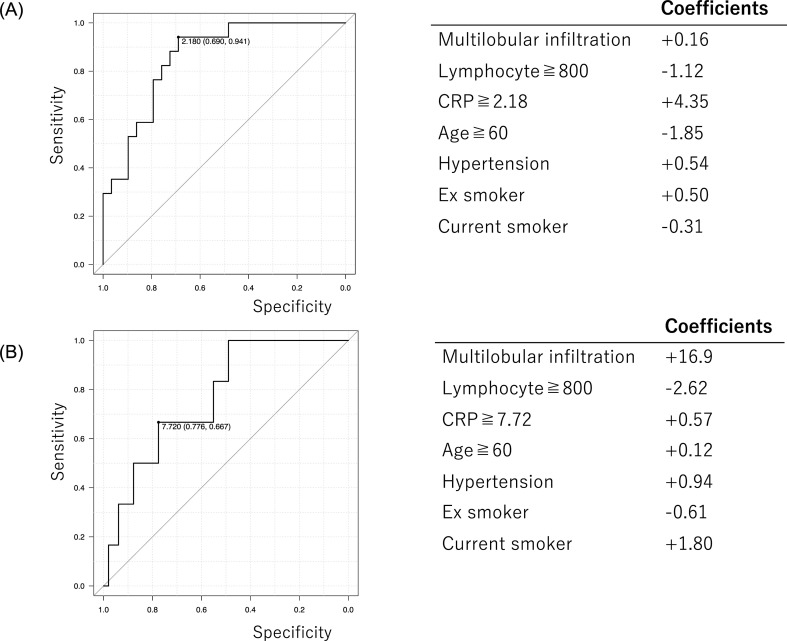
In the process of modification of MuLBSTA score, we removed bacterial coinfection but added elevation of CRP level. This modification is based on a perspective that judgement of bacterial coinfection is inconsistent between the clinicians, making the scoring model impractical for COVID-19 patients, and that elevation of inflammatory marker such as CRP indicates the possibility of complicating cytokine storm which is one of the mechanism of disease worsening. The elevation of CRP level was defined as more than 2.18 mg/dl for prediction of the progression risk, and more than 7.72 mg/dl for prediction of the deterioration risk, determined by ROC curve analysis. As a result, the modified MuLBSTA score seemed to exhibit ROC curve with higher AUC value than MuLBSTA score, especially for prediction of disease progression with significance (Fig. 5 , Supplementary Figure 1).
Fig. 5.
Predictive model with the modified MuLBSTA score. MuLBSTA score is demonstrated in solid line and the modified MuLBSTA score is demonstrated in dashed line. Modified MuLBSTA score seems to be more predictive than MuLBSTA score. Between MuLBSTA score and the modified MuLBSTA score, statistical significance was observed for disease progression (0.70 vs 0.88, p = 0.04) (A) but not observed for disease deterioration (0.87 vs 0.90, p = 0.67) (B).
4. Discussion
This report is the first to validate the utility of MuLBSTA score for predicting disease behavior in patients with COVID-19. We still did not know the recommendations about which patients with mild disease should be hospitalized and which patients with mild or moderate disease is high risk for ICU referral. This scoring system was able to predict these risks and demonstrated that 5 and 11 points were clinically reliable cut-off values. We may use these cut-off values as one of the admission criteria and as a risk estimation of the deterioration to life-threatening status.
Five points, presented as a cut-off value for disease progression in patients with mild disease, seems to be reasonable from clinical perspective. Whether MuLBSTA score exceeds this cut-off value is strongly affected by the presence of any of the high-point-value (more than 4 points) factors, such as multilobular infiltration, lymphopenia, or bacterial coinfection. This result is consistent with those in previous reports that demonstrated that lymphopenia and bacterial coinfection are related to disease severity of patients with influenza pneumonia and SARS-CoV pneumonia [[14], [15], [16]]. These high-point-value factors directly reflect the secondary changes caused by COVID-19, in contrast to the other factors such as smoking history, hypertension, and age, which reflect the background of the patients.
Eleven points, presented as a cut-off value for deterioration to severe disease, is useful for predicting the possibility of ICU referral accompanied by high mortality. In the preceding study, MuLBSTA score of more than 12 points was presented as a standard for the high 90-day mortality risk among patients with viral pneumonia [10]. The 11 points presented in our study was close to the standard, making it reasonable to interpret as cut-off value for deterioration. Different from the preceding study, 90-day mortality was not assessed in our study because the number of patients who died was only 3 and the observational period was short. Regarding the 3 patients who died, 2 patients with 13 points, and 1 patient with only 9 points were included. The patient with 9 points did not have high risk factor of old age, hypertension, or smoking history, resulting in low MuLBSTA score. We speculate his excessive obesity with BMI of 43.7 might be another risk factor concerned.
Among the indexes of MuLBSTA score, the existence of multilobular infiltration, lymphopenia, and bacterial coinfection were significant predictive markers of disease progression and/or deterioration. On the other hand, the other parameters such as smoking history, hypertension, and age, did not reveal clinical significance in either the progression group or deterioration group, though previous reports have presented them as significant mortality risk factors [4,5,17]. Moreover, MuLBSTA score could not predict disease progression and deterioration more accurately than the host risk score with significance. These results may be due to the small sample size in our study. The fact that most of patients with hypertension were already included in the severe group at admission may also have affected the results.
MuLBSTA score sometimes remain problems in terms of practical aspect because the existence of bacterial coinfection is difficult to prove clinically. In the preceding study using MuLBSTA Score, bacterial coinfection was defined as positive bacterial culture of blood and sputum samples. However, characteristic dry cough in COVID-19 sometimes makes difficulty of high-quality sputum sampling and even positive culture cannot exclude the possibility of noninfectious careers. In addition, the judgement of bacterial coinfection may be inconsistent between the clinicians in charge. For these reasons, judging bacterial co-infection is not practical for clinicians to help predicting severity and we removed this factor in modified MuLBSTA score. Alternatively, we included the presence of elevation of CRP value, which indicates high inflammatory status and is related to complicating cytokine storm known as one of the core mechanisms explaining worsening of COVID-19 [[18], [19], [20]]. As a result, the modified model predicted disease deterioration more accurately than MuLBSTA score. It should be noted that the modified model, including each coefficient value shown in supplementary Figure 1, cannot be directly applied in clinical use because no validation is conducted yet. However, this result may be a beacon for constructing a new scoring system based on MuLBSTA score in the future.
The impact of the COVID-19 pandemic has resulted in the collapse of the medical care system in many countries worldwide. In such a situation, it is necessary to establish a proper criteria for hospital admission and to prepare medical resources as thoroughly as possible for patients with severe disease. From such a viewpoint, this scoring system may help to overcome the limitation. For example, hospital admission may not be considered for patients with scoring less than 5 points, which had a sensitivity of 100%, thus preventing these patients from occupying hospital beds. Moreover, recognizing patients with scoring higher than 11 points as those with high risk of needing ICU referral contributes to the ability to prepare for mechanical ventilation or extracorporeal membrane oxygenation (ECMO) in advance.
The limitations of our study are as follows. First, this was a single-institution retrospective study with a small number of participants. However, this is a future suggestion how to utilize this scoring system clinically. Namely, our results may help to establish hospital admission criteria and predict ICU referring, which lead to preventing from collapse of medical care system. Second, the days from symptom onset to admission was significantly longer in the stable group. This means that patients with mild symptoms are likely to be observed at home longer than those with severe symptoms, which introduces a selection bias leading to the differences in MuLBSTA scores. Third, each patient, even those in the same severity group, received different treatments. Because little evidence for effective treatment regimens has been established [[21], [22], [23], [24], [25]], it is necessary to verify which treatment is effective or ineffective and ultimately influences disease development. These factors were not considered in this study, and additional prospective studies are needed.
5. Conclusion
In conclusion, MuLBSTA score is a useful tool for predicting disease behavior in patients with COVID-19. We may use this scoring system as one of the admission criteria and as a risk estimation of ICU referral. However, partial modification is also needed to increase the availability of this model in the near future.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authorship
All authors meet the ICMJE authorship criteria. YI and TO designed the study, and YI wrote the manuscript. YI, TO, TS, TM, RS, TH, MI, TT, and JA provided medical care to the patients. MT, YO, TA, and KT developed the trial design. YM was the chief investigator and responsible for the data analysis. All authors commented on the manuscript and approved the final version.
Declaration of competing interest
None declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2020.10.013.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Figure 1.
ROC curve analysis of CRP elevation and coefficients of each parameter included in modified MuLBSTA score. For estimating disease progression risk in the mild cases, 2.18 mg/dl was used as cut off value of CRP elevation determined by ROC curve analysis. The coefficient values of each parameters were also shown (A). For estimating disease deterioration risk in the mild and moderate cases, 7.72 mg/dl was used as cut off value of CRP elevation determined by ROC curve analysis. The coefficient values of each parameters were also shown (B).
References
- 1.Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://coronavirusjhuedu/maphtml
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu L., Chen S., Fu Y., Gao Z., Long H., Wang J.M., et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F., et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J.J.Y., Lee K.S., Ang L.W., Leo Y.S., Young B.E. Risk factors of severe disease and efficacy of treatment in patients infected with COVID-19: a systematic review, meta-analysis and meta-regression analysis. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang W., Liang H., Ou L., Chen B., Chen A., Li C., et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo L., Wei D., Zhang X., Wu Y., Li Q., Zhou M., et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Japanese ministry of Health, Labour and Welfare Guide of the medical treatment of the noble coronavirus; the 3rd edition. https://www.mhlw.go.jp/content/000670444.pdf [in Japanese]
- 14.Lalueza A., Folgueira D., Díaz-Pedroche C., Hernández-Jiménez P., Ayuso B., Castillo C., et al. Severe lymphopenia in hospitalized patients with influenza virus infection as a marker of a poor outcome. Infect Dis (Lond) 2019;51:543–546. doi: 10.1080/23744235.2019.1598572. [DOI] [PubMed] [Google Scholar]
- 15.Teng F., Liu X., Guo S.B., Li Z., Ji W.Q., Zhang F., et al. Community-acquired bacterial co-infection predicts severity and mortality in influenza-associated pneumonia admitted patients. J Infect Chemother. 2019;25:129–136. doi: 10.1016/j.jiac.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 16.He Z., Zhao C., Dong Q., Zhuang H., Song S., Peng G., et al. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis. 2005;9:323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 20.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baden L.R., Rubin E.J. Covid-19 - the search for effective therapy. N Engl J Med. 2020;382:1851–1852. doi: 10.1056/NEJMe2005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]