Abstract
Hypertension remains the most important modifiable risk factor for the development of cardiovascular disease. While it is clear that inflammation plays a pivotal role in the development and maintenance of hypertension, several novel discoveries have been made within the past decade that have advanced the field and have provided new mechanistic insights. First, recent studies have identified a central role of sodium-induced immune cell activation in the pathogenesis of hypertension by altering the gut microbiome and formation of products of lipid oxidation known as isolevuglandins. Second, cytokine elaboration by the inflammasome leading to end-organ dysfunction and immune activation has been found to play a role in the genesis of hypertension. Third, novel techniques have identified previously uncharacterized immune cell populations that may play a functional role in these processes. Finally, the role of inflammation in hypertension may be an important mediator of severe COVID-19 infections. In this review, we discuss these recent advances in the study of inflammation and hypertension and highlight topics for future studies.
Current Opinion in Physiology 2021, 19:92–98
This review comes from a themed issue on Inflammation
Edited by Pilar Alcaide and Michael Schnoor
Available online 13th October 2020
For complete overview of the section, please refer the article collection - Inflammation
https://doi.org/10.1016/j.cophys.2020.09.016
2468-8673/© 2020 The Author(s). Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Hypertension accounts for nearly half of all strokes and ischemic cardiovascular events worldwide [1, 2, 3]. In the past 15-years it has become clear that inflammation plays an important role in the development of essential hypertension [4, 5, 6, 7]. The physiologic mechanisms that contribute to the development of hypertension are diverse and include endothelial cell dysfunction, renal abnormalities, and dysregulation of the central nervous system. A discussion of the physiology of hypertension is outside the scope of this review, however, inflammation affects these multiple systems and thus the development of hypertension. Inflammation is a complex process involving multiple cell types and secreted factors many of which have been implicated in hypertension.
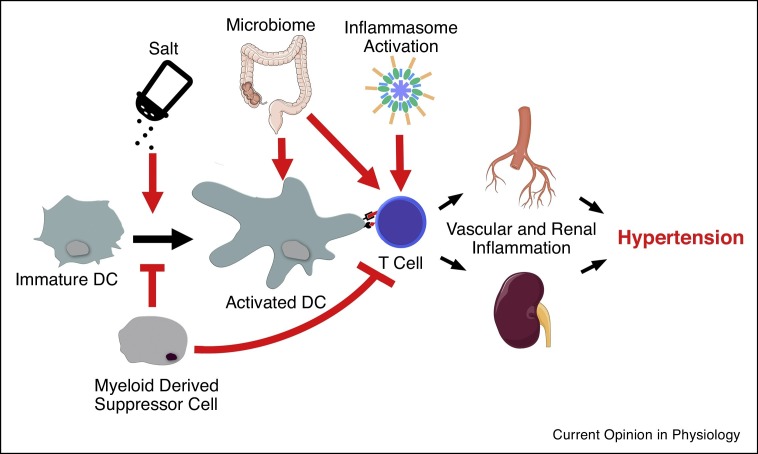
Reactive oxygen species (ROS) production is augmented in dendritic cells from hypertensive patients and in animal models of essential hypertension [8, 9, 10, 11]. This initiates a cascade of events that results in the activation of T-cells. The mechanism of ROS overproduction in this cell type remains incompletely characterized. Here we discuss the various advances that have been recently made in the pathogenesis of immune-mediated hypertension including the role of the inflammasome, excess dietary salt, and COVID-19 infection. These processes and their effects on inflammation in hypertension are represented in Figure 1 .
Figure 1.
Immune activation in hypertension is characterized by activation of dendritic cells (DC) and subsequent activation of T-cells. T-cells then migrate to the vascular tree and the kidney causing inflammation and hypertension. High salt can participate in the activation of immature DCs to activated DCs whereas myeloid derived suppressor cells can inhibit DC and T cell activation. Products of the microbiome and inflammasome both act on innate immune cells and T-cells directly to augment peripheral organ inflammation and exacerbate hypertension. Processes discussed herein are represented by red arrows.
The important role of the spleen in hypertension exemplifies the importance of the interaction of innate and adaptive immune cells in hypertension [12]. Cells of both the innate and adaptive immune systems have been shown to play an important role in hypertension [6,13, 14, 15]. T-cells have been shown to infiltrate the kidney and the vascular tree in animal models of essential hypertension and are hypothesized to directly contribute to endothelial cell dysfunction and renal injury [13,16, 17, 18]. CD8+ ‘killer’ T-cells specifically are hypothesized to mediate direct cellular injury representing a potential autoimmune mechanism of hypertension [13,15,18,19]. Factors effecting T-cell activation and function are therefore important mediators of essential hypertension.
Activation of T-cells occurs by interaction with professional antigen presenting cells such as dendritic cells (DCs). T-cell activation occurs by direct interaction of the major histocompatibility complex (MHC) with the T-cell receptor (TCR). This requires that the antigen presented within the MHC binds with high affinity to the TCR [20]. Inhibition of DC activation of T-cells abrogates the development of hypertension in animal models. Specifically, inhibition of co-stimulation of T cells with the immunoglobulin against CTLA4 blocks angiotensin II (Ang II)-induced and deoxycorticosterone acetate (DOCA)-salt-induced hypertension in mice [21]. Additionally, intrinsic and extrinsic factors that participate in DC development, antigen presentation, and activation play an essential role in hypertension.
Novel immune cell subtypes in hypertension
The characteristics and definition of myeloid cells are ever evolving through technological advances of single cell sequencing and it is now clear that myeloid cells provide both pro-inflammatory and anti-inflammatory roles in the development of hypertension. In a seminal study, Wenzel et al. demonstrated that ablation of myeloid cells expression lysozyme M (LysM iDTR) in mice completely prevented the increase in blood pressure in response to Ang II-induced hypertension [9]. Moreover, mice lacking Macrophage Colony-Stimulating Factor (Op/Op mice), that lack macrophages and other myeloid cells, are protected from inflammation associated vascular injury in both Ang II-induced and DOCA-salt hypertension [22,23]. More recently, Loperena et al. showed that human aortic endothelial cells undergoing hypertensive stretch promoted conversion of monocytes to an intermediate (CD14+/CD16++) phenotype and cause the acquisition of CD209 (DC-SIGN), which is expressed by DCs and activated macrophages [17]. ECs undergoing hypertensive stretch profoundly upregulated the expression of IL-6, IL-1β, IL-23, and TNF-α in adjacent monocytes [17]. Moreover, these activated monocytes markedly stimulate proliferation of T cells obtained from the same volunteers. These studies demonstrate that monocytes and macrophages play a critical role in the development on various forms of hypertension.
There is ample evidence demonstrating a critical role of DCs in promoting T cell proliferation and the development of hypertension and its associated end-organ damage [4,13,24]. In a fundamental study, we discovered that DCs present immunogenic isoLG-modified proteins that lead to T cell activation and the development of hypertension [13]. More recently, we demonstrated a novel mechanism by which excess salt intake drives activation of NADPH oxidase and formation of isoLGs leading to production of IL-6 and IL-1β in DCs [24]. In keeping with this, we found that mice lacking serum-glucocorticoid kinase-1 (SGK1) in DCs abrogates development of salt-sensitive hypertension through an NADPH oxidase-dependent mechanism [4]. These studies demonstrate a critical role of DCs in promoting pro-inflammatory responses during hypertension.
As mentioned above, various myeloid cells promote a pro-inflammatory milieu driving the development of hypertension. However, there are myeloid cell subsets that dampen an inflammatory response limiting blood pressure increase and end-organ damage. Shah et al. demonstrated that a subpopulation of myeloid cells, myeloid-derived suppressor cells (MDSCs), limit the increase in blood and inflammation in response to three murine models of experimental hypertension [25]. In a cyclosporine-A model of hypertension, Chiasson et al. found that adoptive transfer of MSDCs prevented renal and vascular inflammation and improved vascular relaxation [26]. More recently, Crowley et al. demonstrated that DCs expressing A20, a ubiquitin-editing protein known to preserve immune system hemostasis, abrogates Ang II-induced elevations in blood pressures by preventing renal activated T-cell accumulation [20], demonstrating a vital role of myeloid cells in the suppression of inflammatory events during hypertensive insults.
Natural killer (NK) cells or ‘killer’ innate lymphoid cells (ILCs) are activated by and exert cytotoxic effects on targeted cells. ILCs can be classified into three groups based on their surface markers, cytokine production, and transcription factors. NK cells are part of ILC1s and have been shown to play a role in Ang II-induced vascular inflammation and dysfunction through production of INF-γ. Kossman et al. demonstrated that deletion of tbox21 or INF-γ reduced NK cell infiltration into the aortic wall during Ang II-induced hypertension [27]. The role of ILC2s and ILC3s in hypertension remains to be determined and further study is needed.
The role of excess dietary salt in inflammation and hypertension
High salt consumption in the Western diet leads to inflammation and cardiovascular disease but the mechanisms are not known. A major problem with excess dietary intake is salt sensitivity of blood pressure (SSBP). SSBP is an independent risk factor for cardiovascular mortality not only in hypertensive, but also in normotensive adults [28,29]. Most research efforts to understand the mechanisms of SSBP have focused on renal regulation of sodium (Na+). Drs. Guyton and Coleman’s modeling posited that salt would not raise BP unless there was a defect in the regulation of natriuresis and that a multitude of systems including the renin-angiotensin-aldosterone system (RAAS), atrial natriuretic peptide, and sympathetic outflow respond to changes in Na+ intake and control BP [30]. However, salt retention or plasma volume expansion are not enhanced in salt sensitive (SS) versus salt resistant (SR) individuals [31,32]. In addition, over 70% of extracellular fluid is interstitial and therefore not directly controlled by renal salt and water excretion [33]. Thus, further research is needed to understand the extrarenal mechanisms contributing to SSBP including a role of immune cell activation.
Recent studies have found that there is interstitial Na+ storage, which is electrostatically associated with glycosaminoglycans, mostly studied in skin and muscle but could be in other tissues and organs including the kidney and lymphoid organs [33]. Machnik et al. found that excess dietary salt increases interstitial Na+ in the skin without changing plasma concentrations in mice [34]. Subsequent studies using 23Na MRI found that Na+ accumulates in the skin and skeletal muscle of humans with hypertension and during aging [35]. These studies do not contradict proposed mechanisms of pressure natriuresis and renal Na+ regulation. However, they support the premise for existence of extrarenal mechanisms for Na+ homeostasis. It is not known if regulation of interstitial Na+ is different in SS versus SR subjects. This is important because elevated Na+ has been found to activate immune cells, which contribute to hypertension [36, 37, 38]. In addition, these observations regarding tissue Na+ have relevance to circulating monocytes since recent studies have found that these cells can enter and re-emerge from tissues with minimal to no differentiation [39]. There is strong evidence that monocytes contribute to both BP elevation and end-organ damage. Depletion of monocytes markedly reduces experimental hypertension [9]. As described above, antigen presenting cells derived from monocytes; including macrophages and DCs have also been implicated in hypertension [13,24,33]. These monocyte-derived cells activate T cells, which in turn infiltrate the kidneys and perivascular space and release inflammatory cytokines that promote renal and vascular dysfunction leading to elevated BP [33,40].
We recently found that Na+ enters monocyte-derived antigen presenting cells via ENaC, and this is regulated by the Serum/Glucocorticoid regulated Kinase 1 (SGK1) [4,24]. This results in calcium influx and activation of NADPH oxidase, leading to formation of products of lipid oxidation known as isolevuglandins (IsoLGs). IsoLGs adduct to self-proteins forming neoantigens, and activate T cells, which release cytokines that promote Na+ retention, kidney damage, endothelial dysfunction and BP elevation. Adoptive transfer of DCs exposed to elevated salt primes hypertension in response to a subpressor dose of Ang II [24]. IsoLG-protein adduct formation does not occur mice NADPH oxidase deletion and pharmacological scavenging of IsoLGs prevents DC activation, hypertension and end-organ damage [13,24]. These studies suggest that elevated Na+ increases immune cell activation and that therapeutic strategies to reduce Na+ may reduce inflammation and hypertension.
The role of the inflammasome in hypertension
Monocytes and macrophages play a central role in the activation of the inflammatory cascade in hypertension [9,13,17]. Danger-associated molecular pattern receptors (DAMPs) detect intracellular inflammatory agents including ROS [41,42]. This results in the activation of the inflammasome. Inflammasomes are multiprotein complexes with enzymatic activity that cleave and activate pro-inflammatory cytokines IL-1β and IL-18 within innate immune cells. Numerous unique inflammasomes participate in unique cellular functions including the elaboration of cytokines, however, only the NLRP3 inflammasome has been studied in the setting of hypertension. Early studies of hypertensive patients revealed elevated serum IL-1β when compared to controls [43]. Additionally, monocytes from hypertensive patients exhibit augmented secretion of IL-1β [44]. This is attenuated by angiotensin receptor blockade [45].
Krishnan et al. employed uninephrectomized mice treated with DOCA and 0.9% salt in the drinking water as a model of hypertension, urine output, and renal inflammation. They found that treatment with the NLRP3 inflammasome assembly inhibitor MCC950 resulted in attenuation of hypertension. This was accompanied by a reduction in the expression of collagen and pro-inflammatory genes in the kidney. Additionally, total leukocytes and IFN-γ positive T-cells were reduced in the kidney of treated animals [46]. These results support the role of the NLRP3 inflammasome in hypertension, however experimental studies examining the role of non NLRP3 inflammasome subsets in hypertension should be the focus of additional studies.
The importance of the inflammasome in cardiovascular disease is highlighted in the Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) [47]. This study showed the efficacy of Canakinumab, an anti-IL-1β antibody, at reducing recurrent cardiovascular events in patients with elevated high sensitivity C reactive protein levels (CRP). While this study showed no significant change in blood pressure with Canakinumab therapy, an additional analysis was performed that examined the benefit of Canakinumab in groups of participants divided into quartiles based on blood pressure. This analysis revealed a trend of greater reduction in major adverse cardiac events in the highest blood pressure quartile [48]. These studies suggest a role of the inflammasome in the pathogenesis of hypertension. Further studies are needed to identify the intracellular mechanisms and specific cell types involved in these processes.
The microbiome, inflammation, and hypertension
Short chain fatty acids, primarily propionate, acetate, and butyrate, are metabolites of the intestinal microbiome. These are typically metabolized by first pass hepatic metabolism, however, a small fraction of SFCAs are released into the systemic circulation where they are bioactive. SFCA’s modulate immune cell function. Immune cells express the SFCA G-coupled protein receptors FFA2, FFA3, and GPR109A. FFA2 specifically is expressed primarily on cells of the immune system, specifically on dendritic cells and monocytes [49]. FFA2 receptors are potently agonized by 2-carbon acetate and the 3-carbon propionate over the 4-carbon butyrate molecule. These receptors are coupled to Gαi and Gαq subunits which, when activated, result in an anti-inflammatory affects upon agonism of these receptors via inhibition of NF-κB [50]. These studies suggest that alteration of microbial metabolites may directly activate proinflammatory pathways within immune cells.
Next generation bacterial sequencing has led to numerous recent investigations into the role of the microbiome in hypertension. Santisteban et al. showed an increase in gut permeability in hypertensive rats which correlated with augmented gut-neuronal communication [51]. Yang et al. showed an increase in fecal Firmicutes/Bacteroidetes ratio in the spontaneously hypertensive rat model of hypertension compared to Wistar Kyoto control rats [52]. Similarly, Cheema et al. characterized shifts in the microbiome and associated metabolites in the Ang II mouse model of hypertension [53]. Importantly, in the Ang II model, rats treated with the antimicrobial minocycline exhibited attenuated hypertension and reduction of the Firmicutes/Bacteroidetes ratio. In this same study, it was found that hypertensive patients exhibit a reduction in fecal microbial richness [52]. While many studies describing the relationship of the microbiome and hypertension have shown correlations between disease state and metabolites or microbial imbalance, there have been some recent mechanistic insights into these processes. Sharma et al. recently described the effects of intracerebroventricular administration of the antimicrobial derivative tetracycline-3 on hypertension and microbial communities in hypertensive rats. Infusion of tetracycline-3 resulted in attenuation of Ang II induced hypertension, inhibited neuroinflammation, and increased microbial fecal richness indices [54]. These data further suggest a role of the central nervous system in the regulation of the microbiome and the activation of microglial inflammatory cells in the setting of hypertension.
Work from our group has described an important role of excess dietary salt as a modifier of the gut microbiome in humans and mice. This is associated with an increase in blood pressure in humans and predisposition to vascular inflammation, DC activation, and hypertension in a mouse model of hypertension [14]. Wilck et al. found that in both mice and humans, there is a significant increase in blood pressure, Th17-mediated inflammation, and changes in the gut microbiome following a high salt diet [55]. Additionally, Bartolomaeus et al. showed that the SCFA propionate reduced cardiac remodeling and hypertension in apolipoprotein E (ApoE) knockout mice treated with Ang II [56]. This was associated with a marked attenuation of systemic inflammation, T-cell expansion, and cardiac immune cell infiltration [56]. These studies clearly show a correlation of microbiome diversity and metabolism in the development of inflammation and hypertension. Continued mechanistic studies of these processes will likely yield important new insights into the role of the environmental interaction with the immune system in hypertension and cardiovascular disease.
Hypertension, inflammation and COVID-19
Diabetes, cerebrovascular disease, and hypertension are among the most prevalent comorbidities noted in patients with confirmed COVID-19 [57, 58, 59]. Both SARS-CoV and SARS-CoV-2 utilize the angiotensin-converting enzyme 2 (ACE2) as an entry receptor which is mediated by the spike protein for both viruses [60]. ACE2 is a membrane bound carboxypeptidase that cleaves angiotensin (Ang) I into Ang 1-9 and Ang II into Ang 1-7. Yang et al. showed that SARS-CoV exhibits augmented replication in mice that overexpressed the human ACE2 enzyme under the mouse ACE2 promoter [61]. However, multiple studies have shown a potentially protective role of ACE2 activity in lung injury and lung failure [62,63]. These studies suggest potential adverse effects of both ACE2 overexpression and ACE2 inhibition in SARS-CoV-2 infection. Several studies have suggested that treatment with ACE inhibitors may downregulate ACE2 expression [64]. However, Xu et al. in a retrospective single center analysis, showed that COVID-19 patients with pre-existing hypertension who were taking ACE inhibitors did not display significant differences in mortality, mechanical ventilation, or ICU admission [65]. Reynolds et al. examined the relationship between antihypertensive therapy and the likelihood of severe illness in COVID-19 positive patients and found no significant increase in risk with any medication class including ACE inhibitors and angiotensin receptor blockers (ARBs) [66]. In a case-control study, Mancia et al. showed no association of ACE inhibitor or ARB use with COVID-19 positivity, disease severity, or fatality [67]. Based upon these studies and others, clinical guidelines do not support discontinuation of ACE inhibitor or ARB use in COVID-19 patients.
As mentioned above, hypertension is more common in patients with severe COVID-19 infections [68]. The clinical presentation of severe presentations has been compared to cytokine release syndrome and therefore immunomodulation has been suggested as a potential therapeutic strategy in the treatment of severe COVID-19 [69, 70, 71]. The presence of hypertension in patients with severe COVID-19 infections is not isolated and is associated with other comorbidities and advanced age. However, given the essential role of inflammation in the development and pathogenesis of hypertension, it is conceivable that hypertension may represent an immunologically vulnerable condition that predisposes patients to more severe presentations.
Summary and perspectives
Numerous antihypertensive therapies are currently prescribed to patients worldwide, however, despite the availability of therapies, treatment of hypertension remains a challenge. Awareness of hypertension has improved and, in 2015–2016, 48% of patients demonstrated improved control in the United States [72]. Despite these improvements, there remains significant disparities in hypertension control globally, with low income countries exhibiting increases in prevalence and poorer control of hypertension [73]. It is clear that there remains a significant need for continued research and therapeutic development for the treatment of hypertension in addition to a multidisciplinary approach to increase awareness and control. The discovery of novel mechanisms of immune activation suggest new potentially modifiable factors and therapeutic strategies to affect these changes. The regulation of immune activation by both the microbiome and sodium levels suggest that modification of dietary intake may affect inflammation and hypertension. Continued studies focused on these mechanisms may lead to new insights into the role of lifestyle changes and inflammation. These studies also suggest novel cellular targets that may be considered for the development of durable antihypertensive therapies. It is also clear that novel techniques such as next generation microbial sequencing and single cell sequencing are essential tools for these studies moving forward. Finally, focusing on immune activation in hypertension may provide novel insights into the mechanisms of severe COVID-19 infections and should be pursued in focused mechanistic studies.
Conflict of interest statement
Dr. Kirabo was supported by the National Institutes of Health grants K01HL130497 and R01HL147818. Dr. Patrick was funded by the T32 GM007569-44 and is the recipient of a National Institute of Health Ruth L. Kirschstein Individual National Research Service Award (F32HL144050). Dr. Van Beusecum was supported by an F32HL142937.
Dr. Kirabo has a patent entitled “Methods for Treating Inflammation and Hypertension with Gamma-Ketoaldehyde Scavengers (U.S. Patent # 14/232,615).
Dr. Kirabo is an associate editor for circulation research with compensation.
Acknowledgement
Supported by the National Institutes of Health grants K01HL130497 and R01HL147818 to K.A, F32HL142937 to J.P.V.B, T32GM0075699-44 and F32HL144050 to D.M.P.
References
- 1.Rapsomaniki E., Timmis A., George J., Pujades-Rodriguez M., Shah A.D., Denaxas S., White I.R., Caulfield M.J., Deanfield J.E., Smeeth L., et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators GBDRF Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawes C.M., Vander Hoorn S., Rodgers A., International Society of H Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 4.Van Beusecum J.P., Barbaro N.R., McDowell Z., Aden L.A., Xiao L., Pandey A.K., Itani H.A., Himmel L.E., Harrison D.G., Kirabo A. High salt activates CD11c(+) antigen-presenting cells via SGK (Serum Glucocorticoid Kinase) 1 to promote renal inflammation and salt-sensitive hypertension. Hypertension. 2019;74:555–563. doi: 10.1161/HYPERTENSIONAHA.119.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu J., Saleh M.A., Kirabo A., Itani H.A., Montaniel K.R., Xiao L., Chen W., Mernaugh R.L., Cai H., Bernstein K.E., et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126:50–67. doi: 10.1172/JCI80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzik T.J., Hoch N.E., Brown K.A., McCann L.A., Rahman A., Dikalov S., Goronzy J., Weyand C., Harrison D.G. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norlander A.E., Saleh M.A., Pandey A.K., Itani H.A., Wu J., Xiao L., Kang J., Dale B.L., Goleva S.B., Laroumanie F., et al. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norlander A.E., Madhur M.S., Harrison D.G. The immunology of hypertension. J Exp Med. 2018;215:21–33. doi: 10.1084/jem.20171773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenzel P., Knorr M., Kossmann S., Stratmann J., Hausding M., Schuhmacher S., Karbach S.H., Schwenk M., Yogev N., Schulz E., et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 10.Loperena R., Harrison D.G. Oxidative stress and hypertensive diseases. Med Clin North Am. 2017;101:169–193. doi: 10.1016/j.mcna.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montezano A.C., Touyz R.M. Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid Redox Signal. 2014;20:164–182. doi: 10.1089/ars.2013.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lori A., Perrotta M., Lembo G., Carnevale D. The spleen: a hub connecting nervous and immune systems in cardiovascular and metabolic diseases. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18061216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirabo A., Fontana V., de Faria A.P., Loperena R., Galindo C.L., Wu J., Bikineyeva A.T., Dikalov S., Xiao L., Chen W., et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson J.F., Aden L.A., Barbaro N.R., Van Beusecum J.P., Xiao L., Simmons A.J., Warden C., Pasic L., Himmel L.E., Washington M.K., et al. High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. JCI Insight. 2019;5 doi: 10.1172/jci.insight.126241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trott D.W., Thabet S.R., Kirabo A., Saleh M.A., Itani H., Norlander A.E., Wu J., Goldstein A., Arendshorst W.J., Madhur M.S., et al. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension. 2014;64:1108–1115. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y., Wu Y., Zhang C., Li P., Cui W., Hao J., Ma X., Yin Z., Du J. gammadeltaT cell-derived interleukin-17A via an interleukin-1beta-dependent mechanism mediates cardiac injury and fibrosis in hypertension. Hypertension. 2014;64:305–314. doi: 10.1161/HYPERTENSIONAHA.113.02604. [DOI] [PubMed] [Google Scholar]
- 17.Loperena R., Van Beusecum J.P., Itani H.A., Engel N., Laroumanie F., Xiao L., Elijovich F., Laffer C.L., Gnecco J.S., Noonan J., et al. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc Res. 2018;114:1547–1563. doi: 10.1093/cvr/cvy112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J., Thabet S.R., Kirabo A., Trott D.W., Saleh M.A., Xiao L., Madhur M.S., Chen W., Harrison D.G. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res. 2014;114:616–625. doi: 10.1161/CIRCRESAHA.114.302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saleh M.A., McMaster W.G., Wu J., Norlander A.E., Funt S.A., Thabet S.R., Kirabo A., Xiao L., Chen W., Itani H.A., et al. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest. 2015;125:1189–1202. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X., Rudemiller N.P., Wen Y., Ren J., Hammer G.E., Griffiths R., Privratsky J.R., Yang B., Sparks M.A., Crowley S.D. A20 in myeloid cells protects against hypertension by inhibiting dendritic cell-mediated T-cell activation. Circ Res. 2019;125:1055–1066. doi: 10.1161/CIRCRESAHA.119.315343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinh A., Chen W., Blinder Y., Weiss D., Taylor W.R., Goronzy J.J., Weyand C.M., Harrison D.G., Guzik T.J. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Ciuceis C., Amiri F., Brassard P., Endemann D.H., Touyz R.M., Schiffrin E.L. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol. 2005;25:2106–2113. doi: 10.1161/01.ATV.0000181743.28028.57. [DOI] [PubMed] [Google Scholar]
- 23.Ko E.A., Amiri F., Pandey N.R., Javeshghani D., Leibovitz E., Touyz R.M., Schiffrin E.L. Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am J Physiol Heart Circ Physiol. 2007;292:H1789–1795. doi: 10.1152/ajpheart.01118.2006. [DOI] [PubMed] [Google Scholar]
- 24.Barbaro N.R., Foss J.D., Kryshtal D.O., Tsyba N., Kumaresan S., Xiao L., Mernaugh R.L., Itani H.A., Loperena R., Chen W., et al. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 2017;21:1009–1020. doi: 10.1016/j.celrep.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah K.H., Shi P., Giani J.F., Janjulia T., Bernstein E.A., Li Y., Zhao T., Harrison D.G., Bernstein K.E., Shen X.Z. Myeloid suppressor cells accumulate and regulate blood pressure in hypertension. Circ Res. 2015;117:858–869. doi: 10.1161/CIRCRESAHA.115.306539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiasson V.L., Bounds K.R., Chatterjee P., Manandhar L., Pakanati A.R., Hernandez M., Aziz B., Mitchell B.M. Myeloid-derived suppressor cells ameliorate cyclosporine A-induced hypertension in mice. Hypertension. 2018;71:199–207. doi: 10.1161/HYPERTENSIONAHA.117.10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kossmann S., Schwenk M., Hausding M., Karbach S.H., Schmidgen M.I., Brandt M., Knorr M., Hu H., Kroller-Schon S., Schonfelder T., et al. Angiotensin II-induced vascular dysfunction depends on interferon-gamma-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler Thromb Vasc Biol. 2013;33:1313–1319. doi: 10.1161/ATVBAHA.113.301437. [DOI] [PubMed] [Google Scholar]
- 28.Weinberger M.H., Fineberg N.S., Fineberg S.E., Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 29.Weinberger M.H. Salt sensitivity is associated with an increased mortality in both normal and hypertensive humans. J Clin Hypertens (Greenwich) 2002;4:274–276. doi: 10.1111/j.1524-6175.2002.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guyton A.C., Coleman T.G. Quantitative analysis of the pathophysiology of hypertension. Circ Res. 1969;24:1–19. [PubMed] [Google Scholar]
- 31.Dobesova Z., Kunes J., Zicha J. Body fluid alterations and organ hypertrophy in age-dependent salt hypertension of Dahl rats. Physiol Res. 1995;44:377–387. [PubMed] [Google Scholar]
- 32.Qi N., Rapp J.P., Brand P.H., Metting P.J., Britton S.L. Body fluid expansion is not essential for salt-induced hypertension in SS/Jr rats. Am J Physiol. 1999;277:R1392–1400. doi: 10.1152/ajpregu.1999.277.5.R1392. [DOI] [PubMed] [Google Scholar]
- 33.Kirabo A. A new paradigm of sodium regulation in inflammation and hypertension. Am J Physiol Regul Integr Comp Physiol. 2017;313:R706–R710. doi: 10.1152/ajpregu.00250.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machnik A., Neuhofer W., Jantsch J., Dahlmann A., Tammela T., Machura K., Park J.K., Beck F.X., Muller D.N., Derer W., et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 35.Kopp C., Linz P., Dahlmann A., Hammon M., Jantsch J., Muller D.N., Schmieder R.E., Cavallaro A., Eckardt K.U., Uder M., et al. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61:635–640. doi: 10.1161/HYPERTENSIONAHA.111.00566. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W.C., Zheng X.J., Du L.J., Sun J.Y., Shen Z.X., Shi C., Sun S., Zhang Z., Chen X.Q., Qin M., et al. High salt primes a specific activation state of macrophages, M(Na) Cell Res. 2015;25:893–910. doi: 10.1038/cr.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorg S., Kissel J., Manzel A., Kleinewietfeld M., Haghikia A., Gold R., Muller D.N., Linker R.A. High salt drives Th17 responses in experimental autoimmune encephalomyelitis without impacting myeloid dendritic cells. Exp Neurol. 2016;279:212–222. doi: 10.1016/j.expneurol.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Kleinewietfeld M., Manzel A., Titze J., Kvakan H., Yosef N., Linker R.A., Muller D.N., Hafler D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jakubzick C., Gautier E.L., Gibbings S.L., Sojka D.K., Schlitzer A., Johnson T.E., Ivanov S., Duan Q., Bala S., Condon T., et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixon K.B., Davies S.S., Kirabo A. Dendritic cells and isolevuglandins in immunity, inflammation, and hypertension. Am J Physiol Heart Circ Physiol. 2017;312:H368–H374. doi: 10.1152/ajpheart.00603.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banoth B., Cassel S.L. Mitochondria in innate immune signaling. Transl Res. 2018;202:52–68. doi: 10.1016/j.trsl.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui J., Chen Y., Wang H.Y., Wang R.F. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum Vaccin Immunother. 2014;10:3270–3285. doi: 10.4161/21645515.2014.979640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalekos G.N., Elisaf M., Bairaktari E., Tsolas O., Siamopoulos K.C. Increased serum levels of interleukin-1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med. 1997;129:300–308. doi: 10.1016/s0022-2143(97)90178-5. [DOI] [PubMed] [Google Scholar]
- 44.Dorffel Y., Latsch C., Stuhlmuller B., Schreiber S., Scholze S., Burmester G.R., Scholze J. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension. 1999;34:113–117. doi: 10.1161/01.hyp.34.1.113. [DOI] [PubMed] [Google Scholar]
- 45.Li Q.Z., Deng Q., Li J.Q., Yi G.H., Zhao S.P. Valsartan reduces interleukin-1beta secretion by peripheral blood mononuclear cells in patients with essential hypertension. Clin Chim Acta. 2005;355:131–136. doi: 10.1016/j.cccn.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Krishnan S.M., Ling Y.H., Huuskes B.M., Ferens D.M., Saini N., Chan C.T., Diep H., Kett M.M., Samuel C.S., Kemp-Harper B.K., et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc Res. 2019;115:776–787. doi: 10.1093/cvr/cvy252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson P.L., Nidorf S.M. Anti-inflammatory therapy with canakinumab for atherosclerotic disease: lessons from the CANTOS trial. J Thorac Dis. 2018;10:695–698. doi: 10.21037/jtd.2018.01.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothman A.M., MacFadyen J., Thuren T., Webb A., Harrison D.G., Guzik T.J., Libby P., Glynn R.J., Ridker P.M. Effects of interleukin-1beta inhibition on blood pressure, incident hypertension, and residual inflammatory risk: a secondary analysis of CANTOS. Hypertension. 2020;75:477–482. doi: 10.1161/HYPERTENSIONAHA.119.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S., Kim J.H., Park B.O., Kwak Y.S. Perspectives on the therapeutic potential of short-chain fatty acid receptors. BMB Rep. 2014;47:173–178. doi: 10.5483/BMBRep.2014.47.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S.U., In H.J., Kwon M.S., Park B.O., Jo M., Kim M.O., Cho S., Lee S., Lee H.J., Kwak Y.S., et al. beta-Arrestin 2 mediates G protein-coupled receptor 43 signals to nuclear factor-kappaB. Biol Pharm Bull. 2013;36:1754–1759. doi: 10.1248/bpb.b13-00312. [DOI] [PubMed] [Google Scholar]
- 51.Santisteban M.M., Qi Y., Zubcevic J., Kim S., Yang T., Shenoy V., Cole-Jeffrey C.T., Lobaton G.O., Stewart D.C., Rubiano A., et al. Hypertension-linked pathophysiological alterations in the gut. Circ Res. 2017;120:312–323. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang T., Santisteban M.M., Rodriguez V., Li E., Ahmari N., Carvajal J.M., Zadeh M., Gong M., Qi Y., Zubcevic J., et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheema M.U., Pluznick J.L. Gut microbiota plays a central role to modulate the plasma and fecal metabolomes in response to angiotensin II. Hypertension. 2019;74:184–193. doi: 10.1161/HYPERTENSIONAHA.119.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma R.K., Yang T., Oliveira A.C., Lobaton G.O., Aquino V., Kim S., Richards E.M., Pepine C.J., Sumners C., Raizada M.K. Microglial cells impact gut microbiota and gut pathology in angiotensin II-induced hypertension. Circ Res. 2019;124:727–736. doi: 10.1161/CIRCRESAHA.118.313882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilck N., Matus M.G., Kearney S.M., Olesen S.W., Forslund K., Bartolomaeus H., Haase S., Mähler A., Balogh A., Markó L., et al. Salt-responsive gut commensal modulates T(H)17 axis and disease. Nature. 2017;551:585–589. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bartolomaeus H., Balogh A., Yakoub M., Homann S., Marko L., Hoges S., Tsvetkov D., Krannich A., Wundersitz S., Avery E.G., et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139:1407–1421. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., Akdis C.A., Gao Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 60.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang X.H., Deng W., Tong Z., Liu Y.X., Zhang L.F., Zhu H., Gao H., Huang L., Liu Y.L., Ma C.M., et al. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp Med. 2007;57:450–459. [PubMed] [Google Scholar]
- 62.Gandhi C.K., Holmes R., Gewolb I.H., Uhal B.D. Degradation of lung protective angiotensin converting enzyme-2 by meconium in human alveolar epithelial cells: a potential pathogenic mechanism in meconium aspiration syndrome. Lung. 2019;197:227–233. doi: 10.1007/s00408-019-00201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 65.Xu J., Huang C., Fan G., Liu Z., Shang L., Zhou F., Wang Y., Yu J., Yang L., Xie K., et al. Use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in context of COVID-19 outbreak: a retrospective analysis. Front Med. 2020 doi: 10.1007/s11684-020-0800-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., Hausvater A., Newman J.D., Berger J.S., Bangalore S., et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., Liu X.Q., Chen R.C., Tang C.L., Wang T., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pontali E., Volpi S., Antonucci G., Castellaneta M., Buzzi D., Tricerri F., Angelelli A., Caorsi R., Feasi M., Calautti F., et al. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Treon S.P., Castillo J.J., Skarbnik A.P., Soumerai J.D., Ghobrial I.M., Guerrera M.L., Meid K., Yang G. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135:1912–1915. doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roschewski M., Lionakis M.S., Sharman J.P., Roswarski J., Goy A., Monticelli M.A., Roshon M., Wrzesinski S.H., Desai J.V., Zarakas M.A., et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fryar C.D., Ostchega Y., Hales C.M., Zhang G., Kruszon-Moran D. Hypertension prevalence and control among adults: United States, 2015-2016. NCHS Data Brief. 2017:1–8. [PubMed] [Google Scholar]
- 73.Mills K.T., Bundy J.D., Kelly T.N., Reed J.E., Kearney P.M., Reynolds K., Chen J., He J. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]