Conflict of interest disclosures
Drs. Berlow, Zandvakili, and Philip have received or been supported by grants from the National Institute of Mental Health (NIMH R25 MH101076 (YAB)), the National Institute of General Medical Sciences (NIGMS U54GM115677 (AZ) and NIGMS, P20 GM130452 (NSP)), the U.S. Department of Veterans Affairs (I01 RX002450 (NSP)), and the VA RR&D Center for Neurorestoration and Neurotechnology (YAB, AZ, NSP) during the conduct of this study. In the past, Dr. Philip has received grant support from Janssen, Neosync, and Neuronetics through clinical trial contracts and has served as an unpaid scientific advisory board member for Neuronetics. The opinions herein represent those of the authors and not the U.S. Department of Veterans Affairs. The other authors report no financial relationships with commercial interests.
In their recent letter, Miron and colleagues make a cohesive and robust argument for reappraising the role of low frequency (1 Hz), right-sided repetitive transcranial magnetic stimulation (LFR-TMS) for the treatment of depression, highlighting its many potential advantages over high frequency (≥5 Hz) left-sided stimulation (HFL-TMS); these advantages included the ability to use less expensive devices, tolerability, and better safety profile [1]. Such devices may also be better suited to the COVID-19 world where the need for at-home stimulation must carry greater weight. The relative advantages of LFR-TMS become even more critical if it can be shown that this approach has comparable efficacy as HFL-TMS. Consistent with this narrative, multiple studies comparing the efficacy between LFR-TMS and HFL-TMS for depression have failed to detect differences in these approaches, including a meta-analysis of 12 studies (n = 361) [2], and a recent large randomized controlled trial (n = 300) [3]. While the failure to detect differences using null hypothesis significance testing in these studies is insufficient to establish that LFR-TMS is as efficacious as HFL-TMS, formal equivalence testing of this same data can provide quantitative evidence for the absence of a clinically meaningful difference between these treatment approaches. The aim of this study was to perform equivalence testing of the pooled efficacy outcomes from LFR-TMS and HFL-TMS treatments for depression using the currently available evidence.
Randomized controlled trials of LFR-TMS and HFL-TMS for patients with major depressive episodes were identified through PubMed following the methods described by Cao et al. [2], but extending to June 21, 2020 and defining low frequency stimulation as less than 5 Hz. Treatment response was defined as a 50% reduction in depression rating scales. This strategy yielded 12 studies assessing treatment response and six reporting remission rates, including 11 of the studies identified and analyzed by Cao et al. [2] and the two arms of the recent large trial by Fitzgerald et al. [3].
Pooled odds ratios for response and remission rates were calculated using the Mantel-Haenszel (MH) fixed effect method as implemented in the ‘meta’ package from R [4]. Heterogeneity was estimated using the DerSimonian-Laird estimator for tau2. Odds ratios (OR) and the corresponding confidence intervals (CI) were converted to effect sizes (Cohen’s d) and standard error (SE) estimates using the conversion procedures for meta-analysis provided by Chinn [5], where d = ln (OR)∗(√3/π). Equivalence testing for meta-analysis was completed using the two one-sided test (TOST) for meta-analysis in the R ‘TOSTER’ library with alpha (α) set at 0.05 [6]. The equivalence margin was set at d = 0.25, following the work and rationale of Steinert et al. [7] for equivalence testing in meta-analysis of depression treatments. Using the conversion formula noted above [5], this equivalence margin corresponds with OR values between 0.636 and 1.574. Additional analyses were performed using alternative study selection to assess the robustness of these findings. Ninety percent confidence intervals corresponding to 1-2α are presented to illustrate equivalence testing using the TOST [6].
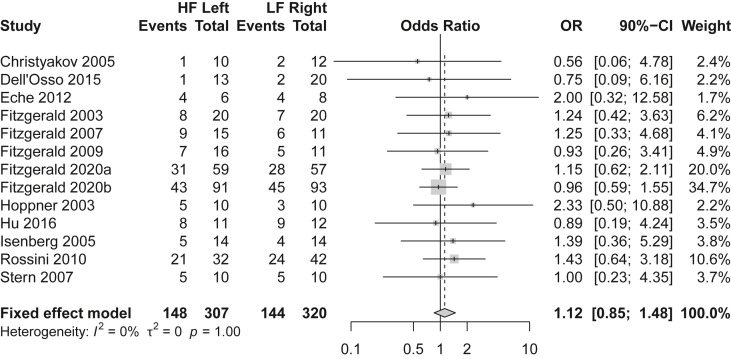
Twelve randomized controlled trials were included in the analysis with a total of 627 subjects with major depressive episodes treated with either LFR-TMS (n = 320) or HFL-TMS (n = 307). Overall, 47% percent (292/627) of these subjects were classified as responders, with reported response rates of 45% (144/320) in subjects treated with LFR-TMS and 48% (148/307) in subjects treated with HFL-TMS. The pooled odds ratio was 1.12 (90% CI: 0.85–1.48), corresponding to an effect size of d = 0.064 (SE = 0.09) nominally favoring HFL-TMS as shown in Fig. 1 . Equivalence testing with a margin of d = 0.25 was significant (Z = −2.02, p = 0.022), indicating evidence to reject the difference hypothesis and accept the equivalence hypothesis for treatment response. A subset of six of these studies (n = 431) reported remission rates in addition to response rates, with 26% (57/217) of patients randomized to LFR-TMS achieving remission compared to 25% (54/214) in the HFL-TMS groups. The pooled odd ratio was 0.92 (90% CI: 0.64–1.33), nominally in favor of LFR-TMS (Supplement 1). Equivalence testing with the same margin (d = 0.25) was significant (Z = 1.687, p = 0.046), indicating evidence for equivalence of remission rates. Additional analyses excluding three studies in which bipolar depression made up the majority of subjects [[8], [9], [10]] yielded similar results supporting equivalence for response rates (pooled OR = 1.10, 90% CI: 0.82–1.49; equivalence testing Z = −1.94, p = 0.026 given margin of d = 0.25) and trend level results for remission rates (pooled OR = 0.87, 90% CI: 0.59–1.28; equivalence testing Z = −1.35, p = 0.088).
Fig. 1.
Meta-Analysis of Depression Response Rates in Subjects Receiving either High Frequency Left-Sided (HF Left) or Low Frequency Right-Sided (LF Right) Transcranial Magnetic Stimulation.
This study provides statistical evidence of the equivalence of LFR-TMS and HFL-TMS efficacy when used to treat major depressive episodes. As illustrated by the confidence intervals in Fig. 1, neither the individual studies nor the pooled estimates comparing LFR-TMS and HFL-TMS demonstrated that either approach has superior efficacy for treating depressive episodes (p > 0.1). These findings confirm previous meta-analyses of LFR-TMS and HFL-TMS outcomes that were unable to detect differences with these two stimulation protocols [2] and suggest there is no statistical evidence that these stimulation protocols differ in efficacy. However, many of these previous studies have been underpowered to demonstrate meaningful equivalence and none have applied the methods of equivalency testing. The current study works to overcome these limitations by combining the statistical power of meta-analysis along with the logic of equivalence testing to define equivalent efficacy outcomes of these two TMS protocols using a conservative margin established for depression treatments [7]. If a true difference between LFR-TMS and HFL-TMS response and remission rates existed larger than the tested equivalency margin (d = 0.25), the current results of significant equivalence would be surprising (p < 0.05). A difference in efficacy outcomes between LFR-TMS and HFL-TMS protocols may still exist, however, this analysis suggests the difference would be small and unlikely to be clinically meaningful. While the evidence base for HFL- TMS has been well established compared to LFR-TMS, the current finding that these two stimulation protocols have equivalent efficacy provides further support of the need for additional studies to re-evaluate the role of LFR-TMS in the treatment of depression.
CRediT authorship contribution statement
Yosef A. Berlow: Conceptualization, Methodology, Visualization, Writing - original draft, preparation. Amin Zandvakili: Conceptualization, Writing - review & editing. Noah S. Philip: Conceptualization, Writing - review & editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brs.2020.10.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Miron J.P., Sheen J., Mansouri F., Blumberger D.M., Daskalakis Z.J., Vila-Rodriguez F., Downar J. The role of low-frequency repetitive transcranial magnetic stimulation in major depression: a call to increase the evidence base. Brain Stimul. 2020;13:1296–1297. doi: 10.1016/j.brs.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao X., Deng C., Su X., Guo Y. Response and remission rates following high-frequency vs. Low-frequency repetitive transcranial magnetic stimulation (rTMS) over right dlpfc for treating major depressive disorder (mdd): a meta-analysis of randomized, double-blind trials. Front Psychiatr. 2018;9:413. doi: 10.3389/fpsyt.2018.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald P.B., Hoy K.E., Reynolds J., Singh A., Gunewardene R., Slack C., Ibrahim S., Daskalakis Z.J. A pragmatic randomized controlled trial exploring the relationship between pulse number and response to repetitive transcranial magnetic stimulation treatment in depression. Brain Stimul. 2020;13:145–152. doi: 10.1016/j.brs.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 5.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19:3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Lakens D. Equivalence tests: a practical primer for t tests, correlations, and meta-analyses. Soc Psychol Personal Sci. 2017;8:355–362. doi: 10.1177/1948550617697177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinert C., Munder T., Rabung S., Hoyer J., Leichsenring F. Psychodynamic therapy: as efficacious as other empirically supported treatments? A meta-analysis testing equivalence of outcomes. Am J Psychiatr. 2017;174:943–953. doi: 10.1176/appi.ajp.2017.17010057. [DOI] [PubMed] [Google Scholar]
- 8.Dell’Osso B., Oldani L., Camuri G., Dobrea C., Cremaschi L., Benatti B., Arici C., Grancini B., Altamura A.C. Augmentative repetitive Transcranial Magnetic Stimulation (rTMS) in the acute treatment of poor responder depressed patients: a comparison study between high and low frequency stimulation. Eur Psychiatr. 2015;30:271–276. doi: 10.1016/j.eurpsy.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Hu S.H., Lai J.B., Xu D.R., Qi H.L., Peterson B.S., Bao A.M., Hu C.C., Huang M.L., Chen J.K., Wei N., Hu J.B., Li S.L., Zhou W.H., Xu W.J., Xu Y. Efficacy of repetitive transcranial magnetic stimulation with quetiapine in treating bipolar II depression: a randomized, double-blinded, control study. Sci Rep. 2016;6:30537. doi: 10.1038/srep30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossini D., Lucca A., Magri L., Malaguti A., Smeraldi E., Colombo C., Zanardi R. A symptom-specific analysis of the effect of high-frequency left or low-frequency right transcranial magnetic stimulation over the dorsolateral prefrontal cortex in major depression. Neuropsychobiology. 2010;62:91–97. doi: 10.1159/000315439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.