Abstract
Purpose
The coronavirus disease 19 (COVID-19) has become a global health event. Cardiac biomarkers like creatine kinase isoenzyme (CK-MB), myoglobin, and high-sensitivity troponin T were usually elevated in early stages. This study aimed to investigate whether the elevated cardiac biomarkers could become effective prognostic predictors for COVID-19 patients.
Methods
The present study involved 357 COVID-19 patients. The potential predictors for two study outcomes (in-hospital death and recovery status) in 28 days were selected by LASSO regression analysis. Prognostic values of cardiac biomarkers selected were evaluated using the receiver operating characteristic curve (ROC) and the area under ROC (AUC).
Results
After 28-day follow-up, overall 357 patients were divided into death group (n = 25) and survival group (n = 332), or non-recovery group (n = 43) and recovery group (n = 314). The LASSO regression analysis showed elevated CK-MB and myoglobin were independent risk predictors for in-hospital death, and CK-MB and myoglobin were also independent risk predictors for non-recovery. The AUC of CK-MB and myoglobin for in-hospital death were 0.862 (95%CL: 0.804–0.920, p < 0.001) and 0.838 respectively (95%CL: 0.729–0.947, p < 0.001). The AUC of CK-MB and myoglobin for non-recovery were 0.839 (95%CL: 0.786–0.892, p < 0.001) and 0.841 (95%CL: 0.765–0.918, p < 0.001) respectively. We also found AUC of combined use of CK-MB and myoglobin for in-hospital death and non-recovery were 0.883 (95CL: 0.813–0.952, p < 0.001), and 0.873 (95%CL: 0.817–0.930, p < 0.001) respectively.
Conclusions
In patients with COVID-19, elevated CK-MB and myoglobin on admission may be effective predictors for adverse outcomes, and combined use of CK-MB and myoglobin had a better performance for prediction.
Keywords: COVID-19, Risk predictors, Creatine kinase isoenzyme, Myoglobin, Prognosis
Abbreviations: COVID-19, coronavirus disease 19; CK-MB, creatine kinase isoenzyme; ROC, receiver operating characteristic curve; AUC, the area under ROC; RT-PCR, real-time reverse transcriptase polymerase chain reaction; CRP, C-reactive protein; SD, standard deviation; LASSO, least absolute shrinkage and selection operator
1. Introduction
The coronavirus disease 2019 due to SARS-CoV-2 infection broke out initially in Wuhan city, China, from December 2019 [1]. And COVID-19 has become a public health event of international concern and spread rapidly. Since now, there are no useful and specific medicines and treatment for COVID-19 [2].
With increasing confirmed number of COVID-19 patients, many studies were conducted to reveal patient clinical characteristics. Abnormal change of characteristics and laboratory tests was more frequent in severe cases or deaths, such as complete blood count, plasma biochemical parameters, and inflammatory marks [3,4]. As the related researches progressing, the researchers gradually discovered infection with SARS-CoV-2 may cause acute myocardial injury. Chaolin Huang and his colleagues reported that part of ICU patients with COVID-19 existed acute myocardial injury, which mainly showed an increase of high-sensitivity troponin I [5]. Some cardiac biomarkers including creatine kinase isoenzyme (CK-MB), myoglobin, and troponin I significantly elevated in long-term hospitalization than short-term hospitalization [6]. Dr. Chirag Bavishi reviewed related studies about COVID-19 and found overall prevalence of acute myocardial injury ranged from 5% to 38% in COVID-19 patients [7]. Therefore, myocardial injury and elevation of relevant cardiac biomarkers were common in patients with SARS-CoV-2 infection [8].
Thus, how about the association between acute myocardial injury or abnormal change of cardiac biomarkers and patient prognosis. Some studies have investigated the relationship between acute myocardial injury and adverse prognosis. Dr. Alvaro Lorente-Ros and his (her) colleagues found myocardial injury was independently associated with adverse outcomes [9]. Dr. Eman A. Toraih also reported assessment of cardiac injury biomarkers may improve the identification of patients at the highest risk [10]. However, the studies to investigate prognostic value of elevated cardiac biomarkers for COVID-19 patient poor outcomes were not many. Therefore, the purpose of the present study was to describe the change of cardiac biomarkers including CK-MB, myoglobin, and high-sensitivity troponin T in patients with COVID-19 and explore the association between elevated cardiac biomarkers and poor outcomes, as well as to investigate whether elevated cardiac biomarkers were effective and valuable prognostic predictors for COVID-19 patient adverse prognosis.
2. Materials and methods
2.1. Study design and participants
This multicenter, observational study was conducted at Sichuan province and Wuhan city from January to March, including 22 tertiary hospitals designated for COVID-19 patients in the local area, and approved by Ethics Committees of West China Hospital of Sichuan University and Renmin Hospital East Campus of Wuhan University. Informed consent was achieved from the patient or the patient's legally authorized representative. Patients confirmed by real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay and diagnosed as COVID-19 according to guidelines for COVID-19 issued by National Health Commission of China were recruited in the present study. Enrolled patients were followed up maximum of 28 days after admission in the designate hospital, or until in-hospital death or recovery, whichever occurred first. Researchers recorded the patient outcomes via medical records. There were two outcomes reflecting prognosis in this study: the primary outcome was in-hospital death in 28 days after admission and the second outcome was non-recovery in 28 days after admission. Non-recovery was defined as the patient died or the patient condition did not recover and still required advanced respiratory support (high-flow nasal oxygen or mechanical ventilation) at the end of 28-day follow-up. Patients receiving mechanical ventilation or high-flow nasal oxygen at the end of 28-day follow-up still received treatment in designate hospital until recovery or death.
2.2. Data collection
Patients confirmed by COVID-19 were transported to designated hospitals in the local areas, and their condition were evaluated on hospital admission. The designated hospitals were set up to only treat COVID-19 patients. Patient clinical information was collected using electronic data capture and analysis system (EDC). Data entry was completed by physicians and nurses who were trained on the use of EDC and were working in the designated hospitals. Data quality was overseen by a team of senior ICU physicians and statisticians. Demographics and characteristics included age, gender, time from symptoms onset to designated hospital, and coexisting disorders. Respiratory rate, blood pressure, and more consisted of signs and symptoms. Laboratory findings including white blood cell count, neutrophil count, lymphocyte count, monocyte count, platelet count, hemoglobin, alanine aminotransferase, aspartate aminotransferase, albumin, total bilirubin, creatinine, procalcitonin, C-reactive protein (CRP), prothrombin time, D-dimer, CK-MB, myoglobin and high-sensitivity troponin T, were examined on admission. Other clinical information about patient treatment was also collected.
2.3. Statistical analyses
Classification variables were presented as number and percentage (%), and compared by Chi-square or Fisher's exact probability test. Normally distributed continuous variables were presented as mean ± standard deviation (SD), and compared by independent t-test. Non-normally distributed continuous variables were presented as medians and interquartile ranges, and compared using Mann–Whitney U test. P < 0.05 was considered statistically significant in comparing differences between survival and death groups, or recovery and non-recovery groups. Spearman correlation analysis was used to investigate the correlation between myoglobin and either CK-MB or high-sensitivity troponin T.
Potential risk predictors for in-hospital death or non-recovery were identified using least absolute shrinkage and selection operator (LASSO) regression analysis, and LASSO regression was a method of machine learning regression, which was used to choose independent risk factors affecting outcomes and applied to minimize the potential collinearity of the variables measured and overfitting of the variables. It is a logistic regression model penalizing the absolute size of the coefficients of a regression model based on the value of λ. With larger penalties, the estimates of weaker factors shrink toward zero, so that only the strongest predictors remain in the predictive model [11]. The ROC and AUC were built to assess the prognostic performance of predictors selected. All related statistics analyses were performed using R software 4.0.1.
3. Results
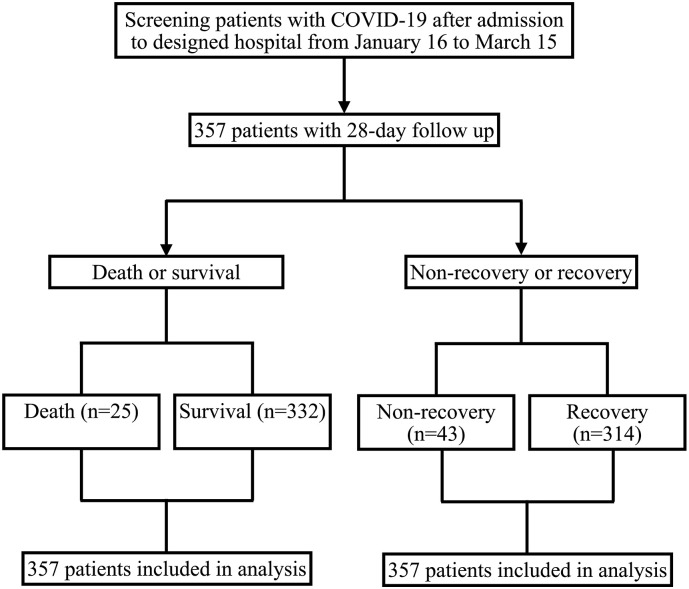
In the present study, a total of 357 patients confirmed by COVID-19 and receiving laboratory test of cardiac biomarkers were enrolled between January 20 and March 15, 2020. According to patient survival status, 357 patients were divided into death group (n = 25) and survival group (n = 332). On the other hand, 43 patients did not recover in 28 days, and 314 patients recovered according to patient recovery status after 28-day follow-up (Fig. 1 ). The median age of all patients was 56.0 (43.0, 68.0) years old, and 164 (45.9%) patients were older than 60 years old. 185 (51.8%) patients were male, and 154 (43.1%) patients were with coexisting disorders. The median time from symptoms onset to designated hospital was 9.0 (5.0, 15.0) days (Table 1, Table 2 ).
Fig. 1.
Study population.
Table 1.
Demographics and baseline clinical characteristics of the COVID-19 patients (Death vs Survivals)
| Total (n = 357) | Death (n = 25) | Survivals (n = 332) | P value | |
|---|---|---|---|---|
| Demographics and characteristics | ||||
| Age, years, median (Q1, Q3) | 56.0 (43.0, 68.0) | 75.0 (61.0, 79.0) | 55.0 (41.0, 67.0) | <0.001 |
| Age ≥ 60, no. (%) | 164 (45.9) | 20 (80.0) | 144 (43.4) | <0.001 |
| Male, no. (%) | 185 (51.8) | 15 (60.0) | 170 (51.2) | 0.396 |
| Time from symptoms onset to designated hospital, days, median (Q1, Q3) | 9.0 (5.0, 15.0) | 7.0 (4.0, 11.0) | 10.0 (5.0, 15.0) | 0.086 |
| Coexisting disordersa, no. (%) | 154 (43.1) | 15 (60.0) | 139 (41.9) | 0.078 |
| Signs and symptoms | ||||
| Respiratory rate, breaths per min, median (Q1, Q3) | 20.0 (19.0, 21.0) | 24.0 (22.0, 30.0) | 20.0 (19.0, 21.0) | <0.001 |
| Systolic pressure, mmHg, mean (SD) | 131.0 (18.6) | 139.8 (21.8) | 130.4 (18.2) | 0.014 |
| Diastolic pressure, mmHg, mean (SD) | 79.2 (11.8) | 81.5 (15.8) | 79.0 (11.5) | 0.452 |
| Fever, no. (%) | 277 (77.6) | 20 (80.0) | 257 (77.4) | 0.765 |
| Fatigue, no. (%) | 138 (38.7) | 12 (48.0) | 126 (38.0) | 0.320 |
| Muscle soreness, no. (%) | 33 (9.2) | 1 (4.0) | 32 (9.6) | 0.493 |
| Headache or dizziness, no. (%) | 35 (9.8) | 1 (4.0) | 34 (10.2) | 0.491 |
| Dyspnea, no. (%) | 112 (31.4) | 16 (64.0) | 96 (28.9) | <0.001 |
| Cough, no. (%) | 226 (63.3) | 17 (68.0) | 209 (63.0) | 0.614 |
| Laboratory findings | ||||
| White blood cell count, ×109/L, median (Q1, Q3) | 5.7 (4.3, 7.7) | 9.9 (7.1, 11.1) | 5.6 (4.2, 7.2) | <0.001 |
| Neutrophil count, ×109/L, median (Q1, Q3) | 3.8 (2.7, 5.8) | 9.1 (5.5, 10.8) | 3.7 (2.6, 5.4) | <0.001 |
| Lymphocyte count, ×109/L, median (Q1, Q3) | 1.0 (0.7, 1.6) | 0.5 (0.3, 0.5) | 1.1 (0.7, 1.6) | <0.001 |
| Monocyte count, ×109/L, median (Q1, Q3) | 0.4 (0.3, 0.6) | 0.4 (0.2, 0.5) | 0.4 (0.3, 0.6) | 0.121 |
| Platelet count, ×109/L, median (Q1, Q3) | 195.0 (142.0, 246.0) | 142.0 (104.0, 239.0) | 196.5 (145.5, 246.0) | 0.046 |
| Hemoglobin, g/L, median (Q1, Q3) | 129.0 (116.0, 141.0) | 122.0 (103.0, 137.0) | 129.0 (117.0, 141.0) | 0.103 |
| Alanine aminotransferase, U/L, median (Q1, Q3) | 25.0 (16.0, 41.0) | 28.0 (20.6, 45.0) | 25.0 (16.0, 41.0) | 0.285 |
| Aspartate aminotransferase, U/L, median (Q1, Q3) | 27.0 (20.0, 39.2) | 42.9 (34.0, 57.0) | 27.0 (20.0, 38.0) | <0.001 |
| Albumin, g/L, median (Q1, Q3) | 38.5 (34.5, 42.1) | 33.2 (29.7, 37.1) | 39.2 (34.9, 42.3) | <0.001 |
| Total bilirubin, μmol/L, median (Q1, Q3) | 10.1 (7.3, 14.4) | 12.6 (8.1, 17.6) | 9.9 (7.1, 14.1) | 0.058 |
| Creatinine, μmol/L, median (Q1, Q3) | 64.0 (51.0, 75.6) | 76.0 (65.0, 112.0) | 63.4 (50.7, 74.0) | 0.001 |
| Procalcitonin, mmol/L, median (Q1, Q3) | 0.05 (0.03, 0.12) | 0.25 (0.17, 0.54) | 0.05 (0.03, 0.09) | <0.001 |
| C-reactive protein, mg/L, median (Q1, Q3) | 27.0 (6.17, 39.4) | 59.5 (39.4, 93.1) | 24.8 (5.2, 39.4) | <0.001 |
| Prothrombin time, s, median (Q1, Q3) | 12.5 (11.7, 13.3) | 13.7 (12.9, 14.5) | 12.4 (11.7, 13.2) | <0.001 |
| D-dimer, mg/L, median (Q1, Q3) | 0.8 (0.5, 1.8) | 5.1 (2.0, 10.9) | 0.7 (0.4, 1.5) | <0.001 |
| CK-MB, ng/mL, median (Q1, Q3) | 1.1 (0.7, 2.1) | 6.9 (2.5, 11.6) | 1.1 (0.6, 1.9) | <0.001 |
| Myoglobin, ng/mL, median (Q1, Q3) | 38.1 (23.0, 63.7) | 194.7 (114.9, 369.3) | 34.9 (21.9, 59.8) | <0.001 |
| High-sensitivity troponin T, ng/mL, median (Q1, Q3) | 0.0 (0.0, 3.0) | 0.1 (0.1, 2.2) | 0.0 (0.0, 3.0) | <0.001 |
| Treatment | ||||
| Lopinavir or ritonavir, no. (%) | 123 (34.5) | 5 (20.0) | 118 (35.5) | 0.115 |
| Ribavirin, no. (%) | 38 (10.6) | 4 (16.0) | 34 (10.2) | 0.323 |
| Abidol, no. (%) | 141 (39.5) | 10 (40.0) | 131 (39.5) | 0.975 |
| Chloroquine phosphate, no. (%) | 14 (3.9) | 0 (0.0) | 14 (4.2) | 0.611 |
| Glucocorticoid, no. (%) | 103 (28.9) | 15 (60.0) | 88 (26.5) | <0.001 |
| Immunoglobulin, no. (%) | 65 (18.2) | 12 (48.0) | 53 (16.0) | <0.001 |
CK-MB, creatine kinase isoenzyme.
Including hypertension, diabetes, chronic pulmonary disease, cardiovascular or cerebrovascular disease, Congestive heart failure, renal disease, AIDS, metastatic malignancy, hepatic disease.
Table 2.
Demographics and baseline clinical characteristics of the COVID-19 patients (Non-recovery vs Recovery)
| Total (n = 357) | Non-recovery (n = 43) | Recovery (n = 314) | P value | |
|---|---|---|---|---|
| Demographics and characteristics | ||||
| Age, years, median (Q1, Q3) | 56.0 (43.0, 68.0) | 75.0 (62.0, 79.5) | 54.0 (40.0, 66.0) | <0.001 |
| Age ≥ 60, no. (%) | 164 (45.9) | 35 (81.4) | 129 (41.1) | <0.001 |
| Male, no. (%) | 185 (51.8) | 28 (65.1) | 157 (50.0) | 0.063 |
| Time from symptoms onset to designated hospital, days, median (Q1, Q3) | 9.0 (5.0, 15.0) | 9.0 (4.5, 15.5) | 9.0 (5.0, 15.0) | 0.658 |
| Coexisting disordersa, no. (%) | 154 (43.1) | 27 (62.8) | 127 (40.4) | 0.006 |
| Signs and symptoms | ||||
| Respiratory rate, breaths per min, median (Q1, Q3) | 20.0 (19.0, 21.0) | 22.0 (19.0, 26.5) | 20.0 (19.0, 21.0) | 0.025 |
| Systolic pressure, mmHg, mean (SD) | 131.0 (18.6) | 137.4 (21.4) | 130.1 (18.0) | 0.016 |
| Diastolic pressure, mmHg, mean (SD) | 79.2 (11.8) | 80.9 (14.4) | 79.0 (11.5) | 0.398 |
| Fever, no. (%) | 277 (77.6) | 34 (79.1) | 243 (77.4) | 0.804 |
| Fatigue, no. (%) | 138 (38.7) | 19 (44.2) | 119 (37.9) | 0.427 |
| Muscle soreness, no. (%) | 33 (9.2) | 3 (7.0) | 30 (9.6) | 0.781 |
| Headache or dizziness, no. (%) | 35 (9.8) | 2 (4.7) | 33 (10.5) | 0.285 |
| Dyspnea, no. (%) | 112 (31.4) | 25 (58.1) | 87 (27.7) | <0.001 |
| Cough, no. (%) | 226 (63.3) | 27 (62.8) | 199 (63.4) | 0.940 |
| Laboratory findings | ||||
| White blood cell count, ×109 /L, median (Q1, Q3) | 5.7 (4.3, 7.7) | 9.7 (7.1, 10.4) | 5.5 (4.1, 6.9) | <0.001 |
| Neutrophil count, ×109/L, median (Q1, Q3) | 3.8 (2.7, 5.8) | 8.1 (5.5, 9.5) | 3.6 (2.6, 5.2) | <0.001 |
| Lymphocyte count, ×109/L, median (Q1, Q3) | 1.0 (0.7, 1.6) | 0.5 (0.4, 0.9) | 1.1 (0.8, 1.6) | <0.001 |
| Monocyte count, ×109/L, median (Q1, Q3) | 0.4 (0.3, 0.6) | 0.4 (0.2, 0.5) | 0.4 (0.3, 0.6) | 0.193 |
| Platelet count, ×109/L, median (Q1, Q3) | 195.0 (142.0, 246.0) | 155.0 (111.5, 239.0) | 197 (147.0, 246.0) | 0.034 |
| Hemoglobin, g/L, median (Q1, Q3) | 129.0 (116.0, 141.0) | 124.0 (104.0, 137.0) | 129.0 (117.0, 141.0) | 0.047 |
| Alanine aminotransferase, U/L, median (Q1, Q3) | 25.0 (16.0, 41.0) | 30.0 (21.3, 45.7) | 24.5 (16.0, 40.0) | 0.029 |
| Aspartate aminotransferase, U/L, median (Q1, Q3) | 27.0 (20.0, 39.2) | 44.0 (33.0, 56.5) | 26.0 (20.0, 36.0) | <0.001 |
| Albumin, g/L, median (Q1, Q3) | 38.5 (34.5, 42.1) | 33.0 (29.8, 36.1) | 39.4 (35.6, 42.8) | <0.001 |
| Total bilirubin, μmol/L, median (Q1, Q3) | 10.1 (7.3, 14.4) | 12.6 (7.9, 19.7) | 9.7 (6.9, 13.7) | 0.003 |
| Creatinine, μmol/L, median (Q1, Q3) | 64.0 (51.0, 75.6) | 74.0 (56.7, 105.8) | 63.0 (51.0, 74.0) | 0.003 |
| Procalcitonin, mmol/L, median (Q1, Q3) | 0.05 (0.03, 0.12) | 0.19 (0.12, 0.45) | 0.05 (0.03, 0.09) | <0.001 |
| C-reactive protein, mg/L, median (Q1, Q3) | 27.0 (6.17, 39.4) | 55.7 (39.4, 108.0) | 22.8 (5.0, 39.4) | <0.001 |
| Prothrombin time, s, median (Q1, Q3) | 12.5 (11.7, 13.3) | 13.4 (12.7, 14.5) | 12.3 (11.7, 13.1) | <0.001 |
| D-dimer, mg/L, median (Q1, Q3) | 0.8 (0.5, 1.8) | 3.8 (1.3, 11.3) | 0.7 (0.4, 1.4) | <0.001 |
| CK-MB, ng/mL, median (Q1, Q3) | 1.1 (0.7, 2.1) | 4.9 (1.9, 8.2) | 1.0 (0.6, 1.8) | <0.001 |
| Myoglobin, ng/mL, median (Q1, Q3) | 38.1 (23.0, 63.7) | 130.9 (65.8, 343.5) | 33.0 (21.7, 57.0) | <0.001 |
| High-sensitivity troponin T, ng/mL, median (Q1, Q3) | 0.0 (0.0, 3.0) | 0.1 (0.0, 2.1) | 0.0 (0.0, 3.0) | <0.001 |
| Treatment | ||||
| Lopinavir or ritonavir, no. (%) | 123 (34.5) | 9 (20.9) | 114 (36.3) | 0.047 |
| Ribavirin, no. (%) | 38 (10.6) | 7 (16.3) | 31 (9.9) | 0.194 |
| Abidol, no. (%) | 141 (39.5) | 21 (48.8) | 120 (38.2) | 0.182 |
| Chloroquine phosphate, no. (%) | 14 (3.9) | 1 (2.3) | 13 (4.1) | 1.000 |
| Glucocorticoid, no. (%) | 103 (28.9) | 25 (58.1) | 78 (24.8) | <0.001 |
| Immunoglobulin, no. (%) | 65 (18.2) | 20 (46.5) | 45 (14.3) | <0.001 |
CK-MB, creatine kinase isoenzyme.
Including hypertension, diabetes, chronic pulmonary disease, cardiovascular or cerebrovascular disease, Congestive heart failure, renal disease, AIDS, metastatic malignancy, hepatic disease.
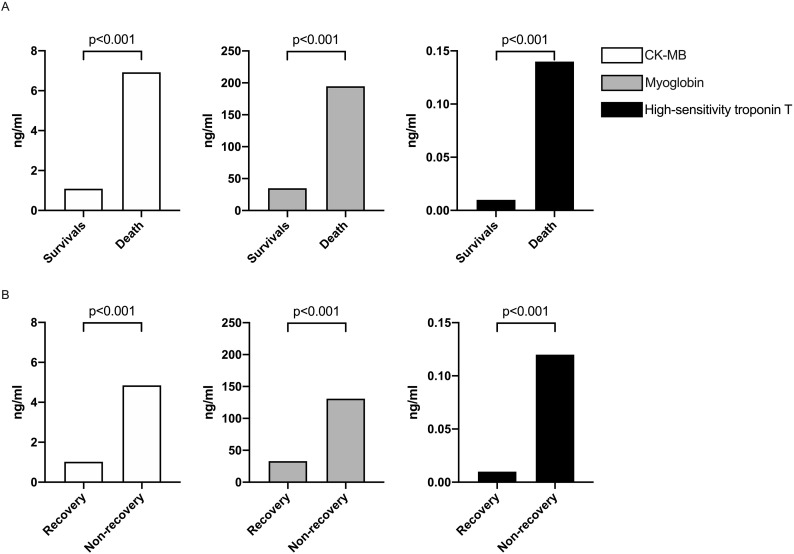
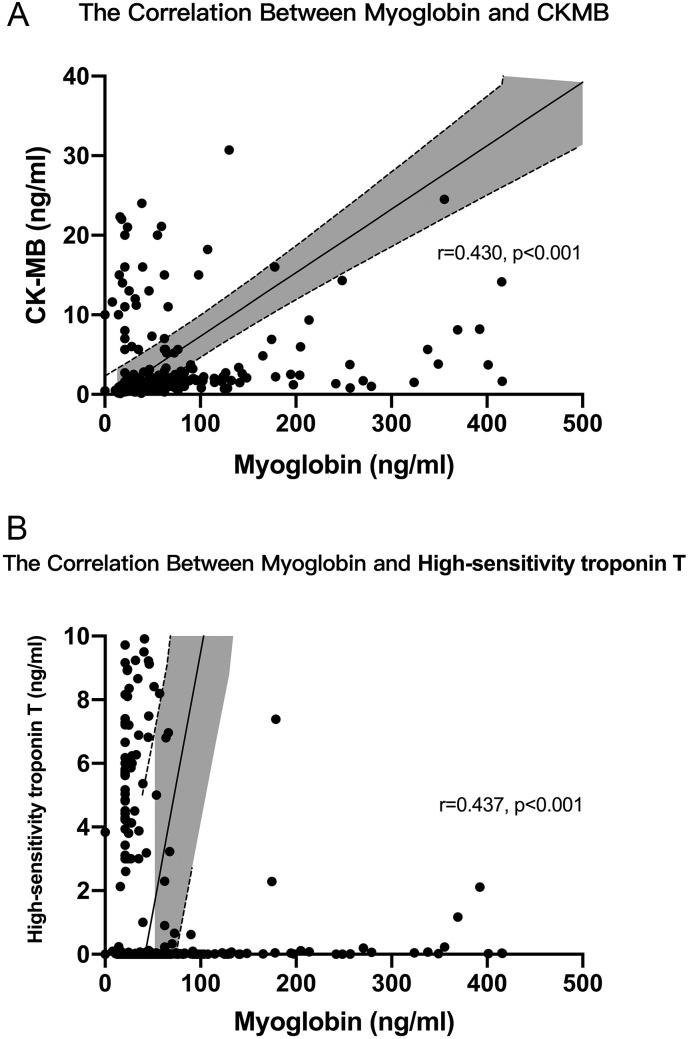
The respiratory rates in death and non-recovery group were both significantly faster than survival and recovery group (24.0 (22.0, 30.0) versus 20.0 (19.0, 21.0), and 22.0 (19.0, 26.5) versus 20.0 (19.0, 21.0)). Dyspnea in death and non-recovery group occurred more frequently than survival and recovery group (64% versus 28.9% and 58.1% versus 27.7%). Laboratory findings like aspartate aminotransferase, total bilirubin, creatinine, procalcitonin, CRP, D-dimer were also significantly higher in death and non-recovery group. However, other laboratory findings including platelet count, hemoglobin, and albumin significantly decreased in these two groups. Cardiac biomarkers including CK-MB, myoglobin, and high-sensitivity troponin T significantly elevated in death and non-recovery group in Fig. 2 . Moreover, admission myoglobin was positively correlated with both CKMB and high-sensitivity troponin T (Fig. 3 ). Died patients or non-recovery patients used glucocorticoid and immunoglobulin more frequently than survival or recovery patients. The other clinical characteristics and their differences between the two groups were also presented in Table 1, Table 2.
Fig. 2.
The level of creatine kinase isoenzyme (CK-MB), myoglobin and high-sensitivity troponin T were compared and analyzed. According to two outcomes in this study, patients were divided into survival group and death group, or recovery group and non-recovery group.
Fig. 3.
The correlation between myoglobin and either creatine kinase isoenzyme (CK-MB) or high-sensitivity troponin T, Shadows in curves indicated the 95% confidence intervals of the corresponding estimates. A Myoglobin was positively correlated with CKMB (r = 0.430, p < 0.001). B Myoglobin was positively correlated with high-sensitivity troponin T (r = 0.437, p < 0.001).
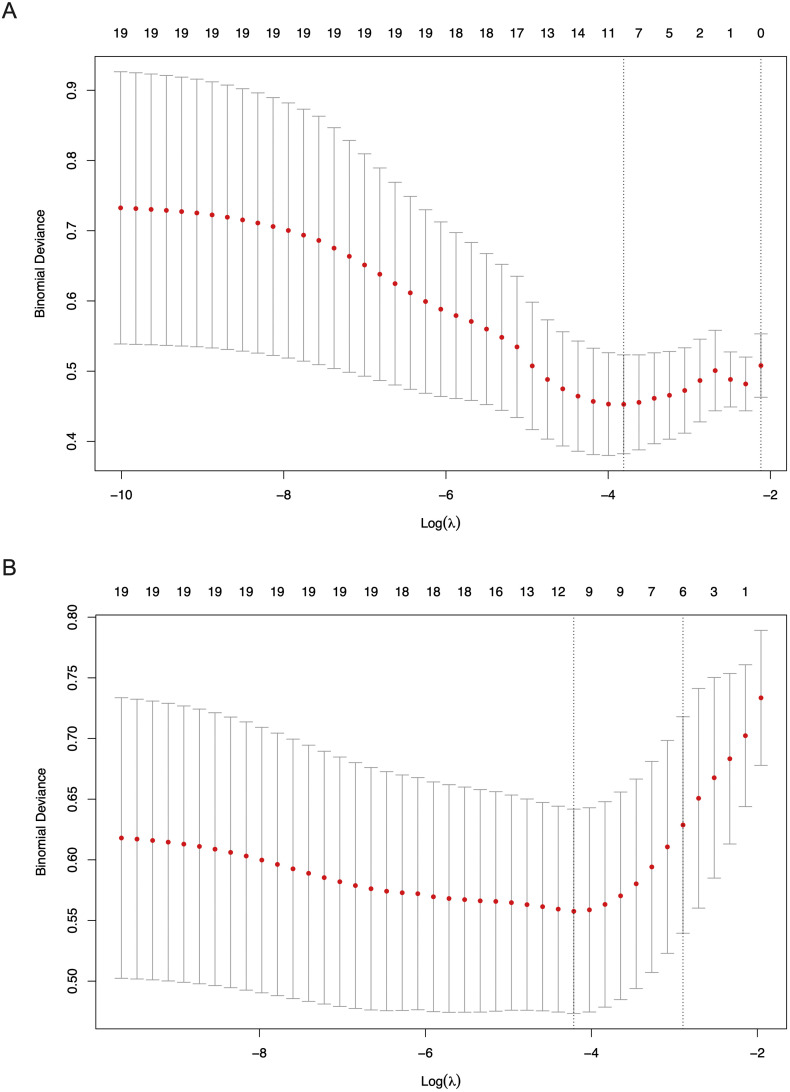
19 variables were included into the LASSO regression analysis to select potential predictors for in-hospital death or non-recovery in 28 days. The included variables were demographics (age ≥ 60 and coexisting disorders), signs and symptoms (respiratory rate and dyspnea), laboratory findings (lymphocyte count, platelet count, hemoglobin, alanine aminotransferase, aspartate aminotransferase, albumin, total bilirubin, creatinine, procalcitonin, CRP, prothrombin time, D-dimer, CK-MB, myoglobin and high-sensitivity troponin T. The LASSO regression results showed age ≥ 60, dyspnea, respiratory rate, aspartate aminotransferase, albumin, total bilirubin, CRP, D-dimer, CK-MB and myoglobin were prognostic factors for in-hospital death when the binomial deviance was the smallest; and age ≥ 60, dyspnea, hemoglobin, albumin, total bilirubin, CRP, D-dimer, CK-MB and myoglobin were prognostic factors for non-recovery when the binomial deviance was the smallest (Fig. 4 ). Therefore, CK-MB and myoglobin were considered as potential predictors for adverse prognosis (in-hospital death or non-recovery) in 28 days. However, high-sensitivity troponin T was not a predictor for adverse prognosis according to LASSO regression analysis in this study.
Fig. 4.
The risk predictors creatine kinase isoenzyme (CK-MB) and myoglobin for in-hospital death or non-recovery in 28 days were selected using LASSO regression analysis. A Binomial deviance plot of the lowest point of red curve (left dash line), which correspond to a ten-variable model for in-hospital death. B Binomial deviance plot of the lowest point of red curve (left dash line), which correspond to a nine-variable model for non-recovery in 28 days. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
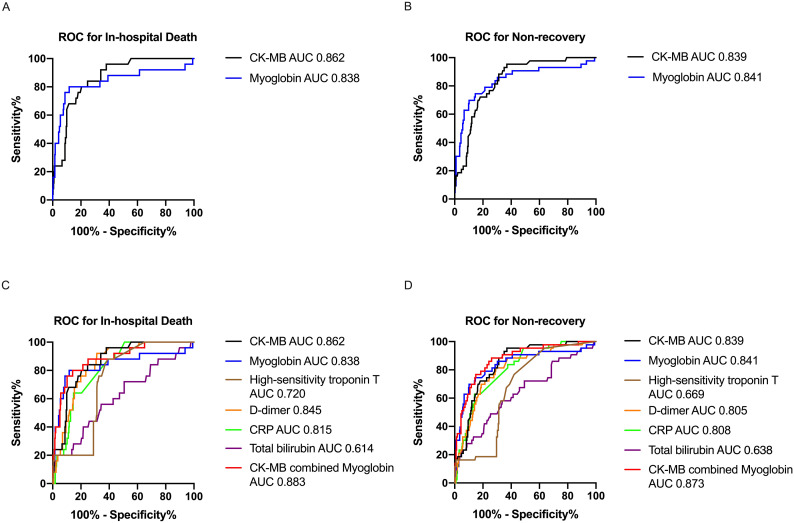
The ROC and AUC of CK-MB and myoglobin for in-hospital death were 0.862 (95%CL: 0.804–0.920, p < 0.001) and 0.838 (95%CL: 0.729–0.947, p < 0.001), and the AUC of CK-MB and myoglobin for non-recovery were 0.839 (95%CL: 0.786–0.892, p < 0.001) and 0.841 (95%CL: 0.765–0.918, p < 0.001), respectively (Fig. 5A, B). Because previous studies as well as the present study showed higher CRP, D-dimer, total bilirubin, and high-sensitivity troponin T might be associated to COVID-19 patient adverse prognosis, we calculated the AUC of these variables and compared the prognostic performance among predictors selected [[12], [13], [14]]. As seen the Fig. 5C, D, the highest AUC for in-hospital death was CK-MB, and the AUC of myoglobin was higher than high-sensitivity troponin T, CRP, and total bilirubin. For non-recovery, the AUC of CK-MB and myoglobin were both higher than high-sensitivity troponin T, D-dimer, CRP, and total bilirubin. With a cut-off value of 2.2, CK-MB exhibited sensitivity 80.0%, specificity 79.5% for in-hospital death. As well as for in-hospital death, with a cut-off value of 90.9, myoglobin exhibited sensitivity 80.0% and specificity 88.3%. CK-MB exhibited sensitivity 95.4% and specificity 63.1% for non-recovery with a cut-off value of 1.4. And myoglobin exhibited sensitivity 74.4% and specificity 85.4% for non-recovery with a cut-off value of 68.9. We also found that combined use of CK-MB and myoglobin showed a better predictive performance for prognosis (AUC for in-hospital death = 0.883 and AUC for non-recovery = 0.873, respectively). Therefore, CK-MB and myoglobin could be combined to early predict patient prognosis better.
Fig. 5.
Receiver operating characteristic curve (ROC) and the area under the curve (AUC) for death or non-recovery in 28 days prediction. A The ROC and AUC for in-hospital death of creatine kinase isoenzyme (CK-MB) and myoglobin were 0.862 and 0.838, respectively. B The ROC and AUC for non-recovery in 28 days of CK-MB and myoglobin were 0.839 and 0.84, respectively. C The ROC and AUC for in-hospital death of CKMB and myoglobin comparing with D-dimer, C-reactive protein (CRP), total bilirubin and high-sensitivity troponin T. D The ROC and AUC for non-recovery in 28 days of CK-MB and myoglobin comparing with D-dimer, CRP, total bilirubin and high-sensitivity troponin T.
4. Discussion
From the outbreak of COVID-19 in Wuhan city, the number of confirmed patients increased to more than 80,000 and spread all over the world. Early studies revealed the clinical features and characteristics of COVID-19 patients [[15], [16], [17]]. Risk factors affecting adverse prognosis were also identified, such as comorbidities, older age, dyspnea, and abnormal laboratory findings [18,19]. More than that, some studies gradually found COVID-19 patients with cardiovascular disease appeared to be severe illness commonly, and COVID-19 may lead to acute myocardial injury with elevated cardiac biomarkers including CK-MB, high-sensitivity troponin T and myoglobin [20,21]. In the present study, these three biomarkers were all elevated on hospital admission. However, the LASSO regression analyses indicated elevated CK-MB and myoglobin were potential risk predictors for adverse prognosis, and high-sensitivity troponin T could not be considered as a significant predictor in this study. The AUC of CK-MB and myoglobin selected for in-hospital death or non-recovery in 28 days were both more than 0.8. Therefore, CK-MB and myoglobin may be effective prognostic predictors for adverse prognosis of COVID-19 patients.
In hospitalized patients with COVID-19, the prevalence of myocardial injury is high, and usually with significant elevation of related cardiac biomarkers [22,23]. Of them, an increase level of CK-MB is a useful cardiac biomarker for acute myocardial injury [24]. Some studies have also revealed the association between CKMB and patient severity or prognosis. CK-MB in ICU patients was higher than non-ICU patients, and the level of CK-MB in non-survivors was significantly higher than survivors [4,25]. The present study showed the level of CK-MB significantly elevated in adverse prognosis groups, and this finding was according with previous studies [26,27]. The AUC of CK-MB for in-hospital death and non-recovery were 0.862 and 0.839, respectively, and both higher than D-dimer, CRP, total bilirubin and high-sensitivity troponin T. These results indicated CK-MB could be an effective and valuable predictive biomarker to early recognize patients with adverse prognosis.
Myoglobin is a type of cytoplasmic protein existing in cardiac and skeletal muscle, and myoglobin in circulation increases rapidly after myocytes damage [28,29]. In severe and critically ill COVID-19 patients, the level of myoglobin was significantly higher than mild patients [30]. And in COVID-19 patients requiring ICU treatment, elevation of myoglobin occurred in 35.2% of patients [31]. Elevated myoglobin might be associated with disease severity and adverse prognosis. Even though myoglobin was not as cardiac specific as troponin measurements were, myoglobin was positively correlative with CK-MB and high-sensitivity troponin T. This study demonstrated the level of myoglobin significantly elevated in death group or non-recovery group, showed in Fig. 2. The AUC of myoglobin for in-hospital death was higher than CRP, total bilirubin and high-sensitivity troponin T, and the AUC of myoglobin for non-recovery was also higher than D-dimer, CRP, total bilirubin and high-sensitivity troponin T. Therefore, these results indicated myoglobin also could be a valuable predictive biomarker for adverse prognosis in COVID-19 patients and might be used as a supplementary biomarker besides CK-MB for diagnosis and prediction. Even though CK-MB or myoglobin showed a good performance for prediction when they used alone, combined use of CK-MB and myoglobin had a better predictive performance for patient prognosis. Therefore, when COVID-19 patients on admission, we could evaluate patient prognosis using combined biomarkers.
High-sensitivity troponin T is a gold-standard biomarker for reflecting the severity of ongoing myocardial damage and previous studies have proved this finding [27,32]. In COVID-19 patients, some of them exhibited elevation of high-sensitivity troponin T and high mortality [33]. Therefore, high-sensitivity troponin T might have a good potential in predicting COVID-19 patient prognosis. However, in the present study, high-sensitivity troponin T could not be considered as a predictor for prognosis. We thought several reasons might be associated with this result. Firstly, only univariate analysis was used to explore high-sensitivity troponin predicting prognosis in previous studies, and multivariate analysis was not used to adjust confounders [5,33]. Secondly, this result might be associated with the data characteristics in this study and the LASSO regression analysis. Thirdly, Dr. Aimo reported high-sensitivity troponin T seemed less predictive than other cardiac biomarker when considering absolute values [34]. Therefore, these three reasons could explain this result to some extent.
This study also has some inevitable limitations due to data collected in the early stage of COVID-19 outbreak. Because of the condition at that time, completed laboratory test including cardiac biomarkers was not available for every patient, and the sample size is limited for this study. Therefore, the present study finally enrolled 357 patients who received laboratory test of cardiac biomarkers on admission to be analyzed. Because the number of events was limited, we did not develop a validation set. Further related large-sample studies are needed to explore and prove our opinion.
5. Conclusion
In patients with COVID-19, CKMB and myoglobin may be considered as effective and valuable predictive biomarkers for patient adverse prognosis, and combined use of CK-MB and myoglobin had a better performance for prediction. Early determination of CK-MB and myoglobin could help clinicians increase awareness of the possibility of adverse outcomes and may improve patient prognosis.
Ethics approval
The present study was initiated by researchers in West China Hospital and approved by Ethics Committees of West China Hospital of Sichuan University and Renmin Hospital of Wuhan University.
Funding
The present work was supported by the Project of Novel Coronavirus Pneumonia in West China Hospital (HX2019nCoV027).
Authors' contributions
Jie Yang and Yan Kang designed the study. Jie yang drafted the manuscript, and Yan Kang revised it. Jie Yang, Xuelian Liao, Wanhong Ying, Bo Wang, Jirong Yue, Lang Bai, Dan Liu, Ting Zhu and Zhixin Huang participated in the data collection and analysis.
CRediT authorship contribution statement
Jie Yang: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Xuelian Liao: Validation, Investigation, Supervision, Project administration. Wanhong Yin: Investigation, Supervision. Bo Wang: Investigation, Supervision. Jirong Yue: Investigation. Lang Bai: Investigation. Dan Liu: Investigation. Ting Zhu: Investigation. Zhixin Huang: Investigation. Yan Kang: Investigation, Validation, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgements
The present study proceeding was supported by involved staff and patients. We would like to thank Desong Qiu from Sichuan Zhikang Technology CO., Chengdu, for his help of establishing the electronic data capture and analysis system. We thanked Ruoran Wang from Department of Critical Care Medicine, West China Hospital of Sichuan University, Chengdu, China, for his useful help and positive advices. And last, we thanked all patients and their families involved in the study.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., Rubin E.J. Covid-19 - the search for effective therapy. N Engl J Med. 2020;382(19):1851–1852. doi: 10.1056/NEJMe2005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Jama; 2020. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y., Hou B., Liu J., Chen Y., Zhong P. Risk factors associated with long-term hospitalization in patients with COVID-19: a single-centered, retrospective study. Front Med (Lausanne) 2020;7:315. doi: 10.3389/fmed.2020.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bavishi C., Bonow R.O., Trivedi V., Abbott J.D., Messerli F.H., Bhatt D.L. Acute myocardial injury in patients hospitalized with COVID-19 infection: a review. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorente-Ros A., Monteagudo Ruiz J.M., Rincón L.M., Ortega Pérez R., Rivas S., Martínez-Moya R., et al. Myocardial injury determination improves risk stratification and predicts mortality in COVID-19 patients. Cardiol J. 2020;27(4) doi: 10.5603/CJ.a2020.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toraih E.A., Elshazli R.M., Hussein M.H., Elgaml A., Amin M.N., El-Mowafy M., et al. Association of cardiac biomarkers and comorbidities with increased mortality, severity, and cardiac injury in COVID-19 patients: a meta-regression and Decision tree analysis. J Med Virol. 2020;92:2473–2488. doi: 10.1002/jmv.26166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang W., Liang H., Ou L., Chen B., Chen A., Li C., et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo X., Zhou W., Yan X., Guo T., Wang B., Xia H., et al. Prognostic value of C-reactive protein in patients with COVID-19. Clin Infect Dis. 2020;XX(XX):1–6. doi: 10.1093/cid/ciaa641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020:1–11. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang A.P., Liu J.P., Tao W.Q., Li H.M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ (Clinical research ed) 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (Clinical research ed) 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H., et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ (Clinical research ed) 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 19.Chen R., Liang W., Jiang M., Guan W., Zhan C., Wang T., et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranard L.S., Fried J.A., Abdalla M., Anstey D.E., Givens R.C., Kumaraiah D., et al. Approach to acute cardiovascular complications in COVID-19 infection. Circ Heart Fail. 2020;13(7):167–176. doi: 10.1161/CIRCHEARTFAILURE.120.007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahajan K., Chand Negi P., Ganju N., Asotra S. Cardiac biomarker-based risk stratification algorithm in patients with severe COVID-19. Diabetes Metab Syndr. 2020;14(5):929–931. doi: 10.1016/j.dsx.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 24.Dickey W., Carson C.A., Andrews W.J., Crowe P.F. Apparent elevation of serum CK-MB not due to acute myocardial infarction. Br J Clin Pract. 1992;46(2):149–150. [PubMed] [Google Scholar]
- 25.Wang D., Yin Y., Hu C., Liu X., Zhang X., Zhou S., et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24(1):188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):1–6. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aboughdir M., Kirwin T., Abdul Khader A., Wang B. Prognostic value of cardiovascular biomarkers in COVID-19: a review. Viruses. 2020;12(5) doi: 10.3390/v12050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mair J., Morandell D., Genser N., Lechleitner P., Dienstl F., Puschendorf B. Equivalent early sensitivities of myoglobin, creatine kinase MB mass, creatine kinase isoform ratios, and cardiac troponins I and T for acute myocardial infarction. Clin Chem. 1995;41(9):1266–1272. [PubMed] [Google Scholar]
- 29.Kocylowski R.D., Dubiel M., Gudmundsson S., Sieg I., Fritzer E., Alkasi O., et al. Biochemical tissue-specific injury markers of the heart and brain in postpartum cord blood. Am J Obstet Gynecol. 2009;200(3):273 e1–273 e25. doi: 10.1016/j.ajog.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Han H., Xie L., Liu R., Yang J., Liu F., Wu K., et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92(7):819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Y., Xu D., Fu S., Zhang J., Yang X., Xu L., et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24(1):219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gohar A., Chong J.P.C., Liew O.W., den Ruijter H., de Kleijn D.P.V., Sim D., et al. The prognostic value of highly sensitive cardiac troponin assays for adverse events in men and women with stable heart failure and a preserved vs. reduced ejection fraction. Eur J Heart Fail. 2017;19(12):1638–1647. doi: 10.1002/ejhf.911. [DOI] [PubMed] [Google Scholar]
- 33.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aimo A., Januzzi J.L., Jr., Mueller C., Mirò O., Pascual Figal D.A., Jacob J., et al. Admission high-sensitivity troponin T and NT-proBNP for outcome prediction in acute heart failure. Int J Cardiol. 2019;293:137–142. doi: 10.1016/j.ijcard.2019.06.005. [DOI] [PubMed] [Google Scholar]