Abstract
Background
We quantified opioid prescribing after the 2014 rescheduling of hydrocodone from schedule III to II in the United States using a state-wide prescription database and studied trends three years before and after the policy change, focusing on certain specialties.
Methods
We used Ohio’s state prescription drug monitoring program database, which includes all filled schedule II and III prescriptions regardless of payer or pharmacy, to conduct an interrupted time series analysis of the nine most prescribed opioids: hydrocodone, oxycodone, tramadol, codeine, and others. We analyzed hydrocodone prescribing trends for the physician specialties of internal medicine, anesthesiology, and emergency medicine. We evaluated trends 37 months before and after the rescheduling change.
Results
Rescheduling was associated with a hydrocodone level change of –26,358 (95% confidence interval [CI] = –36,700 to –16,016) prescriptions (–5.8%) and an additional decrease in prescriptions of –1,568 (95% CI = –2,296 to –839) per month (–0.8%). Codeine prescribing temporarily increased, at a level change of 6,304 (95% CI = 3,003 to 9,606) prescriptions (18.5%), indicating a substitution effect. Hydrocodone prescriptions by specialty were associated with a level change of –805 (95% CI = –1,280 to –330) prescriptions (–8.5%) for anesthesiologists and a level change of –14,619 (95% CI = –23,710 to –5,528) prescriptions (–10.2%) for internists. There was no effect on prescriptions by emergency physicians.
Conclusions
The 2014 federal rescheduling of hydrocodone was associated with declines in hydrocodone prescriptions in Ohio beyond what had already been occurring, and hydrocodone may have been briefly substituted with codeine. These results indicate that rescheduling did have a lasting effect but affected prescribing specialties variably.
Keywords: Opioid, Hydrocodone, Rescheduling, Opioid Prescription, Prescribing, Ohio
Introduction
The modern opioid addiction and overdose epidemic was initially fueled by the prescribing of opioids, which increased from 25.5 billion milligrams of morphine equivalents in 1992 to a peak of 240.3 billion milligrams in 2011 [1]. Although heroin and illicitly manufactured synthetic opioids like fentanyl have overtaken prescribed opioids as the leading cause of drug-related overdoses, prescribed opioids were the leading cause until 2014 and are still an important cause of overdose and death [2]. Federal, state, and local governments have responded by implementing tools like prescription drug monitoring programs and guidelines [3,4].
Another policy change to address the crisis was the rescheduling of hydrocodone from a Drug Enforcement Administration (DEA) class III scheduled medication to a class II scheduled medication in October 2014 [5]. Hydrocodone immediate-release combination products are the most prescribed opioid in the United States and contribute substantially to overall prescription opioid abuse [6]. In 2010, the global production of hydrocodone was 36.3 tons, and the United States consumed more than 99% of that quantity [7]. There are several potential ways that rescheduling hydrocodone could reduce availability of the drug: 1) only a 30-day supply is allowed at a time; 2) prescriptions for schedule II medications cannot be phoned in, but instead required a written or electronically transmitted prescription; 3) refills of schedule II medications are not permitted; and 4) in a handful of states, midlevel providers (i.e., physician assistants and nurse practitioners) are not able to prescribe schedule II medications.
This policy change provides a natural experiment to determine the effects, both short- and long-term, of the rescheduling. Previous work has demonstrated that the policy change did indeed lead to a decline in prescribing. Data from a large commercial health insurance program demonstrated a 26% decrease in hydrocodone products from June 2013 to June 2015, whereas prescribing for nonhydrocodone schedule II opioids and tramadol remained stable [8]. Other studies have found unintended consequences. A health system in Texas discovered an 80.2% decrease in hydrocodone prescriptions but a concomitant 215.1% increase in prescriptions for codeine (still schedule III) in the 180 days after the schedule change [9]. A study of Medicare Part D beneficiaries saw a decrease in the percentage of enrollees who received a hydrocodone prescription—from 21.9% in 2013 to 18.3% in 2015—but an increased rate of opioid-related hospitalizations without a documented opioid prescription, leading the authors to hypothesize that the rescheduling led to an increase in illegal opioid use [10].
Our study focuses on Ohio, a state that has been disproportionately affected by the opioid epidemic. The age-adjusted opioid death rate in Ohio was 32.9 per 100,000 people in 2016, the third highest rate in the country and a 33% increase from the prior year [11]. The data set we use is the Ohio Prescription Drug Monitoring Program (PDMP), also known as the Ohio Automated Rx Reporting System (OARRS). The benefit of OARRS is that it contains information about every opioid prescription filled in the state regardless of payer (including cash payments) or pharmacy and that it also contains the primary specialty of most of the prescribers in the state. We evaluate the effect of hydrocodone rescheduling on prescribing by specialty and its associated effect on prescribing of other opioid pain relievers.
Methods
Study Design and Data Sources
We conducted an interrupted time series analysis using data on monthly counts of opioid prescriptions that were filled in Ohio between October 2011 and November 2017. Data were extracted from OARRS, which includes all prescriptions for schedule II to V medications that are dispensed at pharmacies in the state. However, OARRS does not include prescriptions that were written in Ohio but dispensed in other states, including out-of-state mail-order pharmacies, nor does it include prescriptions that were written but not dispensed. The study was deemed “not human research” by the Partners Healthcare Institutional Review Board.
There are three key dates regarding the scheduling of hydrocodone. The first was the Federal Drug Administration’s recommendation to the DEA to reschedule hydrocodone in December 2013. The second was the DEA’s publication of the rule change into the Federal Register as a Controlled Substances Act in August 2014. The third was the formal date when the rescheduling went into effect in October 2014. Although prescribers were likely aware of the proposed change before the date, we consider October 2014 as the implementation date for the purpose of the study. November 2014 was considered the first month postimplementation.
Study Population and Variables of Interest
We analyzed prescriptions for patients aged five to 99 years of the pill form of the nine most commonly prescribed opioids in Ohio: hydrocodone, oxycodone, tramadol, codeine, hydromorphone, meperidine, methadone, morphine, and oxymorphone. Prescriptions for hydrocodone, oxycodone, tramadol, and codeine were analyzed individually. The remaining five drugs were combined for analysis given their much smaller numbers. The primary outcome of interest was the monthly statewide hydrocodone prescription totals. The secondary outcome was monthly prescription totals for the other opioid groups that we used in order to evaluate a prescribed opioid substitution effect (i.e., that another opioid was used in place of hydrocodone). We further stratified our analysis by specialty, focusing on anesthesiology, emergency medicine, and internal medicine, three specialties that have higher rates of opioid prescriptions [12].
The following fields were extracted from OARRS: name of the drug, its strength, and its formulation; National Drug Code (generic identification of the medication); date the prescription was written by the prescriber; and first listed prescriber specialty. Prescription date, rather than patient fill date, was chosen because the policy change was directed at prescribers. Monthly statewide prescription totals for the studied medications were calculated by summing the number of prescriptions by all prescribers in each month. Both immediate- and extended-release/long-acting formulations were included.
Measures
To measure the effect of the policy on provider specialties, we evaluated prescriptions by providers who identified as anesthesiology, emergency medicine, and internal medicine. During the time of this study, specialty information was provided to the state by the individual when applying for or renewing a medical license. Only providers who had a consistent specialty throughout the study period were included. For example, if a provider was first listed as internal medicine and then reclassified to oncology, they would not be included in the specialty analysis. Our methods for categorizing providers have been previously described [12].
The data set consisted of 74 months of monthly prescription totals: 37 months pre- and 37 months postimplementation. The time window in the postimplementation phase was determined by the maximum months of data available. Although we had more than 37 months of data available in the pre-implementation phase, we chose to use the same time frame as in the postimplementation period for consistency and comparability.
Data Analysis and Statistical Methods
Because the data set included some outliers, which were likely due to pharmacists’ erroneous data entry into OARRS, we excluded prescriptions, as per our prior work [12]. Briefly, the prescriptions with the following characteristics were excluded: 1) pill quantity fewer than four or greater than or equal to the 99th percentile; 2) noninteger pill quantity; 3) days’ supply less than or equal to zero or greater than 90; 4) prescriptions for patients younger than age five years or >99 years of age.
We used an interrupted time series analysis. This analysis projects the trend from the pre-intervention period into the postintervention period to hypothesize what would have happened without the intervention. This analysis works well when there already is a secular trend, as opposed to no change, occurring in the pre-intervention period [13]. We used a segmented ordinary least squares regression with a time variable indicating the number of months from October 2011. To control for fluctuations in prescribing that are due to seasonal changes, we included indicator variables for summer (June to August) and for February (short month) and allowed these effects to vary pre- and postrescheduling by including an interaction of seasonal and postrescheduling indicator variables. We tested for autocorrelation using the Cumby-Huizinga test and, upon finding that autocorrelation was present, estimated the model coefficients specifying an autocorrelation structure with six lags. We determined whether the hydrocodone rescheduling was associated with changes in the outcome’s level (intercept change or level change) and a postintervention change in the outcome’s trend (slope change).
This analysis was performed for each of the four individual medications (hydrocodone, oxycodone, tramadol, codeine) and the combination of the remaining medications (hydromorphone, meperidine, methadone, morphine, and oxymorphone). Next, the analysis for hydrocodone was performed only for prescribers with consistent specialties of anesthesiology, emergency medicine, and internal medicine. Statistical analyses were performed with SAS (version 9.4; SAS Institute, Inc., Cary, NC, USA) and StataMP (version 13; StataCorp, College Station, TX, USA).
Results
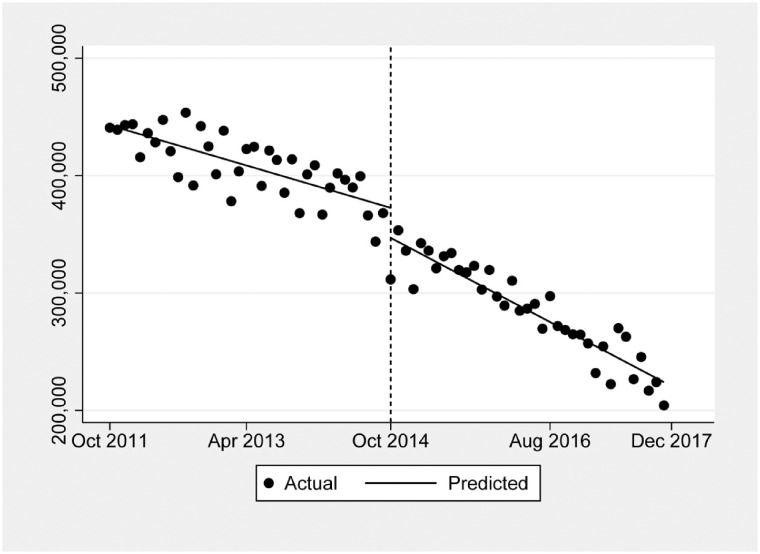
During the 74-month study period, there were 64,717,150 prescriptions for the nine most commonly prescribed opioids in Ohio. Table 1 shows the subsets of prescriptions by active ingredient and by the three individually studied specialties. Figure 1 indicates the overall change in monthly hydrocodone prescribing before and after the schedule change. As shown in Table 2, there was a preexisting downward trend of –1,900 prescriptions per month (–0.5%) before the schedule change, but the implementation was associated with a level change of –26,358 prescriptions (–5.8%) and an additional decrease in prescriptions of –1,568 (–0.8%) per month.
Table 1.
Total prescriptions of opioids before and after the rescheduling of hydrocodone in October 2014
| Total | Oct 2011–Oct 2014 | Nov 2014–Nov 2017 | Percent Change | |
|---|---|---|---|---|
| a) Total numbers of prescriptions by opioid | ||||
| Total opioid prescriptions | 64,717,150 | 35,308,296 | 29,408,854 | –16.7 |
| Hydrocodone | 25,683,086 | 15,119,150 | 10,563,936 | –30.1 |
| Oxycodone | 20,777,414 | 10,563,948 | 10,213,466 | –3.3 |
| Tramadol | 12,051,633 | 6,290,076 | 5,761,557 | –8.4 |
| Hydromorphone, meperidine, methadone, morphine, oxymorphone | 3,635,439 | 1,969,668 | 1,665,771 | –15.4 |
| Codeine | 2,569,576 | 1,365,452 | 1,204,124 | –11.8 |
| b) Total numbers of prescriptions by physician specialty of all opioids and only hydrocodone | ||||
| Prescriptions by specialty: all opioids | ||||
| Internal medicine | 20,875,072 | 12,538,303 | 8,336,769 | –33.5 |
| Emergency medicine | 2,257,678 | 1,445,346 | 812,332 | –43.8 |
| Anesthesiology | 3,173,583 | 1,863,533 | 1,310,050 | –29.7 |
| Prescriptions by specialty: hydrocodone | ||||
| Internal medicine | 8,177,098 | 5,241,426 | 2,935,672 | –44.0 |
| Emergency medicine | 1,182,989 | 770,445 | 412,544 | –46.5 |
| Anesthesiology | 776,105 | 465,631 | 310,474 | –33.3 |
Figure 1.
The overall change in monthly hydrocodone prescription rates by all physician specialties before and after the rescheduling change in October 2014.
Table 2.
Changes in prescription trends from all physicians by opioid
| Total Prescriptions per Month | Hydrocodone, No. Prescriptions (95% CI) | Oxycodone, No. Prescriptions (95% CI) | Tramadol, No. Prescriptions (95% CI) | Hydromorphone, Meperidine, Methadone, Morphine, Oxymorphone, No. Prescriptions (95% CI) | Codeine, No. Prescriptions (95% CI) |
|---|---|---|---|---|---|
| Base trend in the prepolicy segment* | –1,900.0 | 297.6 | 706.7 | –219.8 | –283.4 |
| (–2,334.2 to –1,456.8) | (64.7 to 530.6) | (441.1 to 972.3) | (–253.0 to –186.7) | (–379.1 to –187.7) | |
| Change in level in the postpolicy segment† | –26,357.9 | 18,729.2 | –6,908.7 | 2,079.1 | 6,304.3 |
| (–36,700.3 to –16,015.5) | (–696.0 to 38,154.5) | (–20,595.5 to 6,778.0) | (–538.9 to 4,697.2) | (3,002.9 to 9,605.6) | |
| Additional change in trend in the postpolicy segment‡ | –1,567.6 | –2,273.5 | –1,963.8 | –134.2 | –33.7 |
| (–2,296.3 to –838.8) | (–2,857.1 to –1,689.8) | (–2,613.9 to –1,312.7) | (–231.5 to –36.8) | (–126.6 to 59.2) |
CI = confidence interval.
Monthly prescribing rate from October 2011 to October 2014.
Change in absolute number of prescriptions before and after rescheduling.
Monthly prescribing rate difference before and after rescheduling until November 2017.
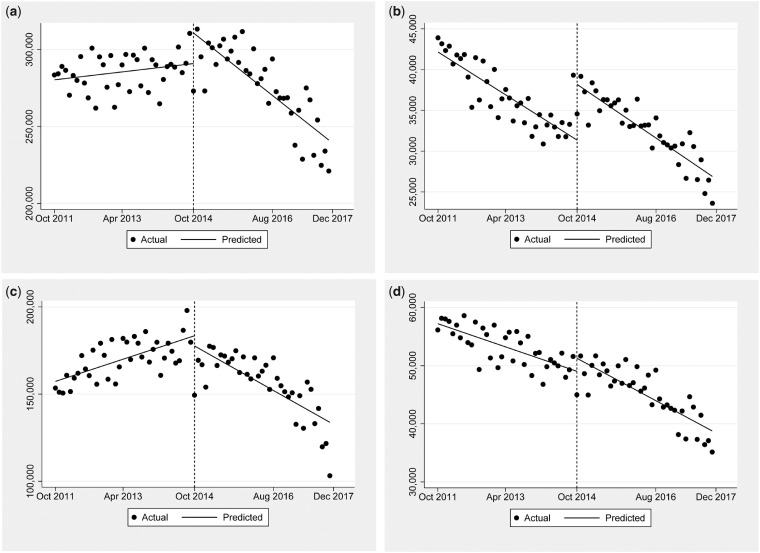
Figure 2 shows the prescribing of the other studied opioids. Figure 2a shows that prescriptions for oxycodone were increasing before the schedule change by 299 (0.1%) per month. The schedule change of hydrocodone led to a non–statistically significant level change of 18,730 (6.9%) additional prescriptions, followed by an additional drop of –2,274 (–0.8%) prescriptions per month. Figure 2b demonstrates prescriptions for codeine during the study period. Prescriptions were decreasing by –283 (–0.8%) per month before the schedule change, but the change was associated with a significant increase in level: an additional 6,304 (18.5%) prescriptions. In the postintervention phase, there was a nonsignificant decrease in codeine prescriptions: –34 (–0.2%) per month.
Figure 2.
The overall change in monthly prescription rates by all physician specialties for different opioids. a, Oxycodone monthly prescription rates were increasing by 298.7 (0.1%) per month before the schedule change. There was a nonsignificant level change of 18,729.2 (6.9%) additional prescriptions, followed by an additional drop of –2,273.5 (–0.8%) prescriptions per month. b, Codeine monthly prescriptions were decreasing by –283.4 (–0.8%) per month before the schedule change. There was a significant level change of 6,304.3 (18.5%) additional prescriptions, followed by a nonsignificant drop of –33.7 (–0.2%) prescriptions per month. c, Tramadol monthly prescriptions were increasing by 706.7 (0.4%) prescriptions per month before the schedule change. There was no significant level change, followed by a drop of –1,963.3 (–1.3%) prescriptions per month. d, Monthly prescription rates for a composite of hydromorphone, meperidine, methadone, morphine, and oxymorphone were decreasing by –219.8 (–0.4%) prescriptions per month before the schedule change. There was no significant level change, followed by a nonsignificant additional decrease of 134.2 (–0.4%) prescriptions per month.
Figure 2c demonstrates prescribing of tramadol. Prescriptions for tramadol were increasing before the schedule change, 707 (0.4%) prescriptions per month. There was no statistically significant change in level, but after the schedule change, the trajectory of tramadol prescribing changed to a decrease of –1,963 (–1.3%) prescriptions per month. This finding demonstrates that tramadol was not apparently used as a substitute for hydrocodone. Finally, Figure 2d shows the composite prescribing for hydromorphone, meperidine, methadone, morphine, and oxymorphone. Prescriptions for these medications were decreasing by –220 (–0.4%) prescriptions per month before the schedule change. There was no statistically significant change in level associated with the schedule change. The downward slope after the schedule change was similar to the pre-intervention phase (an additional decrease of 134 [–0.4%] prescriptions per month).
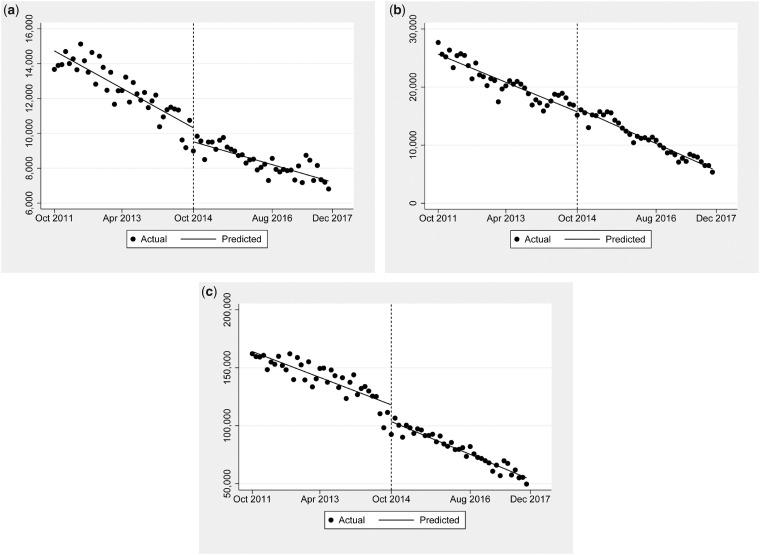
Table 3 and Figure 3 demonstrate opioid prescribing by anesthesiology (Figure 3a), emergency medicine (Figure 3b), and internal medicine (Figure 3c). The level change for anesthesiology was significant, at –805 (–8.5%) prescriptions. For internal medicine, the rescheduling was also associated with a significant level change of –14,619 (–10.2%) prescriptions. The schedule change had no statistically significant effect on prescriptions by emergency physicians.
Table 3.
Changes in prescription trends of hydrocodone by specialty
| Total Hydrocodone Prescriptions per Month | Anesthesiology, No. Prescriptions (95% CI) | Emergency Medicine, No. Prescriptions (95% CI) | Internal Medicine, No. Prescriptions (95% CI) |
|---|---|---|---|
| Base trend in the prepolicy segment* | –120.3 | –274.5 | –1,245.4 |
| (–145.0 to –95.5) | (–333.4 to –215.6) | (–1,630.8 to –860.0) | |
| Change in level in the postpolicy segment† | –804.9 | 804.5 | –14,618.9 |
| (–1,279.9 to –329.9) | (–760.6 to 2,369.5) | (–23,710.2 to –5,527.7) | |
| Additional change in trend in the postpolicy segment‡ | 56.2 | –23.2 | –115.2 |
| (28.0 to 84.5) | (–81.2 to 34.7) | (–564.7 to 334.3) |
CI = confidence interval.
Monthly prescribing rate from October 2011 to October 2014.
Change in absolute number of prescriptions before and after rescheduling.
Monthly prescribing rate difference before and after rescheduling until November 2017.
Figure 3.
The overall change in monthly prescription rates of hydrocodone by physician specialties of anesthesiology, emergency medicine, and internal medicine. a, Monthly hydrocodone prescription rates in anesthesiology showed a significant level change of –804.9 (–8.5%) prescriptions. b, Monthly hydrocodone prescription rates in emergency medicine showed no statistically significant effect on prescriptions. c, Monthly hydrocodone prescription rates in internal medicine showed a significant level change of –14,618.9 (–10.2%) prescriptions.
Discussion
Our results demonstrate that the DEA’s rescheduling of hydrocodone in 2014 was associated with a significant decrease in hydrocodone prescription rates in Ohio beyond what had already been occurring. The 30.1% drop seen for hydrocodone was greater than that of any of the other opioids. This is finding is consistent with studies from institutions [14,15], regionally [16,17] and nationally [8,10,18].
We found that there may have been a temporary substitution effect, in which prescriptions for codeine (a schedule III medication) appeared to increase before declining again. Contrary to previous research that found that tramadol (a schedule IV medication) prescribing increased after the hydrocodone schedule change [14–16], our study showed no substitution effect with tramadol; prescriptions before the policy change were increasing before the change, and then began decreasing. The work by Raji et al., however, found that prescribing of nonhydrocodone schedule II opioids and tramadol for commercially insured patients was stable [8]. As many of the previous studies were conducted on populations in Texas, our differing results indicate that substitution effects may be moderated by regional preferences. Prescriptions for oxycodone also decreased with the intervention, although the curve (Figure 2a) is interesting in that it appears that the upward trend of prescribing did continue beyond the policy change and then started to fall dramatically around mid-2016. We expect that this drop was not associated with hydrocodone rescheduling but rather with the increased attention on prescribing of schedule II opioids overall that occurred later.
Our study also evaluated the effect of the schedule change on three specialties that commonly prescribe opioids: anesthesiology, emergency medicine, and internal medicine. For anesthesiologists, who prescribe most hydrocodone products for patients with chronic pain, the existing rate of decline in hydrocodone prescriptions actually decreased, possibly indicating that hydrocodone may have been used more commonly after the schedule change. The reason for this increase is unclear. For internists, the change was associated with a drop in the level of prescriptions, but the overall slope of decrease was unchanged, indicating that the policy change was associated with a small initial drop, but the secular trend of decreasing prescriptions continued. Finally, for emergency medicine, there was no effect seen, likely because there are essentially no differences between writing a schedule II or schedule III opioid prescription in the emergency department; emergency physicians rarely phone in prescriptions or write for refills.
Our findings on specialty prescription likely differed from previous studies because of our study population. Our study most closely resembles one that used a nationally representative sample estimate [17] that looked at absolute changes and not interrupted time series analysis. They discovered that prescriptions for primary care physicians and emergency physicians dropped after the change, by –22.9% and –17.2%, respectively. Prescriptions by pain medicine actually increased by 7.2%, which was the only specialty that saw an increase. Two other studies found consistent decreases in hydrocodone prescription rates in the emergency department [19,20]. We saw decreases in prescribing, but with the interrupted time series methodology, we determined that the trajectory of opioid prescribing by emergency physicians was not changed by the rescheduling, likely because Ohio had already implemented an additional guideline aimed at reducing inappropriate opioid prescription habits in 2012 that was associated with overall reduced opioid prescription [21].
Our study has limitations that should be considered. This study was a retrospective analysis of a single state’s PDMP data. Although data inputted into the database were required by state law, they still relied on pharmacists’ entering the data at prescription dispensing, and errors may have occurred. For that reason, we eliminated some of the outlier prescriptions that appeared to be nonsensical, such as quantities above the 99th percentile. Although we can show associations of the hydrocodone schedule change, we cannot prove causality, as this policy change did not occur in isolation. For example, an opioid prescribing guideline for emergency physicians was introduced in the fifth month of this study, which likely also influenced that specialty’s opioid prescribing. PDMP data report only on prescriptions that are dispensed and not those that are written but not dispensed. Prescriptions that prescribers wrote but patients decided not to fill were not included. Finally, it is important to acknowledge that PDMP data do not include some important policy-relevant variables such as patient race, educational attainment, or measurements of financial need and poverty. Therefore, detecting if a change in opioid prescribing affects only certain socioeconomic groups is not possible with this analysis.
In conclusion, our research is the first study to look at opioid prescribing habits after the 2014 rescheduling of hydrocodone in a state-wide prescription database, including all payers and studying trends nearly three years after the policy change. Our results are consistent with previous studies that found significant reductions in rates of hydrocodone prescribing as well as a temporary substitution effect with codeine. Future studies should investigate whether misuse or overdoses involving hydrocodone products also decreased after rescheduling.
Funding sources: Support for statistical analysis for this study was provided by an unrestricted grant from the ADK Charities. Dr. Weiner is supported by National Institutes of Health grant 1-R01-DA044167.
Conflicts of interest: There are no conflicts of interest to report.
Prior presentation: Preliminary results of this study were presented at the 2019 Society for Academic Emergency Medicine Annual Meeting, Las Vegas, Nevada.
References
- 1.IQVIA Institute for Human Data Science. Medicine use and spending in the U.S. 2018. Available at: https://www.iqvia.com/institute/reports/medicine-use-and-spending-in-the-us-review-of-2017-outlook-to-2022 (accessed July 14, 2019).
- 2. Seth P, Scholl L, Rudd RA, Bacon S.. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep 2018;67(12):349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fink DS, Schleimer JP, Sarvet A, et al. Association between prescription drug monitoring programs and nonfatal and fatal drug overdoses. A systematic review. Ann Intern Med 2018;168(11):783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA 2016;315(15):1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Rescheduling of hydrocodone combination products from schedule III to schedule II. Final rule. Fed Regist 2014;79(163):49661–82. [PubMed] [Google Scholar]
- 6. Cassidy TA, Oyedele N, Mickle TC, Guenther S, Budman SH.. Patterns of abuse and routes of administration for immediate-release hydrocodone combination products. Pharmacoepidemiol Drug Saf 2017;26(9):1071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United Nations International Narcotic Control Board (INCB). Narcotic drugs: Estimated world requirements for 2012 and statistics for 2010. 2012. Available at: https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2011/Part_FOUR_Comments_NAR-Report-2011_English.pdf (accessed July 14, 2019).
- 8. Raji MA, Kuo YF, Adhikari D, Baillargeon J, Goodwin JS.. Decline in opioid prescribing after federal rescheduling of hydrocodone products. Pharmacoepidemiol Drug Saf 2018;27(5):513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shumway JW, Ran R, McClusky J, et al. Impact of rescheduling hydrocodone-combination products in an urban Texas county healthcare system. J Opioid Manag 2018;14(4):257–64. [DOI] [PubMed] [Google Scholar]
- 10. Kuo YF, Raji MA, Liaw V, Baillargeon J, Goodwin JS.. Opioid prescriptions in older Medicare beneficiaries after the 2014 federal rescheduling of hydrocodone products. J Am Geriatr Soc 2018;66(5):945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser Family Foundation. Opioid overdose death rates and all drug overdose death rates per 100,000 population (age-adjusted). Available at: https://www.kff.org/other/state-indicator/opioid-overdose-death-rates (accessed July 14, 2019).
- 12. Weiner SG, Baker O, Rodgers AF, et al. Opioid prescriptions by specialty in Ohio, 2010–2014. Pain Med 2018;19(5):978–89. [DOI] [PubMed] [Google Scholar]
- 13. Bernal JL, Cummins S, Gasparrini A, Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int J Epidemiol 2017;4(1):348–55.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernhardt MB, Taylor RS, Hagan JL, et al. Evaluation of opioid prescribing after rescheduling of hydrocodone-containing products. Am J Health Syst Pharm 2017;74(24):2046–53. [DOI] [PubMed] [Google Scholar]
- 15. Schultz S, Chamberlain C, Vulcan M, Rana H, Patel B, Alexander JC.. Analgesic utilization before and after rescheduling of hydrocodone in a large academic level 1 trauma center. J Opioid Manag 2016;12(2):119–22. [DOI] [PubMed] [Google Scholar]
- 16. Seago S, Hayek A, Pruszynski J, Newman MG.. Change in prescription habits after federal rescheduling of hydrocodone combination products. Proc (Bayl Univ Med Cent) 2016;29(3):268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tran S, Lavitas P, Stevens K, et al. The effect of a federal controlled substance act schedule change on hydrocodone combination products claims in a Medicaid population. J Manag Care Spec Pharm 2017;23(5):532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones CM, Lurie PG, Throckmorton DC.. Effect of US Drug Enforcement Administration's rescheduling of hydrocodone combination analgesic products on opioid analgesic prescribing. JAMA Intern Med 2016;176(3):399–402. [DOI] [PubMed] [Google Scholar]
- 19. Oehler EC, Day RL, Robinson DB, Brown LH.. Has the rescheduling of hydrocodone changed ED prescribing practices? Am J Emerg Med 2016;34(12):2388–91. [DOI] [PubMed] [Google Scholar]
- 20. Chumpitazi CE, Rees CA, Camp EA, Bernhardt MB.. Decreased opioid prescribing in a pediatric emergency department after the rescheduling of hydrocodone. J Emerg Med 2017;52(4):547–53. [DOI] [PubMed] [Google Scholar]
- 21. Weiner SG, Baker O, Poon SJ, et al. The effect of opioid prescribing guidelines on prescriptions by emergency physicians in Ohio. Ann Emerg Med 2017;70(6):799–808.e1. [DOI] [PubMed] [Google Scholar]