Abstract
Background
Tapentadol is a molecule incorporating mu opioid receptor agonism and norepinephrine reuptake inhibition to provide analgesia, with the potential for a lower incidence of gastrointestinal side effects than full mu opioid agonists. Postmarketing surveillance of tapentadol as an active pharmaceutical ingredient has consistently revealed low levels of abuse and diversion.
Objective
The purpose of the present study was to further characterize the abuse liability of tapentadol extended-release (ER) by evaluating the prevalence of past 30-day tapentadol ER abuse and reported routes of administration as compared with ER opioids with Food and Drug Administration (FDA) abuse-deterrent labeling (“ADF opioids”) and ER opioids without FDA abuse-deterrent labeling (“non-ADF opioids”).
Methods
Data were collected from January 2014 through December 2017 from 776 centers located in 43 states throughout the United States using the Addiction Severity Index–Multimedia Version (ASI-MV), an instrument that is integral to the National Addictions Vigilance Intervention and Prevention Program (NAVIPPRO, Inflexxion, an IBH Company, Costa Mesa, CA, USA).
Results
Tapentadol ER had lower rates of past 30-day abuse than ADF ER and non-ADF ER opioid comparators, both at a population level and when adjusted for drug utilization. Tapentadol ER was primarily abused orally, although it was also abused through alternate routes of administration. Cumulative rates of tapentadol ER abuse by alternative routes of administration were lower than both ADF and non-ADF ER opioid comparators, although large confidence intervals resulting from the small sample size of reported tapentadol ER use limit firm conclusions.
Conclusions
In summary, tapentadol ER was found to have lower rates of both past 30-day abuse and use via alternate routes of administration, specifically snorting and smoking, than ADF and non-ADF ER comparators.
Keywords: Analgesic, Opioid, Prescription Drug Abuse, Pain Management, Tapentadol
Introduction
Tapentadol is a Schedule II, centrally acting, multimechanistic analgesic with a combination of μ-opioid agonist activity and norepinephrine reuptake inhibition (NRI) that is used to treat moderate to severe acute and chronic pain [1–5]. Tapentadol immediate-release (IR; Nucynta) was approved by the Food and Drug Administration (FDA) in December 2008, and the extended-release (ER) formulation (Nucynta ER) was approved by the FDA in August 2011. Between January 2010 and December 2016, tapentadol had the fewest dosage units dispensed, the fewest prescriptions dispensed and individuals filling prescriptions, and the third lowest grams dispensed among hydrocodone, hydromorphone, morphine, oxycodone, oxymorphone, and tramadol [6].
Postmarketing surveillance studies have thus far revealed that, as an active pharmaceutical ingredient (API), tapentadol has low rates of intentional abuse, diversion, and past 30-day abuse or endorsement among comparator opioid APIs at the population level of exposure [7–12]. For instance, from the fourth quarter 2011 to the second quarter 2016 at the population level, tapentadol had event rates of 0.015 of intentional abuse, 0.029 of diversion, and 0.245 of past 30-day use to get high [10]. Comparator opioid APIs (hydrocodone, hydromorphone, morphine, oxycodone, oxymorphone, and tramadol) were reported as being intentionally abused from 7.41 (oxymorphone) to 84.32 (oxycodone) times the rate of tapentadol, diverted from 23.172 (oxymorphone) to 316.862 (oxycodone) times the rate of tapentadol, and used for getting high in the last 30 days from 3.48 (tramadol) to 52.97 (oxycodone) times the rate of tapentadol [10]. Tapentadol was discussed the least of the opioid comparators online and endorsed at similar levels as tramadol (ERo ratio for tramadol = 0.776) and less than oxymorphone (ERo ratio for oxymorphone = 2.35) [11].
Although these are relatively low levels of abuse, when adjusted for drug availability, abuse levels remain clinically relevant. For example, when adjusted for 100,000 dosing units dispensed, tapentadol (API) had a greater rate of intentional abuse than hydrocodone (tapentadol = 0.28, hydrocodone = 0.20) and tramadol (0.22), a similar rate of diversion as tramadol (tapentadol = 0.045, tramadol = 0.047), and greater rates of past-month use to get high (tapentadol = 0.436) than tramadol (0.036), hydrocodone (0.166), or oxycodone (0.337) [10].
This finding may reflect varying patterns of abuse between ER and IR formulations. When tapentadol IR was first introduced, abuse rates were consistently low at the population level; however, when adjusted for drug availability, abuse rates fluctuated, although they were still relatively low overall [8,12]. Abuse and diversion of ER tapentadol, however, have been found to be relatively stable and low at the population level and when adjusted for drug availability [9]. At the population level, tapentadol ER had the lowest level of abuse among opioid comparators. Relative risk of abuse ranged from 1.10 (hydromorphone ER, which did not differ from tapentadol ER) to 315.45 (oxycodone ER) among substance abusers entering treatment [7]. Rate of tapentadol ER diversion ranged from 0 to 0.002 in the first three years of its introduction to the US market (fourth quarter 2011 to third quarter 2014), with an average rate of diversion of 0.001, compared with a range of 1.051 to 2.116, and an average rate of 1.492 for other schedule II opioids at the population level of analysis [9]. When adjusted for prescription volume, relative risk of abuse ranged from 1.92 (hydromorphone ER, which did not differ from tapentadol ER) to 17.02 (oxymorphone ER), and rate of diversion was 0.016, compared with 0.172 for other schedule II opioids [7,9].
Multiple ER opioid formulations exist in the population, including opioids with FDA abuse-deterrent labeling (“ADF opioids”) and opioids without FDA abuse-deterrent labeling (“non-ADF opioids”). To address this complexity, the present study included ER ADF opioids and ER non-ADF opioids as comparators. The purpose of this study was to further characterize the abuse liability of tapentadol ER—a product that, although formulated to make crushing difficult, does not have ADF labeling—by evaluating the prevalence of past 30-day tapentadol ER abuse and reported routes of administration compared with ADF ER opioids and non-ADF ER opioids.
Methods
Data
Data were collected over a four-year period (January 2014 through December 2017) from 776 centers located in 43 states throughout the United States with the National Addictions Vigilance Intervention and Prevention Program (NAVIPPRO; Inflexxion, an IBH Company, Costa Mesa, CA, USA) Addiction Severity Index–Multimedia Version (ASI-MV).
The ASI-MV is an NAVIPPRO data stream that captures person-level data of individuals assessed for substance use problems for clinical treatment planning and triage purposes [13]. The ASI-MV is a structured, self-administered computerized interview that measures the severity of a range of problem areas typically associated with drug and alcohol abuse, including medical, employment status, alcohol and illegal drug use, legal status, family and social status, and psychiatric status. This electronic/multimedia assessment is based on the Addiction Severity Index (ASI), a standard clinical assessment designed for use on admission to drug and alcohol treatment [14]. The ASI has well established reliability and validity [15–18].
Individuals entering treatment centers complete the ASI-MV upon intake; data are self-reported. Respondents who indicate abuse of prescription opioids are asked to endorse the specific prescription opioid products they have used in the past 30 days. More than 60 name brand and generic prescription opioid analgesic products are included in the database, along with photos to assist with product identification. Routes of administration, source of the product, and information regarding pain diagnoses and other treatment are also collected. The ASI-MV provides a validated score indicative of the severity of addiction (as with other indices, the higher the score, the more severe the addiction).
When the respondent has completed the assessment at the treatment site for assessment purposes, individual-level data are de-identified and electronically uploaded to a central server where they are available for analysis [13]. ASI-MV data are collected primarily for clinical purposes; therefore, analyses of de-identified aggregate data for research purposes have been determined to be exempt from institutional review board review by the New England Institutional Review Board.
Definition of Abuse and Routes of Administration
Past 30-day abuse was defined as use of the specified prescription opioid analgesic products in any way not specifically indicated by a prescriber and was evaluated by considering 1) whether the product was used with alternate routes of administration, 2) the source of the product, and 3) whether the respondent had a current pain problem and had taken a prescribed opioid medication for pain in the past 30 days. Routes of administration included swallowing whole, other oral route (an oral route other than swallowing whole, such as chewing or dissolving in mouth), snorting, smoking, injecting, or other route of administration.
Prescription Data Source
Prescription volume data were obtained from the commercial vendor IQVIA (Danbury, CT, USA). The prescription data sample comprises nearly 59,000 pharmacies (over 99% of retail stores) in the United States and includes cash, Medicaid, and third-party transactions. Data are representative of the retail pharmacy universe and do not include other potential channels of distribution, such as long-term care, hospital dispensaries, and mail order.
Study Design and Prescription Opioid Comparators
Among individuals assessed for substance abuse treatment in the ASI-MV network, this observational, cross-sectional study examined the relative prevalence of self-reported abuse of 1) tapentadol ER, 2) ADF ER opioids, and 3) non-ADF ER opioids. Table 1 presents the compounds and associated products assessed in the ASI-MV and included in the analyses.
Table 1.
Comparator groups
| ASI-MV Monitored Products (Description) | Abuse Cases (Jan 2014–Dec 2017) | Tablets Dispensed (Jan 2014–Dec 2017) | |
|---|---|---|---|
| Tapentadol ER |
|
46 | 43,320,490 |
| ADF ER opioids |
|
5,313 | 644,587,2000 |
| Non-ADF ER opioids |
|
7,192 | 1,244,430,081 |
ADF = abuse-deterrent formulation; ASI-MV= Addiction Severity Index–Multimedia Version; ER = extended-release; XR = extended-release.
All generic oxycodone ER products were the same formulation as reformulated OxyContin during the study period.
Data Analyses
Demographic characteristics were assessed using Pearson’s chi-square test for categorical data. Because three comparisons were conducted for each variable (tapentadol ER vs ADF ER opioids, tapentadol ER vs non-ADF ER opioids, ADF ER vs non-ADF ER opioids), only tests where P < 0.01 were considered statistically significant. Abuse prevalence rate estimates were calculated quarterly, annually, and as an aggregate summary measure covering the entire time period (2014–2017). Prevalence rates were calculated using number of abuse cases per 100 ASI-MV assessments and then adjusted for drug utilization (number of abuse cases per 100 ASI-MV assessments per 1,000,000 tablets dispensed). Number of tablets dispensed was chosen as the adjustor for drug availability because each tablet represents an individual opportunity for abuse [19].
The Generalized Linear Model (GENMOD) procedure in SAS Enterprise Guide 7.13 (SAS Institute, Inc., 2016, Cary, NC, USA) was used for these analyses. Data are presented as estimates and 95% confidence intervals (CIs); estimates were interpreted as statistically significantly different if there was no overlap of the 95% CI.
Route of administration was recorded for substances reportedly used in the past 30 days. Estimates of routes of administration were then calculated for each drug as a percentage by dividing the number of participants indicating a particular route by the number of abuse cases of that product. Routes of administration were not mutually exclusive; thus, response categories may sum to greater than 100%.
Results
Between January 2014 through December 2017, a total of 205,189 adults were assessed for substance abuse treatment using the ASI-MV at 776 centers located in 43 states throughout the United States. Table 2 summarizes the patient characteristics for patients who reported past 30-day tapentadol ER abuse (N = 46, 0.02% of the sample), for patients who reported past 30-day abuse of ADF ER opioids (N = 5,313, 2.6% of the sample), and for patients who reported past 30-day abuse of non-ADF ER opioids (N = 7,192, 3.5% of the sample). These groups were not mutually exclusive, as one patient could have reported past 30-day abuse of multiple products. The tapentadol ER group was substantially smaller than the other two composite groups and as a result had greater variability, rendering a need for cautious data interpretation.
Table 2.
Patient characteristics
| Characteristic | 1. Tapentadol ER N = 46 | 2. ADF ER Opioids N = 5,313 | 3. Non-ADF ER Opioids N = 7,192 | 1 vs 2 Χ2 | 1 vs 3 Χ2 | 2 vs 3 Χ2 | |||
|---|---|---|---|---|---|---|---|---|---|
| Age, y | N | % | N | % | N | % | |||
| <21 | 4 | 8.70 | 520 | 9.79 | 601 | 8.36 | 2.52 | 2.75 | 8.59 |
| 21–34 | 23 | 50.00 | 3,090 | 58.16 | 4,243 | 59.00 | |||
| 35–54 | 16 | 34.78 | 1,525 | 28.70 | 2,124 | 29.53 | |||
| 55+ | 3 | 6.52 | 178 | 3.35 | 224 | 3.11 | |||
| Gender | N | % | N | % | N | % | |||
| Male | 30 | 65.22 | 2,741 | 51.59 | 3,417 | 47.51 | 3.39 | 5.73 | 20.08* |
| Female | 16 | 34.78 | 2,572 | 48.41 | 3,771 | 52.43 | |||
| Unknown/missing | 0 | 0.00 | 0 | 0.00 | 4 | 0.06 | |||
| Race | N | % | N | % | N | % | |||
| White | 31 | 67.39 | 4,255 | 80.09 | 6,197 | 86.17 | 6.13 | 16.33* | 101.32* |
| Black | 7 | 15.22 | 482 | 9.07 | 362 | 5.03 | |||
| Hispanic | 3 | 6.52 | 316 | 5.95 | 331 | 4.60 | |||
| Other | 5 | 10.87 | 260 | 4.89 | 302 | 4.20 | |||
| Unknown/missing | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |||
| Education | N | % | N | % | N | % | |||
| Less than high school | 21 | 45.65 | 1,519 | 28.59 | 1,936 | 26.92 | 12.08 | 13.78* | 4.76 |
| High school degree | 14 | 30.43 | 2,185 | 41.13 | 3,064 | 42.60 | |||
| Some college | 6 | 13.04 | 1,330 | 25.03 | 1,810 | 25.17 | |||
| College degree | 2 | 4.35 | 152 | 2.86 | 211 | 2.93 | |||
| >16 y | 3 | 6.52 | 127 | 2.39 | 171 | 2.38 | |||
| Employment | N | % | N | % | N | % | |||
| Professional | 5 | 10.87 | 429 | 8.07 | 538 | 7.48 | 21.37* | 22.52* | 7.80 |
| Administrative | 4 | 8.70 | 636 | 11.97 | 850 | 11.82 | |||
| Skilled/semiskilled | 5 | 10.87 | 1,922 | 36.18 | 2,591 | 36.03 | |||
| Student | 4 | 8.70 | 136 | 2.56 | 160 | 2.22 | |||
| Homemaker | 4 | 8.70 | 463 | 8.71 | 634 | 8.82 | |||
| Other manual/unskilled | 5 | 10.87 | 555 | 10.45 | 723 | 10.05 | |||
| Did not work for pay | 4 | 8.70 | 289 | 5.44 | 417 | 5.80 | |||
| Disabled | 5 | 10.87 | 359 | 6.76 | 544 | 7.56 | |||
| No occupation | 8 | 17.39 | 501 | 9.43 | 696 | 9.68 | |||
| Unknown/missing | 2 | 4.35 | 23 | 0.43 | 39 | 0.54 | |||
| Marital status | N | % | N | % | N | % | |||
| Married | 9 | 19.57 | 1,190 | 22.40 | 1,714 | 23.83 | 0.27 | 0.30 | 9.06 |
| Separated/divorced/widowed | 11 | 23.91 | 1,161 | 21.85 | 1,662 | 23.11 | |||
| Never married | 24 | 52.17 | 2,938 | 55.30 | 3,788 | 52.67 | |||
| Unknown/missing | 2 | 4.35 | 24 | 0.45 | 28 | 0.39 | |||
| Self-reported pain | N | % | N | % | N | % | |||
| Yes | 29 | 63.04 | 3,127 | 58.86 | 4,366 | 60.71 | 0.56 | 0.26 | 6.07 |
| No | 16 | 34.78 | 2,178 | 40.99 | 2,821 | 39.22 | |||
| Unknown/missing | 1 | 2.17 | 8 | 0.15 | 5 | 0.07 | |||
| Chronic medical problem | N | % | N | % | N | % | |||
| Yes | 25 | 54.35 | 2,178 | 40.99 | 3,016 | 41.94 | 3.31 | 2.87 | 1.02 |
| No | 21 | 45.65 | 3,124 | 58.80 | 4,168 | 57.95 | |||
| Unknown/missing | 0 | 0.00 | 11 | 0.21 | 8 | 0.11 | |||
| Criminal justice required substance abuse treatment | N | % | N | % | N | % | |||
| Yes | 20 | 43.48 | 1,204 | 22.66 | 1,718 | 23.89 | 11.09* | 9.54* | 2.45 |
| No | 26 | 56.52 | 4,089 | 76.96 | 5,456 | 75.86 | |||
| Unknown/missing | 0 | 0.00 | 20 | 0.38 | 18 | 0.25 | |||
| Treatment modality | N | % | N | % | N | % | |||
| Residential/inpatient | 22 | 47.83 | 3,214 | 60.49 | 4,814 | 66.94 | 8.57 | 15.98* | 61.20* |
| Outpatient/nonmethadone | 13 | 28.26 | 1,022 | 19.24 | 1,148 | 15.96 | |||
| Methadone/LAAM† | 0 | 0.00 | 278 | 5.23 | 361 | 5.02 | |||
| Corrections | 5 | 10.87 | 288 | 5.42 | 283 | 3.93 | |||
| Other | 6 | 13.04 | 511 | 9.62 | 586 | 8.15 | |||
| Treatment site | N | % | N | % | N | % | |||
| Northeast | 0 | 0.00 | 230 | 4.33 | 154 | 2.14 | 10.91* | 25.87 | 187.23* |
| South | 29 | 63.04 | 3,414 | 64.26 | 5,361 | 74.54 | |||
| West | 9 | 19.57 | 411 | 7.74 | 311 | 4.32 | |||
| Midwest | 8 | 17.39 | 1,258 | 23.68 | 1,366 | 18.99 | |||
| Drug severity score | N | % | N | % | N | % | |||
| 0–1 | 4 | 8.70 | 101 | 1.90 | 147 | 2.04 | 15.06* | 16.24* | 10.00 |
| 2–3 | 0 | 0.00 | 108 | 2.03 | 137 | 1.90 | |||
| 4–5 | 5 | 10.87 | 367 | 6.91 | 401 | 5.58 | |||
| 6–7 | 14 | 30.43 | 1,648 | 31.02 | 2,267 | 31.52 | |||
| 8–9 | 17 | 36.96 | 2,803 | 52.76 | 3,851 | 53.55 | |||
| Unknown/missing | 6 | 13.04 | 286 | 5.38 | 389 | 5.41 | |||
ADF = abuse-deterrent formulation; ER = extended-release; LAAM = levo-α-acetylmethadol.
P < 0.01.
Levo-α-acetylmethadol is a synthetic mu receptor opioid agonist with similar structure to methadone but longer-acting.
As can be seen in Table 2, tapentadol ER abusers were mostly white or black males, age 21–45 years, with a large segment (45.65%) having less than a high school education. Employment was fairly evenly spread across the descriptive categories (ranging from no occupation to professional). Over half (52.17%) were never married. Almost two-thirds (63.04%) had self-reported pain, and over half reported chronic medical problems (54.35%). Close to half (47.83%) reported receiving residential or inpatient treatment, slightly less reported having substance abuse treatment mandated by the criminal justice system (43.48%), and ∼67% had drug severity scores ≥6. There were no systematic differences among the three groups.
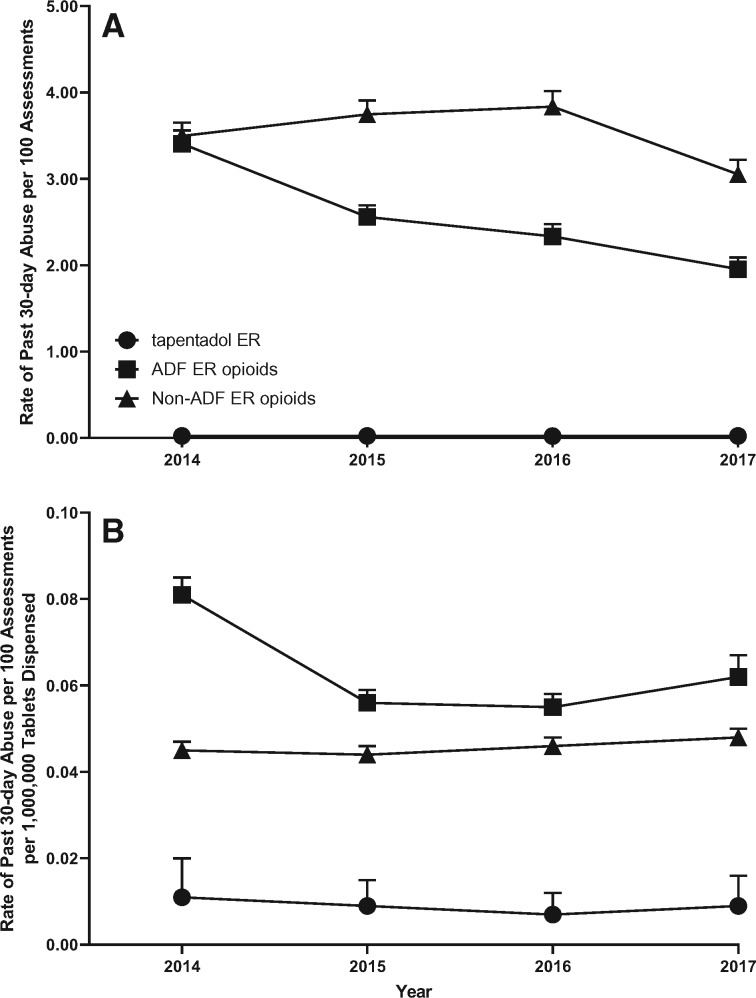
Figure 1A and Table 3A depict the annual rates of past 30-day abuse for tapentadol ER, ADF ER opioids, and non-ADF ER opioids from 2014 through 2017. The past 30-day abuse rate per 100 ASI-MV assessments was greatest for non-ADF ER opioids (aggregate rate = 3.550/100 assessments, 95% CI = 3.470–3.631, quarterly rates ranged from 2.872 to 4.213), followed by ADF ER opioids (aggregate rate = 2.609/100 assessments, 95% CI = 2.541–2.679, quarterly rates ranged from 1.731 to 3.731). Tapentadol ER had the lowest rates of abuse among all comparators (aggregate rate = 0.023/100 assessments, 95% CI = 0.017–0.031, quarterly rate ranged from 0.007 to 0.051), and there was no overlap of CIs.
Figure 1.
Annual rate of past 30-day abuse (A) and annual rate of past 30-day abuse adjusted for tablets dispensed (B). Data are displayed by calendar year. Tapentadol extended-release (ER) is represented by a circle, the abuse-deterrent formulation (ADF) ER opioid comparator group is represented by a square, and the non-ADF ER opioid comparator group is represented by the triangle. Error bars represent the 95% confidence interval.
Table 3A.
Quarterly (Q1 2014–Q4 2017), annual (2014–2017) and cumulative rate of past 30-day abuse per 100 ASI-MV assessments
| Time | Tapentadol ER |
ADF ER Opioids |
Non-ADF ER Opioids |
|||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Quarter | ||||||
| Q1 2014 | 0.015 | 0.004–0.059 | 3.005 | 2.731–3.307 | 3.429 | 3.136–3.749 |
| Q2 2014 | 0.007 | 0.001–0.049 | 3.358 | 3.077–3.665 | 3.492 | 3.206–3.804 |
| Q3 2014 | 0.028 | 0.011–0.075 | 3.537 | 3.246–3.855 | 3.533 | 3.242–3.850 |
| Q4 2014 | 0.045 | 0.020–0.101 | 3.731 | 3.423–4.067 | 3.531 | 3.232–3.857 |
| Q1 2015 | 0.015 | 0.004–0.058 | 3.243 | 2.964–3.548 | 3.567 | 3.274–3.886 |
| Q2 2015 | 0.026 | 0.010–0.069 | 2.045 | 1.836–2.277 | 3.339 | 3.070–3.631 |
| Q3 2015 | 0.027 | 0.010–0.073 | 2.542 | 2.302–2.808 | 3.975 | 3.672–4.304 |
| Q4 2015 | 0.025 | 0.008–0.077 | 2.454 | 2.195–2.744 | 4.213 | 3.871–4.587 |
| Q1 2016 | 0.017 | 0.004–0.068 | 2.451 | 2.191–2.742 | 4.056 | 3.717–4.425 |
| Q2 2016 | 0.033 | 0.013–0.089 | 2.386 | 2.130–2.673 | 3.868 | 3.540–4.228 |
| Q3 2016 | 0.017 | 0.004–0.068 | 2.250 | 2.000–2.533 | 3.747 | 3.421–4.106 |
| Q4 2016 | 0.019 | 0.005–0.075 | 2.243 | 1.980–2.539 | 3.655 | 3.317–4.027 |
| Q1 2017 | 0.017 | 0.004–0.069 | 2.217 | 1.966–2.501 | 3.164 | 2.861–3.499 |
| Q2 2017 | 0.009 | 0.001–0.064 | 1.802 | 1.572–2.065 | 3.208 | 2.896–3.552 |
| Q3 2017 | 0.019 | 0.005–0.076 | 1.731 | 1.502–1.995 | 2.940 | 2.636–3.279 |
| Q4 2017 | 0.051 | 0.021–0.123 | 2.065 | 1.804–2.364 | 2.872 | 2.563–3.218 |
| Annual | ||||||
| 2014 | 0.024 | 0.014–0.040 | 3.407 | 3.260–3.562 | 3.496 | 3.347–3.652 |
| 2015 | 0.023 | 0.014–0.040 | 2.559 | 2.433–2.692 | 3.747 | 3.594–3.907 |
| 2016 | 0.022 | 0.012–0.040 | 2.335 | 2.203–2.476 | 3.837 | 3.666–4.015 |
| 2017 | 0.023 | 0.013–0.040 | 1.956 | 1.830–2.090 | 3.053 | 2.895–3.219 |
| Cumulative | 0.023 | 0.017–0.031 | 2.609 | 2.541–2.679 | 3.550 | 3.470–3.631 |
ADF = abuse-deterrent formulation; ASI-MV= Addiction Severity Index–Multimedia Version; CI = confidence interval; ER = extended-release.
Figure 1B and Table 3B depict the annual rates of past 30-day abuse for tapentadol ER, ADF ER opioids, and non-ADF ER opioids from 2014 through 2017, adjusted for prescription volume (cases/100 assessments/1,000,000 tablets dispensed). When adjusted for prescription volume, the abuse rate was greatest for ADF ER opioids (aggregate rate = 0.064 cases/100 assessments/1,000,000 tablets dispensed, 95% CI = 0.062 to 0.066, quarterly rates ranged from 0.044 to 0.089) followed by non-ADF ER opioids (aggregate rate = 0.045 cases/100 assessments/1,000,000 tablets dispensed, 95% CI = 0.044 to 0.046, quarterly rates ranged from 0.038 to 0.050). Tapentadol ER had the lowest rates of abuse among all comparators (aggregate rate = 0.009 cases/100 assessments/1,000,000 tablets dispensed, 95% CI = 0.006 to 0.011, quarterly rates ranged from 0.003 to 0.021), and there was no overlap of CIs.
Table 3B.
Quarterly (Q1 2014–Q4 2017), annual (2014–2017), and cumulative rate of past 30-day abuse per 100 ASI-MV assessments adjusted for tablets dispensed
| Time | Tapentadol ER |
ADF ER Opioids |
Non-ADF ER Opioids |
|||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Quarter | ||||||
| Q1 2014 | 0.007 | 0.002–0.030 | 0.071 | 0.065–0.078 | 0.046 | 0.042–0.050 |
| Q2 2014 | 0.004 | 0.000–0.025 | 0.082 | 0.075–0.089 | 0.045 | 0.042–0.049 |
| Q3 2014 | 0.013 | 0.005–0.036 | 0.084 | 0.077–0.091 | 0.046 | 0.042–0.050 |
| Q4 2014 | 0.021 | 0.009–0.046 | 0.089 | 0.081–0.097 | 0.045 | 0.041–0.049 |
| Q1 2015 | 0.006 | 0.002–0.025 | 0.074 | 0.067–0.081 | 0.044 | 0.040–0.048 |
| Q2 2015 | 0.010 | 0.004–0.026 | 0.044 | 0.039–0.049 | 0.038 | 0.035–0.041 |
| Q3 2015 | 0.010 | 0.004–0.026 | 0.056 | 0.051–0.062 | 0.046 | 0.043–0.050 |
| Q4 2015 | 0.008 | 0.003–0.025 | 0.054 | 0.048–0.060 | 0.048 | 0.044–0.052 |
| Q1 2016 | 0.006 | 0.001–0.022 | 0.056 | 0.050–0.062 | 0.047 | 0.043–0.051 |
| Q2 2016 | 0.010 | 0.004–0.027 | 0.054 | 0.048–0.060 | 0.045 | 0.041–0.049 |
| Q3 2016 | 0.005 | 0.001–0.020 | 0.052 | 0.046–0.059 | 0.044 | 0.040–0.048 |
| Q4 2016 | 0.006 | 0.001–0.023 | 0.058 | 0.051–0.066 | 0.047 | 0.043–0.052 |
| Q1 2017 | 0.006 | 0.002–0.025 | 0.066 | 0.059–0.074 | 0.047 | 0.042–0.052 |
| Q2 2017 | 0.003 | 0.000–0.024 | 0.057 | 0.050–0.065 | 0.049 | 0.044–0.054 |
| Q3 2017 | 0.007 | 0.002–0.027 | 0.056 | 0.048–0.064 | 0.046 | 0.041–0.051 |
| Q4 2017 | 0.020 | 0.008–0.047 | 0.072 | 0.063–0.082 | 0.050 | 0.044–0.056 |
| Year | ||||||
| 2014 | 0.011 | 0.007–0.020 | 0.081 | 0.078–0.085 | 0.045 | 0.043–0.047 |
| 2015 | 0.009 | 0.005–0.015 | 0.056 | 0.054–0.059 | 0.044 | 0.042–0.046 |
| 2016 | 0.007 | 0.004–0.012 | 0.055 | 0.052–0.058 | 0.046 | 0.044–0.048 |
| 2017 | 0.009 | 0.005–0.016 | 0.062 | 0.058–0.067 | 0.048 | 0.045–0.050 |
| Cumulative | 0.009 | 0.006–0.011 | 0.064 | 0.062–0.066 | 0.045 | 0.044–0.046 |
ADF = abuse-deterrent formulation; ASI-MV= Addiction Severity Index–Multimedia Version; CI = confidence interval; ER = extended-release.
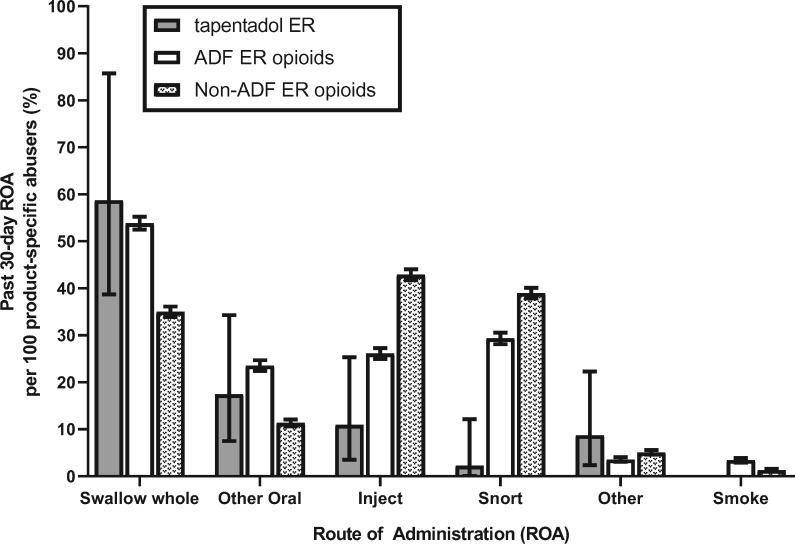
Figure 2 and Table 4 summarize the aggregated percentage of product-specific abusers (per 100 product-specific abusers) reporting use via specific routes of administration in the past 30 days for tapentadol ER and comparators from 2014 through 2017 (tapentadol ER N = 46, ADF ER opioids N = 5,313, non-ADF ER opioids N = 7,192). Here, the effects of the small sample for tapentadol ER were evident: Large confidence intervals limited precision of the estimates in comparison to the ADF ER opioid and non-ADF ER opioid groups. Tapentadol ER and ADF ER opioids were reported to be most often “swallowed whole” (reported by 58.70% and 53.81% of the respective groups; 95% CIs overlapped). The 95% CIs between tapentadol ER and non-ADF ER opioids also overlapped, suggesting that there was no difference between the two groups. Of note, the CIs did not overlap between the ADF ER opioid and non-ADF ER opioid groups, suggesting that ADF ER opioids were “swallowed whole” more than non-ADF ER opioids.
Figure 2.
Cumulative percentage of product-specific abusers reporting past 30-day route of administration per 100 product-specific abusers for tapentadol extended-release (ER), abuse-deterrent formulation (ADF) ER opioids, and non-ADF ER opioids. Data are displayed cumulatively (each bar represents an average across years 2014–2017). Tapentadol ER is represented by the black bar, the ADF ER opioid comparator group is represented by a white bar, and the non-ADF ER opioid comparator group is represented by the patterned bar. Error bars represent the 95% confidence interval.
Table 4.
Cumulative rate of route of administration
| Tapentadol ER N = 46 |
ADF ER Opioids N = 5,313 |
Non-ADF ER Opioids N = 7,192 |
||||
|---|---|---|---|---|---|---|
| ROA | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
| Swallow whole | 58.70 | 38.68–85.70 | 53.81 | 52.48–55.18 | 34.99 | 33.90–36.12 |
| Other oral | 17.39 | 7.50–34.26 | 23.50 | 22.39–24.68 | 11.30 | 10.59–12.06 |
| Inject | 10.87 | 3.52–25.33 | 26.08 | 24.92–27.29 | 42.85 | 41.71–44.02 |
| Snort | 2.17 | 0.06–12.11 | 29.30 | 28.09–30.55 | 38.95 | 37.83–40.10 |
| Other | 8.70 | 2.37–22.26 | 3.51 | 3.04–4.04 | 5.04 | 4.56–5.58 |
| Smoke | 0.00 | 0.00–0.00 | 3.37 | 2.92–3.90 | 1.25 | 1.02–1.54 |
ADF = abuse-deterrent formulation; CI = confidence interval; ER = extended-release; ROA = route of administration.
The second most frequently reported past 30-day route of administration for tapentadol ER was an “other oral” route (17.39%; defined as an oral route other than swallowing whole, such as chewing or dissolving in mouth). Rates of “other oral” use did not differ between tapentadol ER and ADF ER opioids (23.50%), nor between tapentadol ER and non-ADF ER opioids (11.30, 95% CIs of both sets of estimates overlapped). The 95% CIs did not overlap between the ADF ER opioid and the non-ADF ER opioid groups, suggesting that ADF ER opioids were taken via other oral routes more than non-ADF ER opioids.
The proportion of participants reporting past 30-day “injection,” the third most prevalent route of administration for tapentadol ER (10.87%), did not differ from the proportion of individuals reporting past 30-day “injection” of ADF ER opioids (26.08%, 95% CIs overlapped). “Injection” was the most prevalent route of past 30-day administration for non-ADF ER opioids (42.85%), and the 95% CIs did not overlap between the tapentadol ER and ADF ER opioid groups.
Both the ADF ER opioid (29.30%) and non-ADF ER opioid (38.95%) comparator groups had greater percentages of individuals who reported past 30-day “snorting” as a route of administration for those compounds than did the tapentadol ER group (2.17%). No 95% CIs crossed among the three comparator groups. Lastly, no individuals reported past 30-day “smoking” of tapentadol ER. More past 30-day “smoking” was reported in the ADF ER opioid group than in the non-ADF ER opioid comparator group. No 95% CIs crossed among the three groups.
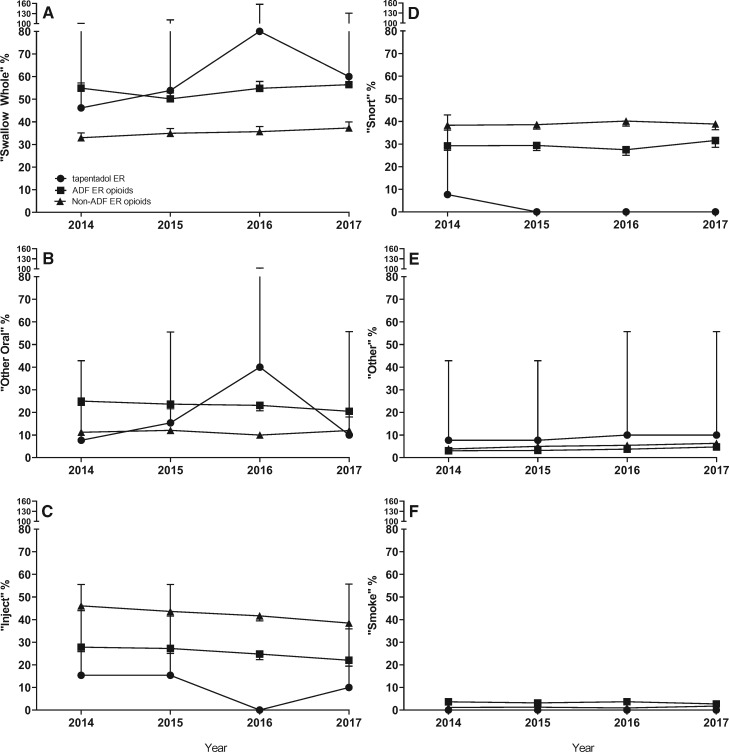
Figure 3 depicts the annual percentages of product-specific abusers (per 100 product-specific abusers) reporting use via specific routes of administration in the past 30 days for tapentadol ER and comparators from 2014 through 2017 by year. Here again, large CIs limit the precision of the estimates of tapentadol ER in comparison with the ADF ER opioid and non-ADF ER opioid groups. Patterns of route of administration within each comparator stayed fairly consistent across the study period. There was an increase of “swallowing whole” and “other oral” routes of administration between 2015 and 2016 and a subsequent decrease between 2016 and 2017 in the tapentadol ER group (Figure 3A and B); however, the 95% CI overlapped with both the ADF ER opioid and non-ADF ER opioid estimates. There was no tapentadol ER injection reported in 2016 (Figure 3C). Snorting of tapentadol ER was only reported in the first quarter of 2014 (Figure 3D).
Figure 3.
Percentage of product-specific abusers reporting each past 30-day route of administration. Data are displayed by calendar year. Routes of administration include swallow whole (A), other oral (B), inject (C), snort (D), other (E), and smoke (F) for tapentadol extended-release (ER; circle), the abuse-deterrent formulation (ADF) ER opioid comparator group (square), and the non-ADF ER opioid comparator group (triangle). Error bars represent the 95% confidence interval.
Discussion
Tapentadol has been reported to have low levels of abuse and diversion relative to other prescription opioids [7,9–11]. The present study continues this line of investigation by updating the time period of surveillance, presenting the characteristics of tapentadol ER abusers and motives for abuse, and also detailing the abuse-related routes of administration. Comparator opioid groups were defined in such a way to position tapentadol ER within the current ER opioid landscape.
Similar to the opioid comparators, more than half of the individuals reporting tapentadol ER abuse also reported pain (63.04%) or chronic medical problems (54.35%). This finding reveals that much of the tapentadol ER abuse, and in fact much of the ER opioid abuse found in this sample, was conducted by individuals with compromised health. Concerns are being raised by providers and patients about the undertreatment of chronic pain in compromised individuals resulting from decisions surrounding the management of the prescription opioid epidemic [20–24]. These data support the present need for supportive health care, both for pain and other health problems, in this vulnerable population.
Rates of tapentadol ER past 30-day abuse were lower than both non-ADF ER opioids and ADF opioids at the population level. This finding is in keeping with other postmarketing surveillance studies: When analyzed as an API at the population level between the fourth quarter of 2011 and the second quarter of 2016, tapentadol had the lowest event rates of past 30-day abuse, in addition to the lowest rates of intentional abuse and diversion [10]. Butler et al. [7] found that much of the variability (60%) in the estimates of unadjusted abuse prevalence were explained by average quarterly prescription volume. Tapentadol currently has the lowest average quarterly prescriptions among the nine APIs that are prescribed in the United States (tapentadol, oxymorphone, hydromorphone, fentanyl, morphine, buprenorphine, tramadol, oxycodone, and hydrocodone) [7], and this may account for why the population rate of abuse remains among the lowest. Measures that have been adjusted for drug utilization are therefore critical to consider [25].
When adjusting the rate of ER abuse for drug utilization, tapentadol ER continued to have the lowest rate of abuse. When adjusted for drug utilization, ADF ER opioids were abused slightly more than non-ADF ER opioids. This difference decreased between 2014 and 2015 and increased somewhat between 2016 and 2017. Of note, ADF ER opioids accounted for 44.8% of the ER opioid prescriptions dispensed during the study period. Although this pattern of ADF ER opioid abuse should be monitored, recent studies suggest that, as a whole, ADFs likely have a beneficial effect [26–28].
This study was not designed to directly address whether the structure of the tapentadol molecule is the underlying reason for the present findings [1,2,5,29,30]. However, in the context of ER comparator rates of past 30-day abuse when adjusted for drug availability, there were only two of the 16 quarters where tapentadol ER did not have the absolute lowest rate of abuse. In Q4 2014 and Q4 2017, the CIs for tapentadol ER overlapped with the non-ADF ER opioid CIs. The rate of tapentadol ER abuse was significantly lower than the rate of abuse of ADF ER opioids in every quarter.
Analysis of routes of administration reveals that the majority of participants who reported tapentadol ER abuse and ADF ER opioid abuse swallowed tablets whole (58.70% and 53.81%, respectively). Swallowing tablets whole is recognized as the primary pathway of nonmedical use [31], and ADFs do not surmount this route of administration. Taking more opioids than prescribed, generally, or swallowing more tapentadol than prescribed can result in side effects and possibly death [4,5,28,32–35]. Symptoms such as drowsiness and respiratory depression have resulted from tapentadol exposure [32], whereas postmarketing safety studies report adverse reactions from supratherapeutic doses [4]. Even so, the toxicity of tapentadol has been characterized as less than conventional opioids [33].
The majority of those who abused non-ADF ER opioids reported injecting as the most common route of administration (42.85%), followed by snorting (38.95%). These data perhaps accentuate signs of accomplishment of one of the goals of employing ADFs: Abuse by alternate routes of administration, which carries greater risks than abuse by oral routes of administration [36], is reduced.
There are limitations to this study. The sample is not a randomized sample of all substance abusers, but rather a convenience sample of treatment-seeking individuals, with most living in the South and Midwest. Even though photos are shown of all drugs to ensure accurate drug identification and there are safeguards in place to ensure proper labeling, it is possible that some drugs are misidentified. Every effort is made to ensure proper understanding of the instrument question and proper handling of the data. The number of tapentadol ER abusers is a small percentage of the larger sample (N = 46, 0.02%). It is not yet clear whether this finding indicates that tapentadol ER is not prescribed as often as other opioids or, simply, that it is not of interest for abuse. Statistically, the size of the CI is related to sample size, so the smaller sample of tapentadol ER abusers leads to larger CIs of outcome measures and therefore less precise estimates. Continued surveillance with this medication will clarify this question.
In conclusion, tapentadol ER is primarily abused orally, although it is also abused by alternate routes of administration. Tapentadol ER was found to have lower rates of snorting and smoking than ADF and non-ADF ER comparators, whereas rates of swallowing whole, other oral, and injecting did not differ from those of ADF ER opioids. Tapentadol ER had lower rates of past 30-day abuse than ADF ER and non-ADF ER opioid comparators, both at a population level and when adjusted for drug utilization.
Funding sources: Funding for this research was provided initially by Depomed, Inc., and later continued by Collegium.
Conflicts of interest: JB, TDG, and JLG are employees of and SFB is a consultant to Inflexxion, an IBH Company. SKV is an independent scientific writer and consultant who contracts with multiple companies. Inflexxion contracts with the Food and Drug Administration and multiple companies with interests in some of the products included in the compounds evaluated for this article. Although the sponsor was involved in reviewing the content of this article, all data collection, analysis, and data interpretation were made by the authors without sponsor influence.
References
- 1. Tzschentke TM, Christoph T, Kogel BY.. The mu-opioid receptor agonist/noradrenaline reuptake inhibition (MOR-NRI) concept in analgesia: The case of tapentadol. CNS Drugs 2014;28(4):319–29. [DOI] [PubMed] [Google Scholar]
- 2. Tzschentke TM, Jahnel U, Kogel B, Christoph T, Englberger W, De Vry J.. Tapentadol hydrochloride: A next-generation, centrally acting analgesic with two mechanisms of action in a single molecule. Drugs Today 2009;45(7):483–96. [DOI] [PubMed] [Google Scholar]
- 3. Raffa RB, Elling C, Tzschentke TM.. Does ‘strong analgesic’ equal ‘strong opioid’? Tapentadol and the concept of ‘micro-load’. Adv Ther 2018;35(10):1471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stollenwerk A, Sohns M, Heisig F, Elling C, von Zabern D.. Review of post-marketing safety data on tapentadol, a centrally acting analgesic. Adv Ther 2018;35(1):12–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hartrick CT, Rozek RJ.. Tapentadol in pain management: A mu-opioid receptor agonist and noradrenaline reuptake inhibitor. CNS Drugs 2011;25(5):359–70. [DOI] [PubMed] [Google Scholar]
- 6. Murphy DL, Lebin JA, Severtson SG, Olsen HA, Dasgupta N, Dart RC.. Comparative rates of mortality and serious adverse effects among commonly prescribed opioid analgesics. Drug Saf 2018;41(8):787–95. [DOI] [PubMed] [Google Scholar]
- 7. Butler SF, McNaughton EC, Black RA.. Tapentadol abuse potential: A postmarketing evaluation using a sample of individuals evaluated for substance abuse treatment. Pain Med 2015;16(1):119–30. [DOI] [PubMed] [Google Scholar]
- 8. Dart RC, Cicero TJ, Surratt HL, Rosenblum A, Bartelson BB, Adams EH.. Assessment of the abuse of tapentadol immediate release: The first 24 months. J Opioid Manag 2012;8(6):395–402. [DOI] [PubMed] [Google Scholar]
- 9. Dart RC, Surratt HL, Le Lait MC, et al. Diversion and illicit sale of extended release tapentadol in the United States. Pain Med 2016;17(8):1490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vosburg SK, Severtson SG, Dart RC, et al. Assessment of tapentadol API abuse liability with the researched abuse, diversion and addiction-related surveillance system. J Pain 2018;19(4):439–53. [DOI] [PubMed] [Google Scholar]
- 11. McNaughton EC, Black RA, Weber SE, Butler SF.. Assessing abuse potential of new analgesic medications following market release: An evaluation of Internet discussion of tapentadol abuse. Pain Med 2015;16(1):131–40. [DOI] [PubMed] [Google Scholar]
- 12. Dart RC, Bartelson BB, Adams EH.. Nonmedical use of tapentadol immediate release by college students. Clin J Pain 2014;30(8):685–92. [DOI] [PubMed] [Google Scholar]
- 13. Butler SF, Budman SH, Licari A, et al. National Addictions Vigilance Intervention and Prevention Program (NAVIPPRO): A real-time, product-specific, public health surveillance system for monitoring prescription drug abuse. Pharmacoepidemiol Drug Saf 2008;17(12):1142–54. [DOI] [PubMed] [Google Scholar]
- 14. Butler SF, Budman SH, Goldman RJ, et al. Initial validation of a computer-administered addiction severity index: The ASI-MV. Psychol Addict Behav 2001;15(1):4–12. [DOI] [PubMed] [Google Scholar]
- 15. Hendriks VM, Kaplan CD, van Limbeek J, Geerlings P.. The Addiction Severity Index: Reliability and validity in a Dutch addict population. J Subst Abuse Treat 1989;6(2):133–41. [DOI] [PubMed] [Google Scholar]
- 16. Kosten TR, Rounsaville BJ, Kleber HD.. Concurrent validity of the Addiction Severity Index. J Nerv Ment Dis 1983;171(10):606–10. [DOI] [PubMed] [Google Scholar]
- 17. McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat 1992;9(3):199–213. [DOI] [PubMed] [Google Scholar]
- 18. Butler SF, Black RA, Fleming AB.. Relative abuse of crush-resistant prescription opioid tablets via alternative oral modes of administration. Pain Med 2018;19(8):1613–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Secora AM, Dormitzer CM, Staffa JA, Dal Pan GJ.. Measures to quantify the abuse of prescription opioids: A review of data sources and metrics. Pharmacoepidemiol Drug Saf 2014;23(12):1227–37. [DOI] [PubMed] [Google Scholar]
- 20. Comerci G Jr, Katzman J, Duhigg D.. Controlling the swing of the opioid pendulum. N Engl J Med 2018;378(8):691–3. [DOI] [PubMed] [Google Scholar]
- 21. Becker-Leckrone M, Lucas M, Start K, et al. Narrative symposium: Living with chronic pain in the midst of the opioid crisis. Narrat Inq Bioeth 2018;8(3):193–224. [DOI] [PubMed] [Google Scholar]
- 22. Huang CJ. On being the “right” kind of chronic pain patient. Narrat Inq Bioeth 2018;8(3):239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rieder TN. There's never just one side to the story: Why America must stop swinging the opioid pendulum. Narrat Inq Bioeth 2018;8(3):225–31. [DOI] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services. Pain Management Best Practices Inter-Agency Task Force Report: Updates, Gaps, Inconsistencies, and Recommendations. 2019. https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf
- 25. Secora A, Trinidad JP, Zhang R, Gill R, Dal Pan G.. Drug availability adjustments in population-based studies of prescription opioid abuse. Pharmacoepidemiol Drug Saf 2017;26(2):180–91. [DOI] [PubMed] [Google Scholar]
- 26. Kumar VM, Agboola F, Synnott PG, et al. Impact of Abuse deterrent formulations of opioids in patients with chronic Pain in the United States: A cost-effectiveness model. Value Health 2019;22(4):416–22. [DOI] [PubMed] [Google Scholar]
- 27. Yenikomshian MA, White AG, Carson ME, Jia ZB, Mendoza MR, Roland CL.. Modelling the potential impact of abuse-deterrent opioids on medical resource utilization. J Med Econ 2019;22(10):1073–9. [DOI] [PubMed] [Google Scholar]
- 28. Vietri J, Masters ET, Barsdorf AI, Mardekian J.. Cost of opioid medication abuse with and without tampering in the USA. Clinicoecon Outcomes Res 2018;10:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tzschentke TM, Christoph T, Kogel B, et al. (-)-(1R, 2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): A novel mu-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther 2007;323(1):265–76. [DOI] [PubMed] [Google Scholar]
- 30. Vadivelu N, Chang D, Helander EM, et al. Ketorolac, oxymorphone, tapentadol, and tramadol: A comprehensive review. Anesthesiol Clin 2017;35(2):e1–20. [DOI] [PubMed] [Google Scholar]
- 31. Ellis MS, Cicero TJ, Dart RC, Green JL.. Understanding multi-pill ingestion of prescription opioids: Prevalence, characteristics, and motivation. Pharmacoepidemiol Drug Saf 2019;28(1):117–21. [DOI] [PubMed] [Google Scholar]
- 32. Tsutaoka BT, Ho RY, Fung SM, Kearney TE.. Comparative toxicity of tapentadol and tramadol utilizing data reported to the National Poison Data System. Ann Pharmacother 2015;49(12):1311–6. [DOI] [PubMed] [Google Scholar]
- 33. Channell JS, Schug S.. Toxicity of tapentadol: A systematic review. Pain Manag 2018;8(5):327–39. [DOI] [PubMed] [Google Scholar]
- 34. Faria J, Barbosa J, Moreira R, Queirós O, Carvalho F, Dinis-Oliveira RJ.. Comparative pharmacology and toxicology of tramadol and tapentadol. Eur J Pain 2018;22(5):827–44. [DOI] [PubMed] [Google Scholar]
- 35. Barbosa J, Faria J, Queiros O, Moreira R, Carvalho F, Dinis-Oliveira RJ.. Comparative metabolism of tramadol and tapentadol: A toxicological perspective. Drug Metab Rev 2016;48(4):577–92. [DOI] [PubMed] [Google Scholar]
- 36. Green JL, Bucher Bartelson B, Le Lait MC, et al. Medical outcomes associated with prescription opioid abuse via oral and non-oral routes of administration. Drug Alcohol Depend 2017;175:140–5. [DOI] [PubMed] [Google Scholar]