Abstract
Aims
The hospitalization of patients with MI has decreased during global lockdown due to the COVID-19 pandemic. Whether this decrease is associated with more severe MI, e.g. MI-CS, is unknown. We aimed to examine the association of Corona virus disease (COVID-19) pandemic and incidence of acute myocardial infarction with cardiogenic shock (MI-CS).
Methods
On March 11, 2020, the Danish government announced national lock-down. Using Danish nationwide registries, we identified patients hospitalized with MI-CS. Incidence rates (IR) and incidence rate ratios (IRR) were used to compare MI-CS before and after March 11 in 2015–2019 and in 2020.
Results
We identified 11,769 patients with MI of whom 696 (5.9%) had cardiogenic shock in 2015–2019. In 2020, 2132 MI patients were identified of whom 119 had cardiogenic shock (5.6%). The IR per 100,000 person years before March 11 in 2015–2019 was 9.2 (95% CI: 8.3–10.2) and after 8.9 (95% CI: 8.0–9.9). In 2020, the IR was 7.5 (95% CI: 5.8–9.7) before March 11 and 7.7 (95% CI: 6.0–9.9) after. The IRRs comparing the 2020-period with the 2015–2019 period before and after March 11 (lockdown) were 0.81 (95% CI: 0.59–1.12) and 0.87 (95% CI: 0.57–1.32), respectively. The IRR comparing the 2020-period during and before lockdown was 1.02 (95% CI: 0.74–1.41). No difference in 7-day mortality or in-hospital management was observed between study periods.
Conclusion
We could not identify a significant association of the national lockdown on the incidence of MI-CS, along with similar in-hospital management and mortality in patients with MI-CS.
Keywords: Corona virus, COVID-19, Myocardial infarction, Cardiogenic shock, Incidence
Abbreviations: CABG, Coronary artery bypass grafting; CAG, Coronary angiography; COVID-19, Corona Virus disease; ECMO, Extra-corporeal membrane oxygenation; IABP, Intra-aortic balloon pump; ICD, International Classification of Diseases; MI, Acute myocardial infarction; MI-CS, Acute myocardial infarction-related cardiogenic shock; PCI, Percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction
1. Introduction
During the outbreak of Corona virus disease (COVID-19) and subsequent national lockdown, a concerning ~40% reduction in acute myocardial infarction (MI) has been reported [1], [2], [3], [4], along with a delay in presentation to hospital compared to before the COVID-19 era [5]. Professional societies have expressed concern that patients with relevant symptoms of MI do not present to the hospital system or that patients present too late. Hence, the COVID-19 pandemic lockdown has been associated with a lower incidence of MI hospitalizations, but whether this in turn is associated with more severe hemodynamic deranged MI, e.g. MI with cardiogenic shock (MI-CS), is unknown. The impact of the COVID-19 pandemic on incidence of MI-CS is very sparse documented in only one prior single-centre study from Hong Kong with a limited number of patients [6].
The leading cause of death in MI is cardiogenic shock with an in-hospital mortality reaching 40–50% [7], [8], [9]. The improved pre-hospital diagnosis and referral to immediate invasive revascularization has led to a decrease in incidence and mortality of cardiogenic shock during the last decade [7], [8]. Treatment recommendations for MI with and without cardiogenic shock is unaffected by the global lockdown [10], so potential aetiologies to the decrease in MI could be a misguided consideration to the health care system, a fear of contracting COVID-19 at hospital, or even a misdiagnosis of MI in the mist of COVID-19 [11]. Delayed or even missed diagnosis of MI leads to increased morbidity and mortality, hereunder a suspected increased risk of cardiogenic shock.
The aim of this study was to examine the incidence of MI-CS during the COVID-19 era (January 9–May 13, 2020) compared with a corresponding control period (January 8–May 13, 2015–2019).
2. Methods
2.1. Design and setting
In Denmark, the first COVID-19 positive patient was found February 27, 2020, and on March 11, the national government announced national lock-down in order to limit spread of infection (e.g. social distancing, closed borders, schools, day-cares, and workplaces). Moreover, the national health care system was re-organized with relocation of resources from non-critical functions to emergency rooms and intensive care units.
Using data from nationwide Danish registries [12], we conducted this nationwide cohort study. The National Health Services provides tax-supported healthcare with equal availability unaffected by socioeconomic status [12]. All Danish citizens are assigned a unique personal identifier code at birth or immigration, which allows cross linkage of data from registries at an individual level [13].
2.2. Study population and outcome
With use of the Danish Civil Population Registry we included the entire Danish population as the study population in a combined period from January 8 through May 13, 2015–2019 (control period) and in the COVID-19 era from January 9 through May 13, 2020. The calendar period was chosen based on 6 periods in 3-week intervals with 3 periods on each side of the lockdown date March 11. The accumulation of data within 3 weeks was necessary due to the small number of patients with MI-CS. We included 2015–2019 years as the control period since the number of patients with MI-CS in the year 2019 was markedly different compared with the 4 preceding years.
The Danish Civil Population Registry was established in 1968 and contains information on date of birth, sex, residence, immigration, and vital status, with daily updates [13]. The total length of follow-up was 126 days for every period, and due to leap year in 2020 the end dates differed between study periods. The study population consisted of persons alive and ≥18 years at index date (January 8, 2015–2019 and 2020) without a previous MI.
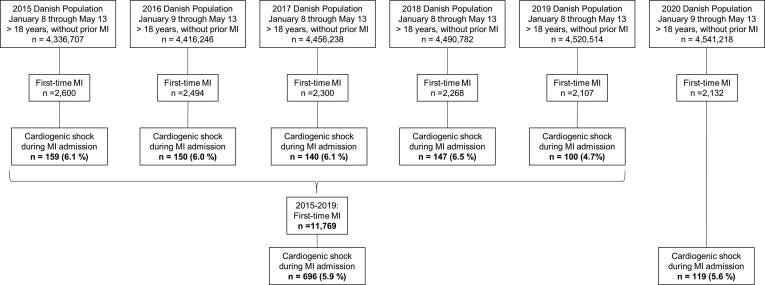
In order to identify the outcome MI-CS, we used the Danish National Patient Registry to identify all patients with a first-time hospitalization (as a measure of incidence) with MI [14]. We chose first-time to create homogenous cohorts. The Danish National Patient Registry contains data on all hospital admissions since 1977 and on all hospital outpatient specialist clinic and emergency room contacts since 1995. Each admission is assigned one primary diagnosis code and one or more secondary codes classified according to the International Classification of Diseases, 8th revision (ICD-8) until the end of 1993 and 10th revision (ICD-10) thereafter [14]. We used validated definitions of MI (positive predictive value: 97%) [15]. The admission with MI had to be at least 24 h, unless the patients died within first 24 h of admission. This was done to increase the specificity of the MI diagnosis. We used a partially validated definition of cardiogenic shock: death within first day of admission, a concurrent diagnosis code of cardiogenic shock and/or by any medical treatment with vasoactive drugs during the MI hospitalization [16]. Patients treated with vasoactive drugs, but without a diagnosis code for cardiogenic shock, were excluded if they had a diagnosis code for septic shock, hypovolemic shock, or shock without further specification during the admission. Moreover, if the need for vasoactive drugs only was in relation to a non-acute coronary artery bypass grafting (CABG) surgery, the patients was classified as MI without cardiogenic shock. in A flowchart of the cohorts is provided in Fig. 1. The cohorts were followed from date of entry until: MI-CS, death, or end of study periods, whichever came first.
Fig. 1.
Flow chart of study-population. The figure displays the selection of the study population. Abbreviations: CABG: Coronary artery bypass grafting surgery, CS: Cardiogenic shock, MI: Myocardial infarction. *Patients with vasoactive drugs only in relation to non-acute CABG: 2015: 36, 2016: 31, 2017: 18, 2018: n = 24, 2019: n = 19, and 2020: n = 29. †Patients excluded due to an ICD-10 code with septic shock, hypovolemic shock, or shock unspecified during MI admission: 2020: n = 2.
2.3. Covariates
Data on age, sex, and marital status was collected from the Danish Civil Registration System [13]. We obtained data on previous comorbidities from the Danish National Patient Registry using both primary and secondary in- and outpatient diagnosis codes (eTable 1 for ICD-8 and ICD-10 codes). We included congestive heart failure, peripheral vascular disease, stroke, chronic obstructive lung disease (COPD), hypertension, atrial fibrillation/flutter, chronic kidney disease, liver disease, diabetes, and cancer. Validated definitions of the comorbidities were used [15], [17]. Beside a diagnosis codes, diabetes was defined by any redeemed prescribed anti-diabetics and within 180 days before admission, and hypertension by ≥2 anti-hypertensive drugs. The Danish National Prescription Registry provided information on filled preadmission prescriptions within 180 days prior MI admission for beta blockers, calcium channel blockers, renin-angiotensin-aldosterone system inhibitors, loop-diuretics, lipid lowering drugs, anti-platelets, anti-coagulants, and anti-diabetics. Among others, we identified in-hospital procedure codes of coronary angiography (CAG), percutaneous coronary intervention (PCI), CABG, thrombolysis, and mechanical circulatory support from the Danish National Patient Registry. High validity has been reported for cardiac procedures (98–100%) [15]. All codes are provided in eTable 1.
2.4. Statistical analyses
We characterized patients according to sex, age, comorbidities, subtypes of MI, concomitant pharmacotherapy, and in-hospital procedures during lockdown (March 12–May 13, 2020) and in a corresponding period in 2015–2019 (March 12–May 13), along with a comparison before and after lockdown within each study period (before: January 8–March 11, after: March 12–May 13). Baseline characteristics were described as frequencies and percentages or medians with interquartile range as appropriate. Differences between periods were tested with use of χ2-test for categorical variables and Kruskal-Wallis test for continuous.
With MI-CS as the numerator and total MI patients as the denominator, we computed cumulative incidence proportions of patients with MI-CS in 3-week intervals from January 8/9-May 13, 2015–2019 and January 9–May 13, 2020. Ninety-five percent confidence intervals were estimated using Clopper-Pearson exact method.
Incidence rates (IR) for MI-CS were estimated in 3-week intervals from index date until end of study period. The IR were computed with person years as the denominator and MI-CS as the numerator. The 3-weeks rates for 2015–2019 were compared with the 3-week rates in 2020-rates as incidence rate ratios (IRR) using a Poisson regression model. In addition, we estimated IRRs comparing 2015–2019 with 2020 in complete periods before (January 8/9–March 11) and after (March 12–May 13) lockdown, respectively. Lastly, the period before and after lockdown in 2020 was compared.
We computed 7-day cumulative mortality for MI-CS patients during lockdown and the corresponding period in 2015–2019 with use of 1-Kaplan Meier functions. Differences in mortality were tested with a log-rank test between 2015 and 2019 and 2020 and before and after lockdown in 2020. Crude and adjusted logistic regression models were created comparing 7-day mortality among patients with MI-CS during lockdown in 2015–2019 and 2020 and comparing mortality in 2020 before and after lockdown. In the adjusted analyses we included: groups of age (<50, 50–59, 60–69, 70–79, 80<), sex, diabetes, hypertension, and chronic renal disease. A P value <0.05 was considered statistically significant. The analyses were performed using SAS version 9.4 and R version 3.5.1.
2.5. Ethics approval
This study complies with the Declaration of Helsinki. Observational register studies do not require ethical permission in Denmark. The use of data for the study was approved by the Danish Data Protection Agency (Approval number: P-2019-191).
3. Results
3.1. Patients characteristics
We identified 11,769 first-time MI patients in the study period 2015–2019 of whom 696 (5.9%) had cardiogenic shock (Fig. 1). In comparison, 2132 first time MI patients were identified in the 2020-period of whom 119 had cardiogenic shock (5.6%). No differences were observed concerning age, sex, co-morbidities, subtypes of MI, or prior prescribed medication between patients with MI-CS during lockdown in 2015–2019 and 2020 (Table 1). The baseline characteristics were also similar in in periods before and after lockdown in 2015–2019 and 2020, respectively (eTable 2).
Table 1.
Baseline characteristics for patients with first-time acute myocardial infarction-related cardiogenic shock during global lockdown, by calendar period.
| 2015–2019 March 12–May 13 n (%) |
2020 March 12–May 13 n (%) |
P value | |
|---|---|---|---|
| Total | 342 (1 0 0) | 60 (1 0 0) | |
| Male gender | 257 (75) | 43 (71) | 0.68 |
| Median age, years [IQR] | 70 [60–77] | 69 [62–76] | 0.82 |
| Comorbidities | |||
| Heart failure | 23 (7) | 4 (7) | 1.00 |
| Peripheral vascular disease | 29 (9) | 4 (7) | 0.82 |
| Stroke | 30 (9) | 3 (5) | 0.46 |
| Chronic obstructive lung disease | 29 (9) | 4 (7) | 0.83 |
| Hypertension* | 153 (45) | 26 (43) | 0.95 |
| Atrial fibrillation/flutter | 25 (7) | 6 (10) | 0.65 |
| Chronic kidney disease | 29 (9) | <3 | >0.05 |
| Liver disease | 15 (4) | 5 (8) | 0.33 |
| Diabetes† | 77 (23) | 10 (17) | 0.40 |
| Cancer | 41 (12) | 8 (13) | 0.94 |
| Subtypes of MI | |||
| STEMI | 152 (44) | 28 (47) | 0.86 |
| NSTEMI | 59 (17) | 9 (15) | 0.81 |
| Unspecified | 133 (39) | 23 (38) | |
| Medication | |||
| Beta blockade | 75 (22) | 10 (17) | 0.45 |
| Ca-channel blocker | 68 (20) | 13 (22) | 0.89 |
| RASi | 131 (38) | 19 (32) | 0.40 |
| Loop-diuretics | 38 (11) | 4 (7) | 0.42 |
| Lipid lowering drugs | 115 (34) | 17 (28) | 0.51 |
| Anti-platelets‡ | 90 (26) | 14 (23) | 0.58 |
| Anti-coagulants | 25 (7) | 8 (13) | 0.19 |
| Anti-diabetics | 64 (19) | 9 (15) | 0.61 |
Abbreviations: ADPi: Adenosine diphosphate inhibitor, COPD: Chronic obstructive pulmonary disease, IQR: Inter quartile range, NSTEMI: Non-ST segment elevation myocardial infarction, STEMI: ST segment elevation myocardial infarction, RASi: Renin-angiotensin system inhibitor.
Defined as redemption of ≥2 antihypertensive drugs 180 days before MI admission.
Defined as an ICD-10 code with diabetes or any redeemed prescribed anti-diabetic drug 180 days before MI admission.
Anti-platelets: Aspirin, adenosine-di-phosphate inhibitors, dipyridamole.
3.2. Incidence of MI with cardiogenic shock
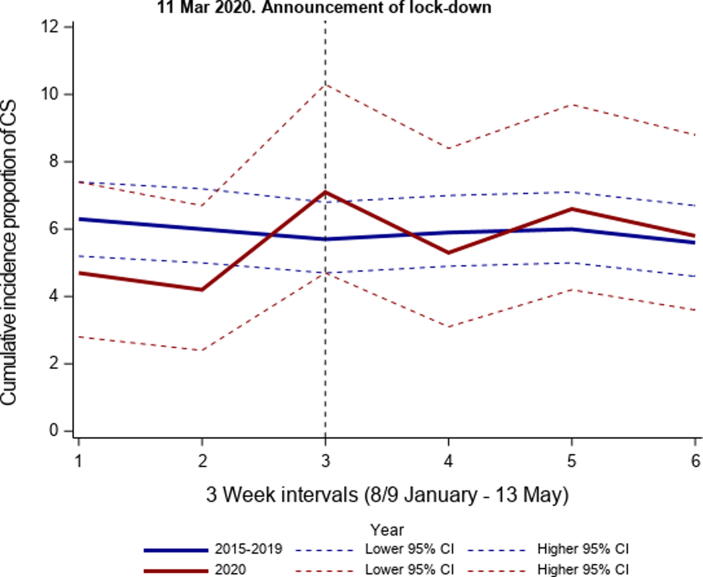
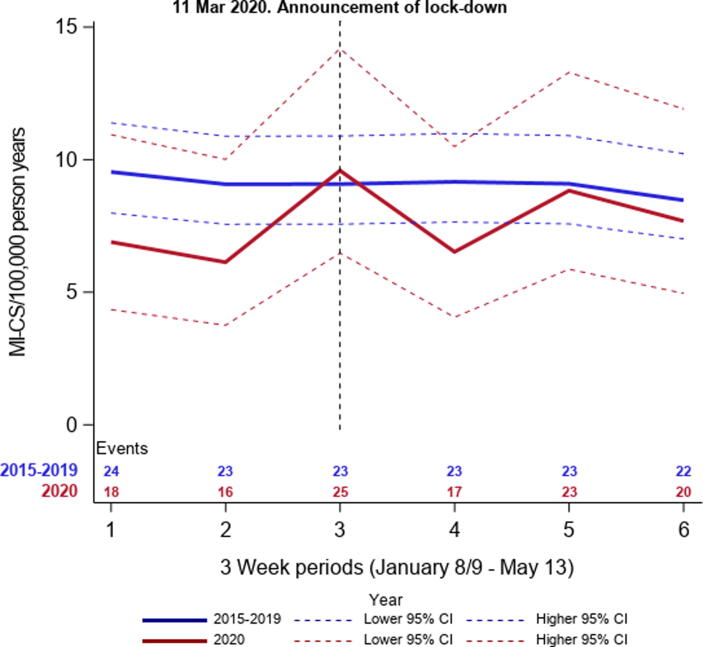
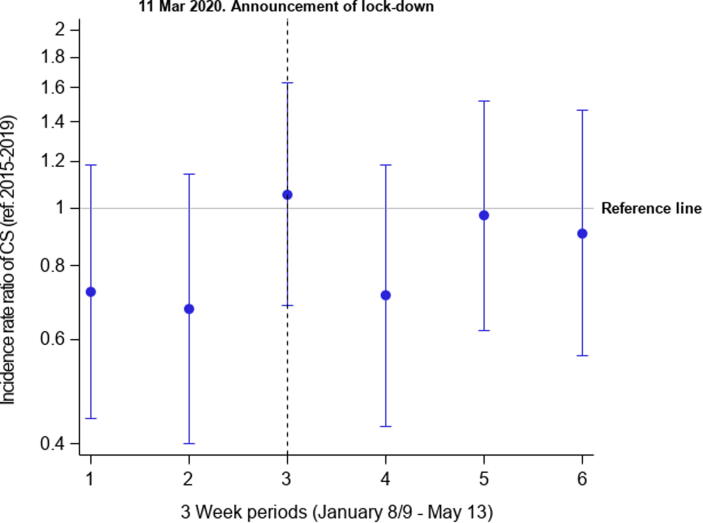
The total number of MI patients decreased with 15% during lockdown comparing the average number of MI admission in 2015–2019 (n = 1171) with the number of MI in 2020 (n = 996). The incidence proportions of MI-CS were similar during lockdown comparing 2015–2019 and 2020 (5.8% (95% CI: 5.2–6.5) vs. 5.9% (95% CI: 4.6–7.6)), and no difference was observed over time in the 2015–2019 and 2020 periods (Fig. 2). The total number of patients with MI-CS, person years, and the following IR in 3-week intervals are presented in Fig. 3 (eTable 3). No differences in IR over time was observed comparing 2015–2019 and 2020. The IR per 100,000 person years in 2015–2019 before lockdown (January 8–March 11) was 9.2 (95% CI: 8.3–10.2) and after lockdown (March 12–May 13) 8.9 (95% CI: 8.0–9.9). In 2020, the IRs before and after lockdown were 7.5 (95% CI: 5.8–9.7) and 7.7 (95% CI:6.0–9.9). In the regression analyses, the corresponding IRRs were 0.81 (95% CI: 0.0.59–1.12) and 0.87 (95% CI: 0.57–1.32) for periods before and after lockdown comparing 2020 with 2015–2019. The IRRs for the complete period from 8 January through May 13 per 3-week comparing 2020 with 2015–2019 are displayed in Fig. 4. The IRR comparing before and after lockdown in 2020 was 1.02 (95% CI: 0.74–1.41).
Fig. 2.
Cumulative incidence proportions of MI-CS, by study years. The figure presents the cumulative incidence proportion of first time MI-CS of the total number of MI per 21st days from January 8–May 13, 2015–2019 and January 9–May 13, 2020. MICS: Myocardial infarction-related cardiogenic shock.
Fig. 3.
Incidence rates as numbers of MI-CS per 100,000 person years, by study years. displays the incidence rates of MI-CS per 21st days from January 8–May 13, 2015–2019 and January 9–May 13, 2020. MICS: Myocardial infarction-related cardiogenic shock.
Fig. 4.
Incidence rate ratios of MI-CS in 2020 compared with 2015–2019. The figure shows the incidence rate ratios per 21st days in 2020 compared with 2015–2019. MICS: Myocardial infarction-related cardiogenic shock.
3.3. Seven-day mortality during national lockdown
In unadjusted analyses, we observed a higher 7-day mortality during lockdown in 2020 compared with 2015–2019 (45% (95% CI: 32–58) vs. 36%, (95% CI: 31–41)) and the same was observed comparing the periods before and after lockdown in 2020 (34% (95% CI: 22–46) vs. 45% (95% CI: 32–58). However, in the age, sex, and comorbidity adjusted analysis comparing 2020 with 2015–2019 during lockdown the mortality was similar (OR: 1.04 (95% CI: 0.68–1.59), and the same result was seen in the analysis comparing before and after lockdown in 2020 (OR: 1.56 (95% CI: 0.76–3.34)).
3.4. In-hospital procedures
The proportion of patients who underwent CAG, PCI, CABG, and extra corporeal membrane oxygenation (ECMO) were similar between 2015 and 2019 and 2020 during lockdown (p > 0.05) (Table 2). Although limited by small numbers, the use of left ventricular assist device seemed lower in 2020 compared with 2015–2020 during lockdown, however, it did not differ before and after lockdown in 2020 (eTable 4). The use of thrombolysis was non-existent in all three years, along with the use of intra-aortic balloon pump (IABP). The results were similar comparing the periods before and after lockdown in 2020 (eTable 4).
Table 2.
In-hospital procedures in myocardial infarction-related cardiogenic shock during national lockdown, by calendar period.
| 2015–2019 March 12–May 13 n (%) |
2020 March 12–May 13 n (%) |
P value | |
|---|---|---|---|
| Total | 342 (1 0 0) | 60 (1 0 0) | |
| Revascularization | |||
| Coronary angiography | 239 (70) | 43 (72) | 0.91 |
| PCI | 208 (61) | 37 (62) | 1.0 |
| CABG | 23 (7) | 4 (7) | 1.0 |
| Thrombolysis | <3 | 0 (0.0) | >0.05 |
| Mechanical circulatory support | |||
| IABP* | <3 | 0 (0.0) | >0.05- |
| Left ventricular assist device | 44 (13) | <3 | <0.05 |
| ECMO | 26 (8) | 4 (7) | 1.0 |
| Intensive care | |||
| Renal replacement therapy | 48 (14) | 8 (13) | 1.0 |
| Mechanical ventilation | 261 (76) | 38 (63) | 0.05 |
Abbreviations: CABG: Coronary artery bypass grafting surgery, ECMO: Extra corporeal membrane oxygenation, IABP: Intra-aortic balloon pump, PCI: Percutaneous coronary intervention.
Due to the data permissions we are not allowed to report <3 observation.
4. Discussion
Despite a decrease in MI overall, the incidence of MI-CS was not associated with the national lockdown during the COVID-19 era compared with a corresponding calendar period from 2015 to 2019. The 7-day mortality and in-hospital procedures in patients with MI-CS were similar between the 2015–2019 period and the 2020-period, and before and after lockdown in 2020.
On February 27 the first Danish citizen was diagnosed with COVID-19, and on March 11 the national government introduced nationwide lockdown of the Danish society with closed schools, day-cares, public workplaces, social distancing, and March 15 the borders were closed. Along with these initiatives in order to limit the infection of the COVID-19, the health care system was reorganized to obtain resources for the management of COVID-19 patients. Non-essential visits were postponed, and emergency rooms and intensive care units were upgraded. Still, in Denmark the resources in the health care systems was enough, and especially were the intensive care units never near maximum capacity.
It was speculated that the increased level of physiological stress could lead to an increase in MI [18], along with increased risk of viral induced MI[19] and peri-myocarditis mimicking the course of an MI [20], [21]. In addition, recent reports indicated that COVID-19 infection was associated with increased thromboembolic risk [22], hereunder myocardial infarction, which would affect MI incidence further during the COVID-19 pandemic. Nevertheless, a 30–40% reduction in the incidence of MI was observed worldwide by reports from the U.S, Spain, Denmark, Italy and Hong Kong [6], [1], [2], [3], [4]. The decrease was observed for both patients with STEMI and NSTEMI with the largest decrease among patients with NSTEMI [4].
In addition, a recent single centre study from study from Hong Kong observed a marked delay in the time from symptom onset until seeking medical aid among 7 ST-segment elevation myocardial infarction patients emphasizing the changed behaviour among MI patients [5]. Due to potential missed or delayed diagnosis and treatment of MI, one would expect more extensive myocardial damage with more compromised hemodynamic status, and thus higher risk of cardiogenic shock and mortality, among MI patients during the COVID-19 pandemic. Based on data from a single tertiary cardiac centre in Hong Kong limited by a few number of observations (n = 149), the cumulative incidence proportion of MI-CS before and after the outbreak of COVID-19 did not differ (8% vs. 13%, p = 0.42) [6]. In a multicentre Italian study with data from intensive care units, the rate of MI with severe complications, including MI-CS, increased in the first week after lockdown [4]. The incidence of cardiogenic shock in both the 2015–2019 and 2020-period in this study was consistent with previous studies (3–7%) [7], [8], [23], [24], and current observations suggest a decreasing trend in MI-CS incidence during latest years [7], [9], [23]. The incidence proportions of MI-CS in 2019 and 2020 was lower compared with previous years, however, no consistent trend in MI-CS incidence was observed. Still, we observed no difference in the incidence of MI-CS during national lockdown in 2020 compared with incidence of cardiogenic shock before lockdown in 2020 or compared with a corresponding calendar period 2015–2019, despite the decrease in MI overall. Neither was any difference in subtypes of MI, e.g. STEMI and NSTEMI, observed comparing lockdown with non-lockdown.
Based on the marked reduction in incidence of MI during COVID-19 pandemic [6], [1], [2], [3], [4], we hypothesized that the incidence of MI-CS would increase as a result of missed or delayed treatment, thus, prolonged ischemia and progressively left-ventricular dysfunction. A concern exists whether the unaffected incidence of MI-CS could be due to misdiagnosis of cardiogenic shock when all focus is diverted towards the COVID-19 disease [11]. Cardiovascular manifestations of COVID-19 is acute heart failure, myocarditis, arrythmias, and elevated troponins, and along with severe systemic inflammation, multi-organ dysfunction, and acute respiratory distress this course mimics MI-CS [21].
In a recent study from Hong Kong comparing patients with MI before and after COVID-19 outbreak, no difference in MI-related in-hospital mortality was observed (6% vs 13%. P = 0.24) [6]. However, the results were based on a limited number of observations. On the contrary, an Italian study observed a marked increase in MI mortality comparing one week after lockdown in 2020 with 2019 (relative risk: 3.6 (2.0–6.4)) [4]. In this study, 7-day absolute mortality in patients with MI-CS was higher in the lockdown period compared before lockdown in 2020 and the corresponding period in 2015–2019. However, the results were similar in the 2015–2019 period and the 2020-period when age, sex, and comorbidities were accounted for. Few studies reported 7-day mortality, however, the results of this study are comparable with current evidence [7], [9], [23], [25].
As a result of the decrease in MI, a recent study reported a 40% reduction in PCI procedures and a small increase in use of thrombolysis among MI patients [1]. During the COVID-19 pandemic, the early revascularization remains the standard recommendations for the treatment of acute MI whenever possible [10], [26]. The management of patients with MI-CS was similar in the 2020-period compared with the 2015–2019-period, and the proportion of patients with MI and cardiogenic shock who underwent CAG and PCI were consistent with previous reporting from MI-CS cohorts [7], [9], [23], [27]. We have no knowledge on potential delays from symptom onset to medical contact or in-hospital timing of revascularization in this study.
4.1. Strengths and limitations
The study data were obtained from nationwide registries in a country with universal and free tax-supported access to health care, unaffected by socioeconomic status or insurance, thus, minimizing the potential of selection bias [12]. Moreover, the registries provide complete and long-term follow-up [13]. The diagnosis codes for MI, CS, comorbidities, and the procedure code of inotropes/vasopressors have been validated with high positive predictive values [15], [16], [17].
Due to the definition of CS based upon need for inotropes/vasopressors in MI patients, we cannot exclude misclassification of pressor-stabilized OHCA patients without manifest CS in the study population. However, at time of hospital arrival these patients present with similar clinical parameters [28] and therefore immediate indistinguishable, why the results of this study reflect the real life setting.
We cannot exclude unmeasured or residual confounding due to the observational design. Uncomplicated cases of hypertension and diabetes are treated only by general practitioners and we may lack completeness of these data; though, we sought to increase completeness of diabetes by adding information on filled prescriptions for anti-diabetic drugs. Additionally, we lacked clinical data such as lactate levels, creatinine clearance, ejection fraction, out-of-hospital-cardiac-arrest status, in addition to complete data on subtypes of MI (STEMI and NSTEMI).
5. Clinical perspectives
A concern exists whether the delay in treatment and decrease in incidence of acute MI have led to increased mortality and morbidity, however, this study did not observe any differences in incidence of cardiogenic shock during the COVID-19 pandemic compared with the 2015–2019 period. The results of this study may be generalizable to other Western countries with similar health care system and guideline-based approach to the management of MI-CS. However, it must be emphasized, that Denmark was a low incidence COVID-19 country, and the Danish health care system was not at any time in threat of break down. In the blur of the COVID-19 pandemic, the modern health care system needs to continuously detect and treat other acute medical conditions.
6. Conclusion
We could not identify a significant association of the national lockdown on the incidence of MI with cardiogenic shock, along with similar in-hospital management and mortality in patients with MI and cardiogenic shock before and after lockdown. Our study does not support the fear that the reduction in MI incidence during the pandemic lockdown translates into worse cases of MI in the Danish context.
Contributorship of authors
MD, JB, LØ, TG, CTP, GG, LK, and EF conceived the study idea and de-signed the study. MD, JB, LØ, and EF established and designed the cohort. The analyses were carried out by MD, JB, LØ, and EF. All authors participated in the discussion and interpretation of the results. MD organized the writing and wrote the initial drafts. All authors critically analysed the results and revised the manuscript for content and approved the final version. EF is the guarantor.
Funding
This work was supported by Rigshospitalets Research Foundation, Master cabinetmaker Sophus Jacobsen and Wife Astrid Jacobsen Foundation, and Director Jakob Madsen and Wife Olga Madsen Foundation (all to MDL). The funding sources had no role in the design, conduct, analysis, or reporting of the study.
Disclosures
Dr. Møller reports personal fees from Abiomed, outside the submitted work;
Dr. Hassager reports personal fees from Abiomed and grant from Lundbeck Foundation (R186-2015-2132), outside the submitted work;
Dr. Torp-Pedersen reports grants from Bayer and Novo Nordisk, outside the submitted work;
Dr. Køber reports personal fees from Novartis, BMS, and AstraZeneca, outside the submitted work;
All other authors have nothing to disclose.
Footnotes
All author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100659.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Rodríguez-Leor O., Cid-Alavrez B., Soledad O., Martin-Moreiras M., Rumoroso J.R., Lopez-Palop R., Serrador A., Cequier A., Romanguera R., Cruz I., Perez de Prado A., Moreno R. Impacto de la pandemia de COVID-19 sobre la actividad asistencial en cardiología ntervencionista en España. REC Interv. Cardiol. 2020;2:82–89. [Google Scholar]
- 2.Garcia S., Albaghdadi M.S., Meraj P.M., Schmidt C., Garberich R., Jaffer F.A., Dixon S., Rade J.J., Tannenbaum M., Chambers J., Huang P.P., Henry T.D. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flensted Lassen J., Carl-Johan J., Winther Jensen J., Cerqueira C., Plambeck Hansen C., Storm L., Nakano Hansen A. Aktivitet På Hjerteområdet under COVID-19 Epidemien Særrapport Fra RKKP I Samarbejde Med Vestdansk Hjertedatabase Og. Dansk Hjerteregister. 2020 [Google Scholar]
- 4.De Rosa S., Spaccarotella C., Basso C., Calabrò M.P., Curcio A., Filardi P.P., Mancone M., Mercuro G., Muscoli S., Nodari S., Pedrinelli R., Sinagra G., Indolfi C. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur. Heart J. 2020;41(22):2083–2088. doi: 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tam C.-C.F., Cheung K.-S., Lam S., Wong A., Yung A., Sze M., Lam Y.-M., Chan C., Tsang T.-C., Tsui M., Tse H.-F., Siu C.-W. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circ. Cardiovasc. Qual. Outcomes. 2020 doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tam C.-C.F., Cheung K.-S., Lam S., Wong A., Yung A., Sze M., Fang J., Tse H.-F., Siu C.-W. Impact of coronavirus disease 2019 (COVID-19) outbreak on outcome of myocardial infarction in Hong Kong, China. Catheter Cardiovasc. Interv. 2020 doi: 10.1002/ccd.28943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunziker L., Radovanovic D., Jeger R., Pedrazzini G., Cuculi F., Urban P., Erne P., Rickli H., Pilgrim T., Hess F., Simon R., Hangartner P.J., Hufschmid U., Hornig B., Altwegg L., Trummler S., Windecker S., Rueff T., Loretan P., Roethlisberger C., Evéquoz D., Mang G., Ryser D., Müller P., Jecker R., Kistler W., Hongler T., Stäuble S., Freiwald G., Schmid H.P., Stauffer J.C., Cook S., Bietenhard K., Roffi M., Wojtyna W., Schönenberger R., Simonin C., Waldburger R., Schmidli M., Federspiel B., Weiss E.M., Marty H., Weber K., Zender H., Poepping I., Hugi A., Koltai E., Iglesias J.F., Erne P., Heimes T., Jordan B., Pagnamenta A., Feraud P., Beretta E., Stettler C., Repond F., Widmer F., Heimgartner C., Polikar R., Bassetti S., Iselin H.U., Giger M., Egger P., Kaeslin T., Fischer A., Herren T., Eichhorn P., Neumeier C., Flury G., Girod G., Vogel R., Niggli B., Yoon S., Nossen J., Stoller U., Veragut U.P., Bächli E., Weber A., Schmidt D., Hellermann J., Eriksson U., Fischer T., Peter M., Gasser S., Fatio R., Vogt M., Ramsay D., Wyss C., Bertel O., Maggiorini M., Eberli F., Christen S. Twenty-year trends in the incidence and outcome of cardiogenic shock in AMIS plus registry. Circ. Cardiovasc. Interv. 2019;12(4):e007293. doi: 10.1161/CIRCINTERVENTIONS.118.007293. [DOI] [PubMed] [Google Scholar]
- 8.Helgestad O.K.L., Josiassen J., Hassager C., Jensen L.O., Holmvang L., Sørensen A., Frydland M., Lassen A.T., Udesen N.L.J., Schmidt H., Ravn H.B., Møller J.E. Temporal trends in incidence and patient characteristics in cardiogenic shock following acute myocardial infarction from 2010 to 2017: a Danish cohort study. Eur. J. Heart Fail. 2019;21(11):1370–1378. doi: 10.1002/ejhf.1566. [DOI] [PubMed] [Google Scholar]
- 9.Redfors B., Angerås O., Råmunddal T., Dworeck C., Haraldsson I., Ioanes D., Petursson P., Libungan B., Odenstedt J., Stewart J., Lodin E., Wahlin M., Albertsson P., Matejka G., Omerovic E. 17-year trends in incidence and prognosis of cardiogenic shock in patients with acute myocardial infarction in western Sweden. Int. J. Cardiol. 2015;2015(185):256–262. doi: 10.1016/j.ijcard.2015.03.106. [DOI] [PubMed] [Google Scholar]
- 10.Welt F.G.P., Shah P.B., Aronow H.D., Bortnick A.E., Henry T.D., Sherwood M.W., Young M.N., Davidson L.J., Kadavath S., Mahmud E., Kirtane A.J. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: from ACC’s interventional council and SCAI. J. Am. Coll. Cardiol. 2020;75(18):2372–2375. doi: 10.1016/j.jacc.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousefzai R., Bhimaraj A. Misdiagnosis in the COVID era: when zebras are everywhere, don’t forget the horses. JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt M., Schmidt S.A.J., Adelborg K. The Danish healthcare system and epidemiological research: from healthcare contacts to database records. Clin. Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt M., Pedersen L., Sorensen H.T. The Danish civil registration system as a tool in epidemiology. Eur. J. Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M., Schmidt S.A.J., Sandegaard J.L., Ehrenstein V., Pedersen L., Sorensen H.T. The danish national patient registry: a review of content, data quality, and research potential. Clin. Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundboll J., Adelborg K., Munch T., Froslev T., Sorensen H.T., Botker H.E., Schmidt M. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6(11):e012832. doi: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauridsen M.D., Gammelager H., Schmidt M., Nielsen H., Christiansen C.F. Positive predictive value of International Classification of Diseases, 10th revision, diagnosis codes for cardiogenic, hypovolemic, and septic shock in the Danish National Patient Registry. BMC Med. Res. Method. 2015;15:23. doi: 10.1186/s12874-015-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thygesen S.K., Christiansen C.F., Christensen S., Lash T.L., Sorensen H.T. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med. Res. Method. 2011;11:83. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moller J., Theorell T., de Faire U., Ahlbom A., Hallqvist J. Work related stressful life events and the risk of myocardial infarction. Case-control and case-crossover analyses within the Stockholm heart epidemiology programme (SHEEP) J. Epidemiol. Commun. Health. 2005;59(1):23–30. doi: 10.1136/jech.2003.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwong J.C., Schwartz K.L., Campitelli M.A., Chung H., Crowcroft N.S., Karnauchow T., Katz K., Ko D.T., McGeer A.J., McNally D., Richardson D.C., Rosella L.C., Simor A., Smieja M., Zahariadis G., Gubbay J.B. Acute myocardial infarction after laboratory-confirmed influenza infection. N. Engl. J. Med. 2018;378(4):345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 20.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A., Sepe P.A., Resasco T., Camporotondo R., Bruno R., Baldanti F., Paolucci S., Pelenghi S., Iotti G.A., Mojoli F., Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aissaoui N., Puymirat E., Delmas C., Ortuno S., Durand E., Bataille V., Drouet E., Bonello L., Bonnefoy-Cudraz E., Lesmeles G., Guerot E., Schiele F., Simon T., Danchin N. Trends in cardiogenic shock complicating acute myocardial infarction. Eur. J. Heart Fail. 2020;22(4):664–672. doi: 10.1002/ejhf.1750. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen H.L., Yarzebski J., Lessard D., Gore J.M., McManus D.D., Goldberg R.J. Year (2001–2011) Trends in the incidence rates and short-term outcomes of early versus late onset cardiogenic shock after hospitalization for acute myocardial infarction. J. Am. Heart Assoc. 2017;6(6) doi: 10.1161/JAHA.117.005566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostenfeld S., Lindholm M.G., Kjaergaard J., Bro-Jeppesen J., Moller J.E., Wanscher M., Hassager C. Prognostic implication of out-of-hospital cardiac arrest in patients with cardiogenic shock and acute myocardial infarction. Resuscitation. 2015;87:57–62. doi: 10.1016/j.resuscitation.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Mahmud E., Dauerman H.L., Welt F.G., Messenger J.C., Rao S.V., Grines C., Mattu A., Kirtane A.J., Jauhar R., Meraj P., Rokos I.C., Rumsfeld J.S., Henry T.D. Management of acute myocardial infarction during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awad H.H., Anderson F.A., Gore J.M., Goodman S.G., Goldberg R.J. Cardiogenic shock complicating acute coronary syndromes: insights from the global registry of acute coronary events. Am. Heart J. 2012;163(6):963–971. doi: 10.1016/j.ahj.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 28.J. Josiassen, O.K.L. Helgestad, J.E. Møller, J. Kjaergaard, H. Hoejgaard, H. Schmidt, L.O. Jensen, L. Holmvang, H.B. Ravn, C. Hassager, Cardiogenic shock patients: those with and those without out-of-hospital-cardiac arrest are different clinical entities. In: Poster at ESC Congress 2019, Session 2, Acute Cardiac Care - Cardiogenic Shock. Paris; 2019. https://esc365.escardio.org/Congress/ESC-CONGRESS-2019/Poster-Session-2-Cardiogenic-shock/198469-cardiogenic-shock-patients-those-with-and-those-without-out-of-hospital-cardiac-arrest-are-different-clinical-entities.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.