Abstract
Objectives
To examine whether patients with multisystem inflammatory syndrome in children (MIS-C) demonstrated well-defined clinical features distinct from other febrile outpatients, given the difficulties of seeing acute care visits during the severe acute respiratory syndrome coronavirus 2 pandemic and the risks associated with both over- and underdiagnosis of MIS-C.
Study design
This case-controlled study compared patients diagnosed with and treated for MIS-C at a large urban children's hospital with patients evaluated for fever at outpatient acute care visits during the peak period of MIS-C. Symptomatology and available objective data were extracted. Comparisons were performed using t tests with corrections for multiple comparisons, and multivariable logistic regression to obtain ORs.
Results
We identified 44 patients with MIS-C between April 16 and June 10, 2020. During the same period, 181 pediatric patients were evaluated for febrile illnesses in participating outpatient clinics. Patients with MIS-C reported greater median maximum reported temperature height (40°C vs 38.9, P < .0001), and increased frequency of abdominal pain (OR 12.5, 95% CI [1.65-33.24]), neck pain (536.5, [2.23-129,029]), conjunctivitis (31.3, [4.6-212.8]), oral mucosal irritation (11.8, [1.4-99.4]), extremity swelling or rash (99.9, [5-1960]), and generalized rash (7.42, [1.6-33.2]). Patients with MIS-C demonstrated lower absolute lymphocyte (P < .0001) and platelet counts (P < .05) and greater C-reactive protein concentrations (P < .001).
Conclusions
Patients treated for MIS-C due to concern for potential cardiac injury show combinations of features distinct from other febrile patients seen in outpatient clinics during the same period.
Keywords: multisystem inflammatory syndrome in children, SARS-CoV-2, Kawasaki disease, myocarditis
Abbreviations: ED, Emergency department; MIS-C, Multisystem inflammatory syndrome in children; KD, Kawasaki disease; ntBNP, N-terminal brain natriuretic peptide; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Multisystem inflammatory syndrome (MIS-C) is a novel inflammatory disease that emerged worldwide between April and May of 2020.1, 2, 3, 4, 5 Characterized by fever and a broad array of signs and symptoms and frequently presenting with shock, a major morbidity associated with MIS-C is cardiac injury with cardiac dysfunction and occasionally with aneurysms.6 , 7 MIS-C has been temporally linked with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and appears to be related to a dysregulated immunologic response to this infection that occurs primarily in children.8
Critical questions and challenges have emerged since the first descriptive reports of MIS-C. First, we do not know if this is the “tip of the iceberg”9 and if admitted cases to date represent a small, but severe, fraction of a similar phenotype that was observed in the outpatient setting. Second, we are confronted with the difficulty of discriminating patients with MIS-C requiring early referral for cardiac evaluation from the broad number of children coming to medical attention to primary care and other outpatient settings because of fever. Further amplifying the challenge, patients with MIS-C have presented with and without shock, and with and without features overlapping with Kawasaki disease (KD) and toxic shock syndrome. Neither the presence of shock nor KD features have been helpful in predicting cardiac complications, including aneurysms, cardiac inflammation, and mechanical dysfunction.5 , 7 , 9 In addition, with the expansion of telehealth, the limited hours and capacity in pediatricians' offices, and the reluctance of families to seek in-person visits in offices and emergency departments (EDs), pediatricians often are evaluating patients remotely with fractured care from other urgent care or telemedicine services.
In recognition of these challenges, we analyzed outpatient records to determine whether clinical presentations of patients admitted and treated for MIS-C were similar or distinguishable from other febrile patients in our outpatient clinics. Given the broad nature of some diagnostic guidelines for MIS-C, our intention was to seek “red-flags” to indicate need for requiring further referral and evaluation.
Methods
We retrospectively reviewed the charts of patients with MIS-C presenting to a tertiary care hospital during the peak of MIS-C cases and all sick-visit encounters for febrile patients at a subset of affiliated pediatric primary care clinics during the same period. Study staff reviewed each encounter of patients meeting inclusion criteria related to the febrile episode of interest.
To construct the MIS-C cohort, we reviewed the records of patients reported as possible MIS-C to the New York City Department of Health by our institution from April 16 to June 10, 2020.1 Patients with MIS-C were then defined as patients who were treated with corticosteroid and intravenous immunoglobulin after consulting rheumatologists and infectious disease physicians determined cases as highly concerning for MIS-C. We identified patients with KD who were assessed by the same consulting services at our institution, who were determined, based on American Heart Association 2017 guidelines10 and clinical judgment, to have focused symptoms only consistent with KD; these cases were excluded from most analysis. Our analysis focuses on symptoms reported at presentation for patients with MIS-C, and although outpatient records were not available for MIS-C cases, pertinent information from ED documentation was reviewed and used.
The febrile outpatient cohort was constructed using a comprehensive list of all acute outpatient visits to participating hospital-affiliated primary care clinics during the same time period. Patients were included if they were brought for evaluation of a febrile illness. Because no patients with MIS-C were infants, patients younger than 12 months of age were excluded. A subset of the febrile outpatients also was evaluated after clinic visit in our ED for the same febrile illness. These encounters were included as both part of the larger febrile outpatient group and as a separate subset (“ED group”) for some analyses. Three children in the febrile outpatient group had more than 1 febrile episode during the study period, with documented periods of wellness in between; for ease of interpretation, only the final febrile episode was included in the main analysis. All reviewed encounters included demographic data, reported symptomatology over the course of their illness up to the point of presentation, findings on examination, results of basic laboratory tests, suspected or confirmed exposure to SARS-CoV-2, and final documented diagnosis.
Historical clinical variables that were not documented as being present by providers were presumed negative. For purposes of analysis, each patient's febrile episode was represented as one clinical encounter. For patients with multiple encounters for the same illness, each visit was reviewed and all information through the last encounter was included in the patient's symptomatology. Objective data, including vital signs and laboratory test results, were captured as repeated measures if there were multiple outpatient or ED encounters/tests.
Fever was defined as a temperature of ≥38°C. Maximum reported temperature was based on parental report. If a patient's chart documented reported fever but did not document a measured temperature, or the only measured temperature was <38°C, the patient was deemed febrile for data analysis, but their maximum fever height was recorded as “subjective.”
Statistical Analyses
Clinical and demographic variables were described using summary statistics and compared using univariable analyses among febrile outpatients, febrile outpatients referred to the ED, and patients with MIS-C. For temperature height and duration comparisons, the Student t test was performed with false discovery analysis using the 2-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli, with Q = 1%. Each row was analyzed individually. For temperature comparisons for duration and height, second-order polynomial curves were fitted to data points from all outpatients and patients referred to the ED, and all patients with MIS-C, and 95% CI bands were plotted. For patient characteristics associated with MIS-C, significance levels of ≤0.1 were tested in logistic regression via stepwise, forward procedure. Patient characteristics with significance levels <.05 in multivariable analyses were retained in the final models. Models were tested for confounding and effect modification. In sensitivity analyses, we then repeated analyses, including the 3 patients with more than 1 febrile episode, and clustered SEs for repeat patients. For collinear variables, adjusted R2 was used to determine variable inclusion. For ORs, a non-parsimonious logistic regression model without stepwise procedure using all variables was done. Statistical analysis was performed using STATA, version 16 (StataCorp LLC) and figures/additional statistics using PRISM 8.3 (GraphPad Software).
This study was approved by the Columbia University Irving Medical Center institutional review board. A waiver of informed consent was provided.
Results
We identified 59 patients meeting New York City Department of Health–reportable criteria for possible MIS-C11 who were admitted to our institution during the study time frame. Of these, 44 patients were diagnosed with and treated for MIS-C; 7 met criteria for only KD and were excluded. Overall, 181 control patients were identified with 184 separate febrile episodes. Of patients initially seen in ambulatory clinics, 23 patients also had ED visits.
The demographics of each group are described in Table I . Patients with MIS-C generally were older than patients with other febrile illnesses and KD, with a median age of 8.2 years (IQR 5.3-13 years) vs 3.5 years (IQR 1.4-6.9 years) vs 2.4 years (1.3-3.9 years, P < .001), consistent with previous reports.1, 2, 3, 4, 5 , 7 , 11 There were no significant differences between MIS-C and febrile outpatient groups in regard to sex, race, or ethnicity. Greater than 50% of the patients in both groups identified as Hispanic, consistent with the background population our institution serves.
Table I.
Patient demographics
| Variables | All groups N = 232 |
Febrile controls (outpatient) N = 158 |
Febrile controls (ED) N = 23 |
MIS-C N = 44 |
KD∗ N = 7 |
|---|---|---|---|---|---|
| Median age, mo (IQR) | 64 (28-109) | 61 (27-97) | 27 (16-70) | 99 (64-157) | 29 (15-47) |
| Female, % | 47% | 46% | 43% | 52% | 71% |
| Ethnicity | |||||
| Hispanic (%) | 116 (50) | 83 (53) | 14 (61) | 18 (41) | 1 (14) |
| Unknown | 36 (15) | 16 (10) | 4 (17) | 11 (25) | 5 (71) |
| Race | |||||
| White | 109 (47) | 79 (50) | 9 (39) | 17 (39) | 4 (57) |
| Black | 43 (19) | 27 (17) | 4 (17) | 12 (27) | 0 (0) |
| Other | 46 (20) | 32 (20) | 7 (30) | 7 (16) | 0 (0) |
| Unknown | 34 (15) | 20 (13) | 3 (13) | 8 (18) | 3 (43) |
| SARS-Cov-2 PCR + (tested in our institution)† | 12/75 (16) tested | 1/8 (13) tested | 1/17 (6) tested | 9/43 (21) tested | 1/7 (14) tested |
| SARS-Cov-2 serology + (tested in our institution)† | 14/60 (23) tested | 3/10 (30) tested | 0/3 (0) tested | 40/42 (95) tested | 2 positive, 1 indeterminate/5 tested |
| Reported COVID-19 exposure | 77/232 (33) | 44/158 (28) | 5/23 (23) | 25/44 (57) | 3/7 (43) |
| Outside test positive | 48 | 30 | 3 | 14 | 1 |
| Suspected/family history (exclusive) | 29 | 14 | 2 | 11 | 2 |
COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction.
Seven patients admitted with prolonged fever did not have typical features of MIS-C (such as abdominal pain or neurologic features). These patients were treated following American Heart Association KD criteria only.10
Nasopharyngeal SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) testing (Roche Cobas SARS-CoV-2 assay), and COVID-19 antibody testing (New York State Department of Health–approved combined assay for IgM and IgG antibodies against SARS-CoV-2 spike trimer or nucleocapsid protein).
Clinical and Symptom Complex of Patients with MIS-C vs Febrile Controls
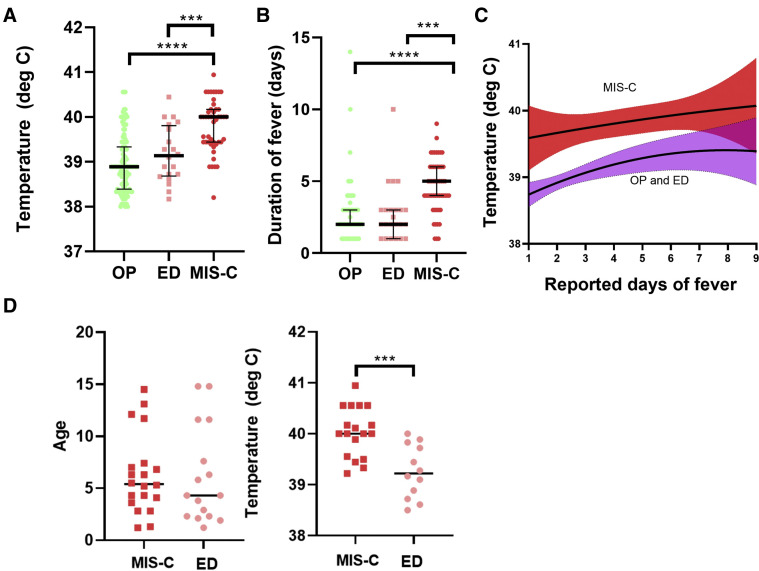
Patients admitted and treatment for MIS-C had greater levels of SARS-CoV-2 association (reported contact and laboratory confirmed), significantly greater maximum reported temperatures (40.0°C vs 38.9°C, P < .001) (Figure 1 , A) and a greater total duration of fever reported at the time of seeking medical attention (5 days vs 2 days, P < .001) (Figure 1, B) than other febrile outpatients. In addition, 42 febrile outpatient controls, and 1 who was referred to the ED, had fever that had resolved before medical attention. None of the patients in the MIS-C group had fever that resolved before treatment, including a patient who was only recognized to have MIS-C 15 days into his hospitalization and had persistent fevers during this entire period. Figure 1, C illustrates a reported duration of fever before seeking care and demonstrates that the patients who required admission and treatment for MIS-C had a greater temperature at all time points compared with febrile outpatients. When matching for age and location of presentation (ED and patients with MIS-C only), the finding that patients with MIS-C had significantly greater temperatures compared with other febrile children persisted (Figure 1, D).
Figure 1.
Characteristics of fever and clinical symptoms in MIS-C vs febrile outpatients. A, Height of fever was significantly elevated in patients with MIS-C vs febrile outpatients and febrile outpatients referred to the ED. B, Duration of fever was longer at time of presentation in patients with MIS-C compared with both febrile outpatient groups, and C, maximum reported temperature height was greater in patients with MIS-C when comparing with reported days of fever. D, Matching for age and site of care (ED) by randomly selecting closely age-matched patients from each group demonstrates that fever height in MIS-C remains elevated as compared with febrile outpatients presenting at the ED. OP, outpatient.
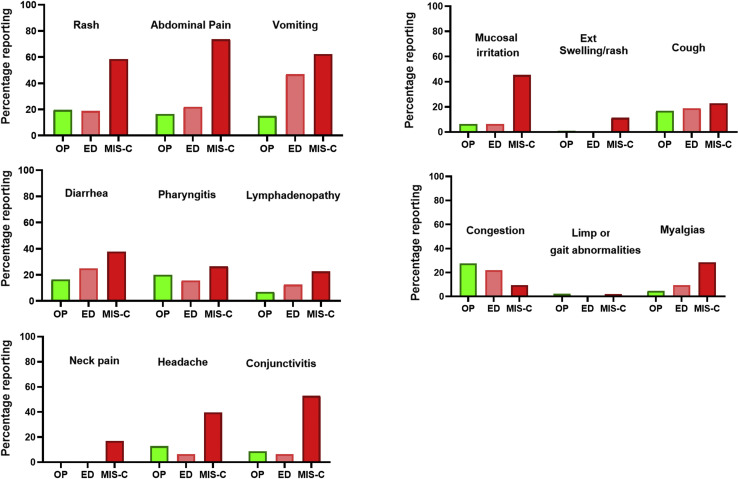
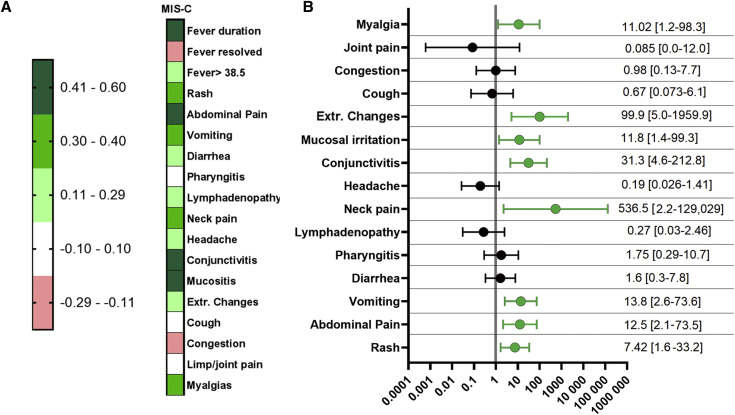
Figure 2 (available at www.jpeds.com) shows reported major symptomatology and other features of patients in each of the groups. Symptomatology correlation of all variables (Figure 3 , A) and multivariable regression were performed to look at correlations and significant symptoms. Resolution of both fever and presence of upper respiratory congestion demonstrated the trend of a modest negative correlation with the absence of MIS-C requiring treatment due to evidence of possible cardiac injury. The presence of abdominal pain, vomiting, mucosal irritation, neck pain or stiffness, and rash all were associated with a significant (P < .05) correlation with MIS-C requiring treatment (Figure 3, B). A list of most common diagnoses for the febrile outpatients is presented in Table II (available at www.jpeds.com).
Figure 2.
Descriptive bar graphs of symptomatology in patients with MIS-C vs febrile outpatients.
Figure 3.
A, Symptomatology correlation and B, OR of patients with MIS-C vs febrile outpatients and patients referred to the ED. Correlation number refers to R values using obtained by Pearson-type correlation. ORs were calculated using a non-parsimonious multivariable regression.
Laboratory and Echocardiographic Features Comparisons
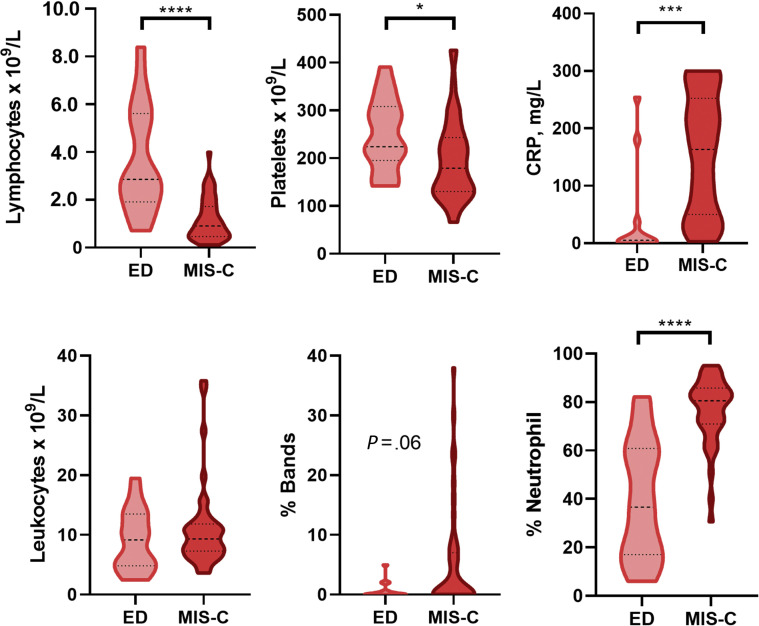
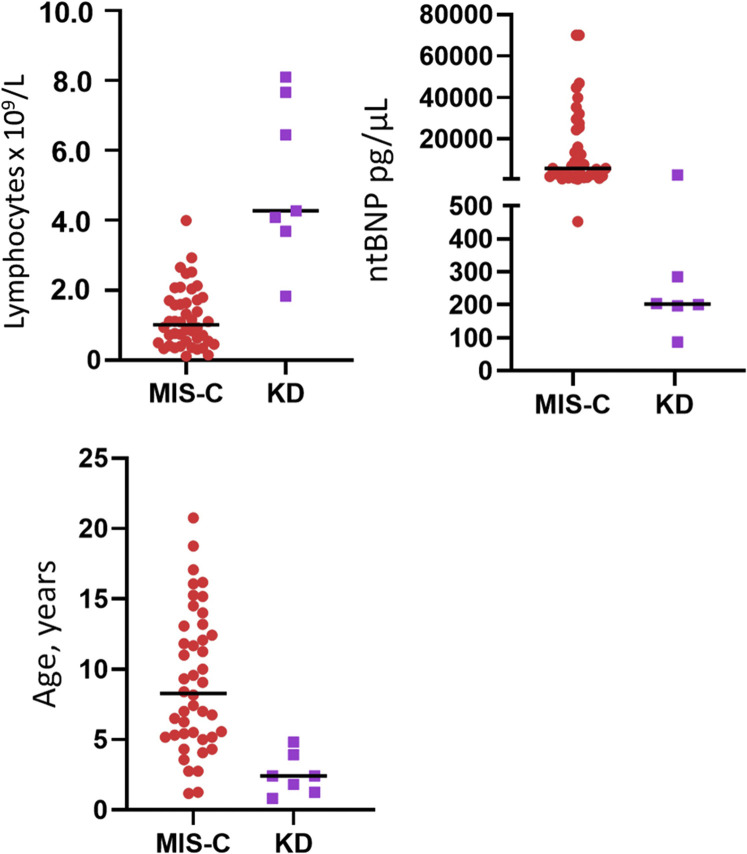
Of 181 febrile outpatients, most were seen as telehealth visits, which did not include laboratory testing; only patients referred to the ED, and in rare cases, patients seen in person in an outpatient clinic had blood or urine testing performed. Therefore, we only compared laboratory test results for the ED group's for MIS-C (Figure 4 ). At the time of presentation, our MIS-C cohort compared with febrile patients in the ED demonstrated profound lymphopenia (absolute lymphocyte counts 900 cells/μL, 490-1700 vs 2860, 1900-5400, respectively; P < .001) and greater percentage of neutrophils (80.5%, 71.6-85.7 vs 40.1%, 19.0-60.0, respectively; P < .001). Patients with MIS-C requiring admission and treatment also tended to have a lower initial platelet count (179 000/μL, 130 000-243 000 vs 224 000/μL, 195 000-308 000; P < .05) than other febrile patients. Finally, our patients with MIS-C demonstrated markedly elevated C reactive protein serum concentrations (164 mg/L, 52-250) and N-terminal brain natriuretic peptide (ntBNP) levels (6700 pg/μL, 2509-25 550). These were statistically greater than observed in febrile outpatients (4 mg/L, IQR <1-14.8 and 98, IQR 65-194; P < .001 for both); however, these tests were performed only in 14 and 9 outpatients, respectively. Patients with KD had greater absolute lymphocyte counts and lower ntBNPs than patients with MIS-C but made up a very small number of patients (Figure 5; available at www.jpeds.com).
Figure 4.
Laboratory testing discriminators for patients with MIS-C vs patients referred to the ED.
Figure 5.
Differences in laboratory findings and age in patients with KD vs patients with MIS-C.
The majority of patients treated for MIS-C showed some evidence of end-organ dysfunction in the form of shock and/or echocardiographic evidence of cardiac involvement or dysfunction (Table III; available at www.jpeds.com).
Discussion
We found a clear difference in presenting symptomatology and basic laboratory test findings in patients diagnosed and treated for MIS-C compared with febrile outpatients who received other diagnoses. We do not yet know the consequences of a missed diagnosis of MIS-C, but in a number of patient series (with the total number of patients described to date being <1000), the incidence of cardiac damage in the form of aneurysm is small but present (approximately 8%-10%), and few patients have died.2 , 3 , 5 , 7 , 11 At the same time, there is risk in overdiagnosing and overtreating presumed patients with MIS-C. Unpublished communications suggest that patients with other serious conditions, including malignancies, have been treated with corticosteroids and intravenous immunoglobulin for presumed MIS-C before a correct diagnosis. Although patients presenting in shock are clearly distinct from than those presenting with low-grade fevers, “rule-out” MIS-C had to be considered for the majority of children coming to attention because of fever. The question of when to refer patients for full laboratory and cardiac evaluation plagued outpatient providers. Our study demonstrates some useful “red-flags.” For instance, an older patient with high-level and prolonged fever, rash, severe abdominal pain, and neck pain or stiffness, with a history of suspected SARS-CoV-2 exposure, should be referred. A younger patient with a low-grade fever and upper respiratory congestion should have follow-up but not further evaluation.
Difficulty clearly identifying MIS-C in a clinical setting extends to difficulty in studying and understanding the disease in a research setting. This issue was raised by Rowley, who noted that the “very broad case definition for MIS-C is of concern…because inclusion of children who have other conditions will skew results and obscure accurate conclusions.”12 Thus, we defined our MIS-C cohort for study as patients who received treatment not only after meeting suggested MIS-C criteria but also having evidence of potential cardiac involvement by either cardiac dysfunction on echocardiogram, coronary abnormalities, or sustained, progressive or markedly elevated ntBNP. Elevated ntBNP has been reported to be associated with cardiac dysfunction or cardiogenic shock in the context of MIS-C.13 , 14 However, it should be noted in our cohort that all 44 patients in the MIS-C cohort who were treated had abnormal ntBNP and only 56% had abnormal function on echocardiogram and 5% had coronary abnormalities. This emphasizes the nonspecific nature of ntBNP as a marker of cardiac dysfunction, but suggests it may have a role in prompting further cardiac evaluation for suspected MIS-C.
Our study has significant limitations. Perhaps most importantly, MIS-C cases were initially observed in the ED, whereas most controls were seen at outpatient telehealth visits. Although this presents some challenges to interpretation, 2 points are of particular importance. First, records from both groups were upon initial encounter with our healthcare system and thus, we had a full history potentially providing effectively the same information for both groups. Second, in light of the limitations introduced by the pandemic, outpatient telehealth visits replaced in-person urgent care visits. MIS-C and febrile outpatients had similar distributions of sex, race, and ethnicity, were drawn from within our hospital referral area, and during the same time period. Third, laboratory data in the febrile outpatients are limited. Fourth, data on the history of present illness and exposures are limited to a retrospective chart review. Such data were likely more robust in those hospitalized for MIS-C. Fifth, there was no formal follow-up of febrile children. However, none of our control patients subsequently were admitted in our system with a diagnosis of MIS-C.
Symptomatology at one point in time may be under-reported in retrospective studies. Abdominal pain, however, associated with MIS-C typically is severe and suggestive of peritoneal irritation, with guarding and rebound almost always present. Rash in these patients on the other hand is generally polymorphous, at times evanescent, often diffuse with a predominance of the trunk, with macular erythematous and blanching lesions. Similarly, some laboratory features seen at initial presentation of MIS-C are not static. Although platelet counts are depressed during initial presentation, they tend to rise dramatically (2- or 3-fold above normal range) in patients at later time points.
Although KD and MIS-C can have overlapping features,9 , 15 , 16 for our study, we excluded patients with clearly recognizable KD and no extended features as seen in MIS-C from formal comparisons. Findings in our KD patients were included to give readers the full view of the data.
Our data suggest that children who will require intervention for MIS-C are clinically distinguishable from other febrile children and that red flags can be sought and patients identified who will require further evaluation. Further long-term study of children exposed to SARS-COV-2 is needed to clarify whether a spectrum of MIS-C phenotypes exists that also could be associated with cardiac injury or long-term cardiac sequelae.
Footnotes
The authors declare no conflicts of interest.
Supplementary Data
Appendix
Table II.
Most common and notable outpatient or ED diagnoses
| Diagnoses | Number of patients |
|---|---|
| Viral illness/syndrome/infection | 62 |
| COVID-19/suspected COVID-19 | 18 |
| Fever not otherwise specified | 14 |
| Pharyngitis (non-streptococcal) | 12 |
| Conjunctivitis | 10 |
| Streptococcal pharyngitis | 9 |
| Gastroenteritis | 9 |
| Atopic dermatitis | 7 |
| Acute otitis media | 6 |
| Appendicitis | 1 |
| Intussusception | 1 |
COVID-19, coronavirus disease 2019.
Table III.
Evidence of end-organ dysfunction in the MIS-C group. STROBE Statement—Checklist of items that should be included in reports of case-control studies
| Evidence | MIS-C N = 44 |
|---|---|
| Elevated ntBNP | 44/44, 100% |
| Median ntBNP | 6700 pg/μL |
| Abnormal echo | 25/44 (56%) |
| Thereof: “Low normal”/Mild dysfunction | 15/44 (34%) |
| Moderate dysfunction | 10/44 (22%) |
| Aneurysms | 2/44 (1 moderate, 1 mild) (5%) |
| Hypotension/dehydration requiring saline boluses | 28/44 (67%) |
| Shock requiring pressor support | 12/44 (27%) |
References
- 1.Cheung E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdoni L.M.A., Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blondiaux E., Parisot P., Redheuil A., Tzaroukian L., Levy Y., Sileo C. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology. 2020;9:202288. doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diorio C., Henrickson S.E., Vella L.A., McNerney K.O., Chase J.M., Burudpakdee C. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130:5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin M. Childhood multisystem inflammatory syndrome - a new challenge in the pandemic. N Engl J Med. 2020;383:393–395. doi: 10.1056/NEJMe2023158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the american heart association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 11.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowley A.H. Diagnosing SARS-CoV-2 related multisystem inflammatory syndrome in children (MIS-C): focus on the gastrointestinal tract and the myocardium. Clin Infect Dis. 2020 Jul 27 doi: 10.1093/cid/ciaa1080. ciaa1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee P.Y., Day-Lewis M., Henderson L.A., Friedman K., Lo J., Roberts J.E. Distinct clinical and immunological features of SARS-COV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belhadjer Z., Meot M., Bajolle F., Khraiche D., Legendre A., Abakka S. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 15.McCrindle B.W., Manlhiot C. SARS-CoV-2-related inflammatory multisystem syndrome in children: different or shared etiology and pathophysiology as Kawasaki disease? JAMA. 2020;324:246–248. doi: 10.1001/jama.2020.10370. [DOI] [PubMed] [Google Scholar]
- 16.Rowley A.H. Multisystem inflammatory syndrome in children and Kawasaki disease: two different illnesses with overlapping clinical features. J Pediatr. 2020;224:129–132. doi: 10.1016/j.jpeds.2020.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.