Abstract
Acute kidney injury causes significant morbidity and mortality. This experimental animal study investigated the simultaneous impact of iron and vitamin D on acute kidney injury induced by iohexol, an iodinated, non-ionic monomeric radiocontrast agent in Wistar rats. Out of 36 healthy male Wistar rats, saline was injected into six control rats (group 1) and iohexol into the remaining 30 experimental rats (groups 2 to 6 comprising six rats each). Biochemical, renal histological changes, and gene expression of iron-regulating proteins and 1 α-hydroxylase were analyzed. Urinary neutrophil gelatinase-associated lipocalin (NGAL), serum creatinine, urine protein, serum and urine catalytic iron, 25-hydroxyvitamin D3, 1,25-dihydroxyvitamin D3, and tissue lipid peroxidation were assayed. Rats injected with iohexol showed elevated urinary NGAL (11.94 ± 6.79 ng/mL), serum creatinine (2.92 ± 0.91 mg/dL), and urinary protein levels (11.03 ± 9.68 mg/mg creatinine) together with histological evidence of tubular injury and iron accumulation. Gene expression of iron-regulating proteins and 1 α-hydroxylase was altered. Serum and urine catalytic iron levels were elevated (0.57 ± 0.17; 48.95 ± 29.13 µmol/L) compared to controls (0.49 ± 0.04; 20.7 ± 2.62 µmol/L, P < 0.001). Urine catalytic iron positively correlated with tissue peroxidation (r = 0.469, CI 0.122 to 0.667, P = 0.004) and urinary NGAL (r = 0.788, CI 0.620 to 0.887, P < 0.001). 25-hydroxyvitamin D3 (61.58 ± 9.60 ng/mL) and 1,25-dihydroxyvitamin D3 (50.44 ± 19.76 pg/mL) levels increased simultaneously. In a multivariate linear regression analysis, serum iron, urine catalytic iron, and tissue lipid peroxidation independently and positively predicted urinary NGAL, an acute kidney injury biomarker. This study highlights the nephrotoxic potential of catalytic iron besides demonstrating a concurrent induction of vitamin D endogenously for possible renoprotection in acute kidney injury.
Impact statement
This work provides in-depth insights on catalytic iron-induced cytotoxicity and the resultant triggering of endogenous vitamin D synthesis in experimental acute kidney injury. Our results reveal significantly elevated levels of catalytic iron culminating in oxidant-mediated renal injury and a concomitant increase in 1,25-dihdyroxyvitamin D3 levels. Also, changes in other iron-related proteins including transferrin, ferritin, and hepcidin were observed both in the serum as well as in their mRNA expression. We consider all these findings vital since no connection between catalytic iron and vitamin D has been established so far. Furthermore, we believe that this work provides new and interesting results, with catalytic iron emerging as an important target in ameliorating renal cellular injury, possibly by timely administration of vitamin D. It also needs to be seen if these observations made in rats could be translated to humans by means of robust clinical trials.
Keywords: Catalytic iron, transferrin, ferritin, hepcidin, vitamin D, oxidative stress, acute kidney injury
Introduction
Acute kidney injury causes significant mortality and morbidity, as well as being responsible for a tremendous burden on health care costs.1,2 The pathogenesis of AKI is complex,3 and presently the treatment is mainly supportive in nature, there being no specific therapeutic modality with proven efficacy.4 Recent studies have identified catalytic iron in inducing AKI and poor outcomes.5–10 However, knowledge of changes in iron metabolism during the course of kidney injury is limited, thereby hampering the development of strategies to counteract AKI.11,12
Meanwhile, the kidneys have both endogenous and exogenous (antioxidant) nephroprotective agents to ameliorate cellular injury.13 Vitamin D has been shown to have a cytoprotective effect owing to its antioxidant property.14–16 Therefore, we sought to investigate the combined roles of catalytic iron and vitamin D in relation to oxidative stress and nephrotoxicity in a rat model of iohexol-induced AKI. The primary goal was to characterize catalytic iron, vitamin D, and oxidative stress over time following iohexol administration, and the secondary goals were to determine the relationship between catalytic iron, vitamin D, and oxidative stress markers, and to determine if these variables collectively predicted AKI.
Materials and methods
Experimental animals
Healthy adult male albino Wistar rats (8–12 weeks, weighing 180–200 g) were used for the study. All animal maintenance and experiments were carried out according to the ethical guidelines issued by the Institutional Animal Ethics Committee of the VIT, Vellore (Registration No. VIT/IAEC/9th/2nd March 2015). Animals were housed in polypropylene metabolic cages at constant temperature (27 ± 1°C) and relative humidity (55 ± 10%) under a 12-h light/dark cycle and with regular access to feed (standard diet consisting of 4.1% fat, 22.2% protein and 12.1% carbohydrates, as a percentage of total kcal, 0.9% calcium per 100 grams and 600 IE vitamin D3 per kg) and water ad libitum.
Study design
Thirty-six healthy rats were randomly divided into six groups of six rats each. Six rats in Group 1 (control group) were injected with normal saline (Sodium chloride injection B.P. 0.9% w/v, Schwitz Biotech, India) and sacrificed together under general anaesthesia (overdose of Sodium thiopental 100 mg/kg IP) as per the IACUC guidelines.17 The remaining 30 animals were injected with iohexol (OMNIPAQUE™, 350 mg I/mL, GE Healthcare, India) at a dose of 3 g of iodine per kg intraperitoneally (IP)18 and sacrificed groupwise sequentially at 2, 4, 6, 12, and 24 h (experimental groups 2 to 6, respectively) following contrast administration. Blood samples were obtained from the inferior vena cava without the anticoagulant, incubated at room temperature, allowed to clot completely and centrifuged (REMI instruments Vasai, India) at 4000 r/min for 20 min at 4°C to separate the serum. Aliquots of serum and urine were then transported frozen on dry ice and stored at −80°C for biochemical analyses.
Histopathological examination of renal tissue
Each kidney harvested at different time points (0, 2, 4, 6, 12, and 24 h post-iohexol) was washed with phosphate-buffered saline (PBS) at pH 7.4, fixed in 10% neutral-buffered formalin, embedded on paraffin blocks, and cut into 4 µm sections using Leica RM 2126 microtome (Leica Inc., Allendale, NJ) for the evaluation of histopathological changes using hematoxylin and eosin (H&E) and Periodic acid-Schiff (PAS) stains and for iron accumulation by Perls’ Prussian blue stain (Sigma-Aldrich, St. Louis, MO, USA). The sections were photographed under a microscope (Olympus BX51; Olympus Optical, Tokyo, Japan) at a magnification of 400×. Ultrastructural examination was performed by transmission electron microscopy (FEI-TECNAI G2-20 TWIN, Netherland) on 2.5% glutaraldehyde-fixed and uranyl acetate-stained sections.19,20 The specimens were evaluated by an experienced pathologist who was blinded to the data.
Quantitative real-time polymerase chain reaction analysis
Gene expression of transferrin receptors 1 and 2, ferritin, hepcidin, and ferroportin proteins was analyzed by qRT-PCR.21 Additional data about the primer sequences used are mentioned in Online Supplementary Table 1.
Serum and urine biochemistry of iron, vitamin D, and AKI biomarker
Serum creatinine, blood urea nitrogen (BUN), serum iron, and total iron binding capacity (TIBC) were determined using the commercial kits (Beckman Coulter Inc., USA) on the Beckman Coulter AU480 fully automated Clinical Chemistry Analyzer. Serum ferritin (Cat: MBS564109), hepcidin (Cat: MBS774771), haptoglobin (Cat: MBS564114), creatine phosphokinase [CPK] (Cat: MBS9344823), 25-hydroxyvitamin D3 [25(OH)D3] (Cat: MBS703860), and 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] (Cat: MBS2602146) were measured by ELISA kits (MyBiosource, USA). An automated ELISA washer (PW40, Bio-Rad, USA) and reader (PR4100, Bio-Rad, USA) were used for the purpose. Serum hemoglobin was measured using the assay kit (MAK115, Sigma-Aldrich, USA) by the colorimetric method at 400 nm. Urine protein (Cat: TP0400, Sigma Aldrich, USA) was measured by the micro Pyrogallol Red method. Urinary neutrophil gelatinase-associated lipocalin (NGAL) was assayed using direct ELISA (Cat: sc-80561, Santa Cruz Biotechnology Inc., USA).22,23
The serum and urine bleomycin detectable iron (BDI), also known as catalytic iron or labile iron, were quantified by following the assay described by Halliwel and Gutteridge24 after suitable modification. The assay is based on the activity of antitumor agent bleomycin that binds to and degrades DNA in the presence of non-transferrin bound iron to produce thiobarbituric acid reactive chromogen. The color intensity of the chromogen was measured at 532 nm wavelength light in a Beckman Coulter UV Visible spectrophotometer, DU800.
Measurement of oxidative stress
Lipid (tissue) peroxidation was evaluated by the TBARS (Thiobarbituric acid reactive species) assay using a spectrophotometric method reported by Ohkawa et al.25 Antioxidants including total thiol content were estimated by Elmman’s method,26 catalase by colorimetric method,27 and superoxide dismutase (SOD) by assessing its ability to inhibit the reduction of nitro-blue tetrazolium by superoxide.28
Statistical analysis
Data were analyzed using SPSS (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp). Descriptive statistics were expressed as mean ± standard deviation. Continuous data (iron indices, vitamin D, oxidative stress and AKI markers) were compared over time following iohexol administration in Wistar rats by performing the one-way repeated measures analysis of variance (ANOVA) with the Greenhouse–Geisser correction. Subsequently, a Bonferroni post hoc test was performed to compute the individual variances in comparison with the control group. The association between the various study parameters was estimated by Pearson correlation. Multiple linear regression analysis was conducted to test if iron, vitamin D, and oxidative stress markers independently predicted urinary NGAL and thereby, AKI. Statistical significance was defined as P < .05 for two-tailed tests. GraphPad Prism Version 8.3.0 and GIMP Version 2.10.12 were used to create the artwork and illustrations.
Results
Histopathological examination of renal tissue
Findings consistent with acute tubular necrosis were evident 4 to 6 hours after contrast administration (Figures 1to 3; Table 1). Punctate bluish inclusions were visible on Perls’ staining indicating the presence of iron (hemosiderin) in the cytosol (Figure 3). Characteristic subcellular modifications such as loss of brush border, nuclear condensation, swollen mitochondria as well as cytoplasmic electron-dense radiocontrast inclusions were conspicuous ultrastructurally (Figure 4). These changes were more prominent at 6 hours post-contrast. Subsequently, there was a progressive decrease in the cytoplasmic inclusions after 12 hours. Reversal of most of these histological changes leading to near-normal morphology of the renal tubules were discernible at 24 hours.
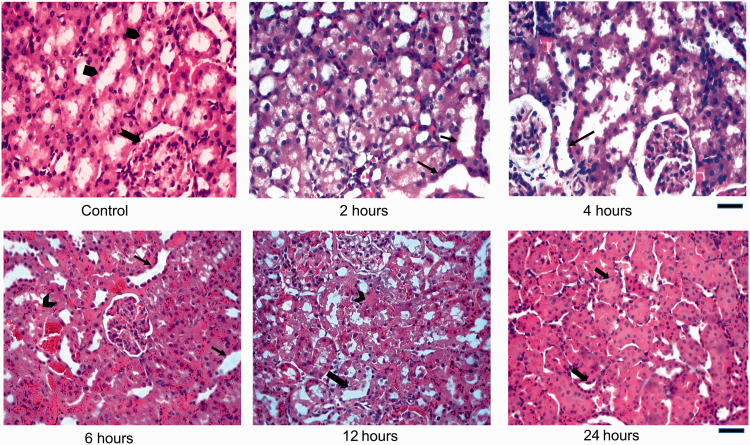
Figure 1.
Histological sections of Control and iohexol-treated rat kidneys stained with haematoxylin and eosin (H&E) stain. Control rat displays a normal morphology with a well-preserved glomerulus (notched black arrow) surrounded by clearly demarcated tubules (arrow pentagon). Experimental rats (2 h through 12 h) demonstrate acute tubular injury with tubular dilatation and degeneration of segments of epithelial cells (thin black arrows). Others have flattened, regenerating-type epithelial cells (thick black arrow) and some show frank necrosis (notched black arrow). Most tubules appear normal at 24 h. Scale bar = 450 µm. (A color version of this figure is available in the online journal.)
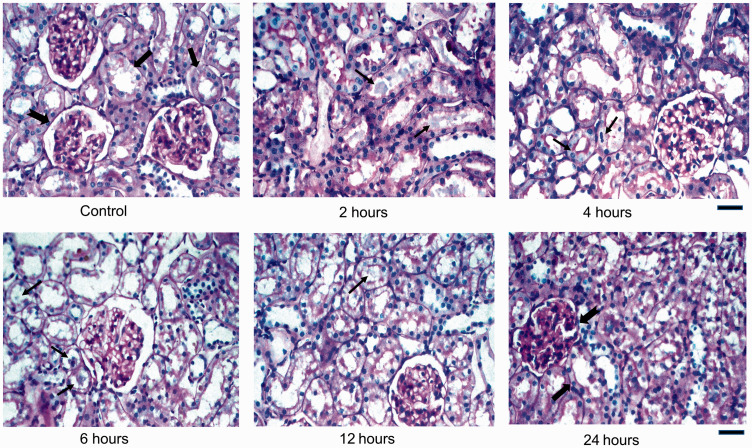
Figure 2.
Histological sections of Control and iohexol-treated rat kidneys stained with periodic acid-Schiff (PAS) stain. Control rat shows normal brush borders of the proximal renal tubular cells (thick black arrow), normal glomeruli (notched black arrow), and preserved corticomedullary junction. Experimental rats (2 h through 12 h) show proximal tubular lumen filled with varying amounts of PAS-negative bluish, acellular contrast material deposits (thin black arrows) within the cytoplasm and tethered to the brush borders of tubules. Glomerulus and tubules appear normal at 24 h. Scale bar = 450 µm. (A color version of this figure is available in the online journal.)
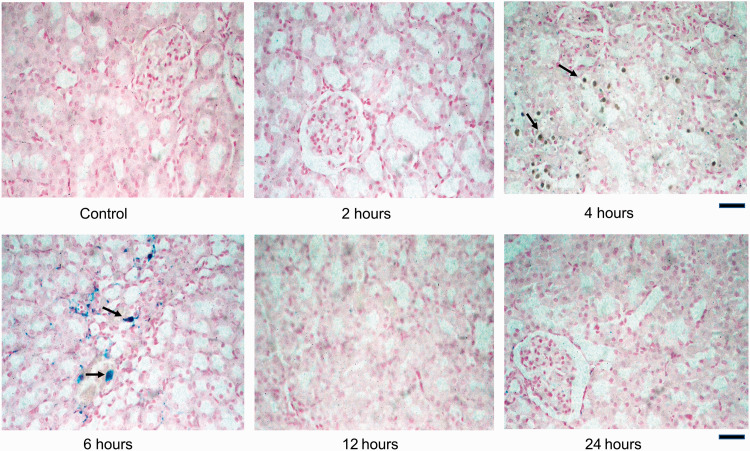
Figure 3.
Histological sections of Control and iohexol-treated rat kidneys stained with Perls’ Prussian blue. Iron deposits (black arrows) are visible as dark brownish green and bluish inclusions in the cytoplasm of renal tubular epithelial cells in experimental rats (4 to 6 h). No iron deposition is evident in Control rat. At 24 h, near-normal morphology is conspicuous. Scale bar = 450 µm. (A color version of this figure is available in the online journal.)
Table 1.
Biochemical findings of iron, vitamin D, oxidative stress, and AKI markers in Control and iohexol-treated rats
| Control rats 0 h |
Time since iohexol injection |
|||||
|---|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 12 h | 24 h | ||
| Serum iron (µg/dL) | 195 ± 4.87 | 212 ± 7.8** | 202 ± 15.1 | 225 ± 26.6 | 135 ± 13.9*** | 180 ± 30 |
| TSAT (%) | 37.5 ± 1.37 | 38.5 ± 4.49 | 32.8 ± 2.57 | 45.9 ± 10.3 | 41.3 ± 15.9 | 39.7 ± 7.71 |
| Serum ferritin (ng/mL) | 901 ± 114 | 1924 ± 252*** | 2143 ± 405** | 1825 ± 122*** | 1399 ± 287* | 1373 ± 267* |
| Serum hepcidin (pg/mL) | 114 ± 6.71 | 48.4 ± 13.7*** | 72 ± 12.8** | 101 ± 11.9 | 106 ± 3.15 | 110 ± 10.8 |
| Serum catalytic iron (µmoI/L) | 0.487 ± 0.039 | 0.913 ± 0.01*** | 0.583 ± 0.014* | 0.547 ± 0.021** | 0.465 ± 0.012 | 0.427 ± 0.025 |
| Urine catalytic iron (µmoI/L) | 20.7 ± 2.62 | 64.9 ±5.07*** | 95.4 ± 4.1*** | 65.2 ± 4.38*** | 29.9 ± 8.25 | 17.7 ± 5.87 |
| Hemoglobin (g/dL) | 16.4 ± 0.34 | 15.2 ± 0.21*** | 19.2 ± 0.29*** | 15.1 ± 0.18** | 14.9 ± 0.16*** | 14.7 ± 0.39** |
| Haptoglobin (µg/mL) | 488 ± 40.6 | 466 ± 78.4 | 500 ± 84 | 457 ± 133 | 411 ± 61.5 | 406 ± 57.9 |
| Serum CPK (units/L) | 95.5 ± 1.87 | 84.5 ± 1.52*** | 68.5 ± 2.43*** | 91.5 ± 1.87** | 70.5 ± 1.38*** | 102 ± 2.74 |
| 25(OH)D3 (ng/mL) | 51 ± 0.74 | 62.7 ± 9.01 | 64.3 ± 2.36*** | 63.8 ± 8.46 | 74.9 ± 4.27*** | 52.6 ± 2.76 |
| 1,25(OH)2D3 (pg/mL) | 19 ± 3.22 | 81.7 ± 2.58*** | 59.7 ± 3.93*** | 52 ± 0.89*** | 52 ± 3.79*** | 38.3 ± 6.71** |
| Tissue peroxidation(mg/mg protein) | 0.102 ± 0.001 | 0.086 ± 0.001 | 0.108 ± 0.001 | 0.157 ± 0.001*** | 0.090 ± 0.007 | 0.074 ± 0.004** |
| Tissue thiol(units/mg protein) | 0.038 ± 0.004 | 0.011 ± 0.001*** | 0.008 ± 0.001*** | 0.006 ± 0.001*** | 0.012 ± 0.001*** | 0.022 ± 0.001** |
| Catalase (units/mg protein) | 2.09 ± 0.06*** | 1.41 ± 0.03*** | 0.96 ± 0.01*** | 0.69 ± 0.01*** | 0.79 ± 0.01*** | 1.81 ± 0.01*** |
| SOD (units/mg protein) | 0.445 ± 0.034 | 0.196 ± 0.001*** | 0.409 ± 0.013 | 0.340 ± 0.005** | 0.306 ± 0.010*** | 0.238 ± 0.015*** |
| Urinary NGAL (ng/mL) | 1 ± 0*** | 13.1 ± 0.1*** | 14.6 ± 0.5*** | 22.3 ± 0.7*** | 7.0 ± 0.1*** | 2.8 ± 0.2*** |
Note: Data shown as mean ± SD. *P < 0.05, ** P < 0.01 and ***P < 0.001 versus control group.
TSAT: transferrin saturation; CPK: creatine phosphokinase; 25(OH)D3: 25-hydroxyvitamin D3; 1,25(OH)2D3: 1,25-dihydroxyvitamin D3; SOD: superoxide dismutase; NGAL: neutrophil gelatinase-associated lipocalin.
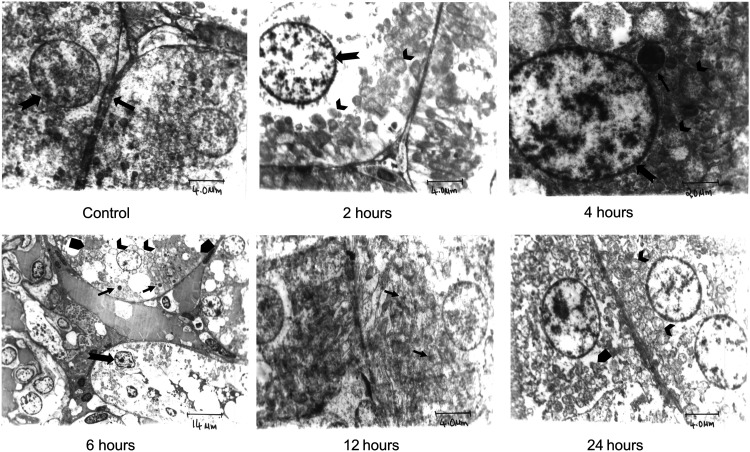
Figure 4.
Transmission electron micrograph of Control and iohexol-treated rats stained with Uranyl acetate. Ultrastructure of proximal convoluted tubular epithelium of the Control rat shows normal cellular constituents with normal distribution of chromatin in nucleus (notched arrow). Intercellular junction is within normal limits (thick arrow) (4 µm). Experimental rats (2 h through 12 h) display loss of brush border, nuclear condensation with irregular clumping and margination of chromatin to periphery (notched arrow), swollen mitochondria (arrow heads), and rough endoplasmic reticulum (arrow pentagon). Multiple electron-dense inclusion bodies (thin arrows) are also observed. At 24 h, recovery of mitochondria with mild swelling and intact cristae is seen; the endoplasmic reticulum is unremarkable. Scale bar = 4 to 20 µm.
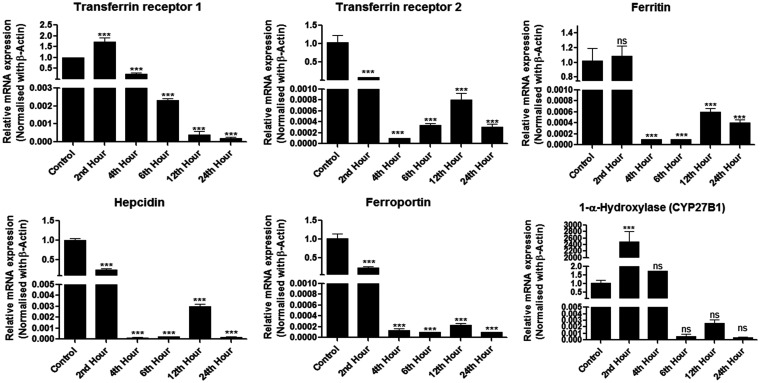
Gene expression of iron-regulating proteins and 1 α-hydroxylase (CYP27B1)
There existed a significant alteration in the gene expression of iron-regulating proteins as well as that of 1α-hydroxylase. Genes of transferrin receptors 1 and 2, ferritin, hepcidin, and ferroportin were significantly down-regulated, notably at 6 hours which coincided with the period of maximal renal injury. On the other hand, 1α-hydroxylase gene was up-regulated quite rapidly following the insult mediated by iohexol (Figure 5).
Figure 5.
Relative mRNA expression of iron-regulating and vitamin D genes normalized to β-actin in Control and iohexol-treated rats. The values represent mean ± SD. *P < 0.05, ** P < 0.01, ***P < 0.001 versus control.
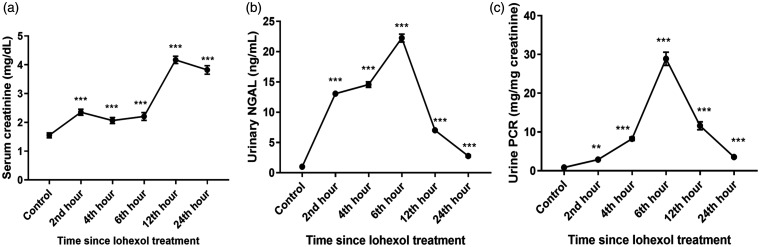
Renal injury
The urinary NGAL levels increased immediately following the use of iohexol, reaching a peak at around 6 hours, a time coinciding with the maximum histologic damage, and thereafter returned to the pre-contrast levels at around 24 hours, during which time most of the tissue damage had resolved, indicating recovery from AKI. Serum creatinine and urinary protein levels also showed a similar trend (Figure 6).
Figure 6.
Levels of (a) Serum creatinine, (b) Urinary NGAL (neutrophil gelatinase-associated lipocalin), and (c) Urine protein-creatinine ratio (PCR) in Control and iohexol-treated rats. The values represent mean ± SD (n = 6 rats per group). *P < 0.05, ** P < 0.01, ***P < 0.001 versus control.
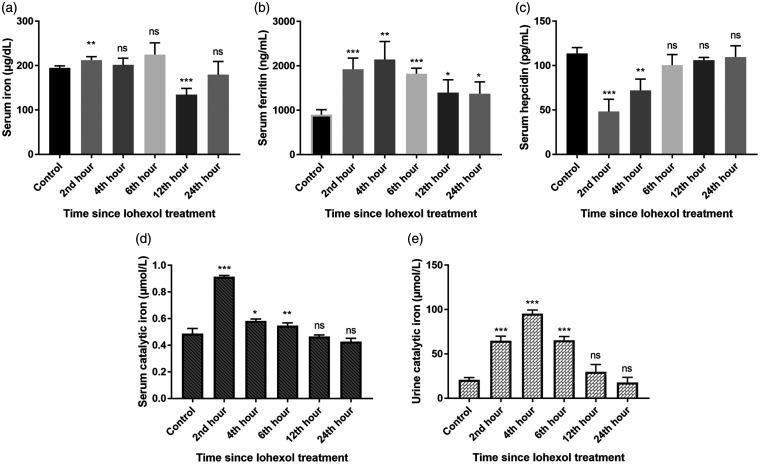
Serum iron, iron-regulating proteins, and catalytic iron
During the renal injury phase, the levels of serum iron, serum ferritin, and both serum and urine catalytic iron were noted to increase, while the serum hepcidin levels declined in a significant manner. Later, these levels gradually returned to their baseline values at the recovery phase (12 to 24 h post-contrast) (Figure 7).
Figure 7.
Demonstrating (a) Serum iron, (b) Serum ferritin, (c) Serum hepcidin, (d) Serum catalytic iron, and (e) Urine catalytic iron levels in Control and iohexol-treated rats, respectively. The values represent mean ± SD (n = 6 rats per group). *P < 0.05, ** P < 0.01, ***P < 0.001 versus control.
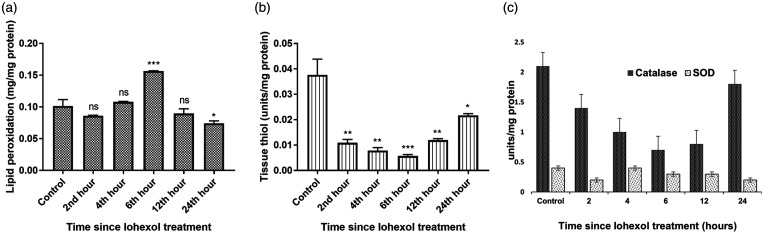
Oxidative stress
There was a significant increase in the tissue lipid peroxidation levels and a corresponding reduction in the levels of antioxidants, namely tissue thiol, catalase, and superoxide dismutase, immediately following contrast administration (Figure 8).
Figure 8.
Illustrating (a) Tissue lipid peroxidation, (b) Tissue thiol, and (c) Catalase and superoxide dismutase (SOD) levels in Control and iohexol-treated rats, respectively. The values represent mean ± SD (n = 6 rats per group). *P < 0.05, ** P < 0.01, ***P < 0.001 versus control.
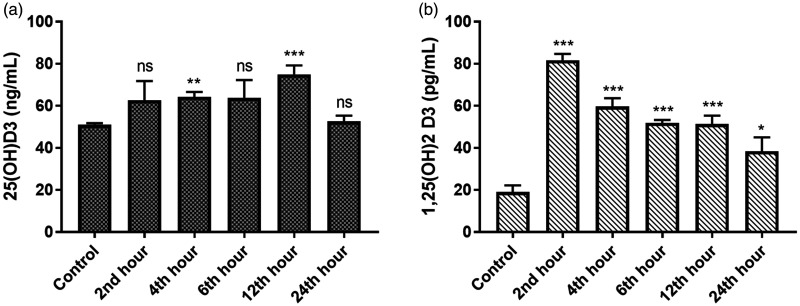
Vitamin D levels
With the onset of AKI, the concentration of both 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 increased abruptly and remained elevated throughout the recovery phase of AKI (Figure 9).
Figure 9.
Displaying (a) 25-hydroxyvitamin D3 and (b) 1,25-dihydroxyvitamin D3 levels in Control and iohexol-treated rats, respectively. The values represent mean ± SD (n = 6 rats per group). *P < 0.05, ** P < 0.01, ***P < 0.001 versus control.
Correlation between iron indices, oxidative stress, and AKI
Increased levels of serum iron were associated with increased tissue lipid peroxidation (r = 0.489, CI 0.166 to 0.691, P = 0.002). In contrast, increased serum ferritin levels were associated with decreased antioxidant levels including tissue thiol (r = −0.720, CI −0.867 to −0.564, P < 0.001) and catalase (r = −0.558, CI −0.749 to −0.281, P < 0.001). Similarly, decreased serum hepcidin levels were associated with a decrease in the levels of antioxidants, namely tissue thiol (r = 0.489, CI 0.175 to 0.696, P = 0.002) and superoxide dismutase (r = 0.363, CI 0.035 to 0.615, P = 0.030). Although there was no correlation between serum catalytic iron and oxidative stress, urine catalytic iron was shown to increase the oxidative stress (r = 0.469, CI 0.122 to 0.667, P = 0.004). Besides, both urine and serum catalytic iron were associated with a decrease in the antioxidant levels. No meaningful relationship existed between TSAT, haptoglobin, and oxidative stress markers (Table 2).
Table 2.
Correlation matrix of iron indices, vitamin D, oxidative stress, and AKI markers (Pearson correlation)
| Variables |
Iron parameters |
Vitamin D |
Oxidative stress |
AKI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| Iron parameters | 1. Serum iron | 1 | |||||||||||||||
| 2. TSAT | 0.232 | 1 | |||||||||||||||
| 3. Serum ferritin | .379* | 0.052 | 1 | ||||||||||||||
| 4. Serum hepcidin | −0.316 | 0.216 | −.667** | 1 | |||||||||||||
| 5. Hemoglobin | 0.197 | −.345* | .346* | −0.287 | 1 | ||||||||||||
| 6. Haptoglobin | 0.241 | −0.008 | 0.012 | −0.205 | 0.307 | 1 | |||||||||||
| 7. Serum CPK | 0.258 | 0.124 | −.452** | .385* | −.503** | −0.07 | 1 | ||||||||||
| 8. Serum catalytic iron | .439** | −0.065 | .472** | −.856** | 0.026 | 0.188 | −0.19 | 1 | |||||||||
| 9. Urine catalytic iron | .454** | −0.15 | .806** | −.679** | .633** | 0.235 | −.563** | .517** | 1 | ||||||||
| Vit D | 10. 25(OH)D3 | −.374* | −0.083 | 0.304 | −0.171 | −0.015 | −0.24 | −.685** | 0.123 | 0.293 | 1 | ||||||
| 11. 1,25(OH)2D3 | 0.233 | 0.029 | .765** | −.795** | 0.027 | −0.045 | −.498** | .784** | .649** | .481** | 1 | ||||||
| Oxidative stress | 12. Tissue peroxidation | .489** | 0.225 | 0.301 | 0.045 | 0.12 | 0.19 | −0.024 | 0.004 | .469** | 0.175 | 0.05 | 1 | ||||
| 13. Tissue thiol | −0.01 | −0.078 | −.720** | .489** | 0.009 | 0.076 | .569** | −.368* | −.611** | −.647** | −.814** | −0.243 | 1 | ||||
| 14. Catalase | 0.035 | −0.149 | −.558** | 0.195 | −0.085 | 0.038 | .658** | −0.074 | −.580** | −.734** | −.528** | −.556** | .832** | 1 | |||
| 15. SOD | 0.06 | −0.146 | −0.218 | .363* | .635** | 0.234 | −0.153 | −.453** | 0.1 | −0.138 | −.591** | .366* | .494** | 0.048 | 1 | ||
| AKI | 16. Urinary NGAL | .506** | 0.142 | .699** | −.432** | 0.144 | 0.119 | −0.308 | .410* | .788** | .401* | .609** | .787** | −.712** | −.780** | −0.074 | 1 |
TSA: transferrin saturation; CPK: creatine phosphokinase; Vit D: vitamin D; 25(OH)D3: 25-dihyrdoxyvitamin D3; 1,25(OH)2D3: 1,25-dihydroxyvitamin D3; SOD: superoxide dismutase; NGAL: neutrophil gelatinase-associated lipocalin
*P < 0.05, ** P < 0.01, ***P < 0.001 (2-tailed), Pearson correlation coefficients (n = 36).
Increased levels of serum iron (r = 0.506, CI 0.213 to 0.716, P = 0.002), serum ferritin (r = 0.506, CI 0.213 to 0.716, P = 0.002), serum catalytic iron (r = 0.410, CI 0.094 to 0.651, P = 0.013), urine catalytic iron (r = 0.788, CI 0.620 to 0.887, P < 0.001), and decreased serum hepcidin levels (r = 0.432, CI −0.666 to −0.121, P = 0.008) were associated with increased urinary NGAL levels indicating AKI (Table 2). Similarly, increased oxidative stress as reflected by an increase in the tissue peroxidation level and a simultaneous reduction in the antioxidant level were associated with AKI (Table 2).
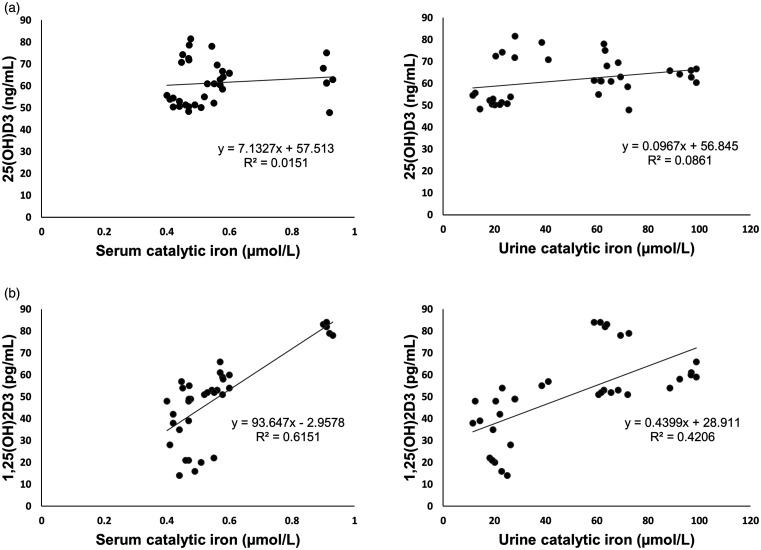
Correlation of vitamin D with catalytic iron, oxidative stress, and AKI markers
25-hydroxyvitamin D3 showed no clear relationship with catalytic iron. Contrarily, there existed a strong, positive linear association between 1,25-dihydroxyvitamin D3 and catalytic iron levels (Figure 10). Although, both 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 showed no correlation with lipid peroxidation, they exhibited an inverse relationship with the antioxidant levels. Furthermore, they correlated positively with urinary NGAL, indicating that their levels increased with the onset of renal failure (Table 2).
Figure 10.
Scatter plot depicting correlation between vitamin D and catalytic iron. (a) 25(OH)D3: 25-hydroxyvitamin D3 versus serum catalytic iron and urine catalytic iron. No clear relationship is noted between 25-dihydroxyvitamin D and catalytic iron levels. (b) 1,25(OH)2D3: 1,25-dihydroxyvitamin D3 versus serum catalytic iron and urine catalytic iron. There exists a strong, positive linear association between 1,25-dihydroxyvitamin D3 and both serum and urine catalytic iron levels.
Prediction of AKI (urinary NGAL) by the collective effect of catalytic iron and iron-regulatory proteins, vitamin D, and oxidative stress markers
Results of the multiple regression analysis indicated that urine catalytic iron, serum iron, and tissue lipid peroxidation were associated with a higher likelihood of developing AKI, whereas the antioxidants including the catalase and superoxide dismutase were associated with increased urinary NGAL levels (Table 3).
Table 3.
Multivariate linear regression analysis of the relationship between urinary NGAL and its potential determinants
|
Unstandardized Coefficients |
Standardized Coefficients |
t-test | P value |
95% confidence interval for B |
|||
|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | Lower bound | Upper bound | |||
| (Constant) | 3.738 | 4.573 | 0.817 | 0.422 | −5.722 | 13.198 | |
| SI (µg/dL) | 0.03 | 0.011 | 0.139 | 2.855 | 0.009*** | .008 | .052 |
| TSAT (%) | −0.038 | 0.025 | −0.046 | −1.539 | 0.138 | −.090 | .013 |
| Ferritin (ng/mL) | 0 | 0.001 | 0.013 | 0.189 | 0.852 | −.002 | .002 |
| Hepcidin (pg/mL) | −0.003 | 0.02 | −0.009 | −0.128 | 0.899 | −.044 | .039 |
| Serum catalytic iron (µmoI/L) | 1.425 | 4.563 | 0.032 | 0.312 | 0.758 | −8.014 | 10.864 |
| Urine catalytic iron (µmoI/L) | 0.098 | 0.023 | 0.382 | 4.318 | 0.001*** | .051 | .144 |
| 25(OH)D3 (ng/mL) | 0.003 | 0.037 | 0.004 | 0.091 | 0.928 | −.074 | .080 |
| 1,25(OH)2D3 (pg/mL) | −0.016 | 0.054 | −0.044 | −0.305 | 0.763 | −.128 | .095 |
| Tissue peroxidation(mg/mg protein) | 128.352 | 15.957 | 0.483 | 8.044 | 0.001*** | 95.343 | 161.362 |
| Tissue thiol (units/mg protein) | 142.651 | 89.114 | 0.206 | 1.601 | 0.123 | −41.694 | 326.997 |
| Catalase (units/mg protein) | −6.484 | 1.545 | −0.462 | −4.196 | 0.001*** | −9.681 | −3.288 |
| SOD (units/mg protein) | −31.757 | 6.615 | −0.389 | −4.801 | 0.001*** | −45.441 | −18.073 |
NGAL: neutrophil gelatinase-associated lipocalin; SI serum iron; TSAT: transferrin saturation; 25(OH)D3: 25-hydroxyvitamin D3; 1,25(OH)2D3: 1,25-dihydroxyvitamin D3; SOD: superoxide dismutase.
*P < 0.05, ** P < 0.01, ***P < 0.001 (2-tailed).
Discussion
In a case-control experimental study using an iohexol-induced AKI rat model, we found that higher catalytic iron levels were associated with increased oxidative stress and development of AKI. There occurred a corresponding increase in the endogenous vitamin D levels. In addition, a substantial accumulation of iron in the injured kidney was detected along with an altered gene expression of iron-regulatory proteins and CYPB21 (1 α-hydroxylase).
Impact of catalytic iron and other iron-regulating proteins on iohexol-treated rat kidneys
Iohexol, an iodinated, non-ionic monomeric radiocontrast agent causes acute kidney injury by a variety of mechanisms.29,30 One of the suggested mechanisms is the nephrotoxic role of iron in contrast-induced AKI.7 The unique property of iron to undergo redox cycling between its ferrous and ferric states is vital for many biological processes. However, traces of free catalytic iron can also have a detrimental effect by participating in the generation of powerful reactive oxygen species (ROS) such as hydroxyl radicals via the Fenton/Haber–Weiss reaction and iron-oxygen complexes such as ferryl or perferryl ions that cause lipid peroxidation of cellular macromolecules and influence local inflammation and vasoconstriction.24,31
Iron-mediated oxidative stress (ferroptosis) has been shown to induce tubular injury in several murine and rat models of AKI.10,32–37 Besides, living cells protect themselves from the harmful effects of oxidative stress by tightly controlling iron homeostasis, consisting of iron uptake, utilization, and storage. In the process, iron is usually bound to intracellular ferritin, an iron-sequestering protein and circulating transferrin for transport or utilization.38 Additionally, the renal tubular cells contain an endogenous antioxidant defense system consisting of superoxide dismutase, catalase, and glutathione S transferase that helps mitigate oxidant-mediated damage.39
This study illustrated that increased levels of serum iron and serum ferritin and lower hepcidin levels were associated with raised levels of both serum and urine catalytic iron levels. Also, elevated serum catalytic iron levels tended to be associated with higher urine catalytic iron levels (Table 2). These were accompanied by an increase in the lipid peroxidation and a simultaneous decrease in the antioxidant levels and a positive association with urinary NGAL reflecting AKI. This is consistent with other studies which showed that catalytic iron increased oxidative stress40 and that an iron chelator reduced lipid peroxidation and improved renal functions in an animal model of AKI.41
Although the mechanism of catalytic iron induction is not investigated in the present study, it is known that the source of catalytic iron is either from the systemic circulation following hemolysis, rhabdomyolysis,33,35 or from the mitochondria within the cells.42,43 Despite an initial raise in the hemoglobin levels, there was no biochemical evidence to support hemolysis or rhabdomyolysis in this study as demonstrated by normal haptoglobin levels and absence of raised serum CPK levels, respectively. The renal cellular injury induced by iohexol is thought to have triggered mitochondrial release of free iron into the cytosol followed by secretion into the tubular lumen and urine. In line with this view, we found urinary catalytic iron to be considerably high. Specifically, iron has been shown to be excreted in urine along with increased free iron in the kidney tissue in several animal models of AKI.10,33,34
In addition, our study also revealed elevated serum iron levels without any change in the transferrin saturation. Because of a high iron-binding capacity, transferrin plays a protective scavenger role of sequestering free iron44 and transferrin saturation increases with elevated iron levels, possibly reflecting an adaptive response to minimize iron-mediated renal tubular toxicity.32 It could, therefore, be speculated that absence of elevated transferrin levels in our study presumably accounted for the increased availability of non-transferrin bound free catalytic iron in the serum.
Similarly, we found a significant increase in the serum ferritin levels which correlated with the decrease in the antioxidant levels and AKI. This is likely to be an effect rather than the cause, indicating probably an attempt to sequester the free catalytic iron. This has been pointed out by some researchers who have documented increased oxidant-mediated iron release from storage proteins and a parallel increase in the ferritin synthesis.45,46
Further, the expression of the hepcidin gene was significantly downregulated along with a reduction in the serum hepcidin levels in this study. This probably is due to renal hypoxia resulting from iohexol-induced renal vasoconstriction and ischemia.47,48 Hepcidin is a negative regulator of intestinal iron absorption and iron release from macrophages, by inactivating the iron export protein ferroportin.49,50 Hepcidin expression is induced by iron storage and inflammation and suppressed by hypoxia and anemia.51 It is thought to confer renoprotection by sequestering intracellular iron, thereby limiting oxidative stress and free radical injury52 as well as by inducing H-ferritin.53 Lower serum hepcidin levels in our study presumably contributed to AKI due to inadequate sequestration of free iron.
Of note, serum catalytic iron and serum iron levels showed an abrupt rise at 2 hours following iohexol use. On the other hand, urinary catalytic iron reached a peak concentration only at 4 hours. This delay could be explained partly by the time taken by the serum catalytic iron to enter the proximal tubular cells following glomerular filtration.10 It is also probable that some catalytic iron could have formed within the cytosol of the renal tubular cells from the iron released from the injured mitochondria containing the cytochrome complex42,43 causing a further time lapse in the appearance of urinary catalytic iron.
On a univariate analysis, increased levels of serum catalytic iron and urine catalytic iron were associated with elevated urinary NGAL levels reflecting AKI. However, in a multivariate regression analysis, only urinary catalytic iron was shown to independently predict AKI. Statistically, this could be attributed to the effect of modification and interaction between the various independent factors considered in the multiple regression model, including serum and urine catalytic iron and urinary NGAL. Importantly, statistical significance does not necessarily imply practical significance.54 Applying the relationship of the mathematical model to a biological (mechanistic) system, where there is multicausality, and the events or cellular metabolic processes change continuously over time, could be misleading.55 In this study, serum catalytic iron did not correlate with lipid peroxidation, whereas urinary catalytic iron showed a positive correlation with lipid peroxidation. This hints at the possibility that the urinary catalytic iron released locally in the kidney as part of the pathophysiology of AKI could have induced oxidative stress in the renal cells rather than by the catalytic iron present in the serum. Importantly, urinary NGAL, a 25-kDa lipocalin-2, and a scavenger of labile iron siderophore56 readily bind urine catalytic iron, while the unbound catalytic iron is reabsorbed by the thick ascending limb of the loop of Henle (TAL) and cortical collecting tubule.57 Consequently, as AKI progresses, less iron is reabsorbed and excess catalytic iron is released into the urine.58 These facts perhaps account for the positive prediction of AKI by urinary catalytic iron.
Taking all these findings into consideration, it is possible to establish that catalytic iron and iron regulatory pathways play a crucial role in AKI pathophysiology.6
Induction of endogenous vitamin D
Vitamin D is a prohormone synthesized endogenously from 7-dehydrocholesterol in the skin. Upon exposure to ultraviolet rays (270–300 nm), 7-dehydrocholesterol is photolytically converted to previtamin D3 which undergoes thermal isomerization to vitamin D3.59,60 Vitamin D3 is then hydroxylated by the cytochrome P450s (CYP2R1 and CYP27A1)61 in the liver to 25-hydroxyvitamin D3, the major circulating form of vitamin D. This inactive metabolite binds to vitamin D-binding protein (DBP), gets filtered in the glomerulus, and subsequently undergoes endocytosis by megalin-cubulin62 in the apical membrane of the renal proximal tubular cells.63,64 Intracellularly, 25-hydroxyvitamin D3 is hydroxylated further by 1 α-hydroxylase to form the active metabolite, 1,25-dihydroxyvitamin D.65–67
1 α-hydroxylase, also a part of the cytochrome P450 complex (CYP27B1), is the rate-limiting enzyme located in the inner membrane of the mitochondria, and functions as a mixed-function oxidase.68,69 Although, predominantly expressed in the proximal renal tubules, its activity has been detected in the distal nephron70 as well as in extrarenal tissues including monocytes,71 keratinocytes,72 colon, lung, and parathyroid cells.73,74 It is worth noting that, while the renal 1 α-hydroxylase performs endocrine functions, the extrarenal 1 α-hydroxylase principally acts in an autocrine or paracrine fashion with cell-specific functions.75 The activity of the 1 α-hydroxylase gene is tightly regulated by the serum levels of calcium, phosphorus, 1,25-dihydroxyvitamin D3, parathyroid hormone (PTH), calcitonin, and bone-derived fibroblast growth factor 23 (FGF23).76–80
Bioactive vitamin D3, also known as Calcitriol, is a secosteroid hormone with pleiotropic effects81,82 and exerts its actions through the vitamin D receptor. In addition to the direct calciotropic effect of enhancing calcium absorption from the gut by modulating parathyroid hormone secretion,83 it also possesses antioxidant,15,16anti-inflammatory, and antiproliferative properties84–86 and plays a role in the prevention of cancer.87
Based on these facts, it is apparent that vitamin D offers cytoprotection in contrast to catalytic iron which causes cytotoxicity. Moreover, unlike in chronic kidney disease, the role of vitamin D in AKI is not well defined88 and it remains unclear whether vitamin D can ameliorate contrast-induced AKI in Wistar rats.13
This study noticed that both 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 levels tended to increase with an increase in the catalytic iron and urinary NGAL levels as well as with a decrease in the antioxidant levels. At the same time, there was a significant upregulation in the CYPB21 (1 α-hydroxylase) gene in the renal tubular cells. Since it is known that vitamin D has a cytoprotective effect, it is conjectured that the injured cells generated endogenous vitamin D to protect themselves from the iron-mediated oxidant damage. In support of these findings, vitamin D and its synthetic analogues have been shown to prevent renal injury and exert a renoprotective effect in several in vitro and in vivo models of glomerular and tubular injury, both in acute and chronic situations. For instance, calcitriol has been demonstrated to exhibit antiproliferative effects in vitro using opossum kidney (OK) cells having characteristics of proximal tubular cells.89 In a study by Ari et al.,90 Paricalcitol, a novel synthetic vitamin D analogue mitigated contrast-induced nephropathy by inhibiting renal and systemic oxidative stress. Vitamin D, by virtue of its antioxidant effects, offers protection against aminoglycoside-induced nephrotoxicity,91,92cyclosporine-mediated kidney injury,93rhabdomyolysis-induced AKI,94 and lipopolysaccharide-induced AKI.95 Additionally, vitamin D deficiency seemed to aggravate renal inflammation, cell proliferation, and cell injury in experimental ischemia-reperfusion injury, indicating the need for sufficient vitamin D levels.96 Apart from these findings, vitamin D has also been documented to prevent active Heymann nephritis97 and experimental murine lupus,98 decrease podocyte loss99 and glomerulosclerosis100 in subtotally nephrectomized rats, lower albuminuria in rats with diabetic nephropathy,101 attenuate renal interstitial fibrosis in obstructive nephropathy,102 and retard the progression of renal insufficiency in uremic rats.103
There is some proof to suggest the protective effects of vitamin D against catalytic iron-induced damage in non-renal cells as well, yet the evidence regarding the same appears to be weak.104,105 Current data indicate that the levels of vitamin D also have an influence on the occurrence and severity of AKI in humans. The study of Zapatero et al.106 has demonstrated renal failure to be significantly more frequent and to cause more mortality in patients with 25-hydroxyvitamin D < 10.9 ng/mL than in those with concentrations of ≥10.9 ng/mL (29% vs.13%). In another clinical study, patients with a severe form of AKI necessitating hemodialysis were noted to have low concentrations of both 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3.107 Similar findings still need to be demonstrated in experimental animals.
Interestingly, 1,25-dihydroxyvitamin D3 displayed a strongly positive correlation with catalytic iron in this study, while 25-hydroxyvitamin D3 lacked such an association (Figure 9) despite the fact that intrarenal 25-hydroxyvitamin D3 is the precursor of 1,25-dihydroxyvitamin D3. Nevertheless, both were noted to correlate strikingly with urinary NGAL and AKI. Analysis of the temporal relationship (Figures 7 and 9) reveals that 1,25-dihydroxyvitamin D3 levels increased rapidly along with serum and urine catalytic iron within the first 2 to 4 hours of iohexol use. 25-hydroxyvitamin D3, on the other hand, attained a maximum concentration only at 12 hours. This disparity in the timing of maximal concentration likely explains the presence of an association between catalytic iron and 1,25-dihydroxyvitamin D3 but not with 25-hydroxyvitamin D3, albeit not statistically evident. Besides, it can be hypothesized that 25-hydroxyvitamin D3, being inactive, might not have had any meaningful direct impact on the catalytic iron accounting for the absence of an abrupt increase in its concentration, and this needs to be proven further. It is also conceivable that the already existing cytosolic 25-hydroxyvitamin D3 was used by the injured renal cells as the substrate to synthesize active 1,25-dihdyroxyvitamin D3 leading to relatively higher concentrations of 1,25-hydroxyvitamin D3 compared to 25-hydroxyvitamin D3. In this context, it is worth mentioning that 25-hydroxyvitamin D3 has a half-life of about 14–20 days and circulates in nmol/L concentrations in marked contrast to 1,25-dihdyroxyvitamin D3 which has a shorter half-life of only 4–15 hours and is present in much lower concentrations (pmol/L)108 in humans. Therefore, variations in the 1,25-dihydroxyvitamin D3 levels can theoretically occur more quickly than 25-hydroxyvitamin D3 levels.
A few studies have described the reduction of malondialdehyde levels by vitamin D,90,109 but our study revealed no such association with lipid peroxidation. Nonetheless, both 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 exhibited an inverse relationship with the antioxidants. These data conflict with findings from other studies which have reported vitamin D to increase antioxidants such as glutathione and gamma glutamine transferase (GSH).92 It can be conceptualized that during the early phase of renal injury, the pre-existing cytosolic antioxidants were utilized by the injured cells to overcome ferrotoxicity, thereby lowering their concentrations. Simultaneously, the cells also synthesized active 1,25-hydroxyvitamin D3 leading to excess quantities. Accordingly, this discrepancy in the vitamin D and antioxidant levels during the early phase of injury is reflected statistically by a negative correlation. However, during the later recovery phase of AKI, the antioxidants increased persistently along with both 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 as evident from Figures 8 and 9, respectively.
To the best of our knowledge, this study is the first to describe the dynamic relationship between both iron and vitamin D in the context of AKI in Wistar rats. Distinctly, the biochemical findings were corroborated by histopathological evidence of tubular injury and repair. Also, this experimental animal study helps one to visualize these renal histological changes induced by iohexol which otherwise would be difficult to observe in patients with contrast-induced acute kidney injury in whom renal biopsy is usually not performed. Next, the determination of changes in the major iron-regulating proteins including transferrin, hepcidin, and ferritin in addition to the changes in the catalytic iron adds more value to this study. Lastly, despite its widespread use in diagnosing clinical AKI, the levels of serum creatinine tend to raise a few days following significant renal damage.110 To overcome this caveat, urinary NGAL which is an early and sensitive AKI marker was used for correlation and regression analyses in this study, particularly to conform to the rapid changes in relation to the iron, vitamin D, and oxidative stress levels occurring within hours after renal insult.111 Moreover, by its ability to bind siderophores which are the small iron-binding molecules, NGAL is involved in the transport of iron to and from cells112 and, thereby by sequestering iron, it may abate iron-induced injury.113,114 Hence, its utility seems most ideal in this study involving iron regulation.
The present study has some limitations. Firstly, it is important to realize that the findings of animal studies do not always translate to human subjects.115,116 Next, the radiocontrast agent, iohexol causes acute tubular necrosis by several mechanisms including alterations in renal hemodynamics and viscosity leading to renal medullary ischemia and by direct tubulotoxicity.29 The relative contribution of each mechanism alone is, however, not known.30,117 Hence, assessing the potential mechanism of catalytic iron-induced cytotoxicity alone using cultured renal cells in vitro in the absence of several confounding variables found in vivo may have been more informative. Furthermore, although the activity of endogenous vitamin D3 was demonstrated along with the clear upregulation of its mRNA and protein in this animal model of AKI, it would have been worthwhile to explore the effects of exogenously administered vitamin D on the functional and histological protection of kidneys in AKI.
Lastly, this study, by and large, shows an association between vitamin D and catalytic iron in an experimental AKI model. It cannot, however, be concluded that vitamin D has a protective role against ferrotoxicity merely based on this finding. Unless supported by a plausible proof, these limitations could preclude the utility of outcomes of this study for translational purposes. Regardless of this shortcoming, taking into consideration the pleiotropic effects of calcitriol and its protective role in the various settings of AKI described above as well as our own study findings of upregulation of 1 α-hydroxylase gene and an association of 1,25-dihydroxyvitamin D3 with catalytic iron, antioxidants, and urinary NGAL, it seems logical to implicate vitamin D in maintaining resistance against iron-induced nephrotoxicity. A definitive conclusion in this regard would entail demonstrating the link between iron and vitamin D pathophysiologic mechanisms and validation in experimental and clinical studies.
Based on these observations, there are a few recommendations for further research. The severity of AKI is likely to be increased in vitamin D-depleted rats than in non-depleted animals. It is also possible that the efficiency of renal recovery after injury is slower in the case of vitamin D deficiency. Necessary investigations are required in order to confirm or refute these hypotheses. Crucially, given the complexity of the pathogenesis of AKI, it is hard to envision a “silver bullet” for its optimal treatment. Rather, drugs targeting multiple pathways might prove more effective. Considering this, it seems desirable to investigate the beneficial effects of vitamin D in comparison with iron chelators to attenuate ferrotoxicity, preferably in various settings and severity of AKI. It might also be helpful to examine pre- and post-AKI vitamin D levels to understand if vitamin D uptrends among several other endogenous factors in AKI and to ascertain its preventive or causative potential. Finally, it needs to be seen if the findings of this animal study could be translated to humans and to facilitate therapeutic interventions by validating in large-scale clinical trials. Future research involving interaction between vitamin D and iron at a critical juncture in AKI timeline is warranted.
Conclusions
In a rat model of iohexol-induced AKI, a considerable increase in the catalytic iron, tissue lipid peroxidation, and vitamin D levels was observed together with deposition of iron and altered gene expression of iron-regulating proteins and CYPB21 (1 α-hydroxylase) in the injured renal tubular cells. In a multivariate linear regression analysis, serum iron, catalytic iron, and lipid peroxidation independently and positively predicted urinary NGAL, the AKI biomarker. Notably, the vitamin D levels remained elevated until the functional and histologic recovery of AKI that ensued a progressive reduction in the catalytic iron levels.
Overall, this study underscores the nephrotoxic potential of catalytic iron besides documenting a concomitant induction of endogenous vitamin D for possible renoprotection. Additional research involving cross-talk between vitamin D and iron, preferably in vitro and in large clinical trials is warranted.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220946271 for Ferrotoxicity and its amelioration by endogenous vitamin D in experimental acute kidney injury by Chandrashekar Annamalai, Rajesh N Ganesh and Pragasam Viswanathan in Experimental Biology and Medicine
ACKNOWLEDGEMENTS
The authors would like to thank the Participating Investigators, Dr. Mohan Rajapurkar and Dr. Banibrata Mukhopadhyay, Muljibhai Patel Urological Hospital, Nadiad, Gujarat, India for carrying out the biochemical analysis of iron indices including catalytic iron and vitamin D as well as for critically reviewing the study proposal and manuscript. They also thank Badrinathan Sridharan, Gokulakannan Ragavan, Ashish Rao and Subhadip Das, VIT, Vellore, India for participating in the animal experiments and gene expression analyses. They express their gratitude to Dr. Lakshman, Professor and Officer-in-charge of Electron Microscope Laboratory, Ruska Labs, Hyderabad, India for performing electron microscopic examination of the rat kidneys. Last but not the least, they are indebted to Dr. Sachin Pandey, Associate Professor (Biostatistics), Department of Community Medicine, Chhattisgarh Institute of Medical Sciences, Bilaspur, India and Dr. K.P. Suresh, Principal Scientist and Biostatistician, National Institute of Veterinary Epidemiology and Disease Informatics (NIVEDI), Bangalore, India for their help with statistical analyses.
Authors’ contributions
CA and PV conceived and designed the study. CA carried out experiments and was assisted by RG. The results were interpreted by CA, RG, and PV. The manuscript was written by CA with inputs from all the authors. PV supervised the project. All authors provided critical feedback and approved the final version of the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partly supported by Vellore Institute of Technology and an educational grant from Novartis, India [114885].
ORCID iD
Pragasam Viswanathan https://orcid.org/0000-0003-3796-5589
SUPPLEMENTAL MATERIAL
Supplemental material for this article is available online.
References
- 1.Sawhney S, Mitchell M, Marks A, Fluck N, Black C. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open 2015; 5:e006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009; 53:961–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinsey GR, Okusa MD. Pathogenesis of acute kidney injury: foundation for clinical practice. Am J Kidney Dis 2011; 58:291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagshaw SM, Wald R. Acute kidney injury: timing of renal replacement therapy in AKI. Nat Rev Nephrol 2016; 12:445–6 [DOI] [PubMed] [Google Scholar]
- 5.Walker VJ, Agarwal A. Targeting iron homeostasis in acute kidney injury. Semin Nephrol 2016; 36:62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Rawn JD, Frendl G, Waikar SS. Increased plasma catalytic iron in patients may mediate acute kidney injury and death following cardiac surgery. Kidney Int 2015; 87:1046–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lele SS, Mukhopadhyay BN, Mardikar MM, Patel TA, Vasavada AK, Banker DN, Kapasi KD, Chauhan VC, Chawla KC, Raju SR, Hiremath SS, Chinchole SS, Rajapurkar MM. Impact of catalytic iron on mortality in patients with acute coronary syndrome exposed to iodinated radiocontrast-The Iscom study. Am Heart J 2013; 165:744–51 [DOI] [PubMed] [Google Scholar]
- 8.Akrawinthawong K, Akrawinthawong K, Shaw MK, Kachner J, Apostolov EO, Basnakian AG, Shah S, Tilak J, McCullough PA. Urine catalytic iron and neutrophil gelatinase-associated lipocalin as companion early markers of acute kidney injury after cardiac surgery: a prospective pilot study. Cardiorenal Med 2013; 3:7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah SV, Rajapurkar MM, Baliga R. The role of catalytic iron in acute kidney injury. Clin J Am Soc Nephrol 2011; 6:2329–31 [DOI] [PubMed] [Google Scholar]
- 10.Baliga R, Ueda N, Shah SV. Increase in bleomycin-detectable iron in ischaemia/reperfusion injury to rat kidneys. Biochem J 1993; 291:901–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martines AM, Masereeuw R, Tjalsma H, Hoenderop JG, Wetzels JF, Swinkels DW. Iron metabolism in the pathogenesis of iron-induced kidney injury. Nat Rev Nephrol 2013; 9:385–98 [DOI] [PubMed] [Google Scholar]
- 12.Swaminathan S. Iron homeostasis pathways as therapeutic targets in acute kidney injury. Nephron 2018; 140:156–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perco P, Mayer G. Endogenous factors and mechanisms of renoprotection and renal repair. Eur J Clin Invest 2018; 48:e12914. [DOI] [PubMed] [Google Scholar]
- 14.Lips P. Vitamin D physiology. Prog Biophys Mol Biol 2006; 92:4–8 [DOI] [PubMed] [Google Scholar]
- 15.Zhong W, Gu B, Gu Y, Groome LJ, Sun J, Wang Y. Activation of vitamin D receptor promotes VEGF and CuZn-SOD expression in endothelial cells. J Steroid Biochem Mol Biol 2014; 140:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sardar S, Chakraborty A, Chatterjee M. Comparative effectiveness of vitamin D3 and dietary vitamin E on peroxidation of lipids and enzymes of the hepatic antioxidant system in Sprague–Dawley rats. Int J Vitam Nutr Res 1996; 66:39–45 [PubMed] [Google Scholar]
- 17.National Research Council Committee for the Update of the Guide for the C, Use of Laboratory Animals. The National Academies Collection: Reports funded by National Institutes of Health In: Guide for the care and use of laboratory animals. Washington (DC): National Academies Press, 2011, pp.11--40. [Google Scholar]
- 18.Singh AP, Junemann A, Muthuraman A, Jaggi AS, Singh N, Grover K, Dhawan R. Animal models of acute renal failure. Pharmacol Rep 2012; 64:31–44 [DOI] [PubMed] [Google Scholar]
- 19.Suvarna KS, Layton C, Bancroft JD. Bancroft's theory and practice of histological techniques E-book. Amsterdam: Elsevier Health Sciences, 2018 [Google Scholar]
- 20.Sridharan B, Michael ST, Arya R, Mohana Roopan S, Ganesh RN, Viswanathan P. Beneficial effect of citrus Limon peel aqueous methanol extract on experimentally induced urolithic rats. Pharm Biol 2016; 54:759–69 [DOI] [PubMed] [Google Scholar]
- 21.Biassoni R, Raso A. Quantitative real-time PCR: methods and protocols. New York: Springer, 2014 [Google Scholar]
- 22.Wilson K, Walker J. Principles and techniques of biochemistry and molecular biology. Cambridge: Cambridge University Press, 2010. [Google Scholar]
- 23.Orsonneau JL, Douet P, Massoubre C, Lustenberger P, Bernard S. An improved pyrogallol red-molybdate method for determining total urinary protein. Clin Chem 1989; 35:2233–6 [PubMed] [Google Scholar]
- 24.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Meth Enzymol 1990; 186:1–85 [DOI] [PubMed] [Google Scholar]
- 25.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analyt Biochem 1979; 95:351–8 [DOI] [PubMed] [Google Scholar]
- 26.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82:70–7 [DOI] [PubMed] [Google Scholar]
- 27.Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972; 47:389–94 [DOI] [PubMed] [Google Scholar]
- 28.Winterbourn CC, Hawkins RE, Brian M, Carrell RW. The estimation of red cell superoxide dismutase activity. J Lab Clin Med 1975; 85:337–41 [PubMed] [Google Scholar]
- 29.Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J 2012; 33:2007–15 [DOI] [PubMed] [Google Scholar]
- 30.McCullough PA, Stacul F, Davidson C, Becker CR, Adam A, Lameire N, Tumlin J. Contrast-induced nephropathy: clinical insights and practical guidance – a report from the CIN consensus working panel – overview. Am J Cardiol 2006; 98:2K–4K [Google Scholar]
- 31.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2012; 2:1303–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Weinlich R, Vanden Berghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Brasen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR, Krautwald S. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A 2014; 111:16836–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baliga R, Zhang Z, Baliga M, Shah SV. Evidence for cytochrome P-450 as a source of catalytic iron in myoglobinuric acute renal failure. Kidney Int 1996; 49:362–9 [DOI] [PubMed] [Google Scholar]
- 34.Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int 1998; 53:394–401 [DOI] [PubMed] [Google Scholar]
- 35.Paller MS. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am J Physiol 1988; 255:F539–44 [DOI] [PubMed] [Google Scholar]
- 36.Kirschner RE, Fantini GA. Role of iron and oxygen-derived free radicals in ischemia-reperfusion injury. J Am Coll Surg 1994; 179:103–17 [PubMed] [Google Scholar]
- 37.Shah SV, Walker PD. Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am J Physiol 1988; 255:F438–43 [DOI] [PubMed] [Google Scholar]
- 38.Zhao N, Enns CA. Iron transport machinery of human cells: players and their interactions. Curr Top Membr 2012; 69:67–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobashi K, Ghosh B, Orak JK, Singh I, Singh AK. Kidney ischemia-reperfusion: modulation of antioxidant defenses. Mol Cell Biochem 2000; 205:1–11 [DOI] [PubMed] [Google Scholar]
- 40.Bakris GL, Lass N, Gaber AO, Jones JD, Burnett JC., Jr., Radiocontrast medium-induced declines in renal function: a role for oxygen free radicals. Am J Physiol 1990; 258:F115–20 [DOI] [PubMed] [Google Scholar]
- 41.Paller MS, Hedlund BE. Role of iron in postischemic renal injury in the rat. Kidney Int 1988; 34:474–80 [DOI] [PubMed] [Google Scholar]
- 42.Ueda N, Guidet B, Shah SV. Gentamicin-induced mobilization of iron from renal cortical mitochondria. Am J Physiol 1993; 265:F435–F39 [DOI] [PubMed] [Google Scholar]
- 43.Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. Role of cytochrome P-450 as a source of catalytic iron in cisplatin-induced nephrotoxicity. Kidney Int 1998; 54:1562–9 [DOI] [PubMed] [Google Scholar]
- 44.Cazzola M, Huebers HA, Sayers MH, MacPhail AP, Eng M, Finch C. Transferrin saturation, plasma iron turnover, and transferrin uptake in normal humans. Blood 1985; 66:935–9 [PubMed] [Google Scholar]
- 45.Bando Y, Aki K. Superoxide-mediated release of iron from ferritin by some flavoenzymes. Biochem Biophys Res Commun 1990; 168:389–95 [DOI] [PubMed] [Google Scholar]
- 46.Lin F, Girotti AW. Elevated ferritin production, iron containment, and oxidant resistance in hemin-treated leukemia cells. Arch Biochem Biophys 1997; 346:131–41 [DOI] [PubMed] [Google Scholar]
- 47.Heyman SN, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis of radiocontrast nephropathy. Clin J Am Soc Nephrol 2008; 3:288–96 [DOI] [PubMed] [Google Scholar]
- 48.Sendeski MM, Persson AB, Liu ZZ, Busch JF, Weikert S, Persson PB, Hippenstiel S, Patzak A. Iodinated contrast media cause endothelial damage leading to vasoconstriction of human and rat vasa recta. Am J Physiol Renal Physiol 2012; 303:F1592–F98 [DOI] [PubMed] [Google Scholar]
- 49.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004; 306:2090–3 [DOI] [PubMed] [Google Scholar]
- 50.Ramey G, Deschemin J-C, Durel B, Canonne-Hergaux F, Nicolas G, Vaulont S. Hepcidin targets ferroportin for degradation in hepatocytes. Haematologica 2010; 95:501–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 2002; 110:1037–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho J, Reslerova M, Gali B, Gao A, Bestland J, Rush DN, Nickerson PW, Rigatto C. Urinary hepcidin-25 and risk of acute kidney injury following cardiopulmonary bypass. Clin J Am Soc Nephrol 2011; 6:2340–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scindia Y, Dey P, Thirunagari A, Liping H, Rosin DL, Floris M, Okusa MD, Swaminathan S. Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis. J Am Soc Nephrol 2015; 26:2800–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leppink J, Winston K, O'Sullivan P. Statistical significance does not imply a real effect. Perspect Med Educ 2016; 5:122–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siemiatycki J, Thomas DC. Biological models and statistical interactions: an example from multistage carcinogenesis. Int J Epidemiol 1981; 10:383–7 [DOI] [PubMed] [Google Scholar]
- 56.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 2007; 18:407–13 [DOI] [PubMed] [Google Scholar]
- 57.Yang J, Mori K, Li JY, Barasch J. Iron, lipocalin, and kidney epithelia. Am J Physiol Renal Physiol 2003; 285:F9–18 [DOI] [PubMed] [Google Scholar]
- 58.Takebayashi S, Jimi S, Segawa M, Takaki A. Mitochondrial DNA deletion of proximal tubules is the result of itai-itai disease. Clin Exp Nephrol 2003; 7:18–26 [DOI] [PubMed] [Google Scholar]
- 59.Holick MF, Frommer JE, McNeill SC, Richtand NM, Henley JW, Potts JT., Jr., Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem Biophys Res Commun 1977; 76:107–14 [DOI] [PubMed] [Google Scholar]
- 60.Okano T, Yasumura M, Mizuno K, Kobayashi T. Photochemical conversion of 7-dehydrocholesterol into vitamin D3 in rat skins. J Nutr Sci Vitaminol 1977; 23:165–8 [DOI] [PubMed] [Google Scholar]
- 61.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A 2004; 101:7711–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaseda R, Hosojima M, Sato H, Saito A. Role of megalin and cubilin in the metabolism of vitamin D(3). Ther Apheres Dial 2011; 15:14–7 [DOI] [PubMed] [Google Scholar]
- 63.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999; 96:507–15 [DOI] [PubMed] [Google Scholar]
- 64.Takemoto F, Shinki T, Yokoyama K, Inokami T, Hara S, Yamada A, Kurokawa K, Uchida S. Gene expression of vitamin D hydroxylase and megalin in the remnant kidney of nephrectomized rats. Kidney Int 2003; 64:414–20 [DOI] [PubMed] [Google Scholar]
- 65.Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature 1970; 228:764–6 [DOI] [PubMed] [Google Scholar]
- 66.Takeyama K, Kato S. The vitamin D3 1alpha-hydroxylase gene and its regulation by active vitamin D3. Biosci Biotechnol Biochem 2011; 75:208–13 [DOI] [PubMed] [Google Scholar]
- 67.Zehnder D, Hewison M. The renal function of 25-hydroxyvitamin D3-1alpha-hydroxylase. Mol Cell Endocrinol 1999; 151:213–20 [DOI] [PubMed] [Google Scholar]
- 68.Paulson SK, DeLuca HF. Subcellular location and properties of rat renal 25-hydroxyvitamin D3-1 alpha-hydroxylase. J Biol Chem 1985; 260:11488–92 [PubMed] [Google Scholar]
- 69.Henry HL. Vitamin D hydroxylases. J Cell Biochem 1992; 49:4–9 [DOI] [PubMed] [Google Scholar]
- 70.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab 2001; 86:888–94 [DOI] [PubMed] [Google Scholar]
- 71.Smith SJ, Rucka AK, Berry JL, Davies M, Mylchreest S, Paterson CR, Heath DA, Tassabehji M, Read AP, Mee AP, Mawer EB. Novel mutations in the 1alpha-hydroxylase (P450c1) gene in three families with pseudovitamin D-deficiency rickets resulting in loss of functional enzyme activity in blood-derived macrophages. J Bone Miner Res 1999; 14:730–9 [DOI] [PubMed] [Google Scholar]
- 72.Bikle DD, Nemanic MK, Whitney JO, Elias PW. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry 1986; 25:1545–8 [DOI] [PubMed] [Google Scholar]
- 73.Hewison M, Zehnder D, Chakraverty R, Adams JS. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol 2004; 215:31–8 [DOI] [PubMed] [Google Scholar]
- 74.Delvin EE, Arabian A. Kinetics and regulation of 25-hydroxycholecalciferol 1 alpha-hydroxylase from cells isolated from human term decidua. Eur J Biochem 1987; 163:659–62 [DOI] [PubMed] [Google Scholar]
- 75.Bouillon R, Garmyn M, Verstuyf A, Segaert S, Casteels K, Mathieu C. Paracrine role for calcitriol in the immune system and skin creates new therapeutic possibilities for vitamin D analogs. Eur J Endocrinol 1995; 133:7–16 [DOI] [PubMed] [Google Scholar]
- 76.Friedlander EJ, Henry HL, Norman AW. Studies on the mode of action of calciferol. Effects of dietary calcium and phosphorus on the relationship between the 25-hydroxyvitamin D3-1alpha-hydroxylase and production of chick intestinal calcium binding protein. J Biol Chem 1977; 252:8677–83 [PubMed] [Google Scholar]
- 77.Fraser DR, Kodicek E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nature 1973; 241:163–6 [DOI] [PubMed] [Google Scholar]
- 78.Kawashima H, Torikai S, Kurokawa K. Calcitonin selectively stimulates 25-hydroxyvitamin D3-1 alpha-hydroxylase in proximal straight tubule of rat kidney. Nature 1981; 291:327–9 [DOI] [PubMed] [Google Scholar]
- 79.Brenza HL, DeLuca HF. Regulation of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys 2000; 381:143–52 [DOI] [PubMed] [Google Scholar]
- 80.Perwad F, Portale AA. Vitamin D metabolism in the kidney: regulation by phosphorus and fibroblast growth factor 23. Mol Cell Endocrinol 2011; 347:17–24 [DOI] [PubMed] [Google Scholar]
- 81.Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin D endocrine system. Endocr Rev 1995; 16:200–57 [DOI] [PubMed] [Google Scholar]
- 82.Bland R, Zehnder D, Hewison M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase along the nephron: new insights into renal vitamin D metabolism. Curr Opin Nephrol Hypertens 2000; 9:17–22 [DOI] [PubMed] [Google Scholar]
- 83.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res 1998; 13:325–49 [DOI] [PubMed] [Google Scholar]
- 84.Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev 1992; 13:719–64 [DOI] [PubMed] [Google Scholar]
- 85.Bouillon R, Bischoff-Ferrari H, Willett W. Vitamin D and health: perspectives from mice and man. J Bone Miner Res 2008; 23:974–9 [DOI] [PubMed] [Google Scholar]
- 86.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 2005; 26:662–87 [DOI] [PubMed] [Google Scholar]
- 87.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014; 14:342–57 [DOI] [PubMed] [Google Scholar]
- 88.Liu WC, Wu CC, Hung YM, Liao MT, Shyu JF, Lin YF, Lu KC, Yeh KC. Pleiotropic effects of vitamin D in chronic kidney disease. Clin Chim Acta 2016; 453:1–12 [DOI] [PubMed] [Google Scholar]
- 89.Weih M, Orth S, Weinreich T, Reichel H, Ritz E. Inhibition of growth by calcitriol in a proximal tubular cell line (OK). Nephrol Dial Transplant 1994; 9:1390–4 [PubMed] [Google Scholar]
- 90.Ari E, Kedrah A, Alahdab Y, Bulut G, Eren Z, Baytekin O, Odabasi D. Antioxidant and renoprotective effects of paricalcitol on experimental contrast-induced nephropathy model. Br J Radiol 2012; 85:1038–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bulut G, Basbugan Y, Ari E, Erten R, Bektas H, Alp HH, Bayram I. Paricalcitol may improve oxidative DNA damage on experimental amikacin-induced nephrotoxicity model. Renal Fail 2016; 38:751–8 [DOI] [PubMed] [Google Scholar]
- 92.Hur E, Garip A, Camyar A, Ilgun S, Ozisik M, Tuna S, Olukman M, Narli Ozdemir Z, Yildirim Sozmen E, Sen S, Akcicek F, Duman S. The effects of vitamin D on gentamicin-induced acute kidney injury in experimental rat model. Int J Endocrinol 2013; 2013:313528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, Kim SW. Paricalcitol attenuates cyclosporine-induced kidney injury in rats. Kidney Int 2010; 77:1076–85 [DOI] [PubMed] [Google Scholar]
- 94.Reis NG, Francescato HDC, de Almeida LF, Silva C, Costa RS, Coimbra TM. Protective effect of calcitriol on rhabdomyolysis-induced acute kidney injury in rats. Sci Rep 2019; 9:7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Du J, Jiang S, Hu Z, Tang S, Sun Y, He J, Li Z, Yi B, Wang J, Zhang H, Li YC. Vitamin D receptor activation protects against lipopolysaccharide-induced acute kidney injury through suppression of tubular cell apoptosis. Am J Physiol Renal Physiol 2019; 316:F1068–f77 [DOI] [PubMed] [Google Scholar]
- 96.de Bragança AC, Volpini RA, Canale D, Gonçalves JG, Shimizu MH, Sanches TR, Seguro AC, Andrade L. Vitamin D deficiency aggravates ischemic acute kidney injury in rats. Physiol Rep 2015; 3:e12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Branisteanu D, Leenaerts P, Van Damme B, Bouillon R. Partial prevention of active heymann nephritis by lα, 25 dihydroxyvitamin D3. Clin Exp Immunol 1993; 94:412–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lemire JM, Ince A, Takashima M. 1, 25-dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/1 mice. Autoimmunity 1992; 12:143–8 [DOI] [PubMed] [Google Scholar]
- 99.Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, Ritz E, Amann K. 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol 2004; 286:F526–33 [DOI] [PubMed] [Google Scholar]
- 100.Schwarz U, Amann K, Orth SR, Simonaviciene A, Wessels S, Ritz E. Effect of 1, 25 (OH) 2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int 1998; 53:1696–705 [DOI] [PubMed] [Google Scholar]
- 101.Eren Z, Günal MY, Bakir EA, Coban J, Çağlayan B, Ekimci N, Ethemoglu S, Albayrak O, Akdeniz T, Demirel GY, Kiliç E, Kantarci G. Effects of paricalcitol and aliskiren combination therapy on experimental diabetic nephropathy model in rats. Kidney Blood Press Res 2014; 39:581–90 [DOI] [PubMed] [Google Scholar]
- 102.Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 2006; 17:3382–93 [DOI] [PubMed] [Google Scholar]
- 103.Mizobuchi M, Morrissey J, Finch JL, Martin DR, Liapis H, Akizawa T, Slatopolsky E. Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol 2007; 18:1796–806 [DOI] [PubMed] [Google Scholar]
- 104.Uberti F, Morsanuto V, Bardelli C, Molinari C. Protective effects of 1α, 25‐dihydroxyvitamin D3 on cultured neural cells exposed to catalytic iron. Physiol Rep 2016; 4:e12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uberti F, Morsanuto V, Lattuada D, Colciaghi B, Cochis A, Bulfoni A, Colombo P, Bolis G, Molinari C. Protective effects of vitamin D 3 on fimbrial cells exposed to catalytic iron damage. J Ovarian Res 2016; 9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zapatero A, Dot I, Diaz Y, Gracia MP, Pérez-Terán P, Climent C, Masclans JR, Nolla J. Severe vitamin D deficiency upon admission in critically ill patients is related to acute kidney injury and a poor prognosis. Med Intens 2018; 42:216–24 [DOI] [PubMed] [Google Scholar]
- 107.Druml W, Schwarzenhofer M, Apsner R, Hörl WH. Fat-soluble vitamins in patients with acute renal failure. Mineral Electrolyte Metab 1998; 24:220–6 [DOI] [PubMed] [Google Scholar]
- 108.Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, Schoenmakers I. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab 2014; 99:3373–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Husain K, Suarez E, Isidro A, Ferder L. Effects of paricalcitol and enalapril on atherosclerotic injury in mouse aortas. Am J Nephrol 2010; 32:296–304 [DOI] [PubMed] [Google Scholar]
- 110.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005; 16:3365–70 [DOI] [PubMed] [Google Scholar]
- 111.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003; 14:2534–43 [DOI] [PubMed] [Google Scholar]
- 112.Yang J, Goetz D, Li J-Y, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R. An iron delivery pathway mediated by a lipocalin. Mol Cell 2002; 10:1045–56 [DOI] [PubMed] [Google Scholar]
- 113.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 2004; 15:3073–82 [DOI] [PubMed] [Google Scholar]
- 114.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 2005; 115:610–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roberts I, Kwan I, Evans P, Haig S. Does animal experimentation inform human healthcare? observations from a systematic review of international animal experiments on fluid resuscitation. BMJ 2002; 324:474–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA 2006; 296:1727–32 [DOI] [PubMed] [Google Scholar]
- 117.McCullough PA, Soman SS. Contrast-induced nephropathy. Crit Care Clin 2005; 21:261–80 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220946271 for Ferrotoxicity and its amelioration by endogenous vitamin D in experimental acute kidney injury by Chandrashekar Annamalai, Rajesh N Ganesh and Pragasam Viswanathan in Experimental Biology and Medicine