Abstract
Portal hypertension is one of the most important cirrhosis-associated complications of chronic liver disease, leading to significant morbidity and mortality. After chronic liver injury, hepatic stellate cells reside in the perisinusoidal space activted and acquire a myofibroblast-like phenotype. The activated hepatic stellate cells act as both sources as well as the target for a potent vasoconstrictor endothelin-1. Activation of hepatic stellate cells plays a vital role in the onset of cirrhosis by way of increased extracellular matrix production and the enhanced contractile response to vasoactive mediators such as endothelin-1. In fibrotic/cirrhotic liver, activated hepatic stellate cells produce endothelin-1 leading to an imbalance between pro and antifibrotic factors responsible for enormous extracellular matrix synthesis. Thus, extracellular matrix deposition in the perisinusoidal space further augments liver stiffness and elevates the vascular tone and portal hypertension. Portal hypertension is a complex process modulated by several cell types like hepatic stellate cells, liver sinusoidal endothelial cells, Kupffer cells, injured hepatocytes, immune cells, and biliary epithelial cells. Therefore, targeting a single cell type may not be useful for regression of cirrhosis and portal hypertension. Nevertheless, numerous findings indicate that functionally liver sinusoidal endothelial cells and hepatic stellate cells closely regulate the sinusoidal blood flow via synthesis of several vasoactive molecules including endothelin-1, and hence targeting these cells with novel pharmacological agents may offer promising results.
Impact statement
Portal hypertension is pathologically defined as increase of portal venous pressure, mainly due to chronic liver diseases such as fibrosis and cirrhosis. In fibrotic liver, activated hepatic stellate cells increase their contraction in response to endothelin-1 (ET-1) via autocrine and paracrine stimulation from liver sinusoidal endothelial cells and injured hepatocytes. Clinical studies are limited with ET receptor antagonists in cirrhotic patients with portal hypertension. Hence, studies are needed to find molecules that block ET-1 synthesis. Accumulation of extracellular matrix proteins in the perisinusoidal space, tissue contraction, and alteration in blood flow are prominent during portal hypertension. Therefore, novel matrix modulators should be tested experimentally as well as in clinical studies. Specifically, tumor necrosis factor-α, transforming growth factor-β1, Wnt, Notch, rho-associated protein kinase 1 signaling antagonists, and peroxisome proliferator-activated receptor α and γ, interferon-γ and sirtuin 1 agonists should be tested elaborately against cirrhosis patients with portal hypertension.
Keywords: Endothelin, hepatic stellate cells, portal hypertension, cirrhosis, hepatic fibrosis
Introduction
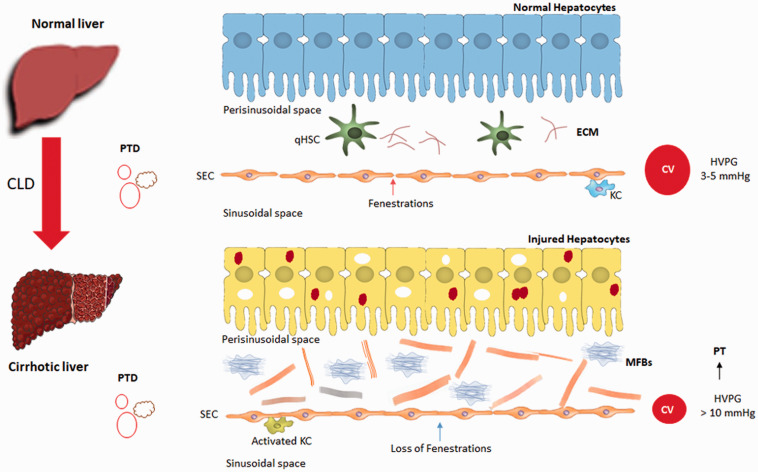
Portal hypertension develops as a consequence of increased intrahepatic vascular resistance often caused by chronic liver disease (CLD) that leads to structural distortion by fibrosis, microvascular thrombosis, dysfunction of liver sinusoidal endothelial cells (LSECs), and activation of hepatic stellate cell (HSC).1–3 The resistance to blood flow through the liver is extremely low with pressure gradients between the portal venous inflow and a normal hepatic venous pressure gradient (HVPG) is 3–5 mmHg.4 But in cirrhosis conditions, the HVPG increased to > 5 mmHg. Clinically significant portal hypertension is defined as HVPG of >10 mmHg.5 Gastroesophageal varices, variceal hemorrhage, ascites, hepatic encephalopathy, and hepatorenal syndrome are common and direct consequences of clinically significant portal hypertension, which lead to significant morbidity and mortality.6,7 The increased intrahepatic resistance in cirrhosis and changes in perisinusoidal space are mediated by the transdifferentiation of quiescent HSCs into myofibroblasts-like phenotype and accumulation of extracellular matrix (ECM), formation of regenerative nodules, and vasoconstriction in the intrahepatic circulation because of decreased production of vasodilators and increased production of vasoconstrictors from LSECs, HSCs, and Kupffer cells.5,8 Hepatic stellate cells are one of the important intrahepatic components of portal hypertension pathophysiology. During CLD, HSCs regulate a variety of signaling molecules including endothelin, responsible for fibrosis/cirrhosis and portal hypertension. Therefore, the aim of this review is to discuss the intricate role of HSCs in endothelin signaling in portal hypertension.
Hepatic stellate cells
Hepatic stellate cells (also called as Ito cells, perisinusoidal cells, fat-storing cells, lipocytes) are liver-specific mesenchymal cells that have features of resident fibroblasts and pericytes, residing in the perisinusoidal space or space of Disse between hepatocytes and LSECs.9–12 HSCs account for 5 to 10% of total resident cells in the normal human liver.13 Under normal physiological conditions, HSCs exhibit a quiescent phenotype (qHSC) with lipid-rich granules and are responsible for vitamin A storage and diverse roles like regulation of epithelial cell fate, immune modulation, tissue health, and matrix degradation, etc.14,15 In the injured liver, qHSCs undergo sequential morphological and physiological changes to maintain the pathophysiological status of the injured liver.16 (Figure 1). Firstly, in response to liver injury, qHSCs undergo transdifferentiation and acquire proliferative, contractile, and myofibroblast (MFBs)-like phenotype, a process termed as initiation. Secondly, the activated MFBs-like phenotypes secrete several pro-fibrotic cytokines and chemokines to maintain the pathophysiological status of the injured liver, and is termed as perpetuation.8,17 For instance, in perpetuation process, the activated HSCs (aHSCs or MFBs) express α-smooth muscle actin (α-SMA) and respond to endothelin-1, transforming growth factor-beta (TGF-β), platelet-derived growth factor (PDGF), Krüppel-like factor 6 (KLF6), matrix metalloproteinases (MMPs), tissue inhibitor of metalloproteinases (TIMPs), and several other signaling molecules from injured hepatocytes, LSECs, and Kupffer cells. This process leads to HSCs contraction, migration, proliferation, synthesis, and accumulation of a variety of ECM proteins including fibril forming collagens 1α1, and 3α1 in the perisinusoidal space that causes portal hypertension and metabolic hindrance.16,18,19 Regardless of etiology, aHSCs are recognized as one of the major contributors for fibrosis/cirrhosis. In injured liver, aHSCs are known to express/respond to endothelin-1 by an autocrine and paracrine fashion.20Endothelin-1 induces HSCs contractility and thereby triggers the pathological chain of events like ECM accumulation, metabolic hindrance, and portal hypertension. Increase in ECM deposition further elevates the vascular tone and augments liver stiffness.8
Figure 1.
Portal hypertension-associated changes in the perisinusoidal space of cirrhotic liver. qHSC-quiescent hepatic stellate cells; aHSC-activated hepatic stellate cells; ECM: extracellular matrix; SEC: sinusoidal endothelial cells; PTD: portal triad; CV: central vein; PT: portal hypertension; HVPG: hepatic venous pressure gradient; CLD: chronic liver diseases; KC: Kupffer cells. (A color version of this figure is available in the online journal.)
Endothelin-1: Synthesis, receptors, and function
Endothelin-1 (ET-1), a 21 amino-acid bioactive peptide first identified by Yanagisawa et al., in 1988.21ET-1, is a potent endogenous vasoconstrictor peptide, which induces contraction, proliferation, and collagen synthesis of aHSCs and is a potent mediator of portal hypertension.22 In normal conditions, hormones, other vascular mediators, and blood flow conditions seem to modulate precursor ET-1 synthesis in endothelial cells (including LSECs). The prepro-ET-1 is a biological precursor of ET-1 and is converted into active ET-1 via two steps. Firstly, cleavage of two basic amino acids by a furin-like enzyme results in the formation of big-ET-1. Secondly, the big ET-1 is further processed by cleavage of a Trp-Val bond by endothelin-converting enzyme-1 (ECE-1), a membrane-bound, phosphoramidon-sensitive metalloproteinase, into biologically active ET-1.22,23 Endothelin has three subtypes such as ET-1, -2, and -3. All ET subtypes act through G protein-coupled receptors (GPCR) such as ETA and ETB.22,24 However, ET-1 has high affinity and mediates long-lasting vasoconstriction effect mainly via the ETA receptor. ETA and ETB receptors are present on HSCs and hepatocytes, while ETB receptors are present on LSECs and Kupffer cells.25 The ETB receptor has two subtypes such as ETB1 and ETB2. ETB1 receptor is responsible for the induction of endothelial cell nitric oxide synthetase resulting in nitric oxide (NO) release and vasodilation,25 while ETB2 receptors are identified in HSCs and are responsible for vasoconstriction.22 Increased plasma ET-1 and ET-3 levels were observed in patients with CLD and portal hypertension.26–28 The role of ET-2 is not reported in CLD conditions. In liver microenvironment, ET-1 synthesized by LSECs acts on several cell types nearby such as hepatocytes, HSCs, and Kupffer cells via paracrine fashion. Kupffer cells induce thromboxane A2 (TXA2)-mediated ET-1 synthesis.29 Particularly, ET-1 stimulates the contraction of qHSCs and myofibroblasts, which contribute to portal hypertension in the cirrhotic liver.30
Hepatic stellate cells in endothelin-1-associated portal hypertension
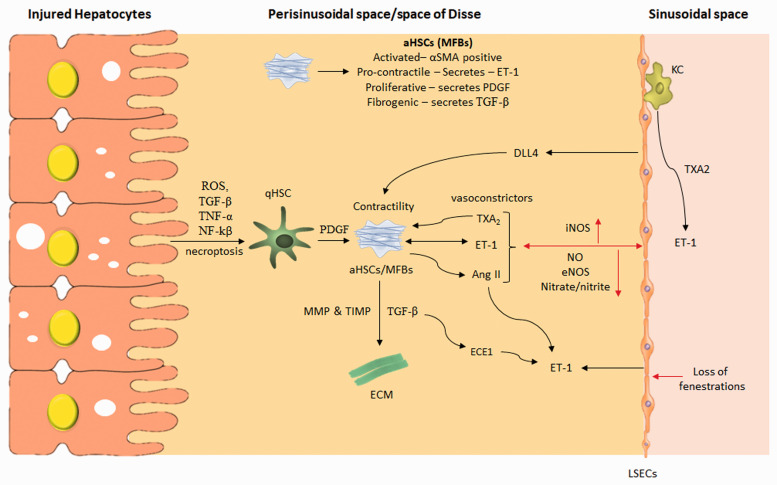
In the perisinusoidal space, HSCs function as liver-specific pericytes surrounding LSECs, and thus their contractility is controlled by the exposure to LSECs-derived vasoconstrictor like ET-1. Other vasoconstrictors like angiotensin II (Ang-II), TXA2, carbon monoxide, and vasodilators like NO also control the diameter of sinusoids, fenestrae and regulate the hepatic microcirculation.31Endothelin-1 synthesized by LSEC, acts on HSCs in a paracrine fashion. After the liver injury, aHSCs are responsible for increased ET-1 synthesis, which contributes to significant vasoconstriction and portal hypertension.30 Experimental studies have shown that aHSCs but not qHSCs are responsible for ET-1 synthesis.22,32 However, in a study, endotoxin (lipopolysaccharide, LPS) treatment significantly increased ET-1 synthesis and its receptor activation in qHSCs and a non-significant ET-1 increase were also observed in aHSCs after LPS treatment.33 The preproET-1 mRNA expression was reported to highly increase during cell culture-induced HSCs activation.32 The ECE is responsible for the conversion of big ET-1 to the mature peptide.22 The increased activity of ECE in aHSC is directly correlated with the up-regulation of ET-1. Upon chronic liver injury, hepatocytes secrete TGF-β, tumor necrosis factor-alpha (TNF-α), lipopolysaccharides, platelet-activating factor (PAF), and several other pro-inflammatory markers which, in turn, stimulate LSECs to secrete ET-1. ET-1 has prominent effects on key cellular effectors, including HSCs in an autocrine fashion.20 (Figure 2). Chronic liver injury induce ET-1 from LSECs to act on HSCs via ETA receptor and induces the secretion of profibrogenic cytokine, i.e. TGF-β1 and ECM-related markers such as collagen 1α1, collagen 3α1, TIMP 1, 2, and MMPs.34 Regardless of etiology, ET-1 is implicated in HSCs activation and portal hypertension. For instance, high fat/methionine-choline-deficient diet and hyperleptinemia-induced NASH cirrhotic rats enhanced hepatic vasoconstrictive response to ET-1 and aggravated hepatic microcirculatory dysfunction which led to increased intrahepatic resistance and portal hypertension.35
Figure 2.
Portal hypertension-mediated changes in the perisinusoidal space of the liver. ROS: reactive oxygen species; TNF-α: tumor necrosis factor alpha; qHSC: quiescent hepatic stellate cells; MFBs: myofibroblasts or activated HSCs; PDGF: platelet-derived growth factor; ET-1: endothelin 1; TGF-β: transforming growth factor-β; MMPs: matrix metalloproteinases; TIMPs: tissue inhibitors of metalloproteinases; ECM: extracellular matrix; LSECs: liver sinusoidal endothelial cells; ECE1: endothelial converting enzyme 1; KC: Kupffer cells; Ang-II: angiotensin-II; DLL4: delta-like Ligand 4; TXA2: thromboxane A2; iNOS: inducible nitric oxide synthase; eNOS: endothelial nitric oxide synthase. (A color version of this figure is available in the online journal.)
Molecular signaling associated with endothelin synthesis and HSCs contraction
At a molecular level, several signaling pathways tightly regulate ET-1 synthesis and function. The transcriptional regulation of preproET-1 synthesis is well established in HSCs. Upon profibrogenic stress, ET-1 acts on qHSCs via its ETA receptor and phosphorylates c-Jun N-terminal kinase (JNK) intracellularly. The phosphorylated JNK subsequently activates and phosphorylates its downstream effectors, i.e. Smad 3 and c-jun. The phosphorylated Smad 3 translocates to the nucleus to induce the activation of Smad3/4 present in the Smad3 binding site at the preproET-1 promoter. Similarly, phosphorylated c-jun translocates to the nucleus to induce the activation of c-fos/c-jun in the activator protein-1 (AP-1) binding site at the preproET-1 promoter. These signaling events trigger the preproET-1 at a transcription level which subsequently converts into active ET-1.32 Thus, after liver injury, aHSCs and LSECs secrete more preproET-1 and convert to active ET-1. In a study, TGF-β1, a potent profibrogenic cytokine was shown to induce ET-1 secretion mediated by the activation of rho-associated protein kinase 1 (ROCK 1) in human HSCs.36 Fibronectin plays an important role in the development of hepatic fibrosis.37 Accumulation of fibronectin along with the different type of fibril forming collagens in the perisinusoidal space increases intrahepatic resistance.8,38 Fibronectin was shown to induce ERK-mediated ET-1 synthesis in aHSCs. Briefly, fibronectin promotes activation of Src via their α5β1 and αVβ3 integrin receptors. The activated Src directly phosphorylates Shc present in Shc/Grb2/Sos complex. The phosphorylated Shc, in turn, activates ERK singling pathway, which increases the preproET-1 expression at the transcription level. Thus, increased ET-1 activates α-SMA expression in aHSCs and induces their contraction.39
Interestingly, unlike the hepatocytes, HSCs are not highly equipped with intracellular antioxidant defense, and hence they are highly susceptible to oxidative and pro-inflammatory mediators’ attack.40 The reactive oxygen species (ROS) play a significant role in the activation HSCs in the injured liver.41 Unsurprisingly, liver inflammation results in the liberation of several pro-inflammatory molecules from injured hepatocytes that directly activate HSCs.42 For instance, TNF-α, a common proinflammatory cytokine secreted by injured hepatocytes promotes ET-1 production by activating the inhibitor of kappa B kinase (IKKα, β, and γ) complex via TNF receptor-associated factor 1, which, in turn, directly induces the phosphorylation of the extracellular-signal-regulated kinase (ERK) and JNK. Subsequently, the phosphorylated JNK translocates to the nucleus to activate c-Jun present in the preproET-1 promoter and triggers preproET-1 mRNA and protein expression.43
In a study, Ang-II was reported to induce ET-1 expression via Ang-II type 1 receptor by the phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway in human HSCs. This study suggests that ET-1/ETA receptor axis can promote the Ang-II-mediated transdifferentiation of qHSCs into MFBs.44 The GPCR-mediated signaling, via ET-1 and Ang II, increases HSCs contraction, migration, and fibrogenic potential. Regulator of G-protein signaling-5 (RGS5), an inhibitor of vasoactive GPCR agonists functions to control GPCR-mediated contraction in smooth muscle cells. In the context of HSCs, RGS5 controls GPCR signaling in aHSCs of CCl4-induced fibrotic mice liver, while RGS5 knockdown enhances ET-1-mediated signaling in aHSCs in vitro.45 The G protein-coupled bile acid receptor TGR5 (Gpbar1) is expressed in aHSCs, LSECs, and Kupffer cells.46,47 The TGR5 receptor activation reduces hepatic vascular resistance through several mechanisms, both in LSECs and in HSCs. A recent finding showed negative effect of Gpbar1 on ET-1 signaling in HSCs.47 Activation of Notch signaling pathway is implicated in many liver diseases and is also responsible for HSCs activation in the injured liver.48,49Delta-like ligand 4 (DLL4), a ligand of the Notch signaling pathway, is predominantly expressed in LSECs and it is one of the factors responsible for the maintenance of liver sinusoidal homeostasis.50,51 Higher levels of DLL4 were detected in LSECs of CCl4-induced fibrotic liver in mice and in fibrotic human liver.52,53 The overexpression of DLL4 caused the defenestration of LSECs and the accumulation of ECM in the injured liver. Overexpression of DLL4 stimulates the aHSCs to induce ET-1 synthesis which is further responsible for pathological hepatic sinusoidal remodeling and this study also suggests that DLL4/Notch signaling could activate ET-1 synthesis via LSECs and aHSCs in the fibrotic liver.53
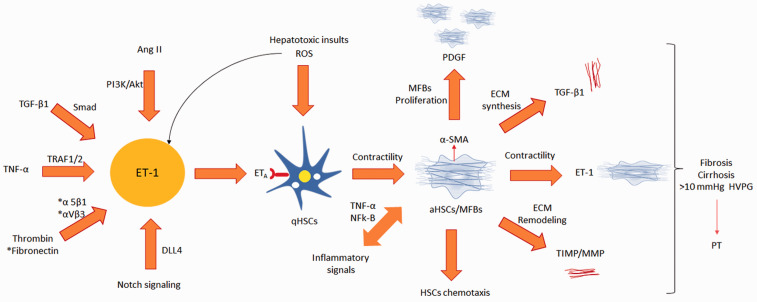
The farnesoid X receptor (FXR) is a ligand-activated transcriptional factor.54 Hepatic stellate cells express FXR and it negatively regulates their phenotype transdifferentiation and TGF-β-mediated collagen synthesis.55,56 In a study, treatment with GW4064, a synthetic FXR agonist inhibited the qHSCs transdifferentiation by an inhibition of the upregulation of ET-1 expression. The FXR agonist treatment also reduced contractile response to ET-1 as compared to untreated HSCs.55 Apelin, a peptide, is overexpressed in HSCs of rat and human cirrhotic livers and studies have also found high levels of this peptide in the serum of cirrhotic patients.57,58 Apelin promotes hepatic fibrosis/cirrhosis through ERK signaling in LX-2 cells.59 In a study, apelin has been reported to mediate fibrogenic effects predominantly via ET-1 activation. Both ET-1 and Ang II enhanced apelin expression in HSCs in vitro.60ET-1 receptor antagonist (RO 48–5695) treatment reduced the apelin synthesis, collagen-I, and PDGF-β in fibrotic rats. 60Endothelin-1 synthesis-associated signaling and their impact on HSCs are depicted in Figure 3.
Figure 3.
Enothelin-1 synthesis-associated signaling and its impact on HSCs. qHSC-quiescent hepatic stellate cells; aHSC: activated hepatic stellate cells; MFBs: myofibroblasts; ET-1: entdothelin-1; ETA: endothelin receptor A; Ang II: angiotensin II; TGF-β1: transforming growth factor-β1; TNF-α: tumor necrosis factor-α; TRAF1/2: TNF receptor-associated factor 1/2; DLL4: DLL4 delta-like canonical Notch ligand 4; ROS: reactive oxygen species; α-SMA: α-smooth muscle actin; PDGF: platelet-derived growth factor; TIMP: tissue inhibitor metalloproteinase-1; MMP: matrix metalloproteinase; NFkB: nuclear factor kB; ECM: extracellular matrix; PT: portal hypertension; HVPG: hepatic venous pressure gradient. (A color version of this figure is available in the online journal.)
Modulators of ET-1 signaling and portal hypertension
There are two types of ET-1 receptor antagonists such as BQ-123 and BQ-788 and they act on ETA and ETB receptors, respectively, as identified so far. Several studies have tried these ET-antagonists in experimental and human portal hypertension. Interestingly, inhibition of ET signaling using ET receptor antagonists reduces the hepatic fibrogenic response in experimental models.61,62 When BQ-123 and BQ-788 were continuously infused at the rate of 10 nmol/min for 10 min into normal rats, only BQ-123, an ETA receptor antagonist has produced a gradual fall in portal pressure and also caused a marked dilatation of the sinusoidal endothelium fenestrae as compared to BQ-788 indicating the potential role of ETA in portal hypertension.63 The tissue mRNAs levels of ECE-1a and –b, ET-1, and ETA and ETB receptors expressions were significantly upregulated in bile duct ligated (BDL) and CCl4-induced fibrotic liver. This study suggested that ECE-1a and -b isoforms are responsible for the ET-1 generation during biliary and hepatic fibrogenesis and portal hypertension.22 High concentration of plasma ET-1 was also reported in cirrhotic rats and intraportal infusion of ET receptor antagonists such as BQ-123, BQ-788, and bosentan administration resulted in decreased portal venous pressure in these rats.64 In an experimental study, BQ-123 infusion in CCl4-induced cirrhotic rats markedly reduced the portal pressure by causing pores in sinusoidal endothelial fenestrae.65 A randomized double-blind study demonstrated the limited role of ETA and ETB antagonists in HVPG patients.66 The release of ET-1 was increased after HSCs are challenged with hypoxanthine/xanthine oxidase, a ROS-generating system in vitro and ETB receptors were significantly expressed than ETA in oxidative stress conditions. Consistently, the ETB receptor antagonist, BQ788 significantly inhibited ET-1 expression in oxidative stress condition.67 This study also indicates ETB is responsible for the activation of ET-1 in oxidative stress condition.
It is known that ET-1 causes smooth muscle cell contraction through phosphorylation of myosin and activation of the actin-myosin contractile apparatus in smooth muscle cells, and thereby leads to narrowing of vascular structures and an increase in resistance to blood flow.68 In vitro, aHSCs showed increased expression of contractile regulatory proteins myosin light chain kinase (MLCK), ROCK2, and 17-kDa PKC-potentiated protein phosphatase 1 inhibitor protein during their activation.69 Activated HSCs induce hepatic sinusoids constriction via ET-1 stimulation in the cirrhotic liver through both Ca2+-dependent MLCK pathway and Ca2+-sensitization mechanism.69ET-1-mediated HSCs contraction is mainly induced by the phosphorylation of myosin light chain, actin stress fiber assembly, and reorganization of myosin to stress fibers.69BQ-123 treatments inhibited ET-1 stimulated myosin phosphorylation and contractility in rat HSCs. Similarly, Y-27632 (a selective ATP-competitive inhibitor of ROCK) treatments also blocked ET-1-induced myosin phosphorylation and contractile force generation in rat HSCs.70 Xu et al. evaluated the effect of salvianolic acid B (Sal B) on HSCs contractility and portal hypertension. In their in vivo study, Sal B administration reduced ET-1-associated portal pressure in dimethylnitrosamine-induced cirrhotic rats. In vitro, Sal B treatments reduced ET-1-induced HSCs contraction by inhibiting the activation of RhoA, ROCK II, and the downstream myosin phosphatase target subunit 1 phosphorylation at Thr(696).71 Another study by the same group revealed that Sal B is capable to inhibit ET-1 and thereby reduces the contraction of primary rat HSCs through its suppressive effects on myosin light chain 2 phosphorylation in HSCs.72
Darusentan, a potent blocker of ETA receptor induced hepatic sinusoidal vasodilation, decreased hepatic ischemia, endothelial injury, and improved liver repopulation after cell transplantation in rats. However, in vitro ETA receptor blockade failed to improve the engraftment of subsequently transplanted hepatocytes.73 Treatment with Octreotide, an analogue of somatostatin, caused downregulation of ET-1 and fibrosis markers in aHSCs in culture.74 Silent information regulator (SIRT) plays an important role in many liver diseases including cirrhosis.75 Resveratrol, a SIRT1 activator treatment in aHSCs and LSECs resulted in a significant downregulation of ET-1 expression along with other profibrogenic markers. This study suggests that SIRT1 activators can restore the LSECs function and inhibit HSCs activation.76 However, the exact mechanism of SIRT 1 action on ET-1 downregualtion is not reported so far. Therefore, further studies are needed on these lines. Interestingly, HSCs-specific vitamin A-decorated nanoparticles with NO donor molecules (S-nitrosoglutathione) released NO specifically in liver cells. Further, HSCs-specific nano construction inhibited ET-1 synthesis, HSCs contraction, and attenuated hemodynamic disorders in BDL-mediated portal hypertension evidenced by decreased portal pressure (≈20%) and unchanging mean arterial pressure.77
TNF-α reportedly induces ET-1 gene expression in human dermal microvascular endothelial cells.78 A previous study has reported that peroxisome proliferator-activated receptor activators directly inhibit thrombin-induced ET-1.79 In liver inflammation context, TNF-α activates HSCs in the injured liver and causes elevated imbalance of ET-1 MMPs/TIMPs.80 Therefore, in a study, aleglitazar, a PPARα or PPARγ agonist has been tested experimentally. This study reported the increased systemic and hepatic TNF-α levels in cirrhotic rats. Further, in vitro, the hyper-expression of hepatic ET-1 in rat perfused livers along with TNF-α coincubation enhanced ET-1-induced primary rat HSCs contraction. The aleglitazar treatments induced the inhibition of ET-1 and TNF-α coincubation-mediated HSCs contraction. Aleglitazar treatments also reduced vasoconstrictor hyper-responsiveness, splanchnic vasodilatation, portal hypertension in BDL, and thioacetamide-induced cirrhotic rats.81 Interferon-γ (IFN-γ), a Th1 cytokine produced by T cells caused significant downregulation of ET-1 precursor, i.e. preproET-1 mRNA expression and ET-1 peptide production. In HSCs, IFN-γ treatment resulted in decreased ET-1 expression via downregulation of JNK phosphorylation and its downstream targets c-Jun and Smad3 which downregulated the activation of c-fos/c-jun in the activator protein-1 (AP-1) binding site at the preproET-1 promoter.32 Collectively, these studies showed promising results of TNF-α antagonists, PPARα, γ agonists, and IFN-γ against ET-1-induced hepatic cirrhosis and portal hypertension.
Therapeutic avenues and future directions
Undoubtedly, activation of HSCs results in fibrosis, cirrhosis leading to portal hypertension due to increased intrahepatic resistance. ET-1 exhibits an autocrine effect on HSCs and is involved in their activation and contractile response.55,82 Clinically, very few studies were conducted with ET receptor antagonists in cirrhotic patients with portal hypertension. More studies specifically using ETA receptor antagonists in portal hypertension conditions are warranted in the near future. In recent studies, the TGR5 receptor activation showed negative regulation of ET-1 signaling in HSCs, suggesting that TGR5 activators may contribute to a reduction in hepatic vascular resistance47 and portal hypertension via modulation of ET-1 singling in cirrhotic liver. The DLL4 inhibitors and other Notch signaling modulators may also have a beneficial effect in portal hypertension through modulation of ET-1 synthesis by aHSCs as well as LSECs. There are several pro-inflammatory mediators like TNF-α and TGF-β1 which directly activates LSECs and HSCs to secrete ET-1. Therefore, their antagonists can reduce inflammation-mediated portal hypertension, though ET-1 is predominantly responsible for HSCs contraction and it is not the only factor for HSCs activation in the injured liver. There are several other molecules like Ang II, thrombin, VEGF, fibronectin, etc. which also acts on HSCs and modulates ET-1 level in cirrhotic liver. Once ET-1 is synthesized by LSEC, it acts on HSCs via paracrine signaling. Hence, it is suggested that more studies are warranted on molecules that block ET-1 synthesis rather than its receptor blockers. Finally, TNF-α, TGF-β1, Wnt, Notch, ROCK antagonists, and PPARα, γ, IFN-γ, SIRT1 agonists should be tested elaborately against portal hypertension. From a pathological point of view, portal hypertension is a direct consequence of increased hepatic vascular resistance due to both ECM accumulation and microvascular dysfunction. Therefore, novel matrix modulators should also be tested experimentally as well as in clinical studies. In a recent phase 2 randomized controlled trial, serelaxin has been tested against a small group of cirrhotic patients (n = 17), in which serelaxin infusion did not induce significant adverse effects and showed a neutral effect in patients with portal hypertension.83 Indeed, portal hypertension occurs due to multifactorial conditions, and hence combinational therapy with novel vasodilators like serelaxin along with anti-inflammatory agents and matrix modulators may be effective and these combinations should be tested experimentally as well as against patients with portal hypertension.
Conclusions
The role of HSCs in the onset of cirrhosis and portal hypertension is complex. The activated HSCs secrete ET-1 and they also respond to ET-1 from LSECs due to their unique anatomic location. In the injured liver, aHSCs receive stress and inflammatory signals from both sides. Firstly, from the parenchymal domain, injured hepatocytes can release proinflammatory markers from one side. Secondly, from the sinusoidal domain, HSCs receive a paracrine signal via ET-1 from LSECs and Kupffer cells. After receiving these pleiotropic signals, qHSCs become contractile, acquire MFBs like transition, and synthesize an enormous amount of ECM, which subsequently causes cirrhosis and its associated complications like a hindrance in hepatic metabolism, loss of fenestration of LSECs, increased hepatic vascular resistance, and portal hypertension. Thus, cirrhotic complications and portal hypertension are not a single entity and it is a complex process involving HSCs, LSECs, Kupffer cells, injured hepatocytes, immune cells, and biliary epithelial cells. Therefore, targeting a single cell type may not be useful to effectively regress cirrhosis and its associated complications like portal hypertension. Hence, targeting these cells with novel pharmacological agents’ like matrix modulators may offer promising results.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Devaraj Ezhilarasanhttps://orcid.org/0000-0002-5068-2383
References
- 1.Bosch J. Portal hypertension and cirrhosis: from evolving concepts to better therapies. Clin Liver Dis 2020; 15:S8–S12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McConnell M, Iwakiri Y. Biology of portal hypertension. Hepatol Int 2018; 12:11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis 2014; 18:281–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lautt WW. Hepatic circulation: physiology and pathophysiology. San Rafael (CA): Morgan & Claypool Life Sciences, 2009 [PubMed] [Google Scholar]
- 5.Mauro E, Gadano A. What's new in portal hypertension? Liver Int 2020; 40:122–7 [DOI] [PubMed] [Google Scholar]
- 6.Simonetto DA, Liu M, Kamath PS. Portal hypertension and related complications: diagnosis and management. Mayo Clin Proc 2019; 94:714–26 [DOI] [PubMed] [Google Scholar]
- 7.Bari K, Garcia-Tsao G. Treatment of portal hypertension. World J Gastroenterol 2012; 18:1166–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezhilarasan D, Sokal E, Najimi M. Hepatic fibrosis: it is time to go with hepatic stellate cell-specific therapeutic targets. Hepatobiliary Pancreat Dis Int 2018; 17:192–7 [DOI] [PubMed] [Google Scholar]
- 9.Ezhilarasan D, Evraerts J, Brice S, Buc-Calderon P, Karthikeyan S, Sokal E, Najimi M. Silibinin inhibits proliferation and migration of human hepatic stellate LX-2 cells. J Clin Exp Hepatol 2016; 6:167–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezhilarasan D, Evraerts J, Sid B, Calderon PB, Karthikeyan S, Sokal E, Najimi M. Silibinin induces hepatic stellate cell cycle arrest via enhancing p53/p27 and inhibiting Akt downstream signaling protein expression. Hepatobiliary Pancreat Dis Int 2017; 16:80–7 [DOI] [PubMed] [Google Scholar]
- 11.Shang L, Hosseini M, Liu X, Kisseleva T, Brenner DA. Human hepatic stellate cell isolation and characterization. J Gastroenterol 2018; 53:6–17 [DOI] [PubMed] [Google Scholar]
- 12.Ezhilarasan D. Lead compounds with the potentials for the treatment of chronic liver diseases In: C Egbuna, S Kumar, J Ifemeje, S Ezzat, S Kaliyaperumal. (eds) Phytochemicals as lead compounds for new drug discovery: recent advances. Amsterdam: Elsevier, 2019, pp.195–210 [Google Scholar]
- 13.Li J, Zhao YR, Tian Z. Roles of hepatic stellate cells in acute liver failure: from the perspective of inflammation and fibrosis. World J Hepatol 2019; 11:412–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haaker MW, Vaandrager AB, Helms JB. Retinoids in health and disease: a role for hepatic stellate cells in affecting retinoid levels. Biochim Biophys Acta Mol Cell Biol Lipids 2020; 1865:158674. [DOI] [PubMed] [Google Scholar]
- 15.Sherman MH. Stellate cells in tissue repair, inflammation, and cancer. Annu Rev Cell Dev Biol 2018; 34:333–55 [DOI] [PubMed] [Google Scholar]
- 16.Gandhi CR. Hepatic stellate cell activation and pro-fibrogenic signals. J Hepatol 2017; 67:1104–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 2017; 14:397–411 [DOI] [PubMed] [Google Scholar]
- 18.Dhar D, Baglieri J, Kisseleva T, Brenner DA. Mechanisms of liver fibrosis and its role in liver cancer. Exp Biol Med 2020; 245:96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev 2017; 121:27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rockey DC. Hepatic fibrosis, stellate cells, and portal hypertension. Clin Liver Dis 2006; 10:459–79 [DOI] [PubMed] [Google Scholar]
- 21.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332:411–5 [DOI] [PubMed] [Google Scholar]
- 22.Cho TJ, Kim HJ, Cho J. Endothelin-converting enzyme-1 expression in acute and chronic liver injury in fibrogenesis. Anim Cells Syst 2019; 23:170–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez-Pascual F, Busnadiego O, González-Santamaría J. The profibrotic role of endothelin-1: is the door still open for the treatment of fibrotic diseases? Life Sci 2014; 118:156–64 [DOI] [PubMed] [Google Scholar]
- 24.Swigris JJ, Brown KK. The role of endothelin-1 in the pathogenesis of idiopathic pulmonary fibrosis. BioDrugs 2010; 24:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynaert H, Thompson MG, Thomas T, Geerts A. Hepatic stellate cells: role in microcirculation and pathophysiology of portal hypertension. Gut 2002; 50:571–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore K, Wendon J, Frazer M, Karani J, Williams R, Badr K. Plasma endothelin immunoreactivity in liver disease and the hepatorenal syndrome. N Engl J Med 1992; 327:1774–8 17 [DOI] [PubMed] [Google Scholar]
- 27.Ohara N, Futagawa S, Watanabe S, Fukasawa M, Takamori S. Clinical investigation of endothelin-1 and nitric oxide in patients with portal hypertension focusing on plasma levels and immunohistological staining of liver tissues. Hepatol Res 2001; 21:40–54 [DOI] [PubMed] [Google Scholar]
- 28.Wereszczynka-Siemiatkowska U, Swidnicka-Siergiejko A, Siemiatkowski A, Bondyra Z, Wasielica-Berger J, Mroczko B, Janica J, Dabrowski A. Endothelin 1 and transforming growth factor-β1 correlate with liver function and portal pressure in cirrhotic patients. Cytokine 2015; 76:144–51 [DOI] [PubMed] [Google Scholar]
- 29.Miller AM, Zhang JX. Altered endothelin-1 signaling in production of thromboxane A2 in Kupffer cells from bile duct ligated rats. Cell Mol Immunol 2009; 6:441–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrone G, Shah VH, Gracia-Sancho J. Sinusoidal communication in liver fibrosis and regeneration. J Hepatol 2016; 65:608–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol 2014; 61:912–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li T, Shi Z, Rockey DC. Preproendothelin-1 expression is negatively regulated by IFNγ during hepatic stellate cell activation. Am J Physiol Gastrointest Liver Physiol 2012; 302:G948–G957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gandhi CR, Uemura T, Kuddus R. Endotoxin causes up-regulation of endothelin receptors in cultured hepatic stellate cells via nitric oxide-dependent and -independent mechanisms. Br J Pharmacol 2000; 131:319–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo J, Liang Y, Kong F, Qiu J, Liu X, Chen A, Luxon BA, Wu HW, Wang Y. Vascular endothelial growth factor promotes the activation of hepatic stellate cells in chronic schistosomiasis. Immunol Cell Biol 2017; 95:399–407 [DOI] [PubMed] [Google Scholar]
- 35.Yang YY, Tsai TH, Huang YT, Lee TY, Chan CC, Lee KC, Lin HC. Hepatic endothelin-1 and endocannabinoids-dependent effects of hyperleptinemia in nonalcoholic steatohepatitis-cirrhotic rats. Hepatology 2012; 55:1540–50 [DOI] [PubMed] [Google Scholar]
- 36.Shimada H, Staten NR, Rajagopalan LE. TGF-β1 mediated activation of rho kinase induces TGF-β2 and endothelin-1 expression in human hepatic stellate cells. J Hepatol 2011; 54:521–8 [DOI] [PubMed] [Google Scholar]
- 37.Mòdol T, Brice N, Ruiz de Galarreta M, García Garzón A, Iraburu MJ, Martínez-Irujo JJ, López-Zabalza MJ. Fibronectin peptides as potential regulators of hepatic fibrosis through apoptosis of hepatic stellate cells. J Cell Physiol 2015; 230:546–53 [DOI] [PubMed] [Google Scholar]
- 38.Sharma A, Verma AK, Kofron M, Kudira R, Miethke A, Wu T, Wang J, Gandhi CR. Lipopolysaccharide reverses hepatic stellate cell activation via modulation of cMyb, SMADs and C/EBP transcription factors. Hepatology 2020. DOI:10.1002/hep.31188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhan S, Chan CC, Serdar B, Rockey DC. Fibronectin stimulates endothelin-1 synthesis in rat hepatic myofibroblasts via a src/ERK-regulated signaling pathway. Gastroenterology 2009; 136:2345–55.e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ezhilarasan D, Karthikeyan S, Vivekanandan P. Ameliorative effect of silibinin against N-nitrosodimethylamine-induced hepatic fibrosis in rats. Environ Toxicol Pharmacol 2012; 34:1004–13 [DOI] [PubMed] [Google Scholar]
- 41.Ezhilarasan D. Oxidative stress is bane in chronic liver diseases: clinical and experimental perspective. Arab J Gastroenterol 2018; 19:56–64 [DOI] [PubMed] [Google Scholar]
- 42.Cubero FJ. Shutting off inflammation: a novel switch on hepatic stellate cells. Hepatology 2016; 63:1086–9 [DOI] [PubMed] [Google Scholar]
- 43.Zhan S, Rockey DC. Tumor necrosis factor α stimulates endothelin-1 synthesis in rat hepatic stellate cells in hepatic wound healing through a novel IKK/JNK pathway. Exp Cell Res 2011; 317:1040–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He C, Miao X, Li J, Qi H. Angiotensin II induces endothelin-1 expression in human hepatic stellate cells. Dig Dis Sci 2013; 58:2542–9 [DOI] [PubMed] [Google Scholar]
- 45.Bahrami AJ, Gunaje JJ, Hayes BJ, Riehle KJ, Kenerson HL, Yeung RS, Stempien-Otero AS, Campbell JS, Mahoney WM., Jr. Regulator of G-protein signaling-5 is a marker of hepatic stellate cells and expression mediates response to liver injury. PLoS One 2014; 9:e108505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kordes C, Sawitza I, Götze S, Häussinger D. Bile acids and stellate cells. Dig Dis 2015; 33:332–7 [DOI] [PubMed] [Google Scholar]
- 47.Klindt C, Reich M, Hellwig B, Stindt J, Rahnenführer J, Hengstler JG, Köhrer K, Schoonjans K, Häussinger D, Keitel V. The G Protein-Coupled bile acid receptor TGR5 (Gpbar1) modulates endothelin-1 signaling in liver. Cells 2019; 8:1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geisler F, Strazzabosco M. Emerging roles of notch signaling in liver disease. Hepatology 2015; 61:382–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aimaiti Y, Yusufukadier M, Li W, Tuerhongjiang T, Shadike A, Meiheriayi A, Gulisitan Abudusalamu A, Wang H, Tuerganaili A, Shao Y, Wen H. TGF-β1 signaling activates hepatic stellate cells through notch pathway. Cytotechnology 2019; 71:881–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bansal R, van Baarlen J, Storm G, Prakash J. The interplay of the notch signaling in hepatic stellate cells and macrophages determines the fate of liver fibrogenesis. Sci Rep 2015; 5:18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen Z, Liu Y, Dewidar B, Hu J, Park O, Feng T, Xu C, Yu C, Li Q, Meyer C, Ilkavets I, Müller A, Stump-Guthier C, Munker S, Liebe R, Zimmer V, Lammert F, Mertens PR, Li H, Ten Dijke P, Augustin HG, Li J, Gao B, Ebert MP, Dooley S, Li Y, Weng HL. Delta-like ligand 4 modulates liver damage by down-regulating chemokine expression. Am J Pathol 2016; 186:1874–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duan JL, Ruan B, Yan XC, Liang L, Song P, Yang ZY, Liu Y, Dou KF, Han H, Wang L. Endothelial notch activation reshapes the angiocrine of sinusoidal endothelia to aggravate liver fibrosis and blunt regeneration in mice. Hepatology 2018; 68:677–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen L, Gu T, Li B, Li F, Ma Z, Zhang Q, Cai X, Lu L. Delta-like ligand 4/DLL4 regulates the capillarization of liver sinusoidal endothelial cell and liver fibrogenesis. Biochim Biophys Acta Mol Cell Res 2019; 1866:1663–75 [DOI] [PubMed] [Google Scholar]
- 54.Hoofnagle JH. FXR agonists as therapy for liver disease. Hepatology 2020;72:1-3 [DOI] [PubMed] [Google Scholar]
- 55.Li J, Kuruba R, Wilson A, Gao X, Zhang Y, Li S. Inhibition of endothelin-1-mediated contraction of hepatic stellate cells by FXR ligand. PLoS One 2010; 5:e13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou J, Cui S, He Q, Guo Y, Pan X, Zhang P, Huang N, Ge C, Wang G, Gonzalez FJ, Wang H, Hao H. SUMOylation inhibitors synergize with FXR agonists in combating liver fibrosis. Nat Commun 2020; 11:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yokomori H, Oda M, Yoshimura K, Machida S, Kaneko F, Hibi T. Overexpression of apelin receptor (APJ/AGTRL1) on hepatic stellate cells and sinusoidal angiogenesis in human cirrhotic liver. J Gastroenterol 2011; 46:222–31 [DOI] [PubMed] [Google Scholar]
- 58.Lim YL, Choi E, Jang YO, Cho YZ, Kang YS, Baik SK, Kwon SO, Kim MY. Clinical implications of the serum apelin level on portal hypertension and prognosis of liver cirrhosis. Gut Liver 2016; 10:109–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Song J, Bian H, Bo J, Lv S, Pan W, Lv X. Apelin promotes hepatic fibrosis through ERK signaling in LX-2 cells. Mol Cell Biochem 2019; 460:205–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melgar-Lesmes P, Casals G, Pauta M, Ros J, Reichenbach V, Bataller R, Morales-Ruiz M, Jimenez W. Apelin mediates the induction of profibrogenic genes in human hepatic stellate cells. Endocrinology 2010; 151:5306–14 [DOI] [PubMed] [Google Scholar]
- 61.Feng HQ, Weymouth ND, Rockey DC. Endothelin antagonism in portal hypertensive mice: implications for endothelin receptor-specific signaling in liver disease. Am J Physiol Gastrointest Liver Physiol 2009; 297:G27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rockey DC, Chung JJ. Endothelin antagonism in experimental hepatic fibrosis. Implications for endothelin in the pathogenesis of wound healing. J Clin Invest 1996; 98:1381–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watanabe N, Takashimizu S, Nishizaki Y, Kojima S, Kagawa T, Matsuzaki S. An endothelin a receptor antagonist induces dilatation of sinusoidal endothelial fenestrae: implications for endothelin-1 in hepatic microcirculation. J Gastroenterol 2007; 42:775–82 [DOI] [PubMed] [Google Scholar]
- 64.Cavasin MA, Semus H, Pitts K, Peng Y, Sandoval J, Chapo J, Plato CF. Acute effects of endothelin receptor antagonists on hepatic hemodynamics of cirrhotic and noncirrhotic rats. Can J Physiol Pharmacol 2010; 88:636–43 [DOI] [PubMed] [Google Scholar]
- 65.Takashimizu S, Kojima S, Nishizaki Y, Kagawa T, Shiraishi K, Mine T, Watanabe N. Effect of endothelin a receptor antagonist on hepatic hemodynamics in cirrhotic rats. Implications for endothelin-1 in portal hypertension. Tokai J Exp Clin Med 2011; 36:37–43 [PubMed] [Google Scholar]
- 66.Tripathi D, Therapondos G, Ferguson JW, Newby DE, Webb DJ, Hayes PC. Endothelin-1 contributes to maintenance of systemic but not portal haemodynamics in patients with early cirrhosis: a randomised controlled trial. Gut 2006; 55:1290–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gabriel A, Kuddus RH, Rao AS, Watkins WD, Gandhi CR. Superoxide-induced changes in endothelin (ET) receptors in hepatic stellate cells. J Hepatol 1998; 29:614–27 [DOI] [PubMed] [Google Scholar]
- 68.Rockey DC. Endothelial dysfunction in advanced liver disease. Am J Med Sci 2015; 349:6–16 [DOI] [PubMed] [Google Scholar]
- 69.Iizuka M, Murata T, Hori M, Ozaki H. Increased contractility of hepatic stellate cells in cirrhosis is mediated by enhanced Ca2+-dependent and Ca2+-sensitization pathways. Am J Physiol Gastrointest Liver Physiol 2011; 300:G1010–G1021 [DOI] [PubMed] [Google Scholar]
- 70.Saab S, Tam S, Tran Bi Melton A, Tangkijvanich P, Wong H, Yee H., Jr. Myosin mediates contractile force generation by hepatic stellate cells in response to endothelin-1. J Biomed Sci 2002; 9:607–12 [DOI] [PubMed] [Google Scholar]
- 71.Xu H, Zhou Y, Lu C, Ping J, Xu LM. Salvianolic acid B lowers portal pressure in cirrhotic rats and attenuates contraction of rat hepatic stellate cells by inhibiting RhoA signaling pathway. Lab Invest 2012; 92:1738–48 [DOI] [PubMed] [Google Scholar]
- 72.Xu H, Lu C, Ping J, Zhou Y, Xu L. Effects of salvianolic acid B on endothelin-1-induced contraction and cytoskeleton organization of hepatic stellate cells in rats Zhonghua Gan Zang Bing Za Zhi 2014; 22:281–4 [DOI] [PubMed] [Google Scholar]
- 73.Bahde R, Kapoor S, Viswanathan P, Spiegel HU, Gupta S. Endothelin-1 receptor a blocker darusentan decreases hepatic changes and improves liver repopulation after cell transplantation in rats. Hepatology 2014; 59:1107–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J, Wang L, Song G, Han B. The mechanism through which octreotide inhibits hepatic stellate cell activity. Mol Med Rep 2013; 7:1559–64 [DOI] [PubMed] [Google Scholar]
- 75.Ding RB, Bao J, Deng CX. Emerging roles of SIRT1 in fatty liver diseases. Int J Biol Sci 2017; 13:852–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun LJ, Yu JW, Shi YG, Zhang XY, Shu MN, Chen MY. Hepatitis C virus core protein induces dysfunction of liver sinusoidal endothelial cell by down-regulation of silent information regulator 1. J Med Virol 2018; 90:926–35 [DOI] [PubMed] [Google Scholar]
- 77.Duong HT, Dong Z, Su L, Boyer C, George J, Davis TP, Wang J. The use of nanoparticles to deliver nitric oxide to hepatic stellate cells for treating liver fibrosis and portal hypertension. Small 2015; 11:2291–304 [DOI] [PubMed] [Google Scholar]
- 78.Zhao RZ, Chen X, Yao Q, Chen C. TNF-alpha induces interleukin-8 and endothelin-1 expression in human endothelial cells with different redox pathways. Biochem Biophys Res Commun 2005; 327:985–92 [DOI] [PubMed] [Google Scholar]
- 79.Yakubu MA, Nsaif RH, Oyekan AO. Peroxisome proliferator-activated receptor alpha activation-mediated regulation of endothelin-1 production via nitric oxide and protein kinase C signaling pathways in piglet cerebral microvascular endothelial cell culture. J Pharmacol Exp Ther 2007; 320:774–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robert S, Gicquel T, Bodin A, Lagente V, Boichot E. Characterization of the MMP/TIMP imbalance and collagen production induced by IL-1β or TNF-α release from human hepatic stellate cells. PLoS One 2016; 11:e0153118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsai HC, Li TH, Huang CC, Huang SF, Liu RS, Yang YY, Hsieh YC, Lee KC, Huang YH, Hou MC, Lin HC. Beneficial effects of the peroxisome Proliferator-Activated receptor α/γ agonist aleglitazar on progressive hepatic and splanchnic abnormalities in cirrhotic rats with portal hypertension. Am J Pathol 2018; 188:1608–24 [DOI] [PubMed] [Google Scholar]
- 82.Khimji AK, Rockey DC. Endothelin and hepatic wound healing. Pharmacol Res 2011; 63:512–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gifford FJ, Dunne PDJ, Weir G, Ireland H, Graham C, Tuck S, Hayes PC, Fallowfield JA. A phase 2 randomised controlled trial of serelaxin to lower portal pressure in cirrhosis (STOPP). Trials 2020; 21:260. [DOI] [PMC free article] [PubMed] [Google Scholar]