Abstract
All cells in organisms ranging from yeast to humans utilize iron as a cofactor or structural element of proteins that function in diverse and critical cellular functions. However, deregulation of the homeostatic mechanisms regulating iron metabolism resulting in a reduction or excess of iron within the cell or outside of it can have serious effects to the health of cells and the organism. This review provides a brief overview of the molecular and cellular mechanisms regulating iron physiology, including the molecules and processes regulating iron uptake, its storage and utilization, its recycling, and its release from the cell, such that the cellular iron levels are sufficient to meet metabolic demand but below those that cause permanent damage. The major focus of review is on the pathological consequences of dysregulation of these homeostatic mechanisms, focusing on the brain. Current advances on the role of iron accumulation to the pathogenesis of rare neurological disorders caused by genetic mutations as well as to the more prevalent and age-associated neurodegenerative diseases are described.
Impact statement
Brain degenerative disorders, which include some neurodevelopmental disorders and age-associated diseases, cause debilitating neurological deficits and are generally fatal. A large body of emerging evidence indicates that iron accumulation in neurons within specific regions of the brain plays an important role in the pathogenesis of many of these disorders. Iron homeostasis is a highly complex and incompletely understood process involving a large number of regulatory molecules. Our review provides a description of what is known about how iron is obtained by the body and brain and how defects in the homeostatic processes could contribute to the development of brain diseases, focusing on Alzheimer’s disease and Parkinson’s disease as well as four other disorders belonging to a class of inherited conditions referred to as neurodegeneration based on iron accumulation (NBIA) disorders. A description of potential therapeutic approaches being tested for each of these different disorders is provided.
Keywords: Alzheimer’s diseases, Parkinson’s disease, neurodegenerative disease, neurodegeneration with brain iron accumulation, iron transport, iron homeostasis
Introduction
Iron is a critical metal that acts as an electron donor and acceptor in a plethora of fundamental cellular processes including oxygen transport, cellular respiration, energy metabolism, DNA synthesis, and cell proliferation.1,2 Not surprisingly, iron deficiency can have serious effects on fetal and postnatal development and through adulthood, particularly on organs such as the brain the proper functioning of which critically dependent on it. On the other hand, a major consequence of elevated iron is impairment of the mitochondrial respiratory chain leading to an abnormal increase in the production of excessive levels of reactive oxygen species (ROS), including hydroxyl radical (OH-), hydrogen peroxide (H2O2), and other highly reactive radicals which damage proteins, lipids, and nucleic acids generally resulting in the death of the cell.1,2 Although the most abundant metal in the brain, the brain is also the organ that is most vulnerable to an elevation of iron, in part because of its high utilization of oxygen for cellular respiration. A compelling body of evidence suggest that deregulated iron homeostasis leading to its accumulation is at least a key contributor to disorders of the developing and adult brain. In this review, we summarize the molecules and mechanisms involved in the transport of iron and the homeostatic mechanisms involved in keeping it at physiological levels. We then describe how deregulation of iron accumulation contributes to neurodevelopmental disorders and neurodegenerative diseases.
Iron transport and homeostasis
Iron transport
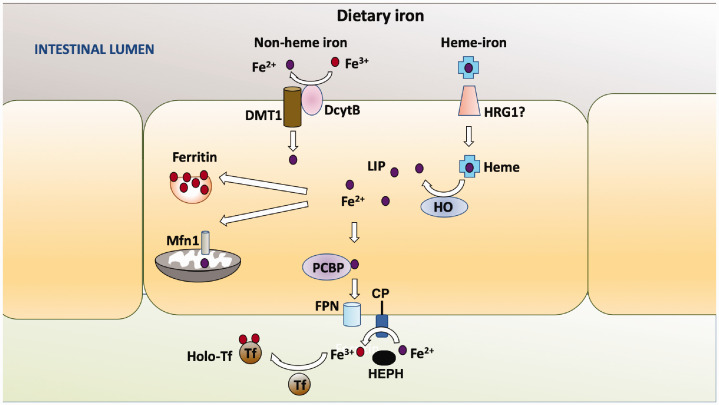
In humans, all iron is obtained from diet where it exists in a heme or non-heme form. Heme-iron comes mostly from the breakdown of hemoglobin and myoglobin in animal products, whereas non-heme iron is derived from plant as well as animal products. Although heme-iron is more bioavailable than non-heme iron, non-heme iron represents over 85% of dietary iron in humans. Dietary iron exists largely in two valency forms: the reduced, reactive, and toxic ferrous ion (Fe2+) form present in heme-iron and the oxidized and relatively non-toxic ferric ion (Fe3+) form in non-heme iron.3–5 Both heme and non-heme are taken up by enterocytes lining the intestinal brush border.3,4 Non-heme iron is transported through the apical membrane of enterocytes through DMT1 (divalent metal transporter-1), a transmembrane transporter (Figure 1).3,5–7 Transport through DMT1 requires reduction of Fe3+ to Fe2+ which is performed by duodenal cytochrome B (Dycb), a ferrireductase also localized at the apical membrane of the enterocytes.8 Because iron absorption in Dycb knockout mice is normal, it is believed that there are other intestinal ferroreductases but these remain to be identified.
Figure 1.
Uptake and release of iron from intestinal enterocytes. Non-heme iron (free Fe3+ or loosely bound to small molecules) is transported through DMT1 after its reduction to Fe2+. Heme-iron (composed of Fe2+) is transported across the apical membrane of intestinal enterocytes through a transporter that has not been firmly identified (HRG1is a candidate). Degradation of heme-iron by HO releases Fe2+ in the cytosol. Fe3+ in non-heme iron is reduced to Fe2+ by DcytB localized at the apical membrane and transported through the membrane by DMT1. As in all cell types, Fe2+ is stored in the cytosol as a complex with ferritin (as Fe3+ following oxidation by H-ferritin), transported into mitochondria through the Mrfn1 transporter, incorporated into iron-requiring enzymes or proteins, or utilized for other cellular purposes. Within enterocytes, transport proteins such as PCBP deliver Fe2+ to the basolateral side of the cell where it is released into the interstitial fluid by the FPN1 transporter. Release by FPN1 involves oxidation of Fe2+ to Fe3+ which is mediated by liver-secreted HEPH or ceruloplasmin (CP). In some cell types, CP is synthesized as a GPI-linked protein as shown in the figure. Fe3+ in interstitial fluid is immediately bound by transferrin, a highly abundant iron transport protein. Fe3+-bound Tf is taken by the endothelial cells of blood vessels which vascularize the microvilli and then delivered to cells that need iron. Most Fe3+-bound Tf is used by erythrocyte precursor cells for the production of heme that becomes part of hemoglobin within RBCs upon differentiation of the precursors. In some cell types. (A color version of this figure is available in the online journal.)
The identity of the intestinal transporter for heme-iron has yet to be resolved. One candidate is heme-responsive gene-1 (HRG1), better known for its role in transporting iron within macrophages, but that is also expressed by enterocytes. Following transport into the enterocyte, heme-iron binds to hemeoxygenase (HO) localized to the cytosolic side of smooth ER which catalyzes the release of Fe2+ (Figure 1).9 Whether originating from heme-iron or non-heme iron, in the cytoplasm, Fe2+ is bound by iron-binding proteins, of which ferritin is the most abundant.10 Ferritin is composed of two proteins, ferritin-light chain (L-ferritin) and ferritin-heavy chain (H-ferritin), that assemble into a hollow, spherical protein cage capable of storing Fe2+ atoms within it.10,11 Sequestration in the ferritin cage requires the oxidation of Fe2+ to Fe3+ which is mediated through ferroxidase activity that H-ferritin possesses.
Fe2+ is transported to subcellular locations for use or to the basolateral side for export by cytosolic chaperones, the best characterized of which is PCBP2, a member of the poly(rC)-binding protein (PCBP) family of metallochaperones (Figure 1).12,13 Export at the basolateral membrane is mediated by ferroportin-1 (FPN1), the only known exporter of iron in all mammals cells.14,15 Efficient export of Fe2+ through FPN1 requires its oxidation back to Fe3+ by hephaestin (HEPH), a soluble ferroxidase secreted by the liver16–18 (Figure 1). Whereas HEPH is the major ferroxidase in enterocytes, another ferroxidase called ceruloplasmin, synthesized and secreted almost exclusively by the liver and abundant in plasma, also participates in the oxidation of Fe2+.19–21
Upon export into interstitial fluid, Fe3+ is bound by transferrin (Tf).3–5 Tf-bound Fe3+ binds to the transferrin receptor-1 (Tfr1) at surface of endothelial cells lining blood vessels after which it is endocytosed as a complex with Tfr1. Fe3+ disassociates from transferrin because of endosome acidification and is exported into the cytosol by transmembrane DMT1 after reduction to Fe2+ a ferric reductase, STEAP3 (six-transmembrane epithelial antigen of prostrate-3).3–5 From the cytosolic pool referred to as labile iron pool (LIP), Fe2+ is transported to different cellular locations for metabolic functions, with much of it going to the mitochondria through the inner membrane proteins mitoferrin-1 or mitoferrin-2 (Mfrn-1/2).22,23 Excess Fe2+ is pumped out of the cell through FPN1 via oxidation to Fe3+ by ceruloplasmin.24 In addition to its ferroxidase function, ceruloplasmin stabilizes FPN1 at the plasma membrane promoting iron efflux into portal blood and facilitates the loading of Fe3+ onto transferrin.19,25 In contrast to HEPH which is soluble, several cell types produce ceruloplasmin as a GPI-anchored protein that localizes to the plasma membrane where it associates with FPN1.19,26,27
Iron exported from blood vessels is taken up by other cell types, an overwhelming majority of which utilize the Tf-Tfr1 system for iron import.
Iron homeostasis
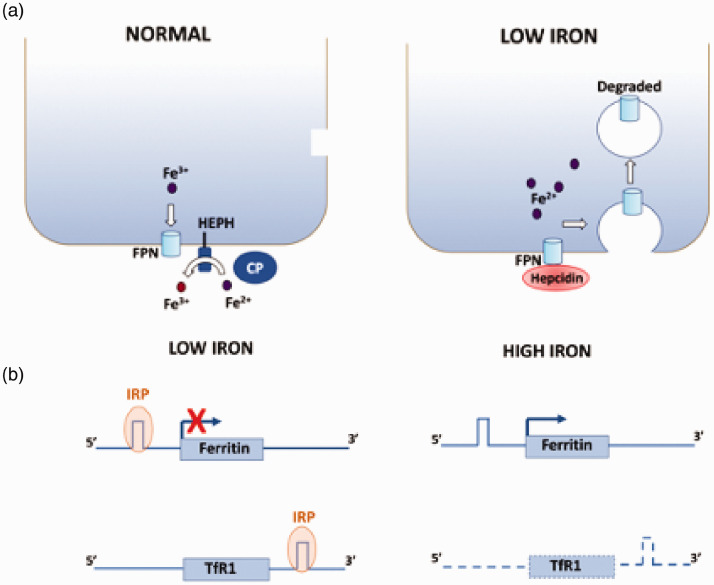
Several mechanisms regulate iron metabolism normally or in response to pathophysiological conditions, such as iron overload, hypoxia, and inflammation.5 Cells of the intestinal epithelium exfoliate when the body requires low iron reducing the transport of iron to cells of the body.28 Internalization of the Tf-Tfr1 complex in endosomes is under control of intracellular iron levels and can be regulated.29,30 Absorption of iron from the intestinal lining or its release is controlled by hepcidin, a small peptide produced in the liver, that acts as the ligand for FPN1.31,32 Binding of hepcidin to FPN1 at membrane of intestinal enterocytes and macrophages causes the internalization of FPN1 and its degradation resulting in reduced release of iron into body fluids33–35 (Figure 2(a)). The synthesis of hepcidin decreases during iron deficiency and is increased by elevation of stored iron.33,34 Downregulation of ceruloplasmin production is another known mechanism of iron homeostasis resulting in reduced cellular iron export.
Figure 2.
Mechanisms of iron homeostasis. Two major mechanisms are shown. (a) Under normal conditions, iron is exported out of the cell through FPN1, a process that is facilitated by HEPH or ceruloplasmin, which oxidize Fe2+. When iron is low, liver-secreted hepcidin binds to FPN1 causing its internalization and degradation. (b) When intracellular iron is low, IRPs bind to the IRE localized at the 5ʹ UTR of the FPN1 mRNA and at the 3ʹ UTR of the TfR1 mRNA. Binding to the 5ʹ UTR IRE of ferritin mRNA results in a block in its translation reducing its intracellular levels when iron storage is not required. In contrast, binding to the 3ʹ UTR of TfR1 mRNA stabilizes it resulting in increased import of iron into the cell. (A color version of this figure is available in the online journal.)
A well-characterized mechanism of cellular iron homeostasis is the upregulation of ferritin and downregulation of Tfr1 mRNA translation in response to increasing level of intracellular iron (Figure 2(b)). Both effects depend on two iron regulatory proteins (IRP1 and IRP2) that bind to RNA hairpin structures called iron-responsive elements (IREs) in the untranslated regions (UTRs) of target mRNAs resulting in effects on the stability or translation of the mRNA. When intracellular iron is high, IRP-1 adopts a conformation that is incapable of binding the IRE, whereas IRP2 is degraded. Thus, the IRE is unbound under high-iron conditions. When iron is low, however, IRP1 acquires the conformation for IRE binding and IRP2 expression is elevated permitting either of these IRPs to bind the IRE (Figure 3(b)). The ferritin mRNA contains an IRE in the 5ʹ-untranslated region (UTR), whereas in the Tfr1 mRNA, multiple IREs are located within the 3ʹ UTR.35–37 Binding of IRPs to ferritin mRNA causes a translational block resulting in reduced synthesis and hence decreased storage, whereas binding of IRP to the TfR1 mRNA protects it from degradation by endonucleases resulting in increased expression of TfR1 and higher iron uptake. When iron is low however, translation of ferritin is blocked, while the stabilized TfR1 mRNA allows its translation permitting efficient iron entry into the cell.35–37 The mRNAs of other RNA transport proteins, including FPN1 and DMT1, also contain IREs and are regulated by the IRE/IRP system35–38 such that when iron is high FPN1 expression is increased due to stabilization of its mRNA, whereas DMT1 expression is reduced via a translation block. These changes increase iron efflux while reducing its influx, respectively.
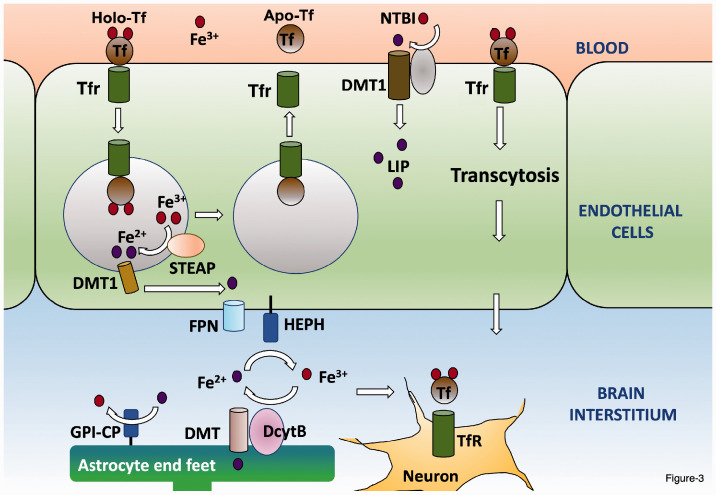
Figure 3.
Delivery to iron from blood vessels of the BBB to the brain. Iron-bound transferrin (holo-Tf) binds to the transferrin receptor (Tfr). The holo-Tf-Tfr1 complex is internalized in endosomes and the acidification of the endosome causes the release of Fe3+ from Tf. The Tf-Tfr1 complex is recycled to the membrane where Tf dissociates from Tfr1 (apo-Tf) and is available for binding to iron. The Fe3+ is reduced to Fe2+ within the endosome by STEAP before it is transported out into the cytosol by DMT. Fe2+ is oxidized by HEPH at the abluminal membrane before it is transported out of the endothelial cell by the FPN1 transporter into the interstitial fluid of the brain. It has been proposed that the Tf-Tfr1 complex can translocate through the apical membrane and be exported out of the abluminal membrane without being internalized in an endosome. Since transferrin is not present at saturating levels, iron exists in the brain parenchyma as NRBI. Neurons take up Fe2+ through the Tf-Tfr1 system, whereas astrocytes, which lack Tfr1 uptake NTBI through DMT1. (A color version of this figure is available in the online journal.)
In response to pathogenic challenge, the expression of hepcidin is induced in macrophage within the site of inflammation resulting in higher uptake of iron. This lowers iron levels in serum depriving pathogens, which depend on iron for their growth, of it.33,34,39
Brain iron transport
Iron transport to the brain
After the liver, which serves as a major storehouse of iron within the body, the brain is the organ with the highest content of iron. In the brain iron is essential for a number of important processes, including energy production, myelination, neurotransmitter synthesis and metabolism, and synaptic activity.40–45 Given its importance to such diverse and important functions, the level of iron in the brain has to be carefully regulated such that there is neither a deficiency nor excess. Iron homeostasis in the brain is regulated separately from the periphery and hence alterations in peripheral iron do not impact the level of iron in the brain. Much of the brain’s homeostatic regulation occurs at the level of the blood–brain barrier (BBB), formed by endothelial cells of the capillary wall have tight junctions between them and with pericytes in their basement membrane. Along with the end-feet of astrocytes that cover the endothelial cell membrane, the BBB is semi-permeable barrier separating circulating blood in the brain from the cerebrospinal fluid and brain parenchyma.46,47 The mechanism by which iron is transported across the endothelial cell lining of BBB vasculature is not well understood. There is some consensus that, as in most other cell types, Tf-bound Fe3+ in blood binds to Tfr1 at the apical side of the endothelial cells, is internalized in endosomes, and following conversion to Fe2+ is released to the cytosol, after which it is exported on the abluminal side of the endothelial cells by FPN1 (Figure 3). Re-oxidation to Fe3+ is performed primarily by soluble ceruloplasmin. Although abundant in serum, liver-secreted ceruloplasmin does not cross the BBB in a healthy brain. Much of the ceruloplasmin in the brain interstitium is synthesized and released by the choroid plexus. In addition to the soluble form, astrocytes (and many cell types outside the brain) synthesize ceruloplasmin in a GPI-anchored form that localizes at their end-feet which is in contact with the FPN1 on the abluminal side of the endothelial cells.26,48–51
Another mechanism that has been proposed for transport of iron across the endothelial cells of the BBB is the translocation of the internalized endosome containing the iron-bound Tf-Tfr1 complex across the cytosol and fusion with the abluminal membrane resulting in the release of iron disassociated from Tf-Tfr1 by exocytosis51,52. Yet, another model posits that rather than being transported in the endosome, the iron-bound Tf-Tfr1 complex travels from the apical membrane to the abluminal membrane by transcytosis where iron-bound Tf is released49, 53,54 (Figure 3). Besides its transport out of the blood vessels of BBB, iron enters the brain interstitium through the epithelial cell lining of the choroid plexus, which has a more permeable than the lining of the BBB.55,56 The choroid plexus expresses the major components for iron transport including high levels of both Tfr11and DMT1 as well as FPN1, DcytB, STEAP3 and FPN1 and, as described above, ceruloplasmin.57,58
Iron transport within the brain
Much of the iron released from endothelial cells of the BBB is taken up by the end-feet of astrocytes and then made available to neurons and other cells types via release in the brain parenchyma59,60 (Figure 3). Because they express ferritin poorly, astrocytes are unlikely to store much iron.61–63 Fe3+ released by astrocytes or the endothelial cells of the BBB is bound by Tf synthesized and secreted predominantly by the epithelial cells of the choroid plexus.55,60,64,65 Although oligodendrocytes also synthesize Tf and may represent another potential source, it is suggested that oligodendrocyte-synthesized Tf lacks the signal peptide for secretion.55,66,67 In contrast to the periphery, however, where Tf is in vast excess, in the brain interstitial fluid, Tf levels are much lower and completely saturated with iron.50,68 Some iron is bound by ferritin, heme, and albumin. But a substantial portion of the iron is unbound and referred to as non-transferrin-bound iron (NTBI). NTBI is loosely bound to small molecules including citrate, acetate, ascorbate, or ATP released by astrocytes. Therefore, while NTBI is not present in plasma of healthy individuals because of the saturating amounts of Tf, it is a normal component of brain interstitial fluid and CSF and is the source of iron for most brain cell types.69 While uptake of Tf-bound iron occurs through the Tf-Tfr1 system, as in other most cells of the body, NTBI is transported into brain cells by DMT1 although the ferrireductases involved in reducing Fe3+ to Fe2+ have not yet been firmly identified. Interestingly, recent studies have shown that the prion protein (PrP), mutations of which causes prion disease, is also involved in transporting NTBI, at least in the liver, kidney, and neuroblastoma cells, by acting as a ferrireductase partner for ZIP14 and DMT1.70–73 As discussed later in this review, α-synuclein, mutations of which cause Parkinson’s disease, also possesses ferrireductase activity and promotes Fe2+ influx.74–77
Neurons and microglia internalize iron from interstitial fluid through the classic Tf-Tfr1 pathway.60,78,79 Neurons also take up iron through another protein called lactoferrin (Lf), which besides binding iron (Fe3+) has other functions including antimicrobial and anti-inflammatory activities.80 In the brain, Lf is synthesized mostly by microglia and generally under conditions when microglia are activated.81 Fe3+-bound Lf binds to the Lf receptor (LrF) expressed by neurons and astrocytes and is internalized by receptor-mediated endocytosis. Expression of LfR in neurons is elevated in disease states.82,83 Several other NTBI transporting proteins have been identified and are expressed by different types of neurons, including the Zrt-Irt-like protein 8 (ZIP8) and ZIP14 transporters and L-type, T-type, and TRPC (transient receptor potential canonical) calcium channels. Therefore, for example, while ZIP8 is the predominant transporter of NTBI in hippocampal neurons, in retinal neurons, it is both ZIP8 and ZIP14.84–86 Ceruloplasmin stabilizes FPN1 in neurons ensuring efflux of excess iron. FPN1 in neurons is also stabilized by amyloid precursor protein (APP) although in contrast to ceruloplasmin and HEPH, it is thought that APP lacks ferroxidase activity.87
The mechanism for iron uptake by astrocytes has not been resolved. Although many studies have reported that astrocytes do not express Tfr1, some recent studies have detected Tfr11 in cultured astrocytes and in vivo.88–91 Binding of Tf to Tfr11 and transport of iron has also been described, at least in cultured astrocytes.31,91,92 It is likely that most iron uptake by astrocytes involves internalization of NTBI through DMT1 which is highly expressed by astrocytes.43,93–95 Additionally, ZIP14 expressed in astrocytes and has been shown to take up Fe3+.96 Finally, uptake by astrocytes through TRPC channels has been proposed.43,97 As in all other cell types in the body, astrocytes release iron through FPN1. However, at least in cultured astrocytes, FPN1 is directly bound by GPI-linked ceruloplasmin and ceruloplasmin is required for the efflux of iron through FPN1.51
While having a high iron content, oligodendrocytes do not express Tfr11 and exactly how iron (which is necessary for myelination) is taken up by these cells is not clear. Internalization in the form of NTBI through DMT1 is one likely mechanism. Oligodendrocytes may also take up iron as a complex with ferritin by binding to a receptor called TIM2 (T cell immunoglobulin domain 2 protein) followed by endocytosis of the iron-bound ferritin/TIM2 complex.98,99 The ferritin/TIM2 system of iron uptake has been described in cell types outside the brain.100 Another difference between the brain cell types with regard to iron is while astrocytes use ceruloplasmin, oligodendrocytes use HEPH.51,101 Like ceruloplasmin, HEPH is thought to stabilize FPN1 in oligodendrocytes.
While widely distributed in the brain, the concentrations of iron in various brain regions vary substantially.102 Most iron is concentrated in the substantia nigra (SN), globus pallidus, and locus coeruleus. At the cellular level, oligodendrocytes contain the highest amount of iron. The level of iron increases in the normal adult brain as a function of age.103 Indeed, it has been suggested that iron accumulation is directly proportional to aging.13 Interestingly, the increase is not uniform across brain regions. For example, whereas in individuals over 80 years of age iron deposition increases in the SN and globus pallidus, there is no increase in the LC.104,105 Besides the increase itself, during aging, iron is converted from its stable and non-toxic ferritin-bound form to hemosiderin and other derivatives in which iron is more reactive.105,106 At the cellular level, although oligodendrocytes contain more iron that other brain cell types, this does not change during aging.105,107 The molecular mechanisms underlying the differences in iron concentration and in their age-related changes are unclear but likely reflect differences in expression of uptake and storage proteins as well as of iron uptake and usage mechanisms.
Deregulated iron homeostasis and neurodegenerative disorders
Deregulation of iron metabolism, its redox activity, or its ability to transition in valency between the Fe2+ and Fe3+ state can have serious consequences to neuronal health and survival, and consequently to brain function.13 In addition to the well-established damaging effects of free radicals on cellular component, iron accumulation/deposition harm the functioning of neurons and other brain cell types through abnormal interactions with proteins, the facilitation of protein aggregation, and disruption of spatially dependent processes, such as axonal transport, release of neurotransmitters, neurotrophic factors and cytokines, and synaptic activity. There are two categories of neurodegenerative disorders to which elevated brain iron is responsible for, or to which it likely contributes. One of these disease categories is neurodegeneration with brain iron accumulation (NBIA) disorders, a group of monogenic inherited disorders characterized by iron deposition in the brain. The second category is composed of a various age-associated neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), Friedreich’s ataxia, ALS, and stroke (for recent reviews, see literature13,81,108–111). We review these two types of iron-associated CNS diseases separately.
Age-associated neurodegenerative diseases
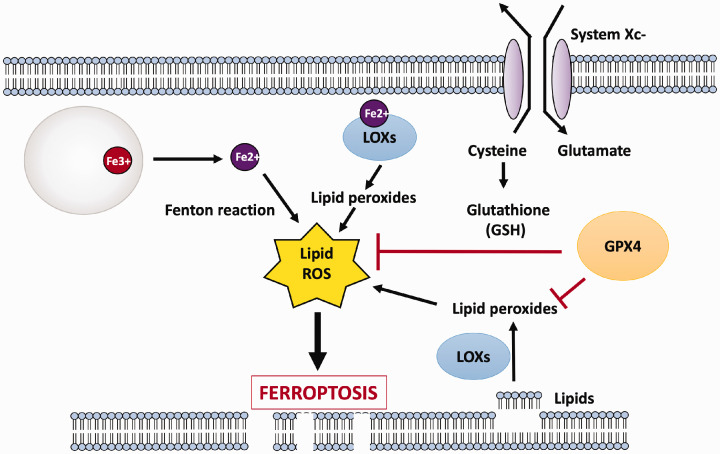
Although there is general consensus that brain iron levels are elevated in several age-associated neurodegenerative diseases, the extent to which this abnormality contributes to neurodegeneration has yet to be resolved. Iron accumulation could promote neuronal loss by different mechanisms. For example, a neuropathological hallmark of many neurodegenerative diseases is the misfolding, fibrillization, and aggregation of specific proteins.112 Iron is often a component of these disease-associated protein aggregates and in the case of some such proteins directly induces their misfolding, fibrillization, and aggregation.113 Another common denominator of age-associated neurodegenerative diseases is oxidative stress. The brain is particularly vulnerable to mitochondrial dysfunction and the generation of toxic free-radicals. A form of iron-dependent cell death that has been described relatively recently is ferroptosis, which is characterized by lipid peroxidation resulting in membrane rupture.114–116 Because of their unique structure composed of axons and dendrites and consequently high plasma membrane composition, neurons could be particularly sensitive to ferroptosis.117,118 Ferroptotic death has been detected in a variety of neurodegenerative diseases.49,118,119 Ferroptosis is often triggered by reduced activity of glutathione peroxidase (GPX4), an enzyme that utilizes glutathione (GSH) as reducing substrate to detoxify highly reactive hydroxyl radical and peroxyl radicals that are produced through the Fenton reaction catalyzed by Fe2+. GSH synthesis, and hence GPX4 activity, depends on the import of cystine through the system Xc-transporter. With a reduction of GPX4 activity, or when the activity of the system Xc-transporter is reduced or inhibited (which results in decreased GSH), harmful levels of hydroxyl, peroxyl radicals, and other ROS accumulate within the cell. Ultimately, peroxidation of membrane lipids results in the rupture of the plasma membrane killing the cell (Figure 4). Ferroptosis can also be triggered by a large or sustained increase in Fe2+ which overwhelms the capacity of GPX4 to reduce ROS and prevent lipid peroxidation. Although GPX4/glutathione activity was believed to be the only defense against ferroptosis, a recent study described that reduction in the activity of another protein, ferroptosis suppressor protein-1 (FSP1), can also trigger ferroptosis acting in a GSH-independent manner.120 This study showed that FSP1 acts downstream of iron to inhibit lipid peroxidation to suppress ferroptosis.120 Iron can also induce death of neurons through apoptosis, a form of cell death triggered by the release of cytochrome c from mitochondria and subsequent activation of caspase-3 or by the activation of caspase-8 through binding of ligands, such as TNF or FasL, to their receptors at the plasma membrane.121–123
Figure 4.
Iron, lipid peroxidation and ferroptosis. Following endocytosis of apo-Tf-Tfr1 complex and the disassociation of Fe3+, the Fe3+ is reduced to Fe2+ and transported to the cytoplasm. Although not shown in the figure, Fe2+ can also accumulate through import of NTBI via DMT1. Fe2+ can produce toxic hydroxyl ion and lipid peroxides through the Fenton reaction. Elevated Fe2+ can also catalyze lipid peroxidation by combining with cytosolic lipoxygenases (LOXs). Peroxidation of membrane lipids damages the plasma membrane rupturing it. Ferroptosis is normally inhibited by glutathione peroxidase (GPX4), which uses glutathione as its substrate to detoxify lipid peroxides, hydroxyl ions, and other ROS by their reduction. Glutathione (GSH) synthesis is critically dependent on the proper functioning of the transporter, system Xc-, which imports cystine which, after reduction to cysteine, is used to produce glutathione (GSH). Although most often resulting from reduced activity system Xc- and/or GPX4/GSH, disruption of iron homeostasis leading to increased free iron can overwhelm the protective activity of GPX4/GSH to promote ferroptosis. (A color version of this figure is available in the online journal.)
Although most effort has been placed on the effects of iron on neurons, it is now well-accepted that dysfunction of glial cells makes key contributions to the pathogenesis of neurodegenerative disorders.124–127 Iron is required for the proper development of oligodendrocytes and a high amount of ferritin-bound iron is necessary for synthesis of myelin and fatty acid synthesis.128 Abnormalities in oligodendrocytes, including effects on the production and maintenance of myelination, are a feature of various neurodegenerative diseases.129–132 Breakdown of myelination has been described in different neurodegenerative disease.133–136 In addition to a direct effect on the functioning of neurons, breakdown of myelin leads to a leakage of iron that not only damages oligodendrocytes (resulting in further myelin destruction) and other cell types, but also promotes the fibrillization of disease-causing proteins, such as α-synuclein and Aβ.134,135,137,138 Additionally, the released iron can be taken up by macrophage and microglia leading to their activation. This has been shown to be the case in multiple sclerosis (MS).133 Neuroinflammation, involving abnormal activation of astrocytes and microglia, is believed to contribute to neuronal loss in several neurodegenerative diseases through the release of toxic cytokines.139–141
There is broad consensus that deregulation of iron homeostasis is involved in the pathogenesis of different neurodegenerative diseases, although whether it triggers disease mechanisms, is a contributor to disease pathogenesis, or is a consequence of pathophysiological alterations that trigger the disease remains to be determined for each disease. The finding that healthy rats fed with a high-iron diet display neurodegeneration is consistent with a causal role for elevated iron in neurodegenerative diseases.142 Moreover, mutations and deregulated expression of specific proteins regulating iron homeostasis can directly cause disorders with neurodegeneration, as observed in NBIA disorders (see below).
Although a role for iron dyshomeostasis has been suggested to be involved in several neurodegenerative diseases, this review focuses on AD and PD where this issue has been most studied, and the results are most convincing. AD and PD are also the two most prevalent neurodegenerative diseases.
Parkinson’s disease
PD is the most common movement disorder and the second most prevalent neurodegenerative disease, afflicting ∼1% of individuals over 65 years of age. The disorder, clinically characterized by bradykinesia, resting tremor, postural instability, and gait and balance issues, results from the selective and progressive degeneration of dopaminergic neurons in the substantial nigra (SN).143–146 In addition to the motor deficits, cognitive decline is also observed in a subset of patients and this is believed to be due to neuronal loss in other brain areas, of which the cortex and other basal ganglia areas are predominant.146–148 Although the reason for selective vulnerability of SN dopaminergic neurons in PD remains unresolved, one widely accepted factor is the normally high level of oxidative stress in these neurons resulting from the degradation of dopamine by monoamine oxidase (MAO) generating H2O2, which in the presence of iron generates toxic hydroxyl radicals and other oxidative species which damage cellular macromolecules.149,150 Dopamine can also be non-enzymatically oxidized in the presence of oxygen to yield harmful metabolites and free radicals.149,150 Additionally, metabolites of dopamine oxidation form adducts with cysteine and other amino acid residues that can be highly harmful to the cell.151,152 As explained below, cysteine is necessary for the synthesis of glutathione (GSH), a potent intracellular antioxidant protects against the build-up of ROS and lipid peroxidation. Dopaminergic neurons of the SN also have high levels of iron under normal conditions rendering them particularly vulnerable to harmful levels of oxidative stress. Another unique feature of most dopaminergic neurons of the SN is that they also contain high levels of neuromelanin, a dark granular pigment that binds Fe3+ with high affinity.104,150,153–155 While neuromelanin can sequester iron preventing toxicity, its levels decrease during aging and such an decrease would leave iron unsequestered.156 In addition to the age-associated reduction, neuromelanin levels are further reduced in the SN of PD patients. While neuromelanin plays a protective role by trapping iron, this same property also concentrates iron in dopaminergic neurons, which can potentiate deregulated oxidative stress resulting from an imbalance of other mechanisms such as dopamine oxidation or GSH synthesis.157 Other studies have described that when Fe3+ is in excess, neuromelanin can itself enhance hydroxyl radical production and oxidative stress, possibly by converting Fe3+ to Fe2+.158–160 These findings provide a plausible explanation for why neuromelanin-containing neurons are selectively lost in PD.
Release of iron from dying neurons is taken up by microglia and astrocytes in the SN and other parts of the basal ganglia activating them resulting in an unwanted inflammatory response.161 Neuromelanin released from degenerating neurons can also promote inflammation.157 Indeed, neuroinflammation is a hallmark of PD and there is strong evidence that it is an essential contributor to disease pathogenesis.162–164
Over 85% of PD represent a sporadic/idiopathic form caused by a combination of environmental factors and genetic susceptibility. Administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or 6-hydroxydopamine (6-OHDA) to rodents as well as monkeys are commonly used models of chemically-induced PD that recapitulate core disease features including robust mitochondrial dysfunction, neuronal loss, motor impairment, and neuroinflammation.165–168 Over the past two decades, several genes have been identified that cause familial PD, the first and best characterized of which is the SNCA gene.169,170 The SNCA gene encodes α-synuclein, a protein abundant in the brain and that localizes predominantly to pre-synaptic terminals. In the PD brain, α-synuclein is found in abnormal oligomeric, fibrillary, and aggregated forms.146,169,170 While oligomeric and fibrillary α-synuclein are neurotoxic, aggregated α-synuclein generally localizes within intracellular inclusions called Lewy bodies. Lewy bodies are distributed in neurons in regions other than the SN and are believed to protect neurons by sequestering oligomeric and fibrillary α-synuclein in an aggregated form. Phosphorylation of α-synuclein at Ser129 enhances its neurotoxicity.143–145 Interestingly, increased expression of normal α-synuclein, as a result of the duplication or triplication of the SNCA gene, is sufficient to cause PD.143–145,171 Consistently, elevating α-synuclein levels in the SN in rodents through viral expression results in neurodegeneration and behavior deficits.172–175 Dysfunction of α-synuclein is widely regarded as central to PD pathogenesis triggering abnormalities, including defective synaptic vesicle fusion and DA release, mitochondrial dysfunction, oxidative stress, endoplasmic reticulum stress, induction of the unfolded protein response, and autophagy-lysosomal pathway impairment.176,177 It has recently been discovered that misfolded α-synuclein can be transferred from one cell to another spreading disease pathology in a prion-like manner.178,179
The normally high level of iron is useful to dopaminergic neurons because it serves as a co-factor for tyrosine hydroxylase, the enzyme that catalyzes the rate-limiting step in dopamine biosynthesis.180 However, many studies have found that iron content is further increased in the SN of patients with PD and have implicated this increase to disease pathogenesis.149,153,181–190 Moreover, within the SN, the increase occurs in neurons that selectively degenerate. Recent studies on PD patients with cognitive impairment describe increased iron accumulation in the caudate and cortex.191–193 Arguing in favor of a causative role for iron in PD are the observations that direct infusion of Fe3+ to the SN in rats results in mitochondrial dysfunction, oxidative stress, neuronal loss, striatal dopamine depletion, and impaired motor function, all characteristic features of PD.194–197 Administration of MPTP or 6-OHDA to rodents and monkeys also increases iron content in the SN and this increase is proportional to the loss of neurons.198–206 When compared, the iron accumulation generally precedes degeneration of the SN.198–201 It deserves mention, however, that in at least one of these studies in which the time-course of events was tracked, the increase in iron occurred after Tf levels had fallen and there was significant neuronal loss.203 Relevant to this point, some human studies have found that the increase of SN iron occurs only in the most severely affected patients.189–195 At least one study utilizing monkeys and the MPTP model also found that the increase of iron in the SN occurs only with severe PD pathology and behavioral deficits.207 Given that the clinical symptoms, neuropathological features, and rate of disease progression display a significant level of variability, it is possible that in a subset of PD cases, iron accumulation may be causally involved in disease progression, whereas it may be associated or result from degeneration in some other cases of PD. Consistent with such heterogeneity, aggregated α-synuclein, which is generally regarded as a biochemical hallmark of PD (see below) is also not seen in some familial forms of the disease.201
The expression or activity of several proteins involved in regulating iron homeostasis is altered in PD.153,184,193,208–210 Both the levels of ceruloplasmin and its ferroxidase activity, the stability of FPN1, and the loading of Fe3+ onto transferrin are severely reduced in the SN and CSF of PD patients and correlate with disease progression. One study reported ∼80% loss of ceruloplasmin in the SN of patients with idiopathic PD. Interestingly, PD patients have a reduced level of copper in the SN and, given the need for copper for the ferroxidase activity of ceruloplasmin, may explain the reduced ceruloplasmin activity and consequently increased iron-accumulation.207–212 Reduced expression of ceruloplasmin in the SN is also seen in rodent model of PD.213 Furthermore, ceruloplasmin knockout mice display motor deficits consistent with its reduction being a pivotal alteration in PD.213,214 FPN1 expression is also reduced in cell and rodent models of chemically-induced PD which would cause accumulation of intracellular iron.213,215,216 Worsening the situation, expression of Tfr11 and more generally DMT1 is increased in the SN in patients and in a variety of cell culture and mouse PD models, an alteration that would increase iron import.217–221 DMT1 upregulation may involve the IRE/IRP system as well as reduced degradation.218 Additionally, the proteasomal degradation of DMT1 by parkin, the E3 ubiquitin ligase parkin mutated in some forms of familial PD, is reduced in PD models. Upregulation of DMT1 in the SN has also been described in PD patients.219 The expression of Lf and the Lf receptor is increased in cell culture and rodent models of PD as well as in patients, alterations that could contribute to iron elevation.82,83,210,222,223 While some studies have described no change in ferritin expression in the SN in PD, other studies have reported a reduction.149,159,189,210,224–227 A reduction in ferritin could increase intracellular free Fe2+ which would have damaging effects on the cell. Dopaminergic neurons are also one of the few cell types in the body that express Tfr2, a transporter related to the plasma membrane transporter, Tfr1, but that transports iron into the mitochondria.228 Additionally, while Tfr1 expression is regulated by intracellular iron and is decreased when iron is high, TfR2 is insensitive to it.228,229 Furthermore, the expression of both Tfr2 and Mfn-2 is significantly upregulated in cell culture models of PD providing an explanation for mitochondrial iron accumulation in the disease.229,230 Such an upregulation would explain both the accumulation of iron in the mitochondria and the well-documented mitochondrial dysfunction in PD.
In addition to promoting oxidative stress, iron accumulation promotes another cardinal feature of PD pathogenesis—fibrillization of α-synuclein. Fe3+ binds α-synuclein with high affinity and this promotes a change of α-synuclein confirmation, converting it to a fibrillar form.137,138,231,232 Based on TEM analyses, addition of micromolar concentration of Fe3+ to wild type α-synuclein generates fibrils that resemble fibrils formed from disease-associated mutant forms of α-synuclein.233 Addition of iron to mutant forms has a stronger effect on fibrillization than on wild type α-synuclein.185 Consistent with binding to α-synuclein, deposits of iron are found in in the core of Lewy bodies where α-synuclein aggregates localize to. Taken together, these results suggest that iron accumulation in the SN promotes α-synuclein fibrilization and oligomerization, which render it neurotoxic.232,234,235 Consistent with the need for iron in α-synuclein toxicity, is the finding that iron-induced toxicity can be blocked by siRNA-mediated knockdown of intracellular α-synuclein expression in neuroblastoma cells.234 As described above, neuroinflammation in the SN is a well-established feature of PD and an occurrence that exacerbates neuronal loss.236,237 Through release of pro-inflammatory cytokines, activated microglia stimulate the uptake of iron by neurons which then can cause additional neurodegeneration through stimulation of α-synuclein aggregation and oxidative stress.238,239 Some studies have shown that oxidation at specific N-terminus residues stabilizes α-synuclein in a non-toxic form that is prevented from fibrilizing. However, iron inhibits this conformational block thus promoting the fibrilization of α-synuclein to a neurotoxic form.187,240–243 The structure of neuromelanin is also altered in the PD brain and in this altered conformation can crosslink with α-synuclein contributing to its aggregation.244 Such cross-linking may reduce the function neuromelanin as well as of α-synucleins.244,245 Indeed, it has been suggested that the association between neuromelanin and iron is reduced in the parkinsonian SN resulting in higher levels of free iron.193
Iron also promotes α-synuclein phosphorylation and in turn the binding affinity of iron is increased in Ser129-phosphorylated α-synuclein.235,246 α-synuclein phosphorylation at Ser129 is also enhanced by oxidative stress indirectly through the oxidation of Fe2+ to Fe3+ .235,247 Elevated iron upregulates α-synuclein expression likely through the IRE/IRP system. The 5ʹ UTR of α-synuclein transcript has a region that folds into a functional IRE and its synthesis can therefore be expected to be increased by elevated iron.248 And lastly, iron-induced oxidative stress promotes cell to cell transmission of pathogenic α-synuclein.235,247,249 Taken together, these results suggest that elevated iron in dopamine-rich SN neurons binds and promotes the pathological phosphorylation and fibrillization of α-synuclein, which besides exerting intracellular toxicity spreads in a prion-like mechanism promoting toxicity in other neurons with the SN and beyond.
It is noteworthy that while elevated iron promotes α-synuclein oligomerization, phosphorylation, and increasing its expression, α-synuclein also regulates iron homeostasis. Tfr1 and α-synuclein colocalize on the plasma membrane.250 Through its interaction with dynamin and modulation of clathrin-coated endocytosis, α-synuclein stimulates the endocytic uptake of iron in neurons.250,251 Depletion of α-synuclein results in an accumulation of Tf/Tfr1 complex within recycling endosomes.250,251 This would reduce iron levels in dopaminergic neurons which would reduce expression of tyrosine hydroxylase and consequently dopamine production. Additionally, ferritin expression is reduced in cells lacking α-synuclein suggesting multiple effects of α-synuclein on iron homeostasis.250 α-synuclein can be post translationally modified in a number of ways and at many residues. Oxidative stress induced by iron and dopamine is enhanced by altered oxidation and phosphorylation of α-synuclein at the membrane.252 As described above, α-synuclein also has ferrireductase activity which, counterintuitively, is reduced in brains of PD patients likely due to its deposition in intracellular aggregates or perhaps a disease-associated posttranslational modification of α-synuclein.74–77 Although much of the ferrireductase activity of α-synuclein is membrane-associated, it is not clear exactly how the reduction in this activity increases intracellular iron. It has been suggested that elevated α-synuclein can change the intracellular distribution of iron.253 Such a redistribution of iron would disrupt its cellular functions.
Results from recent studies suggest that neuronal death in PD is due to ferroptosis.116,254,255 Human induced pluripotent stem cell (iPSC)-derived neurons generated from patients with α-synuclein gene triplication undergo ferroptosis accompanied by oxidative stress and lipid peroxidation, which can be prevented by reducing iron-dependent build-up of free radicals.254 Also supporting the idea that neurodegeneration is due to ferroptosis is the recent finding that DJ-1, loss-of-function mutations of which cause inherited PD, suppresses ferroptosis.255 Loss-of-function mutations of the gene encoding parkin, a E3 ubiquitin ligase, also cause familial PD. Interestingly, overexpression of parkin stimulates the ubiquitination and degradation of DMT1 reducing iron import.256,257 This suggests that parkin may normally serve to control iron import into neurons. Another study found that transgenic mice overexpressing DMT1 had not only selective accumulation of iron in the SN.258 Parkin expression was upregulated in these mice likely as a protective response to the increased DMT1. Surprisingly, the DMT1-overexpressing mice did not display behavioral deficits even when fed with an iron-rich diet.258 Whether the upregulation of parkin expression contributes to the lack of a neurological phenotype in the transgenic mice is unclear.
Based on broad consensus that iron accumulation in the SN causes mitochondrial dysfunction, elevates oxidative stress, and promotes inflammation, which together cause the neurological deficits of PD, administration of iron chelators has been tested as a treatment strategy.259,260 Studies using a variety in vitro systems and cell culture models of PD have found iron chelation be protective.231,260–263 Most of the in vivo testing of iron chelators has used deferiprone because of its ability to cross the BBB. Testing of iron chelators in both chemical and genetic rodent models of PD has revealed the ability to reduce SN degeneration and improve behavioral performance.197,198,204,264–270 Astrocyte and microglial activation are also reduced in the MPTP and 6-OHDA models.204,271 One caveat that deserves noting is that the chelators used in mouse studies and human trials are not specific for iron, instead chelating other metals including copper, aluminum and zinc, dysregulation of which has also been linked to neurodegenerative disease. Therefore, whether the beneficial effects of these inhibitors are solely due to iron chelation, chelation of another metal(s), or a combination of metals is not clear.
In contrast to the success of iron chelators in PD models, a study using deferiprone in PD patients reported a lowering of SN iron in only a small subset of the patients.272 A possible explanation suggested by the authors of this study is that in contrast to other brain regions, iron in the SN is bound to neuromelanin rather than to ferritin rendering its chelation less efficient in the SNc.272 However, results of some other studies suggest possible benefit of iron chelation pointing to the need for additional clinical testing. A potential issue with iron chelators is the depletion of peripheral iron leading to anemia.81,260,273 Also described is that iron depletion in mice causes degeneration of dopaminergic neuron raising another potential difficulty.274 A conservative or moderate regimen of iron chelators has therefore been proposed in human studies.259 One study conducted in a limited number of patients described the ability of moderate doses of deferiprone to reduce iron accumulation in the SN and improve motor performance in PD patients.260
Iron chelators attenuate the motor deficits displayed in ceruloplasmin knockout mice indicating that the behavioral impairment in these mice is iron-dependent.275 Another study that correlated ceruloplasmin activity with efficacy of iron chelation in PD patients described that patients with lower ceruloplasmin activity responded better to iron chelation pointing to reduction in ceruloplasmin activity as being responsible for iron accumulation in the SN that then causes or contributes to disease progression.273 Therefore, targeting ceruloplasmin could be another attractive approach for PD therapy.276 Consistent with this possibility, peripheral infusion of ceruloplasmin has been shown to attenuate neurodegeneration and nigral iron elevation in the MPTP-administered mice.275,276 Exactly how such peripherally-delivered ceruloplasmin crosses the BBB is unclear and whether a similar approach could be effective in PD patients remains to be tested. Tf, which both delivers and removes iron from cells, is depleted in the SN of PD patients.277 Administration of Tf was found to be beneficial in MPTP-treated mice.277 However, like iron chelator administration, Tf supplementation also lowered iron levels in peripheral organs reducing its potential as a therapeutic approach in patients.277 Whether sequestration of elevated iron in the SN by increasing levels of ferritin can reduce neurodegeneration has been examined in the MPTP and 6-OHDA models. Overexpression of ferritin in SN neurons of mice did protect against neurodegeneration in younger animals although prolonged expression resulted in age-associated neurodegeneration.199,278 This age-associated neuronal loss in older transgenic mice could be due to the chronic depletion of iron from the LIP within the neurons, impacting important cellular functions that require iron. Another study descripted that virally-mediated overexpression of hepcidin reduced cellular and mitochondrial iron accumulation and was protective in chemical models of PD.279 Peripherally, hepcidin is best known for promoting FPN1 degradation, an action that would elevate intracellular iron. The mechanism by which hepcidin exerts its neuroprotective is likely through its ability to downregulate expression of DMT1, Tfr1, ferritin-L, and ferritin-H levels in neurons and astrocytes thereby reducing iron uptake and intracellular content in these cell types.31,280,281 Indeed, neuroprotection by elevating brain hepcidin has been described in other models of neurodegenerative disease.282,283 Some studies have described that N-acetyl-cysteine (NAC), a modified form of cysteine (which increases GSH synthesis) can partially protect mice against neurodegeneration in cell culture and the MPTP model of PD.284–287 NAC is likely to act downstream of iron accumulation to prevent oxidative stress. A recent trial in a limited number of PD patients showed efficacy with NAC therapy suggesting the need for larger-scale studies.288
Alzheimer’s disease
AD is the most common neurodegenerative disease, clinically characterized by dementia resulting from the selective loss of neurons in the hippocampus and cortex. One of the two key neuropathological hallmarks in AD is the accumulation of extracellular plaques containing aggregates of the amyloid-β (Aβ) peptide, a cleavage product of the amyloid precursor protein (APP).289–292 The production of Aβ from APP involves sequential proteolysis by two multi-subunit transmembrane proteases, β-secretase and γ-secretase. Aβ levels are elevated in the AD brain through a combination of increased production and reduced clearance.289–292 Although normally Aβ is a 40 amino acid peptide (Aβ40), much of the Aβ generated in the AD brain is 42 amino acids long (Aβ42). Aβ42 has a much higher propensity to oligomerize and aggregate than Aβ40 and is the predominant form of Aβ in amyloid plaques. A second key neuropathological hallmark of the AD brain is cytoplasmic inclusions within neurons called neurofibrillary tangles (NFTs). NFTs are composed of filaments of hyperphosphorylated tau, a microtubule-associated protein. The kinases involved in the pathological phosphorylation of tau include, but are not limited to, the non-mitotic cyclin-dependent kinase-5 (CDK5) and GSK3β.289–292 Hyperphosphorylation of tau not only causes it to disassociate from microtubules, but promotes its mislocalization to dendrites.
Although it is not clear if deregulation of Aβ or of tau is the primary trigger, the growing consensus is that both proteins are central to disease pathogenesis likely acting synergistically.289–292 Also widely accepted is that oligomeric and fibrillar forms of Aβ and tau, rather than the aggregated form in plaques and NFTs, are the neurotoxic species, and that these soluble forms start to form early in disease pathogenesis.293,294 Indeed, plaques and NFTs serve a protective role by sequestering Aβ and hyperphosphorylated tau as insoluble aggregates.293,294 In addition to plaques and NFTs, extensive synaptic loss, neuroinflammation, and oxidative stress are consistent features in the AD brain and contribute to neurodegeneration.289–292 Although the vast majority of AD is sporadic, mutations in the genes encoding APP, presenilin-1 (PS1), and presenilin-2 (PS2) cause familial AD.295,296 PS1 or PS2 is the catalytic subunit of γ-secretase, which includes three other proteins (nicastrin, PEN-2 and APH-1). Mutation or polymorphisms in several genes contributes to susceptibility in sporadic AD. Of these, inheritance of the E4 isoform of ApoE and mutations in the TREM2 genes are the best characterized genetic susceptibility factors.295–297
A growing body of evidence has implicated elevated brain iron in AD pathogenesis.109,298–301 Iron levels, and specifically of Fe3+, are elevated in the hippocampus and cortex of AD patients.109,298,302,303 Iron accumulation in the hippocampus can be observed in early stages of the disease and is considered to be a strong predictor of AD-related cognitive decline.302,304,305 Expression of FPN1 is reduced in the hippocampus of AD patients and likely causes or contributes to the accumulation of iron.306 Other studies have described that increased iron in the cortex correlates with the amount of Aβ plaques, tau pathology, and cognitive decline suggesting that cortical iron accumulation could be used to evaluate AD progression and severity.305,307–311 Histological analyses of the cortex of AD patients reveal that the accumulation of iron deposits is also associated with alterations in the pattern of cortical lamination and in myelination changes.311,312
Consistent with elevated brain iron being an early event in AD pathogenesis, secreted ferritin, which reflects intracellular iron load, is elevated in the CSF of cognitively normal individuals with mild cognitive impairment (MCI). In these pre-AD individuals, the increase either precedes or correlates with Aβ pathology, reduced fluorodeoxyglucose utilization, and most importantly, progression to AD thereafter.313–315 Thus, CSF ferritin could be used as a biomarker to predict near-term risk for disease progression.313–315 Longitudinal imaging studies performed in patients suggest that the increase in CSF ferritin is regulated by ApoE.315,316 The ApoE4 genotype may mediate the negative effects of iron accumulation on cognitive function in AD, although exactly how is unclear.315,317
Although much evidence points to elevated iron triggering Aβ and tau pathology, injection of Aβ oligomers in the hippocampus, which produces Aβ plaques, tau pathology and cognitive decline, causes iron accumulation.318 It is possible that iron and Aβ function in a positive-feedback loop to promote disease pathology. Interestingly, the accumulation of iron as well as the other pathological and cognitive features of AD that are produced by oligomeric Aβ injection does not occur in tau knockout mice raising the possibility that tau plays an essential role in iron accumulation and its effects on AD pathogenesis.318
In contrast to brain tissue, iron level in the CSF of AD patents is not higher from control groups with most studies finding lower levels in AD CSF.319–323 This may be due to an elevation of ceruloplasmin in the CSF of patients in the early stages of AD, which could be expected to inhibit iron export.324 Surprisingly, however, the elevation of CSF ceruloplasmin is associated with accelerated cognitive decline and ventricular volume enlargement.324 It has been suggested that the elevation in ceruloplasmin might be part of a neuroinflammatory response, which would have detrimental effects on neurons.324
Consistent with a causal role for iron in AD, normal rats fed with a high-iron diet display altered expression of DMT1, FPN1, and Tfr1, followed by neuronal loss in the hippocampus and cortex.142 Elevated neuronal iron increases synthesis of APP through IPR binding to an IRE that localizes to the 5-UTR of the APP mRNA.325 In addition to elevated synthesis, processing of APP to generate Aβ is also increased by iron.142,326 This involves the binding of L-ferritin to PEN-2, a component of γ-secretase, stabilizing it.142 However, APP also regulates iron homeostasis as evidenced by the finding that APP knockout mice display an enhancement of age-dependent iron accumulation.327 This suggests that one function of APP is to suppress iron build-up in the brain during normal aging.327 The ability of APP to prevent iron build-up can be explained by its ability to interact with FPN1 at the cell surface and stabilize it consequently facilitating efficient iron efflux.87,328–330 FPN1 expression is reduced and DMT1 expression is increased in mouse models of AD.331 Another study described that reduced FPN1 and increased ferritin protein levels correlated with iron accumulation in the brains of APP knockout mice.327 Altered posttranslational modifications of APP also disrupt iron homeostasis and this has been suggested to be due to reduced association with FPN1.332 A recently-conducted comprehensive study combining mass spectrometry-based proteomics and integrated multi-omics using samples from AD patients and 5XFAD AD mice described the upregulation of four proteins involved in iron homeostasis.333 Although none of the upregulated proteins described above were identified in this particular study, the expression of Lf, the iron-binding protein, and STEAP3, the endosomal ferroreductase that converts insoluble Fe3+ to soluble Fe2+ were among the genes that are substantially elevated in both AD mice and patients.333 Upregulation of these genes would increase cytosolic accumulation of Fe2.
In contrast to APP, which stabilizes FPN1, the amyloidogenic processing of APP to Aβ42 destabilizes FPN1 in neurons and impairs iron export.334 Interestingly, this is not observed in non-amyloidogenic processing of APP, which also stabilizes FPN1.334 Also in contrast to APP, which promotes iron efflux, Aβ42-exposure increases ferritin production in astrocytes suggesting that Aβ42 may promote iron accumulation in astrocytes.335 Such accumulation could be expected to affect the functioning of astrocytes, which would affect the health and functioning of neurons. Indeed, astrocytic dysfunction has been implicated in AD pathogenesis.336–338 Iron accumulation also occurs in microglia in the vicinity of Aβ plaques in mice, a finding that is consistent with the finding of iron accumulation in the vicinity of plaques in the brains of patients.339,340 Experiments in AD mice have found that although promoting neuroinflammation, microglia have a reduced ability to phagocytose Aβ, thus potentiating damage to the brain.339 Intracerebral injection of hepcidin, which, as described above, can reduce intracellular iron in the brain, prevents astrocyte and microglia activation and oxidative damage triggered by Aβ injection supporting a causal role for disrupted iron homeostasis in AD-associated neuroinflammation.341
Iron binds directly to Aβ and tau and promotes their oligomerization/aggregation.342–346 Not surprisingly, iron (and specifically Fe3+) accumulates in Aβ plaques and NFT in the brains of AD patients.224,344,347–352 The colocalization of iron with Aβ is also seen in asymptomatic individuals with the ApoE4 allele and in elderly subjects with MCI suggesting that this interaction occurs early in the pathogenic process causing cognitive decline in AD.353 In cell culture systems the binding of Fe3+ to Aβ increases the neurotoxicity of cell culture.353 In the case of tau, only Fe3+ and not Fe2+, stimulates aggregation.347,354 Indeed, reduction of Fe3+ to Fe2+ reduces tau aggregation rendering it soluble, whereas aggregation increases with chemically-induced transition of Fe2+ to Fe3+.347 It may be noted that this study was performed prior to the realization that oligomeric or fibrillary tau rather than insoluble aggregates were the neurotoxic tau species and therefore the effects of Fe2+ to Fe3+ on soluble oligomeric tau forms were not evaluated. However, these results suggest that Fe3+ is the more dangerous of the two ions with regard to tau neurotoxicity.347 As observed with tau, the addition of Fe3+ to Aβ42 in vitro enhances its aggregation.344,355 Recent studies have shown that oxidative stress, which can be triggered by iron, stimulates formation of soluble Aβ oligomers via Cys–Cys binding between tau molecules.356,357 Besides promoting oligomerization, Fe3+ hyperphosphorylates tau in cultured neurons through activation of specific kinase pathways.358,359 While iron can regulate tau, tau oligomerization and aggregation can also affect iron homeostasis. Tau plays a critical role in the transport of APP to the membrane in neurons where APP stabilizes FPN1.84,360 Depletion of cellular soluble tau decreases FPN1 levels resulting in intracellular iron accumulation.360 Based on all of these findings, it is likely that the dysregulation of iron homeostasis causing its accumulation in neurons can promote pathogenic changes in Aβ and tau, but that Aβ and tau can also disrupt iron homeostasis raising the possibility of a positive-feedback pathogenic process in AD.
As described above, a well-studied mechanism by which elevated iron can cause neuronal death is through oxidative stress, which is a characteristic feature of the AD brain.301,361–363 Like the accumulation of iron, oxidative stress is also an early event in AD pathogenesis.364 Besides iron accumulation due to deregulation, other mechanisms have been proposed to promote oxidative stress. Recent studies have demonstrated that Aβ42 interacts with iron-bound ferritin within plaques.365,366 Both in vitro and in vivo (including within plaques) Aβ42 coverts stored Fe3+ within ferritin to the more unstable and reactive Fe2+, which through Fenton chemistry drives ROS production and oxidative stress.344,365–367 The molecular consequences of uncontrolled oxidative stress, such as abnormal protein oxidation, DNA damage, impaired DNA repair, and lipid peroxidation have all been well-documented in the AD brain. As described above, these alterations are also features of ferroptosis.298,362,368,369 Indeed, ferroptosis has been described in rodent models of AD, and strongly implicated in humans with the disease.370–374 A recent study performed on postmortem human brain tissue found that besides iron dyshomeostasis, the expression of Xc- (the cystine/glutamate transporter) was reduced.374 White matter loss is often among the earliest brain changes in AD, preceding the tangles and plaques that characterize neuronal deficits.375 Loss of oligodendrocytes is preceded by DNA-damage, which occurs in aging and is elevated in AD.376 DNA damage-associated oligodendrocyte degeneration precedes amyloid pathology in AD patients and, through its consequences on neuronal functioning, likely contributes to cognitive impairment.377
A recent hypothesis is that besides causing oxidative stress, iron dyshomeostasis reactivates dormant microbes in the gut and other tissues, which results in systemic inflammation as well as shedding of potent inflammagens, such as lipopolysaccharides, which along with genetic susceptibilities play a key role in the pathogenesis of AD (as well as other neurodegenerative diseases, such as PD).378–382 Inflammation resulting from microbial products causes cell damage releasing ferritin, which may provide an explanation for elevated serum ferritin in AD. Therefore, while serum ferritin can represent a measure of liver iron and intracellular iron content, it may also a be a marker of inflammation.383 While plausible, the Iron Dysregulation and Dormant Microbes (IDDM) hypothesis remains to be rigorously tested.
Increasing hepcidin levels in the brain has been suggested as a potential therapeutic approach for AD and other neurodegenerative diseases.282,283 Consistently, hepcidin expression is reduced in the AD brain.282,306 Administration of iron chelators has been tested as a the therapeutic strategies for AD.326,360,384,385 Iron chelation reduces Fe3+-induced Aβ42 aggregation in vitro.386 Iron chelators also disassociate tau aggregates obtained from the brains of AD patients both in vitro and in AD brain slices.387,388 A number of studies testing the efficacy of iron chelator administration in genetic mouse models of AD have described amelioration of cognitive impairment along with reductions in GSK3β activity, tau phosphorylation, generation of Aβ, and aggregation of Aβ in the hippocampus of AD mice.389–393 Additionally, oxidative stress and microglial activation are reduced with iron chelators.389,391–393 Reduction of cognitive impairment with iron chelation has also been described in a rat model of sporadic AD.394 As observed in rodent models, treatment with iron chelators reduces oxidative stress and increases survival and locomotor activity in an Aβ42-overexpressing fly model of AD.395 Sustained administration of iron chelators to a limited number of patients with early AD has been reported to slow the progression of dementia.396 One study that tested iron chelation in AD patients reported a significant reduction in the rate of decline of daily living skills with intramuscular deferoxamine administration.396 A study using iodochlorhydroxyquin (clioquinol) which blocks metal binding to Aβ lowered the level of Aβ42 in CSF.397 Another pilot study utilizing a more recently developed chelator, PBT2, reported improvement of cognition and decreased Aβ42 in CSF.398
Although not examined in adequate detail, it is possible that the accumulation of iron in the mitochondria is particularly significant in AD pathogenesis. Knockdown of the C. Elegans ortholog of the mitochondrial iron transporter, mitoferrin-1/2, in a worm model of AD reduced mitochondrial iron and mitochondrial ROS and is protective against disease progression.399 Targeting mitoferrin and other molecules involved in mitochondrial iron transport could be another therapeutic avenue for AD.
Neurodegeneration with brain accumulation disorders
NBIAs are a group of at least 12 very rare, clinically and genetically inherited neurodegenerative disorders characterized by deposition of iron generally (but not in all NBIAs) within the basal ganglia, and most specifically, in the globus pallidus and SN (see Table 1).400–403 These are regions of the brain that normally have a high iron content and are therefore selectively vulnerable to any further elevation of iron. Neuropathologically, NBIAs are often associated with cerebral, cerebellar and optic atrophy, and retinal degeneration.400–403 The major clinical manifestations are progressive dystonia, spasticity, parkinsonism, and neuropsychiatric abnormalities. Cognitive impairment is displayed in some NBIAs, but not others. Depending on the disorder, onset ranges from infancy to adulthood. While iron accumulates in the brain in all NBIAs, in most of these disorders the level of systemic iron is not elevated.
Table 1.
List of NBIA disorders.
| Gene | Protein | Protein localization | Disorder |
|---|---|---|---|
| AP4M1 | Adaptor protein complex-4-Subunit M1 | Endosome | |
| ATP13A2 | Cation-transporting ATPase 13A2 | Lysosome, mitochondria | Kubor-Raken disease (KRS) |
| C19orf12 | C19orf12 | Mitochondrial membrane, ER | Mitochondrial membrane protein-associated neurodegeneration (MPAN) |
| CPL | Ceruloplasmin | Plasma membrane | Aceruloplasminaemia |
| CoASY | Coenzyme A synthase | Mitochondria, cytosol | COASY-protein-associated neurodegeneration (CoPAN) |
| CRAT | Carnitine acetyltransferase | Mitochondria | |
| DCAF17 | DDB1- and CUL4-associated factor-17 | Nucleolus | Woodhouse-Sakati syndrome (WSS) |
| GTPBP2 | GTP-binding protein-2 | Cytoplasm | |
| FA2H | Fatty acid 2-hydroxylase | ER | Fatty acid hydroxylase-associated neurodegeneration |
| FTL | Ferritin light chain | Cytoplasm | Neuroferritinopathy (NF) |
| PANK2 | Panthothenate kinase 2 | Mitochondria | |
| PLA2G6 | Calcium-independent phospholipase A2 group VIa (iPLA2VIa) | Mitochondria, ER, cytosol | PLA2G6-associated neurodegeneration |
| REPS1 | RalBP-associated Eps15-homology domain protein | Cytoplasm endosome | |
| SCP2 | Sterol carrier protein 2 | Peroxisomes | |
| WDR45 | WD40-repeat protein 45 | ER | β-propeller protein-associated neurodegeneration (BPAN) |
While brain iron accumulation is the common denominator, only two NBIA disorders are caused by mutations in proteins that directly regulate iron homeostasis (Table 1). These are ceruloplasmin and L-ferritin, which cause aceruloplasminemia and neuroferritinopathy, respectively. A recent case report of iron accumulation, brain atrophy, and severe neurological impairment in a patient with bi-allelic loss of the IRP2 gene suggests the possibility of a third NBIA resulting from mutation of a gene regulating iron homeostasis. However, this remains to be confirmed.404 Of the other known NBIAs, mutations in the PANK2 (pantothenate kinase-2) gene and the PLA2G6 (calcium-independent phospholipase A2, Group VIa) gene together account for a majority of all cases. NBIA disorders caused by mutations in PANK2 and PLA2G6 are referred to as PKAN (PANK-associated neurodegeneration) and PLAN (PLA2-associated neurodegeneration), respectively. Because aceruloplasminemia and neuroferritinopathy are caused by direct disruption of iron homeostasis and because PKAN and PLAN are the best studied of the iron dyshomeostasis-unrelated NBIA disorders, this review will be limited to these four NBIA disorders. For a more comprehensive description of NBIA disorders, their underlying disruptions, and a description of mechanistic studies, the reader is referred to other excellent and recent reviews.400–403
Besides elevated iron and abnormal neuronal loss, NBIA disorders share important clinical and neuropathological features with age-associated neurodegenerative diseases. It is therefore likely that the pathogenesis of these two categories of neurodegenerative disorders share molecular and cellular mechanisms. This issue remains to be systematically investigated, however.
Aceruloplasminemia
Aceruloplasminemia is an autosomal recessive disease caused by mutations in the gene encoding ceruloplasmin.405,406 As described above, ceruloplasmin facilitates export of iron through the FPN1 transporter by oxidizing Fe2+. Indeed, it is the predominant ferroxidase in plasma. Ceruloplasmin also promotes iron export by stabilizing FPN1. Clinical manifestations of aceruloplasminemia, which start at around 50 years of age, are heterogeneous and include ataxia, involuntary movement, dysarthria, retinal degeneration, psychiatric issues, parkinsonism, and cognitive impairment, including dementia.405–409 Retinal degeneration, diabetes, and dementia are three consistent disease features.405,406 Diabetes mellitus precedes brain abnormalities by decades. Because of iron accumulation in tissues, the level of blood iron is lower which is potentiated by microcytosis.13 Microcytic anemia, low transferrin saturation, and paradoxically hyperferritinemia are also seen decades prior to neurological symptoms.405 In most patients, brain iron accumulation is seen in the basal ganglia and cerebellum.405,406,408,409 The extent of brain overload does not correlate with the severity of neurological symptoms suggesting contribution from other genetic or environmental factors. A distinguishing feature of aceruloplasminemia is that it is the only known NBIA in which systemic iron is also elevated.406,409 This is likely to be because ceruloplasmin plays a key role in iron homeostasis at the systemic level, which cannot be fully compensated for by HEPH. Additionally, and for reasons that are not known, patients with aceruloplasminemia exhibit low serum HEPH levels and decreased FPN1 protein expression in the liver.410 Outside the brain, iron accumulates in the liver.405,406,408,409 Individuals heterozygous for the disease mutations have reduced ceruloplasmin activity, but usually display normal iron metabolism and no clinical symptoms.405,406
Within the brain of patients, large iron deposits form first in the epithelial cells of the choroid plexus, which likely represents an important event in the disruption of brain iron homeostasis.411 Depositions are also observed in astrocytes and to a lesser degree in neurons. Oligodendrocytes are unaffected likely because they utilize HEPH and not ceruloplasmin for FPN1-associated ferroxidase activity.
The mechanism by which neurodegeneration occurs in aceruloplasminemia is not known. Unoxidized Fe2+ released into the brain interstitium is taken up through unregulated internalization pathways and can have toxic effects. It has been reported that astrocyte loss precedes neuronal loss suggesting that neurodegeneration could be secondary to the loss of astrocytes.412–414 It is also possible, and perhaps likely, that initial neuronal loss is due to iron starvation resulting from the inability of dysfunctional astrocytes to release iron through FPN1.408 Eventually, and following astrocytic dysfunction or degeneration, neurons might take up excessive amounts of NTBI resulting in oxidative stress and neurotoxicity.405,415 Neuronal loss could also result, in part, from the deprivation of astrocytic neurotrophic factors. Since astrocytes play a key role in glutamate uptake, excitotoxic death of neurons may represent yet another possibility. Finally, several studies have described ceruloplasmin itself has neuroprotective activity and defends against different neurodegenerative conditions.275,416–420 Indeed, antioxidant effects and an ability to inhibit lipid peroxidation have been described.419,421–423 Loss of such neuroprotective activity could also contribute to the loss of neurons in aceruloplasminemia.416
Cultured glial cells overexpressing disease-causing mutant forms of ceruloplasmin display iron overloading and reduced FPN1 stability.410,424 Some mutant forms of ceruloplasmin form aggregates in astrocytes, which likely cause dysfunction and perhaps death in line with the idea that astrocytic dysfunction and degeneration play a key role in disease pathogenesis.409 Mice lacking ceruloplasmin have been analyzed and found to have increased iron levels in the brainstem, cerebellum, and spinal cord as they age. As expected, while iron import is unaffected, iron efflux is severely reduced in ceruloplasmin KO mice.425 Within the cerebellum, DMT1 and ferritin mRNA and protein expression are increased, whereas expression of Tfr1 is reduced in the KO mice.426 Within the cerebellum, astrocytes and Bergman glia display highly elevated iron content and about 60% of astrocytes are lost as the mutant mice age.426 About 50% of the Purkinje neurons also die in an age-dependent manner ceruloplasmin−/− mice, although surprisingly there is no increase of iron in these cells.426 Also somewhat surprisingly, in view of its stabilizing effect on FPN1, the expression of FPN1 is not reduced in the cerebellum of mice lacking ceruloplasmin.426 Active caspase-3 staining, which is used as a marker of apoptosis, is not elevated indicating that cell death in the cerebellum is not apoptotic, pointing to ferroptosis as a likely mechanism.426
Administration of iron chelators to patients with aceruloplasminemia reduces systemic iron overload but convincing evidence that it reduces brain iron accumulation is lacking. Prolonged treatment with iron chelators have not had success because of the anemia resulting from peripheral iron deficiency. Most importantly, neurological symptoms are not reduced by iron chelation treatment.406,427 Some mitigation of neurological symptoms has been described when iron-chelation with deferiprone is used in combination with phlebotomy.427 Intravenous administration of fresh-frozen plasma (FFP) partially and temporarily restores circulating ceruloplasmin, reduces brain iron deposition and reduces neurological symptoms, although exactly how the ceruloplasmin crosses the BBB is not clear.428 Given the heavy iron overload in the epithelial cells of the choroid plexus, one possibility is that ceruloplasmin enters the brain through a damaged choroid plexus lining or of the BBB. In the small number of patients in which iron chelators were administered along with FFP early during the disease, iron deposition and neurological symptoms were reduced raising the possibility of a potential treatment strategy.429
Neuroferritinopathy
Neuroferritinopathy is an autosomal dominant disorder caused by a number of different mutations in the L-ferritin gene that reduce the ability of ferritin to sequester iron resulting in increased free, unbound iron in the cytosol.115,430 The mutated forms of L-ferritin have less stability and act dominant-negatively thus impacting the ability of normal ferritin to sequester iron, explaining the dominant transmission of the disorder.431,432 While variable, symptoms generally appear in adulthood at 35–45 years of age. Extrapyramidal symptoms, including chorea and dystonia, are the major clinical features during early stages of the disease, whereas progressive aphonia, dysphagia, severe motor disability, and minor cognitive impairment are late features.430,433 Patients with some types of mutations display parkinsonism, ataxia, and eventually dementia.434 Iron deposition and lesions are observed in the globus pallidus, putamen, and cerebellum. A small proportion of patients exhibit the “eye of the tiger” sign (low MRI signal in the globus pallidus due to abnormal accumulation of iron that resembles eyes with a vertical stripe of high MRI signal due to gliosis) a feature seen in a few other brain degenerative disorders including PANK and atypical PD.435 Serum levels of ferritin are significantly lower than normal (except in pre-menopausal women) and a key feature of neuroferritinopathy.401,436 Synthesis of ferritin in cells of affected brain areas is paradoxically increased possibly in a failed effort to manage the elevation in intracellular iron.115 A characteristic neuropathological feature of neuroferritinopathy is the presence of swollen and vacuolated nuclei and cytoplasmic bodies in neurons and glia that contain ferritin and iron in an insoluble form, which precede iron deposition.435 Most deposition is in astrocytes and oligodendrocytes.401,436 In the putamen, the increase in iron content is about 4-fold and contains both Fe3+ and Fe2+.435 Abnormalities in mitochondria, lipid peroxidation, and apoptotic death are observed in the putamen but also in the globus pallidus and SN. Iron accumulation along with the presence of lipid peroxidation suggests the possibility of ferroptotic death also. In the cerebellum, degeneration of Purkinje cells and vacuolated Bergmann glia are observed.435 As the animals age, they display accumulation of lipofuscin (lipid-containing granules) in cells with iron aggregates, particularly in the cerebellum and striatum.437 It is possible that rupture of lysosomes releases lipofuscin which seeds iron accumulation.401 This along with the hydrolytic lysosomal enzymes that are also released through rupture likely promotes cell degeneration.401
In vitro analyses of disease-causing mutant forms of L-ferritin indicate an inability to sequester iron. When such mutant forms are overexpressed in cells, they cause the formation of ferritin-iron inclusion bodies (the neuropathological hallmark), increase in free iron, oxidative stress, and cell death.115,438–441 Transgenic mice overexpressing mutant L-ferritin display neurodegeneration and motor deficits.437 Analyses of these mice have revealed decreased expression of Tfr1 protein mRNA and increased iron levels, and increased lipid peroxidation in the brain.115,437,442–444 Several genes regulating iron metabolism are also deregulated. Iron deposits are seen in the brain, which localize mostly in the stratum and cerebellum.115,437,442 IPSCs from patient fibroblasts and differentiated into neuronal precursors and neurons display altered iron homeostasis with increased Fe2+, ferritin aggregates, lipid-containing granules, oxidative stress, and cell death, providing an excellent cellular model to study disease mechanisms and test therapeutic agents.445 Based on their results, the authors of this study proposed that increased Fe2 in these ferritinopathic neurons induces ferritin translation, causing ferritin-iron aggregates which promotes ROS accumulation, lipid peroxidation, and cell death. NAC was found to have protective effects in this paradigm.445
Treatment of cells overexpressing mutant L-ferritin with the iron chelator deferiprone increased cell viability and reduced iron content.444 In mice, deferiprone administration reduced systemic iron substantially. While ferritin deposition was also reduced, overall brain pathology in not affected by iron chelation.444 Likewise, patients administered with iron chelators displayed reduced systemic iron but had no clinical benefit.401,436 Neurons derived from iPSCs obtained from patients exhibit increased cytosolic iron, iron and ferritin aggregates, oxidative damage and reduced viability.445 Death of these iPSC-derived neurons was determined to be by ferroptosis.445 NAC administration has shown efficacy in IPSC-derived ferritinopathic neurons and may represent another treatment approach.
PANK2-associated neurodegeneration
PKAN (previously called Hallervorden-Spatz syndrome) is caused by a number of different autosomal recessive mutations in the PANK2 gene and is the most common of the NBIA disorders accounting for about 50% of all cases.446–448 PANK2 is one of the four isoforms of PANK, the first enzyme in the synthesis of coenzyme A (CoA) from pantothenate (vitamin B5). PANK2 is the only one of the four isoforms that localizes to the mitochondria where it exists as a homodimer in the intermembrane space.449 Onset of symptoms in PKAN can display at as early as three years of age or in adulthood.403 Clinical manifestations include progressive dystonia, dysarthria, and rigidity. Retinal degeneration is often seen in early-onset PKAN, whereas psychiatric disturbances are often observed in the adult-onset form of the disorder.402,450 PKAN is characterized by iron overload in the globus pallidus along with the accumulation of axonal spheroids, axonal degeneration, and neuronal loss.403,447,450,451 Axonal swelling and neurodegeneration are also observed in the SN (where increased iron content is often described in PKAN), the cerebellum, and the dentate nucleus. Although prior to the identification of the gene there were reports of α-synuclein and tau aggregation in the brains of PKAN patients, more recent studies have failed to find α-synuclein aggregation.451 Mild tau pathology was observed in a small proportion of patients.451 In addition to iron, a disruption of calcium homeostasis has been described in iPSC-derived glutaminergic neurons as well as in the basal ganglia of PKAN patients.452 Specifically, elevated calcium influx due to leakage from stores in the ER or mitochondria has been reported and this disruption has been suggested to be due to elevated iron possibly resulting from modification of calcium homeostatic proteins by iron-induced oxidative stress.452 It is well known that intracellular calcium plays key roles in neuronal survival and function and is under tight regulation.
PANK2 knockout mice have been generated but fail to display iron accumulation in the brain or the neurological impairments seen in patients even after 18 months of age.453,454 While normally asymptomatic, when fed with a ketogenic diet PANK2−/− mice display severe weight loss, locomotor impairment reminiscent of patients455 (although another group failed to reproduce this finding456). These abnormalities induced by ketogenic diet were found to be substantially reduced by administration of the compound pantetheine, which is hypothesized to activate an alternative CoA biosynthesis pathway that bypasses the PANK2 defect to restore cellular CoA levels.455 A subsequent study of the PANK2−/− mice confirmed lack of abnormalities in the cerebellum and SN but found reduced CoA, disrupted expression of genes regulating iron homeostasis, mitochondrial dysfunction, and defective dopamine metabolism in the globus pallidus, which displays highest iron accumulation in PKAN patients.457 Feeding of PANK−/− mice with 4ʹ-phosphopantetheine, which lies downstream of pantothenate/vitamin B5 in the CoA biosynthetic pathway restored CoA levels and corrected the other abnormalities in the globus pallidus.457
The lack of a disease phenotype in the PANK2−/− mice along with restriction of cellular abnormalities to the globus pallidus is likely due to functional compensation by PANK1, which is also expressed in the brain in mice. Consistent with this, double-knockout mice lacking PANK2 in all cells along with PANK1-deficiency in neurons and glial cells do develop a severe disease phenotype and die within two to three weeks.456 As observed in patients, CoA levels were substantially reduced in the brains of the mutant mice. Surprisingly given the severe phenotype, both heme levels and total iron levels in the brain of the mutant mice were lower than that in control mice.456 Drosophila heterozygous for fumble (fbl) deletion, the only PANK2 gene in flies, display neurodegeneration, impaired motor function, defective CoA metabolism, increased protein oxidation, disrupted lipid homeostasis, sensitivity to ROS, impaired DNA integrity, and reduced lifespan.458,459 However, iron deposits are not found. Knockdown of PANK2 in zebrafish results in severe impairment of neurodevelopment, particularly in the anterior part of the CNS.460 Neurons have been generated from iPSCs derived from PKAN patients.461 Although iron deposits were not seen, these neurons exhibited elevated free cytosolic iron, highly increased ROS levels, impairment of mitochondrial iron-dependent biosynthesis, impaired energy production, and premature death of the neurons.461 Expression of Tfr1 is increased, while heme and ferritin levels are reduced in patient-derived neurons explaining the elevated free iron content.461 This may reflect the fact that iron deposition occurs mostly in the globus pallidus and not most other neuronal types.402 Furthermore, iron deposition might require much more time and thus difficult to recapitulate in cultured neurons. These possibilities notwithstanding, none of the in vivo or in vitro models of PKAN recapitulate iron deposition, the cardinal feature of the disease.
There is no treatment for PKAN. Although a pilot studies in patients with deferiprone indicated efficacy, a larger study conducted recently failed to show significant improvement even after 18 months of drug administration.462–464 It is possible that treatment may have begun too late in the pathogenic process for it to be stopped. A recently identified blood-permeable, allosteric activator of PANK2 was found to increase CoA levels in the brain and livers.465 Administration of this compound, PZ-2891, reduced weight loss and locomotor impairment and extended life span in PKAN−/− mice.465 Clinical trials testing CoA as a target has yet to be conducted in PKAN patients. In the Drosophila model of PKAN, all abnormalities including the oxidative damage, impaired DNA integrity, and reduced lifespan are prevented by pantetheine or CoA.458 As described above, administration of 4-phosphopantetheine normalized CoA and corrected the iron and other abnormalities in the globus pallidus. Treatment with 4-phosphopantetheine also corrected abnormalities in PKAN patient-derived fibroblasts and may hence represent an effective treatment strategy for this disorder.457
PLA2G6-associated neurodegeneration (PLAN)
PLAN (formerly called Seitelberger disease) is the second-most studied of the iron dyshomeostasis-unrelated NBIA disorders. In this disorder, iron accumulation occurs in the globus pallidum and cerebellum.446,466,467 It is a complex disorder caused by mutations in the PLA2G6 gene, which encodes iPLA2-β, an intracellular Ca2+-independent PLA2 that metabolizes membrane phospholipids and that regulates cellular processes, including vascular relaxation, secretion, inflammation, and apoptosis.468 In the brain, iPLA2-β localizes mainly in dendritic and axon terminals. PLAN-causing mutations in PLA2G6 display genetic heterogeneity and include missense mutations, truncation mutations, and copy number differences. It is believed that the clinical heterogeneity depends on the genetic heterogeneity. Based on age of onset and clinical features, PLAN has been subclassified into three groups: infantile neuroaxonal dystrophy (INAD), atypical neuroaxonal dystrophy (atypical NAD), and PLA2G6-related dystonia-parkinsonism (DP) also referred to as adult-onset dystonia-parkinsonism. INAD usually begins between ages six months and three years with motor deterioration, dystonia, spasticity, and optic atrophy. Iron deposition is seen in the globus pallidus and SN along with cerebellar atrophy. Disease progression is rapid with increased spasticity, ataxia, cognitive impairment and visual impairment, and death generally occurs within the first decade. Similar clinical features are seen in atypical NAD although the symptoms are mild during early childhood with neurological deterioration beginning slightly later. The neuropathological hallmark is neuroaxonal dystrophy and the presence of intracellular spheroids comprised of tubulovesicular structures. Additionally, extensive phosphorylated α-synuclein-positive Lewy bodies, and tau-positive neurofibrillary tangles are found in INAD and atypical NAD.117,469,470 Cerebellar atrophy is a common feature in INAD and atypical NAD. In contrast to INAD and DP, Lewy bodies and NFTs are not always seen in DP brains. DP patients also do not display cerebellar atrophy. The diversity in clinical phenotype may be due to differences in the effect of the various mutations on PLA2G6 enzyme activity. Although not a perfect overlap, the commonalities in clinical and neuropathological features between PLAN and PD suggest common downstream pathogenic mechanisms. In this context it is interesting that compound mutations in the PLA2G6 gene have been suggested to cause autosomal recessive early-onset PD.471,472
PLA2G6 knockout mice develop progressive motor impairment in their second year of life.473–476 Like patients, these mice display neuropathological features including damaged mitochondria, axonal degeneration, cerebellar atrophy, accumulation of α-synuclein-containing and ubiquitin-positive spheroids in axons in nearly all brain regions.473–476 However, there is no iron accumulation in the basal ganglia or any other brain region in PLA2G6 knockout mice generated so far. Although the mechanism is unclear, a-synuclein expression is highly elevated in neurons cultured from PLA2G6 knockout mice as well as in wild type neurons in which is knocked down.477 In these neurons, α-synuclein is contained in granules that localized to the membrane of mitochondria that are damaged.477 The α-synuclein-positive granules are also seen in the brain close to Lewy bodies and prior to the onset of behavioral abnormalities.477 Flies in which iPLA2-VIA (the fly homolog of PLA2G6) is inactivated have a reduced lifespan, display locomotor defects, and exhibit mitochondrial abnormalities, impaired synaptic transmission, and neurodegeneration.478,479 Again, no iron deposition has been found. Interestingly, in flies iPLA2-VIA binds vacuolar protein sorting protein 35 (VPS35), a component of the retromer. Loss of iPLA2-VIA activity resulted in impaired retromer function, a defect also resulting from loss of Vps35 activity or overexpression of a-synuclein, alterations that cause or contribute to PD.479–481 Although best known in the context of PD, results of several studies have implicated impaired retromer in AD pathogenesis also.482–488
Studies conducted using patient-derived iPSCs have revealed that the death of dopaminergic neurons is associated with oxidative stress, ER stress, defective mitophagy, and transcriptional dysregulation.471 Death of these iPSC-generated dopaminergic neurons can be prevented by azoramide, a modulator of the unfolded protein response (UPR) as well as an inhibitor of the CREB transcription factor.489 Treatment with azorimide also reduced ER stress, ROS accumulation and mitochondrial dysfunction, and restored proper gene expression.489 Impaired UPR function and other core cellular perturbations in PLAN are similar to those in PD as well as other age-associated neurodegenerative diseases.490,491 Whether azoramide or other UPR modulators will be useful in the treatment of age-associated neurodegenerative disorders remains to be tested, but has been suggested.490,491
Conclusions
The iron content within a cell has to be carefully regulated to permit the normal functioning of the cell while preventing an elevation that could result in its damage or death through mechanisms including, but not restricted to, the generation of highly-reactive free radicals. Research conducted over the past two decades has identified a large number of important regulators of iron homeostasis. Nowhere in the body is precise regulation of iron homeostasis more critical than in the brain. Elevated iron and its accumulation and depositions within cells of the brain are a defining feature of NBIAs and a common feature in a variety to age-associated neurodegenerative diseases. Besides elevated iron, recent studies have revealed a remarkable overlap in the cellular perturbations between NBIAs and age-associated neurodegeneration, including oxidative stress, lipid peroxidation, mitochondrial dysfunction, ER stress, and transcriptional deregulation. A better understanding of the molecular and cellular mechanisms that cause iron accumulation is necessary and the extent to which iron accumulation contributes to the cellular abnormalities as well as disease onset, severity, and progression deserves careful study. Accumulating evidence for a key role for ferroptosis in neuronal loss in both NBIAs and age-associated neurodegenerative disease point to a causative role for iron dyshomeostasis in disease pathogenesis. Future work will provide additional and much-needed information on how iron interfaces normally with disease-relevant molecules, such as Aβ42, tau, and α-synuclein as well as ceruloplasmin, PLA2G6, and PANK2, and how these relationships are affected by iron accumulation and/or the dysfunction of the disease-relevant molecules. Together, this knowledge will facilitate the development of effective strategies to treat or cure these devastating brain disorders.
Authors’ contributions
SRD and MCK wrote the review.
DECLARATION OF CONFLICTING INTERESTS
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by grants from the National Institutes of Health (R01 ES016774-01, R21AG043718), VA Merit Award (RR&D, I01RX001450), an AHA SFRN grant (15SFDRN25710468), and AHA Transformation Award (19TPA34910015) to MSK. Dr. Kindy is a Senior Research Career Scientist (BLR&D) in the VA. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
ORCID iD
Santosh R D’Mello https://orcid.org/0000-0002-7652-1334
References
- 1.Lieu PT, Heiskala M, Peterson PA, Yang Y. The roles of iron in health and disease. Mol Aspects Med 2001; 22:1–87 [DOI] [PubMed] [Google Scholar]
- 2.The Essential Role of Iron in Biology. In: Iron Metabolism [Internet]. John Wiley & Sons, Ltd; 2016. [cited 2020 Jan 5]. p. 22–70, https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118925645.ch2 (accessed 12 August 2020)
- 3.Yanatori I, Kishi F. DMT1 and iron transport. Free Radic Biol Med 2019; 133:55–63 [DOI] [PubMed] [Google Scholar]
- 4.Philpott CC, Jadhav S. The ins and outs of iron: escorting iron through the mammalian cytosol. Free Radic Biol Med 2019; 133:112–7 [DOI] [PubMed] [Google Scholar]
- 5.Gao G, Li J, Zhang Y, Chang Y-Z. Cellular iron metabolism and regulation. Adv Exp Med Biol 2019; 1173:21–32 [DOI] [PubMed] [Google Scholar]
- 6.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997; 388:482–8 [DOI] [PubMed] [Google Scholar]
- 7.Andrews NC. The iron transporter DMT1. Int J Biochem Cell Biol 1999; 31:991–4 [DOI] [PubMed] [Google Scholar]
- 8.McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A, Peters TJ, Raja KB, Shirali S, Hediger MA, Farzaneh F, Simpson RJ. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001; 291:1755–9 [DOI] [PubMed] [Google Scholar]
- 9.White C, Yuan X, Schmidt PJ, Bresciani E, Samuel TK, Campagna D, Hall C, Bishop K, Calicchio ML, Lapierre A, Ward DM, Liu P, Fleming MD, Hamza I. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab 2013; 17:261–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta 2009; 1790:589–99 [DOI] [PubMed] [Google Scholar]
- 11.Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1996; 1275:161–203 [DOI] [PubMed] [Google Scholar]
- 12.Knutson MD. Iron transport proteins: gateways of cellular and systemic iron homeostasis. J Biol Chem 2017; 292:12735–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thirupathi A, Chang Y-Z. Brain iron metabolism and CNS diseases. Adv Exp Med Biol 2019; 1173:1–19 [DOI] [PubMed] [Google Scholar]
- 14.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 2000; 5:299–309 [DOI] [PubMed] [Google Scholar]
- 15.Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC, Zon LI. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 2000; 403:776–81 [DOI] [PubMed] [Google Scholar]
- 16.Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet 1999; 21:195–9 [DOI] [PubMed] [Google Scholar]
- 17.Agarwal AK, Yee J. Hepcidin. Adv Chronic Kidney Dis 2019; 26:298–305 [DOI] [PubMed] [Google Scholar]
- 18.Ginzburg YZ. Hepcidin-ferroportin axis in health and disease. Vitam Horm 2019; 110:17–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musci G, Polticelli FB, di Patti MC. Ceruloplasmin-ferroportin system of iron traffic in vertebrates. World J Biol Chem 2014; 5:204–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr 2002; 22:439–58 [DOI] [PubMed] [Google Scholar]
- 21.Gaware V, Kotade K, Dhamak K, Somawanshi S. Ceruloplasmin its role and significance: a review. Int J Biomed Res 2010; 1:153–62 [Google Scholar]
- 22.Shaw GC, Cope JJ, Li L, Corson K, Hersey C, Ackermann GE, Gwynn B, Lambert AJ, Wingert RA, Traver D, Trede NS, Barut BA, Zhou Y, Minet E, Donovan A, Brownlie A, Balzan R, Weiss MJ, Peters LL, Kaplan J, Zon LI, Paw BH. Mitoferrin is essential for erythroid iron assimilation. Nature 2006; 440:96–100 [DOI] [PubMed] [Google Scholar]
- 23.Richardson DR, Lane DJR, Becker EM, Huang ML-H, Whitnall M, Suryo Rahmanto Y, Sheftel AD, Ponka P. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci U S A 2010; 107:10775–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drakesmith H, Nemeth E, Ganz T. Ironing out ferroportin. Cell Metab 2015; 22:777–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Domenico I, Ward DM, di Patti MCB, Jeong SY, David S, Musci G, Kaplan J. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J 2007; 26:2823–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel BN, Dunn RJ, David S. Alternative RNA splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. J Biol Chem 2000; 275:4305–10 [DOI] [PubMed] [Google Scholar]
- 27.Fortna RR, Watson HA, Nyquist SE. Glycosyl phosphatidylinositol-anchored ceruloplasmin is expressed by rat sertoli cells and is concentrated in detergent-insoluble membrane fractions. Biol Reprod 1999; 61:1042–9 [DOI] [PubMed] [Google Scholar]
- 28.Conrad ME, Crosby WH. Intestinal mucosal mechanisms controlling iron absorption. Blood 1963; 22:406–15 [PubMed] [Google Scholar]
- 29.Cmejla R, Petrak J, Cmejlova J. A novel iron responsive element in the 3’UTR of human MRCKalpha. Biochem Biophys Res Commun 2006; 341:158–66 [DOI] [PubMed] [Google Scholar]
- 30.Cmejla R, Ptackova P, Petrak J, Savvulidi F, Cerny J, Sebesta O, Vyoral D. Human MRCKalpha is regulated by cellular iron levels and interferes with transferrin iron uptake. Biochem Biophys Res Commun 2010; 395:163–7 [DOI] [PubMed] [Google Scholar]
- 31.Du F, Qian C, Qian ZM, Wu X-M, Xie H, Yung W-H, Ke Y. Hepcidin directly inhibits transferrin receptor 1 expression in astrocytes via a cyclic AMP-protein kinase a pathway. Glia 2011; 59:936–45 [DOI] [PubMed] [Google Scholar]
- 32.De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell 2007; 18:2569–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr 2010; 30:105–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta 2012; 1823:1434–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr 2008; 28:197–213 [DOI] [PubMed] [Google Scholar]
- 36.Anderson CP, Shen M, Eisenstein RS, Leibold EA. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta 2012; 1823:1468–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J 2011; 434:365–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou ZD, Tan E-K. Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. Mol Neurodegener 2017; 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gammella E, Buratti P, Cairo G, Recalcati S. Macrophages: central regulators of iron balance. Metallomics 2014; 6:1336–45 [DOI] [PubMed] [Google Scholar]
- 40.Hidalgo C, Núñez MT. Calcium, iron and neuronal function. IUBMB Life 2007; 59:280–5 [DOI] [PubMed] [Google Scholar]
- 41.Hidalgo C, Carrasco MA, Muñoz P, Núñez MT. A role for reactive oxygen/nitrogen species and iron on neuronal synaptic plasticity. Antioxid Redox Signal 2007; 9:245–55 [DOI] [PubMed] [Google Scholar]
- 42.Salvador GA. Iron in neuronal function and dysfunction. Biofactors 2010; 36:103–10 [DOI] [PubMed] [Google Scholar]
- 43.Codazzi F, Pelizzoni I, Zacchetti D, Grohovaz F. Iron entry in neurons and astrocytes: a link with synaptic activity. Front Mol Neurosci 2015; 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muñoz P, Humeres A. Iron deficiency on neuronal function. Biometals 2012; 25:825–35 [DOI] [PubMed] [Google Scholar]
- 45.Stephenson E, Nathoo N, Mahjoub Y, Dunn JF, Yong VW. Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat Rev Neurol 2014; 10:459–68 [DOI] [PubMed] [Google Scholar]
- 46.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 2004; 16:1–13 [DOI] [PubMed] [Google Scholar]
- 47.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol 2015; 7:a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petillon C, Hergesheimer R, Puy H, Corcia P, Vourc’h P, Andres C, Karim Z, Blasco H. The relevancy of data regarding the metabolism of iron to our understanding of deregulated mechanisms in ALS; hypotheses and pitfalls. Front Neurosci 2018; 12:1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masaldan S, Bush AI, Devos D, Rolland AS, Moreau C. Striking while the iron is hot: iron metabolism and ferroptosis in neurodegeneration. Free Radic Biol Med 2019; 133:221–33 [DOI] [PubMed] [Google Scholar]
- 50.DeGregorio-Rocasolano N, Martí-Sistac O, Gasull T. Deciphering the iron side of stroke: neurodegeneration at the crossroads between iron dyshomeostasis, excitotoxicity, and ferroptosis. Front Neurosci 2019; 13:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeong SY, David S. Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J Biol Chem 2003; 278:27144–8 [DOI] [PubMed] [Google Scholar]
- 52.Wu LJ, Leenders AGM, Cooperman S, Meyron-Holtz E, Smith S, Land W, Tsai RYL, Berger UV, Sheng Z-H, Rouault TA. Expression of the iron transporter ferroportin in synaptic vesicles and the blood-brain barrier. Brain Res 2004; 1001:108–17 [DOI] [PubMed] [Google Scholar]
- 53.Descamps L, Dehouck MP, Torpier G, Cecchelli R. Receptor-mediated transcytosis of transferrin through blood-brain barrier endothelial cells. Am J Physiol 1996; 270:H1149–1158 [DOI] [PubMed] [Google Scholar]
- 54.Raub TJ, Newton CR. Recycling kinetics and transcytosis of transferrin in primary cultures of bovine brain microvessel endothelial cells. J Cell Physiol 1991; 149:141–51 [DOI] [PubMed] [Google Scholar]
- 55.Qian Z-M, Ke Y. Brain iron transport. Biol Rev Camb Philos Soc 2019; 94:1672–84 [DOI] [PubMed] [Google Scholar]
- 56.Qian Z-M, Chang Y-Z, Zhu L, Yang L, Du J-R, Ho K-P, Wang Q, Li L-Z, Wang C-Y, Ge X, Jing NL, Li L, Ke Y. Development and iron-dependent expression of hephaestin in different brain regions of rats. J Cell Biochem 2007; 102:1225–33 [DOI] [PubMed] [Google Scholar]
- 57.Rouault TA, Zhang D-L, Jeong SY. Brain iron homeostasis, the choroid plexus, and localization of iron transport proteins. Metab Brain Dis 2009; 24:673–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCarthy RC, Kosman DJ. Mechanisms and regulation of iron trafficking across the capillary endothelial cells of the blood-brain barrier. Front Mol Neurosci 2015; 8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malecki EA, Devenyi AG, Beard JL, Connor JR. Existing and emerging mechanisms for transport of iron and manganese to the brain. J Neurosci Res 1999; 56:113–22 [DOI] [PubMed] [Google Scholar]
- 60.Moos T, Rosengren Nielsen T, Skjørringe T, Morgan EH. Iron trafficking inside the brain. J Neurochem 2007; 103:1730–40 [DOI] [PubMed] [Google Scholar]
- 61.Connor JR, Boeshore KL, Benkovic SA, Menzies SL. Isoforms of ferritin have a specific cellular distribution in the brain. J Neurosci Res 1994; 37:461–5 [DOI] [PubMed] [Google Scholar]
- 62.Han J, Day JR, Connor JR, Beard JL. H and L ferritin subunit mRNA expression differs in brains of control and iron-deficient rats. J Nutr 2002; 132:2769–74 [DOI] [PubMed] [Google Scholar]
- 63.Cheepsunthorn P, Palmer C, Connor JR. Cellular distribution of ferritin subunits in postnatal rat brain. J Comp Neurol 1998; 400:73–86 [PubMed] [Google Scholar]
- 64.Møllgård K, Balslev Y. The subcellular distribution of transferrin in rat choroid plexus studied with immunogold labelling of ultracryosections. Histochem J 1989; 21:441–8 [DOI] [PubMed] [Google Scholar]
- 65.Tsutsumi M, Skinner MK, Sanders-Bush E. Transferrin gene expression and synthesis by cultured choroid plexus epithelial cells. Regulation by serotonin and cyclic adenosine 3’,5’-monophosphate. J Biol Chem 1989; 264:9626–31 [PubMed] [Google Scholar]
- 66.de Arriba Zerpa GA, Saleh MC, Fernández PM, Guillou F, Espinosa de los Monteros A, de Vellis J, Zakin MM, Baron B. Alternative splicing prevents transferrin secretion during differentiation of a human oligodendrocyte cell line. J Neurosci Res 2000; 61:388–95 [DOI] [PubMed] [Google Scholar]
- 67.Duchange N, Saleh M-C, de Arriba Zerpa G, Pidoux J, Guillou F, Zakin MM, Baron B. Alternative splicing in the brain of mice and rats generates transferrin transcripts lacking, as in humans, the signal peptide sequence. Neurochem Res 2002; 27:1459–63 [DOI] [PubMed] [Google Scholar]
- 68.Bradbury MW. Transport of iron in the blood-brain-cerebrospinal fluid system. J Neurochem 1997; 69:443–54 [DOI] [PubMed] [Google Scholar]
- 69.Moos T, Morgan EH. Evidence for low molecular weight, non-transferrin-bound iron in rat brain and cerebrospinal fluid. J Neurosci Res 1998; 54:486–94 [DOI] [PubMed] [Google Scholar]
- 70.Tripathi AK, Haldar S, Qian J, Beserra A, Suda S, Singh A, Hopfer U, Chen SG, Garrick MD, Turner JR, Knutson MD, Singh N. Prion protein functions as a ferrireductase partner for ZIP14 and DMT1. Free Radic Biol Med 2015; 84:322–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haldar S, Tripathi A, Qian J, Beserra A, Suda S, McElwee M, Turner J, Hopfer U, Singh N. Prion protein promotes kidney iron uptake via its ferrireductase activity. J Biol Chem 2015; 290:5512–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh N, Asthana A, Baksi S, Desai V, Haldar S, Hari S, Tripathi AK. The prion-ZIP connection: from cousins to partners in iron uptake. Prion 2015; 9:420–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh A, Haldar S, Horback K, Tom C, Zhou L, Meyerson H, Singh N. Prion protein regulates iron transport by functioning as a ferrireductase. J Alzheimers Dis 2013; 35:541–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown DR. α-Synuclein as a ferrireductase. Biochem Soc Trans 2013; 41:1513–7 [DOI] [PubMed] [Google Scholar]
- 75.Davies P, Moualla D, Brown DR. Alpha-synuclein is a cellular ferrireductase. PLoS ONE 2011; 6:e15814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDowall JS, Brown DR. Alpha-synuclein: relating metals to structure, function and inhibition. Metallomics 2016; 8:385–97 [DOI] [PubMed] [Google Scholar]
- 77.McDowall JS, Ntai I, Honeychurch KC, Hart JP, Colin P, Schneider BL, Brown DR. Alpha-synuclein ferrireductase activity is detectible in vivo, is altered in Parkinson’s disease and increases the neurotoxicity of DOPAL. Mol Cell Neurosci 2017; 85:1–11 [DOI] [PubMed] [Google Scholar]
- 78.Rathnasamy G, Ling E-A, Kaur C. Consequences of iron accumulation in microglia and its implications in neuropathological conditions. CNS Neurol Disord Drug Targets 2013; 12:785–98 [DOI] [PubMed] [Google Scholar]
- 79.Ward RJ, Crichton RR, Taylor DL, Della Corte L, Srai SK, Dexter DT. Iron and the immune system. J Neural Transm 2011; 118:315–28 [DOI] [PubMed] [Google Scholar]
- 80.Brock JH. Lactoferrin-50 years on. Biochem Cell Biol 2012; 90:245–51 [DOI] [PubMed] [Google Scholar]
- 81.Jiang H, Song N, Jiao Q, Shi L, Du X. Iron pathophysiology in Parkinson diseases. Adv Exp Med Biol 2019; 1173:45–66 [DOI] [PubMed] [Google Scholar]
- 82.Bonn D. Pumping iron in Parkinson’s disease. Lancet 1996; 347:1614. [DOI] [PubMed] [Google Scholar]
- 83.Faucheux BA, Nillesse N, Damier P, Spik G, Mouatt-Prigent A, Pierce A, Leveugle B, Kubis N, Hauw JJ, Agid Y. Expression of lactoferrin receptors is increased in the mesencephalon of patients with Parkinson disease. Proc Natl Acad Sci U S A 1995; 92:9603–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ji C, Kosman DJ. Molecular mechanisms of non-transferrin-bound and transferring-bound iron uptake in primary hippocampal neurons. J Neurochem 2015; 133:668–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sterling J, Guttha S, Song Y, Song D, Hadziahmetovic M, Dunaief JL. Iron importers Zip8 and Zip14 are expressed in retina and regulated by retinal iron levels. Exp Eye Res 2017; 155:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baumann B, Sterling J, Song Y, Song D, Fruttiger M, Gillies M, Shen W, Dunaief JL. Conditional Müller cell ablation leads to retinal iron accumulation. Invest Ophthalmol Vis Sci 2017; 58:4223–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wong BX, Tsatsanis A, Lim LQ, Adlard PA, Bush AI, Duce JA. β-Amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin. PLoS One 2014; 9:e114174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoepken HH, Korten T, Robinson SR, Dringen R. Iron accumulation, iron-mediated toxicity and altered levels of ferritin and transferrin receptor in cultured astrocytes during incubation with ferric ammonium citrate. J Neurochem 2004; 88:1194–202 [DOI] [PubMed] [Google Scholar]
- 89.Zarruk JG, Berard JL, Passos dos Santos R, Kroner A, Lee J, Arosio P, David S. Expression of iron homeostasis proteins in the spinal cord in experimental autoimmune encephalomyelitis and their implications for iron accumulation. Neurobiol Dis 2015; 81:93–107 [DOI] [PubMed] [Google Scholar]
- 90.Qian ZM, To Y, Tang PL, Feng YM. Transferrin receptors on the plasma membrane of cultured rat astrocytes. Exp Brain Res 1999; 129:473–6 [DOI] [PubMed] [Google Scholar]
- 91.Qian ZM, Liao QK, To Y, Ke Y, Tsoi YK, Wang GF, Ho KP. Transferrin-bound and transferrin free iron uptake by cultured rat astrocytes. Cell Mol Biol 2000; 46:541–8 [PubMed] [Google Scholar]
- 92.Huang S, Du F, Li L, Liu Y, Liu Y, Zhang C, Qian ZM. Angiotensin II inhibits uptake of transferrin-bound iron but not non-transferrin-bound iron by cultured astrocytes. Neuropeptides 2014; 48:161–6 [DOI] [PubMed] [Google Scholar]
- 93.Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, Haile DJ, Beard JL, Connor JR. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res 2001; 66:1198–207 [DOI] [PubMed] [Google Scholar]
- 94.Tulpule K, Robinson SR, Bishop GM, Dringen R. Uptake of ferrous iron by cultured rat astrocytes. J Neurosci Res 2010; 88:563–71 [DOI] [PubMed] [Google Scholar]
- 95.Song N, Jiang H, Wang J, Xie J-X. Divalent metal transporter 1 up-regulation is involved in the 6-hydroxydopamine-induced ferrous iron influx. J Neurosci Res 2007; 85:3118–26 [DOI] [PubMed] [Google Scholar]
- 96.Bishop GM, Scheiber IF, Dringen R, Robinson SR. Synergistic accumulation of iron and zinc by cultured astrocytes. J Neural Transm 2010; 117:809–17 [DOI] [PubMed] [Google Scholar]
- 97.Pelizzoni I, Zacchetti D, Campanella A, Grohovaz F, Codazzi F. Iron uptake in quiescent and inflammation-activated astrocytes: a potentially neuroprotective control of iron burden. Biochim Biophys Acta 2013; 1832:1326–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Todorich B, Zhang X, Connor JR. H-ferritin is the major source of iron for oligodendrocytes. Glia 2011; 59:927–35 [DOI] [PubMed] [Google Scholar]
- 99.Todorich B, Zhang X, Slagle-Webb B, Seaman WE, Connor JR. Tim-2 is the receptor for H-ferritin on oligodendrocytes. J Neurochem 2008; 107:1495–505 [DOI] [PubMed] [Google Scholar]
- 100.Han J, Seaman WE, Di X, Wang W, Willingham M, Torti FM, Torti SV. Iron uptake mediated by binding of H-ferritin to the TIM-2 receptor in mouse cells. PLoS One 2011; 6:e23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schulz K, Vulpe CD, Harris LZ, David S. Iron efflux from oligodendrocytes is differentially regulated in gray and white matter. J Neurosci 2011; 31:13301–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koeppen AH. A brief history of brain iron research. J Neurol Sci. 2003; 207:95–7. [DOI] [PubMed] [Google Scholar]
- 103.Pirpamer L, Hofer E, Gesierich B, De Guio F, Freudenberger P, Seiler S, Duering M, Jouvent E, Duchesnay E, Dichgans M, Ropele S, Schmidt R. Determinants of iron accumulation in the normal aging brain. Neurobiol Aging 2016; 43:149–55 [DOI] [PubMed] [Google Scholar]
- 104.Zecca L, Gallorini M, Schünemann V, Trautwein AX, Gerlach M, Riederer P, Vezzoni P, Tampellini D. Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: consequences for iron storage and neurodegenerative processes. J Neurochem 2001; 76:1766–73 [DOI] [PubMed] [Google Scholar]
- 105.Zecca L, Youdim MBH, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 2004; 5:863–73 [DOI] [PubMed] [Google Scholar]
- 106.Crichton RR, Dexter DT, Ward RJ. Brain iron metabolism and its perturbation in neurological diseases. J Neural Transm 2011; 118:301–14 [DOI] [PubMed] [Google Scholar]
- 107.Connor JR, Menzies SL, St Martin SM, Mufson EJ. Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J Neurosci Res 1990; 27:595–611 [DOI] [PubMed] [Google Scholar]
- 108.Li K. Iron pathophysiology in Friedreich’s ataxia. Adv Exp Med Biol 2019; 1173:125–43 [DOI] [PubMed] [Google Scholar]
- 109.Wang T, Xu S-F, Fan Y-G, Li L-B, Guo C. Iron pathophysiology in Alzheimer’s diseases. Adv Exp Med Biol 2019; 1173:67–104 [DOI] [PubMed] [Google Scholar]
- 110.Almutairi MMA, Xu G, Shi H. Iron pathophysiology in stroke. Adv Exp Med Biol 2019; 1173:105–23 [DOI] [PubMed] [Google Scholar]
- 111.Zhao Z. Iron and oxidizing species in oxidative stress and Alzheimer’s disease. Aging Med 2019; 2:82–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kotzbauer PT, Truax AC, Trojanowski JQ, Lee VM-Y. Altered neuronal mitochondrial coenzyme a synthesis in neurodegeneration with brain iron accumulation caused by abnormal processing, stability, and catalytic activity of mutant pantothenate kinase 2. J Neurosci 2005; 25:689–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Joppe K, Roser A-E, Maass F, Lingor P. The contribution of iron to protein aggregation disorders in the Central nervous system. Front Neurosci 2019; 13:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti SM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017; 171:273–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Muhoberac BB, Vidal R. Iron, ferritin, hereditary ferritinopathy, and neurodegeneration. Front Neurosci 2019; 13:1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guiney SJ, Adlard PA, Bush AI, Finkelstein DI, Ayton S. Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochem Int 2017; 104:34–48 [DOI] [PubMed] [Google Scholar]
- 117.Paisán-Ruiz C, Li A, Schneider SA, Holton JL, Johnson R, Kidd D, Chataway J, Bhatia KP, Lees AJ, Hardy J, Revesz T, Houlden H. Widespread Lewy body and tau accumulation in childhood and adult onset dystonia-parkinsonism cases with PLA2G6 mutations. Neurobiol Aging 2012; 33:814–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ratan RR. The chemical biology of ferroptosis in the central nervous system. Cell Chem Biol 2020; 27:479–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu J-R, Tuo Q-Z, Lei P. Ferroptosis, a recent defined form of critical cell death in neurological disorders. J Mol Neurosci 2018; 66:197–206 [DOI] [PubMed] [Google Scholar]
- 120.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Grocin AG, Xavier da Silva TN, Panzilius E, Scheel CH, Mourão A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O'Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JPF, Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019; 575:693–8 [DOI] [PubMed] [Google Scholar]
- 121.Huang XT, Liu X, Ye CY, Tao LX, Zhou H, Zhang HY. Iron-induced energy supply deficiency and mitochondrial fragmentation in neurons. J Neurochem 2018; 147:816–30 [DOI] [PubMed] [Google Scholar]
- 122.Rakshit J, Mallick A, Roy S, Sarbajna A, Dutta M, Bandyopadhyay J. Iron-Induced apoptotic cell death and autophagy dysfunction in human neuroblastoma cell line SH-SY5Y. Biol Trace Elem Res 2020; 193:138–51 [DOI] [PubMed] [Google Scholar]
- 123.Yuan Y, Xu F, Cao Y, Xu L, Yu C, Yang F, Zhang P, Wang L, Shen G, Wang J, Xu Y. Iron accumulation leads to bone loss by inducing mesenchymal stem cell apoptosis through the activation of Caspase3. Biol Trace Elem Res 2019; 187:434–41 [DOI] [PubMed] [Google Scholar]
- 124.de Majo M, Koontz M, Rowitch D, Ullian EM. An update on human astrocytes and their role in development and disease. Glia 2020; 68:685–704 [DOI] [PubMed] [Google Scholar]
- 125.Sidoryk-Węgrzynowicz M, Strużyńska L. Astroglial contribution to tau-dependent neurodegeneration. Biochem J 2019; 476:3493–504 [DOI] [PubMed] [Google Scholar]
- 126.Valori CF, Guidotti G, Brambilla L, Rossi D. Astrocytes in motor neuron diseases. Adv Exp Med Biol 2019; 1175:227–72 [DOI] [PubMed] [Google Scholar]
- 127.Gray M. Astrocytes in Huntington’s disease. Adv Exp Med Biol 2019; 1175:355–81 [DOI] [PubMed] [Google Scholar]
- 128.Cheli VT, Santiago González DA, Marziali LN, Zamora NN, Guitart ME, Spreuer V, Pasquini JM, Paez PM. The divalent metal transporter 1 (DMT1) is required for iron uptake and normal development of oligodendrocyte progenitor cells. J Neurosci 2018; 38:9142–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nasrabady SE, Rizvi B, Goldman JE, Brickman AM. White matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocytes. Acta Neuropathol Commun 2018; 6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cai Z, Xiao M. Oligodendrocytes and Alzheimer’s disease. Int J Neurosci 2016; 126:97–104 [DOI] [PubMed] [Google Scholar]
- 131.LoPresti P. Tau in oligodendrocytes takes neurons in sickness and in health. Int J Mol Sci 2018; 19:2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ferrer I. Oligodendrogliopathy in neurodegenerative diseases with abnormal protein aggregates: the forgotten partner. Prog Neurobiol 2018; 169:24–54 [DOI] [PubMed] [Google Scholar]
- 133.Hametner S, Wimmer I, Haider L, Pfeifenbring S, Brück W, Lassmann H. Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol 2013; 74:848–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and alzheimer’s disease. Neurobiol Aging 2004; 25:5–18; author reply: 49–62 [DOI] [PubMed] [Google Scholar]
- 135.Bartzokis G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging 2011; 32:1341–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bartzokis G, Lu PH, Tishler TA, Fong SM, Oluwadara B, Finn JP, Huang D, Bordelon Y, Mintz J, Perlman S. Myelin Breakdown and iron changes in Huntington’s disease: pathogenesis and treatment implications. Neurochem Res 2007; 32:1655–64 [DOI] [PubMed] [Google Scholar]
- 137.Binolfi A, Rasia RM, Bertoncini CW, Ceolin M, Zweckstetter M, Griesinger C, Jovin TM, Fernández CO. Interaction of alpha-synuclein with divalent metal ions reveals key differences: a link between structure, binding specificity and fibrillation enhancement. J Am Chem Soc 2006; 128:9893–901 [DOI] [PubMed] [Google Scholar]
- 138.Bartels M, Weckbecker D, Kuhn P-H, Ryazanov S, Leonov A, Griesinger C, Lichtenthaler SF, Bötzel K, Giese A. Iron-mediated aggregation and toxicity in a novel neuronal cell culture model with inducible alpha-synuclein expression. Sci Rep 2019; 9:9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chiot A, Lobsiger CS, Boillée S. New insights on the disease contribution of neuroinflammation in amyotrophic lateral sclerosis. Curr Opin Neurol 2019; 32:764–70 [DOI] [PubMed] [Google Scholar]
- 140.Ciccocioppo F, Bologna G, Ercolino E, Pierdomenico L, Simeone P, Lanuti P, Pieragostino D, Del Boccio P, Marchisio M, Miscia S. Neurodegenerative diseases as proteinopathies-driven immune disorders. Neural Regen Res 2020; 15:850–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Frost GR, Jonas LA, Li Y-M. Friend, foe or both? Immune activity in Alzheimer’s disease. Front Aging Neurosci 2019; 11:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li X, Liu Y, Zheng Q, Yao G, Cheng P, Bu G, Xu H, Zhang Y. Ferritin light chain interacts with PEN-2 and affects γ-secretase activity. Neurosci Lett 2013; 548:90–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fahn S. The 200-year journey of Parkinson disease: reflecting on the past and looking towards the future. Parkinsonism Relat Disord 2018; 46(Suppl 1):S1–5 [DOI] [PubMed] [Google Scholar]
- 144.Maetzler W, Berg D. Parkinson disease in 2017: changing views after 200 years of Parkinson disease. Nat Rev Neurol 2018; 14:70–2 [DOI] [PubMed] [Google Scholar]
- 145.McDonald C, Gordon G, Hand A, Walker RW, Fisher JM. 200 Years of Parkinson’s disease: what have we learnt from James Parkinson? Age Age 2018; 47:209–14 [DOI] [PubMed] [Google Scholar]
- 146.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag A-E, Lang AE. Parkinson disease. Nat Rev Dis Primers 2017; 3:17013. [DOI] [PubMed] [Google Scholar]
- 147.Fang C, Lv L, Mao S, Dong H, Liu B. Cognition deficits in Parkinson’s disease: mechanisms and treatment. Parkinsons Dis 2020; 2020:2076942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goldman JG, Sieg E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med 2020; 36:365–77 [DOI] [PubMed] [Google Scholar]
- 149.Jellinger K, Paulus W, Grundke-Iqbal I, Riederer P, Youdim MB. Brain iron and ferritin in Parkinson’s and Alzheimer’s diseases. J Neural Transm Park Dis Dement Sect 1990; 2:327–40 [DOI] [PubMed] [Google Scholar]
- 150.Kaur D, Andersen J. Does cellular iron dysregulation play a causative role in Parkinson’s disease? Ageing Res Rev 2004; 3:327–43 [DOI] [PubMed] [Google Scholar]
- 151.Shen XM, Dryhurst G. Iron- and manganese-catalyzed autoxidation of dopamine in the presence of L-cysteine: possible insights into iron- and manganese-mediated dopaminergic neurotoxicity. Chem Res Toxicol 1998; 11:824–37 [DOI] [PubMed] [Google Scholar]
- 152.Shen XM, Zhang F, Dryhurst G. Oxidation of dopamine in the presence of cysteine: characterization of new toxic products. Chem Res Toxicol 1997; 10:147–55 [DOI] [PubMed] [Google Scholar]
- 153.Gerlach M, Double K, Riederer P, Hirsch E, Jellinger K, Jenner P, Trautwein A, Youdim MB. Iron in the parkinsonian substantia nigra. Mov Disord 1997; 12:258–60 [PubMed] [Google Scholar]
- 154.Double KL, Gerlach M, Schünemann V, Trautwein AX, Zecca L, Gallorini M, Youdim MBH, Riederer P, Ben-Shachar D. Iron-binding characteristics of neuromelanin of the human substantia nigra. Biochem Pharmacol 2003; 66:489–94 [DOI] [PubMed] [Google Scholar]
- 155.Gerlach M, Double KL, Ben-Shachar D, Zecca L, Youdim MBH, Riederer P. Neuromelanin and its interaction with iron as a potential risk factor for dopaminergic neurodegeneration underlying Parkinson’s disease. Neurotox Res 2003; 5:35–44 [DOI] [PubMed] [Google Scholar]
- 156.Xing Y, Sapuan A, Dineen RA, Auer DP. Life span pigmentation changes of the substantia nigra detected by neuromelanin-sensitive MRI. Mov Disord 2018; 33:1792–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zucca FA, Segura-Aguilar J, Ferrari E, Muñoz P, Paris I, Sulzer D, Sarna T, Casella L, Zecca L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol 2017; 155:96–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Youdim MB, Ben-Shachar D, Riederer P. The enigma of neuromelanin in Parkinson’s disease substantia nigra. J Neural Transm Suppl 1994; 43:113–22 [PubMed] [Google Scholar]
- 159.Jellinger KA, Kienzl E, Rumpelmaier G, Paulus W, Riederer P, Stachelberger H, Youdim MB, Ben-Shachar D. Iron and ferritin in substantia nigra in Parkinson’s disease. Adv Neurol 1993; 60:267–72 [PubMed] [Google Scholar]
- 160.Faucheux BA, Martin M-E, Beaumont C, Hauw J-J, Agid Y, Hirsch EC. Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson’s disease. J Neurochem 2003; 86:1142–8 [DOI] [PubMed] [Google Scholar]
- 161.Thomsen MS, Andersen MV, Christoffersen PR, Jensen MD, Lichota J, Moos T. Neurodegeneration with inflammation is accompanied by accumulation of iron and ferritin in microglia and neurons. Neurobiol Dis 2015; 81:108–18 [DOI] [PubMed] [Google Scholar]
- 162.Rocha EM, De Miranda B, Sanders LH. Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol Dis 2018; 109:249–57 [DOI] [PubMed] [Google Scholar]
- 163.Gelders G, Baekelandt V, Van der Perren A. Linking neuroinflammation and neurodegeneration in Parkinson’s disease. J Immunol Res 2018; 2018:4784268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cabezudo D, Baekelandt V, Lobbestael E. Multiple-Hit hypothesis in Parkinson’s disease: LRRK2 and inflammation. Front Neurosci 2020; 14:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Blesa J, Phani S, Jackson-Lewis V, Przedborski S. Classic and new animal models of Parkinson’s disease. J Biomed Biotechnol 2012; 2012:845618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gubellini P, Kachidian P. Animal models of Parkinson’s disease: an updated overview. Rev Neurol 2015; 171:750–61 [DOI] [PubMed] [Google Scholar]
- 167.Schober A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 2004; 318:215–24 [DOI] [PubMed] [Google Scholar]
- 168.Oiwa Y, Eberling JL, Nagy D, Pivirotto P, Emborg ME, Bankiewicz KS. Overlesioned hemiparkinsonian non human primate model: correlation between clinical, neurochemical and histochemical changes. Front Biosci 2003; 8:a155–166 [DOI] [PubMed] [Google Scholar]
- 169.Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med 2020; 36:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bandres-Ciga S, Diez-Fairen M, Kim JJ, Singleton AB. Genetics of Parkinson’s disease: an introspection of its journey towards precision medicine. Neurobiol Dis 2020; 137:104782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-synuclein locus triplication causes Parkinson’s disease. Science 2003; 302:841. [DOI] [PubMed] [Google Scholar]
- 172.Ip CW, Klaus L-C, Karikari AA, Visanji NP, Brotchie JM, Lang AE, Volkmann J, Koprich JB. AAV1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson’s disease. Acta Neuropathol Commun 2017; 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Koprich JB, Johnston TH, Reyes G, Omana V, Brotchie JM. Towards a non-human primate model of alpha-synucleinopathy for development of therapeutics for Parkinson’s disease: optimization of AAV1/2 delivery parameters to drive sustained expression of alpha synuclein and dopaminergic degeneration in macaque. PLoS One 2016; 11:e0167235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Van der Perren A, Casteels C, Van Laere K, Gijsbers R, Van den Haute C, Baekelandt V. Development of an alpha-synuclein based rat model for Parkinson’s disease via stereotactic injection of a recombinant adeno-associated viral vector. J Vis Exp 2016; 108:53670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Landeck N, Buck K, Kirik D. Toxic effects of human and rodent variants of alpha-synuclein in vivo. Eur J Neurosci 2017; 45:536–47 [DOI] [PubMed] [Google Scholar]
- 176.Fields CR, Bengoa-Vergniory N, Wade-Martins R. Targeting alpha-synuclein as a therapy for Parkinson’s disease. Front Mol Neurosci 2019; 12:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zeng X-S, Geng W-S, Jia J-J, Chen L, Zhang P-P. Cellular and molecular basis of neurodegeneration in Parkinson disease. Front Aging Neurosci 2018; 10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 2008; 14:504–6 [DOI] [PubMed] [Google Scholar]
- 179.Ma J, Gao J, Wang J, Xie A. Prion-Like mechanisms in Parkinson’s disease. Front Neurosci 2019; 13:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sian-Hülsmann J, Mandel S, Youdim MBH, Riederer P. The relevance of iron in the pathogenesis of Parkinson’s disease. J Neurochem 2011; 118:939–57 [DOI] [PubMed] [Google Scholar]
- 181.Dexter DT, Wells FR, Agid F, Agid Y, Lees AJ, Jenner P, Marsden CD. Increased nigral iron content in postmortem parkinsonian brain. Lancet 1987; 2:1219–20 [DOI] [PubMed] [Google Scholar]
- 182.Götz ME, Double K, Gerlach M, Youdim MBH, Riederer P. The relevance of iron in the pathogenesis of Parkinson’s disease. Ann N Y Acad Sci 2004; 1012:193–208 [DOI] [PubMed] [Google Scholar]
- 183.Genoud S, Senior AM, Hare DJ, Double KL. Meta-analysis of copper and iron in Parkinson’s disease brain and biofluids. Mov Disord 2019; 35:662–71 [DOI] [PubMed] [Google Scholar]
- 184.Wolozin B, Golts N. Iron and Parkinson’s disease. Neuroscientist 2002; 8:22–32 [DOI] [PubMed] [Google Scholar]
- 185.Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B. The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci 2000; 20:6048–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Golts N, Snyder H, Frasier M, Theisler C, Choi P, Wolozin B. Magnesium inhibits spontaneous and iron-induced aggregation of alpha-synuclein. J Biol Chem 2002; 277:16116–23 [DOI] [PubMed] [Google Scholar]
- 187.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J Biol Chem 2001; 276:44284–96 [DOI] [PubMed] [Google Scholar]
- 188.Lv Z, Jiang H, Xu H, Song N, Xie J. Increased iron levels correlate with the selective nigral dopaminergic neuron degeneration in Parkinson’s disease. J Neural Transm 2011; 118:361–9 [DOI] [PubMed] [Google Scholar]
- 189.Sofic E, Paulus W, Jellinger K, Riederer P, Youdim MB. Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. J Neurochem 1991; 56:978–82 [DOI] [PubMed] [Google Scholar]
- 190.Riederer P, Dirr A, Goetz M, Sofic E, Jellinger K, Youdim MB. Distribution of iron in different brain regions and subcellular compartments in Parkinson’s disease. Ann Neurol 1992; 32(Suppl):S101–104 [DOI] [PubMed] [Google Scholar]
- 191.Uchida Y, Kan H, Sakurai K, Arai N, Kato D, Kawashima S, Ueki Y, Matsukawa N. Voxel-based quantitative susceptibility mapping in Parkinson’s disease with mild cognitive impairment. Mov Disord 2019; 34:1164–73 [DOI] [PubMed] [Google Scholar]
- 192.Tambasco N, Paolini Paoletti F, Chiappiniello A, Lisetti V, Nigro P, Eusebi P, Chiarini P, Romoli M, Brahimi E, Simoni S, Filidei M, Floridi P, Tarducci R, Parnetti L, Calabresi P. T2*-weighted MRI values correlate with motor and cognitive dysfunction in Parkinson’s disease. Neurobiol Aging 2019; 80:91–8 [DOI] [PubMed] [Google Scholar]
- 193.Mochizuki H, Choong C-J, Baba K. Parkinson’s disease and iron. J Neural Transm 2020; 127:181–7 [DOI] [PubMed] [Google Scholar]
- 194.Sengstock GJ, Olanow CW, Menzies RA, Dunn AJ, Arendash GW. Infusion of iron into the rat substantia nigra: nigral pathology and dose-dependent loss of striatal dopaminergic markers. J Neurosci Res 1993; 35:67–82 [DOI] [PubMed] [Google Scholar]
- 195.Sziráki I, Mohanakumar KP, Rauhala P, Kim HG, Yeh KJ, Chiueh CC. Manganese: a transition metal protects nigrostriatal neurons from oxidative stress in the iron-induced animal model of parkinsonism. Neuroscience 1998; 85:1101–11 [DOI] [PubMed] [Google Scholar]
- 196.Wesemann W, Blaschke S, Solbach M, Grote C, Clement HW, Riederer P. Intranigral injected iron progressively reduces striatal dopamine metabolism. J Neural Transm Park Dis Dement Sect 1994; 8:209–14 [DOI] [PubMed] [Google Scholar]
- 197.Ben-Shachar D, Youdim MB. Intranigral iron injection induces behavioral and biochemical “parkinsonism” in rats. J Neurochem 1991; 57:2133–5 [DOI] [PubMed] [Google Scholar]
- 198.Lan J, Jiang DH. Desferrioxamine and vitamin E protect against iron and MPTP-induced neurodegeneration in mice. J Neural Transm 1997; 104:469–81 [DOI] [PubMed] [Google Scholar]
- 199.Kaur D, Yantiri F, Rajagopalan S, Kumar J, Mo JQ, Boonplueang R, Viswanath V, Jacobs R, Yang L, Beal MF, DiMonte D, Volitaskis I, Ellerby L, Cherny RA, Bush AI, Andersen JK. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson’s disease. Neuron 2003; 37:899–909 [DOI] [PubMed] [Google Scholar]
- 200.Mochizuki H, Imai H, Endo K, Yokomizo K, Murata Y, Hattori N, Mizuno Y. Iron accumulation in the substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced hemiparkinsonian monkeys. Neurosci Lett 1994; 168:251–3 [DOI] [PubMed] [Google Scholar]
- 201.Wang J, Xu H-M, Yang H-D, Du X-X, Jiang H, Xie J-X. Rg1 reduces nigral iron levels of MPTP-treated C57BL6 mice by regulating certain iron transport proteins. Neurochem Int 2009; 54:43–8 [DOI] [PubMed] [Google Scholar]
- 202.Wang J, Jiang H, Xie J-X. Time dependent effects of 6-OHDA lesions on iron level and neuronal loss in rat nigrostriatal system. Neurochem Res 2004; 29:2239–43 [DOI] [PubMed] [Google Scholar]
- 203.He Y, Lee T, Leong SK. Time course of dopaminergic cell death and changes in iron, ferritin and transferrin levels in the rat substantia nigra after 6-hydroxydopamine (6-OHDA) lesioning. Free Radic Res 1999; 31:103–12 [DOI] [PubMed] [Google Scholar]
- 204.Guo C, Hao L-J, Yang Z-H, Chai R, Zhang S, Gu Y, Gao H-L, Zhong M-L, Wang T, Li J-Y, Wang Z-Y. Deferoxamine-mediated up-regulation of HIF-1α prevents dopaminergic neuronal death via the activation of MAPK family proteins in MPTP-treated mice. Exp Neurol 2016; 280:13–23 [DOI] [PubMed] [Google Scholar]
- 205.Goto K, Mochizuki H, Imai H, Akiyama H, Mizuno Y. An immuno-histochemical study of ferritin in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced hemiparkinsonian monkeys. Brain Res 1996; 724:125–8 [DOI] [PubMed] [Google Scholar]
- 206.Uitti RJ, Rajput AH, Rozdilsky B, Bickis M, Wollin T, Yuen WK. Regional metal concentrations in Parkinson’s disease, other chronic neurological diseases, and control brains. Can J Neurol Sci 1989; 16:310–4 [DOI] [PubMed] [Google Scholar]
- 207.He Y, Thong PS, Lee T, Leong SK, Mao BY, Dong F, Watt F. Dopaminergic cell death precedes iron elevation in MPTP-injected monkeys. Free Radic Biol Med 2003; 35:540–7 [DOI] [PubMed] [Google Scholar]
- 208.Double KL, Gerlach M, Youdim MB, Riederer P. Impaired iron homeostasis in Parkinson’s disease. J Neural Transm Suppl 2000; 60:37–58 [DOI] [PubMed] [Google Scholar]
- 209.Berg D, Gerlach M, Youdim MB, Double KL, Zecca L, Riederer P, Becker G. Brain iron pathways and their relevance to Parkinson’s disease. J Neurochem 2001; 79:225–36 [DOI] [PubMed] [Google Scholar]
- 210.Hirsch EC. Altered regulation of iron transport and storage in Parkinson’s disease. J Neural Transm Suppl 2006; 71:201–4 [DOI] [PubMed] [Google Scholar]
- 211.Xuan M, Guan X, Gu Q, Shen Z, Yu X, Qiu T, Luo X, Song R, Jiaerken Y, Xu X, Huang P, Luo W, Zhang M. Different iron deposition patterns in early- and middle-late-onset Parkinson’s disease. Parkinsonism Relat Disord 2017; 44:23–7 [DOI] [PubMed] [Google Scholar]
- 212.Huang Y, Cheung L, Rowe D, Halliday G. Genetic contributions to Parkinson’s disease. Brain Res Brain Res Rev 2004; 46:44–70 [DOI] [PubMed] [Google Scholar]
- 213.Wang J, Jiang H, Xie J-X. Ferroportin1 and hephaestin are involved in the nigral iron accumulation of 6-OHDA-lesioned rats. Eur J Neurosci 2007; 25:2766–72 [DOI] [PubMed] [Google Scholar]
- 214.Davies KM, Bohic S, Carmona A, Ortega R, Cottam V, Hare DJ, Finberg JPM, Reyes S, Halliday GM, Mercer JFB, Double KL. Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol Aging 2014; 35:858–66 [DOI] [PubMed] [Google Scholar]
- 215.Zhang Z, Hou L, Song J-L, Song N, Sun Y-J, Lin X, Wang X-L, Zhang F-Z, Ge Y-L. Pro-inflammatory cytokine-mediated ferroportin down-regulation contributes to the nigral iron accumulation in lipopolysaccharide-induced parkinsonian models. Neuroscience 2014; 257:20–30 [DOI] [PubMed] [Google Scholar]
- 216.Song N, Wang J, Jiang H, Xie J. Ferroportin 1 but not hephaestin contributes to iron accumulation in a cell model of Parkinson’s disease. Free Radic Biol Med 2010; 48:332–41 [DOI] [PubMed] [Google Scholar]
- 217.Kalivendi SV, Kotamraju S, Cunningham S, Shang T, Hillard CJ, Kalyanaraman B. 1-Methyl-4-phenylpyridinium (MPP+)-induced apoptosis and mitochondrial oxidant generation: role of transferrin-receptor-dependent iron and hydrogen peroxide. Biochem J 2003; 371:151–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jiang H, Song N, Xu H, Zhang S, Wang J, Xie J. Up-regulation of divalent metal transporter 1 in 6-hydroxydopamine intoxication is IRE/IRP dependent. Cell Res 2010; 20:345–56 [DOI] [PubMed] [Google Scholar]
- 219.Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C, Sazdovitch V, Zhao L, Garrick LM, Nuñez MT, Garrick MD, Raisman-Vozari R, Hirsch EC. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci U S A 2008; 105:18578–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang S, Wang J, Song N, Xie J, Jiang H. Up-regulation of divalent metal transporter 1 is involved in 1-methyl-4-phenylpyridinium (MPP(+))-induced apoptosis in MES23.5 cells. Neurobiol Aging 2009; 30:1466–76 [DOI] [PubMed] [Google Scholar]
- 221.Howitt J, Gysbers AM, Ayton S, Carew-Jones F, Putz U, Finkelstein DI, Halliday GM, Tan S-S. Increased Ndfip1 in the substantia nigra of parkinsonian brains is associated with elevated iron levels. PLoS One 2014; 9:e87119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fillebeen C, Mitchell V, Dexter D, Benaissa M, Beauvillain J, Spik G, Pierce A. Lactoferrin is synthesized by mouse brain tissue and its expression is enhanced after MPTP treatment. Brain Res Mol Brain Res 1999; 72:183–94 [DOI] [PubMed] [Google Scholar]
- 223.Fillebeen C, Ruchoux MM, Mitchell V, Vincent S, Benaïssa M, Pierce A. Lactoferrin is synthesized by activated microglia in the human substantia nigra and its synthesis by the human microglial CHME cell line is upregulated by tumor necrosis factor alpha or 1-methyl-4-phenylpyridinium treatment. Brain Res Mol Brain Res 2001; 96:103–13 [DOI] [PubMed] [Google Scholar]
- 224.Connor JR, Snyder BS, Arosio P, Loeffler DA, LeWitt P. A quantitative analysis of isoferritins in select regions of aged, parkinsonian, and Alzheimer’s diseased brains. J Neurochem 1995; 65:717–24 [DOI] [PubMed] [Google Scholar]
- 225.Faucheux BA, Martin M-E, Beaumont C, Hunot S, Hauw J-J, Agid Y, Hirsch EC. Lack of up-regulation of ferritin is associated with sustained iron regulatory protein-1 binding activity in the substantia nigra of patients with Parkinson’s disease. J Neurochem 2002; 83:320–30 [DOI] [PubMed] [Google Scholar]
- 226.Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 1991; 114:1953–75 [DOI] [PubMed] [Google Scholar]
- 227.Dexter DT, Carayon A, Vidailhet M, Ruberg M, Agid F, Agid Y, Lees AJ, Wells FR, Jenner P, Marsden CD. Decreased ferritin levels in brain in Parkinson’s disease. J Neurochem 1990; 55:16–20 [DOI] [PubMed] [Google Scholar]
- 228.Kawabata H. Transferrin and transferrin receptors update. Free Radic Biol Med 2019; 133:46–54 [DOI] [PubMed] [Google Scholar]
- 229.Mastroberardino PG, Hoffman EK, Horowitz MP, Betarbet R, Taylor G, Cheng D, Na HM, Gutekunst C-A, Gearing M, Trojanowski JQ, Anderson M, Chu CT, Peng J, Greenamyre JT. A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson’s disease. Neurobiol Dis 2009; 34:417–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Carroll CB, Zeissler M-L, Chadborn N, Gibson K, Williams G, Zajicek JP, Morrison KE, Hanemann CO. Changes in iron-regulatory gene expression occur in human cell culture models of Parkinson’s disease. Neurochem Int 2011; 59:73–80 [DOI] [PubMed] [Google Scholar]
- 231.Bjørklund G, Hofer T, Nurchi VM, Aaseth J. Iron and other metals in the pathogenesis of Parkinson’s disease: toxic effects and possible detoxification. J Inorg Biochem 2019; 199:110717. [DOI] [PubMed] [Google Scholar]
- 232.Chen B, Wen X, Jiang H, Wang J, Song N, Xie J. Interactions between iron and α-synuclein pathology in Parkinson’s disease. Free Radic Biol Med 2019; 141:253–60 [DOI] [PubMed] [Google Scholar]
- 233.Null B, Indi SS, Rao KSJ. Copper- and iron-induced differential fibril formation in alpha-synuclein: TEM study. Neurosci Lett 2007; 424:78–82 [DOI] [PubMed] [Google Scholar]
- 234.He Q, Song N, Xu H, Wang R, Xie J, Jiang H. Alpha-synuclein aggregation is involved in the toxicity induced by ferric iron to SK-N-SH neuroblastoma cells. J Neural Transm 2011; 118:397–406 [DOI] [PubMed] [Google Scholar]
- 235.Wang R, Wang Y, Qu L, Chen B, Jiang H, Song N, Xie J. Iron-induced oxidative stress contributes to α-synuclein phosphorylation and up-regulation via polo-like kinase 2 and casein kinase 2. Neurochem Int 2019; 125:127–35 [DOI] [PubMed] [Google Scholar]
- 236.Liu B, Hong J-S. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther 2003; 304:7. [DOI] [PubMed] [Google Scholar]
- 237.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord 2004; 10(Suppl 1):S3–7 [DOI] [PubMed] [Google Scholar]
- 238.Colonna M, Butovsky O. Microglia function in the Central nervous system during health and neurodegeneration. Annu Rev Immunol 2017; 35:441–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Nnah IC, Wessling-Resnick M. Brain iron homeostasis: a focus on microglial iron. Pharmaceuticals 2018; 11:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Uversky VN, Yamin G, Souillac PO, Goers J, Glaser CB, Fink AL. Methionine oxidation inhibits fibrillation of human alpha-synuclein in vitro. FEBS Lett 2002; 517:239–44 [DOI] [PubMed] [Google Scholar]
- 241.Yamin G, Glaser CB, Uversky VN, Fink AL. Certain metals trigger fibrillation of methionine-oxidized alpha-synuclein. J Biol Chem 2003; 278:27630–5 [DOI] [PubMed] [Google Scholar]
- 242.Zhou W, Long C, Reaney SH, Di Monte DA, Fink AL, Uversky VN. Methionine oxidation stabilizes non-toxic oligomers of alpha-synuclein through strengthening the auto-inhibitory intra-molecular long-range interactions. Biochim Biophys Acta 2010; 1802:322–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Glaser CB, Yamin G, Uversky VN, Fink AL. Methionine oxidation, alpha-synuclein and Parkinson’s disease. Biochim Biophys Acta 2005; 1703:157–69 [DOI] [PubMed] [Google Scholar]
- 244.Double KL, Halliday GM. New face of neuromelanin. J Neural Transm Suppl 2006; 70:119–23 [DOI] [PubMed] [Google Scholar]
- 245.Fasano M, Giraudo S, Coha S, Bergamasco B, Lopiano L. Residual substantia nigra neuromelanin in Parkinson’s disease is cross-linked to alpha-synuclein. Neurochem Int 2003; 42:603–6 [DOI] [PubMed] [Google Scholar]
- 246.Lu Y, Prudent M, Fauvet B, Lashuel HA, Girault HH. Phosphorylation of α-synuclein at Y125 and S129 alters its metal binding properties: implications for understanding the role of α-synuclein in the pathogenesis of Parkinson’s disease and related disorders. ACS Chem Neurosci 2011; 2:667–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Li W, Jiang H, Song N, Xie J. Oxidative stress partially contributes to iron-induced α-synuclein aggregation in SK-N-SH cells. Neurotox Res 2011; 19:435–42 [DOI] [PubMed] [Google Scholar]
- 248.Rogers JT, Mikkilineni S, Cantuti-Castelvetri I, Smith DH, Huang X, Bandyopadhyay S, Cahill CM, Maccecchini ML, Lahiri DK, Greig NH. The alpha-synuclein 5’untranslated region targeted translation blockers: anti-alpha synuclein efficacy of cardiac glycosides and posiphen. J Neural Transm 2011; 118:493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Xiao Y, Chen X, Huang S, Li G, Mo M, Zhang L, Chen C, Guo W, Zhou M, Wu Z, Cen L, Long S, Li S, Yang X, Qu S, Pei Z, Xu P. Iron promotes α-synuclein aggregation and transmission by inhibiting TFEB-mediated autophagosome-lysosome fusion. J Neurochem 2018; 145:34–50 [DOI] [PubMed] [Google Scholar]
- 250.Baksi S, Tripathi AK, Singh N. Alpha-synuclein modulates retinal iron homeostasis by facilitating the uptake of transferrin-bound iron: implications for visual manifestations of Parkinson’s disease. Free Radic Biol Med 2016; 97:292–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Vargas KJ, Makani S, Davis T, Westphal CH, Castillo PE, Chandra SS. Synucleins Regulate the kinetics of synaptic vesicle endocytosis. J Neurosci 2014; 34:9364–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Duce JA, Wong BX, Durham H, Devedjian J-C, Smith DP, Devos D. Post translational changes to α-synuclein control iron and dopamine trafficking; a concept for neuron vulnerability in Parkinson’s disease. Mol Neurodegener 2017; 12:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ortega R, Carmona A, Roudeau S, Perrin L, Dučić T, Carboni E, Bohic S, Cloetens P, Lingor P. α-Synuclein over-Expression induces increased iron accumulation and redistribution in Iron-Exposed neurons. Mol Neurobiol 2016; 53:1925–34 [DOI] [PubMed] [Google Scholar]
- 254.Angelova PR, Choi ML, Berezhnov AV, Horrocks MH, Hughes CD, De S, Rodrigues M, Yapom R, Little D, Dolt KS, Kunath T, Devine MJ, Gissen P, Shchepinov MS, Sylantyev S, Pavlov EV, Klenerman D, Abramov AY, Gandhi S. Alpha synuclein aggregation drives ferroptosis: an interplay of iron, calcium and lipid peroxidation. Cell Death Differ 2020. (in press). doi: 10.1038/s41418-020-0542-z [DOI] [PMC free article] [PubMed]
- 255.Cao J, Chen X, Jiang L, Lu B, Yuan M, Zhu D, Zhu H, He Q, Yang B, Ying M. DJ-1 suppresses ferroptosis through preserving the activity of S-adenosyl homocysteine hydrolase. Nat Commun 2020; 11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Roth JA, Singleton S, Feng J, Garrick M, Paradkar PN. Parkin regulates metal transport via proteasomal degradation of the 1B isoforms of divalent metal transporter 1. J Neurochem 2010; 113:454–64 [DOI] [PubMed] [Google Scholar]
- 257.Garrick MD, Zhao L, Roth JA, Jiang H, Feng J, Foot NJ, Dalton H, Kumar S, Garrick LM. Isoform specific regulation of divalent metal (ion) transporter (DMT1) by proteasomal degradation. Biometals 2012; 25:787–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhang C-W, Tai YK, Chai B-H, Chew KCM, Ang E-T, Tsang F, Tan BWQ, Hong ETE, Asad ABA, Chuang K-H, Lim K-L, Soong TW. Transgenic mice overexpressing the divalent metal transporter 1 exhibit iron accumulation and enhanced Parkin expression in the brain. Neuromolecular Med 2017; 19:375–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Moreau C, Duce JA, Rascol O, Devedjian J-C, Berg D, Dexter D, Cabantchik ZI, Bush AI, Devos D. Iron as a therapeutic target for Parkinson’s disease. Mov Disord 2018; 33:568–74 [DOI] [PubMed] [Google Scholar]
- 260.Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, Jonneaux A, Ryckewaert G, Garçon G, Rouaix N, Duhamel A, Jissendi P, Dujardin K, Auger F, Ravasi L, Hopes L, Grolez G, Firdaus W, Sablonnière B, Strubi-Vuillaume I, Zahr N, Destée A, Corvol J-C, Pöltl D, Leist M, Rose C, Defebvre L, Marchetti P, Cabantchik ZI, Bordet R. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid Redox Signal 2014; 21:195–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dexter DT, Statton SA, Whitmore C, Freinbichler W, Weinberger P, Tipton KF, Della Corte L, Ward RJ, Crichton RR. Clinically available iron chelators induce neuroprotection in the 6-OHDA model of Parkinson’s disease after peripheral administration. J Neural Transm 2011; 118:223–31 [DOI] [PubMed] [Google Scholar]
- 262.Carboni E, Tatenhorst L, Tönges L, Barski E, Dambeck V, Bähr M, Lingor P. Deferiprone rescues behavioral deficits induced by mild iron exposure in a mouse model of alpha-synuclein aggregation. Neuromolecular Med 2017; 19:309–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Devos D, Moreau C, Dujardin K, Cabantchik I, Defebvre L, Bordet R. New pharmacological options for treating advanced Parkinson’s disease. Clin Ther 2013; 35:1640–52 [DOI] [PubMed] [Google Scholar]
- 264.Grünblatt E, Mandel S, Gassen M, Youdim MB. Potent neuroprotective and antioxidant activity of apomorphine in MPTP and 6-hydroxydopamine induced neurotoxicity. J Neural Transm Suppl 1999; 55:57–70 [DOI] [PubMed] [Google Scholar]
- 265.Das B, Rajagopalan S, Joshi GS, Xu L, Luo D, Andersen JK, Todi SV, Dutta AK. A novel iron (II) preferring dopamine agonist chelator D-607 significantly suppresses α-syn- and MPTP-induced toxicities in vivo. Neuropharmacology 2017; 123:88–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Finkelstein DI, Billings JL, Adlard PA, Ayton S, Sedjahtera A, Masters CL, Wilkins S, Shackleford DM, Charman SA, Bal W, Zawisza IA, Kurowska E, Gundlach AL, Ma S, Bush AI, Hare DJ, Doble PA, Crawford S, Gautier EC, Parsons J, Huggins P, Barnham KJ, Cherny RA. The novel compound PBT434 prevents iron mediated neurodegeneration and alpha-synuclein toxicity in multiple models of Parkinson’s disease. Acta Neuropathol Commun 2017; 5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Finkelstein DI, Hare DJ, Billings JL, Sedjahtera A, Nurjono M, Arthofer E, George S, Culvenor JG, Bush AI, Adlard PA. Clioquinol improves cognitive, motor function, and microanatomy of the alpha-synuclein hA53T transgenic mice. ACS Chem Neurosci 2016; 7:119–29 [DOI] [PubMed] [Google Scholar]
- 268.Billings JL, Hare DJ, Nurjono M, Volitakis I, Cherny RA, Bush AI, Adlard PA, Finkelstein DI. Effects of neonatal iron feeding and chronic clioquinol administration on the parkinsonian human A53T transgenic mouse. ACS Chem Neurosci 2016; 7:360–6 [DOI] [PubMed] [Google Scholar]
- 269.Gotsbacher MP, Telfer TJ, Witting PK, Double KL, Finkelstein DI, Codd R. Analogues of desferrioxamine B designed to attenuate iron-mediated neurodegeneration: synthesis, characterisation and activity in the MPTP-mouse model of Parkinson’s disease. Metallomics 2017; 9:852–64 [DOI] [PubMed] [Google Scholar]
- 270.Aguirre P, García-Beltrán O, Tapia V, Muñoz Y, Cassels BK, Núñez MT. Neuroprotective effect of a new 7,8-dihydroxycoumarin-Based Fe2+/Cu2+ chelator in cell and animal models of Parkinson’s disease. ACS Chem Neurosci 2017; 8:178–85 [DOI] [PubMed] [Google Scholar]
- 271.Zhu Y, Wang B, Tao K, Yang H, Wang Y, Zhou T, Yang Y, Yuan L, Liu X, Duan Y. Iron accumulation and microglia activation contribute to substantia nigra hyperechogenicity in the 6-OHDA-induced rat model of Parkinson’s disease. Parkinsonism Relat Disord 2017; 36:76–82 [DOI] [PubMed] [Google Scholar]
- 272.Martin-Bastida A, Ward RJ, Newbould R, Piccini P, Sharp D, Kabba C, Patel MC, Spino M, Connelly J, Tricta F, Crichton RR, Dexter DT. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci Rep 2017; 7:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Grolez G, Moreau C, Sablonnière B, Garçon G, Devedjian J-C, Meguig S, Gelé P, Delmaire C, Bordet R, Defebvre L, Cabantchik IZ, Devos D. Ceruloplasmin activity and iron chelation treatment of patients with Parkinson’s disease. BMC Neurol 2015; 15:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Matak P, Matak A, Moustafa S, Aryal DK, Benner EJ, Wetsel W, Andrews NC. Disrupted iron homeostasis causes dopaminergic neurodegeneration in mice. Proc Natl Acad Sci U S A 2016; 113:3428–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ayton S, Zhang M, Roberts BR, Lam LQ, Lind M, McLean C, Bush AI, Frugier T, Crack PJ, Duce JA. Ceruloplasmin and β-amyloid precursor protein confer neuroprotection in traumatic brain injury and lower neuronal iron. Free Radic Biol Med 2014; 69:331–7 [DOI] [PubMed] [Google Scholar]
- 276.Ayton S, Lei P, Duce JA, Wong BXW, Sedjahtera A, Adlard PA, Bush AI, Finkelstein DI. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann Neurol 2013; 73:554–9 [DOI] [PubMed] [Google Scholar]
- 277.Ayton S, Lei P, Mclean C, Bush AI, Finkelstein DI. Transferrin protects against parkinsonian neurotoxicity and is deficient in Parkinson’s substantia nigra. Signal Transduct Target Ther 2016; 1:16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kaur D, Rajagopalan S, Chinta S, Kumar J, Di Monte D, Cherny RA, Andersen JK. Chronic ferritin expression within murine dopaminergic midbrain neurons results in a progressive age-related neurodegeneration. Brain Res 2007; 1140:188–94 [DOI] [PubMed] [Google Scholar]
- 279.Liang T, Qian Z-M, Mu M-D, Yung W-H, Ke Y. Brain hepcidin suppresses major pathologies in experimental parkinsonism. iScience 2020; 23:101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Du F, Qian Z-M, Luo Q, Yung W-H, Ke Y. Hepcidin suppresses brain iron accumulation by downregulating iron transport proteins in iron-overloaded rats. Mol Neurobiol 2015; 52:101–14 [DOI] [PubMed] [Google Scholar]
- 281.Zhou Y-F, Zhang C, Yang G, Qian Z-M, Zhang M-W, Ma J, Zhang F-L, Ke Y. Hepcidin protects neuron from hemin-mediated injury by reducing iron. Front Physiol 2017; 8:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Qian Z-M, Ke Y. Hepcidin and its therapeutic potential in neurodegenerative disorders. Med Res Rev 2019; 40:633–53 [DOI] [PubMed] [Google Scholar]
- 283.Vela D. The dual role of hepcidin in brain iron load and inflammation. Front Neurosci 2018; 12:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sharma A, Kaur P, Kumar V, Gill KD. Attenuation of 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine induced nigrostriatal toxicity in mice by N-acetyl cysteine. Cell Mol Biol 2007; 53:48–55 [PubMed] [Google Scholar]
- 285.Perry TL, Yong VW, Clavier RM, Jones K, Wright JM, Foulks JG, Wall RA. Partial protection from the dopaminergic neurotoxin N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by four different antioxidants in the mouse. Neurosci Lett 1985; 60:109–14 [DOI] [PubMed] [Google Scholar]
- 286.Offen D, Ziv I, Sternin H, Melamed E, Hochman A. Prevention of Dopamine-Induced cell death by thiol antioxidants: possible implications for treatment of Parkinson’s disease. Exp Neurol 1996; 141:32–9 [DOI] [PubMed] [Google Scholar]
- 287.Martínez-Banaclocha MA. N-acetyl-cysteine in the treatment of Parkinson’s disease. What are we waiting for? Med Hypoth 2012; 79:8–12 [DOI] [PubMed] [Google Scholar]
- 288.Monti DA, Zabrecky G, Kremens D, Liang T-W, Wintering NA, Bazzan AJ, Zhong L, Bowens BK, Chervoneva I, Intenzo C, Newberg AB. N-Acetyl cysteine is associated with dopaminergic improvement in Parkinson’s disease. Clin Pharmacol Ther 2019; 106:884–90 [DOI] [PubMed] [Google Scholar]
- 289.Selkoe D, Mandelkow E, Holtzman D. Deciphering Alzheimer disease. Cold Spring Harb Perspect Med 2012; 2:a011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 2016; 8:595–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Holtzman DM, Mandelkow E, Selkoe DJ. Alzheimer disease in 2020. Cold Spring Harb Perspect Med 2012;2:a011585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Iqbal K, Liu F, Gong C-X. Alzheimer disease therapeutics: focus on the disease and not just plaques and tangles. Biochem Pharmacol 2014; 88:631–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Reiss AB, Arain HA, Stecker MM, Siegart NM, Kasselman LJ. Amyloid toxicity in Alzheimer’s disease. Rev Neurosci 2018; 29:613–27 [DOI] [PubMed] [Google Scholar]
- 294.Chen X-Q, Mobley WC. Alzheimer disease pathogenesis: insights from molecular and cellular biology studies of oligomeric Aβ and tau species. Front Neurosci 2019; 13:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Guerreiro R, Hardy J. Genetics of Alzheimer’s disease. Neurotherapeutics 2014; 11:732–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Chouraki V, Seshadri S. Genetics of Alzheimer’s disease. Adv Genet 2014; 87:245–94 [DOI] [PubMed] [Google Scholar]
- 297.Wolfe CM, Fitz NF, Nam KN, Lefterov I, Koldamova R. The role of APOE and TREM2 in Alzheimer’s disease-current understanding and perspectives. Int J Mol Sci 2018; 20:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lane DJR, Ayton S, Bush AI. Iron and Alzheimer’s disease: an update on emerging mechanisms. J Alzheimers Dis 2018; 64:S379–95 [DOI] [PubMed] [Google Scholar]
- 299.Mandel S, Amit T, Bar-Am O, Youdim MBH. Iron dysregulation in Alzheimer’s disease: multimodal brain permeable iron chelating drugs, possessing neuroprotective-neurorescue and amyloid precursor protein-processing regulatory activities as therapeutic agents. Prog Neurobiol 2007; 82:348–60 [DOI] [PubMed] [Google Scholar]
- 300.Bush AI. The metal theory of Alzheimer’s disease. J Alzheimers Dis 2013; 33(Suppl 1):S277–281 [DOI] [PubMed] [Google Scholar]
- 301.Peters DG, Connor JR, Meadowcroft MD. The relationship between iron dyshomeostasis and amyloidogenesis in Alzheimer’s disease: two sides of the same coin. Neurobiol Dis 2015; 81:49–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ding B, Chen K-M, Ling H-W, Sun F, Li X, Wan T, Chai W-M, Zhang H, Zhan Y, Guan Y-J. Correlation of iron in the hippocampus with MMSE in patients with Alzheimer’s disease. J Magn Reson Imaging 2009; 29:793–8 [DOI] [PubMed] [Google Scholar]
- 303.Raven EP, Lu PH, Tishler TA, Heydari P, Bartzokis G. Increased iron levels and decreased tissue integrity in hippocampus of Alzheimer’s disease detected in vivo with magnetic resonance imaging. J Alzheimer Dis 2013; 37:127–36 [DOI] [PubMed] [Google Scholar]
- 304.Brar S, Henderson D, Schenck J, Zimmerman EA. Iron accumulation in the substantia nigra of patients with Alzheimer disease and parkinsonism. Arch Neurol 2009; 66:371–4 [DOI] [PubMed] [Google Scholar]
- 305.van Bergen JMG, Li X, Quevenco FC, Gietl AF, Treyer V, Meyer R, Buck A, Kaufmann PA, Nitsch RM, van Zijl PCM, Hock C, Unschuld PG. Simultaneous quantitative susceptibility mapping and Flutemetamol-PET suggests local correlation of iron and β-amyloid as an indicator of cognitive performance at high age. Neuroimage 2018; 174:308–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Raha AA, Vaishnav RA, Friedland RP, Bomford A, Raha-Chowdhury R. The systemic iron-regulatory proteins hepcidin and ferroportin are reduced in the brain in Alzheimer’s disease. Acta Neuropathol Commun 2013; 1:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.van Duijn S, Bulk M, van Duinen SG, Nabuurs RJA, van Buchem MA, van der Weerd L, Natté R. Cortical iron reflects severity of Alzheimer’s disease. J Alzheimer Dis 2017; 60:1533–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ayton S, Wang Y, Diouf I, Schneider JA, Brockman J, Morris MC, Bush AI. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology Mol Psychiatry 2019. doi: 10.1038/s41380-019-0375-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Gong N-J, Dibb R, Bulk M, van der Weerd L, Liu C. Imaging beta amyloid aggregation and iron accumulation in Alzheimer’s disease using quantitative susceptibility mapping MRI. Neuroimage 2019; 191:176–85 [DOI] [PubMed] [Google Scholar]
- 310.Du L, Zhao Z, Cui A, Zhu Y, Zhang L, Liu J, Shi S, Fu C, Han X, Gao W, Song T, Xie L, Wang L, Sun S, Guo R, Ma G. Increased iron deposition on brain quantitative susceptibility mapping correlates with decreased cognitive function in Alzheimer’s disease. ACS Chem Neurosci 2018; 9:1849–57 [DOI] [PubMed] [Google Scholar]
- 311.Bulk M, Abdelmoula WM, Nabuurs RJA, van der Graaf LM, Mulders CWH, Mulder AA, Jost CR, Koster AJ, van Buchem MA, Natté R, Dijkstra J, van der Weerd L. Postmortem MRI and histology demonstrate differential iron accumulation and cortical myelin organization in early- and late-onset Alzheimer’s disease. Neurobiol Aging 2018; 62:231–42 [DOI] [PubMed] [Google Scholar]
- 312.Kenkhuis B, Jonkman LE, Bulk M, Buijs M, Boon BDC, Bouwman FH, Geurts JJG, van de Berg WDJ, van der Weerd L. 7T MRI allows detection of disturbed cortical lamination of the medial temporal lobe in patients with Alzheimer’s disease. Neuroimage Clin 2019; 21:101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Diouf I, Fazlollahi A, Bush AI, Ayton S, Alzheimer’s disease Neuroimaging Initiative. Cerebrospinal fluid ferritin levels predict brain hypometabolism in people with underlying β-amyloid pathology. Neurobiol Dis 2019; 124:335–9 [DOI] [PubMed] [Google Scholar]
- 314.Ayton S, Diouf I, Bush AI, Alzheimer’s disease Neuroimaging Initiative. Evidence that iron accelerates Alzheimer’s pathology: a CSF biomarker study. J Neurol Neurosurg Psychiatry 2018; 89:456–60 [DOI] [PubMed] [Google Scholar]
- 315.Ayton S, Faux NG, Bush AI. Association of cerebrospinal fluid ferritin level with preclinical cognitive decline in APOE-ε4 carriers. JAMA Neurol 2017; 74:122–5 [DOI] [PubMed] [Google Scholar]
- 316.Ayton S, Faux NG, Bush AI, Alzheimer’s Disease Neuroimaging Initiative. Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun 2015; 6:6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kagerer SM, van Bergen JMG, Li X, Quevenco FC, Gietl AF, Studer S, Treyer V, Meyer R, Kaufmann PA, Nitsch RM, van Zijl PCM, Hock C, Unschuld PG. APOE4 moderates effects of cortical iron on synchronized default mode network activity in cognitively healthy old-aged adults. Alzheimers Dement 2020; 12:e12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Li X, Lei P, Tuo Q, Ayton S, Li Q-X, Moon S, Volitakis I, Liu R, Masters CL, Finkelstein DI, Bush AI. Enduring elevations of hippocampal amyloid precursor protein and iron are features of β-Amyloid toxicity and are mediated by tau. Neurotherapeutics 2015; 12:862–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Gerhardsson L, Lundh T, Londos E, Minthon L. Cerebrospinal fluid/plasma quotients of essential and non-essential metals in patients with Alzheimer’s disease. J Neural Transm 2011; 118:957–62 [DOI] [PubMed] [Google Scholar]
- 320.Gerhardsson L, Blennow K, Lundh T, Londos E, Minthon L. Concentrations of metals, beta-amyloid and tau-markers in cerebrospinal fluid in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 2009; 28:88–94 [DOI] [PubMed] [Google Scholar]
- 321.Hozumi I, Hasegawa T, Honda A, Ozawa K, Hayashi Y, Hashimoto K, Yamada M, Koumura A, Sakurai T, Kimura A, Tanaka Y, Satoh M, Inuzuka T. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J Neurol Sci 2011; 303:95–9 [DOI] [PubMed] [Google Scholar]
- 322.Lavados M, Guillón M, Mujica MC, Rojo LE, Fuentes P, Maccioni RB. Mild cognitive impairment and alzheimer patients display different levels of redox-active CSF iron. J Alzheimers Dis 2008; 13:225–32 [DOI] [PubMed] [Google Scholar]
- 323.Molina JA, Jiménez-Jiménez FJ, Aguilar MV, Meseguer I, Mateos-Vega CJ, González-Muñoz MJ, de Bustos F, Porta J, Ortí-Pareja M, Zurdo M, Barrios E, Martínez-Para MC. Cerebrospinal fluid levels of transition metals in patients with Alzheimer’s disease. J Neural Transm 1998; 105:479–88 [DOI] [PubMed] [Google Scholar]
- 324.Diouf I, Bush AI, Ayton S, Alzheimer’s disease Neuroimaging Initiative. Cerebrospinal fluid ceruloplasmin levels predict cognitive decline and brain atrophy in people with underlying β-amyloid pathology. Neurobiol Dis 2020; 139:104810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Rogers JT, Randall JD, Cahill CM, Eder PS, Huang X, Gunshin H, Leiter L, McPhee J, Sarang SS, Utsuki T, Greig NH, Lahiri DK, Tanzi RE, Bush AI, Giordano T, Gullans SR. An iron-responsive element type II in the 5’-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J Biol Chem 2002; 277:45518–28 [DOI] [PubMed] [Google Scholar]
- 326.Greenough MA, Camakaris J, Bush AI. Metal dyshomeostasis and oxidative stress in Alzheimer’s disease. Neurochem Int 2013; 62:540–55 [DOI] [PubMed] [Google Scholar]
- 327.Belaidi AA, Gunn AP, Wong BX, Ayton S, Appukuttan AT, Roberts BR, Duce JA, Bush AI. Marked age-related changes in brain iron homeostasis in amyloid protein precursor knockout mice. Neurotherapeutics 2018; 15:1055–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, Leong SL, Perez K, Johanssen T, Greenough MA, Cho H-H, Galatis D, Moir RD, Masters CL, McLean C, Tanzi RE, Cappai R, Barnham KJ, Ciccotosto GD, Rogers JT, Bush AI. Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell 2010; 142:857–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.McCarthy RC, Park Y-H, Kosman DJ. sAPP modulates iron efflux from brain microvascular endothelial cells by stabilizing the ferrous iron exporter ferroportin. EMBO Rep 2014; 15:809–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Lahiri DK, Maloney B, Wang R. APPealing for a role in cellular iron efflux. J Biol Chem 2019; 294:9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Xian-Hui D, Wei-Juan G, Tie-Mei S, Hong-Lin X, Jiang-Tao B, Jing-Yi Z, Xi-Qing C. Age-related changes of brain iron load changes in the frontal cortex in APPswe/PS1ΔE9 transgenic mouse model of Alzheimer’s disease. J Trace Elem Med Biol 2015; 30:118–23 [DOI] [PubMed] [Google Scholar]
- 332.Tsatsanis A, Dickens S, Kwok JCF, Wong BX, Duce JA. Post translational modulation of β-amyloid precursor protein trafficking to the cell surface alters neuronal iron homeostasis. Neurochem Res 2019; 44:1367–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Bai B, Wang X, Li Y, Chen P-C, Yu K, Dey KK, Yarbro JM, Han X, Lutz BM, Rao S, Jiao Y, Sifford JM, Han J, Wang M, Tan H, Shaw TI, Cho J-H, Zhou S, Wang H, Niu M, Mancieri A, Messler KA, Sun X, Wu Z, Pagala P, High AA, Bi W, Zhang H, Chi H, Haroutunian V, Zhang B, Beach TG, Yu G, Peng J. Deep multilayer brain proteomics identifies molecular networks in Alzheimer’s disease progression. Neuron 2020; 105:975–91.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Tsatsanis A, Wong BX, Gunn AP, Ayton S, Bush AI, Devos D, Duce JA. Amyloidogenic processing of Alzheimer’s disease β-amyloid precursor protein induces cellular iron retention. Mol Psychiatry 2020. doi: 10.1038/s41380-020-0762-0 [DOI] [PubMed] [Google Scholar]
- 335.Dekens DW, De Deyn PP, Sap F, Eisel ULM, Naudé PJW. Iron chelators inhibit amyloid-β-induced production of lipocalin 2 in cultured astrocytes. Neurochem Int 2020; 132:104607. [DOI] [PubMed] [Google Scholar]
- 336.Verkhratsky A, Parpura V, Rodriguez-Arellano JJ, Zorec R. Astroglia in Alzheimer’s disease. Adv Exp Med Biol 2019; 1175:273–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Trujillo-Estrada L, Gomez-Arboledas A, Forner S, Martini AC, Gutierrez A, Baglietto-Vargas D, LaFerla FM. Astrocytes: from the physiology to the disease. Curr Alzheimer Res 2019; 16:675–98 [DOI] [PubMed] [Google Scholar]
- 338.Liang Z, Valla J, Sefidvash‐Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer’s disease patients. J Neurochem 2002; 80:807–14 [DOI] [PubMed] [Google Scholar]
- 339.McIntosh A, Mela V, Harty C, Minogue AM, Costello DA, Kerskens C, Lynch MA. Iron accumulation in microglia triggers a Cascade of events that leads to altered metabolism and compromised function in APP/PS1 mice. Brain Pathol 2019; 29:606–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Tiepolt S, Schäfer A, Rullmann M, Roggenhofer E, Netherlands Brain Bank Gertz H-J, Schroeter ML, Patt M, Bazin P-L, Jochimsen TH, Turner R, Sabri O, Barthel H. Quantitative susceptibility mapping of amyloid-β aggregates in Alzheimer’s disease with 7T MR. J Alzheimer Dis 2018; 64:393–404 [DOI] [PubMed] [Google Scholar]
- 341.Urrutia PJ, Hirsch EC, González-Billault C, Núñez MT. Hepcidin attenuates amyloid beta-induced inflammatory and pro-oxidant responses in astrocytes and microglia. J Neurochem 2017; 142:140–52 [DOI] [PubMed] [Google Scholar]
- 342.Ahmadi S, Wu B, Song R, Zhu S, Simpson A, Wilson DJ, Kraatz H-B. Exploring the interactions of iron and zinc with the microtubule binding repeats R1 and R4. J Inorg Biochem 2019; 205:110987. [DOI] [PubMed] [Google Scholar]
- 343.Ahmadi S, Zhu S, Sharma R, Wilson DJ, Kraatz H-B. Interaction of metal ions with tau protein. The case for a metal-mediated tau aggregation. J Inorg Biochem 2019; 194:44–51 [DOI] [PubMed] [Google Scholar]
- 344.Everett J, Céspedes E, Shelford LR, Exley C, Collingwood JF, Dobson J, van der Laan G, Jenkins CA, Arenholz E, Telling ND. Ferrous iron formation following the co-aggregation of ferric iron and the Alzheimer’s disease peptide β-amyloid (1-42). J R Soc Interface 2014; 11:20140165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Kim AC, Lim S, Kim YK. Metal ion effects on Aβ and tau aggregation. Int J Mol Sci 2018; 19:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Telling ND, Everett J, Collingwood JF, Dobson J, van der Laan G, Gallagher JJ, Wang J, Hitchcock AP. Iron biochemistry is correlated with amyloid plaque morphology in an established mouse model of Alzheimer’s disease. Cell Chem Biol 2017; 24:1205–15.e3 [DOI] [PubMed] [Google Scholar]
- 347.Yamamoto A, Shin R-W, Hasegawa K, Naiki H, Sato H, Yoshimasu F, Kitamoto T. Iron (III) induces aggregation of hyperphosphorylated τ and its reduction to iron (II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer’s disease. J Neurochem 2002; 82:1137–47 [DOI] [PubMed] [Google Scholar]
- 348.Good PF, Perl DP, Bierer LM, Schmeidler J. Selective accumulation of aluminum and iron in the neurofibrillary tangles of Alzheimer’s disease: a laser microprobe (LAMMA) study. Ann Neurol 1992; 31:286–92 [DOI] [PubMed] [Google Scholar]
- 349.Meadowcroft MD, Connor JR, Smith MB, Yang QX. MRI and histological analysis of beta-amyloid plaques in both human Alzheimer’s disease and APP/PS1 transgenic mice. J Magn Reson Imaging 2009; 29:997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A 1997; 94:9866–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Plascencia-Villa G, Ponce A, Collingwood JF, Arellano-Jiménez MJ, Zhu X, Rogers JT, Betancourt I, José-Yacamán M, Perry G. High-resolution analytical imaging and electron holography of magnetite particles in amyloid cores of Alzheimer’s disease. Sci Rep 2016; 6:24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.van Bergen JMG, Li X, Hua J, Schreiner SJ, Steininger SC, Quevenco FC, Wyss M, Gietl AF, Treyer V, Leh SE, Buck F, Nitsch RM, Pruessmann KP, van Zijl PCM, Hock C, Unschuld PG. Colocalization of cerebral iron with. Sci Rep 2016; 6:35514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Rottkamp CA, Raina AK, Zhu X, Gaier E, Bush AI, Atwood CS, Chevion M, Perry G, Smith MA. Redox-active iron mediates amyloid-beta toxicity. Free Radic Biol Med 2001; 30:447–50 [DOI] [PubMed] [Google Scholar]
- 354.Bader B, Nübling G, Mehle A, Nobile S, Kretzschmar H, Giese A. Single particle analysis of tau oligomer formation induced by metal ions and organic solvents. Biochem Biophys Res Commun 2011; 411:190–6 [DOI] [PubMed] [Google Scholar]
- 355.Galante D, Cavallo E, Perico A, DC. Effect of ferric citrate on amyloid-beta peptides behavior. Biopolymers 2018; 109:e23224. [DOI] [PubMed] [Google Scholar]
- 356.Soeda Y, Yoshikawa M, Almeida OFX, Sumioka A, Maeda S, Osada H, Kondoh Y, Saito A, Miyasaka T, Kimura T, Suzuki M, Koyama H, Yoshiike Y, Sugimoto H, Ihara Y, Takashima A. Toxic tau oligomer formation blocked by capping of cysteine residues with 1,2-dihydroxybenzene groups. Nat Commun 2015; 6:10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Sahara N, Maeda S, Murayama M, Suzuki T, Dohmae N, Yen S-H, Takashima A. Assembly of two distinct dimers and higher-order oligomers from full-length tau. Eur J Neurosci 2007; 25:3020–9 [DOI] [PubMed] [Google Scholar]
- 358.Rao SS, Adlard PA. Untangling tau and iron: exploring the interaction between iron and tau in neurodegeneration. Front Mol Neurosci 2018; 11:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Wan W, Cao L, Kalionis B, Murthi P, Xia S, Guan Y. Iron deposition leads to hyperphosphorylation of tau and disruption of insulin signaling. Front Neurol 2019; 10:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BXW, Adlard PA, Cherny RA, Lam LQ, Roberts BR, Volitakis I, Egan GF, McLean CA, Cappai R, Duce JA, Bush AI. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med 2012; 18:291–5 [DOI] [PubMed] [Google Scholar]
- 361.Baloyannis SJ. Mitochondrial alterations in Alzheimer’s disease. J Alzheimers Dis 2006; 9:119–26 [DOI] [PubMed] [Google Scholar]
- 362.Hofer T, Perry G. Nucleic acid oxidative damage in Alzheimer’s disease-explained by the hepcidin-ferroportin neuronal iron overload hypothesis? J Trace Elem Med Biol 2016; 38:1–9 [DOI] [PubMed] [Google Scholar]
- 363.Praticò D. Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal. Trends Pharmacol Sci 2008; 29:609–15 [DOI] [PubMed] [Google Scholar]
- 364.Peña-Bautista C, Tirle T, López-Nogueroles M, Vento M, Baquero M, Cháfer-Pericás C. Oxidative damage of DNA as early marker of Alzheimer’s disease. Int J Mol Sci 2019; 20:6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Balejcikova L, Siposova K, Kopcansky P, Safarik I. Fe(II) formation after interaction of the amyloid β-peptide with iron-storage protein ferritin. J Biol Phys 2018; 44:237–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Everett J, Brooks J, Lermyte F, O’Connor PB, Sadler PJ, Dobson J, Collingwood JF, Telling ND. Iron stored in ferritin is chemically reduced in the presence of aggregating Aβ(1-42). Sci Rep 2020; 10:10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Everett J, Céspedes E, Shelford LR, Exley C, Collingwood JF, Dobson J, van der Laan G, Jenkins CA, Arenholz E, Telling ND. Evidence of redox-active iron formation following aggregation of ferrihydrite and the Alzheimer’s disease peptide β-amyloid. Inorg Chem 2014; 53:2803–9 [DOI] [PubMed] [Google Scholar]
- 368.Merlo D, Mollinari C, Racaniello M, Garaci E, Cardinale A. DNA double strand breaks: a common theme in neurodegenerative diseases. Curr Alzheimer Res 2016; 13:1208–18 [DOI] [PubMed] [Google Scholar]
- 369.Shichiri M. The role of lipid peroxidation in neurological disorders. J Clin Biochem Nutr 2014; 54:151–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Derry PJ, Hegde ML, Jackson GR, Kayed R, Tour JM, Tsai A-L, Kent TA. Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer’s disease from a ferroptosis perspective. Prog Neurobiol 2020; 184:101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Cong L, Dong X, Wang Y, Deng Y, Li B, Dai R. On the role of synthesized hydroxylated chalcones as dual functional amyloid-β aggregation and ferroptosis inhibitors for potential treatment of Alzheimer’s disease. Eur J Med Chem 2019; 166:11–21 [DOI] [PubMed] [Google Scholar]
- 372.Weiland A, Wang Y, Wu W, Lan X, Han X, Li Q, Wang J. Ferroptosis and its role in diverse brain diseases. Mol Neurobiol 2019; 56:4880–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Yan N, Zhang J. Iron metabolism, ferroptosis, and the links with Alzheimer’s disease. Front Neurosci 2019; 13:1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ashraf A, Jeandriens J, Parkes HG, So P-W. Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer’s disease: evidence of ferroptosis. Redox Biol 2020; 32:101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Butt AM, De La Rocha IC, Rivera A. Oligodendroglial cells in Alzheimer’s disease. Adv Exp Med Biol 2019; 1175:325–33 [DOI] [PubMed] [Google Scholar]
- 376.Tse K-H, Herrup K. DNA damage in the oligodendrocyte lineage and its role in brain aging. Mech Ageing Dev 2017; 161:37–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Tse K-H, Cheng A, Ma F, Herrup K. DNA damage-associated oligodendrocyte degeneration precedes amyloid pathology and contributes to Alzheimer’s disease and dementia. Alzheimers Dement 2018; 14:664–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Kell DB, Pretorius E. No effects without causes: the iron dysregulation and dormant microbes hypothesis for chronic, inflammatory diseases. Biol Rev Camb Philos Soc 2018; 93:1518–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Pretorius E, Bester J, Kell DB. A bacterial component to Alzheimer’s-Type dementia seen via a systems biology approach that links iron dysregulation and inflammagen shedding to disease. J Alzheimers Dis 2016; 53:1237–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Pretorius L, Kell DB, Pretorius E. Iron dysregulation and dormant microbes as causative agents for impaired blood rheology and pathological clotting in Alzheimer’s type dementia. Front Neurosci 2018; 12:851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Adams B, Nunes JM, Page MJ, Roberts T, Carr J, Nell TA, Kell DB, Pretorius E. Parkinson’s disease: a systemic inflammatory disease accompanied by bacterial inflammagens. Front Aging Neurosci 2019; 11:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Olsen I, Kell DB, Pretorius E. Is Porphyromonas gingivalis involved in Parkinson’s disease?. Eur J Clin Microbiol Infect Dis 2020. doi: 10.1007/s10096-020-03944-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014; 6:748–73 [DOI] [PubMed] [Google Scholar]
- 384.Adlard PA, Bush AI. Metal chaperones: a holistic approach to the treatment of Alzheimer’s disease. Front Psychiatry 2012; 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Budimir A. Metal ions, Alzheimer’s disease and chelation therapy. Acta Pharm 2011; 61:1–14 [DOI] [PubMed] [Google Scholar]
- 386.Tahmasebinia F, Emadi S. Effect of metal chelators on the aggregation of beta-amyloid peptides in the presence of copper and iron. Biometals 2017; 30:285–93 [DOI] [PubMed] [Google Scholar]
- 387.Murayama H, Shin RW, Higuchi J, Shibuya S, Muramoto T, Kitamoto T. Interaction of aluminum with PHFtau in Alzheimer’s disease neurofibrillary degeneration evidenced by desferrioxamine-assisted chelating autoclave method. Am J Pathol 1999; 155:877–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Shin R-W, Kruck TPA, Murayama H, Kitamoto T. A novel trivalent cation chelator feralex dissociates binding of aluminum and iron associated with hyperphosphorylated tau of Alzheimer’s disease. Brain Res 2003; 961:139–46 [DOI] [PubMed] [Google Scholar]
- 389.Fine JM, Renner DB, Forsberg AC, Cameron RA, Galick BT, Le C, Conway PM, Stroebel BM, Frey WH, Hanson LR. Intranasal deferoxamine engages multiple pathways to decrease memory loss in the APP/PS1 model of amyloid accumulation. Neurosci Lett 2015; 584:362–7 [DOI] [PubMed] [Google Scholar]
- 390.Guo C, Wang P, Zhong M-L, Wang T, Huang X-S, Li J-Y, Wang Z-Y. Deferoxamine inhibits iron induced hippocampal tau phosphorylation in the Alzheimer transgenic mouse brain. Neurochem Int 2013; 62:165–72 [DOI] [PubMed] [Google Scholar]
- 391.Guo C, Wang T, Zheng W, Shan Z-Y, Teng W-P, Wang Z-Y. Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer’s disease. Neurobiol Aging 2013; 34:562–75 [DOI] [PubMed] [Google Scholar]
- 392.Fine JM, Baillargeon AM, Renner DB, Hoerster NS, Tokarev J, Colton S, Pelleg A, Andrews A, Sparley KA, Krogh KM, Frey WH, Hanson LR. Intranasal deferoxamine improves performance in radial arm water maze, stabilizes HIF-1α, and phosphorylates GSK3β in P301L tau transgenic mice. Exp Brain Res 2012; 219:381–90 [DOI] [PubMed] [Google Scholar]
- 393.Zhang Y, He M-L. Deferoxamine enhances alternative activation of microglia and inhibits amyloid beta deposits in APP/PS1 mice. Brain Res 2017; 1677:86–92 [DOI] [PubMed] [Google Scholar]
- 394.Salkovic-Petrisic M, Knezovic A, Osmanovic-Barilar J, Smailovic U, Trkulja V, Riederer P, Amit T, Mandel S, Youdim MBH. Multi-target iron-chelators improve memory loss in a rat model of sporadic Alzheimer’s disease. Life Sci 2015; 136:108–19 [DOI] [PubMed] [Google Scholar]
- 395.Rival T, Page RM, Chandraratna DS, Sendall TJ, Ryder E, Liu B, Lewis H, Rosahl T, Hider R, Camargo LM, Shearman MS, Crowther DC, Lomas DA. Fenton chemistry and oxidative stress mediate the toxicity of the beta-amyloid peptide in a drosophila model of Alzheimer’s disease. Eur J Neurosci 2009; 29:1335–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.C, McLachlan DR, Dalton AJ, Kruck TP, Bell MY, Smith WL, Kalow W, Andrews DF. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 1991; 337:1304–8 [DOI] [PubMed] [Google Scholar]
- 397.Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li Q-X, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol 2003; 60:1685–91 [DOI] [PubMed] [Google Scholar]
- 398.Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, Ritchie CW. PBT2-201-EURO study group. Safety, efficacy, and biomarker findings of PBT2 in targeting abeta as a modifying therapy for Alzheimer’s disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol 2008; 7:779–86 [DOI] [PubMed] [Google Scholar]
- 399.Huang J, Chen S, Hu L, Niu H, Sun Q, Li W, Tan G, Li J, Jin L, Lyu J, Zhou H. Mitoferrin-1 is involved in the progression of Alzheimer’s disease through targeting mitochondrial iron metabolism in a Caenorhabditis elegans model of Alzheimer’s disease. Neuroscience 2018; 385:90–101 [DOI] [PubMed] [Google Scholar]
- 400.Levi S, Tiranti V. Neurodegeneration with brain iron accumulation disorders: valuable models aimed at understanding the pathogenesis of iron deposition. Pharmaceuticals 2019; 12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Levi S, Cozzi A, Santambrogio P. Iron pathophysiology in neurodegeneration with brain iron accumulation. Adv Exp Med Biol 2019; 1173:153–77 [DOI] [PubMed] [Google Scholar]
- 402.Hayflick SJ, Kurian MA, Hogarth P. Neurodegeneration with brain iron accumulation. Handb Clin Neurol 2018; 147:293–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Wang Z-B, Liu J-Y, Xu X-J, Mao X-Y, Zhang W, Zhou H-H, Liu Z-Q. Neurodegeneration with brain iron accumulation: insights into the mitochondria dysregulation. Biomed Pharmacother 2019; 118:109068. [DOI] [PubMed] [Google Scholar]
- 404.Costain G, Ghosh MC, Maio N, Carnevale A, Si YC, Rouault TA, Yoon G. Absence of iron-responsive element-binding protein 2 causes a novel neurodegenerative syndrome. Brain 2019; 142:1195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Marchi G, Busti F, Zidanes LA, Castagna A, Girelli D. Aceruloplasminemia: a severe neurodegenerative disorder deserving an early diagnosis. Front Neurosci 2019; 13:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Piperno A, Alessio M. Aceruloplasminemia: waiting for an efficient therapy. Front Neurosci 2018; 12:903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Pelucchi S, Mariani R, Ravasi G, Pelloni I, Marano M, Tremolizzo L, Alessio M, Piperno A. Phenotypic heterogeneity in seven italian cases of aceruloplasminemia. Parkinsonism Relat Disord 2018; 51:36–42 [DOI] [PubMed] [Google Scholar]
- 408.Kono S. Aceruloplasminemia: an update. Int Rev Neurobiol 2013; 110:125–51 [DOI] [PubMed] [Google Scholar]
- 409.Kono S. Aceruloplasminemia. Curr Drug Targets 2012; 13:1190–9 [DOI] [PubMed] [Google Scholar]
- 410.Kono S, Yoshida K, Tomosugi N, Terada T, Hamaya Y, Kanaoka S, Miyajima H. Biological effects of mutant ceruloplasmin on hepcidin-mediated internalization of ferroportin. Biochim Biophys Acta 2010; 1802:968–75 [DOI] [PubMed] [Google Scholar]
- 411.Zanardi A, Conti A, Cremonesi M, D’Adamo P, Gilberti E, Apostoli P, Cannistraci CV, Piperno A, David S, Alessio M. Ceruloplasmin replacement therapy ameliorates neurological symptoms in a preclinical model of aceruloplasminemia. EMBO Mol Med 2018; 10:91–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Kaneko K, Yoshida K, Arima K, Ohara S, Miyajima H, Kato T, Ohta M, Ikeda S. Astrocytic deformity and globular structures are characteristic of the brains of patients with aceruloplasminemia. J Neuropathol Exp Neurol 2002; 61:1069–77 [DOI] [PubMed] [Google Scholar]
- 413.Oide T, Yoshida K, Kaneko K, Ohta M, Arima K. Iron overload and antioxidative role of perivascular astrocytes in aceruloplasminemia. Neuropathol Appl Neurobiol 2006; 32:170–6 [DOI] [PubMed] [Google Scholar]
- 414.Kaneko K, Hineno A, Yoshida K, Ohara S, Morita H, Ikeda S. Extensive brain pathology in a patient with aceruloplasminemia with a prolonged duration of illness. Hum Pathol 2012; 43:451–6 [DOI] [PubMed] [Google Scholar]
- 415.Breuer W, Hershko C, Cabantchik ZI. The importance of non-transferrin bound iron in disorders of iron metabolism. Transfus Sci 2000; 23:185–92 [DOI] [PubMed] [Google Scholar]
- 416.Wang B, Wang X-P. Does ceruloplasmin defend against neurodegenerative diseases? Curr Neuropharmacol 2019; 17:539–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Ryan F, Zarruk JG, Lößlein L, David S. Ceruloplasmin plays a neuroprotective role in cerebral ischemia. Front Neurosci 2018; 12:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Zhao Y-S, Zhang L-H, Yu P-P, Gou Y-J, Zhao J, You L-H, Wang Z-Y, Zheng X, Yan L-J, Yu P, Chang Y-Z. Ceruloplasmin, a potential therapeutic agent for Alzheimer’s disease. Antioxid Redox Signal 2018; 28:1323–37 [DOI] [PubMed] [Google Scholar]
- 419.Hineno A, Kaneko K, Yoshida K, Ikeda S. Ceruloplasmin protects against rotenone-induced oxidative stress and neurotoxicity. Neurochem Res 2011; 36:2127–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Rathore KI, Kerr BJ, Redensek A, López-Vales R, Jeong SY, Ponka P, David S. Ceruloplasmin protects injured spinal cord from iron-mediated oxidative damage. J Neurosci 2008; 28:12736–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Shin E-J, Jeong JH, Chung CK, Kim D-J, Wie M-B, Park ES, Chung YH, Nam Y, Tran T-V, Lee SY, Kim H-J, Ong W-Y, Kim H-C. Ceruloplasmin is an endogenous protectant against kainate neurotoxicity. Free Radic Biol Med 2015; 84:355–72 [DOI] [PubMed] [Google Scholar]
- 422.Inoue K, Akaike T, Miyamoto Y, Okamoto T, Sawa T, Otagiri M, Suzuki S, Yoshimura T, Maeda H. Nitrosothiol formation catalyzed by ceruloplasmin. Implication for cytoprotective mechanism in vivo. J Biol Chem 1999; 274:27069–75 [DOI] [PubMed] [Google Scholar]
- 423.Boero L, Cuniberti L, Magnani N, Manavela M, Yapur V, Bustos M, Gómez Rosso L, Meroño T, Marziali L, Viale L, Evelson P, Negri G, Brites F. Increased oxidized low density lipoprotein associated with high ceruloplasmin activity in patients with active acromegaly. Clin Endocrinol 2010; 72:654–60 [DOI] [PubMed] [Google Scholar]
- 424.di Patti MCB, Maio N, Rizzo G, De Francesco G, Persichini T, Colasanti M, Polticelli F, Musci G. Dominant mutants of ceruloplasmin impair the copper loading machinery in aceruloplasminemia. J Biol Chem 2009; 284:4545–54 [DOI] [PubMed] [Google Scholar]
- 425.Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci U S A 1999; 96:10812–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Jeong SY, David S. Age-related changes in iron homeostasis and cell death in the cerebellum of ceruloplasmin-deficient mice. J Neurosci 2006; 26:9810–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Vroegindeweij LHP, Boon AJW, Wilson JHP, Langendonk JG. Effects of iron chelation therapy on the clinical course of aceruloplasminemia: an analysis of aggregated case reports. Orphanet J Rare Dis 2020; 15:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Yonekawa M, Okabe T, Asamoto Y, Ohta M. A case of hereditary ceruloplasmin deficiency with iron deposition in the brain associated with chorea, dementia, diabetes mellitus and retinal pigmentation: administration of fresh-frozen human plasma. Eur Neurol 1999; 42:157–62 [DOI] [PubMed] [Google Scholar]
- 429.Poli L, Alberici A, Buzzi P, Marchina E, Lanari A, Arosio C, Ciccone A, Semeraro F, Gasparotti R, Padovani A, Borroni B. Is aceruloplasminemia treatable? Combining iron chelation and fresh-frozen plasma treatment. Neurol Sci 2017; 38:357–60 [DOI] [PubMed] [Google Scholar]
- 430.Curtis AR, Fey C, Morris CM, Bindoff LA, Ince PG, Chinnery PF, Coulthard A, Jackson MJ, Jackson AP, McHale DP, Hay D, Barker WA, Markham AF, Bates D, Curtis A, Burn J. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet 2001; 28:350–4 [DOI] [PubMed] [Google Scholar]
- 431.Luscieti S, Santambrogio P, Langlois d’Estaintot B, Granier T, Cozzi A, Poli M, Gallois B, Finazzi D, Cattaneo A, Levi S, Arosio P. Mutant ferritin L-chains that cause neurodegeneration act in a dominant-negative manner to reduce ferritin iron incorporation. J Biol Chem 2010; 285:11948–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.McNally JR, Mehlenbacher MR, Luscieti S, Smith GL, Reutovich AA, Maura P, Arosio P. Bou-Abdallah F. Mutant L-chain ferritins that cause neuroferritinopathy alter ferritin functionality and iron permeability. Metallomics 2019; 11:1635–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Chinnery PF, Crompton DE, Birchall D, Jackson MJ, Coulthard A, Lombès A, Quinn N, Wills A, Fletcher N, Mottershead JP, Cooper P, Kellett M, Bates D, Burn J. Clinical features and natural history of neuroferritinopathy caused by the FTL1 460InsA mutation. Brain 2007; 130:110–9 [DOI] [PubMed] [Google Scholar]
- 434.Devos D, Tchofo PJ, Vuillaume I, Destée A, Batey S, Burn J, Chinnery PF. Clinical features and natural history of neuroferritinopathy caused by the 458dupA FTL mutation. Brain 2009; 132:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Mancuso M, Davidzon G, Kurlan RM, Tawil R, Bonilla E, Di Mauro S, Powers JM. Hereditary ferritinopathy: a novel mutation, its cellular pathology, and pathogenetic insights. J Neuropathol Exp Neurol 2005; 64:280–94 [DOI] [PubMed] [Google Scholar]
- 436.Kumar N, Rizek P, Jog M. Neuroferritinopathy: pathophysiology, presentation, differential diagnoses and management. Tremor Other Hyperkinet Mov 2016; 6:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Maccarinelli F, Pagani A, Cozzi A, Codazzi F, Di Giacomo G, Capoccia S, Rapino S, Finazzi D, Politi LS, Cirulli F, Giorgio M, Cremona O, Grohovaz F, Levi S. A novel neuroferritinopathy mouse model (FTL 498InsTC) shows progressive brain iron dysregulation, morphological signs of early neurodegeneration and motor coordination deficits. Neurobiol Dis 2015; 81:119–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Baraibar MA, Muhoberac BB, Garringer HJ, Hurley TD, Vidal R. Unraveling of the E-helices and disruption of 4-fold pores are associated with iron mishandling in a mutant ferritin causing neurodegeneration. J Biol Chem 2010; 285:1950–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Baraibar MA, Barbeito AG, Muhoberac BB, Vidal R. A mutant light-chain ferritin that causes neurodegeneration has enhanced propensity toward oxidative damage. Free Radic Biol Med 2012; 52:1692–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Cozzi A, Rovelli E, Frizzale G, Campanella A, Amendola M, Arosio P, Levi S. Oxidative stress and cell death in cells expressing L-ferritin variants causing neuroferritinopathy. Neurobiol Dis 2010; 37:77–85 [DOI] [PubMed] [Google Scholar]
- 441.Cozzi A, Santambrogio P, Corsi B, Campanella A, Arosio P, Levi S. Characterization of the l-ferritin variant 460InsA responsible of a hereditary ferritinopathy disorder. Neurobiol Dis 2006; 23:644–52 [DOI] [PubMed] [Google Scholar]
- 442.Vidal R, Miravalle L, Gao X, Barbeito AG, Baraibar MA, Hekmatyar SK, Widel M, Bansal N, Delisle MB, Ghetti B. Expression of a mutant form of the ferritin light chain gene induces neurodegeneration and iron overload in transgenic mice. J Neurosci 2008; 28:60–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Capoccia S, Maccarinelli F, Buffoli B, Rodella LF, Cremona O, Arosio P, Cirulli F. Behavioral characterization of mouse models of neuroferritinopathy. PLoS One 2015; 10:e0118990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Garringer HJ, Irimia JM, Li W, Goodwin CB, Richine B, Acton A, Chan RJ, Peacock M, Muhoberac BB, Ghetti B, Vidal R. Effect of systemic iron overload and a chelation therapy in a mouse model of the neurodegenerative disease hereditary ferritinopathy. PLoS One 2016; 11:e0161341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Cozzi A, Orellana DI, Santambrogio P, Rubio A, Cancellieri C, Giannelli S, Ripamonti M, Taverna S, Di Lullo G, Rovida E, Ferrari M, Forni GL, Fiorillo C, Broccoli V, Levi S. Stem cell modeling of neuroferritinopathy reveals iron as a determinant of senescence and ferroptosis during neuronal aging. Stem Cell Rep 2019; 13:832–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Zhou B, Westaway SK, Levinson B, Johnson MA, Gitschier J, Hayflick SJ. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat Genet 2001; 28:345–9 [DOI] [PubMed] [Google Scholar]
- 447.Hartig MB, Prokisch H, Meitinger T, Klopstock T. Pantothenate kinase-associated neurodegeneration. Curr Drug Targets 2012; 13:1182–9 [DOI] [PubMed] [Google Scholar]
- 448.Hartig MB, Hörtnagel K, Garavaglia B, Zorzi G, Kmiec T, Klopstock T, Rostasy K, Svetel M, Kostic VS, Schuelke M, Botz E, Weindl A, Novakovic I, Nardocci N, Prokisch H, Meitinger T. Genotypic and phenotypic spectrum of PANK2 mutations in patients with neurodegeneration with brain iron accumulation. Ann Neurol 2006; 59:248–56 [DOI] [PubMed] [Google Scholar]
- 449.Johnson MA, Kuo YM, Westaway SK, Parker SM, Ching KHL, Gitschier J, Hayflick SJ. Mitochondrial localization of human PANK2 and hypotheses of secondary iron accumulation in pantothenate kinase-associated neurodegeneration. Ann N Y Acad Sci 2004; 1012:282–98 [DOI] [PubMed] [Google Scholar]
- 450.Kruer MC, Hiken M, Gregory A, Malandrini A, Clark D, Hogarth P, Grafe M, Hayflick SJ, Woltjer RL. Novel histopathologic findings in molecularly-confirmed pantothenate kinase-associated neurodegeneration. Brain 2011; 134:947–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Li A, Paudel R, Johnson R, Courtney R, Lees AJ, Holton JL, Hardy J, Revesz T, Houlden H. Pantothenate kinase-associated neurodegeneration is not a synucleinopathy. Neuropathol Appl Neurobiol 2013; 39:121–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Santambrogio P, Ripamonti M, Paolizzi C, Panteghini C, Carecchio M, Chiapparini L, Raimondi M, Rubio A, Di Meo I, Cozzi A, Taverna S, De Palma G, Tiranti V, Levi S. Harmful Iron-Calcium relationship in pantothenate kinase associated neurodegeneration. Int J Mol Sci 2020; 21:3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Kuo Y-M, Duncan JL, Westaway SK, Yang H, Nune G, Xu EY, Hayflick SJ, Gitschier J. Deficiency of pantothenate kinase 2 (Pank2) in mice leads to retinal degeneration and azoospermia. Hum Mol Genet 2005; 14:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Brunetti D, Dusi S, Morbin M, Uggetti A, Moda F, D’Amato I, Giordano C, d’Amati G, Cozzi A, Levi S, Hayflick S, Tiranti V. Pantothenate kinase-associated neurodegeneration: altered mitochondria membrane potential and defective respiration in Pank2 knock-out mouse model. Hum Mol Genet 2012; 21:5294–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Brunetti D, Dusi S, Giordano C, Lamperti C, Morbin M, Fugnanesi V, Marchet S, Fagiolari G, Sibon O, Moggio M, d’Amati G, Tiranti V. Pantethine treatment is effective in recovering the disease phenotype induced by ketogenic diet in a pantothenate kinase-associated neurodegeneration mouse model. Brain 2014; 137:57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Subramanian C, Yao J, Frank MW, Rock CO, Jackowski S. A pantothenate kinase-deficient mouse model reveals a gene expression program associated with brain coenzyme a reduction. Biochim Biophys Acta Mol Basis Dis 2020; 1866:165663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Jeong SY, Hogarth P, Placzek A, Gregory AM, Fox R, Zhen D, Hamada J, van der Zwaag M, Lambrechts R, Jin H, Nilsen A, Cobb J, Pham T, Gray N, Ralle M, Duffy M, Schwanemann L, Rai P, Freed A, Wakeman K, Woltjer RL, Sibon OC, Hayflick SJ. EMBO Mol Med 2019; 11:e10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Rana A, Seinen E, Siudeja K, Muntendam R, Srinivasan B, van der Want JJ, Hayflick S, Reijngoud D-J, Kayser O, Sibon OCM. Pantethine rescues a drosophila model for pantothenate kinase-associated neurodegeneration. Proc Natl Acad Sci U S A 2010; 107:6988–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Wu Z, Li C, Lv S, Zhou B. Pantothenate kinase-associated neurodegeneration: insights from a drosophila model. Hum Mol Genet 2009; 18:3659–72 [DOI] [PubMed] [Google Scholar]
- 460.Zizioli D, Tiso N, Guglielmi A, Saraceno C, Busolin G, Giuliani R, Khatri D, Monti E, Borsani G, Argenton F, Finazzi D. Knock-down of pantothenate kinase 2 severely affects the development of the nervous and vascular system in zebrafish, providing new insights into PKAN disease. Neurobiol Dis 2016; 85:35–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Orellana DI, Santambrogio P, Rubio A, Yekhlef L, Cancellieri C, Dusi S, Giannelli SG, Venco P, Mazzara PG, Cozzi A, Ferrari M, Garavaglia B, Taverna S, Tiranti V, Broccoli V, Levi S. Coenzyme a corrects pathological defects in human neurons of PANK2-associated neurodegeneration. EMBO Mol Med 2016; 8:1197–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Klopstock T, Tricta F, Neumayr L, Karin I, Zorzi G, Fradette C, Kmieć T, Büchner B, Steele HE, Horvath R, Chinnery PF, Basu A, Küpper C, Neuhofer C, Kálmán B, Dušek P, Yapici Z, Wilson I, Zhao F, Zibordi F, Nardocci N, Aguilar C, Hayflick SJ, Spino M, Blamire AM, Hogarth P, Vichinsky E. Safety and efficacy of deferiprone for pantothenate kinase-associated neurodegeneration: a randomised, double-blind, controlled trial and an open-label extension study. Lancet Neurol 2019; 18:631–42 [DOI] [PubMed] [Google Scholar]
- 463.Rohani M, Razmeh S, Shahidi GA, Alizadeh E, Orooji M. A pilot trial of deferiprone in pantothenate kinase-associated neurodegeneration patients. Neurol Int 2017; 9:7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Huebl J, Schneider SA. Iron chelation in pantothenate kinase-associated neurodegeneration: a possible new avenue for slowing down disease progression in neurodegeneration. Mov Disord 2019; 34:1476–7 [DOI] [PubMed] [Google Scholar]
- 465.Sharma LK, Subramanian C, Yun M-K, Frank MW, White SW, Rock CO, Lee RE, Jackowski S. A therapeutic approach to pantothenate kinase associated neurodegeneration. Nat Commun 2018; 9:4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Guo Y-P, Tang B-S, Guo J-F. PLA2G6-associated neurodegeneration (PLAN): review of clinical phenotypes and genotypes. Front Neurol 2018; 9:1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Darling A, Aguilera-Albesa S, Tello CA, Serrano M, Tomás M, Camino-León R, Fernández-Ramos J, Jiménez-Escrig A, Poó P, O’Callaghan M, Ortez C, Nascimento A, Fernández Mesaque RC, Madruga M, Arrabal L, Roldan S, Gómez-Martín H, Garrido C, Temudo T, Jou-Muñoz C, Muchart J, Huisman TAGM, Poretti A, Lupo V, Espinós C, Pérez-Dueñas B. PLA2G6-associated neurodegeneration: new insights into brain abnormalities and disease progression. Parkinsonism Relat Disord 2019; 61:179–86 [DOI] [PubMed] [Google Scholar]
- 468.Ramanadham S, Ali T, Ashley JW, Bone RN, Hancock WD, Lei X. Calcium-independent phospholipases A2 and their roles in biological processes and diseases. J Lipid Res 2015; 56:1643–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Riku Y, Ikeuchi T, Yoshino H, Mimuro M, Mano K, Goto Y, Hattori N, Sobue G, Yoshida M. Extensive aggregation of α-synuclein and tau in juvenile-onset neuroaxonal dystrophy: an autopsied individual with a novel mutation in the PLA2G6 gene-splicing site. Acta Neuropathol Commun 2013; 1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Iodice A, Spagnoli C, Salerno GG, Frattini D, Bertani G, Bergonzini P, Pisani F, Fusco C. Infantile neuroaxonal dystrophy and PLA2G6-associated neurodegeneration: an update for the diagnosis. Brain Dev 2017; 39:93–100 [DOI] [PubMed] [Google Scholar]
- 471.Chiu C-C, Wang H-L, Weng Y-H, Chen R-S, Chen C-M, Yeh T-H, Lu C-S, Chen Y-J, Liu Y-C, Huang Y-Z, Chang K-H. Generation of induced pluripotent stem cells from a young-onset Parkinson’s disease patient carrying the compound heterozygous PLA2G6 p.D331Y/p.M358IfsX mutations. Stem Cell Res. 2019; 40:101552. [DOI] [PubMed] [Google Scholar]
- 472.Shen T, Hu J, Jiang Y, Zhao S, Lin C, Yin X, Yan Y, Pu J, Lai H-Y, Zhang B. Early-Onset Parkinson’s disease caused by PLA2G6 compound heterozygous mutation. A case report and literature review. Front Neurol 2019; 10:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Sumi-Akamaru H, Beck G, Kato S, Mochizuki H. Neuroaxonal dystrophy in PLA2G6 knockout mice. Neuropathology 2015; 35:289–302 [DOI] [PubMed] [Google Scholar]
- 474.Malik I, Turk J, Mancuso DJ, Montier L, Wohltmann M, Wozniak DF, Schmidt RE, Gross RW, Kotzbauer PT. Disrupted membrane homeostasis and accumulation of ubiquitinated proteins in a mouse model of infantile neuroaxonal dystrophy caused by PLA2G6 mutations. Am J Pathol 2008; 172:406–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Shinzawa K, Sumi H, Ikawa M, Matsuoka Y, Okabe M, Sakoda S, Tsujimoto Y. Neuroaxonal dystrophy caused by group via phospholipase A2 deficiency in mice: a model of human neurodegenerative disease. J Neurosci 2008; 28:2212–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Wada H, Kojo S, Seino K. Mouse models of human INAD by Pla2g6 deficiency. Histol Histopathol 2013; 28:965–9 [DOI] [PubMed] [Google Scholar]
- 477.Sumi-Akamaru H, Beck G, Shinzawa K, Kato S, Riku Y, Yoshida M, Fujimura H, Tsujimoto Y, Sakoda S, Mochizuki H. High expression of α-synuclein in damaged mitochondria with PLA2G6 dysfunction. Acta Neuropathol Commun 2016; 4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Iliadi KG, Gluscencova OB, Iliadi N, Boulianne GL. Mutations in the drosophila homolog of human PLA2G6 give rise to age-dependent loss of psychomotor activity and neurodegeneration. Sci Rep 2018; 8:2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Lin G, Lee P-T, Chen K, Mao D, Tan KL, Zuo Z, Lin W-W, Wang L, Bellen HJ. Phospholipase PLA2G6, a Parkinsonism-associated gene, affects Vps26 and Vps35, retromer function, and ceramide levels, similar to α-synuclein gain. Cell Metab 2018; 28:605–18.e6 [DOI] [PubMed] [Google Scholar]
- 480.Morrison BE, D’Mello SR. Polydactyly in mice lacking HDAC9/HDRP. Exp Biol Med 2008; 233:980–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Williams ET, Chen X, Moore DJ. VPS35, the retromer complex and Parkinson’s disease. J Parkinsons Dis 2017; 7:219–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Sullivan CP, Jay AG, Stack EC, Pakaluk M, Wadlinger E, Fine RE, Wells JM, Morin PJ. Retromer disruption promotes amyloidogenic APP processing. Neurobiol Dis 2011; 43:338–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Wen L, Tang F-L, Hong Y, Luo S-W, Wang C-L, He W, Shen C, Jung J-U, Xiong F, Lee D, Zhang Q-G, Brann D, Kim T-W, Yan R, Mei L, Xiong W-C. VPS35 haploinsufficiency increases Alzheimer’s disease neuropathology. J Cell Biol 2011; 195:765–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Ansell-Schultz A, Reyes JF, Samuelsson M, Hallbeck M. Reduced retromer function results in the accumulation of amyloid-beta oligomers. Mol Cell Neurosci 2018; 93:18–26 [DOI] [PubMed] [Google Scholar]
- 485.Young JE, Fong LK, Frankowski H, Petsko GA, Small SA, Goldstein LSB. Stabilizing the retromer complex in a human stem cell model of Alzheimer’s disease reduces TAU phosphorylation independently of amyloid precursor protein. Stem Cell Rep 2018; 10:1046–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Sadigh-Eteghad S, Askari-Nejad MS, Mahmoudi J, Majdi A. Cargo trafficking in Alzheimer’s disease: the possible role of retromer. Neurol Sci 2016; 37:17–22 [DOI] [PubMed] [Google Scholar]
- 487.Tammineni P, Jeong YY, Feng T, Aikal D, Cai Q. Impaired axonal retrograde trafficking of the retromer complex augments lysosomal deficits in Alzheimer’s disease neurons. Hum Mol Genet 2017; 26:4352–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Chu J, Praticò D. The retromer complex system in a transgenic mouse model of AD: influence of age. Neurobiol Aging 2017; 52:32–8 [DOI] [PubMed] [Google Scholar]
- 489.Ke M, Chong C-M, Zeng H, Huang M, Huang Z, Zhang K, Cen X, Lu J-H, Yao X, Qin D, Su H. Azoramide protects iPSC-derived dopaminergic neurons with PLA2G6 D331Y mutation through restoring ER function and CREB signaling. Cell Death Dis 2020; 11:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Guillen C. Azoramide: a new drug for the treatment of type 2 diabetes? Ann Transl Med 2016; 4:S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Rozpędek-Kamińska W, Siwecka N, Wawrzynkiewicz A, Wojtczak R, Pytel D, Diehl JA, Majsterek I. The PERK-Dependent molecular mechanisms as a novel therapeutic target for neurodegenerative diseases. Int J Mol Sci 2020; 21:2108. [DOI] [PMC free article] [PubMed] [Google Scholar]