Abstract
The objective of this study was to elucidate the pathophysiology that underlies severe COVID-19 by assessing the histopathology and the in situ detection of infectious SARS-CoV-2 and viral capsid proteins along with the cellular target(s) and host response from twelve autopsies. There were three key findings: 1) high copy infectious virus was limited mostly to the alveolar macrophages and endothelial cells of the septal capillaries; 2) viral spike protein without viral RNA localized to ACE2+ endothelial cells in microvessels that were most abundant in the subcutaneous fat and brain; 3) although both infectious virus and docked viral spike protein was associated with complement activation, only the endocytosed pseudovirions induced a marked up-regulation of the key COVID-19 associated proteins IL6, TNF alpha, IL1 beta, p38, IL8, and caspase 3 in endothelium. Importantly, this microvasculitis was associated with characteristic findings on hematoxylin and eosin examination that included endothelial degeneration and resultant basement membrane zone disruption and reduplication. It is concluded that serious COVID-19 infection has two distinct mechanisms: 1) a microangiopathy of pulmonary capillaries associated with a high infectious viral load where endothelial cell death releases pseudovirions into the circulation, and 2) the pseudovirions dock on ACE2+ endothelial cells most prevalent in the skin/subcutaneous fat and brain that activates the complement pathway/coagulation cascade resulting in a systemic procoagulant state as well as endothelial expression of cytokines that produce the cytokine storm. The data predicts a favorable response to therapies based on either removal of circulating viral proteins and/or blunting of the endothelial-induced response.
Keywords: COVID-19, In situ, Spike protein, Endothelialitis, Complement
1. Introduction
The severe acute respiratory distress syndrome-associated coronavirus-2 (SARS-CoV-2), etiologic agent of Coronavirus disease 2019 (COVID-19), was initially identified in Wuhan, Hubei, China in December 2010 [1,2] and, by mid August there were over 22.5 million confirmed cases and over 800,000 deaths [3]. Respiratory failure, the so-called “cytokine storm”, cardiovascular collapse, and a coagulopathy/procoagulant state are associated with fatal disease. Well-documented risk factors include increased age, preexisting medical conditions including diabetes and cardiovascular disease [4,5]; obesity is an independent risk factor for severe COVID-19 disease [6,7].
While the majority of SARS-CoV-2 infections are self-limited, 20% of patients are symptomatic, often requiring hospitalization, and about 3% of all documented cases are fatal. In New York City, where this group is centered, the number of deaths per 1000 person-years greatly increased from a nearly flat monthly death rate, average of 7.83, in 2017 to 9.44 in April and May of 2020 [8], a 21% increase in all cause mortality. Serious manifestations typically begin within 1–2 weeks after the onset of symptoms, and are heralded by profound difficulty in breathing with further complications related to a hypercoagulable state [[9], [10], [11]]. Patients who develop severe COVID-19 have extensive microangiopathy and an increased risk of larger vessel thrombosis whereby it has been hypothesized that the complement and coagulation system work synergistically to produce potentially catastrophic sequelae [9,[12], [13], [14], [15], [16]]. It was recently demonstrated that diseased and normal skin are an important source for docked viral protein within ACE2 positive microvasculature especially in the deeper dermis and subcutaneous fat without any evidence of viral replication [17] (Magro, CM, submitted for publication). The extent of viral protein load in patients with severe COVID-19 including those with a fatal outcome may be linked to a poor interferon response [17]. In this study severe COVID-19 is presented as a multifaceted viral-triggered vasculopathy syndrome with the potential to affect multiple organ systems. The extent and location of viral RNA and capsid proteins throughout the body is presented after analysis of diverse tissues from twelve autopsies and correlated with the SARS-CoV2 receptor, ACE2. The results of these analyses, including the demonstration of high copy infectious virus only in the lung and the localization of the SARS CoV-2 capsid proteins including the spike protein without viral RNA (i.e. pseudovirion) within various microvascular beds where ACE2+ endothelia dominate, suggests that severe COVID-19 may have two distinct mechanisms.
2. Methods
2.1. SARS-CoV-2 infected tissues
The formalin fixed, paraffin embedded tissues studied comprised a combination of autopsy cases and surgical pathology specimens from symptomatic patients. A total of 12 autopsies with tissue samples procured from many organs including lung, heart, liver, spleen, brain, lymph nodes, kidney and skin/subcutaneous fat, one pre-mortem open lung biopsy, 13 skin samples, and two below the knee amputations specimens were studied. This study presents archival pathology material where any additional studies were covered under IRB protocol 20-02021524.
2.2. Routine light microscopy
Four-micron sections were cut and stained with hematoxylin and eosin. The histologic evaluations included evidence of cell necrosis, inflammatory infiltrates, thrombi, platelet aggregation, viral inclusions, as well as microvascular damage as defined by endothelial cell denudement, basement membrane zone reduplication, and microthrombi.
2.3. Immunohistochemistry
Immunohistochemical assessment for the deposition of C5b-9 (membranolytic attack complex (MAC)), C3d, and C4d, caspase 3, ACE-2, and myxovirus resistance protein A (MXA) using a diaminobenzidine chromogen was conducted on the formalin-fixed tissues. Further, the presence and distribution of a series of cytokines associated with severe COVID-19, including IL6, TNFα, IL1β, p38, and IL8, were tested after optimization with appropriate positive and negative controls as previously described [16]. Brain related molecular changes were studied by testing for SUR1, TRPM4, PERK, PARP3, NMDAR1, and caspase 3. Identification of C5b-9, C3d, or C4d within epithelial basement membrane zones, elastic fibers, or the elastic lamina of vessels was considered nonspecific staining as previously described [9,17]. The source of the antibodies was: C3d (Cell Marque, Rocklin CA, 403A-78), C4d (Alpco, Salem NH, BI-RC4d), and C5b-9 (Agilent, Santa Clara CA, M077701-5). Each of the other antibodies was from ABCAM (Cambridge, Massachusetts, USA) with the exception of ACE2 (cat # 3215) (PROSCI, Poway, California, USA) and optimal pretreatment in each case was 30 min with the Leica EDTA antigen retrieval solution.
Detection of the SARS-CoV-2 spike glycoprotein, membrane and/or envelope proteins was as previously described [9,16,17]. In brief, the Leica Bond Max automated platform was used with the primary antibodies (PROSCI) at dilutions of 1:500 (membrane, cat # 3527), 1:4500 (spike; cat # 3525) and 1:500 (envelope; cat # 3521) after antigen retrieval for 30 min. The HRP conjugate from Enzo Life Sciences (Farmingdale, New York, USA) was used in place of the equivalent reagent from Leica as this has been shown to substantially reduce background [16].
2.4. In situ hybridization
Detection of SARS-CoV-2 RNA was done using the ACD RNAscope (Newark, California, USA) probe (Cat No. 848561-C3) through a previously published protocol in which only the viral RNA probe is changed [16]. In brief, pretreatment in the ACD RNA retrieval solution and protease digestion is followed by overnight hybridization at 37C and detection using the ACD 2.5 HD DAB detection kit.
2.5. Co-expression analysis
Co-expression analyses were done using the Nuance system whereby each chromogenic signal is separated, converted to a fluorescence based signal, then mixed to determine what percentage of cells were expressing the two proteins of interest as previously described [16].
3. Results
3.1. Clinical correlates
Of the 26 patients, all but two had severe COVID-19 including 23 with respiratory failure; 12 people died of the disease and had complete post-mortems. The patients ranged in age from 28 to 95 years and were represented by 17 males and 9 females. Of the 24 patients with severe or fatal COVID-19, clinical risk factors included obesity (n = 14), arterial hypertension (n = 10), diabetes mellitus (n = 8), cardiopulmonary disease (n = 7), hyperlipidemia (n = 4), sickle cell anemia (n = 2) and deep vein thrombosis (n = 1). Neurologic signs/symptoms were nonspecific and included lethargy and confusion. The two patients with moderate COVID-19 each survived but needed below the knee amputations for deep vein thrombosis; one person was obese and the other had diabetes mellitus.
3.2. Autopsy and light microscopic findings
The primary gross abnormality at autopsy was the lungs that were heavy and diffusely congested. The other gross abnormality was macrothrombi that was evident in three autopsies at times associated with tissue infarction. The brains were grossly normal.
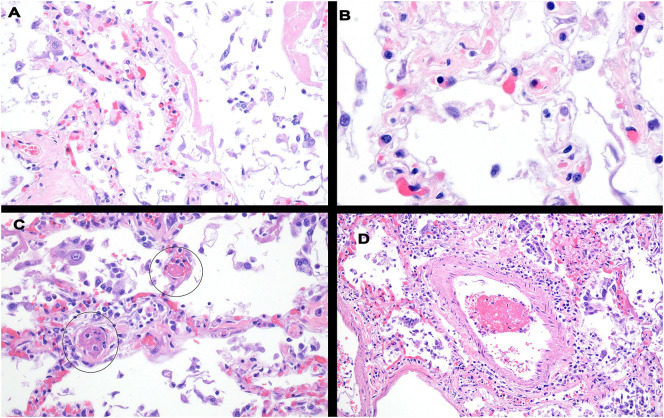
Histologically, in each of the lung samples the most consistent pattern of vascular injury was one characterized by septal capillary walls exhibiting a disrupted and frayed dyshesive appearance (Fig. 1 ) best appreciated under oil-immersion (100× objective) magnification. As seen in Fig. 1, the vessels had a dilated rarefied appearance and were largely devoid of viable endothelium or pneumocytes and exhibited focal thrombosis. There was also focal small vessel and larger vessel thrombosis affecting both the venous and arterial system. In some cases, organizing pneumonitis with interstitial fibrosis and hyaline membrane formation could be seen. The one pre-mortem lung sample showed an end stage pattern of lung injury characterized by pauci-cellular fibrosis with obliteration of the terminal parenchymal architecture.
Fig. 1.
Histologic changes in the lung in fatal COVID-19. A common pattern of septal capillary injury was characterized by septal capillary walls exhibiting a disrupted and frayed dyshesive appearance with or without endothelial cell denudement unaccompanied by significant inflammation best appreciated under oil examination (panel B). There was also significant vessel thrombosis affecting septal capillaries and both the venous and arterial systems (Panels C, D).
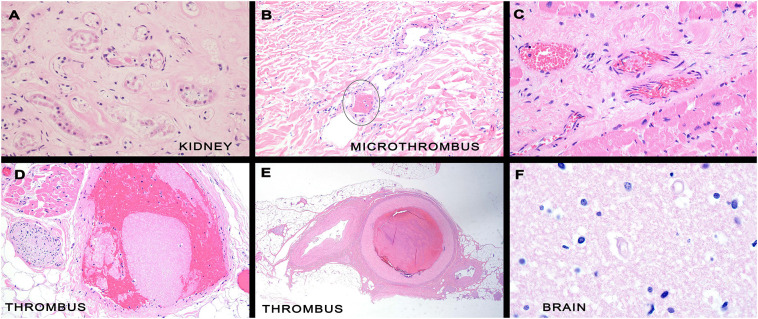
Microvascular thrombi were evident in many organs including the kidney, brain, heart, skin and liver and were seen in capillaries, venules, and arterioles (Fig. 2 ). The pattern of vascular thrombosis varied. One was characterized by bland luminal thrombi or incipient platelet thrombi without endothelial injury (Fig. 2). This pattern was also seen in much larger vessels in the two below the knee amputations specimens where arterial thrombosis involving small and medium sized blood vessels (Fig. 2). The second pattern of thrombosis was one accompanied by significant endothelial injury and resultant basement membrane zone disruption and reduplication seen most prominently in the brain and biopsied skin lesions of thrombotic retiform purpura. Interestingly, this same pattern of microvascular damage was seen in grossly normal skin from people who died of COVID-19. Extravascular inflammation apart from inflammation related to ischemic tissue necrosis was not observed. Viral inclusions were not evident. Histologic sections of brain showed rare luminal thrombi comprising large platelets admixed with fibrin in capillaries and venules. However the most striking abnormality was in the context of mummified capillaries devoid of endothelium with associated basement membrane zone reduplication and perivascular edema (Fig. 2). Red blood cell extravasation and perivascular hemosiderin deposition was noted around capillaries and venules, which is very rare in the normal brain and may be a marker of on-going capillary fragility. The perivascular edema was exemplified by an expanded space surrounding blood vessels and appeared greater around vessels positive for SARS CoV-2 protein compared to normal brain. To quantify this, the perivascular space diameter was measured for 50 microvessels in the gray matter of the normal versus COVID-19 brain blinded to the clinical information. The mean (SEM) values for the perivascular space around microvessels were: normal brain 98.7 μm (9.8), COVID-19 brain, overall 84.1 μm (8.9), COVID-19 brain, microvessels with spike protein 164.7 μm (12.1). The latter was significantly increased versus the normal brain and overall COVID-19 brain using the Tukey-Kramer Multiple Comparisons Test (p < 0.001).
Fig. 2.
Histologic changes in various organs in fatal COVID-19. Many organs, including the kidney, brain, heart, skin and liver, demonstrated focal microvascular thrombi in smaller vessels; among the affected vessels were capillaries, venules, arterioles and small arteries (Panel A/B; kidney). Panels C/D depict cardiac tissue showing loose platelet thrombi without any evidence of endothelial cell injury or vessel wall inflammation. In two below the knee amputations specimens, there was arterial thrombosis involving small and medium sized blood vessels in the deeper dermis and subcutaneous fat (panel E). The other pattern of thrombosis was one accompanied by significant endothelial cell injury and resultant basement membrane zone disruption and reduplication most often seen in the brain and skin. The most striking abnormality was in the context of mummified vessels (i.e. capillaries) devoid of endothelium with associated basement membrane zone reduplication and perivascular edema (panel F, brain).
3.3. Complement studies
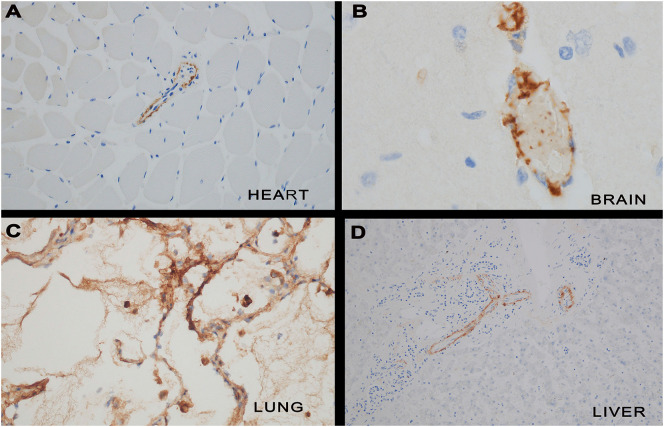
Complement studies were conducted on tissue from the lung (n = 6), heart (n = 4), liver (n = 4), kidney (n = 4), brain (n = 3) and skin (n = 20). There was significant endothelial and subendothelial microvascular deposition of C3d, C4d and/or C5b-9 in all cases tested and in none of the controls (Fig. 3 ). The complement expression was temporal and heterogeneous for a particular case. Hence while there could be extensive C4d likely reflective of mannan binding lectin activation, C5b-9 or C3d could be absent. The key was to document substantial deposition of one component of complement activation such as C3d, C4d, and/or C5b-9, the latter in a punctate granular pattern highlighting endothelium and vessel walls. The C5b-9 was deposited in capillary walls, endothelia and intravascular macrophages; the pattern of immunoreactivity included an intracellular one within the endothelium and a surface punctate granular one that outlined the abluminal surface of the vessels. Intravascular platelets also contained C4d and at times other components of complement. In lung samples showing advanced injury with end stage fibrosis there was no vascular complement deposition. Both lesional and nonlesional clinically normal skin exhibited complement deposition where it was most apparent in the deeper dermis and subcutaneous fat. In larger occluded arteries of the lower extremity the deposition was largely confined to the vessel wall but not the endothelia. The extent of complement deposition was greatest in the lung, skin, brain, kidney, and heart.
Fig. 3.
Complement cascade activation in COVID-19. There was significant endothelial and subendothelial microvascular deposition of C3d, C4d and/or C5b-9 in all severe/fatal COVID-19 cases tested. Representative images of pulmonary and extrapulmonary complement staining is provided (Panel A = C4d heart, Panel B = C4d brain, Panel C = C4d lung and Panel D = C5b-9 liver).
3.4. SARS-CoV-2 RNA in situ hybridization
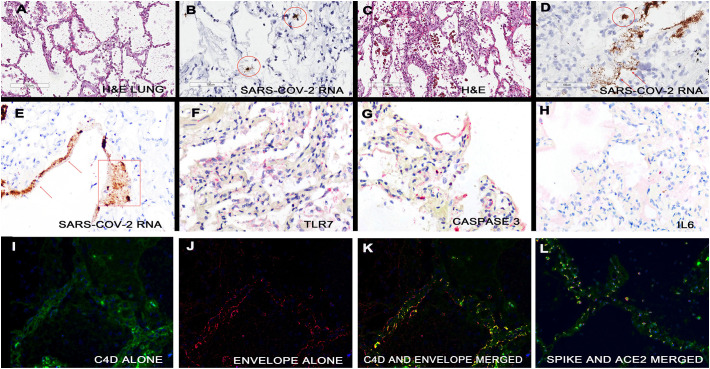
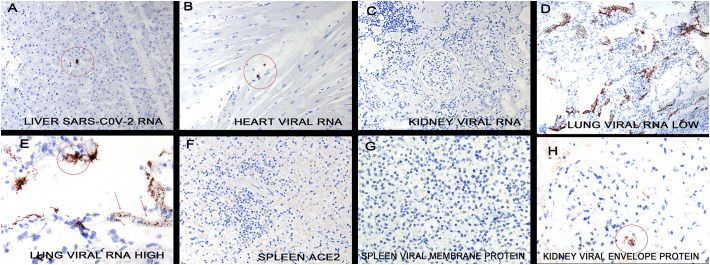
The distribution of SARS-CoV-2 RNA was determined by in situ hybridization. Abundant viral RNA was evident in the lung tissues where it localized to the alveolar macrophages and adjacent septal capillary's endothelia (Fig. 4 ) as evidenced by co-expression with the markers CD68, CD11b, and CD206 as well as the endothelial marker CD31, respectively. Rare signal was evident in alveolar pneumocytes although these cells were often absent from areas with high viral load. Using signal intensity and known standards [16], viral copy number was estimated at >500,000 viral genomes/mm2 in many fields. Viral RNA and capsid proteins (spike, envelope, membrane) strongly co-expressed and co-localized with C3d, C4d, and C5b-9 as well as to the ACE2 receptor (Fig. 4). Interrogation of the spleen, lymph nodes (Fig. 5 ), brain and skin with attached subcutaneous fat did not reveal any cells positive for SARS-CoV-2 RNA. Viral RNA was evident in the liver, heart, and kidney but in rare cells that co-expressed the macrophage marker CD68 (Fig. 5); the highest viral RNA load was evident in the liver but this was many fold lower than in the lung (0–250 genomes/mm2).
Fig. 4.
Detection of SARS-CoV-2 RNA and capsid proteins in the lungs of people who died of COVID-19. The lung in fatal COVID-19 shows marked heterogeneity. Panel A shows a histologically unremarkable area of lung. In the serial section, viral RNA was evident in rare cells (circles) that had the morphology of macrophages (panel B) and did co-express with CD68 (data not shown). In adjacent areas the septal capillaries were markedly expanded (panel C). This was associated with a very high viral load where the viral genomes localized to the macrophages (circles) and septal capillaries (arrows). Although the viral RNA in the septal capillaries often showed a thin, linear pattern, at times the zone containing viral RNA was much expanded and associated with degenerated virus (panel E, rectangle). The areas of high viral load and septal capillary injury were associated with strong expression of TLR7 (panel F; signal red) and caspase 3 (panel G, signal red); however, IL6 was much less evident (panel H, signal red). Co-expression of C4d and the envelope protein of SARS-CoV2 in the areas of septal damage were analyzed by the Nuance system. The C4d is seen as fluorescent green (panel I), the envelope protein as fluorescent red (panel J) and the merged image shows the cells in the septal capillaries that contain both proteins as fluorescent yellow (panel K). Panel L shows co-expression of ACE2 (green) and spike protein (red); note that the spike protein strongly co-localizes with ACE2 on the endothelial (yellow). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Detection of SARS-CoV-2 RNA and capsid proteins in organs other than the lung and skin. SARS-CoV-2 RNA was analyzed in the liver (panel A), heart (panel B), kidney (panel C) and spleen (not shown) in cases where a very high viral load was present in the lung (panels D and E). Note that either no or very rare (no more than 3+ cells/400× field) viral positive cells were present in these other organs (circles); the infected cells had the morphology of macrophages These same organs were interrogated for the ACE2 receptor on endothelial cells and either none were evident (panel F, spleen) or less than 10% of the endothelial cells expressed the viral receptor in these organs. Viral capsid protein was not evident in the spleen microvessels (panel G) and rarely present in the microvessels of other organs (kidney panel H, circle) where they did track with IL6 and caspase 3, data not shown.
3.5. SARS-CoV-2 spike and ACE2 protein immunohistochemistry
Immunohistochemistry for the viral spike protein in the COVID-19 cases and negative controls was analyzed in a blinded fashion. In the skin serial section analysis for evidence of the spike glycoprotein receptor ACE2 showed an equivalent distribution to the viral protein. Among the endothelial cells in the various organs by far the strongest expression/unit area for the ACE2 receptor was in the microvessels of the skin (Fig. 6 ) and the lung where >50% of the microvascular endothelia expressed the viral receptor. There was strong expression of the ACE2 receptor in the microvasculature of the brain (i.e. capillaries and venules), albeit focal. Overall ACE-2 expression in the microvasculature's endothelia, as represented by both number of positive staining vessels and the intensity of staining, from highest to lowest follows: skin, lung, brain, liver, placenta, kidney and heart with no signal evident in the spleen, lymph node, prostate, ovary, bone marrow and esophagus.
Fig. 6.
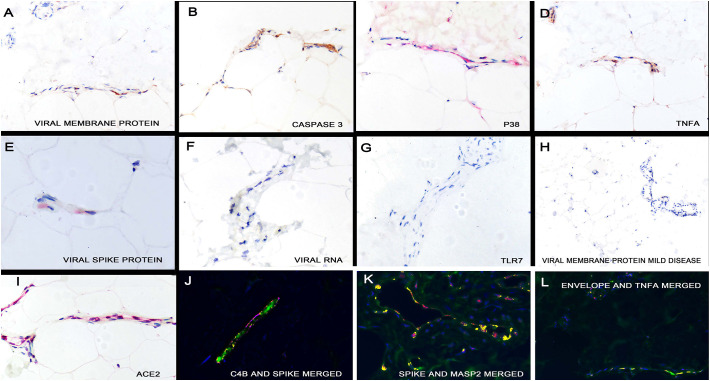
Detection of SARS-CoV-2 RNA and capsid proteins in the skin and subcutaneous fat. Serial section analyses demonstrated that the same microvessels of the skin in people who died of COVID-19 showed endothelial localization of the viral membrane protein (panel A), activated caspase 3 (panel B), p38 (panel C, signal red), TNF α (panel D) and the viral spike protein (panel E, signal red). However, viral RNA was not detected by in situ hybridization (panel F); consistent with this finding was the lack of TLF7 expression (panel G); compare to TLR7 expression in lung in areas with infectious virus (Fig. 1, panel F). Neither viral capsid proteins (panel H) nor caspase 3/IL6/TNF alpha (not shown) were evident in the skin microvessels in people with mild SARS-CoV-2 infection. Panel I (signal red) shows the strong expression of ACE2 in the microvessels of the subcutaneous fat in people who died of COVID-19. Co-expression experiments showed strong co-localization of C4b and the viral spike protein in the endothelial cells of these microvessels (panel J), of the complement cascade activator MASP2 and viral spike protein (panel K) and of the viral envelope protein and TNF α (panel L) as well as the endothelial cell marker CD31 (data not shown). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
There was significant viral protein localization in the microvasculature of the skin especially the deeper dermis and subcutaneous fat procured from both thrombosed lesional skin and clinically normal deltoid skin. Multiple foci of viral protein localization within the microvasculature of the brain was also identified although less dense than that noted in the skin. Co-localization experiments documented in both the skin and brain that the endothelial cells with viral spike protein also strongly expressed ACE-2 and that the spike protein co-localized with both the envelope and membrane proteins, suggesting that the capsid proteins circulated as a unit.
The viral spike protein was not identified in the spleen (Fig. 5) or in any of the negative controls. Rare viral proteins were evident in the endothelia as determined by CD31 co-expression in the heart and kidney from fatal COVID-19 cases but these organs were predominantly negative and reflected the minimal ACE-2 receptor expression. The spike protein was evident in small groups of cells in the liver that were either macrophages or endothelial cells as demonstrated by co-expression with CD68 or CD31, respectively (data not shown). In the heart tissue, the ACE2 receptor and spike protein were more prevalent in the microvessels of the fat surrounding the heart than in the heart muscle proper (data not shown).
3.6. Cellular reaction to SARS-CoV-2 RNA and SARS CoV-2 proteins: viral defense systems and cytokines
The data indicates that circulating SARS-CoV-2 spike protein but not intact infectious virus, dock into various microvascular beds throughout the body that express the ACE-2 receptor being most apparent in the skin, brain and liver. To determine other sequelae of this pseudovirion docking, cases and controls were analyzed for TLR3, TLR7, interferon gamma, PDL1, activated caspase 3 and a series of cytokines that have been associated with severe COVID-19: IL6, TNF α, IL1 β, IL8, and p38. TLR3, TLR7 and interferon gamma were increased in the lung in the areas of viral proliferation but not in the liver, brain, or subcutaneous fat (Fig. 4, Fig. 6). Caspase 3 and PDL1 were each increased in the lung in the endothelia that contained infectious virus as well as in the endothelia of the microvessels of other organs, primarily the skin, where the viral spike protein alone was detected. Each of the cytokines (IL6, TNF α, IL1 β, IL8, and p38) were significantly increased in the endothelia of select extrapulmonary microvascular beds where they each strongly co-localized with the viral spike protein and ACE-2 receptors including the skin (Fig. 6) and brain (Fig. 7 ). However, these cytokines either were not detected, or weakly expressed, in the lung in the areas of active viral replication (Fig. 4).
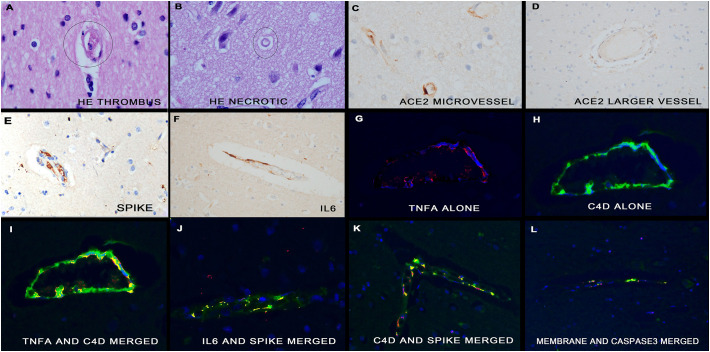
Fig. 7.
Molecular correlates of COVID-19 in the brain. Panels A and B show representative histologic changes in the microvessels in the gray matter of the brain from people who died of COVID-19. Note the microthrombus (panel A) and the degenerated microvessel in which the endothelial nuclei are not evident (panel B). Panels C/D show that ACE2 receptor is expressed robustly in the endothelia of microvessels but not of the larger arterioles. Serial section analysis shows that the endothelial cells of the microvessels also the viral spike protein (panel E), IL6 (panel F) and TNF α (panel G). Note that the latter co-localizes with Cd4 (panels G-I) as does IL6 and the spike protein (panel J), C4d and the spike protein (panel K) and the viral membrane protein with caspase 3 (panel L); all co-localization is evident as fluorescent yellow. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.7. Cellular reaction to microvascular SARS CoV-2 proteins in the brain
Neurological symptoms are common in severe COVID-19 and, thus, the microenvironment in the area of the disease microvessels was explored. Microglia, astrocytes, neurons, and oligodendroglial cells were identified with TMEM, GFAP, pyruvate dehydrogenase, NeuN, and S100, respectively [16]. The endothelial cells, as identified with CD31, that contained the viral capsid proteins strongly expressed SUR1, TRPM4, activated caspase 3, and PARP3 (data not shown); none of these proteins were noted in the viral protein negative endothelial cells nor in the surrounding cells, including neurons. SUR1 and TRPM4 are indicators of disruption in the blood brain barrier and caspase 3/PARP3 expression may signify cell death in the CNS [16]. The glutamate dependent NMDAR1 was expressed in neurons within 100 μm of the microvessels that contained the viral proteins and not in other neurons (data not shown). Taken together, the data suggests that the CNS damage is primarily a microvasculitis with no evidence of neuronal death, but rather of neuronal dysfunction including excitotoxicity as suggested by the increased NMDAR1 expression.
4. Discussion
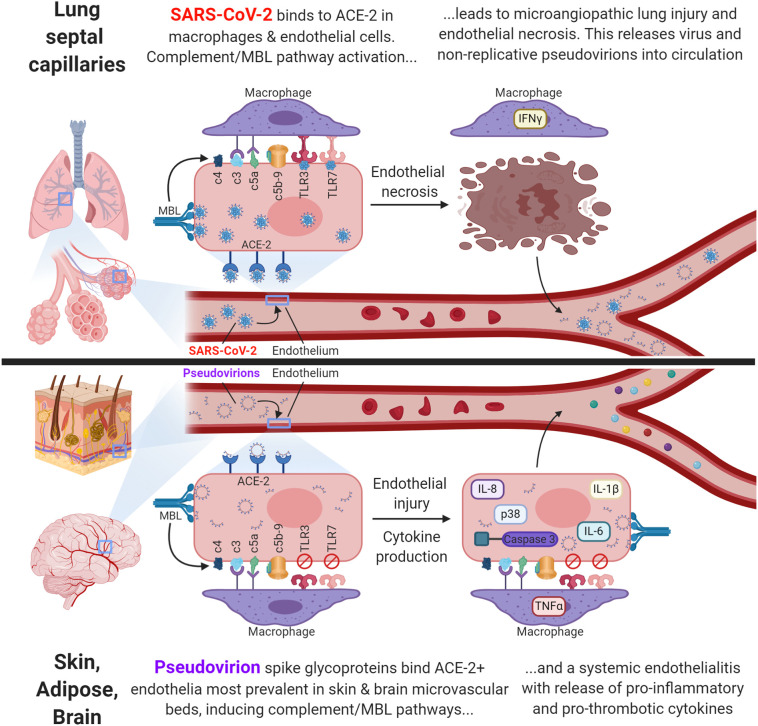
The primary findings of this study are threefold. 1) Histologic. Besides the microangiopathy in the septal capillaries of the lung as previously described [9], there is systemic vascular disease marked either by thrombi in large vessels or endothelial cell damage/necrosis, basement membrane duplication, perivascular edema, and microthrombi in microvessels; the latter are most prominent in the skin, brain, and liver. 2) Viral. SARS-CoV-2 RNA/protein (infectious virus) in high copy number is evident only in the septal capillaries/macrophages in the lung whereas pseudovirions (spike, envelope, membrane proteins without viral RNA) are evident systemically where they are endocytosed by ACE2+ endothelia, which dominate in the skin, brain, and liver. 3) Host response. Complement activation is evident with both the infectious virus and pseudovirions. However, cytokine up regulation including IL6, IL8, IL1 β, TNF a, and p38 as well as caspase 3 expression is evident only with the pseudovirions where they strongly co-express with the viral capsid proteins. In the brain, the microvasculitis is mainly confined to the endothelial cells with minimal evidence of neuronal or other cell death. A graphic summary of these mechanisms is provided in Fig. 8 .
Fig. 8.
Graphic representation of the two distinct mechanisms of severe COVID-19.
In an earlier study we showed that a critical aspect of the pathophysiology of severe COVID-19 is one of complement mediated vascular injury in the lung [9]. In the 5 cases presented in the original study, the lung and skin of all patients had evidence of vascular injury characterized by endothelial necrosis and thrombosis associated with microvascular deposition of complement including C4d, MASP2, and C5b-9 indicative of mannan binding lectin (MBL) pathway activation [9]. By demonstrating that endothelial based SARS CoV-2 viral capsid proteins co-expressed with these complement proteins, it was assumed that infectious virus was inducing the MBL pathway. This prior study did not specifically address whether or not the viral protein present was reflective of active viral replication or merely docked nonreplicative viral proteins (i.e. pseudovirions).
Given the millions of cases worldwide, the clinical correlates of severe COVID-19 are well documented. These include an independent association with obesity, strong correlation with preexisting conditions including diabetes, a hypercoagulable state, so-called cytokine storm and neurologic signs/symptoms that often become manifest 1–2 weeks after pneumonia [[4], [5], [6], [7], [8]]. The current study by correlating the light microscopic, viral, and host response, including cytokine expression and complement analysis of significantly ill COVID-19 patients, has provided data that may help to understand the foundation for these clinical correlates. The extremely high copy viral load in the lung induces a microangiopathy that destroys the infected endothelial cell and, thus, could release large numbers of pseudovirions into the circulation. The spike protein in these pseudovirions will dock on ACE2+ endothelia. The largest reservoir for the latter is the subcutaneous fat, which will be much increased in the obese. The endocytosed viral proteins induce a strong complement cascade activation and marked cytokine response including IL6, IL8, IL1 β, p38, and TNF α. These cellular responses would cause many systemic medical problems, especially in those with pre-existing medical conditions. The observation that the microvascular bed in the liver and brain also contains many ACE2+ endothelial cells that, as demonstrated here, shows much evidence of viral-induced damage, could clearly be related to the hepatic and neurological sequela, respectively.
The complement deposition in the capillaries revealed by C4d and C5b-9 suggested a direct causal association between endotheliotropic viral infection and complement activation. In brief C4d is a byproduct of MBL activation that likely occurs due to MBL engagement with spike glycoprotein [18,19]. Interestingly, substantial deposits of C4d and C5b-9 within the dermal and subcutaneous vasculature were observed in the autopsy cases in grossly normal skin. This reinforces the potential use of deltoid skin biopsies in symptomatic COVID-19 as a means to identify patients in whom substantial viral capsid protein docking has occurred and, thus, may benefit from blood thinning or anti-cytokine therapy.
Prior studies utilizing RT-PCR based methodologies require tissue destruction, which prevents cellular microanatomic localization of the virus. These studies have found active replicating virus in the blood, brain, spleen, placenta, liver, large and small intestine, skeletal muscle, and lymph nodes [[20], [21], [22]]. To our knowledge, replicating virus has not been detected in skin samples [[23], [24], [25]]. What has been reported as viral inclusions have been found in the kidney endothelium, and apoptotic blebbing with microvesicles has been seen in the endothelium of other organ systems as well; active replication was not proven [26,27]. Virus-like particles have also been seen in the endothelium in the brain with concomitant detection of viral RNA from minced FFPE tissue, though localization to the endothelium could not document [20]. Given the widespread expression of ACE-2 receptors across many tissue types, it is possible that the aforementioned studies detected viral replication in non-vascular tissue [28]; this study did document rare macrophages in the heart/liver/kidney that contained infectious virus that could produce a positive result with RTPCR. It is postulated that the absence of viral RNA from the endothelium in these tissues represents a true lack of actively replicating virus. It has been shown that SARS-CoV-2 spike protein expression on non-replicating pseudovirions is sufficient for ACE-2 receptor mediated endocytosis [29], and indeed, prior studies from SARS-CoV have proven that the MBL pathway may be activated by both actively replicating virus as well as by pseudovirions [30].
The rarity of finding viral RNA using an ultrasensitive in situ method but detecting viral capsid proteins in the ACE-2 receptor-expressing endothelia of the skin and brain and, less so, in the liver, heart and kidney was unexpected. Data that supported the conclusion that the absence of viral RNA in the endothelia of these organs was not a false negative result included: 1) viral RNA detected in the heart, liver, and kidney in a few cells with the phenotype of macrophages but not in endothelia; 2) the in situ hybridization method used has a detection threshold of 1 viral genome/cell; 3) detection of viral capsid proteins in the context of infectious virions is invariably associated with high viral copy number, as we have demonstrated in the lung of people who died of COVID-19 [9] and 4) the methodology used in this paper did detect infectious SARS-CoV2 in the skin of people with perniosis [17]. Data that supports the conclusion that viral capsid proteins alone evident in the microvascular endothelia was not a false positive result included: 1) strong co-localization with the ACE-2 receptor; 2) strong co-localization with many cytokines associated with severe COVID-19; 3) strong co-localization with complement cascade proteins; 4) co-localization with caspase 3 that, like the other proteins, would not be present in endothelia without a direct stimulus; and 5) lack of expression of the TLRs that respond to viral RNA and not the proteins.
A critical result in this study was the strong co-expression of ACE2 with both infectious virus and pseudovirions. The ACE-2 is a zinc metalloprotease involved in homeostatic balance of the renin-angiotensin-aldosterone axis. ACE2 deactivates of angiotensin I and angiotensin II, ultimately producing Angiotensin [[1], [2], [3], [4], [5], [6], [7]], a seven-member peptide that opposes the actions of angiotensin I and II through receptor MasR Circulating Angiotensin [[1], [2], [3], [4], [5], [6], [7]] along with apelins, a second product of ACE-2, affects tissues including the endothelium throughout the body [28,[31], [32], [33], [34], [35], [36], [37]]. An important finding in this study that may explain in part some of the clinical manifestations of COVID-19 is that not all organs express ACE-2 in the microvascular bed with many organs showing no detectable staining in endothelia. ACE-2 receptor expression is highest in the microvasculature of the subcutaneous fat and brain though still evident, albeit in lower amounts in the liver, kidney, and heart.
The extent of pseudovirion localization within the microvasculature of the brain with its attendant sequelae being one of complement activation and endothelial cell could account for the encephalitis pattern seen clinically and radiographically in patient with COVID-19 [38]. The findings are reminiscent of the microvascular changes seen in Susac's syndrome where vessels of similar caliber demonstrate complement mediated microvascular injury resulting in encephalitis [39]. In one case report, a CSF analysis found elevated levels of IL-6, IL-8, IL-17A, IP-10, and a unique MCP-10 signature in Covid-19 encephalitis [40,41].
The exact mechanism by which the docked protein leads to this multicytokine expression pattern in endothelium is unclear but could be related to mannan-binding lectin activation best exemplified by enhanced IL-6 expression in endothelium that has been documented with mannan-binding lectin pathway activation [42]. Mannose-binding lectin (MBL) is a glycoprotein that belongs to the family of collect subfamily of the C type lectin. This pathway not only activates the complement pathway but also induces the production of many pro-inflammatory cytokines [43]. The MBL pathway was found to be critical in the viral response in patients during the 2004 SARS-CoV outbreak, wherein deficiency of MBL was associated with SARS, pointing to a front-line role for MBL in the betacoronavirus response [18]. However, MBL activity has also been associated with deleterious inflammatory responses in the case of Influenza H1N1, wherein high serum MBL activity was associated with increased mortality [44]. In one mouse model, binding of SARS-CoV-2 to MASP-2 led to complement over-activation and lung injury, and blockage of this interaction reduced disease severity [45].
A constant theme in severe COVID-19 is a procoagulant state leading to multiorgan thrombosis that is mechanistically different than the complement mediated microvascular endothelial cell injury that also occurs. The basis for the procoagulant milieu is multifactorial. Within the lung there is near-complete destruction of ACE2 positive endothelium through direct infection of the endothelium by SARS CoV-2 [[46], [47], [48], [49], [50], [51], [52], [53]]. In reducing the converting potential of ACE2, the virus disrupts homeostasis, and shifts toward the effects of angiotensin II [52]. ACE2 destruction brings about enhanced endothelial NADPH2 oxidase activity, increasing necrotic cell death and decreased endothelial nitric oxidase activity promoting vasoconstriction [53,54]. Vasoconstriction in a setting of cellular necrosis and endothelial disruption will increase thrombin generation, which acts as an independent C3 and C5 convertase, among other complement-activating changes [14]. Hypoxia induced during endothelial cell damage further compounds a hypercoagulable state through hypoxia inducible factor, down regulation of protein S, and activity of other pro-thrombotic pathways [53,[55], [56], [57]]. The high levels of IL6, as well TNF α, p38, IL8 and IL1 β contribute further to the thrombotic diathesis by virtue of its positive effects on inducing platelet aggregation [47]. Indeed, exposure to serum from severe Covid-19 patients can induce control platelet aggregation, pointing to a critical role in circulating factors in platelet aggregation and thrombosis [58].
Removing circulating pseudovirions may help protect against severe COVID-19. Lectin Affinity Plasmapheresis for the filtration of enveloped viral particles has been used to treat Ebola, and MERS [59,60]. This practice utilizes the natural affinity of viral glycoproteins (such as the SARS-CoV-2 spike protein) to lectin to hemo-adsorb the virus and filter the blood extra corporeally. In the setting of MERS there was reduced infectivity and reduced concentrations of circulating viral particles over time with apheresis. Both MERS-CoV (pseudovirus) and MARV soluble glycoproteins were effectively eliminated by lectin affinity plasmapheresis [60].
With the extensive complement activation and widespread endothelial destruction, inhibition of complement pathways may also represent a favorable therapeutic target [61,62]. Compassionate use of the C3 inhibitor AMY-101, as well as the anti-CD5 monoclonal antibody eculizumab have shown anecdotal success [[63], [64], [65]]. Mannan binding lectin has also been proposed as a therapeutic target given its gatekeeping role in complement activity and endothelial damage. A number of trials are now underway with inhibitors to complement and mannan binding lectin as novel therapeutic targets [66].
In conclusion, COVID-19 represents a viral infection with limited sites of infectious virions but deadly sequelae due to the effective manner in which pseudovirions in the context of released viral proteins activate synergistic microvascular pathways of tissue destruction throughout the body. Through the understanding of the pathophysiology of severe COVID-19, therapeutic trials utilizing Lectin Affinity plasmapheresis could add to the armamentarium of therapeutic options to treat this global pandemic.
CRediT authorship contribution statement
CMM (designed research studies, acquiring data, analyzing data, writing the manuscript).
GJN (designed research studies, conducted experiments, acquiring data, analyzing data, writing the manuscript).
JM, JK, SM (analyzing data, writing the manuscript).
DS, ANC, JL (acquiring data).
Declaration of competing interest
The authors have no competing interests to report.
Acknowledgements
The authors thank Dr. Margaret Nuovo for her excellent help with the photomicrographs and the website “Created with BioRender.com” for help with the graphic figure. We also thank Dr. Steven Salvatore with the procurement of some tissues.
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menachery V.D., Graham R.L., Baric R.S. Jumping species—a mechanism for coronavirus persistence and survival. Curr Opin Virol. 2017;23:1–7. doi: 10.1016/j.coviro.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed]
- 4.Guan W.-J., Ni Z.-Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020 doi: 10.1016/s2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietz W., Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity (Silver Spring) April 2020 doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 7.Simonnet A., Chetboun M., Poissy J. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020 doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heuveline P., Tzen M. Beyond deaths per capita: comparative CoViD-19 mortality indicators. medRxiv. 2020 doi: 10.1101/2020.04.29.20085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magro C., Mulvey J.J., Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. April 2020 doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020 doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruf W. Links between complement activation and thrombosis. Blood. 2019;134(Supplement_1):SCI–40-SCI-40. doi: 10.1182/blood-2019-121113. [DOI] [Google Scholar]
- 13.Subramaniam S., Jurk K., Hobohm L. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood. 2017;129(16):2291–2302. doi: 10.1182/blood-2016-11-749879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson H.-L., Gebhard F., Lambris J.D. Complement and coagulation systems molecular intercommunication between the. J Immunol Ref. 2010;185:5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peerschke E.I.B., Yin W., Grigg S.E., Ghebrehiwet B. Blood platelets activate the classical pathway of human complement. J Thromb Haemost. 2006 doi: 10.1111/j.1538-7836.2006.02065.x. [DOI] [PubMed] [Google Scholar]
- 16.Nuovo G.J. 2nd ed. Elsevier; San Diego CA: 2020. In situ molecular pathology and co-expression analyses. [Google Scholar]
- 17.Magro C., Mulvey J., Laurence J., Sanders S., Crowson N., Grossman M. Two polarizing cutaneous manifestations of COVID-19: perniosis and thrombotic retiform purpura and their correlation with cutaneous type 1 interferon inducible protein myxovirus resistance protein A expression. Brit J Dermatol. 2020 doi: 10.1111/bjd.19415. [DOI] [Google Scholar]
- 18.Ip W.K.E., Chan K.H., Law H.K.W. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005;191:1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerrigan A.M., Brown G.D. C-type lectins and phagocytosis. Immunobiology. 2009;214(7):562–575. doi: 10.1016/j.imbio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paniz-Mondolfi A., Bryce C., Grimes Z. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92(7):699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sekulic M., Harper H., Nezami B.G. Molecular detection of SARS-CoV-2 infection in FFPE samples and histopathologic findings in fatal SARS-CoV-2 cases. Am J Clin Pathol. 2020:1–11. doi: 10.1093/ajcp/aqaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vivanti A.J., Vauloup-Fellous C., Prevot S. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11(3572):1–7. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wollina U., Karadağ A.S., Rowland-Payne C., Chiriac A., Lotti T. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;e13549(May):1–6. doi: 10.1111/dth.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahouach B., Harant S., Ullmer A. Cutaneous lesions in a patient with COVID-19: are they related? Br J Dermatol. 2020;e31:183. doi: 10.1111/bjd.19168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marzano A.V., Cassano N., Genovese G., Moltrasio C., Vena G.A. Cutaneous manifestations in patients with COVID-19: a preliminary review of an emerging issue. Br J Dermatol. 2020:0–2. doi: 10.1111/bjd.19264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung F., Krüger-Genge A., Franke R.P., Hufert F., Küpper J.-H. COVID-19 and the endothelium. Clin Hemorheol Microcirc. 2020;75:1–5. doi: 10.3233/ch-209007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hikmet F., Mear L., Uhlen M., Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16(e9610):1–16. doi: 10.1101/2020.03.31.016048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shang J., Wan Y., Luo C. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117(21) doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y., Lu K., Pfefferle S. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J Virol. 2010;84(17):8753–8764. doi: 10.1128/jvi.00554-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penninger J., Imai Y., Kuba K. The discovery of ACE2 and its role in acute lung injury. Exp Physiol. 2008;93:543–548. doi: 10.1113/expphysiol.2007.040048. [doi:expphysiol.2007.040048 [pii]r10.1113/expphysiol.2007.040048] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srivastava P., Badhwar S., Chandran D.S., Jaryal A.K., Jyotsna V.P., Deepak K.K. Imbalance between angiotensin II - angiotensin (1-7)system is associated with vascular endothelial dysfunction and inflammation in type 2 diabetes with newly diagnosed hypertension. Diabetes Metab Syndr Clin Res Rev. 2019 doi: 10.1016/j.dsx.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 33.Li W., Moore M.J., Vasllieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003 doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imai Y., Kuba K., Rao S. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005 doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos R.A.S., Sampaio W.O., Alzamora A.C. The ACE2/angiotensin-(1-7)/mas axis of the renin-angiotensin system: focus on angiotensin-(1-7) Physiol Rev. 2018 doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iannelli A., Favre G., Frey S. Obesity and COVID-19: ACE 2, the missing tile. Obes Surg. 2020:6–8. doi: 10.1007/s11695-020-04734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kremer S., Lersy F., Anheim M. Neurologic and neuroimaging findings in COVID-19 patients: a retrospective multicenter study. Neurology. 2020 doi: 10.1212/WNL.0000000000010112. [DOI] [PubMed] [Google Scholar]
- 39.Magro C.M., Poe J.C., Lubow M., Susac J.O. Susac syndrome: an organ-specific autoimmune endotheliopathy syndrome associated with anti-endothelial cell antibodies. Am J Clin Pathol. 2011;136(6):903–912. doi: 10.1309/AJCPERI7LC4VNFYK. [DOI] [PubMed] [Google Scholar]
- 40.Farhadian S., Farhadian S., Glick L.R. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020;20(1):1–5. doi: 10.1186/s12883-020-01812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang H.J., Lee S.M., Lee H.H. Mannose-binding lectin without the aid of its associated serine proteases alters lipopolysaccharide-mediated cytokine/chemokine secretion from human endothelial cells. Immunology. 2007;122(3):335–342. doi: 10.1111/j.1365-2567.2007.02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han H., Ma Q., Li C. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Zayat S.R., Sibaii H., Mannaa F.A. Toll-like receptors activation, signaling, and targeting: an overview. Bull Natl Res Cent. 2019;43(1) doi: 10.1186/s42269-019-0227-2. [DOI] [Google Scholar]
- 44.Jani P.K., Kajdácsi E., Megyeri M. MASP-1 induces a unique cytokine pattern in endothelial cells: a novel link between complement system and neutrophil granulocytes. PLoS One. 2014;9(1):10–13. doi: 10.1371/journal.pone.0087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zogheib E., Nyga R., Cornu M. Prospective observational study on the association between serum mannose-binding lectin levels and severe outcome in critically ill patients with pandemic influenza type A (H1N1) infection. Lung. 2018;196(1):65–72. doi: 10.1007/s00408-017-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noris M., Benigni A., Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98:314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bester J., Pretorius E. Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep. 2016;6(June):1–10. doi: 10.1038/srep32188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlino M.V., Valenti N., Cesaro F. Predictors of intensive care unit admission in patients with coronavirus disease 2019 (COVID-19) Monaldi Arch chest Dis Mal del torace. 2020;90(1410):430–436. doi: 10.4081/monaldi.2020.1410. [DOI] [PubMed] [Google Scholar]
- 49.Vultaggio A., Vivarelli E., Virgili G. Prompt predicting of early clinical deterioration of moderate-to-severe COVID-19 patients: usefulness of a combined score using IL-6 in a preliminary study. J Allergy Clin Immunol Pract. 2020 doi: 10.1016/j.jaip.2020.06.013. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bourgonje A.R., Abdulle A.E., Timens W. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wysocki J., Ortiz-Melo D.I., Mattocks N.K. ACE2 deficiency increases NADPH-mediated oxidative stress in the kidney. Physiol Rep. 2014;2(3):1–12. doi: 10.1002/phy2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lovren F., Pan Y., Quan A. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol. 2008;295(4):1377–1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 55.Marchetti M. COVID-19-driven endothelial damage: complement, HIF-1, and ABL2 are potential pathways of damage and targets for cure. Ann Hematol. 2020;99(8):1701–1707. doi: 10.1007/s00277-020-04138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta N., Sahu A., Prabhakar A. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci U S A. 2017;114(18):4763–4768. doi: 10.1073/pnas.1620458114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pilli V.S., Datta A., Afreen S., Catalano D., Szabo G., Majumder R. Hypoxia downregulates protein S expression. Blood. 2018;132(4):452–455. doi: 10.1182/blood-2018-04-841585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L. Platelet activation and platelet-monocyte aggregates formation trigger tissue factor expression in severe COVID-19 patients. Blood. 2020 doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Büttner S., Koch B., Dolnik O. Extracorporeal virus elimination for the treatment of severe ebola virus disease - first experience with lectin affinity plasmapheresis. Blood Purif. 2014;38(3–4):286–291. doi: 10.1159/000375229. [DOI] [PubMed] [Google Scholar]
- 60.Koch B., Schult-Dietrich P., Büttner S. Lectin affinity plasmapheresis for Middle East respiratory syndrome-coronavirus and Marburg virus glycoprotein elimination. Blood Purif. 2018;46(2):126–133. doi: 10.1159/000487224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Risitano A.M., Mastellos D.C., Huber-Lang M. Complement as a target in COVID-19? Nat Rev Immunol. 2020;20(6):343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Polycarpou A., Howard M., Farrar C.A. Rationale for targeting complement in COVID-19. EMBO Mol Med. 2020 doi: 10.15252/emmm.202012642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diurno F., Numis F.G., Porta G. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24(7):4040–4047. doi: 10.26355/EURREV_202004_20875. [DOI] [PubMed] [Google Scholar]
- 64.Mastaglio S., Ruggeri A., Risitano A.M. The first case of COVID-19 treated with the complement C3 inhibitor AMY- 101. Clin Immunol. 2020;215:108450. doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giudice V., Pagliano P., Vatrella A. Combination of ruxolitinib and eculizumab for treatment of severe SARS-CoV-2-related acute respiratory distress syndrome: a controlled study. Front Pharmacol. 2020;11(June):1–6. doi: 10.3389/fphar.2020.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stahel P.F., Barnum S.R. vol. 11. 2020. Complement inhibition in coronavirus disease (COVID) -19: a neglected therapeutic option; pp. 1–4. (July) [DOI] [PMC free article] [PubMed] [Google Scholar]