Abstract
Despite scientific evidence proving that inhaled β2-adrenergic receptor (β2-AR) agonists can reverse bronchoconstriction in all ages, current guidelines advocate against the use of β2-AR bronchodilators in infants with viral bronchiolitis because clinical trials have not demonstrated an overall clinical benefit. However, there are many different types of viral bronchiolitis, with variations occurring at an individual and viral level. To discard a potentially helpful treatment from all children regardless of their clinical features may be unwarranted. Unfortunately, the clinical criteria to identify the infants that may benefit from bronchodilators from those who do not are not clear. Thus, we summarised the current understanding of the individual factors that may help clinicians determine the highest probability of response to β2-AR bronchodilators during viral bronchiolitis, based on the individual immunobiology, viral pathogen, host factors and clinical presentation.
Short abstract
There are several factors that may help clinicians determine the highest probability of response to β2-AR bronchodilators during viral bronchiolitis, based on the individual immunobiology, viral pathogen, host factors and clinical presentation https://bit.ly/30CoHcH
Introduction
Viral bronchiolitis is the most important cause of lower respiratory tract infection (LRTI) in children during the first 2 years of life and is the leading cause of hospitalisation among infants younger than 1 year [1–3]. Despite the high incidence of viral bronchiolitis, there is not yet a unified definition nor international agreement on diagnostic criteria of the disease: while in North America the presence of wheezing in infants aged up to 24 months is usually a criterion used for defining bronchiolitis, in the UK, the presence of inspiratory crackles in infants aged up to 12 months is the diagnostic criterion [4]. The latter is a major issue because it makes comparison of therapeutic studies difficult.
β2-Adrenergic receptor (β2-AR) agonists are essential for the management of many conditions causing lower airway obstruction in adults and children [5, 6]. β2-AR agonists prevent bronchial airway smooth muscle (ASM) constriction increasing the production of cyclic AMP, the primary mediator of relaxation in the ASM cell [5, 6]. Given that viruses and pro-asthmatic type 2 cytokines (e.g. interleukin (IL)-4/IL-13) directly elicit bronchial ASM constriction [7–14], β2-AR agonists are primarily used as a “rescue” bronchodilator therapy during virus or allergen induced exacerbations of asthma, and other causes of episodic wheezing (e.g. exercise-induced ASM bronchoconstriction). Clinically, there is no doubt that with proper use of inhaler devices, β2-AR agonists can deposit in the lower airways and can induce potent bronchodilation in all age groups, including newborns and infants [15–19].
Despite scientific evidence proving that the ASM is present and fully functional during early human life [20, 21], and that β2-AR agonists can reverse bronchoconstriction in newborns and young children [15–19], clinical trials (all of them with albuterol by nebuliser but not by metered dose inhaler- MDI with a spacer) have failed to demonstrate an overall clinical benefit of β2-AR agonists in infants with “viral bronchiolitis” [22]. As a result, viral bronchiolitis guidelines advocate against the use, or even a therapeutic trial, of bronchodilators [23, 24]. However, the presence of multiple viral bronchiolitis phenotypes [4] indicates that while β2-AR agonists may be ineffective in some cases, bronchodilators are potentially useful in in infants with a phenotype in which bronchial ASM hyperreactivity is a primary component. Unfortunately, there are no criteria to identify infants with viral bronchiolitis that may benefit from β2-AR agonists from those who do not. Thus, we aimed to perform a narrative review to summarise and analyse the current scientific evidence that may help to identify possible phenotypes or subgroups of responders to β2-AR agonist bronchodilators among infants with “viral bronchiolitis”.
Evidence-based medicine guidelines and the need for phenotype-specific treatments in viral bronchiolitis
For decades, the treatment of bronchiolitis has been mostly supportive, focussing only on observation, hydration and oxygen supplementation. Although prior evidence-based medicine clinical practice guidelines (CPGs), such as the 2006 American Academy of Pediatrics (AAP) bronchiolitis CPG, recommended the use of β2-AR agonist bronchodilators on a trial basis [25], the latest evidence-based CPGs on viral bronchiolitis no longer recommend a trial of bronchodilators [23, 24]. The most commonly argued reasons for these new recommendations are the greater strength of the evidence demonstrating no benefit in the viral bronchiolitis population as a whole, and that there is no “objective method” of determining response [23]. However, the implicit assumption of these evidence-based medicine guidelines is that the group of infants with “viral bronchiolitis” is homogeneous, which is no longer considered true [4]. Recent studies have identified multiple viral bronchiolitis phenotypes with high heterogeneity in clinical presentation and molecular pathobiology, indicating that we also need to consider heterogeneity in the response to different therapeutic options (phenotype-specific treatment strategies) [4]. Despite this novel understanding of the disease, it is currently unknown which patients are most likely to benefit from the available respiratory therapies (i.e. β2-AR agonist bronchodilators such as albuterol) [26]. Just as it is inappropriate to use β2-AR agonist indiscriminately in all patients with the diagnosis of “viral bronchiolitis”, we would contend that it is also inappropriate to not administer it to patients who could benefit from this medication. Indeed, inhaled β2-AR agonists are effective inducing bronchodilation in newborns and infants according to objective clinical and functional respiratory parameters [15–19]. Thus, the lack of response in prior clinical trials might be related to issues with the study design (e.g. definition of bronchiolitis, inappropriate outcomes, nebulised normal saline treatment rather than placebo effect [27], and not trials with albuterol by MDI) and the fact that some infants with viral respiratory illnesses may have bronchial ASM hyperactivity but others do not. Using inhaled β2-AR agonist based on specific patient characteristics or biomarkers, instead of a “one-size-fits-all” guideline-based approach [4] can lead to a more cost-effective treatment strategy centred on personalised and precision medicine, which may ultimately improve the outcomes of all subtypes of “viral bronchiolitis”.
β2-AR agonist responsiveness and virus-induced type 2 immune signatures
The most appropriate way to identify infants with viral bronchiolitis who may benefit from β2-AR agonists from those who do not, is to conduct randomised clinical trials using stratified randomisation based on the presence or absence of certain clinical characteristics or biomarkers that could be plausibly associated with a clinical response to β2-AR agonists. In the meanwhile, current practice requires that clinicians integrate scientific evidence with individual clinical and molecular signatures to select the patient profile with the greatest likelihood of response to β2-AR agonists. This profile could provide a scientific rationale for bronchodilator administration in a subset of patients with “viral bronchiolitis”, at least on a therapeutic trial basis [28].
Notably, there is a strong scientific rationale to link the bronchiolitis profile characterised by ASM hyperactivity and responsiveness to β2-AR agonists with the presence of T-helper cell type 2 (Th2 cell) responses, which are also defined as “type 2” to encompass other cells (e.g. innate lymphoid cells type 2 and the airway epithelium) [29]. Indeed, several studies have shown that Th2/type 2 cytokines such as IL-4/IL-13 can directly induce ASM alterations in calcium homeostasis [9–14], activation of the mitogen-activated protein kinases [9–14], changes in phosphodiesterase activity and cAMP metabolism [30–32], and activation/desensitization to β2-AR and G protein-coupled receptor signalling [30–32].
Collectively, these Th2/type 2-driven molecular changes create an ASM “pro-asthmatic” phenotype that is hyperresponsive to contractile agonists and viruses [9–14]. In view of the latter mechanisms, Th2/type 2 inflammation, a common feature of the atopic and asthmatic condition [33], is often considered a molecular signature of β2-AR agonist-responsive airway obstruction [34]. For example, Seumois et al. [34] found a significant association between bronchodilator reversibility post-albuterol and a Th2 transcriptional profile in adult asthmatics. The presence of bronchodilator reversibility has been associated with asthma in the paediatric population as well [15]. In addition, Debley et al. [17] identified that exhaled nitric oxide fraction, which is considered a biomarker of Th2/type 2 airway inflammation [35], predicts changes in lung function and risk of future wheezing in wheezy infants and toddlers.
In the context of viral bronchiolitis, it is important to mention that several studies have described that respiratory syncytial virus (RSV) and rhinovirus, the most common causes of bronchiolitis [36], are more likely to elicit Th2/type 2 airway responses and bronchial ASM hyperactivity in neonatal mice than in mature animals [35, 37]. Similarly, studies in human infants have reported that early-life rhinovirus infections are associated with robust Th2/type 2 airway responses [38, 39], and that RSV-infected infants with severe disease exhibit a Th2 polarisation in their respiratory secretions [40, 41].
Causative virus, viral bronchiolitis seasonality, and potential implications for β2-AR agonist responsiveness
Respiratory viruses can promote Th2/type 2 responses during early life [35, 37–41], but the degree in which this occurs seems to be modified by the virus type. Studies suggest that rhinovirus-infected infants display a different acquired immunological response compared to infants with RSV bronchiolitis, with a stronger predominance of Th2 polarisation (Th2/Th1 ratios) [42]. There are also reported differences in the airway transcriptomic profiles of infants with rhinovirus and RSV infections [43]. Using network analysis, Turi et al. [43] identified that type-2 and type-17 cytokines were central to the immune response to RSV, whereas growth factors and chemokines were central to the immune response to rhinovirus. Hasegawa et al. [44] demonstrated that infants with rhinovirus have higher levels of nuclear factor (NF)-κB signalling responses and type-2 cytokines compared to those with RSV infection.
In the same line, there is evidence showing that RSV and rhinovirus bronchiolitis are associated with significantly different nasopharyngeal metabolome, and bacterial metagenome [45, 46]. For instance, Stewart et al. [45] demonstrated that RSV and rhinovirus are associated with different metabolic pathways and that the associated bacterial functional capacity is derived primarily from Streptococcus pneumoniae in RSV bronchiolitis and from Haemophilus influenzae in rhinovirus bronchiolitis. In addition to these airway molecular differences, prior studies have also found that rhinovirus and RSV infections exhibit distinct clinical features in young children [47, 48]. In comparison to infants with RSV bronchiolitis, those infected with rhinovirus are more likely to be older, to have a prior history of eczema, to be treated with systemic corticosteroids [48]; have a significantly shorter length of stay [49]; and have an increased risk of subsequent development of childhood asthma [50, 51]. However, it is worth mentioning that respiratory viral testing could not be available for routine clinical use, especially for patients with mild to moderate bronchiolitis. This fact could limit the clinical utility of the knowledge of the causative virus as a potential predictor of response to β2-AR agonists.
These data indicate that young children with rhinvirus-bronchiolitis are more likely than their counterparts with RSV infection to have asthma-like characteristics (i.e. wheezing, atopic characteristics, Th2/type 2 signatures) [47, 48], which in turn may be associated with a greater component of ASM hyperreactivity and responsiveness to β2-AR agonists bronchodilators.
Closely related to the causative virus, another factor that modifies the interplay between early viral infection and the development of airway hyperreactivity is the season of presentation. Cangiano et al. [52] divided infants according to hospitalisation during the peak months or non-peak months (with RSV infections predominating during the peak months) and found significant differences in terms of risk factors for respiratory diseases. They found that infants hospitalised during peak months had a lower family history of asthma, more smoking mothers during pregnancy, were slightly more breastfed, had a lower number of blood eosinophils, and had higher clinical severity scores [52]. The authors hypothesised that infants hospitalised during the peak months of bronchiolitis epidemics and those hospitalised in non-peak months might reflect two different populations of infants [52]. The same group performed additional analyses aimed at testing the hypothesis that the balance of type 1/type 2 immune responses differs between these two populations of infants. They found that infants hospitalised during the non-peak months had a significantly higher percentage of CD4 T-cells producing IL-4, a slightly lower percentage of CD8 T-cells producing interferon γ, and a significantly higher Th2 polarisation than infants hospitalised during the peak months. In addition, other studies have estimated a population-based 25% increased risk of early childhood asthma following infant bronchiolitis occurring during rhinovirus-predominant months compared to asthma following infant bronchiolitis during RSV-predominant months [53]. Taking together, these studies suggest two different bronchiolitis phenotypes: previously healthy full-term infants hospitalised with RSV bronchiolitis during the peak months, and infants with a possible genetic predisposition to atopy, hospitalised during the non-peak months [53].
Host factors potentially linked to ASM hyperactivity and β2-AR agonist responsiveness during viral bronchiolitis
There are multiple viral bronchiolitis phenotypes characterised by distinct host factors [54], and likely different probability of ASM hyperactivity and response to β2-AR agonists. Dumas et al. [54] analysed data from two prospective, multi-centre cohorts of children younger than 2 years hospitalised with viral bronchiolitis to define individual profiles using latent class analysis based on clinical factors and viral aetiology. Among the four clinical profiles (phenotypes) identified, it is worth highlighting the “profile A”: patients characterised by history of wheezing and eczema, wheezing at presentation and rhinovirus infection. Children in this profile were also more often boys, older (>6 months), and had more often a parental history of asthma [54]. These results suggest that there is a subset of viral bronchiolitis characterised by early signs of asthma/atopy and older age that may have increased probability of response to β2-AR agonists. In support of this notion, a classical study that measured the respiratory resistance and the thoracic gas volume before and after nebulised salbutamol therapy in 32 wheezing children, using a modification of the forced oscillation technique and total body plethysmograph, found that while no child under 18 months of age showed a greater than 5% fall in resistance or any fall in thoracic gas volume, the majority of children over 20 months showed a fall in resistance greater than 20% [55]. Additionally, a previous meta-analyses of the efficacy of bronchodilator therapy in viral bronchiolitis showed modest short-term improvements in older infants (>12 months) [56, 57]. Unfortunately, more recent meta-analysis have failed to include age subgroup analyses to confirm these previous findings [22, 58]. However, when analysing the results of studies included in the National Institute for Health and Care Excellence (NICE) guidance on the diagnosis and management of bronchiolitis that recruited children 24 months or younger (as compared to those studies that included younger infants) [59], β2-AR agonists use was associated with significant improvements in outcomes such as accessory muscle score, oxygen saturation, respiratory rate [60], clinical respiratory scores [61, 62] and respiratory distress [63]. It is worth mentioning that two independent studies aimed at better understanding of predictors of prescription of albuterol in viral bronchiolitis identified age as an independent predictor, with the older patients being more likely to be prescribed with this β2-AR agonist therapy [64, 65]. Altogether, these results indicate that the older the patient with viral bronchiolitis, the higher the probability of obtaining clinical benefit with β2-AR agonists.
Atopy (e.g. eczema) is another host factor that may be associated with a higher probability of airway hyperactivity and β2-AR agonist responsiveness during viral bronchiolitis. Alansari et al. [66] reported that dexamethasone with salbutamol shortened the time for readiness for infirmary discharge during viral bronchiolitis episodes in patients with eczema or a family history of asthma in a first-degree relative. Additionally, eczema was identified as an independent predictor of inappropriate use of diagnostic test and management of bronchiolitis, defined as not following the recommendations given in the main CPGs on how to diagnose and manage patients with viral bronchiolitis. Notably, the lack of adherence to these recommendations was mainly due to the use of β2-AR agonists (in 89.4% of patients) [67]. There is also evidence showing that atopic dermatitis aggravates allergic airway inflammation in patients with acute viral bronchiolitis [68]. Notably, there is strong evidence suggesting that the interplay between early viral infection and the development of airway hyperreactivity and asthma is also influenced by the individual genetic background. Indeed, genome-wide association studies (GWAS) have shown that the ORMDL3 locus in chromosome 17q, the most highly replicated GWAS finding for asthma to date, seems to exert its effects by increasing susceptibility to rhivovirus wheezing illnesses in early life [69].
Bedside parameters and viral bronchiolitis respiratory phenotypes
The clinical presentation of viral bronchiolitis is heterogeneous and individual bedside parameters may also be used to assess airway hyperactivity and possible β2-AR agonist responsiveness. Specifically, while wheezing and sub-costal retractions most likely represent underlying bronchial ASM hyperactivity (airway obstruction and hyperinflation/diaphragm flattening, respectively) [70, 71], hypoxaemia is primarily a clinical manifestation of ventilation/perfusion mismatching and/or diffusion abnormalities due to lung parenchyma compromise, particularly in the absence of wheezing and sub-costal retractions [72]. This concept was recently investigated by Arroyo et al. [72] using a weighted score system to integrate cardinal bedside parameters of viral bronchiolitis (wheezing, sub-costal retractions, hypoxaemia). Notably, this weighted scored system predicted the risk of recurrence of virus-induced LRTI illnesses, suggesting that bedside clinical parameters may help to identify the presence of recurrent virus-induced bronchial ASM hyperreactivity [72]. In addition, wheezing and family history of asthma showed the best predictive model for recurrence after viral LRTI hospitalisation [72], and young children with recurrent viral-induced wheezing had higher nasal airway levels of type 2 cytokines (IL-4/IL-13) [73].
Concluding remarks
Despite the CPGs recommendation against the use of β2-AR agonist bronchodilators in infants with viral bronchiolitis, even on a trial basis [23–25], there is evidence that this has not had a major impact on physicians’ behaviour [74, 75]. The rates of bronchodilator use in viral bronchiolitis range from 18% to 90% with substantial differences between countries and even among hospitals in the same country [64, 67, 76]. At first glance these data may be interpreted simply as inappropriate compliance with the bronchiolitis CPGs. However, the real issue is more complex and likely reflects the lack of criteria to identify infants with viral bronchiolitis that may benefit from bronchodilators from those who do not.
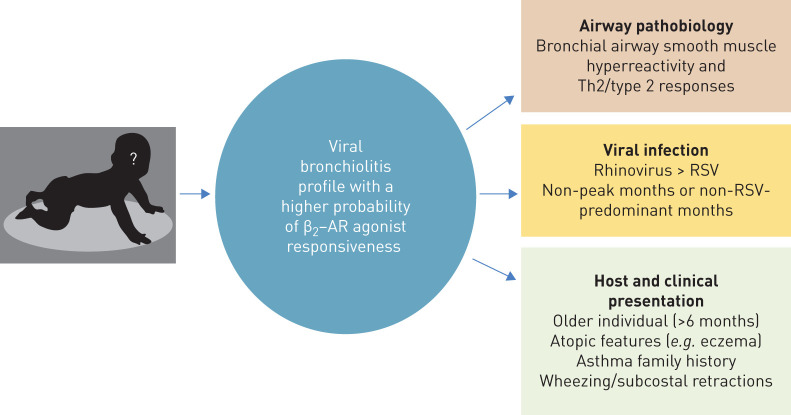
Accordingly, although acknowledging some speculation (due to the fact that responsiveness to β2-AR agonists does not automatically imply improvement in clinically important outcomes, and to the lack of current randomised data to fully support their routine usage), based on our review and analysis of the literature we propose the following features (figure 1) to identify the subset of infants with viral bronchiolitis that most likely will benefit from β2-AR agonist: 1) older infants (>6 months) with rhinovirus-bronchiolitis, 2) viral bronchiolitis occurring during non-peak months or during non- RSV-predominant months, 3) viral bronchiolitis presenting predominantly with wheezing/subcostal retractions, and 4) infants with viral bronchiolitis and atopic features (e.g. eczema) or a family history of asthma in a first-degree relative. Although at the moment this patient's profile could serve as a basis for rational administration of bronchodilators in patients with viral bronchiolitis, at least on a therapeutic trial basis, we believe that this β2-AR agonist-responsive profile, in combination with robust airway biomarkers, could be the starting point for future targeted randomised clinical trials to rationalise the use of bronchodilators and improve outcomes in all the subsets of infants with viral bronchiolitis.
FIGURE 1.
Viral bronchiolitis profile with a higher probability of β2-adrenergic receptor (β2-AR) agonist responsiveness. Th2: T-helper cell type 2; RSV: respiratory syncytial virus.
Footnotes
Conflict of interest: G. Nino has nothing to disclose.
Conflict of interest: C.E. Rodriguez-Martinez has nothing to disclose.
Conflict of interest: J.A. Castro-Rodriguez has nothing to disclose.
References
- 1.Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr 2003; 143: S127–S132. doi: 10.1067/S0022-3476(03)00510-9 [DOI] [PubMed] [Google Scholar]
- 2.Paramore LC, Ciuryla V, Ciesla G, et al. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. PharmacoEconomics 2004; 22: 275–284. doi: 10.2165/00019053-200422050-00001 [DOI] [PubMed] [Google Scholar]
- 3.Scheltema NM, Gentile A, Lucion F, et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health 2017; 5: e984–e991. doi: 10.1016/S2214-109X(17)30344-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez-Martínez CE, Castro-Rodriguez JA, Nino G, et al. The impact of viral bronchiolitis phenotyping: is it time to consider phenotype-specific responses to individualize pharmacological management? Paediatr Respir Rev 2020; 34: 53–58. doi: 10.1016/j.prrv.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nino G, Grunstein MM. Current concepts on the use of glucocorticosteroids and beta-2-adrenoreceptor agonists to treat childhood asthma. Curr Opin Pediatr 2010; 22: 290–295. doi: 10.1097/MOP.0b013e328337cb0c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wendell SG, Fan H, Zhang C. G protein-coupled receptors in asthma therapy: pharmacology and drug action. Pharmacol Rev 2020; 72: 1–49. doi: 10.1124/pr.118.016899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunstein MM, Hakonarson H, Hodinka RL, et al. Mechanism of cooperative effects of rhinovirus and atopic sensitization on airway responsiveness. Am J Physiol Lung Cell Mol Physiol 2001; 280: L229–L238. doi: 10.1152/ajplung.2001.280.2.L229 [DOI] [PubMed] [Google Scholar]
- 8.Hakonarson H, Maskeri N, Carter C, et al. Mechanism of rhinovirus-induced changes in airway smooth muscle responsiveness. J Clin Invest 1998; 102: 1732–1741. doi: 10.1172/JCI4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakonarson H, Whelan R, Leiter J, et al. T lymphocyte-mediated changes in airway smooth muscle responsiveness are attributed to induced autocrine release and actions of IL-5 and IL-1beta. J Allergy Clin Immunol 2002; 110: 624–633. doi: 10.1067/mai.2002.128529 [DOI] [PubMed] [Google Scholar]
- 10.Hu A, Fatma S, Cao J, et al. Th2 cytokine-induced upregulation of 11beta-hydroxysteroid dehydrogenase-1 facilitates glucocorticoid suppression of proasthmatic airway smooth muscle function. Am J Physiol Lung Cell Mol Physiol 2009; 296: L790–L803. doi: 10.1152/ajplung.90572.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Josephson MB, Jiao J, Xu S, et al. IL-13-induced changes in endogenous glucocorticoid metabolism in the lung regulate the proasthmatic response. Am J Physiol Lung Cell Mol Physiol 2012; 303: L382–L390. doi: 10.1152/ajplung.00125.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laporte JC, Moore PE, Baraldo S, et al. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med 2001; 164: 141–148. doi: 10.1164/ajrccm.164.1.2008060 [DOI] [PubMed] [Google Scholar]
- 13.Shore SA. Cytokine regulation of beta-adrenergic responses in airway smooth muscle. J Allergy Clin Immunol 2002; 110: S255–S260. doi: 10.1067/mai.2002.129947 [DOI] [PubMed] [Google Scholar]
- 14.Shore SA, Moore PE. Effects of cytokines on contractile and dilator responses of airway smooth muscle. Clin Exp Pharmacol Physiol 2002; 29: 859–866. doi: 10.1046/j.1440-1681.2002.03756.x [DOI] [PubMed] [Google Scholar]
- 15.Busi LE, Restuccia S, Tourres R, et al. Assessing bronchodilator response in preschool children using spirometry. Thorax 2017; 72: 367–372. doi: 10.1136/thoraxjnl-2015-207961 [DOI] [PubMed] [Google Scholar]
- 16.Castro-Rodriguez JA. Rodrigo GJ. β-agonists through metered-dose inhaler with valved holding chamber versus nebulizer for acute exacerbation of wheezing or asthma in children under 5 years of age: a systematic review with meta-analysis. J Pediatr 2004; 145: 172–177. doi: 10.1016/j.jpeds.2004.04.007 [DOI] [PubMed] [Google Scholar]
- 17.Debley JS, Stamey DC, Cochrane ES, et al. Exhaled nitric oxide, lung function, and exacerbations in wheezy infants and toddlers. J Allergy Clin Immunol 2010; 125: 1228–1234.e1213. doi: 10.1016/j.jaci.2010.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein AB, Castile RG, Davis SD, et al. Bronchodilator responsiveness in normal infants and young children. Am J Respir Crit Care Med 2001; 164: 447–454. doi: 10.1164/ajrccm.164.3.2005080 [DOI] [PubMed] [Google Scholar]
- 19.Yuksel B, Greenough A, Maconochie I. Effective bronchodilator treatment by a simple spacer device for wheezy premature infants. Arch Dis Child 1990; 65: 782–785. doi: 10.1136/adc.65.7.782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danopoulos S, Bhattacharya S, Mariani TJ, et al. Transcriptional characterisation of human lung cells identifies novel mesenchymal lineage markers. Eur Respir J 2020; 55: 1900746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roesler AM, Wicher SA, Ravix J, et al. Calcium sensing receptor in developing human airway smooth muscle. J Cell Physiol 2019; 234: 14187–14197. doi: 10.1002/jcp.28115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev 2014; 6: CD001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134: e1474–e1502. doi: 10.1542/peds.2014-2742 [DOI] [PubMed] [Google Scholar]
- 24.Ricci V, Delgado Nunes V, Murphy MS, et al. Bronchiolitis in children: summary of NICE guidance. BMJ 2015; 350: h2305. doi: 10.1136/bmj.h2305 [DOI] [PubMed] [Google Scholar]
- 25.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis Diagnosis and management of bronchiolitis. Pediatrics 2006; 118: 1774–1793. doi: 10.1542/peds.2006-2223 [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Martinez CE, Castro-Rodriguez JA. Bronchodilators should be considered for all patients with acute bronchiolitis, but closely monitored for objectively measured clinical benefits. Acta Paediatr 2015; 104: 858–860. doi: 10.1111/apa.13051 [DOI] [PubMed] [Google Scholar]
- 27.House SA, Gadomski AM, Ralston SL. Evaluating the placebo status of nebulized normal saline in patients with acute viral bronchiolitis: a systematic review and meta-analysis. JAMA Pediatr 2020; 174: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Martinez CE, Castro-Rodriguez JA. Children under 12 months could benefit from a therapeutic trial with bronchodilators if the clinical response is positive. Acta Paediatr 2015; 104: e540. doi: 10.1111/apa.13218 [DOI] [PubMed] [Google Scholar]
- 29.Lloyd CM, Snelgrove RJ. Type 2 immunity: expanding our view. Sci Immunol 2018; 3: eaat1604. doi: 10.1126/sciimmunol.aat1604 [DOI] [PubMed] [Google Scholar]
- 30.Hu A, Nino G, Grunstein JS, et al. Prolonged heterologous beta2-adrenoceptor desensitization promotes proasthmatic airway smooth muscle function via PKA/ERK1/2-mediated phosphodiesterase-4 induction. Am J Physiol Lung Cell Mol Physiol 2008; 294: L1055–L1067. doi: 10.1152/ajplung.00021.2008 [DOI] [PubMed] [Google Scholar]
- 31.Nino G, Hu A, Grunstein JS, et al. Mechanism regulating proasthmatic effects of prolonged homologous beta2-adrenergic receptor desensitization in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2009; 297: L746–L757. doi: 10.1152/ajplung.00079.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nino G, Hu A, Grunstein JS, et al. Mechanism of glucocorticoid protection of airway smooth muscle from proasthmatic effects of long-acting beta2-adrenoceptor agonist exposure. J Allergy Clin Immunol 2010; 125: 1020–1027. doi: 10.1016/j.jaci.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parulekar AD, Diamant Z, Hanania NA. Role of T2 inflammation biomarkers in severe asthma. Curr Opin Pulm Med 2016; 22: 59–68. doi: 10.1097/MCP.0000000000000231 [DOI] [PubMed] [Google Scholar]
- 34.Seumois G, Zapardiel-Gonzalo J, White B, et al. Transcriptional profiling of Th2 cells identifies pathogenic features associated with asthma. J Immunol 2016; 197: 655–664. doi: 10.4049/jimmunol.1600397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malinczak CA, Fonseca W, Rasky AJ, et al. Sex-associated TSLP-induced immune alterations following early-life RSV infection leads to enhanced allergic disease. Mucosal Immunol 2019; 12: 969–979. doi: 10.1038/s41385-019-0171-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Midulla F, Scagnolari C, Bonci E, et al. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child 2010; 95: 35–41. doi: 10.1136/adc.2008.153361 [DOI] [PubMed] [Google Scholar]
- 37.Han M, Rajput C, Hong JY, et al. The innate cytokines IL-25, IL-33, and TSLP cooperate in the induction of type 2 innate lymphoid cell expansion and mucous metaplasia in rhinovirus-infected immature mice. J Immunol 2017; 199: 1308–1318. doi: 10.4049/jimmunol.1700216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caballero MT, Hijano DR, Acosta PL, et al. Interleukin-13 associates with life-threatening rhinovirus infections in infants and young children. Pediatr Pulmonol 2018; 53: 787–795. doi: 10.1002/ppul.23998 [DOI] [PubMed] [Google Scholar]
- 39.Perez GF, Pancham K, Huseni S, et al. Rhinovirus infection in young children is associated with elevated airway TSLP levels. Eur Respir J 2014; 44: 1075–1078. doi: 10.1183/09031936.00049214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caballero MT, Serra ME, Acosta PL, et al. TLR4 genotype and environmental LPS mediate RSV bronchiolitis through Th2 polarization. J Clin Invest 2015; 125: 571–582. doi: 10.1172/JCI75183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vu LD, Siefker D, Jones TL, et al. Elevated levels of type 2 respiratory innate lymphoid cells in human infants with severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 2019; 200: 1414–1423. doi: 10.1164/rccm.201812-2366OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fedele G, Schiavoni I, Nenna R, et al. Analysis of the immune response in infants hospitalized with viral bronchiolitis shows different Th1/Th2 profiles associated with respiratory syncytial virus and human rhinovirus. Pediatr Allergy Immunol 2018; 29: 555–557. doi: 10.1111/pai.12919 [DOI] [PubMed] [Google Scholar]
- 43.Turi KN, Shankar J, Anderson LJ, et al. Infant viral respiratory infection nasal immune-response patterns and their association with subsequent childhood recurrent wheeze. Am J Respir Crit Care Med 2018; 198: 1064–1073. doi: 10.1164/rccm.201711-2348OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasegawa K, Perez-Losada M, Hoptay CE, et al. RSV vs. rhinovirus bronchiolitis: difference in nasal airway microRNA profiles and NFκB signaling. Pediatr Res 2018; 83: 606–614. doi: 10.1038/pr.2017.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart CJ, Hasegawa K, Wong MC, et al. Respiratory syncytial virus and rhinovirus bronchiolitis are associated with distinct metabolic pathways. J Infect Dis 2018; 217: 1160–1169. doi: 10.1093/infdis/jix680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Zhang X, Zhang N, et al. Airway microbiome, host immune response and recurrent wheezing in infants with severe respiratory syncytial virus bronchiolitis. Pediatri Allergy Immunol 2020; 31: 281–289. [DOI] [PubMed] [Google Scholar]
- 47.Korppi M, Kotaniemi-Syrjanen A, Waris M, et al. Rhinovirus-associated wheezing in infancy: comparison with respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J 2004; 23: 995–999. doi: 10.1097/01.inf.0000143642.72480.53 [DOI] [PubMed] [Google Scholar]
- 48.Mansbach JM, Clark S, Teach SJ, et al. Children hospitalized with rhinovirus bronchiolitis have asthma-like characteristics. J Pediatr 2016; 172: 202–204.e201. doi: 10.1016/j.jpeds.2016.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med 2012; 166: 700–706. doi: 10.1001/archpediatrics.2011.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 2008; 178: 667–672. doi: 10.1164/rccm.200802-309OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubner FJ, Jackson DJ, Evans MD, et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol 2017; 139: 501–507. doi: 10.1016/j.jaci.2016.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cangiano G, Nenna R, Frassanito A, et al. Bronchiolitis: analysis of 10 consecutive epidemic seasons. Pediatr Pulmonol 2016; 51: 1330–1335. doi: 10.1002/ppul.23476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nenna R, Frassanito A, Petrarca L, et al. RSV bronchiolitis in infants hospitalized during the epidemic peak and non-peak months: different Th1/Th2 response. Am J Respir Crit Care Med 2018; 197: A2865. [Google Scholar]
- 54.Dumas O, Mansbach JM, Jartti T, et al. A clustering approach to identify severe bronchiolitis profiles in children. Thorax 2016; 71: 712–718. doi: 10.1136/thoraxjnl-2016-208535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lenney W, Milner AD. At what age do bronchodilator drugs work? Arch Dis Child 1978; 53: 532–535. doi: 10.1136/adc.53.7.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flores G, Horwitz RI. Efficacy of beta2-agonists in bronchiolitis: a reappraisal and meta-analysis. Pediatrics 1997; 100: 233–239. doi: 10.1542/peds.100.2.233 [DOI] [PubMed] [Google Scholar]
- 57.Kellner JD, Ohlsson A, Gadomski AM, et al. Efficacy of bronchodilator therapy in bronchiolitis. A meta-analysis. Arch Pediatr Adolesc Med 1996; 150: 1166–1172. doi: 10.1001/archpedi.1996.02170360056009 [DOI] [PubMed] [Google Scholar]
- 58.Cai Z, Lin Y, Liang J. Efficacy of salbutamol in the treatment of infants with bronchiolitis: A meta-analysis of 13 studies. Medicine 2020; 99: e18657. doi: 10.1097/MD.0000000000018657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.National Institute for Health and Care Excellence. Bronchiolitis in children: diagnosis and management. NICE guideline NG9. 2015. Available from: www.nice.org.uk/guidance/ng9/. [PubMed]
- 60.Schuh S, Canny G, Reisman JJ, et al. Nebulized albuterol in acute bronchiolitis. J Pediatr 1990; 117: 633–637. doi: 10.1016/S0022-3476(05)80706-1 [DOI] [PubMed] [Google Scholar]
- 61.Can D, Inan G, Yendur G, et al. Salbutamol or mist in acute bronchiolitis. Acta Paediatr Jpn 1998; 40: 252–255. doi: 10.1111/j.1442-200X.1998.tb01922.x [DOI] [PubMed] [Google Scholar]
- 62.Klassen TP, Rowe PC, Sutcliffe T, et al. Randomized trial of salbutamol in acute bronchiolitis. J Pediatr 1991; 118: 807–811. doi: 10.1016/S0022-3476(05)80051-4 [DOI] [PubMed] [Google Scholar]
- 63.Khashabi J, Salari Lak S, Karamiyar M, et al. Comparison of the efficacy of nebulized l-epinephrine, salbutamol and normal saline in acute bronchiolitis: a randomized clinical trial. Med J Islam Repub Iran 2005; 19: 119–125. [Google Scholar]
- 64.Condella A, Mansbach JM, Hasegawa K, et al. Multicenter study of albuterol use among infants hospitalized with bronchiolitis. West J Emerg Med 2018; 19: 475–483. doi: 10.5811/westjem.2018.3.35837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piña-Hincapie SM, Sossa-Briceño MP, Rodriguez-Martinez CE. Predictors for the prescription of albuterol in infants hospitalized for viral bronchiolitis. Allergol Immunopathol 2020; 48: 469–474. doi: 10.1016/j.aller.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 66.Alansari K, Sakran M, Davidson BL, et al. Oral dexamethasone for bronchiolitis: a randomized trial. Pediatrics 2013; 132: e810–e816. doi: 10.1542/peds.2012-3746 [DOI] [PubMed] [Google Scholar]
- 67.Sarmiento L, Rojas-Soto GE, Rodriguez-Martinez CE. Predictors of inappropriate use of diagnostic tests and management of bronchiolitis. Biomed Res Int 2017; 2017: 9730696. doi: 10.1155/2017/9730696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marchand D, Tayara N, Choukroun ML, et al. La dermatite atopique aggrave l'inflammation allergique dans la bronchiolite aiguë virale [Atopic dermatitis aggravates the allergic airways inflammation in acute viral bronchiolitis]. Rev Mal Respir 2008; 25: 1087–1093. doi: 10.1016/S0761-8425(08)74978-7 [DOI] [PubMed] [Google Scholar]
- 69.Martinez FD. Childhood asthma inception and progression: role of microbial exposures, susceptibility to viruses and early allergic sensitization. Immunol Allergy Clin North Am 2019; 39: 141–150. doi: 10.1016/j.iac.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Florin TA, Ludwig S, Netter FH. Netter's pediatrics. Chapter 37: Wheezing and Bronchiolitis. Philadelphia, Elsevier Saunders, 2011. [Google Scholar]
- 71.Finder JD. Understanding airway disease in infants. Curr Probl Pediatr 1999; 29: 65–81. doi: 10.1016/S0045-9380(99)80040-1 [DOI] [PubMed] [Google Scholar]
- 72.Arroyo M, Salka KP, Perez GF, et al. Bedside clinical assessment predicts recurrence after hospitalization due to viral lower respiratory tract infection in young children. J Investig Med 2020; 68: 756–761. doi: 10.1136/jim-2019-001024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arroyo M, Salka K, Perez GF, et al. Phenotypical sub-setting of the first episode of severe viral respiratory infection based on clinical assessment and underlying airway disease: a pilot study. Front Pediatr 2020; 8: 121. doi: 10.3389/fped.2020.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carande EJ, Pollard AJ, Drysdale SB. Management of respiratory syncytial virus bronchiolitis: 2015 survey of members of the european society for paediatric infectious diseases. Can J Infect Dis Med Microbiol 2016; 2016: 9139537. doi: 10.1155/2016/9139537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carroll WD, Srinivas J. Bronchodilators in wheezy under 2-year-olds: when and which (if any)? Arch Dis Child Educ Pract Ed 2013; 98: 113–118. doi: 10.1136/archdischild-2012-303078 [DOI] [PubMed] [Google Scholar]
- 76.Elenius V, Bergroth E, Koponen P, et al. Marked variability observed in inpatient management of bronchiolitis in three Finnish hospitals. Acta Paediatr 2017; 106: 1512–1518. doi: 10.1111/apa.13931 [DOI] [PMC free article] [PubMed] [Google Scholar]